ABSTRACT
The field of immuno-oncology has revolutionized cancer patient care and improved survival and quality of life for patients. Much of the focus in the field has been on exploiting the power of the adaptive immune response through therapeutic targeting of T cells. While these approaches have markedly advanced the field, some challenges remain, and the clinical benefit of T cell therapies does not extend to all patients or tumor indications. Alternative strategies, such as engaging the innate immune system, have become an intense area of focus in the field. In particular, the engagement of natural killer (NK) cells as potent effectors of the innate immune response has emerged as a promising modality in immunotherapy. Here, we review therapeutic approaches for selective engagement of NK cells for cancer therapy, with a particular focus on targeting the key activating receptors NK Group 2D (NKG2D) and cluster of differentiation 16A (CD16A).
KEYWORDS: ADCC, CD16A, MICA, MICB, monoclonal antibody, NK cell, NKG2D
Introduction
In contrast to T cells, natural killer (NK) cells act as a first line of defense and have the ability to detect and clear tumor or virally infected cells without any requirement for prior antigen-specific activation or differentiation. As such, NK cells possess several attractive properties justifying the development of agents that harness their therapeutic potential. Here, we review recent advances in the use of antibody-based therapies targeting the NKG2D/ligand axis to deliver a key activating signal that can synergize with CD16A signaling, allowing NK cells to mediate tumor cell lysis. The intricate balance between activating versus inhibitory signals, which is a hallmark of NK cells, calls for the engagement of multiple activating receptors, a combination of receptor agonists with inhibitors of NK cell checkpoints, and/or combination with T cell therapies, in order to maximize therapeutic efficacy in patients.
Like T cells, NK cells are increasingly recognized as having high cytotoxic potential when appropriately engaged. This is most evident when NK cells are transfected to express a chimeric antigen receptor (CAR) identical to those used to engineer CAR-T cells. When equipped with a strong activating receptor specific for a cell surface antigen, NK cell therapies have been shown to drive complete responses in patients.1 Likewise, a T cell receptor complex (TCR) in NK cells enhanced their effector function in a similar manner to T-cells.2,3
NK cell therapies have several advantages over T cell therapies. Most notable is their potential for allogenic, off-the-shelf use because they avoid graft-versus-host reactivity due to the lack of a TCR. In addition, NK cell therapies are typically associated with less severe toxicities, including immune effector cell-associated neurotoxicity syndrome (ICANs) and cytokine release syndrome (CRS), as compared to certain T cell-based therapies.4–6 However, there remain several key challenges for NK cell-based cell therapies, including inefficient transgene delivery, NK cell manufacturing complexities, exhaustion and limited persistence of genetically engineered NK cells.7
One of the most significant revolutions in cancer therapy is the development of monoclonal antibodies (mAbs) that target T cells rather than cancer cells to effectively treat, and in some cases, enable durable responses, in cancer patients. Unlike cell therapies, mAbs engaging NK cells, much like those targeting T cells, have the potential to reach every effector NK cell, rely on standardized mAb manufacturing methods, and enable predictable pharmacokinetic properties.
NK cell engagement has long been leveraged in the clinic by IgG1 mAb-based cancer therapies exerting antibody-dependent cellular cytotoxicity (ADCC) activity. ADCC is primarily mediated by an interaction between the CD16A/Fc-gamma receptor IIIA (FcγRIIIa) expressed on NK cells and the Fc gamma domain 1 (Fcγ1) of mAbs that recognize tumor-associated cell surface antigens on cancer cells. Many chimeric, human, and humanized IgG1 antibody therapeutics, such as rituximab (targeting CD20), daratumumab (CD38), and trastuzumab (human epidermal growth factor receptor 2- HER2), engage CD16A.8 However, these antibodies leave another key NK cell-activating receptor, namely NKG2D, untapped. NKG2D is a potent modulator of NK cell activation that has recently emerged as an important target in the immuno-oncology field.9,10 Strategies to therapeutically harness the activity of NKG2D are still in early development.
The biology of NK cells versus T cells in the context of cancer therapy
Among immune cells, NK cells are most closely related to cytotoxic T cells. The latter encompasses T cell subsets, including CD8+ and CD4+ T cells, gamma-delta T cells, and natural killer T (NKT) cells, all of which contain cytotoxic granules filled with cysteine proteases, called granzymes, and a pore-forming protein called perforin. NK cells, NKT cells and gamma-delta T cells belong to the innate immune system and serve as a first line of defense against pathogens, while CD8+ and CD4+ T cells are elements of the adaptive immune system, which are highly specific for pathogenic antigens, but first need to be primed, selected, and expanded in response to peptide antigen stimuli.11 Like T cells, NK cells are cytotoxic by virtue of having secretory granules filled with the same granzymes and a variant of perforin.12 Once delivered into a cytolytic synapse formed between NK cell and target cell, perforin forms a pore in the target cell membrane that enables transmembrane delivery of granzymes. Inside the target cell, granzyme B activates pro-caspases 3 and 7, eliciting programmed cell death, or apoptosis, while other granzymes such as granzyme A, H, K and M cleave numerous other protein substrates, causing target cell damage.13 Like T cells, NK cells also release inflammatory cytokines and chemokines upon activation.14
What is fundamentally different between cytotoxic T cells and NK cells is the mechanism by which they recognize and are activated by target cells. T cells recognize their target cells via their TCR, which has six distinct subunits and is among the most intricate receptor complexes in our body.15,16 The TCRs of CD8+ and CD4+ T cells utilize their variant alpha and beta subunits to recognize specific peptide/major histocompatibility complexes (MHC) displayed on target cells. A density as low as five copies of a specific peptide/MHC complex on a target cell is sufficient for a specific T cell clone to recognize target cells and induce their lysis.17 This relies on a powerful signal amplification downstream of the TCR. Some effector cells of the innate immune system, including gamma-delta T cells and NKT cells, instead utilize an invariant TCR to recognize metabolites presented by butyrophilins and lipids presented by CD1d, respectively.18,19 In contrast, NK cell activation does not depend on one “master” activating receptor equivalent to the TCR; instead, NK cell activity is regulated by the balance of activating and inhibitory signals transduced by several cell surface receptors.20 A comprehensive description of these receptors is found in several recent reviews.21–23 The interactions between tumor cells and T cells or NK cells that are the focus of this review are shown in Figure 1. Briefly, NK cell-activating and inhibitory receptors fall into two classes, human leukocyte antigen (HLA) and non-HLA specific. HLA-specific activating receptors include the activating killer Ig-like receptors (aKIR)s and Natural killer group 2C (NKG2C)/CD94 heterodimer. Activation of these receptors via ligand engagement triggers NK-mediated effector functions.24 Non-HLA-specific activating receptors include CD16A and NKG2D, as well the natural cytotoxicity receptors (NCRs), NKp30, NKp44 and NKp46. NCRs play a primary role in cytotoxicity of tumor cells and in regulating both innate and adaptive immune responses.25 Lastly, there are several activating co-receptors, such as DNAX accessory molecule-1 (DNAM-1), 2B4 and NKp80, that promote immune cell cross talk and tumor cell killing through amplification of signals coming from NCRs and NKG2D.26–28
Figure 1.
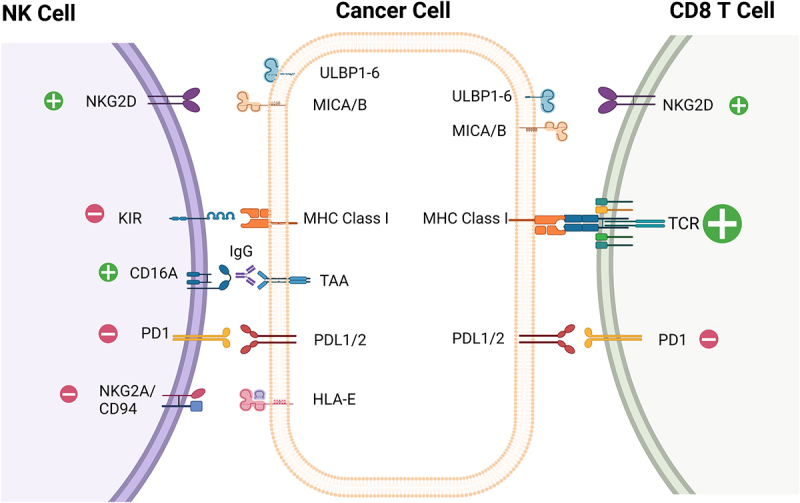
Select activating and inhibitory receptors on NK cells and CD8 T cells and their corresponding ligands.
NK cell activation is regulated by the balance of signals from activating and inhibitory receptors present on the cell surface, whereas T cell signalling is driven primarily through a master activating receptor, the TCR, in addition to multiple co-stimulatory and co-inhibitory receptors. Select activating receptors (+) are presented as green positive signal; select inhibitory receptors (-) are depicted by a red negative signal.
HLA-specific inhibitory receptors present on NK cells are important for negatively regulating cellular activity upon engagement with HLA molecules, including the inhibitory killer Ig-like receptors (iKIRs)/CD158 family and the CD94/Natural killer group 2A (NKG2A) (CD94/CD159a) heterodimer.29 Several clinical trials are ongoing with mAbs designed to reinvigorate NK cells by blocking inhibitory ligands on tumor cells or the inhibitory receptor, including anti-NKG2A monalizumab and anti-killer cell immunoglobulin like receptor, three Ig domains and long cytoplasmic tail 2 (KIR3DL2) lacutamab, which are under development by Innate Pharma (NCT05321147, NCT04984837).30–32 Antibodies targeting killer cell immunoglobulin like receptor, three Ig domains and long cytoplasmic tail 1–3 (KIR2DL1-L3) have shown limited success in the clinic. Lirilumab, a mAb that targets multiple iKIRs, has failed to meet its clinical endpoints.33,34
In addition to the HLA-specific inhibitory receptors, non-HLA inhibitory receptors also provide important regulatory control for NK cells. This includes the immune checkpoint molecules programmed cell death 1 receptor (PD-1), T cell immunoreceptor with Ig and ITIM domains (TIGIT), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), cluster of differentiation 96 (CD96), sialic acid binding Ig like lectin 7 (siglec-7), leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1), and inhibitor receptor protein (Irp60).29,35–39 PD-1, considered the prototypic immune checkpoint molecule, negatively regulates T cell function upon interaction with programmed death-ligand 1 and 2 (PD-L1 and PD-L2), on cancer cells and on immune cells in the tumor microenvironment (TME) and this axis has been targeted with great success in the clinic with antagonist mAb therapies.40,41 Tumor-experienced NK cells have also been shown to express PD-1 and PD-L1 in both preclinical and clinical settings.42–49 Preclinical data have demonstrated the importance of this pathway in suppressing NK cell activity and may contribute to the activity of anti-PD-1/PD-L1 mAb therapies in the clinic.42,48
In summary, NK cell activity is kept in a delicate balance and tightly controlled by a network of multiple regulatory proteins. Given the inhibitory milieu for NK cell signals in the TME, it is attractive to engage NK cell-activating receptors, such as CD16A and NKG2D, and an inhibitory receptor, such as PD-1, to unleash tumor cell lysis by NK cells.
Leveraging the CD16A/Fc-gamma receptor IIIA to engage NK cells
Fc gamma receptors (FcγRs) are a family of proteins expressed on the surface of immune cells which bind the Fc portion of antibodies to mediate immune effector functions.50 All FcγRs are activating, with the exception of FcγRIIB (CD32B), which is the only known inhibitory receptor and is the predominant FcγR present on B cells where it serves as a critical mediator of B-cell homeostasis.51 Activating receptors include the high-affinity FcγRI (CD64), a receptor primarily responsible for mediating antibody-dependent cellular phagocytosis (ADCP), the highly abundant FcγRIIA (CD32A), a receptor that facilitates phagocytosis and endocytosis, and FcγRIIIb (CD16B), a receptor present on granulocytes that mediates opsonization of microbes.52,53 Importantly, all of these receptors are notably absent in NK cells.
The most well-characterized and predominant activating receptor on NK cells is FcγRIIIA, or CD16A, which binds to the Fcγ domain of various IgG antibody isotypes. Importantly, CD16A is expressed only on mature NK cells, which is the CD56dim subset present primarily in the periphery, whereas the majority of NK cells present in tissues are the less mature, CD56bright population and express low levels of CD16A.20,54,55 NK cells are naturally coated with serum IgG bound to their CD16A receptors via a low-affinity interaction.56 CD16A, transmembrane receptor with two extracellular Ig-like domains and a short cytoplasmic tail, lacks an intrinsic signaling domain and thus requires immunoreceptor tyrosine-based activation motif (ITAM)-bearing signaling proteins, CD3 zeta (CD3ζ) and/or Fc epsilon RI (FcϵRiγ), to transduce signals upon binding to IgG. Upon CD16A engagement, recruitment and phosphorylation of other kinases result in calcium influx and degranulation. Calcium influx is one of the major signals for triggering degranulation and ADCC and enables nuclear factor of activated T-cells (NFAT) translocation into the nucleus to induce the transcriptional program to drive an inflammatory response.57
MAbs for cancer therapy are designed to simultaneously bind a cell surface antigen on cancer cells and CD16A on NK cells. These mAbs function like an NK cell-engaging bispecific antibody by bridging NK cells with cancer target cells for redirected lysis via ADCC. It is the human IgG1 isotype (and murine IgG2a/c ortholog) that most effectively mediates ADCC, as well as complement-dependent cytotoxicity (CDC) and ADCP.58 Support for a role of CD16A in the anti-tumor activity of hIgG1 mAb therapies comes from a polymorphism of CD16A that reduces its affinity for the Fc gamma domain of mAbs.59 The lower affinity isoform of CD16A is associated with decreased response rates for rituximab in non-Hodgkin lymphoma.60 Additional evidence for the role of CD16A in NK cell-mediated ADCC comes from modifications of the Fcγ1 domain of mAbs that can enhance their affinity for FcγRs resulting in more potent target cell lysis.61 These modifications include specific mutations in the Fcγ1 domain or prevention of fucosylation in the N-linked carbohydrate moiety of hIgG1. Amino acid (AA) substitutions in the Fcγ1 domain of IgG1, for example S239D/I332E and S298A/Q333A/K334A, have been shown to increase the affinity of binding to CD16A and enhance ADCC, and have been covered in detail in recent reviews.61–66 Prevention of fucosylation in the N-linked carbohydrate moiety of hIgG1 is another way to augment Fc-effector functions, as the presence of fucose has been shown to sterically hinder optimal interaction between IgG1 and CD16A.67 Afucosylation can be achieved by genetic modification of the host biosynthesis pathways, post-translational enzymatic modification or through the use of small molecule inhibitors.68–71 The most robust methods of complete afucosylation include deletions of either the fucosyltransferse (FUT8) enzyme or the enzyme responsible for generating a key intermediate of fucose, GDP mannose 4,6-dehydratase (GMD), in mammalian host systems.70,71
Approved Fc-enhanced antibodies include the Fcγ1-modified antibody margetuximab (targeting HER-2) and the afucosylated antibody mogamulizumab (targeting C-C chemokine receptor type 4-CCR4).72,73 In addition to introducing antibodies to enhance Fc functionality, other anti-tumor agents, such as lenalidomide, a degrader of the multifunctional protease cereblon have been reported to enhance NK cell activity by lowering the threshold required for NK cell activation by CD16A and other receptors.74,75 The combination of lenalidomide and the anti-CD38 IgG1 mAb, daratumumab, is now standard-of-care treatment for multiple myeloma (MM) patients.76
Activating CD16A through an anti-CD16A-specific antibody rather than modification of the Fcγ domain binding is an alternate approach to activating NK cells. This approach has the advantage of preventing co-engagement of CD16B, the competitive and highly abundant glycosylphosphatidylinositol (GPI)-anchored FcγR expressed on granulocytes, which has been shown to act as a decoy receptor on neutrophils, reducing ADCC activity.50,77 Furthermore, engaging CD16A with an engineered binder reduces competitive binding of excess native serum IgG1 antibodies to CD16A. Affimed’s AFM13, a CD30/CD16A-bispecific tandem diabody (Tandab) that exemplifies this approach, has shown encouraging clinical activity in Hodgkin lymphoma (HL) patients when administered in combination with the anti-PD-1 mAb pembrolizumab.78,79 Intriguingly, a cell therapy generated by coating cord blood-derived NK cells with the Tandab has shown a very high rate of complete responses in HL patients.80 RO7297089, a bispecific antibody from Genentech that targets both B-cell maturation antigen (BCMA) and a unique binding site on CD16A resulting in increased tumor cell killing compared to Fc-enhanced tumor-targeting antibodies, was tested in a phase 1 study but does not appear to be under further development.81,82 GT-Biopharma has generated both bi- and tri-specific NK cell engagers (NKCEs) using camelid nanobody technology. These molecules contain a camelid nanobody that binds CD16A, a single-chain variable fragment (scFv) that recognizes a tumor antigen and a human wild-type interleukin 15 molecule to enhance NK cell activity. GTB-3560 targeting CD33 is currently in IND-enabling development (NCT03214666).83,84
Since NK cell activation is governed by multiple receptors, efforts are underway to simultaneously engage other activating receptors beyond CD16A, such as NKp30 and NKp46, as reviewed by Phung et al and Demaria et al.85,86 Compass Therapeutics generated CTX-8573, a bispecific engager which contains an anti-NKp30 fragment antigen-binding (Fab) fragment linked to the C-terminus of an anti-BCMA IgG1 antibody containing an afucosylated Fc gamma domain for enhanced CD16A engagement, but development has been terminated.87 Innate Pharma aims to target activating receptors NKp46, CD16A, and tumor-associated antigens via their Antibody-based NK cell Engager Therapeutics (ANKET) platform, which consists of both trivalent and tetravalent NKCEs. Trivalent engagers include IPH6401, a BCMA-targeting ANKET, and IPH6101, which targets CD123 and is currently in Phase 1 clinical trials in hematological malignancies. The tetravalent ANKET IPH6501, which targets CD20 and contains an interleukin-2 variant (IL-2 v), is currently in preclinical development (NCT05086315).88,89
Role of the NKG2D/ligand axis in immune surveillance
Another key activating receptor is NKG2D, which is not only expressed on NK cells, but also on certain T cell populations, including subpopulations of CD8+, NKT and gamma/delta T cells.90,91 NKG2D recognizes eight distinct ligands that are upregulated on tumor cells: MHC class I chain-related protein A (MICA), MHC class I chain-related protein sequence B (MICB), and UL-16- binding proteins (ULBPs) named ULBP1 through ULBP6.91,92
Upon engagement with one or more of these ligands, NKG2D triggers a downstream signaling cascade in effector cells leading to the formation of a cytolytic synapse. NKG2D is expressed as a homodimer and lacks its own signaling domain. It therefore relies on binding to the adaptor protein DNAX-activating protein 10 (DAP10), which recruits phosphoinositide 3-kinase(PI3K) and growth factor receptor bound 2 protein (Grb2).93 Activation of these signaling proteins triggers an increase in intracellular calcium concentration, actin cytoskeleton rearrangement, and activation of transcription factors, including NFAT and nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB). In NK cells, these signals culminate in cytotoxicity by release of granzymes, perforin and cytokines. Direct target cell killing by NKG2D ligation on NK cells has been demonstrated using NKG2D ligands, such as MICA or ULPB2, fused to a mAb that recognizes a tumor-associated antigen.94–96 In the case of T cell populations that express NKG2D, the axis may serve a costimulatory role in lowering the threshold for lysis while the primary recognition of target cells is via the TCR.97
NKG2D ligation serves an important role in clearing cells with stress-induced damage, including those undergoing nascent carcinogenesis. NKG2D ligands are not expressed on healthy cells, but appear on the cell surface when cells are exposed to a variety of stresses, including metabolic stress, genotoxic stress, irradiation, viral infection, or malignant transformation.91,98 As shown in Figure 2, gene expression profiling of 32 different human cancer tissues from The Cancer Genome Atlas (TCGA) database revealed that, compared to other NKG2D ligands, MICA is the most consistently and highly expressed across all solid tumors and hematological malignancies. The second broadest expression pattern is exhibited by MICB, which is closely related in sequence to MICA. This makes both MICA and MICB (MICA/B) attractive pan-cancer targets.
Figure 2.
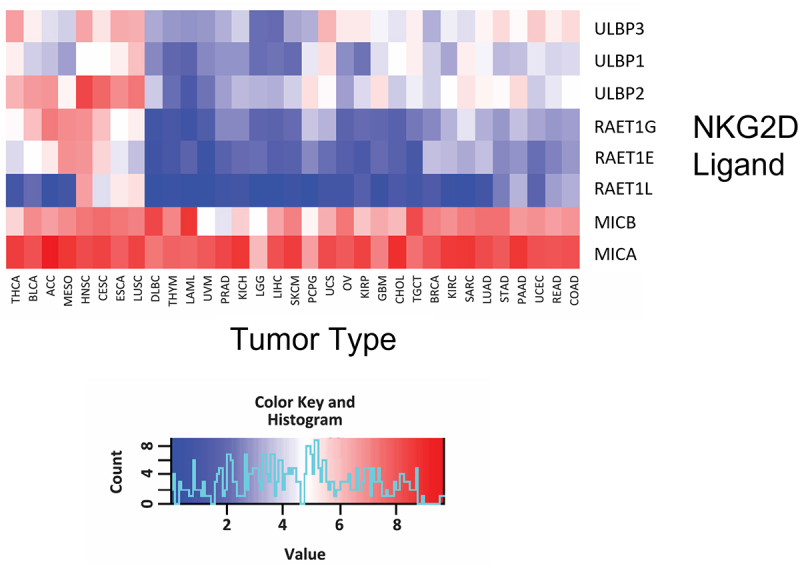
Broad expression of MICA and MICB in human cancers.
mRNA gene expression analysis of thirty-two cancer indications from the TCGA database. NKG2D ligands analyzed include MICA, MICB and ULBP1-6. Abbreviations for indications can be found here: https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations. Abbreviations are captured in the order they appear starting from left to right. THCA: thyroid carcinoma, BLCA: bladder urothelial carcinoma, ACC: adrenocortical carcinoma, MESO: mesothelioma, HNSC: head and neck squamous cell carcinoma, CESC: cervical squamous cell carcinoma and endocervical carcinoma, ESCA: esophageal carcinoma, LUSC: lung squamous cell carcinoma, DLBC: lymphoid neoplasm diffuse large B-cell lymphoma, THYM: thymoma, LAML: acute myeloid leukaemia, UVM: uveal melanoma, PRAD: prostate adenocarcinoma, KICH: kidney chromophobe, LGG: brain lower grade glioma, LIHC: liver hepatocellular carcinoma, SKCM: skin cutaneous melanoma, PCPG: pheochromocytoma and paraganglioma, UCS: Uterine carcinosarcoma, OV: ovarian serous cystadenocarcinoma, KIRP: kidney renal papillary carcinoma, GMB: glioblastoma multiforme, CHOL: cholangiocarcinoma, TGCT: testicular germ cell tumors, BRCA: breast invasive carcinoma, KIRC: kidney renal clear cell carcinoma, SARC: sarcoma, LUAD: lung adenocarcinoma, STAD: stomach adenocarcinoma, PAAD: pancreatic adenocarcinoma, UCEC: uterine corpus endometrial carcinoma, READ: rectum adenocarcinoma, COAD: colon adenocarcinoma.
MICA/B contain key structural differences compared to other members of the NKG2D ligand family. While all ligands consist of MHC class I-related polypeptide chains that lack beta-2-microglobulin and peptide association, MICA/B ligands contain alpha 1, alpha 2 and alpha 3 domains, whereas the ULBPs only contain the alpha 1 and alpha 2 domains and have low (25%) sequence identity to MICA/B. Despite differences in protein sequence, co-crystal structures of NKG2D with MICA or with ULBP3 revealed that NKG2D binds to its ligands through topologically similar but distinct sets of interface residues of the alpha 1/alpha 2 domains using a rigid adaptation mode.99 Of note, the alpha 3 domain of MICA, which connects the other two domains to the plasma membrane of NK cells, is not involved in this interaction (Figure 3).100
Figure 3.
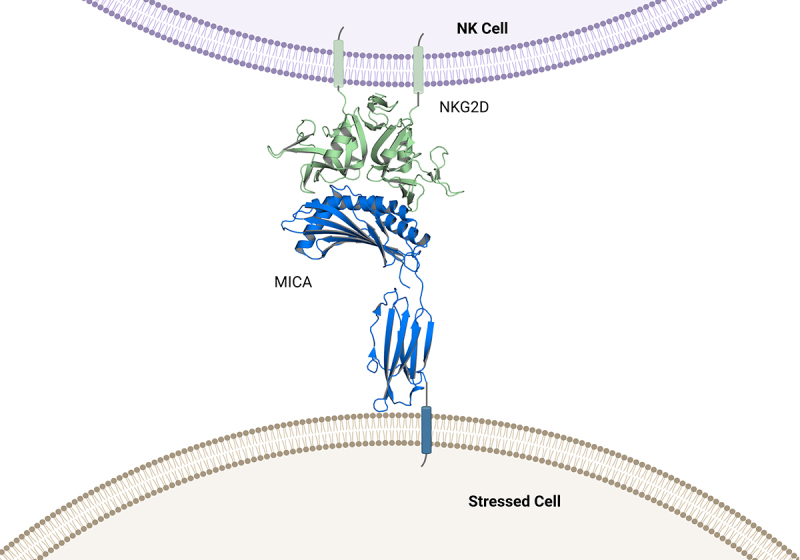
Structure of the NKG2D-MICA complex. .
MICA is shown in blue. The alpha 1 and alpha 2 domains of MICA form the interface with the NKG2D receptor, depicted in green. The arrow indicates the region containing proteolytic cleavage sites of MICA which are proximal to the cell membrane. (Figure adapted from https://www.rcsb.org/, PDB 1HYR (complex of MICA and NKG2D).
Cancer cells avoid NKG2D-mediated NK cell lysis by exploiting the unique alpha 3 region of MICA/B, which is the target for proteolytic shedding of the extracellular domain (ECD) from the cancer cell surface. Shedding is enabled by multiple proteases that most cancer cells express on their cell surface or release into the TME. It has been reported that matrix metalloproteinase-9 (MMP-9)/ matrix metalloproteinase −14, (MMP-14), a disintegrin and metalloproteinase-10 (ADAM10), and a disintegrin and metalloproteinase-17 (ADAM17) are all able to recognize and cleave sequences located in the stalk region between the alpha 3 domain and the transmembrane domain of MICA.101–103 Several cleavage sites have been mapped by mutational analysis that are proximal to the transmembrane domain of MICA (Figure 3). A prerequisite for cleavage of MICA/B appears to be the reduction of a single disulfide bridge in the alpha 3 domain by protein disulfide isomerase endoplasmic reticulum protein 5 (ERP5), which may enable MICA/B to adopt a protease-sensitive conformation.104
Shedding of MICA/B from the tumor cell membrane leads to an accumulation of soluble MICA/B in the blood of cancer patients.105,106 The potentially pivotal role of the NKG2D/ligand axis in immune surveillance is evident from the negative prognostic value of soluble MICA in a variety of cancers, including solid tumors, such as hepatocellular carcinoma (HCC), as well as hematological malignancies, like MM. This has been corroborated in a meta-analysis including many other cancer indications.107 Of note, expression of MICA on cancer cells, as observed in tumor biopsies, is a positive prognostic factor for improved overall survival.108
Collectively, these data suggest that therapies preventing MICA/B shedding, and thereby preserving MICA/B on the cell surface to promote NKG2D signaling, may have therapeutic potential in treating diverse cancers. However, MHC-related proteins MICA/B exhibit a high degree of polymorphism, which poses a potential challenge with this approach. Nearly 150 alleles have been identified for MICA and 50 alleles for MICB across the population.109 When polymorphic amino acid residues are present in target epitopes of mAbs, they may negatively impact antibody binding affinity across allelic variants and reduce target recognition in the diverse patient population. Great care must therefore be taken to identify mAbs that broadly recognize MICA/B alleles. Given the high sequence homology between MICA/B, it is possible to discover cross-reactive mAbs that recognize both NKG2D ligands. A MICA/B cross-reactive mAb with coverage of the most common MICA/B alleles should be able to overcome the challenges posed by polymorphisms, since each tumor may express up to four relevant target alleles for MICA and MICB.
Nevertheless, given the large number of activating and inhibitory receptors on NK cells, even if NKG2D signaling is preserved or enhanced, its activity is contextually dependent on the NK cell activation status. This justifies the development of therapeutic strategies with multi-modal mechanisms of action that combine NKG2D agonism with additional activating signals for NK cells.
Therapies leveraging the NKG2D-ligand axis for cancer therapy
The compelling role of the NKG2D-MICA/B axis in cancer biology has triggered numerous attempts to leverage these pathways for therapeutic targeting. Approaches include engineered T or NK cells, NK cell engagers, antibody-drug conjugates (ADCs), vaccines and mAbs (Figure 4). Therapeutics in active clinical development targeting the axis are highlighted in Table 1.
Figure 4.
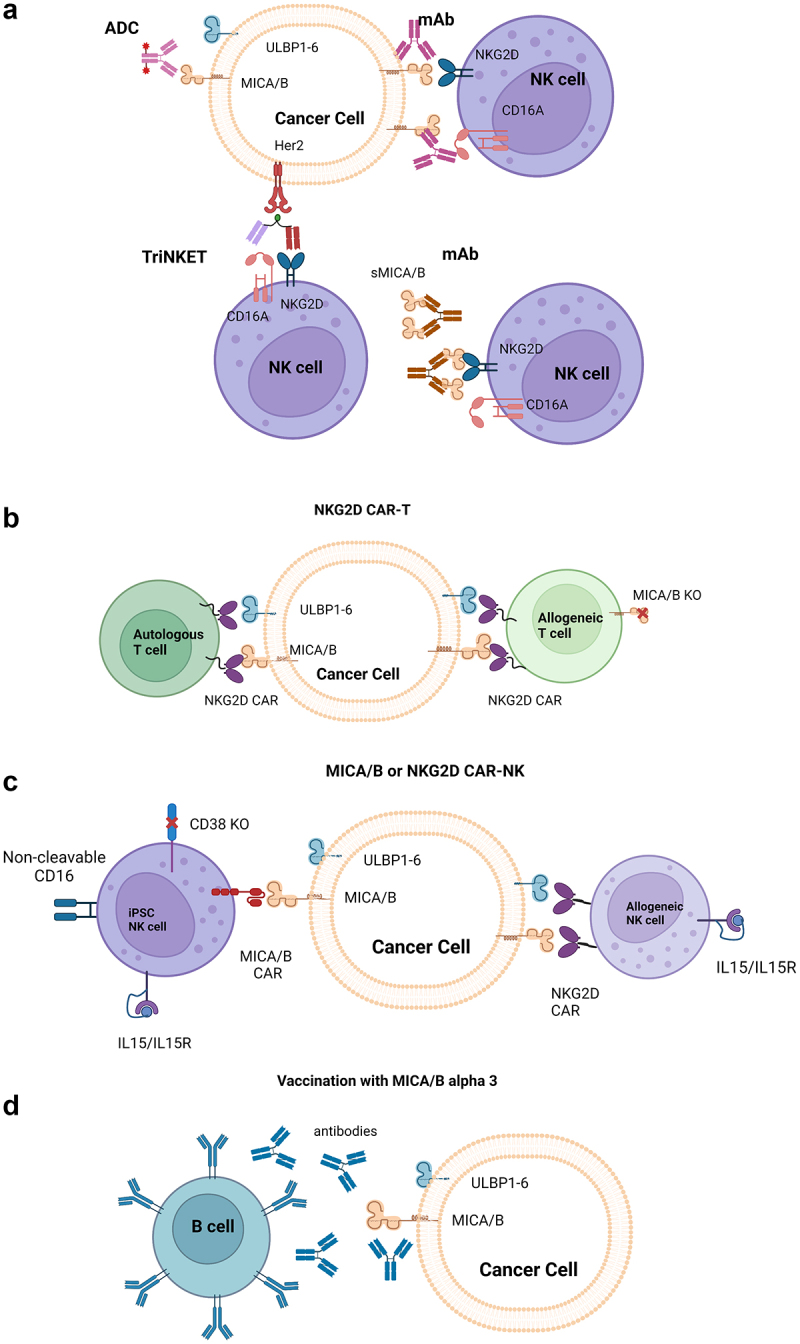
Schematic representation of therapeutic modalities targeting the MICA/B pathway.
(a) Protein-based therapeutics targeting the MICA/B pathway including an ADC that delivers a cytotoxic payload to MICA/B-expressing tumor cells, mAbs that induce immune-mediated tumor cell killing by preventing shedding of cell surface MICA/B or by binding to sMICA/B to form a NKG2D-activating complex, and a TriNKET that simultaneously binds NKG2D and CD16A on NK cells and targets Her2-expressing tumor cells. (b) Allogeneic or autologous NKG2D based r CAR)T-cell therapies activated by NKG2D ligands including MICA/B and (c) an allogeneic NKG2D based CAR NK cell therapy and iPSC-derived allogeneic MICA/B scFV-based CAR NK cell therapy activated by NKG2D ligands including MICA/B. (d) Vaccine-induced production of antibodies targeting the MICA/B alpha 3 domain.
Table 1.
Therapeutics targeting NKG2D in active clinical development. Abbreviations used: CAR: Chimeric antigen receptor, NKG2D: Natural Killer Group 2D.
| Company (Asset) | Modality | Molecule properties | Structure | Indication | Development Phase/Clinical trial number |
|---|---|---|---|---|---|
| Cullinan Oncology (CLN-619) |
Monoclonal antibody | Tumor target: MICA/B NK cell target: NKG2D, CD16A |
 |
Advanced solid tumors | Phase 1 NCT05117476 |
| Dragonfly Therapeutics (DF1001) | NK cell engager | Tumor target : HER2 NK cell target: NKG2D NK cell target: CD16A |
 |
Advanced solid tumors | Phase 1/2 NCT04143711 |
| Celyad (CYAD-02) |
Allogeneic CAR T-cell therapy | Target: All NKG2D ligands CAR: NKG2D extracellular domain Additional modification: MICA/B knockdown to reduce fratricide |
 |
Acute myeloid leukemia Myelodysplastic syndrome |
Phase 1 NCT04167696 |
| Nkarta (NKX101) | Human donor allogeneic CAR NK cell therapy | Target: All NKG2D ligands CAR: NKG2D extracellular domain Additional modification: IL-15 R/IL15 fusion |
 |
Acute myeloid leukemia Myelodysplastic syndrome |
Phase 1 NCT04623944 |
Celyad Oncology has developed two autologous (CYAD-01, CYAD-02) and one allogenic (CYAD-101) T cell therapies that express a CAR based on the ECD of NKG2D. Although CYAD-01 and CYAD-101 are no longer in clinical development, CYAD-02 is being evaluated in acute myeloid leukemia (AML) and myeloid dysplastic syndrome (MDS) patients in a Phase 1 study where two patients were reported to have achieved a complete marrow response (NCT04167696).110–114
Nkarta Therapeutics is developing NKX101, an allogenic NK cell therapy based on NK cells collected from normal human donors by apheresis and expanded ex vivo. Like Celyad, the CAR utilizes the ECD of NKG2D to target NKG2D ligands on tumor cells; the CAR-NK cells also express an interleukin-15 (IL-15) receptor/IL-15 fusion protein, which allows for the enhancement of NK cell proliferation, cytotoxicity, cytokine release and maintenance.115 NKX101 is being evaluated in a Phase 1 study of relapsed or refractory AML and MDS patients (NCT04623944).116 Of five AML patients treated, three were recently reported to have achieved a complete response with hematological recovery.117 Other clinical trials of CAR T or CAR gamma-delta T cells that incorporate the ECD of NKG2D are ongoing and are reviewed by Curio et al.118
Fate Therapeutics has developed an allogenic MICA/B-directed NK cell therapy, FT536, based on the company’s human-induced pluripotent stem cell (iPSC) platform. Rather than using the ECD of NKG2D to target MICA/B, FT536 expresses a CAR harboring a scFv domain with specificity for the alpha 3 domain of MICA/B, which prevents shedding of MICA/B from the tumor cell surface. This approach was shown to have superior anti-tumor activity as compared to an NKG2D-based CAR in preclinical models.119 The engineered NK cells are further enhanced by expression of a high-affinity, non-cleavable version of CD16A (for combination with mAb therapies), as well as an IL-15 receptor/IL-15 fusion protein and knockout of the CD38 gene for enhanced NK cell activity and augmented metabolic fitness and persistence120. Fate recently announced that it is discontinuing development of the program.121
While these cell therapies speak to the industry’s interest in targeting NKG2D ligands for cancer therapy and have shown initial signs of efficacy, all involve complex manufacturing of genetically engineered cells and will likely be limited by the number of cells that can be safely administered to patients. Furthermore, therapies using the ECD of NKG2D for MICA/B targeting will be hampered by the frequent shedding of the two NKG2D ligands. In addition, there is a potential for lack of specificity given the ability of the NKG2D ECD to interact with other NKG2D ligands for which the safety consequences are unknown. Lastly, MICA/B can be expressed on cellular products as a consequence of stressful cell isolation and expansion, which may trigger fratricide and thereby limit the manufacturing yield of engineered cells.
Biologics, such as mAbs and antibody constructs like NKCEs, represent an alternative approach to leveraging the NKG2D pathway. NKCEs consisting of the ECD of NKG2D fused to Fab fragments directed against CD16A (NKG2D-CD16) have demonstrated potent target cell lysis in preclinical studies.122 NKCEs that use the MICA ECD fused to scFvs against tumor- or TME-associated antigens, such as vascular endothelial growth factor receptor 2 (VEGFR2), CD24, CD20 and BCMA, have also been generated.96,123–125 Such constructs were tested preclinically and demonstrated specific NK cell activation and target cell lysis. However, the potential cleavage of the ECD of MICA by proteases in the TME is a liability. This is the case for those NKCEs that incorporate full-length, unmodified MICA ECD, such as the VEGR2 and CD24 constructs. In addition, the affinity of MICA for NKG2D is weak and may limit the potency of these molecules, supporting the use of higher-affinity/avidity constructs such as those from Dragonfly Therapeutics and Xencor, which simultaneously bind to tumor-associated antigens and NK cells via CD16A and NKG2D. DF1001, a Tri-specific NKCE (TriNKET) from Dragonfly Therapeutics, is composed of three antibody fragments simultaneously targeting tumor cells via HER2 and NK cells via both CD16A and NKG2D. DF1001 is currently being evaluated in Phase 1/2 clinical studies of patients with advanced solid tumors (NCT04143711). Xencor’s XmAb® bispecific NKE molecule, which targets B7 homolog 3 protein (B7H3) and NKG2D, and is currently in preclinical development, is another example of a NKCE that leverages NKG2D and CD16A to promote NK cell activation and lysis of tumor cells.126 These approaches are similar to the ANKET platform described above, with the addition of an NKG2D-targeting arm.
Several mAbs have been generated that directly target MICA/B. These antibodies all aim to augment the NKG2D signaling axis and have diverse mechanisms of action. One strategy involves targeting soluble MICA/B (sMICA/B) as a means to modulate the interaction of sMICA/B with NKG2D, which is thought to be detrimental to NK cell function via impairment of NKG2D functionality.106,127–130 For example, the antibody B10G5 binds to shed sMICA/B to stimulate NK cells through two proposed mechanisms of action, namely clearance of sMICA/B and formation of antibody/MICA/B complexes that may agonize NKG2D on NK and T cells. B10G5 has been demonstrated to restore NK cell function and remodel the TME, resulting in tumor growth inhibition in preclinical models.131–133
Innate Pharma recently reported results for the MICA/B targeted ADC MICAB1. The mAb used to generate the ADC was selected due to its ability to stimulate a high level of MICA/B internalization and engineered to be Fc-silent. The antibody is coupled to first-generation pyrrolobenzodiazepine dimers (PBD) as a cytotoxic payload. Treatment with MICAB1 in preclinical models was effective at single, low doses in both human xenograft and patient-derived xenograft models.134 The current development status of this agent is unknown.
The generation of therapeutics that inhibit shedding of MICA/B is an emerging strategy to leverage NKG2D ligands.135,136 One approach to achieve this is the induction of antibodies in response to vaccination with the alpha 3 domain of MICA/B. These antibodies can prevent shedding by binding MICA/B at the proteolytic cleavage site. Robust anti-tumor responses dependent upon both NK and T cells were generated in response to vaccination in preclinical studies.137 Furthermore, engaging multiple effector cells mediated protective immunity against tumors with common escape mechanisms, such as loss of MHC-I expression.137 A potential limitation to this approach is the lack of control over how much antibody is produced and the functionality of the antibody response.
A promising approach to targeting inhibition of MICA/B shedding is being undertaken by Cullinan Oncology with the generation of CLN-619, a humanized IgG1 antibody that is currently the only MICA/B-targeted mAb in the clinic. CLN-619 prevents shedding of MICA/B from cancer cells by binding to the alpha 3 domain and contains an active Fcγ1 domain to drive ADCC and ADCP. As described above, the MICA/B genes are highly polymorphic, yet CLN-619 has been demonstrated to have broad reactivity to all allelic variants tested. Reduced levels of shed MICA and a concomitant increase in MICA on the surface of tumor cells has been observed with CLN-619 treatment. Importantly, the alpha 1 and alpha 2 domains of MICA, which bind to NKG2D, are unencumbered by the bound antibody. In fact, CLN-619 enhances the binding of MICA to NKG2D, which appears to be dependent on the Fcγ1 domain of the antibody that can concomitantly bind to CD16A on NK cells. Notably, CLN-619 shows compelling single-agent activity at low doses in tumor xenograft models, where its activity critically relies upon a functional Fcγ1 domain.138,139
Stabilization of MICA/B by CLN-619 leads to accumulation of these NKG2D ligands on the tumor cell surface, thereby overcoming immune evasion by MICA/B shedding. This likely maximizes ADCC and ADCP functions of the antibody due to increased target antigen density. Importantly, CLN-619 has the potential to activate NK cells via simultaneous and perhaps synergistic engagement of two key activating receptors: NKG2D and CD16A. Cooperation between the two receptors is supported by published data showing engagement NKG2D can lower the activation threshold of several receptors including CD16A.140 Cooperation between the two receptors may be important when considering NK cell-activating receptors in the TME are often downmodulated141–143. CLN-619 is currently in a Phase 1 clinical trial in patients administered alone or in combination with pembrolizumab in patients with advanced solid tumors (NCT05117476). Given the broad tumor expression of the pathway (Figure 2), it is anticipated that CLN-619 may have the potential for pan-cancer activity.
Conclusions and outlook
While therapeutic success has been achieved with engagement of T cells using multiple modalities, this review highlights the challenges and opportunities to accomplish this by engaging NK cells. Although certain NK cell therapies are beginning to show some promise in the clinic, an off-the-shelf mAb therapy has the advantage of ease of manufacturing and the potential to engage most if not all NK cells in a patient for lysis of cancer cells. However, treatment with mAbs comes with potential liabilities, including the possibility of recruiting exhausted, dysfunctional NK cells. In addition, the intricate interplay between negative and positive regulatory receptors on NK cells makes it difficult to simply engage a single receptor to achieve therapeutic benefit. Nevertheless, one approach that has met with success is leveraging activation of the CD16A receptor on NK cells via tumor antigen-targeted mAbs mediating ADCC. Another attractive target on NK cells is the activating receptor NKG2D. However, the shedding of its most abundantly expressed ligands MICA/B must be addressed in order to maximize the therapeutic potential of engaging this pathway. To fully unleash their killing potential, NK cells in the immunosuppressive TME may require more than one activating stimulus, for example simultaneous engagement of both CD16A and NKG2D. The ultimate therapeutic potential for NK cell-activating therapeutics in the clinic may be enabled when delivered in combination with therapies that enhance or complement NK cell activation, such as lenalidomide, cytokines or checkpoint inhibitors. Combination with checkpoint inhibitors provides the opportunity for dual activation of both the innate and adaptive immune response and may attenuate exhaustion of both T cells and NK cells expressing PD-1. In summary, engagement of NK cells, particularly via stimulatory mAbs, holds great promise for the future development of novel immuno-oncology therapeutics.
Acknowledgments
Figures 1, 3 , and 4 were created using Biorender.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
- AA
Amino acid
- ADAM10
a disintegrin and metalloproteinase-10
- ADAM17
a disintegrin and metalloproteinase-17
- ADCC
Antibody-dependent cellular cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- ADC(s)
antibody-drug conjugates
- aKIR(s)
activating killer Ig-like receptors
- AML
acute myeloid leukemia
- ANKET
Antibody-based NK cell Engager Therapeutics
- B7H3
B7 homolog 3 protein
- BCMA
B-cell maturation antigen
- CAR
Chimeric antigen receptor
- CCR4
C-C chemokine receptor type 4
- CD
Cluster of differentiation
- CD16A
Cluster of differentiation 16A
- CD3ζ
CD3 zeta
- CD96
cluster of differentiation 96
- CDC
complement-dependent cytotoxicity
- CRS
Cytokine release syndrome
- DAP10
DNAX-activating protein 10
- DNAM-1
DNAX accessory molecule-1
- ECD
extracellular domain
- ERP5
endoplasmic reticulum protein 5
- Fab
fragment antigen-binding
- FcϵRIγ
Fc epsilon RI
- Fcγ1
Fc gamma domain 1
- FcγRIIIa
Fc-gamma receptor IIIA
- FcγRs
Fc gamma receptors
- FUT8
fucosyltransferase
- GMD
GDP mannose 4,6-dehydratase
- GPI
glycosylphosphatidylinositol
- Grb
growth factor receptor bound 2 protein
- HCC
hepatocellular carcinoma
- HER2
human epidermal growth factor receptor 2
- HL
Hodgkin lymphoma
- HLA
human leukocyte antigen
- ICANs
Immune effector cell-associated neurotoxicity syndrome
- iKIR(s)
inhibitory killer Ig-like receptors
- IL-15
interleukin-15
- IL-2 v
interleukin-2 variant
- Ipsc
induced pluripotent stem cell
- IRp60
inhibitor receptor protein
- ITAM
immunoreceptor tyrosine-based activation motif
- KIR2DL1-L3
killer cell immunoglobulin like receptor, three Ig domains and long cytoplasmic tail 1–3
- KIR3DL2
killer cell immunoglobulin like receptor three Ig domains and long cytoplasmic tail 2
- LAIR-1
leukocyte-associated immunoglobulin-like receptor 1
- mAb
monoclonal antibody
- MDS
myeloid dysplastic syndrome
- MHC
Major histocompatibility complexes
- MICA
MHC class I chain-related protein A
- MICB
MHC class I chain-related protein sequence B
- MICA/B
MICA and MICB
- MM
Multiple myeloma
- MMP-9
matrix metalloproteinase-9
- MMP-14
matrix metalloproteinase −14
- NCR
natural cytotoxicity receptors
- NFAT
nuclear factor of activated T-cells
- NFKB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural Killer
- NKCEs
NK cell engagers
- NKG2A
Natural Killer Group 2A
- NKG2C
Natural Killer group 2C
- NKG2D
Natural Killer Group 2D
- NKT
Natural Killer T cell
- PBD
pyrrolobenzodiazepine dimers
- PD-1
programmed cell death 1 receptor
- PD-L1 and PD-L2
programmed death-ligand 1 and 2
- PI3K
phosphoinositide 3-kinase
- scFv
single-chain variable fragment
- siglec-7
sialic acid binding Ig like lectin 7
- sMICA/B
soluble MICA/B
- Tandab
tandem diabody
- TCGA
The Cancer Genome Atlas
- TCR
T cell receptor complex
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TME
tumor microenvironment
- TriNKET
Tri-specific NKCE
- ULBPs
UL-16- binding proteins
- VEGFR2
vascular endothelial growth factor receptor 2.
References
- 1.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-Transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–15. PMID: 32023374. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mensali N, Dillard P, Hebeisen M, Lorenz S, Theodossiou T, Myhre MR, Fane A, Gaudernack G, Kvalheim G, Myklebust JH, et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine. 2019;40:106–17. PMID: 30665853. doi: 10.1016/j.ebiom.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton LT, Wachsmann TLA, Meeuwsen MH, Wouters AK, Remst DFG, van Loenen MM, Falkenburg JHF, Heemskerk MHM, van Loenen MM.. T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion. J ImmunoTher Cancer. 2022;10(3):e003715. PMID: 35288464. doi: 10.1136/jitc-2021-003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. PMID: 32853984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marofi F, Abdul-Rasheed OF, Rahman HS, Budi HS, Jalil AT, Yumashev AV, Hassanzadeh A, Yazdanifar M, Motavalli R, Chartrand MS, et al. CAR-NK cell in cancer immunotherapy; a promising frontier. Cancer Sci. 2021;112(9):3427–36. PMID: 34050690. doi: 10.1111/cas.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laskowski TJ, Biederstadt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22(10):557–75. PMID: 35879429. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heipertz EL, Zynda ER, Stav-Noraas TE, Hungler AD, Boucher SE, Kaur N, Vemuri MC. Current perspectives on “off-the-shelf” allogeneic NK and CAR-NK cell therapies. Front Immunol. 2021;12:732135. PMID: 34925314. doi: 10.3389/fimmu.2021.732135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto S, Pahl J, Schottelius A, Carter PJ, Koch J. Reimagining antibody-dependent cellular cytotoxicity in cancer: the potential of natural killer cell engagers. Trends Immunol. 2022;43(11):932–46. PMID: 36306739. doi: 10.1016/j.it.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Fuertes MB, Domaica CI, Zwirner NW. Leveraging NKG2D ligands in immuno-oncology. Front Immunol. 2021;12:713158. PMID: 34394116. doi: 10.3389/fimmu.2021.713158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarova M, Steinle A. The NKG2D axis: an emerging target in cancer immunotherapy. Expert Opin Ther Targets. 2019;23(4):281–94. PMID: 30732494. doi: 10.1080/14728222.2019.1580693. [DOI] [PubMed] [Google Scholar]
- 11.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2):S3–23. PMID: 20176265. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol. 2012;3:335. PMID: 23162553. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319–29. PMID: 31107565. doi: 10.1002/JLB.MR0718-269R. [DOI] [PubMed] [Google Scholar]
- 14.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. PMID: 18425106. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang JR, Byeon Y, Kim D, Park SG. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Experimental & Molecular Medi. 2020;52(5):750–61. PMID: 32439954. doi: 10.1038/s12276-020-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtney AH, Lo WL, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43(2):108–23. PMID: 29269020. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5(5):524–30. PMID: 15048111. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 18.Krovi SH, Gapin L. Invariant natural killer T cell subsets-more than just developmental intermediates. Front Immunol. 2018;9:1393. PMID: 29973936. doi: 10.3389/fimmu.2018.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10(1):21–29. PMID: 23085947. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. PMID: 30150991. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quatrini L, Della Chiesa M, Sivori S, Mingari MC, Pende D, Moretta L. Human NK cells, their receptors and function. Eur J Immunol. 2021;51(7):1566–79. PMID: 33899224. doi: 10.1002/eji.202049028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16(5):430–41. PMID: 30778167. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology. 2018;154(3):383–93. PMID: 29512837. doi: 10.1111/imm.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debska-Zielkowska J, Moszkowska G, Zielinski M, Zielinska H, Dukat-Mazurek A, Trzonkowski P, Stefanska K. KIR receptors as key regulators of NK cells activity in health and disease. Cells. 2021;10(7):1777. PMID: 34359951. doi: 10.3390/cells10071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol. 2019;10:909. PMID: 31134055. doi: 10.3389/fimmu.2019.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–81. PMID: 8673704. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 27.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, Moretta L, Moretta A. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–93. PMID: 10741393. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Vitale M, Falco M, Castriconi R, Parolini S, Zambello R, Semenzato G, Biassoni R, Bottino C, Moretta L, Moretta A. Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur J Immunol. 2001;31:233–42. PMID: 11265639. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Sivori S, Della Chiesa M, Carlomagno S, Quatrini L, Munari E, Vacca P, Tumino N, Mariotti FR, Mingari MC, Pende D, et al. Inhibitory receptors and checkpoints in human NK cells, implications for the immunotherapy of cancer. Front Immunol. 2020;11:2156. PMID: 33013909. doi: 10.3389/fimmu.2020.02156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Hall T, Andre P, Horowitz A, Ruan DF, Borst L, Zerbib R, Narni-Mancinelli E, van der Burg SH, Vivier E, van der Burg SH. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J ImmunoTher Cancer. 2019;7(1):263. PMID: 31623687. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Innate P . Safety and efficacy of lacutamab in patients with relapse peripheral T-cell lymphoma that express KIR3DL2 Safety and Efficacy of Lacutamab in Patients With Relapse Peripheral T-cell Lymphoma That Express KIR3DL2. 2022. (NCT05321147). [Google Scholar]
- 32.The lymphoma academic research O, Innate P. study of lacutamab in peripheral T-cell lymphoma. Study of Lacutamab in Peripheral T-Cell Lymphoma. 2025. (NCT04984837). [Google Scholar]
- 33.Vey N, Dumas P-Y, Recher C, Gastaud L, Lioure B, Bulabois C-E, Pautas C, Marolleau J-P, Leprêtre S, Raffoux E, et al. Randomized Phase 2 Trial of Lirilumab (anti-KIR monoclonal antibody, mAb) as maintenance treatment in elderly Patients (pts) with acute myeloid leukemia (AML): results of the effikir trial. Blood. 2017;130(Suppl_1):889. doi: 10.1182/blood.V130.Suppl_1.889.889. [DOI] [Google Scholar]
- 34.Grivas P, Yin J, Koshkin VS, Cole S, Jain RK, Dreicer R, Cetnar JP, Sundi D, Gartrell BA, Galsky MD, et al. PrE0807: a phase Ib feasibility trial of neoadjuvant nivolumab (N) without or with lirilumab (L) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J Clin Oncol. 2021;39(15_suppl):4518. doi: 10.1200/JCO.2021.39.15_suppl.4518. [DOI] [PubMed] [Google Scholar]
- 35.Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–85. PMID: 31676858. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108–19. PMID: 31828774. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao JY, Yin WW, Zhang QF, Liu Q, Peng ML, Hu HD, Hu P, Ren H, Zhang DZ. Siglec-7 Defines a highly functional natural killer cell subset and inhibits cell-mediated activities. Scand J Immunol. 2016;84(3):182–90. PMID: 27312286. doi: 10.1111/sji.12455. [DOI] [PubMed] [Google Scholar]
- 38.Lankry D, Simic H, Klieger Y, Levi-Schaffer F, Jonjic S, Mandelboim O. Expression and function of CD300 in NK cells. J Immunol. 2010;185(5):2877–86. PMID: 20656921. doi: 10.4049/jimmunol.0903347. [DOI] [PubMed] [Google Scholar]
- 39.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–90. PMID: 9285412. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 40.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. PMID: 32433532. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16(1):223–49. PMID: 33197221. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 42.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–68. PMID: 30198904. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasim MS, Marotel M, Hodgins JJ, Vulpis E, Makinson OJ, Asif S, Shih HY, Scheer AK, MacMillan O, Alonso FG, et al. When killers become thieves: trogocytosed PD-1 inhibits NK cells in cancer. Sci Adv. 2022;8(15):eabj3286. PMID: 35417234. doi: 10.1126/sciadv.abj3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H, Quek C, Silva I, Tasker A, Batten M, Rizos H, Lim SY, Nur Gide T, Shang P, Attrill GH, et al. Integrated molecular and immunophenotypic analysis of NK cells in anti-PD-1 treated metastatic melanoma patients. Oncoimmunology. 2019;8(2):e1537581. PMID: 30713793. doi: 10.1080/2162402X.2018.1537581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 mediates dysfunction in activated PD-1(+) NK cells in head and neck cancer patients. Cancer Immunol Res. 2018;6(12):1548–60. PMID: 30282672. doi: 10.1158/2326-6066.CIR-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood. 2010;116(13):2286–94. PMID: 20460501. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trefny MP, Kaiser M, Stanczak MA, Herzig P, Savic S, Wiese M, Lardinois D, Laubli H, Uhlenbrock F, Zippelius A. PD-1(+) natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol Immunother. 2020;69(8):1505–17. PMID: 32296919. doi: 10.1007/s00262-020-02558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, Zhang J, Benson DM, He K, Caligiuri MA, et al. The mechanism of anti–PD-L1 antibody efficacy against PD-L1–Negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9(10):1422–37. PMID: 31340937. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sierra JM, Secchiari F, Nunez SY, Iraolagoitia XLR, Ziblat A, Friedrich AD, Regge MV, Santilli MC, Torres NI, Gantov M, et al. Tumor-Experienced Human NK cells express high levels of PD-L1 and Inhibit CD8(+) T Cell Proliferation. Front Immunol. 2021;12:745939. PMID: 34616407. doi: 10.3389/fimmu.2021.745939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. PMID: 18064051. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 51.Phillips NE, Parker DC. Cross-linking of B lymphocyte Fc gamma receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984;132(2):627–32. PMID: 6228594. https://www.ncbi.nlm.nih.gov/pubmed/6228594. [PubMed] [Google Scholar]
- 52.Anania JC, Chenoweth AM, Wines BD, Hogarth PM. The human FcgammaRII (CD32) family of leukocyte FcR in health and disease. Front Immunol. 2019;10:464. PMID: 30941127. doi: 10.3389/fimmu.2019.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192(5):2252–60. PMID: 24489098. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47(5):820–33. PMID: 29166586. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashemi E, Malarkannan S. Tissue-resident NK cells: development, maturation, and clinical relevance. Cancers (Basel). 2020;12(6):1553. PMID: 32545516. doi: 10.3390/cancers12061553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, Baeuerle PA, Prang NS, Silva AJD. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol. 2006;43(8):1183–93. PMID: 16102830. doi: 10.1016/j.molimm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Coenon L, Villalba M. From CD16a biology to antibody-dependent cell-mediated cytotoxicity improvement. Front Immunol. 2022;13:913215. PMID: 35720368. doi: 10.3389/fimmu.2022.913215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268(1):25–51. PMID: 26497511. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100(5):1059–70. PMID: 9276722. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99(3):754–58. PMID: 11806974. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 61.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-engineering for modulated effector functions—improving antibodies for cancer treatment. Antibodies (Basel). 2020;9(4):64. PMID: 33212886. doi: 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711. PMID: 29733746. doi: 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein & Cell. 2018;9(1):63–73. PMID: 28986820. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103(11):4005–10. PMID: 16537476. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276(9):6591–604. PMID: 11096108. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 66.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Vijh S, Johnson S, Bonvini E, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67(18):8882–90. PMID: 17875730. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 67.Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcγRIIIa. J Mol Biol. 2004;336(5):1239–49. PMID: 15037082. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Li T, Wang LX. Chemoenzymatic defucosylation of therapeutic antibodies for enhanced effector functions using bacterial alpha-fucosidases. Methods Mol Biol. 2018;1827:367–80. PMID: 30196507. doi: 10.1007/978-1-4939-8648-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, Jeffrey SC, Klussman K, Law CL, Sussman D, et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A. 2013;110(14):5404–09. PMID: 23493549. doi: 10.1073/pnas.1222263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanda Y, Imai-Nishiya H, Kuni-Kamochi R, Mori K, Inoue M, Kitajima-Miyama K, Okazaki A, Iida S, Shitara K, Satoh M. Establishment of a GDP-mannose 4,6-dehydratase (GMD) knockout host cell line: a new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol. 2007;130(3):300–10. PMID: 17559959. doi: 10.1016/j.jbiotec.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 71.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87(5):614–22. PMID: 15352059. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 72.Musolino A, Gradishar WJ, Rugo HS, Nordstrom JL, Rock EP, Arnaldez F, Pegram MD. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J ImmunoTher Cancer. 2022;10(1):e003171. PMID: 34992090. doi: 10.1136/jitc-2021-003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4(4):419–25. PMID: 22699226. doi: 10.4161/mabs.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG Jr.. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–09. PMID: 24292623. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015;126(1):50–60. PMID: 26002964. doi: 10.1182/blood-2015-01-625004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–15. PMID: 31141632. doi: 10.1056/NEJMoa1817249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Treffers LW, van Houdt M, Bruggeman CW, Heineke MH, Zhao XW, van der Heijden J, Nagelkerke SQ, Verkuijlen P, Geissler J, Lissenberg-Thunnissen S, et al. FcγRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front Immunol. 2018;9:3124. PMID: 30761158. doi: 10.3389/fimmu.2018.03124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024–31. PMID: 25887777. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartlett NL, Herrera AF, Domingo-Domenech E, Mehta A, Forero-Torres A, Garcia-Sanz R, Armand P, Devata S, Izquierdo AR, Lossos IS, et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2020;136(21):2401–09. PMID: 32730586. doi: 10.1182/blood.2019004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nieto Y, Banerjee P, Kaur I, Bassett R, Kerbauy L, Basar R, Kaplan M, Griffin L, Esqueda D, Ganesh C, et al. Abstract CT003: innate cell engager (ICE®) AFM13 combined with preactivated and expanded cord blood (CB)-derived NK cells for patients with refractory/relapsed CD30+ lymphoma. Cancer Res. 2022;82(12_Supplement):CT003. doi: 10.1158/1538-7445.Am2022-ct003. [DOI] [Google Scholar]
- 81.Kakiuchi-Kiyota S, Ross T, Wallweber HA, Kiefer JR, Schutten MM, Adedeji AO, Cai H, Hendricks R, Cohen S, Myneni S, et al. A BCMA/CD16A bispecific innate cell engager for the treatment of multiple myeloma. Leukemia. 2022;36(4):1006–14. PMID: 35001074. doi: 10.1038/s41375-021-01478-w. [DOI] [PubMed] [Google Scholar]
- 82.Plesner T, Harrison SJ, Quach H, Lee C, Bryant A, Vangsted A, Estell J, Delforge M, Offner F, Twomey P, et al. Phase I study of safety and pharmacokinetics of RO7297089, an anti-BCMA/CD16a bispecific antibody, in patients with relapsed, refractory multiple myeloma. Clin Hematol Int. 2023;5(1):43–51. PMID: 36656461. doi: 10.1007/s44228-022-00023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gt Biopharma I. GTB-3550 tri-specific killer engager (TriKE®) for high risk hematological malignancies. In: GTB-3550 tri-specific killer engager (TriKE®) for high risk hematological malignancies. 2021. (NCT03214666). [Google Scholar]
- 84.Vallera DA, Ferrone S, Kodal B, Hinderlie P, Bendzick L, Ettestad B, Hallstrom C, Zorko NA, Rao A, Fujioka N, et al. NK-Cell-mediated targeting of various solid tumors using a B7-H3 tri-specific killer engager in vitro and in vivo. Cancers (Basel). 2020;12(9):2659. PMID: 32961861. doi: 10.3390/cancers12092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phung SK, Miller JS, Felices M. Bi-specific and Tri-specific NK Cell engagers: the new avenue of targeted NK cell immunotherapy. Molecular Diagnosis & Therapy. 2021;25(5):577–92. PMID: 34327614. doi: 10.1007/s40291-021-00550-6. [DOI] [PubMed] [Google Scholar]
- 86.Demaria O, Gauthier L, Debroas G, Vivier E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021;51(8):1934–42. PMID: 34145579. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 87.Watkins-Yoon J, Guzman W, Oliphant A, Haserlat S, Leung A, Chottin C, Ophir M, Vekeria J, Nelson AP, Frye Z, et al. CTX-8573, an innate-cell engager targeting BCMA, is a highly potent multispecific antibody for the treatment of multiple myeloma. Blood. 2019;134(Supplement_1):3182. doi: 10.1182/blood-2019-128749. [DOI] [Google Scholar]
- 88.Sanofi . First-in-human study of sar443579 infusion in male and female participants of at least 12 years of age with relapsed or refractory acute myeloid leukemia (R/R AML), B-cell acute lymphoblastic leukemia (B-ALL) or high risk-myelodysplasia (HR-MDS). First-In-Human Study of SAR443579 Infusion in Male and Female Participants of at Least 12 Years of Age with Relapsed or Refractory Acute Myeloid Leukemia (R/R AML), B-Cell Acute Lymphoblastic Leukemia (B-ALL) or High Risk-Myelodysplasia (HR-MDS). 2024. (NCT05086315). [Google Scholar]
- 89.Demaria O, Habif G, Le Floch F, Chiossone L, Remark R, Vetizou M, Maurel N, Gauthier L, Morel Y, Paturel C, et al. IPH6501 is a Novel NKp46-targeting tetraspecific antibody-based natural killer cell engager therapeutic (ANKET) armed with a non-alpha IL-2 variant and developed for the treatment of CD20-positive malignancies. Blood. 2022;140(Supplement 1):11559. doi: 10.1182/blood-2022-163561. [DOI] [Google Scholar]
- 90.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–29. PMID: 10426993. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 91.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31(1):413–41. PMID: 23298206. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology. 2013;2(10):e26097. PMID: 24353908. doi: 10.4161/onci.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wensveen FM, Jelencic V, Polic B. NKG2D: a master regulator of immune cell responsiveness. Front Immunol. 2018;9:441. PMID: 29568297. doi: 10.3389/fimmu.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. Nkg2D-DAP10 triggers human NK cell–mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4(6):557–64. PMID: 12740575. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 95.Rothe A, Jachimowicz RD, Borchmann S, Madlener M, Kessler J, Reiners KS, Sauer M, Hansen HP, Ullrich RT, Chatterjee S, et al. The bispecific immunoligand ULBP2-Acea redirects natural killer cells to tumor cells and reveals potent anti-tumor activity against colon carcinoma. Int J Cancer. 2014;134(12):2829–40. PMID: 24242212. doi: 10.1002/ijc.28609. [DOI] [PubMed] [Google Scholar]
- 96.Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, van de Winkel JG, Parren PW, Stauch M, Valerius T, et al. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia. 2012;26(4):830–34. PMID: 22005785. doi: 10.1038/leu.2011.288. [DOI] [PubMed] [Google Scholar]
- 97.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2(3):255–60. PMID: 11224526. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 98.Spies T. Regulation of NKG2D ligands: a purposeful but delicate affair. Nat Immunol. 2008;9(9):1013–15. PMID: 18711442. doi: 10.1038/ni0908-1013. [DOI] [PubMed] [Google Scholar]
- 99.McFarland BJ, Strong RK. Thermodynamic analysis of degenerate recognition by the NKG2D immunoreceptor: not induced fit but rigid adaptation. Immunity. 2003;19(6):803–12. PMID: 14670298. doi: 10.1016/s1074-7613(03)00320-0. [DOI] [PubMed] [Google Scholar]
- 100.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I–like ligand MICA. Nat Immunol. 2001;2(5):443–51. PMID: 11323699. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 101.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368–76. PMID: 18676862. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 102.Liu G, Atteridge CL, Wang X, Lundgren AD, Wu JD. The membrane type matrix metalloproteinase MMP14 mediates constitutive shedding of MHC class I chain-related molecule a independent of a disintegrin and metalloproteinases. J Immunol. 2010;184(7):3346–50. PMID: 20208009. doi: 10.4049/jimmunol.0903789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78(2):120–29. PMID: 23679194. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 104.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447(7143):482–86. PMID: 17495932. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 105.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169(8):4098–102. PMID: 12370336. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 106.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–38. PMID: 12384702. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 107.Zhao Y, Chen N, Yu Y, Zhou L, Niu C, Liu Y, Tian H, Lv Z, Han F, Cui J. Prognostic value of MICA/B in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96384–95. PMID: 29221214. doi: 10.18632/oncotarget.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fang L, Gong J, Wang Y, Liu R, Li Z, Wang Z, Zhang Y, Zhang C, Song C, Yang A, et al. MICA/B expression is inhibited by unfolded protein response and associated with poor prognosis in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33(1):76. PMID: 25228093. doi: 10.1186/s13046-014-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klussmeier A, Massalski C, Putke K, Schafer G, Sauter J, Schefzyk D, Pruschke J, Hofmann J, Furst D, Carapito R, et al. High-throughput MICA/B genotyping of over two million samples: workflow and allele frequencies. Front Immunol. 2020;11:314. PMID: 32153595. doi: 10.3389/fimmu.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zelkovic S Celyad oncology provides strategic update., Celyad oncology provides strategic update. Company Website: Celyad Oncology. 2022 [Google Scholar]
- 111.Zelkovic S. Celyad oncology provides updates on allogeneic and autologous CAR T programs at 62nd ASH annual meeting and exposition. In: Celyad oncology. Celyad oncology provides updates on allogeneic and autologous CAR T programs at 62nd ASH annual meeting and exposition. Belgium: Mont-Saint-Guibert; 2020. p. 4. [Google Scholar]
- 112.Sallman DA, Kerre T, Havelange V, Poire X, Lewalle P, Wang ES, Brayer JB, Davila ML, Moors I, Machiels JP, et al. CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): haematological cohorts of the dose escalation segment of a phase 1 trial. Lancet Haematol. 2023;10(3):e191–202. PMID: 36764323. doi: 10.1016/S2352-3026(22)00378-7. [DOI] [PubMed] [Google Scholar]
- 113.Celyad Oncology SA. Study in relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome patients to determine the recommended dose of CYAD-02. In: Study in relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome patients to determine the recommended dose of CYAD-02. 2021. (NCT04167696). [Google Scholar]
- 114.Deeren D, Maertens JA, Lin TL, Beguin Y, Alcantar-Orozco E, Dheur M-S, Breman E, Braun N, Lonez C, Gilham D, et al. Co-expression of an shRNA targeting MICA/Micb improves the clinical activity of a NKG2D-Based CAR T in patients with relapsed/refractory AML/MDS. Blood. 2021;138(Supplement 1):408. doi: 10.1182/blood-2021-152413. [DOI] [Google Scholar]
- 115.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. PMID: 11133738. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 116.Nkarta I. NKX101, intravenous allogeneic CAR NK cells, in adults with AML or MDS NKX101, Intravenous Allogeneic CAR NK Cells, in Adults With AML or MDS. 2023. (NCT04623944). [Google Scholar]
- 117.Mann G. Nkarta announces positive preliminary dose finding data for two lead engineered natural killer cell programs. In: Nkarta announces positive preliminary dose finding data for two lead engineered natural killer cell programs. Company website; 2022. [Google Scholar]
- 118.Curio S, Jonsson G, Marinovic S. A summary of current NKG2D-based CAR clinical trials. Immunother Adv. 2021;1(1):ltab018. PMID: 34604863. doi: 10.1093/immadv/ltab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goulding J, Hancock B, Blum R, Yeh W-I, Chang C-W, Pribadi M, Pan Y, Chu H-Y, Sikaroodi S, Dailey T, et al. 204 combining FT536, a pan-tumor targeting CAR NK cell therapy, with CD16 engagers provides a coordinated targeting strategy to overcome tumor heterogeneity. J ImmunoTher Cancer. 2022;10:A217. doi: 10.1136/jitc-2022-SITC2022.0204. [DOI] [Google Scholar]
- 120.Woan KV, Kim H, Bjordahl R, Davis ZB, Gaidarova S, Goulding J, Hancock B, Mahmood S, Abujarour R, Wang H, et al. Harnessing features of adaptive NK cells to generate Ipsc-derived NK cells for enhanced immunotherapy. Cell Stem Cell. 2021;28(12):2062–75 e2065. PMID: 34525347. doi: 10.1016/j.stem.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tartaglia C. Fate therapeutics announces termination of collaboration agreement with janssen, pipeline prioritization, next-generation programs, and key 2023 initiatives. In: Fate therapeutics announces termination of collaboration agreement with janssen, pipeline prioritization, next-generation programs, and key 2023 initiatives. Fate Therapeutics Company Website; 2023. [Google Scholar]
- 122.Hagelstein I, Lutz MS, Schmidt M, Heitmann JS, Malenke E, Zhou Y, Clar KL, Kopp HG, Jung G, Salih HR, et al. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins as novel treatment option in advanced soft tissue sarcomas. Front Immunol. 2021;12:653081. PMID: 33936075. doi: 10.3389/fimmu.2021.653081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Acheampong DO, Tang M, Wang Y, Zhao X, Xie W, Chen Z, Tian W, Wang M, Zhang J. A novel fusion antibody exhibits antiangiogenic activity and stimulates NK cell-mediated immune surveillance through fused NKG2D ligand. J Immunother (1991). 2017;40(3):94–103. PMID: 28234666. doi: 10.1097/CJI.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Li H, Xu W, Pan M, Qiao C, Cai J, Xu J, Wang M, Zhang J. BCMA-targeting bispecific antibody that simultaneously stimulates NKG2D-enhanced efficacy against multiple myeloma. J Immunother (1991). 2020;43(6):175–88. PMID: 32349046. doi: 10.1097/CJI.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 125.Wang T, Sun F, Xie W, Tang M, He H, Jia X, Tian X, Wang M, Zhang J. A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett. 2016;372(2):166–78. PMID: 26791237. doi: 10.1016/j.canlet.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Bykova K, Faber M, Liu K, Siddiqi N, Bernett M, Bonzon C, Diaz J, Nam DH, Avery K, Qi J, et al. 787 Natural killer cell engagers activate innate and adaptive immunity and show synergy with proinflammatory cytokines. J ImmunoTher Cancer. 2021;9(Suppl 2):A822. doi: 10.1136/jitc-2021-SITC2021.787. [DOI] [Google Scholar]
- 127.Du C, Bevers J 3rd, Cook R, Lombana TN, Rajasekaran K, Matsumoto M, Spiess C, Kim JM, Ye Z. MICA immune complex formed with alpha 3 domain-specific antibody activates human NK cells in a Fc-dependent manner. J ImmunoTher Cancer. 2019;7(1):207. PMID: 31387641. doi: 10.1186/s40425-019-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arreygue-Garcia NA, Daneri-Navarro A, Del Toro-Arreola A, Cid-Arregui A, Gonzalez-Ramella O, Jave-Suarez LF, Aguilar-Lemarroy A, Troyo-Sanroman R, Bravo-Cuellar A, Delgado-Rizo V, et al. Augmented serum level of major histocompatibility complex class I-related chain a (MICA) protein and reduced NKG2D expression on NK and T cells in patients with cervical cancer and precursor lesions. BMC Cancer. 2008;8(1):16. PMID: 18208618. doi: 10.1186/1471-2407-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kloss S, Chambron N, Gardlowski T, Arseniev L, Koch J, Esser R, Glienke W, Seitz O, Kohl U. Increased sMICA and TGFβ 1 levels in HNSCC patients impair NKG2D-dependent functionality of activated NK cells. Oncoimmunology. 2015;4(11):e1055993. PMID: 26451327. doi: 10.1080/2162402X.2015.1055993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain a in advanced human hepatocellular carcinomas. J Hepatol. 2005;43(6):1013–20. PMID: 16168521. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 131.Basher F, Dhar P, Wang X, Wainwright DA, Zhang B, Sosman J, Ji Z, Wu JD. Antibody targeting tumor-derived soluble NKG2D ligand sMIC reprograms NK cell homeostatic survival and function and enhances melanoma response to PDL1 blockade therapy. J Hematol Oncol. 2020;13(1):74. PMID: 32517713. doi: 10.1186/s13045-020-00896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang J, Larrocha PS, Zhang B, Wainwright D, Dhar P, Wu JD. Antibody targeting tumor-derived soluble NKG2D ligand sMIC provides dual co-stimulation of CD8 T cells and enables sMIC(+) tumors respond to PD1/PD-L1 blockade therapy. J ImmunoTher Cancer. 2019;7(1):223. PMID: 31446896. doi: 10.1186/s40425-019-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu S, Zhang J, Liu D, Li G, Staveley-O’Carroll KF, Li Z, Wu JD. Nonblocking monoclonal antibody targeting soluble MIC revamps endogenous innate and adaptive antitumor responses and eliminates primary and metastatic tumors. Clin Cancer Res. 2015;21(21):4819–30. PMID: 26106076. doi: 10.1158/1078-0432.CCR-15-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blery M, Mrabet-Kraiem M, Morel A, Lhospice F, Bregeon D, Bonnafous C, Gauthier L, Rossi B, Remark R, Cornen S, et al. Targeting MICA/B with cytotoxic therapeutic antibodies leads to tumor control [version 2; peer review: 2 approved]. Open Res Eur. 2021;1:107. PMID: 35967081. doi: 10.12688/openreseurope.13314.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferrari de Andrade L, Kumar S, Luoma AM, Ito Y, da Silva PH A, Pan D, Pyrdol JW, Yoon CH, Wucherpfennig KW. Inhibition of MICA and MICB shedding elicits NK-Cell–Mediated immunity against tumors resistant to cytotoxic T cells. Cancer Immunol Res. 2020;8(6):769–80. PMID: 32209637. doi: 10.1158/2326-6066.CIR-19-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Andrade L F, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, Tsoucas D, Franz B, May KF HC Jr., Harvey CJ, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. Science. 2018;359(6383):1537–42. PMID: 29599246. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Badrinath S, Dellacherie MO, Li A, Zheng S, Zhang X, Sobral M, Pyrdol JW, Smith KL, Lu Y, Haag S, et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature. 2022;606(7916):992–98. PMID: 35614223. doi: 10.1038/s41586-022-04772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whalen K, Naveen M, Meetze K, Gibson N, Michaelson J, Baeuerle P. 1395 CLN 619 a clinical stage MICA B specific IgG1 antibody which restores the MICA B-NKG2D axis requires Fc function for potent anti-tumor activity. J ImmunoTher Cancer. 2022;10:A1450. doi: 10.1136/jitc-2022-SITC2022.1395. [DOI] [Google Scholar]
- 139.Whalen KA, Mehta NK, Yalcin S, Meetze K, Gibson NW, Michaelson JS, Baeuerle PA. Abstract 3506: cLN-619, a clinical-stage MICA/MICB-specific IgG1 antibody, restores the MICA/MICB-NKG2D axis to promote NK-mediated tumor cell lysis. Cancer Res. 2022;82(12_Supplement):3506. doi: 10.1158/1538-7445.Am2022-3506. [DOI] [Google Scholar]
- 140.Parsons MS, Richard J, Lee WS, Vanderven H, Grant MD, Finzi A, Kent SJ. NKG2D acts as a co-receptor for natural killer cell-mediated anti-HIV-1 antibody-dependent cellular cytotoxicity. AIDS Res Hum Retroviruses. 2016;32(10–11):1089–96. PMID: 27487965. doi: 10.1089/AID.2016.0099. [DOI] [PubMed] [Google Scholar]
- 141.Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 2019;10:3038. PMID: 32038612. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. 2019;16(5):415–22. PMID: 30911118. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, Wang Y, Xiong F, Guo C, Li Y, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. 2019;18(1):29. PMID: 30813924. doi: 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


