Abstract
The widespread use of portable ultrasound scanners has promoted the concept of point of care ultrasound (POCUS), namely “ultrasound performed bedside and interpreted directly by the clinician.” The purpose of this short review is to outline how POCUS can be used in patients with diseases of the gastrointestinal (GI) tract. POCUS is not a replacement for comprehensive ultrasound, but rather allows physicians immediate access to clinical imaging for rapid diagnosis and efficient work-up and treatment of the patients. There are many indications for doing POCUS of the GI tract, including abdominal pain, diarrhea, palpable masses, and to detect fluid or free air in the abdominal cavity. To improve the visibility of deeper parts of the abdomen, the graded compression technique with the scan head is useful. During POCUS, the operator should look for signs of severe pathology including target lesions, the pseudo-kidney sign, the onion sign, dilated bowel loops, gastric retention, free fluid, and free air, depending on the actual clinical problem. We conclude that POCUS of the GI tract is very useful to provide a rapid diagnosis in many clinical scenarios.
Keywords: Abdominal diseases, gastrointestinal ultrasound, inflammatory bowel disease (IBD), point of care ultrasound, ultrasonography
INTRODUCTION
The utilization of portable scanners has paved the way for bedside scanning and the use of ultrasound in emergency settings,[1,2,3] leading up to the concept of point-of-care ultrasound (POCUS).[4] POCUS examinations differ from ordinary, more comprehensive examinations.[5,6] Ordinary abdominal ultrasound examinations cover several anatomical regions performed in a systematic way, for example, “6+” #15930[7] and result in a full report of the examination. On the contrary, the focus of POCUS examinations is to answer specific questions rapidly (e.g., does my patient have dilated bowels?), and the findings are often included in the general patient report.
Ultrasound of the gastrointestinal (GI) tract requires an experienced operator as systematic scanning of tiny details is often necessary to detect pathological changes and reach the correct diagnosis. Furthermore, pattern recognition of well-known clinical ultrasound signs of severe pathology is important to identify during scanning. Therefore, seven guidelines were published to establish a sound scientific and clinical foundation for the use of GI ultrasound (GIUS).[8,9,10,11,12,13,14] There are numerous indications for doing POCUS of the GI tract, including abdominal pain, diarrhea, palpable masses, and also to detect fluid or free air in the abdominal cavity.[15] In these cases, POCUS is often indicated as a first diagnostic procedure to guide further work-up and treatment.[10,16]
The aim of this short review paper is to present how POCUS can be applied in patients which have signs of diseases of the GI tract.
EXAMINATION TECHNIQUES AND NORMAL FINDINGS
Ultrasound B-mode and Doppler
The thickness of the bowel wall is usually <2 mm in a healthy subject,[17,18] Accordingly, the frequency of the transducer should be at least 5 megahertz (MHz) for wall layers to be well discriminated,[18,19] A low-frequency transducer is preferred to obtain a good overview of the bowels, whereas a high-frequency linear scan head (9–12 MHz) is recommended for detailed studies of the GI wall layers and its pathology.
Doppler US can assess the flow velocity of visceral vessels that supply the GI tract and evaluate the vascularity of smaller vessels in the intestinal wall.[20] Color Doppler can be used to determine the direction of flow and to semi-quantitatively estimate bowel wall vascularity,[21] as shown in Figure 1. It is important to optimize the color Doppler parameters to increase the sensitivity for low-velocity flow in the bowel wall to avoid false-negative results.[22]
Figure 1.
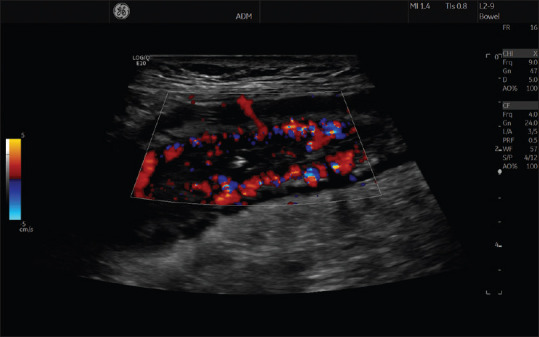
The image shows multiple color Doppler signals inside the intestinal wall of a patient with Crohn's disease, indicating increased vascularity, dilated vessels, and inflammatory activity
Scanning procedure
To improve the visibility of deeper parts of the intestines, graded compression is performed by using the US probe much in the same way as when performing palpation with the fingertips.[23] Manual force onto the scan head is used to compress the abdominal wall to push away overlying bowel segments with gas or intra-abdominal fat and in this way enable the examiner to reach deeper, for example, in the pelvic region.[24,25,26]
The rectum can be scanned with a low-frequency probe using a well-filled urinary bladder as an acoustic window. The cecum, ileocecal valve, and terminal ileum can be identified lying over the iliopsoas muscle in the right iliac region using a high-frequency linear probe. Furthermore, this is a convenient location to start a systematic scan of both the large and small intestines.[6] The hallmark of the large bowel is the haustrations, which are best viewed with the probe oriented in the bowel's longitudinal direction. After the cecum has been identified in the right iliac fossa, the large bowel is followed in the distal direction through the ascending colon, transverse colon, descending colon, sigmoid colon, and finally the rectum.[27]
Scanning of the small bowel can start by returning the probe to the right iliac fossa and identifying the terminal ileum. The examiner should then follow the terminal ileum as far as possible in a proximal direction. The rest of the small bowel should be scanned in parallel overlapping lanes cranially and caudally almost like “mowing the lawn” searching for signs of pathology. If the dorsal wall of the abdominal cavity can be seen clearly throughout the scanning, it could reduce the risk of missing pathology and increase the examiner's confidence in the examination.
Bowel wall thickness and layers
The GI wall consists of five sonographic layers, which correlate well with histological sections,[28,29] Bowel wall thickness (BWT) is measured from the outer border of the hypoechoic proper muscle layer and into the lumen interface shown as a bright line between the mucosa and the lumen, often with small air bubbles [Figure 2]. Typically, both the normal small and large intestine is <2 mm when distended,[30,31] The exceptions are the gastric antrum, the duodenal bulb, sigmoid colon (thicker proper muscle layer), and rectum which normally have BWT <3–4 mm.[18]
Figure 2.
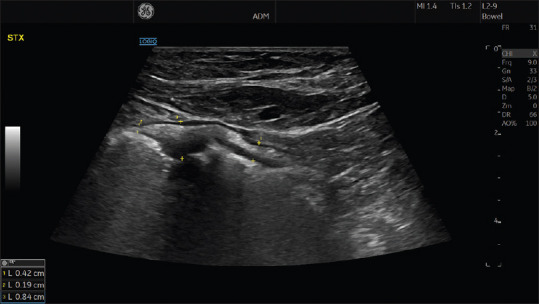
Measurement of BWT is performed by locating the cursor at the outer point of the proper muscle layer and moving through the wall layers to the interface echo between the mucosa and lumen, see yellow markers. The ultrasonogram demonstrates three different measurements in a pathological segment in a patient with Crohn's disease. BWT: Bowel wall thickness
Signs of gastrointestinal pathology
When performing POCUS of the GI tract, the operator should look for signs of severe pathology, which includes the following: Target lesions, the pseudo-kidney sign, the onion sign, dilated bowel loops, gastric retention, free fluid, and free air.
Target lesion and pseudo-kidney sign
A target lesion or the pseudo-kidney sign are sign of significant pathology, most often inflammation or malignancy in a short segment of the GI tract.[32]
In a patient with Crohn's disease, a target lesion, where the bowel is scanned in a transverse section, most commonly represents a stenosis [Figure 3]. In a longitudinal section of the bowel, a stenosis may appear more like a pseudo-kidney lesion [Figure 4]. However, a pseudo-kidney lesion or a target lesion may also indicate colonic cancer, acute appendicitis or diverticulitis, but also other conditions can induce a significant BWT. If the operator has access to a high-end scanner with a high-frequency transducer more subtle changes in the GI-wall can be detected, for example, discerning between normal and slightly thickened intestinal wall.
Figure 3.
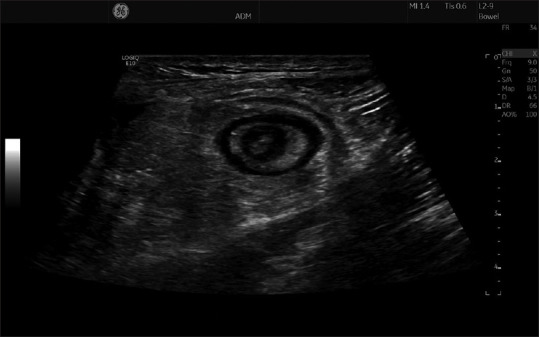
A target lesion with concentric rings is observed when the wall layers are markedly thickened and projected in a transverse section, like in this patient with inflammatory bowel disease
Figure 4.
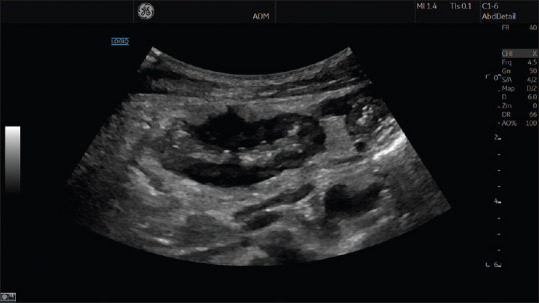
This image shows a pseudo-kidney lesion, depicting a longitudinal section of the intestine in a patient with Crohn's disease. Note in the central upper part of image that the transmural inflammatory activity is creeping out through the proper muscle layer into the surrounding bright fatty tissue
Findings in inflammatory bowel disease
Typical complications of Crohn's disease are fistulas, phlegmons, and abscesses. Fistulas from the GI tract are seen as hypoechoic tract connecting with the bowel lumen and its endpoint. Sometimes the lumen is gas-filled and identified as a hyperechoic tract within. An abscess is a rounded, hypoechoic lesion sometimes with hyperechoic gas contents floating to the top [Figure 5]. It can be confused with a phlegmon but a phlegmon does not contain gas and often vessels are detected within using color Doppler.[33,34]
Figure 5.
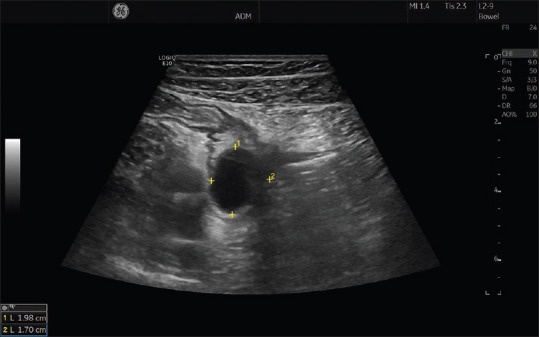
An abscess cavity is depicted between the four markers in a patient with Crohn's disease. Note the bowel segment to the upper left of the abscess with a twisted fistula leading down into the left side of the abscess cavity
Although there is so far less evidence indicating the accuracy of POCUS for detecting these lesions, it appears useful.[35,36]
Patients with inflammatory bowel diseases require frequent follow up to evaluate and adjust medical treatment. GIUS in a point-of-care setting has been shown to be accurate,[37] and the inclusion of POCUS in the decision-making leads to significant changes in disease management.[35,38,39] Evidence also suggests that the introduction of POCUS reduces the need for magnetic resonance (MR) enterography and colonoscopy.[40]
The onion sign
The onion sign denotes the classical finding of bowel invagination, most often observed in children using ultrasound.[41] The characteristic pattern arises when the proximal part of a bowel segment is feeding into the bowel distally located, thus generating multiple layers resembling a section through an onion [Figure 6]. If both the anterior and posterior walls are seen in a section through the bowel invagination, then 20 layers may be observed. Furthermore, one may also observe mesentery inside the lumen, as well as a polyp or tumor, which is often the cause of the invagination in adults.[42]
Figure 6.
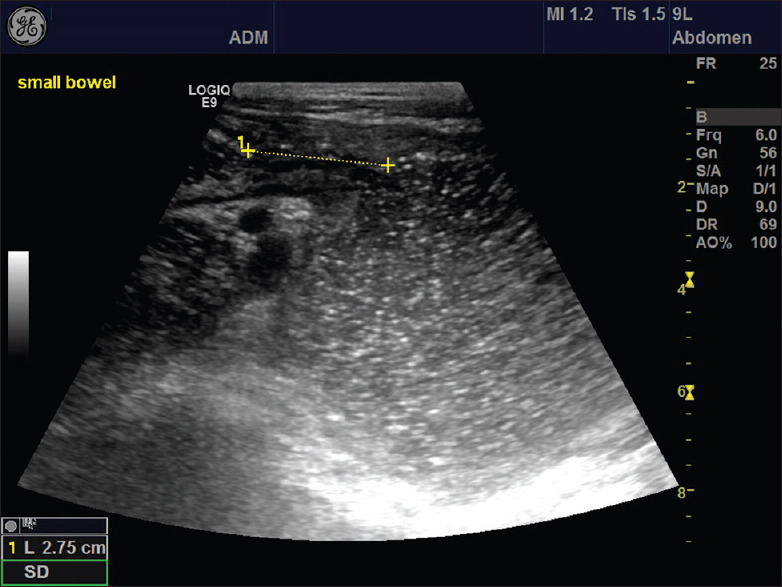
A marked dilatation (7 cm) of the small bowel is observed in this image of a young boy with chronic abdominal pain, weight loss and diarrhea. The yellow cursors depict the length of the narrow stenosis giving rise to the dilatation caused by Crohn's disease
Dilated bowel loops
Dilated bowel loops are a marker of intestinal obstruction, which in turn may have several causes.[43] POCUS can easily demonstrate dilated fluid-filled loops of the intestines [Figure 7], changes in peristaltic activity, and often a collapsed distal bowel.[44] Furthermore, POCUS may confirm or exclude bowel obstruction, decide whether small bowel dilatation is mechanical or functional, identify the site of obstruction, and point toward the cause of the obstruction.[13] Small bowel obstruction is usually diagnosed if the dilatation exceeds 2.5–3 cm over a length of at least 2–3 loops or more than 10 cm. Passage of the bowel content may be absent, normal or increased with to-and-fro motion, depending on the cause of occlusion.
Figure 7.
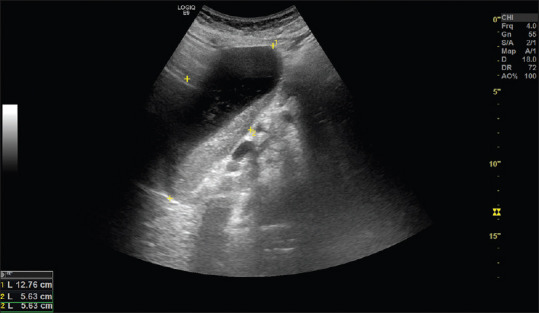
The ultrasonogram shows a major expansion of the gastric antrum in a patient with a stricturing tumor in the proximal small intestine. The content of the stomach has both liquid and solid components and represent significant gastris retention
Initially, mechanical obstruction is evident by increased peristaltic activity that may diminish later with potential progression toward a chronic condition. Looking for collapsed bowel loops distally to a stenosis is important to locate the exact position of the obstruction. Typically, the site is detected by observing a contracted descending colon or terminal ileum.
For a systematic scanning approach, a 3-step examination technique was developed: 1st step scanning of epigastrium (stomach); 2nd step scanning of the left mid abdomen (jejunum and descending colon); 3rd step right lower abdomen (ileocecal junction). Particularly for inexperienced operators, this method may help to get a first overview whether bowel obstruction is present or not, and which segments are involved.[45] Moreover, using POCUS to diagnose bowel obstruction may save lives and reduce costs significantly.[46]
Gastric retention – dilated stomach
Severe delayed gastric emptying may result in the retention of food and fluids in the gastric compartment. The distal part of the stomach is readily available for ultrasound scanning both in vertical and horizontal sections in the epigastrium.[47,48] Depending on the body habitus of the patient, the antrum may be located at various positions in the craniocaudal direction. Classically, a tall, slim female will have the antrum located in the caudal part of the epigastrium whereas a corpulent male will tend to have the antrum in the uppermost part of the abdomen. The proximal part of the stomach is more difficult to scan but is easier to obtain in a fluid-filled stomach,[49,50] By positioning the probe in the upper epigastrium and tilting it cranially, the proximal stomach can be scanned. Alternatively, in a left lateral, intercostal approach, the spleen can be used as an acoustic window to allow for scanning of the proximal gastric compartment.[51]
The etiology of gastric retention is manifold, ranging from severe dysmotility of the antro-pyloric segment to malignancies of the upper GI tract giving rise to stenosis, as shown in Figure 8. Pyloric stenosis often result is severe gastric retention and both the thickened muscle layer of the pylorus and the dilatation of the stomach can be observed by POCUS. In patients with clinical signs of small bowel obstruction, the presence of a dilated and fluid-filled stomach gives further evidence to the correct diagnosis. Moreover, POCUS of the stomach may also be useful to assess the need of prompt placement of a nasogastric tube to prevent lung aspiration of gastric content.
Figure 8.
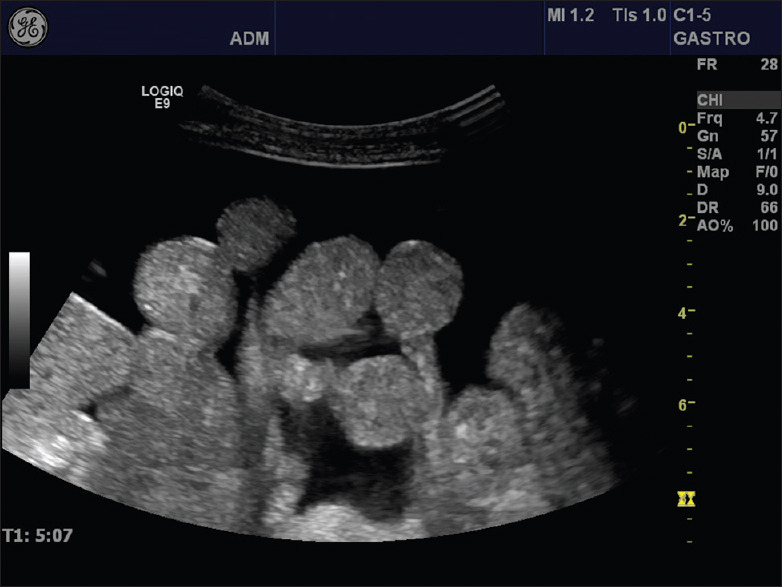
Many small bowel loops with mesenteric attachment can be observed in the abdominal cavity surrounded by ascites. POCUS is highly sensitive to detect even the smallest amount of free abdominal fluid. POCUS: Point of care ultrasound
Free fluid in the peritoneal space
Free fluid in the abdominal cavity is typically detected in peritoneal spaces such as the perihepatic space (Morrison's pouch), the perisplenic space (Koller's pouch), and the deep pelvic space (pouch of Douglas),[52] as shown in [Figure 9]. Ultrasonography is highly sensitive to detect even the smallest amount of liquid. Accordingly, examination for free fluid is used by POCUS, for example, with the focused assessment with sonography in trauma (FAST) protocol.[53,54,55,56,57] Furthermore, to scan for ascites, for example, in chronic liver diseases or abdominal malignancies, is highly feasible by applying POCUS.[3,58] Moreover, POCUS is used to guide the aspiration of intraperitoneal fluid by needle drainage or catheter placement.[59]
Figure 9.
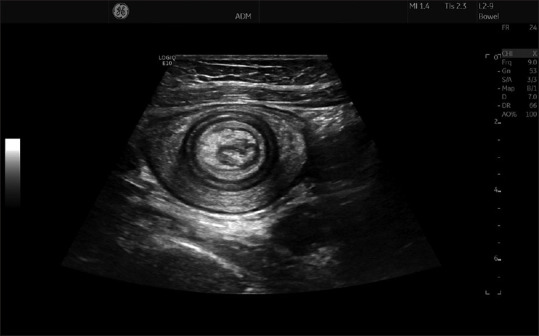
The onion sign is depicted in this ultraonogram of a patient with invagination due to a large polyp in the small intestine. The onion sign refers to the similarity between all the visible bowel wall layers and a section through an onion. Note that the polyp and parts of the mesentery can be seen on the luminal side of the lesion
Free air in the abdominal cavity
Pneumoperitoneum is a rare cause of acute abdominal pain and its etiology encompasses perforation of peptic ulcer, diverticulitis, ischemic bowel disease, trauma, or neoplasm.[60] The classical symptom is a sudden onset of severe abdominal pain. Free abdominal air is the main sign of GI perforation and rapid diagnosis is very important due to its high mortality. Ultrasound can be used as the initial diagnostic tool for the evaluation of patients with acute GI perforation.[61] Although the presence of gas sometimes may limit the ultrasonic field of view, detection of gas collections is an important element of ultrasound imaging in acute abdominal conditions.[62]
Examination protocols may include scanning of the epigastrium and the right hypochondrium.[63,64] Pneumoperitoneum is often described as an accentuation of the peritoneal stripe, similar to a sharp echogenic line, and as hyperechoic foci with reverberations and so-called “dirty shadowing.”[65,66] This phenomenon is usually best visible between the abdominal wall and the anterior surface of the liver [Figure 10]. Typically, free gas is easily moved around by pressure of the scan head or movement of the patient (the shifting phenomenon) and this indicates free intraperitoneal air. Furthermore, simple application and release of probe pressure can displace free gas in the epigastrium and subsequently the liver appears and disappears (like opening and closing of a curtain).[67] However, note that detection of pneumoperitoneum is a difficult task because free intraperitoneal air is easily confused with intraluminal air of the bowels.
Figure 10.
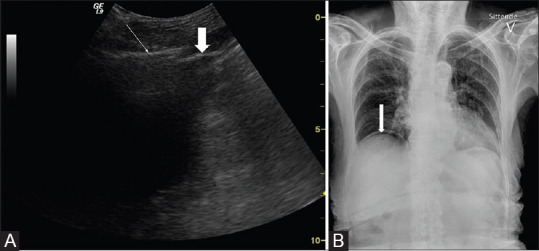
A 100-year-old female was admitted to the hospital with nausea and acute abdominal pain in the upper epigastrium. Panel A shows an ultrasonogram with free air (white thin arrow) above the liver as a bright line with a dirty shadow, and the thick arrow depicts the liver capsule. In panel B, the X-ray image demonstrates free air between the liver and the diaphragm (white arrow)
DISCUSSION/CONCLUSION
In this review paper, we have elaborated on the use of POCUS to diagnose acute and severe diseases of the GI tract. The great advantage of POCUS compared to computed tomography and MR is the immediate availability, inexpensive cost, and high level of safety. Accordingly, the use of POCUS at first glance when the patient meets the doctor has a great potential to streamline further work-up and treatment. Patients with symptoms like acute abdominal pain, diarrhea or findings like palpable masses or distended abdomen may all profit by the application of POCUS. However, scanning of the GI tract and particularly if looking for pneumoperitoneum requires an experienced operator. Therefore, education of doctors in ultrasonography and providing hands-on training is key to enable the efficient use of POCUS.[68,69] We conclude that POCUS of the GI tract is very useful to provide a rapid diagnosis in many clinical scenarios.
Financial support and sponsorship
Nil.
Conflicts of interest
Odd Helge Gilja has received speaker honoraria from the following companies: AbbVie, Bracco, Almirall, GE Healthcare, Takeda AS, Meda AS, Ferring AS, Allergan, and Janssen-Cilag. He has served as a consultant for Bracco, GE Healthcare, Takeda and Samsung, but not during the past 3 years.
Kim Nylund has received speaker honoraria from Takeda and Janssen Cilag AS.
REFERENCES
- 1.Gilja OH, Hausken T, Ødegaard S, Wendelbo Ø, Thierley M. Mobile ultrasonography in a medical department. Tidsskr Nor Laegeforen. 2003;123:2713–4. [PubMed] [Google Scholar]
- 2.Piscaglia FD, Nolsoe C, Gilja OH, Gaitini D. Birth of echoscopy – The efsumb point of view. Ultraschall Med. 2013;34:92. [Google Scholar]
- 3.Barreiros AP, Cui XW, Ignee A, De Molo C, Pirri C, Dietrich CF. Echo scopy in scanning abdominal diseases: Initial clinical experience. Z Gastroenterol. 2014;52:269–75. doi: 10.1055/s-0033-1350114. [DOI] [PubMed] [Google Scholar]
- 4.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–57. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 5.Torres-Macho J, Aro T, Bruckner I, Cogliati C, Gilja OH, Gurghean A, et al. Point-of-care ultrasound in internal medicine: A position paper by the ultrasound working group of the European federation of internal medicine. Eur J Intern Med. 2020;73:67–71. doi: 10.1016/j.ejim.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich CF, Goudie A, Chiorean L, Cui XW, Gilja OH, Dong Y, et al. Point of care ultrasound: A WFUMB position paper. Ultrasound Med Biol. 2017;43:49–58. doi: 10.1016/j.ultrasmedbio.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Furset IM, Gilja OH. A structured systematic ultrasound examination of abdominal organs (6+technique) In: Nurnberg DC, Chammas C, Gilja OH, Sporea I, Sirli R, editors. WFUMB Course Book. London: World Federation for Ultrasound in Medicine and Biology; 2021. pp. 61–70. [Google Scholar]
- 8.Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med. 2017;38:273–84. doi: 10.1055/s-0042-115410. [DOI] [PubMed] [Google Scholar]
- 9.Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF, et al. EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall Med. 2018;39:304–17. doi: 10.1055/s-0043-125329. [DOI] [PubMed] [Google Scholar]
- 10.Dirks K, Calabrese E, Dietrich CF, Gilja OH, Hausken T, Higginson A, et al. EFSUMB position paper: Recommendations for gastrointestinal ultrasound (GIUS) in acute appendicitis and diverticulitis. Ultraschall Med. 2019;40:163–75. doi: 10.1055/a-0824-6952. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich CF, Hollerweger A, Dirks K, Higginson A, Serra C, Calabrese E, et al. EFSUMB gastrointestinal ultrasound (GIUS) task force group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med Ultrason. 2019;21:299–315. doi: 10.11152/mu-2162. [DOI] [PubMed] [Google Scholar]
- 12.Nuernberg D, Saftoiu A, Barreiros AP, Burmester E, Ivan ET, Clevert DA, et al. EFSUMB recommendations for gastrointestinal ultrasound part 3: Endorectal, endoanal and perineal ultrasound. Ultrasound Int Open. 2019;5:E34–51. doi: 10.1055/a-0825-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollerweger A, Maconi G, Ripolles T, Nylund K, Higginson A, Serra C, et al. Gastrointestinal ultrasound (GIUS) in intestinal emergencies – An EFSUMB position paper. Ultraschall Med. 2020;41:646–57. doi: 10.1055/a-1147-1295. [DOI] [PubMed] [Google Scholar]
- 14.Maconi G, Hausken T, Dietrich CF, Pallotta N, Sporea I, Nurnberg D, et al. Gastrointestinal ultrasound in functional disorders of the gastrointestinal tract – EFSUMB consensus statement. Ultrasound Int Open. 2021;7:E14–24. doi: 10.1055/a-1474-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puylaert JB. Ultrasound of acute GI tract conditions. Eur Radiol. 2001;11:1867–77. doi: 10.1007/s003300101076. [DOI] [PubMed] [Google Scholar]
- 16.Puylaert JB. Ultrasonography of the acute abdomen: Gastrointestinal conditions. Radiol Clin North Am. 2003;41:1227–42. doi: 10.1016/s0033-8389(03)00120-9. vii. [DOI] [PubMed] [Google Scholar]
- 17.Haber HP, Busch A, Ziebach R, Stern M. Bowel wall thickness measured by ultrasound as a marker of Crohn's disease activity in children. Lancet. 2000;355:1239–40. doi: 10.1016/S0140-6736(00)02092-4. [DOI] [PubMed] [Google Scholar]
- 18.Nylund K, Hausken T, Ødegaard S, Eide GE, Gilja OH. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med. 2012;33:E225–32. doi: 10.1055/s-0031-1299329. [DOI] [PubMed] [Google Scholar]
- 19.Aibe T, Fuji T, Okita K, Takemoto T. A fundamental study of normal layer structure of the gastrointestinal wall visualized by endoscopic ultrasonography. Scand J Gastroenterol Suppl. 1986;123:6–15. doi: 10.3109/00365528609091857. [DOI] [PubMed] [Google Scholar]
- 20.Patriquin HB, Garcier JM, Lafortune M, Yazbeck S, Russo P, Jequier S, et al. Appendicitis in children and young adults: Doppler sonographic-pathologic correlation. AJR Am J Roentgenol. 1996;166:629–33. doi: 10.2214/ajr.166.3.8623640. [DOI] [PubMed] [Google Scholar]
- 21.Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, et al. Doppler US in patients with crohn disease: Vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787–91. doi: 10.1148/radiology.217.3.r00dc19787. [DOI] [PubMed] [Google Scholar]
- 22.Drews BH, Barth TF, Hänle MM, Akinli AS, Mason RA, Muche R, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn's disease. Eur Radiol. 2009;19:1379–86. doi: 10.1007/s00330-008-1290-5. [DOI] [PubMed] [Google Scholar]
- 23.Puylaert JB. Mesenteric adenitis and acute terminal ileitis: US evaluation using graded compression. Radiology. 1986;161:691–5. doi: 10.1148/radiology.161.3.3538138. [DOI] [PubMed] [Google Scholar]
- 24.Puylaert JB. Acute appendicitis: US evaluation using graded compression. Radiology. 1986;158:355–60. doi: 10.1148/radiology.158.2.2934762. [DOI] [PubMed] [Google Scholar]
- 25.Schwerk WB, Schwarz S, Rothmund M. Sonography in acute colonic diverticulitis. A prospective study. Dis Colon Rectum. 1992;35:1077–84. doi: 10.1007/BF02252999. [DOI] [PubMed] [Google Scholar]
- 26.Nylund K, Ødegaard S, Hausken T, Folvik G, Lied GA, Viola I, et al. Sonography of the small intestine. World J Gastroenterol. 2009;15:1319–30. doi: 10.3748/wjg.15.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saevik F, Maconi G, Gilja OH. Gastrointestinal ultrasound (GIUS) – Normal findings. In: Nurnberg DC, Chammas C, Gilja OH, Sporea I, Sirli R, editors. WFUMB Course Book. Vol. 1. London: WFUMB; 2021. pp. 127–34. [Google Scholar]
- 28.Odegaard S, Kimmey MB, Martin RW, Yee HC, Cheung AH, Silverstein FE. The effects of applied pressure on the thickness, layers, and echogenicity of gastrointestinal wall ultrasound images. Gastrointest Endosc. 1992;38:351–6. doi: 10.1016/s0016-5107(92)70431-3. [DOI] [PubMed] [Google Scholar]
- 29.Odegaard S, Nesje LB, Hoff DA, Gilja OH, Gregersen H. Morphology and motor function of the gastrointestinal tract examined with endosonography. World J Gastroenterol. 2006;12:2858–63. doi: 10.3748/wjg.v12.i18.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haber HP, Stern M. Intestinal ultrasonography in children and young adults: Bowel wall thickness is age dependent. J Ultrasound Med. 2000;19:315–21. [PubMed] [Google Scholar]
- 31.Huh CH, Bhutani MS, Farfán EB, Bolch WE. Individual variations in mucosa and total wall thickness in the stomach and rectum assessed via endoscopic ultrasound. Physiol Meas. 2003;24:N15–22. doi: 10.1088/0967-3334/24/4/401. [DOI] [PubMed] [Google Scholar]
- 32.Nylund KG, Gilja OH, Dietrich CF. Gastrointestinal ultrasound. In: Nurnberg DC, Chammas C, Gilja OH, Sporea I, Sirli R, editors. WFUMB Course Book. Vol. 1. London: WFUMB; 2021. pp. 251–64. [Google Scholar]
- 33.Parente F, Maconi G, Bianchi PG. Bowel ultrasound in Crohn disease: Current role and future applications. Scand J Gastroenterol. 2002;37:871–6. doi: 10.1080/003655202760230801. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese E, Maaser C, Zorzi F, Kannengiesser K, Hanauer SB, Bruining DH, et al. Bowel ultrasonography in the management of Crohn's disease.A review with recommendations of an international panel of experts. Inflamm Bowel Dis. 2016;22:1168–83. doi: 10.1097/MIB.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 35.Gonen C, Surmelioglu A, Kochan K, Ozer S, Aslan E, Tilki M. Impact of intestinal ultrasound with a portable system in the management of Crohn's disease. Gastroenterol Rep (Oxf) 2021;9:418–26. doi: 10.1093/gastro/goaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Voogd FA, Verstockt B, Maaser C, Gecse KB. Point-of-care intestinal ultrasonography in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:209–10. doi: 10.1038/s41575-021-00418-4. [DOI] [PubMed] [Google Scholar]
- 37.Sathananthan D, Rajagopalan A, Van De Ven L, Martin S, Fon J, Costello S, et al. Point-of-care gastrointestinal ultrasound in inflammatory bowel disease: An accurate alternative for disease monitoring. JGH Open. 2020;4:273–9. doi: 10.1002/jgh3.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak K, Tanyingoh D, Petersen F, Kucharzik T, Panaccione R, Ghosh S, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn's disease: Impact on clinical decision making. J Crohns Colitis. 2015;9:795–801. doi: 10.1093/ecco-jcc/jjv105. [DOI] [PubMed] [Google Scholar]
- 39.Friedman AB, Asthana A, Knowles SR, Robbins A, Gibson PR. Effect of point-of-care gastrointestinal ultrasound on decision-making and management in inflammatory bowel disease. Aliment Pharmacol Ther. 2021;54:652–66. doi: 10.1111/apt.16452. [DOI] [PubMed] [Google Scholar]
- 40.Bots S, De Voogd F, De Jong M, Ligtvoet V, Löwenberg M, Duijvestein M, et al. Point-of-care intestinal ultrasound in IBD patients: Disease management and diagnostic yield in a real-world cohort and proposal of a point-of-care algorithm. J Crohns Colitis. 2022;16:606–15. doi: 10.1093/ecco-jcc/jjab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel I, Anand R, Choudhury SR, Agarwal S. Evolving concepts in ultrasonography of pediatric intussusceptions: Unequivocal differentiation of ileocolic, obstructive and transient small-bowel intussusceptions. Ultrasound Med Biol. 2020;46:589–97. doi: 10.1016/j.ultrasmedbio.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Klinger C, Riecken B, Dietrich CF, Dirks K, Caca K, Fröhlich E. Use of ultrasound in the diagnostic work-up of adult intussusception – A multicenter retrospective analysis. Ultraschall Med. 2020;41:418–27. doi: 10.1055/a-0604-2676. [DOI] [PubMed] [Google Scholar]
- 43.Miller G, Boman J, Shrier I, Gordon PH. Etiology of small bowel obstruction. Am J Surg. 2000;180:33–6. doi: 10.1016/s0002-9610(00)00407-4. [DOI] [PubMed] [Google Scholar]
- 44.Singer D, Larson J, Reardon L, Lohse M, Fernandes R, Sbayi S, et al. Can point-of-care ultrasound speed up time to diagnosis for small bowel obstruction? Acad Emerg Med. 2022;29:1379–80. doi: 10.1111/acem.14570. [DOI] [PubMed] [Google Scholar]
- 45.Leung AM, Vu H. Factors predicting need for and delay in surgery in small bowel obstruction. Am Surg. 2012;78:403–7. [PubMed] [Google Scholar]
- 46.Brower CH, Baugh CW, Shokoohi H, Liteplo AS, Duggan N, Havens J, et al. Point-of-care ultrasound- first for the evaluation of small bowel obstruction: National cost savings, length of stay reduction, and preventable radiation exposure. Acad Emerg Med. 2022;29:824–34. doi: 10.1111/acem.14464. [DOI] [PubMed] [Google Scholar]
- 47.Hveem K, Hausken T, Berstad A. Ultrasonographic assessment of fasting liquid content in the human stomach. Scand J Gastroenterol. 1994;29:786–9. doi: 10.3109/00365529409092511. [DOI] [PubMed] [Google Scholar]
- 48.Gilja OH. Ultrasound of the stomach – The EUROSON lecture 2006. Ultraschall Med. 2007;28:32–9. doi: 10.1055/s-2007-962866. [DOI] [PubMed] [Google Scholar]
- 49.Gilja OH, Hausken T, Odegaard S, Berstad A. Monitoring postprandial size of the proximal stomach by ultrasonography. J Ultrasound Med. 1995;14:81–9. doi: 10.7863/jum.1995.14.2.81. [DOI] [PubMed] [Google Scholar]
- 50.Gilja OH, Detmer PR, Jong JM, Leotta DF, Li XN, Beach KW, et al. Intragastric distribution and gastric emptying assessed by three-dimensional ultrasonography. Gastroenterology. 1997;113:38–49. doi: 10.1016/s0016-5085(97)70078-7. [DOI] [PubMed] [Google Scholar]
- 51.Hata T, Kato M, Kudo T, Nishida M, Nishida U, Imai A, et al. Comparison of gastric relaxation and sensory functions between functional dyspepsia and healthy subjects using novel drinking-ultrasonography test. Digestion. 2013;87:34–9. doi: 10.1159/000343935. [DOI] [PubMed] [Google Scholar]
- 52.Dolich MO, McKenney MG, Varela JE, Compton RP, McKenney KL, Cohn SM. 2,576 ultrasounds for blunt abdominal trauma. J Trauma. 2001;50:108–12. doi: 10.1097/00005373-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Scalea TM, Rodriguez A, Chiu WC, Brenneman FD, Fallon WF, Jr, Kato K, et al. Focused assessment with sonography for trauma (FAST): Results from an international consensus conference. J Trauma. 1999;46:466–72. doi: 10.1097/00005373-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Sisley AC, Rozycki GS, Ballard RB, Namias N, Salomone JP, Feliciano DV. Rapid detection of traumatic effusion using surgeon-performed ultrasonography. J Trauma. 1998;44:291–6. doi: 10.1097/00005373-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Blackbourne LH, Soffer D, McKenney M, Amortegui J, Schulman CI, Crookes B, et al. Secondary ultrasound examination increases the sensitivity of the FAST exam in blunt trauma. J Trauma. 2004;57:934–8. doi: 10.1097/01.ta.0000149494.40478.e4. [DOI] [PubMed] [Google Scholar]
- 56.Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: The extended focused assessment with sonography for trauma (EFAST) J Trauma. 2004;57:288–95. doi: 10.1097/01.ta.0000133565.88871.e4. [DOI] [PubMed] [Google Scholar]
- 57.Kirkpatrick AW, Simons RK, Brown R, Nicolaou S, Dulchavsky S. The hand-held FAST: Experience with hand-held trauma sonography in a level-I urban trauma center. Injury. 2002;33:303–8. doi: 10.1016/s0020-1383(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 58.Colli A, Prati D, Fraquelli M, Segato S, Vescovi PP, Colombo F, et al. The use of a pocket-sized ultrasound device improves physical examination: Results of an in- and outpatient cohort study. PLoS One. 2015;10:e0122181. doi: 10.1371/journal.pone.0122181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nolsøe CP, Lorentzen T, Skjoldbye BO, Bachmann Nielsen M. The basics of interventional ultrasound. Ultraschall Med. 2007;28:248–63. doi: 10.1055/s-2007-963226. [DOI] [PubMed] [Google Scholar]
- 60.Hefny AF, Abu-Zidan FM. Sonographic diagnosis of intraperitoneal free air. J Emerg Trauma Shock. 2011;4:511–3. doi: 10.4103/0974-2700.86649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nazerian P, Tozzetti C, Vanni S, Bartolucci M, Gualtieri S, Trausi F, et al. Accuracy of abdominal ultrasound for the diagnosis of pneumoperitoneum in patients with acute abdominal pain: A pilot study. Crit Ultrasound J. 2015;7:15. doi: 10.1186/s13089-015-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson SR, Burns PN, Wilkinson LM, Simpson DH, Muradali D. Gas at abdominal US: Appearance, relevance, and analysis of artifacts. Radiology. 1999;210:113–23. doi: 10.1148/radiology.210.1.r99ja12113. [DOI] [PubMed] [Google Scholar]
- 63.Lee DH, Lim JH, Ko YT, Yoon Y. Sonographic detection of pneumoperitoneum in patients with acute abdomen. AJR Am J Roentgenol. 1990;154:107–9. doi: 10.2214/ajr.154.1.2104691. [DOI] [PubMed] [Google Scholar]
- 64.Kuzmich S, Harvey CJ, Fascia DT, Kuzmich T, Neriman D, Basit R, et al. Perforated pyloroduodenal peptic ulcer and sonography. AJR Am J Roentgenol. 2012;199:W587–94. doi: 10.2214/AJR.11.8292. [DOI] [PubMed] [Google Scholar]
- 65.Muradali D, Wilson S, Burns PN, Shapiro H, Hope-Simpson D. A specific sign of pneumoperitoneum on sonography: Enhancement of the peritoneal stripe. AJR Am J Roentgenol. 1999;173:1257–62. doi: 10.2214/ajr.173.5.10541100. [DOI] [PubMed] [Google Scholar]
- 66.Alami N, Farahmand S, Akhgar A. Pneumoperitoneum by ultrasonography: Clinical imaging. Intern Emerg Med. 2019;14:183–4. doi: 10.1007/s11739-018-1937-4. [DOI] [PubMed] [Google Scholar]
- 67.Goudie A. Detection of intraperitoneal free gas by ultrasound. Australas J Ultrasound Med. 2013;16:56–61. doi: 10.1002/j.2205-0140.2013.tb00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puylaert JB, van Rijn RR. No hocus POCUS: High standards should be set for point-of-care ultrasonography. Ned Tijdschr Geneeskd. 2020;164:D5121. [PubMed] [Google Scholar]
- 69.Dietrich CF, Hoffmann B, Abramowicz J, Badea R, Braden B, Cantisani V, et al. Medical student ultrasound education: A WFUMB position paper, Part I. Ultrasound Med Biol. 2019;45:271–81. doi: 10.1016/j.ultrasmedbio.2018.09.017. [DOI] [PubMed] [Google Scholar]


