Abstract
Phytocannabinoids (and synthetic analogs thereof) are gaining significant attention as promising leads in modern medicine. Considering this, new directions for the design of phytocannabinoid-inspired molecules is of immediate interest. In this regard, we have hypothesized that axially-chiral-cannabinols (ax-CBNs), unnatural and unknown isomers of cannabinol (CBN) may be valuable scaffolds for cannabinoid-inspired drug discovery. There are two main factors directing our interest to these scaffolds: (a) ax-CBNs would have ground-state three-dimensionality; ligand–receptor interactions can be more significant with complimentary 3D-topology, and (b) ax-CBNs at their core structure are biaryl molecules, generally attractive platforms for pharmaceutical development due to their ease of functionalization and stability. Herein we report a synthesis of ax-CBNs, examine physical properties experimentally and computationally, and perform a comparative analysis of ax-CBN and THC in mice behavioral studies.
Keywords: Cannabinoids, Cannabinol, Atropisomerism, Axial-Chirality, Total Synthesis
Graphical Abstract
A new twist for cannabinoid research. We report a strategy to conformationally bias cannabinol (CBN) scaffolds in a three-dimensional orientation. CBN C-9 to C-10 methyl transposition yields axially-chiral cannabinols (ax-CBNs), unnatural isomers of CBN that are comprised of the pharmaceutically relevant biaryl framework and display ground state three-dimensionality due to steric hinderance. The conformationally biased scaffolds may provide new directions for cannabinoid-inspired drug discovery.
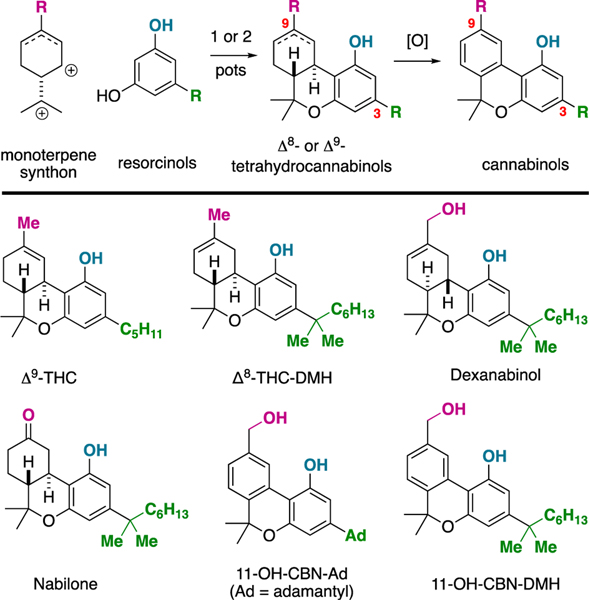
Cannabinoids are under intense investigation in modern medicine as leads for treating pain,2a epilepsy,2b nausea,2c and as appetite stimulants and suppressants.2d,2e Drug discovery efforts rely on the accessibility of cannabinoid natural products and analogues,3 which can be obtained from natural sources, by semisynthesis, or by total synthesis.4 Regarding the latter, the most common strategy to synthesize cannabinoids for drug discovery is through the union of monoterpene derivatives with resorcinols by a Friedel-Crafts alkylation then etherification pathway (Figure 1A).5 This route’s origins date back to the 1940’s (Adams5a,b) and 1960’s (Mechoulam5c) and is still utilized extensively in modern cannabinoid drug discovery. While efficient, molecular diversity about the cannabinoid core using this route is limited to the C3- and the C9-positions, generally speaking.6
Figure 1.
A: The standard route to synthesize cannabinoids. B: Representative natural products and analogs prepared by this method.
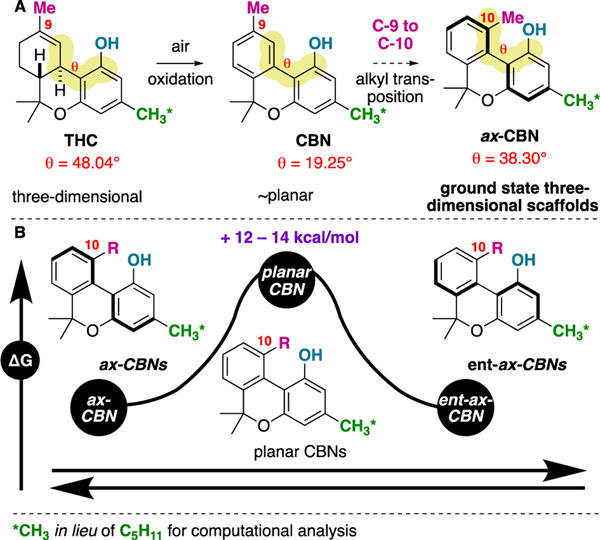
One potential approach to increase the diversity of synthetically accessible cannabinoid analogs is to devise novel synthetic routes to the natural products. In this regard, tetrahydrocannabinol (THC) has been extensively studied7 and the Carreira route is being utilized in analog synthesis,8 but there are also routes to cannabinol (CBN),9 cannabidiol (CBD),10 cannabichromene (CBC),11 and other major and minor cannabinoids. While total synthesis will continue to pave way for better understanding of cannabinoids, we wished to devise a novel scaffold that could potentially yield new research directions for cannabinoid-inspired drug discovery. We have conceived axially-chiral cannabinols (ax-CBNs); previously unknown and unnatural cannabinols. While natural cannabinol (CBN) and axially-chiral cannabinol (ax-CBN) have many similarities (they are isomeric), it is hypothesized that the C9 (natural) to C10 (unnatural) methyl transposition will yield unique three-dimensional molecules. Ax-CBNs are comprised of the pharmaceutically relevant biaryl framework12 and would be three-dimensional due to steric hindrance.13 On this line, it is often significant that a ligand and its receptors have complimentary 3D-topology.14 Supporting the proposed structure of ax-CBN, it was found that the dihedral angle between the aromatic rings was significantly non-planar (θ = 38.30°) compared to CBN (θ = 19.25°) (Figure 2A).15 It was also found that the barrier to atropisomerism is ~14.5 kcal/mol (R = CH2OH) (Figure 2B). The calculated barrier supports that ax-CBN would be biased to a three-dimensional conformer in its ground state but would be readily atropisomerizing.13 In other words, if binding cannabinoids to receptors is preferred in a three-dimensional arrangement, then ax-could provide uniquely tuned molecular conformations. Furthermore, because the atropisomers are calculated to be readily equilibrating,15 both enantiomers would be present in the biological system.
Figure 2.
A: A comparison of THC, CBN, and ax-CBN, including calculated dihedral angles about the carbocycle linkages B: ax-CBN is three-dimensional in its ground-state.
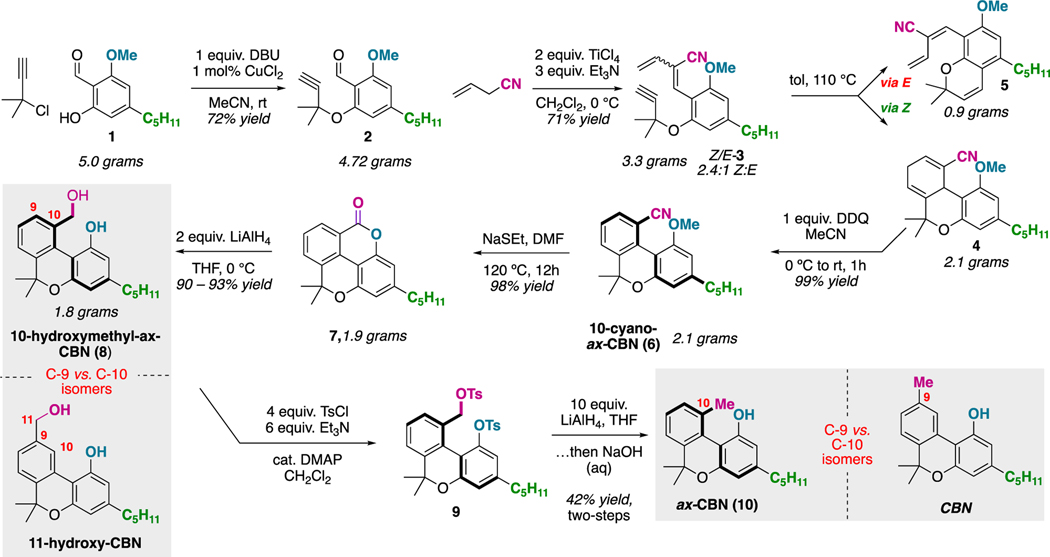
The hypothesis and the computational data related to ax-CBNs is compelling and supports that such scaffolds could be attractive new directions for cannabinoid-inspired drug discovery. As such, we set out to establish a concise route to access the targets. We have uncovered the following straightforward six- to eight- step route to axially-chiral cannabinols (ax-CBNs, Figure 3): Salicylaldehyde 116 can react with 1,1-dimethylpropargyl chloride via copper-catalysis to yield the phenyl propargyl ether 2.17 Condensation of 2 with allyl nitrile yielded an inseparable 2.5:1 Z:E diene 3 mixture. This Et3N and TiCl4 mediated condensation is based on a related protocol for the coupling of acrylates and benzaldehydes.18 Notably, the conditions and choice of the nitrile functional group were crucial for successful coupling (vide infra, Figure 4). Upon heating this mixture of Z:E dienes to promote the Diels-Alder reaction, it was found that only the Z-diene diastereomer undergoes the desired cycloaddition. The minor E-diene isomer converts to the chromene 5 by a propargyl Claisen rearrangement/intramolecular etherification.19 The two different scaffolds (4 and 5) are easily separated by silica gel chromatography. From the Diels-Alder adduct 4, biaryl scaffold 6 is prepared by DDQ-promoted oxidation.20 Lactone intermediate 7 is established whereby ethanethiolate promotes a demethylation and intramolecular Pinner sequence. The 10-hydroxymethyl-ax-CBN 8 is prepared by LiAlH4 reduction. The overall synthesis of 10-hydroxymethyl-ax-CBN 8 is six-steps from salicylaldehyde 1, dimethyl propargyl chloride, and allyl nitrile and is scalable: From 5 grams of the salicylaldehyde 1, 1.8 grams of 10-hydroxymethyl-ax-CBN (8) was prepared in a single pass under the optimized procedure. Additionally, 8 is a C-9 vs C-10 isomer of 11-hydroxy-CBN, a natural cannabinoid. From here, the parent ax-CBN (10) (the C-9 vs C-10 isomer of natural CBN) is accessed by benzylic reduction, which is accomplished over a two-step procedure involving bis-tosylation (9) and LiAlH4 reduction. The phenolic tosylate is partially removed during the reduction and fully removed by a basic work-up.
Figure 3:
Six- to eight- step de novo synthesis of ax-CBNs from abundant starting materials.
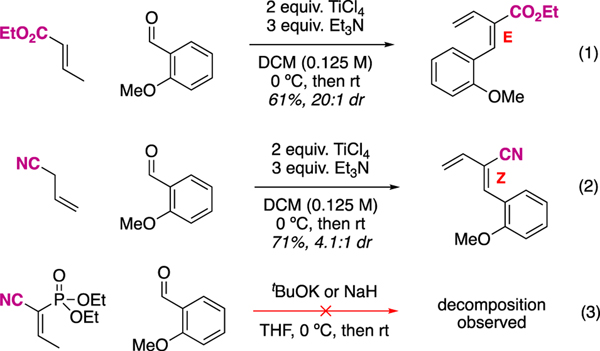
Figure 4.
The nitrile is significant to achieving the necessary Z-diene stereoisomer.
The use of allyl nitrile as the carbon source in this synthetic route is notable. Under the best conditions we have found to date (reported in Figure 3), allyl nitrile and the aldehyde yield a 2.4:1 mixture of Z:E diene isomers and only the major Z isomer proceeds to the desired product. In related attempts to optimize this diene synthesis, we explored crotonate-type nucleophiles (Figure 4, equation 1). With a model salicylaldehyde, undesired (for our synthesis) E-dienes are exclusively prepared. Thus, the sterically smaller nitrile results in a stereochemical switch to the desired Z-dienes (Figure 4, equation 2 and the applied version to ax-CBN synthesis in Figure 3). The different stereochemical outcomes for crotonates vs allyl nitriles are likely a thermodynamic preference. Finally, in limited attempts, vinylogous Horner-Wadsworth Emmons (HWE) reactions were unsuccessfully explored (Figure 4, equation 3). Furthermore, it is known in the literature that such alkylidene cyanophosphonates react by vinylogous HWE reaction, resulting in regioisomeric products. This could be a contributing factor to the decomposition observed.21
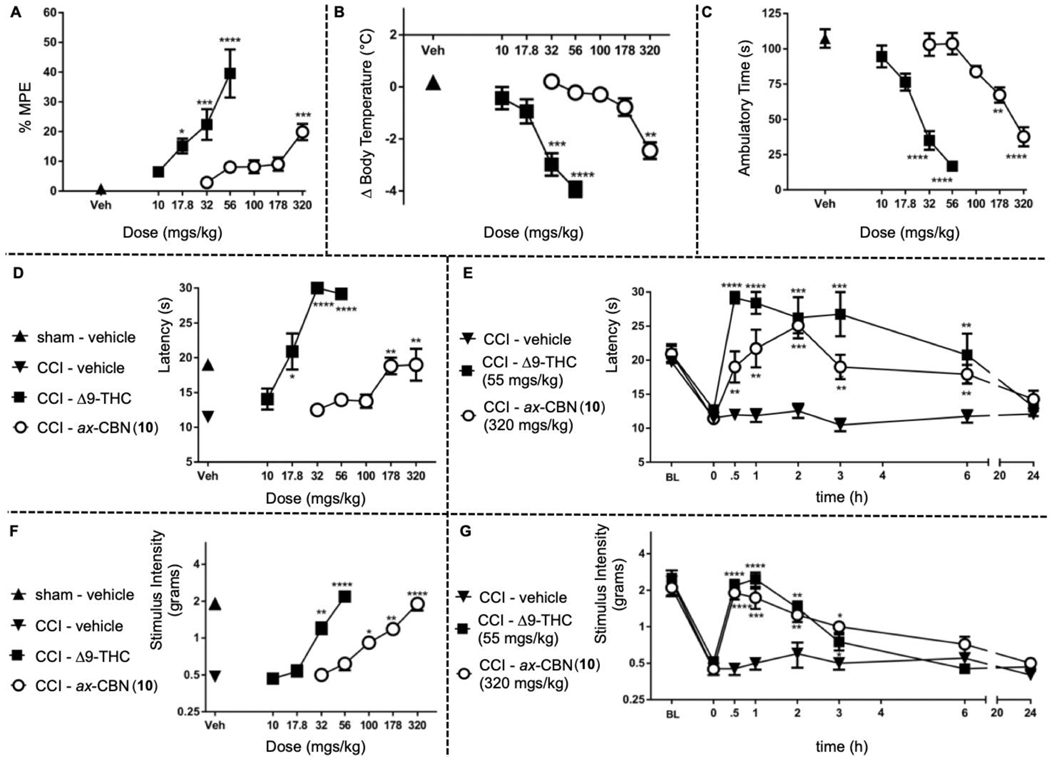
While the main goal of this work is to conceptualize ax-CBNs and develop a synthetic route to access the architecture, we also wished to provide preliminary support for their bioactivity. In this regard, we next performed a comparative analysis of ax-CBNs vs. THC in mice behavioral and analgesic studies. To examine whether ax-CBN produces overt physiological effects similar to THC, we assessed ax-CBN in a modified version of the tetrad assay, which consists of measuring acute thermal antinociception, body temperature, as well as locomotion and is generally used to screen CB1 receptor agonists.22,23 Mice were given vehicle, THC (10 – 56 mg/kg) or ax-CBN (56 – 320 mg/kg) and were tested in the three assays (Figure 5). Both THC and ax-CBN dose-relatedly produced thermal antinociception, hypothermia and decreased locomotion (see the supporting information for statistical analysis results). Given that THC is well-established to produce anti-pain behavioral effects in numerous animal studies,24,25 we next tested ax-CBN in a mouse model of neuropathic pain. Chronic constriction injury (CCI) of the sciatic nerve is widely used as a model of neuropathic pain and produces increased sensitivity to thermal heat, termed thermal hyperalgesia, as well as an increase in light touch sensitivity, termed mechanical allodynia.22,23,25,26 Both THC and ax-CBN dose-relatedly reversed CCI-induced thermal hyperalgesia within 30 minutes of injection, which persisted for at least 6 hours (Figure 5, see the supporting information for statistical analysis results). THC and ax-CBN also dose-relatedly reversed CCI-induced mechanical allodynia within 30 minutes of intraperitoneal administration, which persisted for at least 3 hours (Figure 5). Analysis reveals that ax-CBN reverses mechanical allodynia and thermal hyperalgesia in an equipotent manner. THC is less potent in the reversal of mechanical allodynia than in the reversal of thermal hyperalgesia. Further, THC produces cannabimimetic effects at doses required to reverse mechanical allodynia (see the supporting information for potency ratio analysis). Meanwhile, the doses of ax-CBN needed to reverse mechanical allodynia are about 2-fold lower than those that produce cannabimimetic effects. Therefore, ax-CBN and analogs may hold therapeutic promise in the treatment of chronic pain with fewer dose-limiting cannabimimetic effects.
Figure 5.
Behavioral and Biological Studies A: Tail-Flick Antinociception measured as percent maximum possible effect (% MPE) B: Body Temperature measured as change from baseline C: Locomotion measured as ambulatory time. D,E: Neuropathic Pain-Induced Thermal Hyperalgesia, measured as latency in seconds to respond F,G: Neuropathic Pain-Induced Mechanical Allodynia measured as grams required to produce a stimulus response.
In conclusion, we have developed an 8-step synthesis of axially-chiral cannabinol (ax-CBN), an unnatural isomer of cannabinol (CBN), whereby C-9 to C-10 methyl transposition results in an isomer with substantially unique ground-state three-dimensionality. This controlled structural change will provide new directions for cannabinoid-inspired drug discovery. On this line, we validated physical and biological properties, which support our hypothesis. Future studies include the identification of ax-CBN biological targets and the synthesis of designed analogs based on the synthetic route disclosed herein.
Supplementary Material
Acknowledgements
We thank the College of Liberal Arts and Sciences and the Department of Chemistry at the University of Florida for start-up funds. We thank the Mass Spectrometry Research and Education Center and their funding source: NIH S10 OD021758-01A1.
References
- [1].Zuardi AW, Brazilian J. Psychiatry 2006, 28, 153–157. [Google Scholar]
- [2].(a) Elikkottil J, Gupta P, Gupta K, Opioid Manag J. 2009, 5, 341–357. [PMC free article] [PubMed] [Google Scholar]; (b) Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, et al. , Epilepsia 2014, 55, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Parker LA, Rock EM, Limebeer CL, Br. J. Pharmacol. 2011, 163, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Padwal RS, Majumdar SR, Lancet 2007, 369, 71–77. [DOI] [PubMed] [Google Scholar]; (e) Kirkham TC, Int. Rev. Psychiatry 2009, 21, 163–171. [DOI] [PubMed] [Google Scholar]
- [3].Bow EW, Rimoldi JM, Perspect. Medicin. Chem. 2016, 8, 17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].(a) Schafroth MA, Carreira EM, Prog. Chem. Org. Nat. Prod. 2017, 103, 37–59. [DOI] [PubMed] [Google Scholar]; (b) Reekie TA, Scott MP, Kassiou M, Nat. Rev. Chem. 2017, 2, 101. [Google Scholar]; (c) Mechoulam R, Hanuš L, Chem. Phys. Lipids 2000, 108, 1–13. [DOI] [PubMed] [Google Scholar]
- [5].(a) Mechoulam R, Gaoni Y, J. Am. Chem. Soc. 1965, 87, 3273–3275. [DOI] [PubMed] [Google Scholar]; (b) Adams R, Harfenist M, Loewe S, J. Am. Chem. Soc. 1949, 71, 1624–1628. [Google Scholar]; (c) Adams R, Baker BR, Wearn RB, J. Am. Chem. Soc. 1940, 62, 2204–2207. [Google Scholar]
- [6].(a) Kulkarni S, Nikas SP, Sharma R, Jiang S, Paronis CA, Leonard MZ, Zhang B, Honrao C, Mallipeddi S, Raghav JG, et al. , J. Med. Chem. 2016, 59, 6903–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sharma R, Nikas SP, Paronis CA, Wood JT, Halikhedkar A, Guo JJ, Thakur GA, Kulkarni S, Benchama O, Raghav JG, et al. , J. Med. Chem. 2013, 56, 10142–10157. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Thakur GA, Bajaj S, Paronis C, Peng Y, Bowman AL, Barak LS, Caron MG, Parrish D, Deschamps JR, Makriyannis A, J. Med. Chem. 2013, 56, 3904–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dixon DD, Sethumadhavan D, Benneche T, Banaag AR, Tius MA, Thakur GA, Bowman A, Wood JT, Makriyannis A, J. Med. Chem. 2010, 53, 5656–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lu D, Guo J, Duclos RI, Bowman AL, Makriyannis A, J. Med. Chem. 2008, 51, 6393–6399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Khanolkar AD, Lu D, Ibrahim M, Duclos RI Jr., Thakur GA, Malan TP Jr., Porreca F, Veerappan V, Tian X, George C, et al. , J. Med. Chem. 2007, 50, 6493–6500. [DOI] [PubMed] [Google Scholar]; (g) Lu D, Meng Z, Thakur GA, Fan P, Steed J, Tartal CL, Hurst DP, Reggio PH, Deschamps JR, Parrish DA, et al. , J. Med. Chem. 2005, 48, 4576–4585. [DOI] [PubMed] [Google Scholar]; (h) Mahadevan A, J. Med. Chem. 2000, 43, 3778–3785. [DOI] [PubMed] [Google Scholar]
- [7].(a) Ametovski A, Lupton DW, Org. Lett. 2019, 21, 1212–1215. [DOI] [PubMed] [Google Scholar]; (b) Shultz ZP, Lawrence GA, Jacobson JM, Cruz EJ, Leahy JW, Org. Lett. 2018, 20, 381–384. [DOI] [PubMed] [Google Scholar]; (d) Schafroth MA, Zuccarello G, Krautwald S, Sarlah D, Carreira EM, Angew. Chemie Int. Ed. 2014, 53, 13898–13901. [DOI] [PubMed] [Google Scholar]; (e) Emma L P, Nicholas K, W. Anthony C, P. Michael N, S. Michael S, Chem. – A Eur. J. 2010, 16, 8280–8284. [Google Scholar]; (f) Trost BM, Dogra K, Org. Lett. 2007, 9, 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chicca A, Schafroth MA, Reynoso-Moreno I, Erni R, Petrucci V, Carreira EM, Gertsch J, Sci. Adv. 2018, 4, eaat2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].(a) Caprioglio D, Mattoteia D, Minassi A, Pollastro F, Lopatriello A, Munoz E, Taglialatela-Scafati O, Appendino G, Org. Lett. 2019, 21, 6122–6125. [DOI] [PubMed] [Google Scholar]; (b) Fan F, Adv. Synth. Catal. 2014, 356, 1337–1342. [Google Scholar]; (c) Minuti L, Temperini A, Ballerini E, J. Org. Chem. 2012, 77, 7923–7931. [DOI] [PubMed] [Google Scholar]; (d) Teske JA, Deiters A, Org. Lett. 2008, 10, 2195–2198. [DOI] [PubMed] [Google Scholar]
- [10].Jung B, Lee JK, Kim J, Kang EK, Han SY, Lee H-Y, Choi IS, Chem. - An Asian J. 2019, Ahead of Print. [DOI] [PubMed] [Google Scholar]
- [11].Yeom H-S, Li H, Tang Y, Hsung RP, Org. Lett. 2013, 15, 3130–3133. [DOI] [PubMed] [Google Scholar]
- [12].Privil. Struct. Drug Discov. 2018, 83–154. [Google Scholar]
- [13].(a) Toenjes ST, Gustafson JL, Future Med. Chem. 2018, 10, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith DE, Marquez I, Lokensgard ME, Rheingold AL, Hecht DA, Gustafson JL, Angew. Chemie Int. Ed. 2015, 54, 11754–11759. [DOI] [PubMed] [Google Scholar]; (c) LaPlante SR, Fader LD, Fandrick KR, Fandrick DR, Hucke O, Kemper R, Miller SPF, Edwards PJ, J. Med. Chem. 2011, 54, 7005–7022. [DOI] [PubMed] [Google Scholar]
- [14].(a) Lovering F, Bikker J, Humblet C, J. Med. Chem. 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (b) Salo OMH, Lahtela-Kakkonen M, Gynther J, Jaervinen T, Poso A, J. Med. Chem. 2004, 47, 3048–3057. [DOI] [PubMed] [Google Scholar]
- [15].Dihedral angles were determined by computational analysis optimized with the ωB97X DFT functional and the 6–31G(d) basis set, using the Gaussian 09 electronic structure package, in the presence of water as the solvent. It was also found computationally that the dihedral angle can be increased by adjusting the steric size of the “R” group. See the supporting information for additional details.
- [16].Yamaguchi S, Nedachi M, Yokoyama H, Hirai Y, Tetrahedron Lett. 1999, 40, 7363–7365. [Google Scholar]
- [17].Godfrey JD, Mueller RH, Sedergran TC, Soundararajan N, Colandrea VJ, Tetrahedron Lett. 1994, 35, 6405–6408. [Google Scholar]
- [18].Sun R, Song W, Ma C, Zhang H, Yu X, Adv. Synth. Catal. 2016, 358, 3977–3982. [Google Scholar]
- [19].Tejedor D, Mendez-Abt G, Cotos L, Garcia-Tellado F, Chem. Soc. Rev. 2013, 42, 458–471. [DOI] [PubMed] [Google Scholar]
- [20].Walker D, Hiebert JD, Chem. Rev. 1967, 67, 153–195. [DOI] [PubMed] [Google Scholar]
- [21].Date SM, Ghosh SK, Angew. Chem. Int. Ed. 2006, 46, 386–388. [DOI] [PubMed] [Google Scholar]
- [22].L Wilkerson. J, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, Maldonado RL, Cravatt BF, Lichtman AH, Neuropharmacology 2017, 114, 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wilkerson JL, Niphakis MJ, Grim TW, Mustafa MA, Abdullah RA, Poklis JL, Dewey WL, Akbarali H, Banks ML, Wise LE, Cravatt BF, Lichtman AH, J Pharmacol Exp Ther 2016, 357, 145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ, Br J Pharmacol 2017, 174, 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH, Neuropsychopharmacology 2018, 43, 52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bennett GJ, Xie KY, Pain 1988, 33, 87–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.