Abstract
Background/objectives
Regular exercise such as aerobic exercise has been shown to reduce the risk of some diseases such as cardiovascular disease (CVD). However, only few studies have investigated the impact of regular aerobic exercise on non-obese and overweight/obese persons. Therefore, this study was designed to compare the effect of a 12-week 10,000 steps a day walking intervention on the body composition, serum lipids, adipose tissue function, and obesity-associated cardiometabolic risk between normal weight and overweight/obese female college students.
Methods
Ten normal weight (NWCG) and 10 overweight/obese (AOG) individuals were recruited in this study. Both groups performed a regular 10,000 steps a day walk for 12 weeks. Their blood pressure, body mass index, waist-to-hip ratio, and blood lipid profiles were evaluated. Moreover, serum leptin and adiponectin levels were measured using an enzyme-linked immunosorbent assay.
Results
Our results revealed that triglyceride (TG), TG/high-density lipoprotein cholesterol (HDL-C) ratio and leptin were significantly reduced in the AOG group after the 12-week walking intervention. However, total cholesterol, HDL-C, and adiponectin/leptin ratio were significantly increased in the AOG group. There was little or no change in these variables in the NWCG group after the 12-week walking intervention.
Conclusions
Our study demonstrated that a 12-week walking intervention may help improve cardiorespiratory fitness and obesity-associated cardiometabolic risk by decrease resting heart rate, modulating blood lipid profiles, and inducing adipokine alterations in obese individuals. Therefore, our research encourages obese young adults to improve their physical health by participating in a 12-week walking program of 10,000 steps a day.
Keywords: Regular walking, Adipokine, Obesity-associated cardiometabolic risk, Obesity, Young adults
1. Introduction
A growing number of overweight and obese persons have been described globally.1 Obesity, especially in young adults, increases the risk of developing health problems, such as sleep apnea,2 type 2 diabetes mellitus,3 cancers,4 and particularly, cardiovascular diseases. Obesity increases the cardiovascular risk not only through unfavorably disturbing the circulating lipid profile5 but also by altering secretion patterns of adipokines.6 Characteristically, the serum lipid levels, such as total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) levels, are abnormally high in an obese state. Two important adipocytokines, leptin and adiponectin, which are produced excessively by the adipocytes, have opposite effects on obesity-associated cardiometabolic risk. The circulating adiponectin level is considered cardioprotective, while leptin may act as a risk factor in the pathophysiological link between increased adiposity and cardiometabolic alterations. Thus, the adiponectin/leptin (A/L) ratio constitutes a biomarker that may influence obesity-related inflammation and increased cardiovascular risk.7,8 Meanwhile, adiposopathy-related alterations of circulating adipokine patterns (low adiponectin and high leptin levels) contribute to a spectrum of obesity-associated cardiovascular diseases.9 A recent study demonstrated that the circulating adiponectin level has an inverse relationship with the LDL-C and TG levels, whereas it has a positive correlation with the HDL-C level.10 Because of the fundamental role of the circulating adipokine level in obesity and also the importance of dyslipidemia in the risk of developing obesity-associated cardiovascular diseases, the aim of the present study was to evaluate whether regular walking exercise decreases the cardiovascular risk through improving the adipokine level and plasma lipid profile of obese subjects.
Low physical activity is a crucial contributor to the youth obesity epidemic and increased morbidity in young adults in Taiwan.11 Among the Taiwanese youth, less than 30 percent of high school students and none of the college-aged students in Taiwan met the current physical activity guidelines of at least 60 min of moderate-to-vigorous physical activity per day.12,13 Regular aerobic exercises are suggested for obese young adults to improve their body composition, skeletal muscle mass, maximal oxygen consumption, and cardiovascular fitness.14,15 Aerobic exercise (such as running, walking, and cycling) is a repetitive, large muscle group exercise that is associated with an increase in the skeletal muscle capillary density, aerobic enzyme concentrations, and mitochondrial density.16, 17, 18 However, the requirements of effective aerobic exercise are usually strict and inflexible, thus setting a comparatively difficult goal for most sedentary obese young adults. Among the aerobic exercises, walking is the most popular physical activity that can be performed easily without the need for expensive equipment or specialist skills,19 and it provides a promising population-level strategy for increased physical activity.20,21 Growing evidence suggests that walking exercise leads to improved physical health in various populations. A systematic review suggested that patients with chronic diseases after a Nordic walking program had a significantly improved resting heart rate, blood pressure, exercise capacity, maximal oxygen consumption (VO2 max), and quality of life.22 Ruchat et al. (2012) demonstrated that a prenatal walking program with a low or vigorous intensity could increase the oxygen pulse, thus improving maternal cardiorespiratory responses.23 Moreover, Wong et al. (2003) showed that older adults with habitual walking had a lower body fat composition, better VO2 max, and stronger handgrip, and suggested that habitual walking was beneficial for preventing physical disability in older adults.24 Hence, regular walking can be an effective exercise and may serve as preventive medicine for the elderly. For younger persons, differences in exercise interventions and participant characteristics, such as their gender and body mass index (BMI), among the different studies may have contributed to the inconsistent results. The Black Women's Health Study followed 20,259 African American women aged <40 years and who were not obese at baseline. After 14 years of follow-up, vigorous exercise may have reduced the incidence of obesity among young African American women. Results for brisk walking were inconclusive.25
Several studies have associated adolescent or young-adulthood obesity with a high long-term mortality risk, regardless of race, sex, and obesity status in later life.26, 27, 28, 29 Based on the evidence of the positive effect of walking on physical health, Tudor-Locke et al. (2011) suggested that a walking exercise of 4000 to 18,000 steps per day is a reasonable daily goal for healthy adults.30 Over the last few years, 10,000 steps a day walk has become popularized as a key to physical health. The American College of Sports Medicine (ACSM) recommended a minimum of 2.5 h per week of moderate-to-vigorous-intensity physical activity for overweight and obese adults to improve their health.31 However, the intensity and duration requirements to achieve weight loss, and other health benefits may differ among different adult populations.32,33 Thus, it is necessary to evaluate whether a 12-week, 10,000 steps per day, walking exercise meets the current physical activity guidelines. In this study, the general hypothesis was that a 12-week, 10,000 steps per day, walking exercise would improve the body composition, serum lipids, adipose tissue function, and obesity-related cardiometabolic risk in overweight or obese female college students.
Males are more active and tend to participate in more sports than females,34 and the problem of physical inactivity appears to be more prevalent among females. A lack of motivation was reported by participants as one of the barriers to engaging in high-intensity exercises among female undergraduates.35 The causes of physical inactivity are multifactorial, including a lack of access to the necessary facilities, time, exercise partner, and confidence.36 Thus, the present study sought to investigate how a 12-week, 10,000 steps per day, walking exercise influenced the body composition, serum lipids, adipose tissue function, and obesity-associated cardiometabolic risk in young adult women from two weight groups, overweight and normal.
2. Methods
2.1. Study design and participants
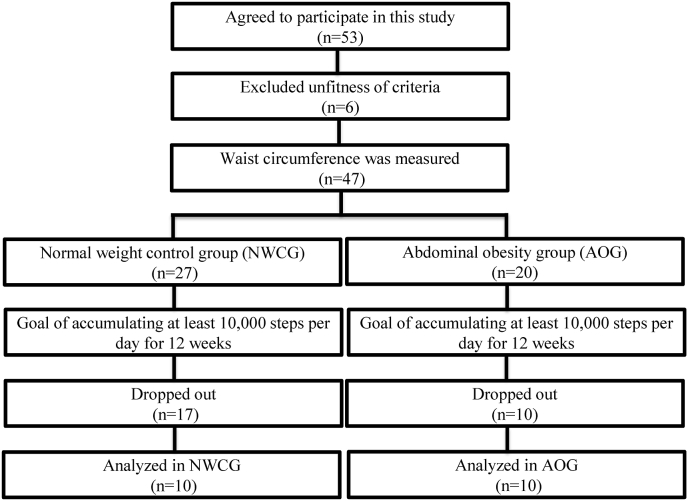
Fifty-three female college students (aged 18–25 years) were recruited for the study. Of them, 6 did not meet the study requirements, and the remaining 47 were further measured for the waist circumference before the exercise intervention. According to the WHO cut-off recommendation for Asian populations, Chinese women with a waist circumference ≥80 cm were considered to have abdominal obesity.37, 38, 39 Thus, the 47 participants were divided into an abdominal obesity group (AOG) comprising 20 participants (waist circumference ≥80 cm) and a normal weight control group (NWCG) comprising participants (waist circumference <80 cm) (Fig. 1). Twenty-seven participants withdrew from the study because of health reasons, unavailability, lack of perseverance, or other unknown excuses. Twenty participants (ten in each group) were eventually assessed (Fig. 1). Participants with cardiovascular disease, diabetes, liver dysfunction, renal impairment, an endocrine disorder, and a smoking habit were excluded to reduce the influence of other confounding factors. The baseline characteristics of the participants are shown in Table 1. The average number of daily steps was defined as the sum of the steps taken each day and calculated using smartphone-based records from the past 12 months. Each participant provided written informed consent after receiving full information about the study. The experimental protocol was approved by the Human Research Ethics Committee of Mackay Memorial Hospital (research number 12MMHIS119).
Fig. 1.
Flow chart of participant recruitment and completion of the 12-week 10,000 steps/day walking exercise program.
Table 1.
Baseline characteristics of the participants included in this study.
| Variable | NWCG (n = 10) | AOG (n = 10) | p |
|---|---|---|---|
| Age (yrs) | 19.87 ± 1.68 | 19.38 ± 2.22 | 0.835 |
| Height (cm) | 161.74 ± 4.18 | 161.07 ± 4.44 | 0.732 |
| Weight (kg) | 54.21 ± 4.44 | 60.28 ± 5.42 | 0.014 |
| BMI (kg/m2) | 20.69 ± 0.91 | 23.24 ± 1.94 | 0.003 |
| Body fat (%) | 26.22 ± 1.10 | 31.23 ± 4.42 | 0.006 |
| Resting SBP (mmHg) | 103.55 ± 7.91 | 109.45 ± 12.37 | 0.219 |
| Resting DBP (mmHg) | 62.00 ± 5.45 | 67.15 ± 6.16 | 0.031 |
| Resting Pluse (beat/min) | 73.10 ± 10.19 | 83.65 ± 7.06 | 0.008 |
| Waist Circumference (cm) | 74.60 ± 3.66 | 83.94 ± 3.13 | <0.001 |
| Hip Circumference (cm) | 93.12 ± 3.63 | 94.27 ± 14.43 | 0.812 |
| W/H Ratio | 0.80 ± 0.04 | 0.92 ± 0.23 | 0.134 |
| Average daily steps (steps) | 4192.60 ± 1483.84 | 3814.20 ± 1688.55 | 0.301 |
p determined using the independent t-test. Data were presented as the mean ± standard deviation. Abbreviations: NWCG=Normal Waist Circumference Group; AOG = Abdominal Obesity Group; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure.
2.2. Exercise intervention
Pre-intervention assessments were completed prior to participants engaging in any of the walking exercise interventions. The exercise protocol was performed as described in the literature, with slight modifications.40 Participants were encouraged to take at least 8000 steps per day for seven consecutive days and achieve 9000 steps per day for another seven consecutive days. Finally, participants were prescribed a physical activity of at least 10,000 steps per day for 12 successive weeks.
All participants were given a waist-mounted Digi-Walker FP2001 electronic pedometer (Agoss Health Business, Co., Ltd, Taiwan) and educated on its correct use. The Digi-Walker FP series pedometers were used to steadily record the data accurately. The participants were asked to wear the pedometer each day during waking hours and record their steps in their diary and to not change their normal activities during the 12-week intervention. Participants were asked to participate in six counseling interventions at 2, 4, 6, 8, 10 and 12 weeks, and researchers collected all activity data from participants throughout the study. Where appropriate and depending on the participant's condition, the observational data were validated.
2.3. Characteristics, body composition, and anthropometric measures
Demographic parameters (such as age, alcohol consumption, smoking, and family history of diseases), body composition (such as height, weight, BMI, body fat, waist, and hip circumference), and physiological data (such as systolic blood pressure and diastolic blood pressure) were recorded before and after the 12 weeks of walking.
Age, alcohol consumption, smoking, family history of diseases, and other health characteristics were obtained using a questionnaire. Body composition and physiological variables were measured according to the guidelines of the International Biological Program. The height was measured, without shoes, to the nearest 0.1 cm by a metric measuring tape. The body weight was measured, in light, indoor clothing, to the nearest 0.01 kg using a digital scale. The BMI was calculated by dividing the weight (kg) by the square of the height (m). The percent body fat was assessed using an eight-contact electrode system, the BC-418 Segmental Body Composition Analyzer (Tanita BC418, Tanita Corp, Tokyo, Japan). The waist circumference (WC) was measured in the standing position, at the midpoint between the anterior iliac crest and below the lowest rib margin, at the end of gentle respiration, by an inelastic flexible tape.41 The hip circumference (HC) was measured to the nearest 0.1 cm, at the end of gentle expiration, around the maximum circumference of the buttocks. The mean of two determinations was calculated for both WC and HC.
The resting pulse and the systolic and diastolic blood pressures after at least 5 min of rest were obtained twice from the right arm by placing the cuff of the automatic sphygmomanometer (HEM-7320, Omron, Kyoto, Japan) on the right brachial artery for a relative measurement. The second measurements of the resting pulse and the systolic and diastolic blood pressures were taken after a 2-min rest after the first measurements. The average of the two evaluations was used to determine the resting pulse and systolic and diastolic blood pressures. In addition, all participants were informed not to change their typical diet and daily physical activity during the study.
2.4. Blood sample analysis
Blood samples were taken to determine the biochemical parameters, blood lipid profile, and adipokine levels before and after the 12 weeks of walking. After an overnight fasting, venous blood samples (10 mL) were collected after a resting period of 10 min between 08:00 a.m. and 10:00 a.m. Blood samples were drawn from the antecubital vein in vacutainer tubes containing EDTA as an anticoagulant to obtain plasma or in dry tubes to obtain serum. The blood was allowed to coagulate for 60 min (10 min at room temperature and 50 min on ice). The plasma and serum were centrifuged at 3000 rpm, for 10 min at 4 °C and then stored at −80 °C until analysis. Serum biochemical parameters and the lipid profile, including the fasting blood glucose, hemoglobin A1c (HbA1c), total cholesterol (CHOL), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) levels, were measured using a Roche Cobas c501 auto analyzer with the original reagents (Roche Diagnostics, Germany). TG/HDL-C, LDL-C/HDL-C, and CHOL/HDL-C ratios were used to index the extent of cardiovascular disease (CVD) risk markers.42,43 Total adiponectin concentrations in the plasma were determined using the Quantikine® Human Total Adiponectin/Acrp30 Immunoassay kit (R&D Systems, Inc.; Minneapolis, MN, USA), following the manufacturer's protocol. The mean minimum detectable dose was 0.891 ng/mL. The levels of leptin in the plasma were detected by a commercially available enzyme-linked immunosorbent assay (ELISA; Leptin, R&D Systems, Inc.), following the manufacturer's protocol. The mean minimum detectable dose was 7.8 pg/mL.
2.5. Statistical analysis
All data were analyzed using SPSS/PC + Version 18.0 for Windows (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to evaluate the normality of the distributions of continuous random variables. Variables with a non-normal distribution were transformed before hypothesis testing. The independent-sample t-test was used to compare baseline variables between groups (AOG vs. NWCG). A two-way repeated analysis of variance (ANOVA) was conducted to assess the differences in each dependent variable and group before and after the exercise training course, followed by Bonferroni post hoc comparison analysis. An independent t-test was executed to investigate the differences between NWCG and AOG at baseline. p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Body composition and anthropometric measurements
A total of 20 participants, aged 18–25 years were recruited. Comparisons between NWCG and AOG body composition and physiological data are summarized in Table 1. Participants in the AOG initially had a higher body weight, BMI, body fat content, and WC than those of the NWCG (p < 0.05). No significant differences were observed in age, height, HC, and WC/HC (W/C) ratio between the two groups.
There was a slight reduction in weight, BMI, body fat, WC, and HC values in the AOG after the 12-week walking intervention; however, no significant reduction in weight, BMI, body fat content, WC, and HC were observed when the values were compared to the AOG baseline values (p > 0.05), as presented in Table 2. Conversely, there was a general increase in weight, BMI, and HC values in the NWCG after the 12-week intervention period, while there was a non-significant increase in weight, BMI, WC, and HC in the NWCG (p > 0.05, Table 2).
Table 2.
Change in body composition and anthropometric assessments baseline and after training (mean ± SD).
| Variable | NWCG (n = 10) |
AOG (n = 10) |
Time × group interaction |
|||
|---|---|---|---|---|---|---|
| Baseline | After training | Baseline | After training | F | p | |
| Weight (kg) | 52.92 ± 3.58 | 53.29 ± 3.53 | 60.28 ± 5.42∗ | 59.85 ± 6.73 | 1.045 | 0.302 |
| BMI (kg/m2) | 20.54 ± 0.81 | 20.61 ± 0.77 | 23.24 ± 1.94∗ | 23.01 ± 2.48 | 0.860 | 0.366 |
| Body fat (%) | 26.13 ± 0.91 | 25.79 ± 1.00 | 31.23 ± 4.42∗ | 30.58 ± 3.37 | 0.148 | 0.71 |
| Resting SBP (mmHg) | 104.85 ± 5.08 | 104.20 ± 7.67 | 109.45 ± 12.37 | 110.95 ± 9.01 | 0.614 | 0.44 |
| Resting DBP (mmHg) | 63.05 ± 3.88 | 61.15 ± 4.09 | 67.15 ± 6.16 | 67.10 ± 6.26 | 0.204 | 0.66 |
| Resting Pulse (beat/min) | 73.15 ± 10.30 | 73.45 ± 8.12 | 83.65 ± 7.05 | 75.70 ± 7.31# | 5.791 | 0.03 |
| Waist Circumference (cm) | 75.62 ± 2.24 | 74.28 ± 3.39 | 83.94 ± 3.13∗ | 82.86 ± 3.92 | 0.069 | 0.80 |
| Hip Circumference (cm) | 91.84 ± 3.51 | 92.49 ± 2.76 | 98.70 ± 3.66∗ | 98.50 ± 4.22 | 1.216 | 0.29 |
| W/H Ratio | 0.80 ± 0.04 | 0.79 ± 0.41 | 0.92 ± 0.23 | 0.90 ± 0.16 | 0.027 | 0.872 |
∗p < 0.05, compared with corresponding values in NWCG group; #p < 0.05, compared with baseline values within AOG group. Abbreviations: BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure. W/H Ratio = Waist Circumference/Hip Circumference ratio; NWCG = Normal waist circumference group; AOG = Abdominal Obesity group.
The resting pulse in the AOG was reduced from 83.65 ± 7.05 to 75.70 ± 7.31 beats/min after the 12-week walking intervention. A two-way repeated ANOVA test showed a significant difference in the effect of the interaction for the resting pulse (F = 5.791, p = 0.03). The post hoc test results revealed that participants in the AOG had a lower resting pulse after training than at baseline (p < 0.05); however, participants in the NWCG had no such phenomenon.
3.2. Lipid profiles
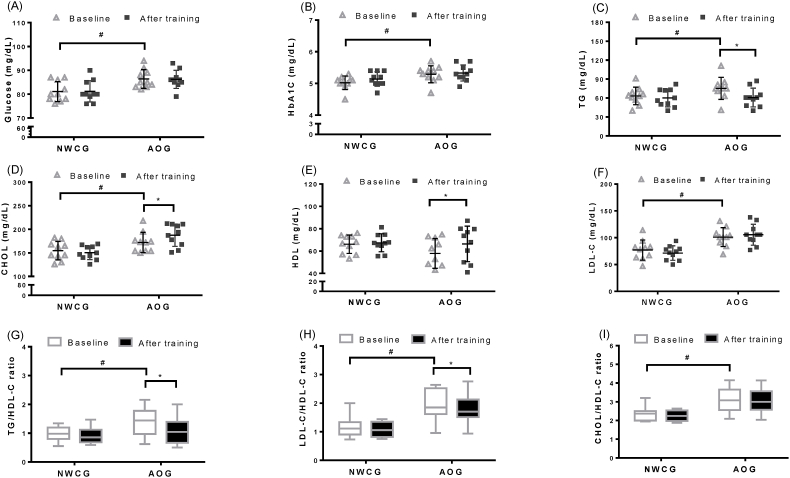
Fig. 2 shows the alterations in fasting blood glucose, blood lipid profile (CHOL, TG, LDL-C, and HDL-C), and CVD risk markers (TG/HDL-C, LDL-C/HDL-C, and TC/HDL-C) of participants in both groups before and after the 12-week walking intervention. In the AOG, the 12-week walking intervention was positively associated with an increase in CHOL (from 171.30 ± 21.40 to 187.44 ± 25.53, p < 0.05) and HDL-C (from 57.88 ± 13.12 to 66.38 ± 16.11, p < 0.05); however, a significant decrease in TG (from 75.290 ± 22.68 to 60.86 ± 17.14, p < 0.05) was observed. By contrast, a statistically significant difference was not observed in the NWCG before and after the 12-week walking intervention.
Fig. 2.
Plots illustrating alterations in individual fasting blood glucose and the blood lipid profile. Dot-plots indicate the variation of (A)plasma glucose, (B) hemoglobin A1c(HbA1c), (C) triglyceride (TG), (D) total cholesterol (CHOL), (E) High-density lipoprotein (HDL-C), and (F) low-density lipoprotein(LDL-C) at baseline and after 12-weeks walking intervention in NWCG and AOG g. Box-plots indicates relative (G) TG/HDL-C ratio, (H) LDL-C/HDL-C ratio, and (I) CHOL/HDL-C ratio at baseline and after 12-weeks of walking intervention in NWCG and AOG. The median serum lipid ratio and the interquartile ranges have been indicated in the plot. ∗p < 0.05 (significant difference baseline/after training in the same group); #p < 0.05 (significant difference baseline training between NWCG and AOG).
The mean values of the CVD risk markers TG/HDL-C and LDL-C/HDL-C were significantly reduced from 1.44 ± 0.61 to 1.04 ± 0.57 and 1.85 ± 0.54 to 1.70 ± 0.55, respectively, in the AOG (p < 0.05; Fig. 2G & H). However, no differences in the mean TG/HDL-C and LDL-C/HDL-C ratio were observed in the NWCG after the 12-week walking intervention when compared with baseline values, indicating that the CVD risk markers were not noted (p > 0.05).
3.3. Circulating adipokines
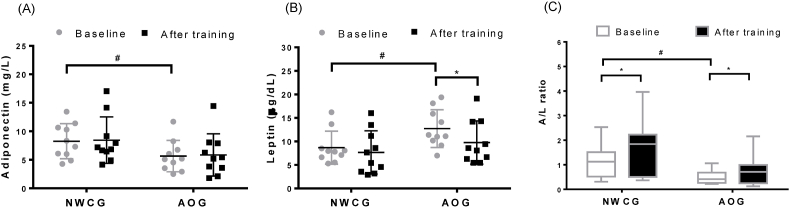
Fig. 3 shows the alterations in circulating levels of adiponectin, leptin, and A/L ratio in the NWCG and AOG before and after the 12-week walking intervention. The AOG exhibited significantly lower and higher circulating adiponectin and leptin levels, respectively, and a lower baseline A/L ratio than those of the NWCG (p < 0.05). However, after the 12-week walking intervention, the A/L ratio significantly increased (from 5.65 ± 1.86 to 5.86 ± 2.27; p < 0.05; Fig. 3C), with a decreasing circulating level of leptin in the AOG (from 12.74 ± 4.20 to 9.76 ± 4.74; p < 0.05; Fig. 3B). The NWCG participants experienced a significant increase in the A/L ratio after the 12-week walking intervention (p < 0.05; Fig. 3C).
Fig. 3.
Plots illustrating changes in individual circulatory adipokines levels. Dot-plots indicate serum concentration of (A) adiponectin and (B) leptin at baseline and after a 12-week walking intervention in NWCG and AOG groups. (C) Box-plot indicates relative adiponectin/leptin ratio (A/L ratio) according to serum concentrations of adiponectin and leptin at baseline and after a 12-week walking intervention in NWCG and AOG. The median A/L ratio and the interquartile ranges have been indicated in the plot. ∗p < 0.05; ∗∗p < 0.05 (significant difference baseline training between NWCG and AOG).
4. Discussion
Walking is an easy and popular form of physical activity; however, some barriers discourage obese adults from walking.44 The results of our study showed that no significant changes in anthropometric parameters (body weight, BMI, fat mass content, fat percentage, WC, HC, and W/C ratio) in the NWCG and AOG after the 12-week walking intervention. This is inconsistent with the results of previous studies, which showed that walking interventions improved the anthropometric parameters and blood lipid profiles.45 Brandon & Elliott-Lloyd (2006) found that sedentary and obese American women, excluding African Americans, experienced reductions in the body weight and fat content after a 16-week brisk walking intervention,46 which was another inconsistency in the reports of effects of walking interventions on weight loss across many studies. The ACSM recommends walking as a popular aerobic exercise and suggests a 40–59% heart rate reserve for 150 min per week at the beginning to a 60% heart rate reserve for 250–300 min per week at a vigorous intensity as an appropriate intensity to reduce obesity. However, whether the participants in our study achieved the optimal exercise intensity is still in doubt. The participants in our study were female college students who were predominantly overweight and obese. A lack of motivation was reported by the participants as one of the barriers to engaging in high-intensity exercises,47 which may explain the relatively little time spent in moderate-to-vigorous intensity during the intervention and the insignificant effect on anthropometric parameters.
The resting heart rate, the most direct response to cardiorespiratory fitness, is normally between 60 and 100 beats per minute. The resting heart rate can differ with an individual's fitness level, and people with better cardiorespiratory fitness tend to generally have a lower resting heart rate.48,49 In order to improve cardiorespiratory fitness, the 2007 ACSM/AHA guidelines recommend a 45%–55% heart rate reserve of 150 min per week or a 65%–75% heart rate reserve of 60 min per week as an appropriate intensity for sedentary adults. Anton et al. (2011) showed that clinically meaningful improvements in cardiorespiratory fitness were observed in participants who walked a minimum of 60 min per week at a fast-pace and not in those who walked at a leisurely-pace.50 The participants in our study were encouraged to achieve the goal of a minimum of 10,000 steps per day for 12 successive weeks. However, we did not request the participants to walk at a fast-pace or high intensity. Our results showed that participants in the AOG have a lower resting pulse after training, which was not observed in the NWCG. In agreement with the previous study, our results suggested that a 12-week leisurely-walk can be optimal to better improve the cardiorespiratory endurance level in obese persons than in persons with normal weight. Our study encourages obese adults to improve their cardiorespiratory endurance by participating in a regular program of unstressed walking exercise, while higher-intensity exercise training is needed for improving cardiorespiratory fitness for NWCG participants.
The results of our study showed higher baseline plasma glucose, HbA1c, TG, total cholesterol, and LDL-C levels in the AOG than in the NWCG and suggested that poor glycemic control could be a higher risk of diabetic complications in obese female college students.51 Obesity is well known to be associated not only with an abnormal lipid metabolism but also with poor cardiorespiratory fitness.52,53 Lipid parameters (TG/HDL-C, LDL-C/HDL-C, and CHOL/HDL-C ratios) have been linked to the risk of CVD.35,36 Urbina et al. (2013) found that a higher TG/HDL-C value was correlated with an increase in weight, heart rate, and risk of diabetic complications in adolescents and young adults, especially in obese youth.54 In addition, the study showed a relationship between the TG/HDL-C ratio and arterial stiffness in identifying young adults at risk for obesity-related atherosclerosis.20 A study that evaluated the effects of a 12-week modified lower-calorie diet and Nordic walking interventions on the anthropometric parameters and lipid profiles of overweight and obese retired miners found that the participants’ TC and LDL-C/HDL-C values were significantly reduced. However, large differences were observed between the baseline and week-12 levels of lipid parameters for the modified lower-calorie diet compared to those for walking interventions.45 Our study agrees with the literature by showing that the walking intervention was positively associated with an increase in HDL-C levels and negatively associated with a decrease in TG, TG/HDL-C, LDL-C/HDL-C values in the AOG, which was not observed in the NWCG, suggesting that the 12-week walking intervention was more effective in improving the lipid profiles and cardiorespiratory fitness of participants in the AOG.
Adipokines, such as leptin and adiponectin, are secreted almost exclusively by the adipose tissue.55,56 Obese individuals are characterized as having leptin resistance, with a high circulating level of leptin.57 Conversely, adiponectin has cardioprotective functions and protects against insulin resistance58,59; thus, adiponectin and the A/L ratio tend to be lower in obese than in lean persons. In agreement with the literature, our study showed the AOG had significantly higher and lower circulating leptin and adiponectin levels, respectively, and the AOG had a lower A/L ratio at baseline, when compared with the NWCG. However, after the 12-week walking intervention, all participants, regardless of obesity status, appeared to have a significantly increased A/L ratio. Obesity-associated alterations in adipokines play an important role in the development of dysfunctional adipose tissue and the pathogenesis of cardiometabolic complications.60,61 Our results showed that the 12-week walking intervention was beneficial in decreasing the cardiometabolic risk in both the AOG and NWCG.
5. Limitations
As with all work, this study was not without limitations, which should be acknowledged. First, the study was based on self-reporting by participants regarding their daily walking steps. There is evidence suggesting there is decreased compliance when subjects are asked to wear a pedometer or similar device for an extended period. The records may be inaccurate because of “decreased compliance” responses or recall difficulties. However, there is no alternative source of information available regarding participants’ records of daily walking steps. To enhance the reliability of self-reporting daily walking steps by participants, participants were asked to receive one counseling intervention every two weeks, for a total of six times. Researchers collected and validated all activity data from participants during that timeframe.
Second, this study elected to use the resting heart rate as a surrogate measure to assess the effect of the 12-week walking intervention on the cardiorespiratory fitness of obese young adults. During exercise, the heart rate responds by increasing, as does the delivery of oxygen to the body. Because of the dependent relationship between VO2 and resting heart rate, both are valuable tools to assess cardiovascular fitness. However, VO2 is the most accurate measure of cardiorespiratory fitness, and several meta-analyses have shown that exercise training improves VO2 max in overweight or obese adults.
Finally, our results are inconsistent with the results of previous studies, which showed that walking intervention improved the anthropometric parameters and blood lipid profiles in AOG and NWCG subjects. We speculate the inconsistency stemmed from an insufficient exercise intensity of participants. However, the lack of intensity information prohibits drawing conclusions about the cause and effect; therefore, we refer only to an association between lacking positive beliefs and an insufficient exercise intensity.
6. Conclusion
This research partly revealed that a 12-week walking intervention might help combat cardiorespiratory fitness and obesity-associated cardiometabolic risk in three possible ways. First, the intervention decreased the resting heart rate in obese persons and further improved inadequate cardiorespiratory endurance. Second, the intervention effectively modulated circulating lipids in obese persons and further improved the CVD risk. Third, the intervention induced adipokine alterations by raising the A/L ratio with a declining circulating level of leptin and further improved the cardiometabolic risk. However, the effect of the 12-week walking intervention on strengthening the cardiorespiratory health in the normal-weight population was not as pronounced as it was in obese individuals.
CRediT author statement
∗Yi Han Chiu: Writing - Original Draft preparation, Conceptualization, Visualization, Formal analysis.
∗Shiow-Chwen Tsai: Formal analysis, Investigation, Data curation.
∗Chen-Si Lin: Software, Resources, Data curation.
∗Li-Yu Wang: Funding acquisition, Conceptualization, Data curation.
∗Kuo-Chin Huang: Conceptualization, Methodology, Data curation, Project administration, Formal analysis, Writing- Reviewing and Editing.
Funding
This work was supported by National Science and Technology Council [MOST 107-2410-H-715-005]; and the MacKay Medical College, Taiwan [MMC-RD-109-1C-02, MMC-RD-111-1B-P007].
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are grateful to the participants for their valuable contribution to achieve the walking program.
Contributor Information
Yi Han Chiu, Email: chiuyiham@scu.edu.tw.
Shiow-Chwen Tsai, Email: sctsai6@gmail.com.
Chen-Si Lin, Email: cslin100@ntu.edu.tw.
Li-Yu Wang, Email: yannbo@mmc.edu.tw.
Kuo-Chin Huang, Email: kchsports@mmc.edu.tw.
References
- 1.Jebeile H., Kelly A.S., O'Malley G., Baur L.A. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022;10:351–365. doi: 10.1016/S2213-8587(22)00047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sever O., Kezirian E.J., Gillett E., Davidson Ward S.L., Khoo M., Perez I.A. Association between REM sleep and obstructive sleep apnea in obese and overweight adolescents. Sleep Breath. 2019;23:645–650. doi: 10.1007/s11325-018-1768-6. [DOI] [PubMed] [Google Scholar]
- 3.Amuta A.O., Barry A.E., McKyer E.L.J. Risk perceptions for developing type 2 diabetes among overweight and obese adolescents with and without a family history of type 2 diabetes. Am J Health Behav. 2015;39:786–793. doi: 10.5993/AJHB.39.6.6. [DOI] [PubMed] [Google Scholar]
- 4.Bendor C.D., Bardugo A., Pinhas-Hamiel O., Afek A., Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19:79. doi: 10.1186/s12933-020-01052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmaillzadeh A., Azadbakht L. Increased levels of inflammation among women with enlarged waist and elevated triglyceride concentrations. Ann Nutr Metab. 2010;57:77–84. doi: 10.1159/000318588. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento H., Silva L., Lourenço P., et al. Lipid profile in Portuguese obese children and adolescents: interaction of apolipoprotein E polymorphism with adiponectin levels. Arch Pediatr Adolesc Med. 2009;163:1030–1036. doi: 10.1001/archpediatrics.2009.190. [DOI] [PubMed] [Google Scholar]
- 7.Zhao S., Kusminski C.M., Scherer P.E. Adiponectin, leptin and cardiovascular disorders. Circ Res. 2021;128:136–149. doi: 10.1161/CIRCRESAHA.120.314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frühbeck G., Catalán V., Rodríguez A., et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11:454. doi: 10.3390/nu11020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Supriya R., Tam B.T., Yu A.P., et al. Adipokines demonstrate the interacting influence of central obesity with other cardiometabolic risk factors of metabolic syndrome in Hong Kong Chinese adults. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara M., Maruoka S., Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 11.Chang H.C., Yang H.C., Chang H.Y., et al. Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.K., Wu C.L. Results from the Chinese Taipei (Taiwan) 2022 report card on physical activity for children and youth. J Exerc Sci Fit. 2023;21:6–13. doi: 10.1016/j.jesf.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health and Welfare 1111 Walking for health and low carbohydrate: 40% of the population chooses walking as their daily exercise. MOHW web site. https://www.mohw.gov.tw/cp-3159-23931-1.html
- 14.Chiu C.H., Ko M.C., Wu L.S., et al. Benefits of different intensity of aerobic exercise in modulating body composition among obese young adults: a pilot randomized controlled trial. Health Qual Life Outcome. 2017;15:168. doi: 10.1186/s12955-017-0743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinckard K., Baskin K.K., Stanford K.I. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69. doi: 10.3389/fcvm.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philp A.M., Saner N.J., Lazarou M., Ganley I.G., Philp A. The influence of aerobic exercise on mitochondrial quality control in skeletal muscle. J Physiol. 2021:599. doi: 10.1113/JP279411. [DOI] [PubMed] [Google Scholar]
- 17.Meinild Lundby A.K., Jacobs R.A., Gehrig S., et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 2018;222 doi: 10.1111/apha.12905. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin M.H., Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with intervalsprint training versus aerobic endurance training. J Physiol Pharmacol. 2008;59:71–88. [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson S., Jones A. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br J Sports Med. 2015;49:710–715. doi: 10.1136/bjsports-2014-094157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravata D.M., Smith-Spangler C., Sundaram V., et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 21.Haskell W.L., Lee I.M., Pate R.R., et al. Physical activity and public health:updated recommendation for adults from the American college of sports medicine and the American heart association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 22.Tschentscher M., Niederseer D., Niebauer J. Health benefits of nordic walking: a systematic review. Am J Prev Med. 2013;44:76–84. doi: 10.1016/j.amepre.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Ruchat S.M., Davenport M.H., Giroux I., et al. Walking program of low or vigorous intensity during pregnancy confers an aerobic benefit. Int J Sports Med. 2012;33:661–666. doi: 10.1055/s-0032-1304635. [DOI] [PubMed] [Google Scholar]
- 24.Wong C.H., Wong S.F., PangW S., Azizah M.Y., Dass M.J. Habitual walking and its correlation to better physical function: implications for prevention of physical disability in older persons. J Gerontol A Biol Sci Med Sci. 2003;58:555–560. doi: 10.1093/gerona/58.6.m555. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg L., Kipping-Ruane K.L., Boggs D.A., Palmer J.R. Physical activity and the incidence of obesity in young African-American women. Am J Prev Med. 2013;45:262–268. doi: 10.1016/j.amepre.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S.Y., Wilkens L.R., Murphy S.P., Monroe K.R., Henderson B.E., Kolonel L.N. Body mass index and mortality in an ethnically diverse population:The multiethnic cohort study. Eur J Epidemiol. 2012;27:489–497. doi: 10.1007/s10654-012-9695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanDam R.M., Willett W.C., Manson J.A.E., Hu F.B. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145:91–97. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 28.Hirko K.A., Kantor E.D., Cohen S.S., Blot W.J., Stampfer M.J., Signorello L.B. Body mass index in young adulthood, obesity trajectory, and premature mortality. AJE (Am J Epidemiol) 2015;182:441–450. doi: 10.1093/aje/kwv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strand B.H., Kuh D., Shah I., Guralnik J., Hardy R. Childhood, adolescent and early adult body mass index in relation to adult mortality: results from the British 1946 birth cohort. J Epidemiol Community Health. 2012;66:225–232. doi: 10.1136/jech.2010.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tudor-Locke C., Craig C.L., Brown W.J., et al. How many steps/day are enough? for adults. Int J Behav Nutr Phys Activ. 2011;8:79. [Google Scholar]
- 31.Piercy K.L., Troiano R.P., Ballard R.M., et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stensvold D., Viken H., Steinshamn S.L., et al. Effect of exercise training for five years on all cause mortality in older adults-The Generation 100 study: randomised controlled trial. Br Med J. 2020;371:m3485. doi: 10.1136/bmj.m3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chodzko-Zajko W.J., Proctor D.N., Fiatarone Singh M.A., et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 34.Kee Y.H., Wang C.K.J., Chen M.H., Arjunan S.P. Physical inactivity and activity patterns among Taiwanese secondary students. Int J Sport Exerc Psychol. 2018;16:577–589. [Google Scholar]
- 35.Othman M.S., Ludin A.F.M., Chen L.L., et al. Motivations, barriers and exercise preferences among female undergraduates: a need assessment analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0264158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baillot A., Chenail S., Polita N.B., et al. Physical activity motives, barriers, and preferences in people with obesity: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Waist circumference and waist–hip ratio. WHO Expert. 2011;64:2–5. http://www.nature.com/doifinder/10.1038/ejcn.2009.139 [Google Scholar]
- 38.Xi B., Liang Y., He T., et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. 2012;13:287–296. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberti K.G.M.M., Zimmet P., Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 40.Schneider P.L., Bassett D.R., Thompson D.L., Pronk N.P., Bielak K.M. Effects of a 10,000 steps per day goal in overweight adults. Am J Health Promot. 2006;21:85–89. doi: 10.4278/0890-1171-21.2.85. [DOI] [PubMed] [Google Scholar]
- 41.Ma W.Y., Yang C.Y., Shih S.R., et al. Measurement of Waist Circumference: midabdominal or iliac crest? Diabetes Care. 2013;36:1660–1666. doi: 10.2337/dc12-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gharipour M., Sadeghi M., Nezafati P., Dianatkhah M., Sarrafzadegan N. Cardiovascular disease risk assessment: triglyceride/high-density lipoprotein versus metabolic syndrome criteria. Int J Health Sci. 2019;19 [PMC free article] [PubMed] [Google Scholar]
- 43.Girona J., Amigó N., Ibarretxe D., et al. HDL triglycerides: a new marker of metabolic and cardiovascular risk. Int J Mol Sci. 2019;20:3151. doi: 10.3390/ijms20133151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadir M.A., Kubacki K., Rundle-Thiele S. Perceived benefits and barriers of walking among overweight and obese adults. Health Market Q. 2019;36:54–70. doi: 10.1080/07359683.2019.1567004. [DOI] [PubMed] [Google Scholar]
- 45.Sadowska-Krȩpa E., Gdańska A., Rozpara M., Pilch W., Přidalová M., Bańkowski S. Effect of 12-week interventions involving nordic walking exercise and a modified diet on the anthropometric parameters and blood lipid profiles in overweight and obese ex-coal miners. Obes Facts. 2020;13:201–212. doi: 10.1159/000506403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandon L.J., Elliott-Lloyd M.B. Walking, body composition, and blood pressure dose-response in African American and White women. Ethn Dis. 2006;16:675–681. [PubMed] [Google Scholar]
- 47.Frimpong E., Dafkin C., Donaldson J., Millen A.M.E., Meiring R.M. The effect of home-based low-volume, high-intensity interval training on cardiorespiratory fitness, body composition and cardiometabolic health in women of normal body mass and those with overweight or obesity: protocol for a randomized controlled trial. BMC Sports Sci Med Rehabilitation. 2019;11:39. doi: 10.1186/s13102-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aladin A.I., Whelton S.P., Al-Mallah M.H., et al. Relation of resting heart rate to risk for all-cause mortality by gender after considering exercise capacity (the henry ford exercise testing project) Am J Cardiol. 2014;114:1701–1706. doi: 10.1016/j.amjcard.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 49.Dimopoulos S., Manetos C., Panagopoulou N., Karatzanos L., Nanas S. The prognostic role of heart rate recovery after exercise in health and disease. Austin J Cardio Dis Atherosclerosis. 2015;2:1014. [Google Scholar]
- 50.Anton S.D., Duncan G.E., Limacher M.C., Martin A.D., Perri M.G. How much walking is needed to improve cardiorespiratory fitness? An examination of the 2008 physical activity guidelines for americans. Res Q Exerc Sport. 2011;82:365–370. doi: 10.1080/02701367.2011.10599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Babic N., Valjevac A., Zaciragic A., Avdagic N., Zukic S., Hasic S. The triglyceride/HDL ratio and triglyceride glucose index as predictors of glycemic control in patients with diabetes mellitus type 2. Med Arch. 2019;73:163–168. doi: 10.5455/medarh.2019.73.163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drozdz D., Alvarez-Pitti J., Wójcik M., et al. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. 2021;13:4176. doi: 10.3390/nu13114176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamer M., O'Donovan G. Cardiorespiratory fitness and metabolic risk factors in obesity. Curr Opin Lipidol. 2010;21:1–7. doi: 10.1097/MOL.0b013e328331dd21. [DOI] [PubMed] [Google Scholar]
- 54.Urbina E.M., Khoury P.R., McCoy C.E., Dolan L.M., Daniels S.R., Kimball T.R. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013:131. doi: 10.1542/peds.2012-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 56.Kita S., Maeda N., Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129:4041–4049. doi: 10.1172/JCI129193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.KönnerAC BrüningJC. Selective insulin and leptin resistance in metabolic disorders. Cell Metabol. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Vu V., Sweeney G. Examining the potential of developing and implementing use of adiponectin-targeted therapeutics for metabolic and cardiovascular diseases. Front Endocrinol. 2019;10:842. doi: 10.3389/fendo.2019.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yadav A., Kataria M.A., Saini V., Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Luordi C., Maddaloni E., Bizzarri C., et al. Wrist circumference is a biomarker of adipose tissue dysfunction and cardiovascular risk in children with obesity. J Endocrinol Invest. 2020;43:101–107. doi: 10.1007/s40618-019-01086-7. [DOI] [PubMed] [Google Scholar]
- 61.Zhang P., Konja D., Wang Y. Adipose tissue secretory profile and cardiometabolic risk in obesity. Endoc Metab Sci. 2020;1 [Google Scholar]