Abstract
Background
The Omicron variant of SARS-CoV-2 led to a steep rise in transmissions, and emerging variants continue to influence case rates across the US. As public tolerance for isolation abated, CDC guidance on duration of at-home isolation of COVID-19 cases was shortened to five days if no symptoms, with no laboratory test requirement, despite more cautious approaches advocated by other federal experts.
Methods
We conducted a decision tree analysis of alternative protocols for ending COVID-19 isolation, estimating net costs (direct and productivity), secondary infections, and incremental cost-effectiveness ratios. Sensitivity analyses assessed the impact of input uncertainty.
Results
Per 100 individuals, five-day isolation had 23 predicted secondary infections and a net cost of $33,000. Symptom check on day five (CDC guidance) yielded a 23% decrease in secondary infections (to 17.8), with a net cost of $45,000. Antigen testing on day six yielded 2.9 secondary infections and $63,000 in net costs. This protocol, compared to the next best protocol of antigen testing on day five of a maximum eight-day isolation, cost an additional $1,300 per secondary infection averted. Antigen or polymerase chain reaction testing on day five were dominated (more expensive and less effective) versus antigen testing on day six. Results were qualitatively robust to uncertainty in key inputs.
Conclusions
A six-day isolation with antigen testing to confirm the absence of contagious virus appears the most effective and cost-effective de-isolation protocol to shorten at-home isolation of individuals with COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-15762-0.
Keywords: Cost-effectiveness, COVID-19, SARS-CoV-2, Antigen test, Isolation
Introduction
The SARS-CoV-2 B.1.1.529 (Omicron) variant was designated a variant of concern by the World Health Organization in November 2021, as it had several mutations that are suspected to impact its transmissibility and disease severity [1, 2]. In late December, while vaccination coverage was above 60% in the US,[3] public health guidance from the Centers for Disease Control and Prevention (CDC) regarding isolation of COVID-19 cases were relaxed. The recommended duration of isolation was decreased from ten to five days, with no laboratory testing required to end isolation [4]. Individuals were asked to evaluate their symptoms on day five to determine whether to continue isolating for the full ten days. This guidance was received with skepticism [5–7] given the understanding that Omicron, constituting 95% of COVID-19 cases in the US, [8] was potentially more transmissible than earlier variants and less susceptible to vaccines [1, 2]. Shortages of rapid antigen tests in the US, [7, 9] economic losses associated with extended periods of isolation, [10] and the psychological effects of longer at-home isolation durations [11] were suggested as possible reasonings behind the updated guidance. However, fully elaborated scientific evidence supporting the decision was lacking [12].
While quantitative studies on the viral kinetics and pathophysiology of the Omicron variant are underway, decision makers must offer timely guidance that balances public health and economic considerations in their COVID-19 isolation recommendations. As new variants appear and influence case rates, antigen testing could offer benefits over using symptom status as a marker of infectivity given the high rate of asymptomatic COVID-19 infections [13]. Furthermore, over half of those with COVID-19 may continue to shed infectious doses of SARS-CoV-2 on day five of isolation, [14–16] suggesting that antigen tests may be informative for the decision to de-isolate. We aimed to evaluate the trade-offs between costs (including lost productivity) and secondary infections averted when adopting different protocols to end COVID-19 isolation in order to provide an evidence-base for such decisions.
Materials and methods
Model design
We used a cost-effectiveness study design to model six different protocols for ending COVID-19 isolation. Using a decision tree adapted from our previous work [17], we compared the number of secondary COVID-19 infections that occurred when individuals followed each of the different protocols. We adopted a societal perspective and a two-week time horizon to capture all costs and secondary infections. The hypothetical cohort consisted of 100 individuals in the US who had COVID-19 (confirmed by polymerase chain reaction (PCR) and/or antigen test) and were on the fifth day of isolation. We modeled only individuals with asymptomatic or mild COVID-19; those with more severe disease would be hospitalized rather than isolating at home and therefore were not included.
De-isolation protocols
Interventions were selected to demonstrate current policy options as well as alternatives that might reduce transmissions while also shortening isolation duration. While not exhaustive, these protocols represent a variety of options that might be acceptable to policymakers and the general public alike and warrant further evaluation. In all strategies, we assumed that individuals leaving isolation would follow best practices for infection prevention, which at the time of the analysis included mask wearing. Individuals could leave their home the day after their isolation ended (i.e., for a five-day isolation, they spent five full days at home and could leave on day six if cleared).
Five-day isolation. Person with confirmed COVID-19 stays at home for five days, then can leave without any further consideration.
Ten-day isolation with symptom check on day five (i.e., the CDC guidance). Person with confirmed COVID-19 stays at home for five days. On day five, they review their symptoms. Those who were asymptomatic or fever free for 24 h can end isolation, while those with persisting symptoms continue to isolate until day ten.
Ten-day isolation with antigen test on day five. Person with confirmed COVID-19 stays at home for five days. On day five, they perform a rapid antigen test. Those who test negative can end isolation while those who test positive continue to isolate until day ten.
Ten-day isolation with PCR test on day five. Person with confirmed COVID-19 stays at home for five days. On day five, they conduct a PCR test. Those who test negative can end isolation while those who test positive continue to isolate until day ten. We assumed results are obtained within 24 h.
Ten-day isolation with antigen test on day six. Person with confirmed COVID-19 stays at home for six days. On day six, they perform a rapid antigen test. Those who test negative can end isolation while those who test positive continue to isolate until day ten.
Eight-day isolation with antigen test on day five. Person with confirmed COVID-19 stays at home for five days. On day five, they perform a rapid antigen test. Those who test negative can end isolation while those who test positive continue to isolate until day eight instead of day ten (no re-test is done).
Key assumptions
We assumed that no one in the cohort was SARS-CoV-2-naïve (i.e., all had begun isolation based on true-positive test results). As such, individuals were either still carrying contagious virus or had cleared all viable virus. We defined a frontloaded distribution for infectivity over ten days following symptom onset (or positive test, if asymptomatic), based on empirical data on culture-positivity of patient samples [15, 16, 18, 19]. For those remaining in isolation after day five, we assumed imperfect isolation effectiveness such that continued isolation led to a 95% reduction in the risk of transmission. Finally, for illustration purposes, we assumed 100% testing coverage (i.e., everyone had access to the tests necessary). This was varied in sensitivity analyses.
Model inputs
We used data specific to the Omicron variant when available to parameterize the model. Otherwise, we used data generated during the wildtype (Alpha) and B.1.617.2 (Delta) variant waves. Key model inputs are presented in Table 1, with uncertainty ranges and sources.
Table 1.
Model parameters, uncertainty ranges, and sources
| Parameter | Base-case input | Uncertainty range | Source |
|---|---|---|---|
| Health parameters | |||
| % still infectious on day 5 | 90% | 45 – 100% | Wolfel 2020 [16] |
| Reduction in portion with infectious virus from day 5 to 6 | 22% | 0 – 50% | Wolfel 2020 [16] |
| Secondary reproduction number | 1.2 | 0.96–1.4 | CMMID 2022 [20] |
| Intervention parameters | |||
| Symptom check sensitivity | 23.8% | 18.4 – 33.3% | Ma 2021 [21], Dinh 2021 [22] |
| Antigen test sensitivity | 79.3% | 65.3 – 93.3% | Pilarowski 2021 [23]; see text |
| PCR test sensitivity | 89.0% | 83.0 – 93.0% | Mallett 2020 [24], Singanayagam 2020 [13] |
| Intervention reach or adherence (same for all interventions) | 100% | 0 – 100% | Assumed |
| Effectiveness of isolation for reducing transmission | 95% | Assumed | |
| Cost parameters (per person) | |||
| Antigen test cost | $10 | $5 – $15 | URMC 2022 [25], Krouse 2020 [26] |
| PCR test cost | $150 | $100 – $200 | URMC 2022 [25], Kurani 2021 [27] |
| Direct medical cost per secondary infection | $1436 | $500 – $2000 | Rae 2020 [29] |
| Daily productivity | $200 | Assumed; $25 per hour | |
| Productivity drop in isolation | 90% | Assumed | |
Health inputs. The probability of carrying viable SARS-CoV-2 was 90% on day five and 70% on day six, quickly dropping to zero by day ten from the start of isolation, based on studies of previous variants [13, 15, 16, 18, 19]. We used the effective secondary reproduction number (Reff), which implicitly accounts for infection prevention measures that were in place such as mask wearing, to calculate the number of secondary infections that occur per index case. As of January 16th, 2022, Reff was estimated as 1.2 in the US [20]. We adjusted this value based on the probability of carrying infectious virus on each day of infection to reflect the reduced transmissibility five days after the start of isolation; this “residual R” was 0.26 over days six to ten if isolation was discontinued (See Supplement for calculations). Similar calculations were made to adjust Reff for de-isolation protocols requiring longer isolation periods.
Test performance. 40% of relevant COVID-19 cases were asymptomatic, [21] and of those who develop symptoms, 60% had symptom resolution by day five post-symptom onset, [22] yielding approximately 24% sensitivity for the symptom check protocol. Previous studies among mildly symptomatic and asymptomatic individuals showed antigen tests had over 93% sensitivity for viral loads high enough to be transmissible [23]. We reduced this value by 15% to account for the suspected reduction in sensitivity for the Omicron variant, [1] which resulted in approximately 80% antigen test sensitivity. PCR tests had 89% sensitivity [24].
Cost inputs. Costs were calculated from a societal perspective and in 2022 US dollars. A rapid antigen test cost $10, while a PCR test cost $150 [25–27]. We included productivity loss due to isolation as the indirect cost, which was $900 over five days assuming a 90% decrease in productivity and $200 wage per day. This is likely an overestimate of productivity loss since many individuals with asymptomatic COVID-19 isolating at home can continue working remotely with no or minimal loss in productivity. We therefore calculated base-case outputs both with and without productivity loss. While the societal economic toll of isolation is vast [28], productivity loss is a more relevant indirect impact in the short-term, and importantly it is most likely to be sensitive to relatively small differences (2–5 days) in isolation duration. Direct medical costs incurred for secondary infections were $1436 on average; this accounted for varying costs for different disease severity levels (e.g., $0 if asymptomatic or no healthcare is sought vs. $61,000 if intensive care admission is required; see Supplement) [29, 30]. We assumed all medical costs were incurred in year one and did not require discounting.
Model outputs and sensitivity analyses
For each de-isolation protocol, we calculated the number of secondary infections, societal net costs, and, when appropriate, incremental cost per secondary infections averted. We first calculated path probabilities along each decision tree branch by multiplying the probability of carrying transmissible virus on test day with the probability of a positive or negative test conditional on true viral status. We then multiplied this by the residual R appropriate for the day of de-isolation to estimate the number of secondary infections. Net costs included direct medical costs due to these secondary infections, the cost of the test used in the de-isolation protocol, and productivity loss due isolating depending on duration. De-isolation protocols that led to fewer net costs and fewer secondary infections than their comparator were dominant; no cost per infection averted were calculated.
We conducted deterministic and probabilistic sensitivity analyses to assess uncertainty in key inputs in Table 1. Since test availability and protocol adherence was arbitrarily set as 100%, these two inputs were not included in one-way and multivariate (Monte Carlo) sensitivity analyses. Instead, we performed threshold analyses and two-way sensitivity analyses (where inputs were varied two at a time) to determine minimum necessary adherence and test availability for de-isolation protocols to be effective. When test availability was not 100%, individuals left isolation after the day on which they would have otherwise taken a test.
Additionally, we simulated three separate risk scenarios to evaluate how the environment individuals are re-entering upon ending isolation would affect outcomes. The change in the risk of infection due to varying vaccination rate, mask-wearing [3, 31–33], and number of contacts [34] from base-case were used to adjust the transmission rate, Reff (see Supplement 1). We defined a low-risk scenario representing the infected individual re-joining the household where everyone was fully vaccinated and continued to wear masks for the next five days (Reff=0.35, residual R after de-isolation = 0.07). A medium-risk scenario reflected individuals starting to see few non-household members, all of whom were fully vaccinated, but mask-wearing was inconsistent (Reff=1.11, residual R = 0.24). Finally, a high-risk scenario was defined in which both vaccination and mask-wearing was inconsistent, and a greater number of social contacts were occurring (e.g., going to the movies, eating at restaurants, attending school; Reff=3.78, residual R = 0.81). We did not conduct multivariate sensitivity analyses on the scenarios.
Statistical analysis
The model was built in Excel® (Office 365, Microsoft Corporation) and sensitivity analyses were conducted using @RISK® (version 8.2, Palisade Corporation) [35]. The decision tree and all data are available upon request.
Results
We present base-case results from a societal perspective (i.e., including productivity loss due to isolation) in Table 2; all outcomes are given per 100 individuals. Ending isolation at day five without further testing led to 23.0 secondary infections and $33,100 in direct medical costs. Symptom check at day five (17.8 secondary infections) reduced transmissions by 23% with a $11,900 increase in net costs; this cost an additional $2,282 per secondary infection averted.
Table 2.
Base-case results from (a) societal perspective (including productivity loss) and (b) with direct costs only (no productivity loss)
| Optiona | Testing cost | Medical cost | Productivity loss for index infection | Net cost | Secondary infections | Incremental cost | Secondary infections averted | Incremental cost per secondary infection averted |
|---|---|---|---|---|---|---|---|---|
| 2a. Societal perspective (including productivity loss) | ||||||||
| 5-day isolation, no test | $0 | $33,086 | $0 | $33,086 | 23.04 | n/a | n/a | n/a |
| Symptom check on day 5 | $0 | $25,605 | $19,368 | $44,973 | 17.83 | $11,887 | 5.21 | Extended dominatedb |
| Antigen test on day 5 (8-day isolation) | $1,000 | $14,391 | $38,564 | $53,954 | 10.02 | $20,868 | 13.02 | Extended dominated b |
| Antigen test on day 6 | $1,000 | $4,132 | $58,056 | $63,189 | 2.88 | $30,103 | 20.16 | $1,493 |
| Antigen test on day 5 | $1,000 | $8,159 | $64,273 | $73,432 | 5.68 | $10,243 | -2.80 | Dominated |
| PCR test on day 5 | $15,000 | $5,112 | $72,099 | $92,211 | 3.56 | $29,022 | -0.68 | Dominated |
| 2b. Direct costs only (no productivity loss) | ||||||||
| Antigen test on day 6 | $1,000 | $4,132 | - | $5,132 | 2.88 | n/a | n/a | Dominant |
| Antigen test on day 5 | $1,000 | $8,159 | - | $9,159 | 5.68 | $4,027 | -2.80 | Dominated |
| Antigen test on day 5, (8-day isolation) | $1,000 | $14,391 | - | $15,391 | 10.02 | $10,258 | -7.14 | Dominated |
| PCR test on day 5 | $15,000 | $5,112 | - | $20,112 | 3.56 | $14,979 | -0.68 | Dominated |
| Symptom check on day 5 | $0 | $25,605 | - | $25,605 | 17.83 | $20,472 | -14.95 | Dominated |
| 5-day isolation, no test | $0 | $33,086 | - | $33,086 | 23.04 | $27,953 | -20.16 | Dominated |
Values are per 100 people. De-isolation protocols are compared to previous non-dominated protocol
a Isolation duration is up to 10 days unless otherwise noted
b Strategy is extended dominated, i.e., a more expensive strategy (lower in the table) has a lower cost/infection averted ratio. Ratios that are not shown due to weak dominance are as follows: $2,282 for symptom check on day five versus no test; $1,150 for antigen test on day five of eight-day isolation versus symptom check; $1,603 for antigen test on day five of eight-day isolation versus no test; and $1,293 for antigen test on day six versus antigen test on day five of eight-day isolation
Antigen testing on day five of an eight-day isolation period cost an additional $1,150 per secondary infection averted compared with symptom check. This drop in the additional cost per infection averted represents extended dominance [36] over the symptom check. The additional cost for day five antigen test versus no test was $1,603 per secondary infection averted.
The most cost-effective de-isolation protocol was performing an antigen test on day six of a ten-day isolation period. This protocol led to $63,200 in net costs and 2.9 secondary infections, costing an additional $1,293 per secondary infection averted versus an antigen test on day five of an eight-day isolation, again representing extended dominance. Both antigen and PCR testing on day five were dominated by antigen testing on day six; they led to greater net costs and more secondary infections.
If productivity losses were omitted, leaving just direct costs, antigen testing on day six was strictly dominant (i.e., lowest net cost and fewest secondary infections) over all other de-isolation protocols (Table 2).
Sensitivity analyses
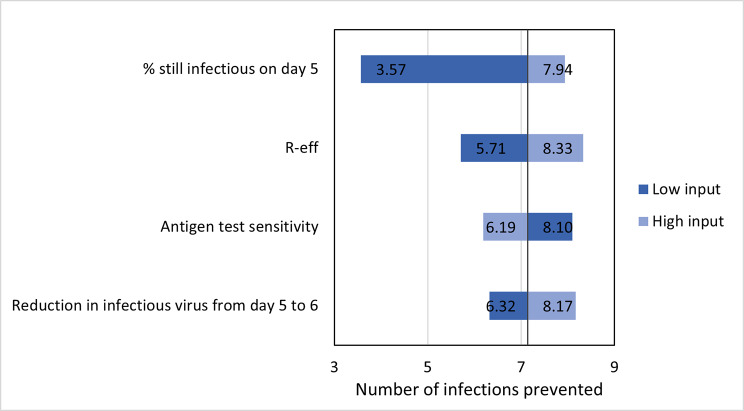
In one-way sensitivity analyses where key inputs were varied one at a time, antigen testing on day five prevented between 51 and 85% secondary infections over symptom check, depending primarily on antigen test sensitivity for transmissible viral loads. Secondary infections prevented with an antigen test on day six versus day five was mostly related to the relative reduction in viable viral load from day five to six and varied between 35 and 67%. Antigen test on day six, compared to the next most cost-effective option (antigen test on day five of eight-day isolation) prevented between 3.6 and 8.0 secondary infections. This value was most sensitive to the probability of having transmissible virus on day five (Fig. 1).
Fig. 1.
One-way sensitivity analyses on the number of secondary infections averted with antigen test on day six versus next most cost-effective strategy (eight-day isolation with antigen test on day five) Base-case output is 7.14
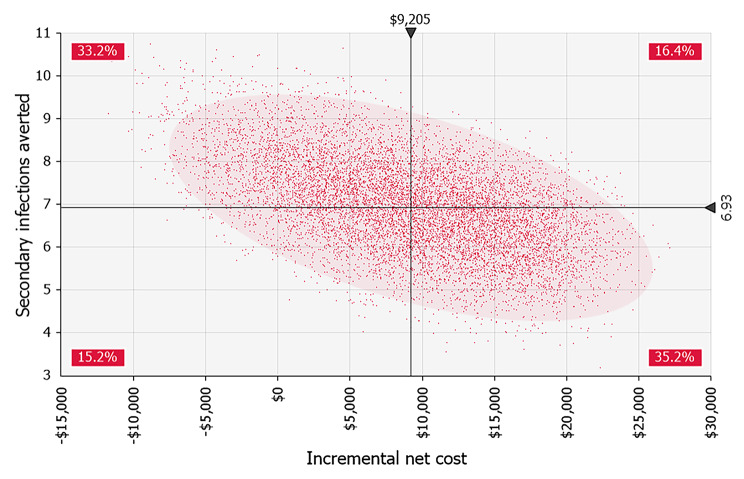
Probabilistic Monte Carlo analyses with 10,000 iterations showed that antigen testing on day six was either dominant or cost-effective with up to an increment of $3,759 per secondary infection averted, given varying inputs. This outcome was most sensitive to uncertainty in the probability of a persistent high viral load, followed by the community transmission rate and the direct medical cost per COVID-19 infection. Antigen test on day six always prevented more secondary infections than antigen test on day five of eight-day isolation but had a nearly 90% probability of having greater net costs (Fig. 2).
Fig. 2.
Simulated incremental costs and secondary infections averted with antigen testing on day six of isolation versus next most cost-effective strategy (eight-day isolation with antigen test on day five). 10,000 iterations. Shaded ellipse represents 95% confidence area for results. Percentages are the probabilities of the result being in each of the quadrants
In all three of the risk scenarios considered, antigen testing on day six remained the optimal de-isolation protocol (Table 3). Both the low- and medium-risk scenario results followed base-case findings: symptom check at day five, antigen test on day five of eight-day isolation, and antigen test on day six were all cost-effective with greater incremental costs per infections averted as transmission risk decreases. Antigen testing on day six was had an incremental cost of $8,050 and $1,500 per secondary infection averted in the low- and medium-risk scenarios, respectively. In the high-risk scenario, antigen testing on day six was strictly dominant, leading to $72,000 in net costs and 9.1 secondary infections, as opposed to $100,000 net costs and 72.6 secondary infections with a symptom check on day five.
Table 3.
Analyses of different risk scenarios. Values per 100 people
| Optiona | Testing cost | Medical cost | Productivity loss | Net cost | Secondary infections | Incremental cost | Secondary infection averted | Incremental cost per secondary infection averted |
|---|---|---|---|---|---|---|---|---|
| Low Risk (R = 0.35) | ||||||||
| 5-day isolation, no test | $0 | $9,516 | $0 | $9,516 | 6.63 | n/a | n/a | n/a |
| Symptom check on day 5 | $0 | $7,364 | $19,368 | $26,732 | 5.13 | $17,217 | 1.50 | Extended dominatedb |
| Antigen test on day 5 (8-day isolation) | $1,000 | $4,139 | $38,564 | $43,703 | 2.88 | $34,187 | 3.75 | Extended dominatedb |
| Antigen test on day 6 | $1,000 | $1,189 | $58,056 | $60,245 | 0.83 | $50,729 | 5.80 | $8,748b |
| Antigen test on day 5 | $1,000 | $2,347 | $64,273 | $67,620 | 1.63 | $7,375 | -0.81 | Dominated |
| PCR test on day 5 | $15,000 | $1,470 | $72,099 | $88,569 | 1.02 | $28,325 | -0.20 | Dominated |
| Medium Risk (R = 1.11) | ||||||||
| 5-day isolation, no test | $0 | $30,728 | $0 | $30,728 | 21.40 | n/a | n/a | n/a |
| Symptom check on day 5 | $0 | $23,780 | $19,368 | $43,148 | 16.56 | $12,421 | 4.84 | Extended dominatedb |
| Antigen test on day 5 (8-day isolation) | $1,000 | $13,365 | $38,564 | $52,929 | 9.31 | $22,201 | 12.09 | Extended dominatedb |
| Antigen test on day 6 | $1,000 | $3,838 | $58,056 | $62,894 | 2.67 | $32,166 | 18.73 | $1,718b |
| Antigen test on day 5 | $1,000 | $7,577 | $64,273 | $72,851 | 5.28 | $9,956 | -2.60 | Dominated |
| PCR test on day 5 | $15,000 | $4,747 | $72,099 | $91,846 | 3.31 | $28,952 | -0.63 | Dominated |
| High Risk (R = 3.78) | ||||||||
| Antigen test on day 6 | $1,000 | $13,021 | $58,056 | $72,077 | 9.07 | n/a | n/a | Dominant |
| Antigen test on day 5 (8-day isolation) | $1,000 | $45,344 | $38,564 | $84,908 | 31.58 | $12,831 | -22.51 | Dominated |
| Antigen test on day 5 | $1,000 | $25,709 | $64,273 | $90,982 | 17.90 | $18,904 | -8.84 | Dominated |
| Symptom check on day 5 | $0 | $80,680 | $19,368 | $100,048 | 56.18 | $27,971 | -47.12 | Dominated |
| PCR test on day 5 | $15,000 | $16,107 | $72,099 | $103,206 | 11.22 | $31,128 | -2.15 | Dominated |
| 5-day isolation, no test | $0 | $104,251 | $0 | $104,251 | 72.60 | $32,174 | -63.53 | Dominated |
Values are per 100 people. De-isolation protocols are compared to previous non-dominated protocol
a Isolation duration is up to 10 days unless otherwise noted
b Strategy is extended dominated, i.e., a more expensive strategy (lower in the table) has a lower cost/infection averted ratio. Ratios that are not shown due to extended dominance are as follows: In the low risk scenario, $11,491 for symptom check versus no test, $7,556 for antigen test on day five of eight-day isolation versus symptom check, $91,131 for antigen test on day five of eight-day isolation versus no test, and $8,051 for antigen test on day six versus antigen test on day five of eight-day isolation. In the medium risk scenario, $2,567 for symptom check versus no test, $1,349 for antigen test on day five of eight-day isolation versus symptom check, $1,836 for antigen test on day five of eight-day isolation versus no test, and $1,502 for antigen test on day six versus antigen test on day five of eight-day isolation
Under the base-case assumption of 100% adherence to a symptom check protocol, at least 30% antigen test availability (i.e., 30% of those in isolation can access a test) was necessary for antigen testing to prevent more secondary infections than the symptom check if done on day five. Similarly, PCR tests had greater benefit than symptom check when test availability was greater than approximately 27%. In a two-way sensitivity analysis, if symptom check adherence was below 70%, then < 20% antigen test or < 19% PCR test availability was sufficient for these tests to prevent a greater number of transmissions than the symptom check protocol on day five. Notably, antigen testing on day six prevented more secondary infections than symptom check on day five even when test availability was as low as 1%, due to the added day of isolation.
Discussion
We compared health and cost outcomes associated with different de-isolation protocols to end COVID-19 isolation for those with confirmed asymptomatic or mild COVID-19. All testing strategies had favorable cost-effectiveness ratios except for antigen or PCR test on day five of 10-day isolation. We found that while symptom check without testing on day five of isolation did reduce secondary transmissions after de-isolation by 23% compared to no testing, it still led to nearly 18 secondary infections per 100 individuals and had the least favorable cost-effectiveness ratio due to high medical costs for secondary infections. The most cost-effective protocol was to remain in isolation through day six and then perform an antigen test, which dominated both antigen testing and PCR testing on day five. Antigen testing on day six led to an overall 87% decrease in secondary infections compared to no testing and cost an additional $1,300 per secondary infection averted compared to the next best option.
Notably, a threshold analysis on antigen test availability suggested that the benefit of antigen testing on day six might be primarily due to the extra day of isolation (during which the probability of still carrying infectious virus quickly begins to drop), rather than the ability of the test to identify those who still might have a transmissible viral load. This insight warrants further evaluation using emerging data on the viral dynamics of variants. Moreover, antigen testing on day six was associated with lower productivity loss than antigen testing on day five; even though everyone remained in isolation for one more day, more individuals were cleared for de-isolation on day six than would have been on day five. The four days gained by this portion of index cases offset the extra day lost by everyone. Workforce shortages have been an important adverse effect of COVID-19 isolation [37–41]. Antigen testing on day six generated both health and economic benefits; it minimized post-isolation transmissions while allowing individuals to return to work sooner on average.
By modeling different risk scenarios, we demonstrated that the de-isolation environment had a considerable impact on the cost-effectiveness of testing strategies. Regardless of the risk scenario, the optimal protocol remained antigen testing on day six, which became dominant over other protocols in high-risk situations and remained cost-effective, although cost-effectiveness was less favorable in low-risk situations. Nevertheless, these findings suggest that much like other public health policy decisions throughout the pandemic, de-isolation guidelines must evolve as the context of the pandemic shifts. For example, potential new surges may call for more stringent policies with longer minimum isolation and more sensitive tests, while declining transmissions may allow more lenient approaches. It is plausible that the CDC has reached this same conclusion and proposed a symptom check rather than an antigen test because of an expectation that transmissions would subside in the weeks following the announcement of the new guidance. While reasonable at first glance, this could be a risky approach; loosening infection prevention measures may have prevented the expected drop in transmissions, leading instead to a quick rise in cases that would have prohibited the loosened guidance being put in place to begin with. Indeed, our modeling of the CDC guidance resulted in a substantial number of secondary COVID-19 cases given the transmission rate at the time the guidance was issued, some of which were avoided with a different de-isolation approach.
Evidence from earlier SARS-CoV-2 variants suggest that transmissibility of the virus peaks by approximately the fifth day from symptom onset, and swiftly drops afterward [13, 15, 16, 18, 19, 42]. However, the risk of further transmission after day five is not zero, and it is highly dependent on health behavior following de-isolation (e.g., continuing to wear masks, limiting the number of social contacts etc.) Given the high probability of asymptomatic infection and the possibility of short-lived symptoms, a symptom check to end isolation on the fifth day does not substantially reduce the risk of further transmission. Antigen tests, on the other hand, allow a more accurate measure of ongoing risk. There is now evidence that rapid tests have good sensitivity for detecting high viral loads that are most likely to be transmissible [19, 23, 42, 43]. As such, antigen tests are an important public health tool that can help mitigate the health harms of the COVID-19 pandemic, and should be incorporated into public health responses as resources allow.
Limitations
This study had important limitations, especially regarding uncertainty in key inputs such as the viral kinetics of SARS-CoV-2 and sensitivity of antigen tests. First, we distributed Reff over the 10 days following COVID-19 confirmation, but a portion of transmissions occur prior to the index case learning their COVID-19 status and entering isolation. As such, we have overestimated the number of secondary infections in our model and underestimated cost-effectiveness ratios. Decreasing the residual R would increase costs per secondary infection averted, but given favorable cost/infection averted ratios even in the low-risk scenario, we believe the implications of our findings would not be affected. More importantly, given the novelty of the Omicron variant at the time of analysis, we had to rely on studies of prior variants for these two important factors. While variance in neither of these inputs changed cost-effectiveness results qualitatively, they did have an impact on the number of further transmissions after isolation and thus the level of cost-effectiveness. Additional studies on the viral kinetics of recent variants are necessary to refine these estimates.
Conclusions
The Omicron and following variants of SARS-CoV-2 continue to present a threat to public health due to high transmissibility and potential ability to evade vaccine-induced immunity. Cost-effectiveness analyses can help decision makers assess the trade-offs between the economic disadvantages and health risks of adopting different COVID-19 de-isolation guidance. Using a decision tree model and Omicron-specific data when available, we found that ending isolation in five days given a negative symptom check left substantial risk of transmission and was not the most cost-effective strategy even when high productivity losses of longer isolation were accounted for. Instead, our findings suggest a baseline isolation duration of six days, at which time an antigen test, if available, can be conducted to confirm that the individual no longer carries transmissible SARS-CoV-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Elliot Marseille for review.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- COVID-19
Coronavirus Disease 2019
- PCR
polymerase chain reaction
- Reff
effective secondary reproduction number
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Authors contributions
SM contributed to methodology, formal analysis and investigation, data curation, and wrote the original draft of the manuscript. JGK conceptualized the study, provided supervision, and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
Authors received funding from the National Institute on Drug Abuse grant number 5R37DA015612-170. The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data Availability
All data generated and/or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participant
Not applicable. The data to parameterize model inputs came entirely from summary measures in published literature, therefore ethics approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rao S, Singh M: The Newly Detected B.1.1.529 (Omicron) Variant of SARS-CoV-2 With Multiple Mutations Implications for transmission, diagnostics, therapeutics, and immune evasion. DHR Proceedings 2021, 1(S5):7–10.
- 2.Abdool Karim SS, Abdool Karim Q. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Projections [https://covid19.healthdata.org/united-states-of-america]
- 4.Centers for Disease Control and Prevention: CDC Updates and Shortens recommended isolation and Quarantine Period for General Population. In.; 2021.
- 5.Harmon GE: AMA: CDC quarantine and isolation guidance is confusing, counterproductive. In.: American Medical Association; 2022.
- 6.LaFraniere S, Stolberg SG, Weiland N: For C.D.C.’s Walensky, a Steep Learning Curve on Messaging. In: The New York Times Washington, D.C.; 2022.
- 7.Tufekci Z: The C.D.C. Is Hoping You’ll Figure Covid Out on Your Own. In: The New York Times 2022.
- 8.COVID Data Tracker [https://covid.cdc.gov/covid-data-tracker/#variant-proportions]
- 9.Heyward G, Kasakove S: Americans Hunt for Virus Tests and the Assurance of Safe Holiday Gatherings. In: The New York Times 2021.
- 10.Hirsch L: The C.D.C.’s decision to halve isolation will ease staffing woes for airlines, but concerns linger. In: The New York Times 2021.
- 11.Sharfstein J: Bonus - the COVID-19 pandemic’s transition phase with Dr. Monica Gandhi: what questions do we need to ask and what answers do we need to find in 2022? In: Public Health on Call. Johns Hopkins Bloomberg School of Public Health; 2022.
- 12.MDHHS statement on CDC guidelines [https://www.michigan.gov/coronavirus/0,9753,7-406-98163-574710--,00.html]
- 13.Singanayagam A, Patel M, Charlett A, Lopez Bernal J, Saliba V, Ellis J, Ladhani S, Zambon M, Gopal R: Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020, 25(32). [DOI] [PMC free article] [PubMed]
- 14.Lefferts B, Blake I, Bruden D, Hagen MB, Hodges E, Kirking HL, Bates E, Hoeldt A, Lamont B, Saydah S, et al. Antigen Test Positivity after COVID-19 isolation - Yukon-Kuskokwim Delta Region, Alaska, January-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(8):293–298. doi: 10.15585/mmwr.mm7108a3. [DOI] [PubMed] [Google Scholar]
- 15.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 16.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 17.Maya S, Padda G, Close V, Wilson T, Ahmed F, Marseille E, Kahn JG. Optimal strategies to screen health care workers for COVID-19 in the US: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2022;20(1):2. doi: 10.1186/s12962-021-00336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, Ahern S, Carty PG, O’Brien KK, O’Murchu E, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81(3):357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera RAPM, Tso E, Tsang OTY, Tsang DNC, Fung K, Leung YWY, Chin AWH, Chu DKW, Cheng SMS, Poon LLM, et al. SARS-CoV-2 Virus Culture and Subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg Infect Dis. 2020;26(11):2701–2704. doi: 10.3201/eid2611.203219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National and Subnational estimates for the United States of America [https://epiforecasts.io/covid/posts/national/united-states/]
- 21.Ma Q, Liu J, Liu Q, Kang L, Liu R, Jing W, Wu Y, Liu M. Global percentage of asymptomatic SARS-CoV-2 infections among the Tested Population and individuals with confirmed COVID-19 diagnosis: a systematic review and Meta-analysis. JAMA Netw Open. 2021;4(12):e2137257. doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinh A, Jaulmes L, Dechartres A, Duran C, Mascitti H, Lescure X, Yordanov Y, Jourdain P. Collaboration AP-HUIC-r, data-sciences c et al: time to resolution of respiratory and systemic coronavirus disease 2019 symptoms in community setting. Clin Microbiol Infect. 2021;27(12):1862. doi: 10.1016/j.cmi.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilarowski G, Lebel P, Sunshine S, Liu J, Crawford E, Marquez C, Rubio L, Chamie G, Martinez J, Peng J, et al. Performance characteristics of a Rapid severe Acute Respiratory Syndrome Coronavirus 2 Antigen Detection Assay at a Public Plaza Testing Site in San Francisco. J Infect Dis. 2021;223(7):1139–1144. doi: 10.1093/infdis/jiaa802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallett S, Allen AJ, Graziadio S, Taylor SA, Sakai NS, Green K, Suklan J, Hyde C, Shinkins B, Zhelev Z, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18(1):346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID-19 Related Testing Costs [https://www.urmc.rochester.edu/patients-families/bill-pay/cost-estimates-and-pricing/covid-19-related-testing-charges.aspx]
- 26.Krouse S: Abbott’s $5 Covid-19 Rapid Antigen Test gets emergency-use Status from FDA. In: The Wall Street Journal. 2020.
- 27.COVID-19 test prices and payment policy [https://www.healthsystemtracker.org/brief/covid-19-test-prices-and-payment-policy/]
- 28.Maya S, Kahn JG, Lin TK, Jacobs LM, Schmidt LA, Burrough WB, Ghasemzadeh R, Mousli L, Allan M, Donovan M, et al. Indirect COVID-19 health effects and potential mitigating interventions: cost-effectiveness framework. PLoS One. 2022;17(7):e0271523. doi: 10.1371/journal.pone.0271523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potential costs of COVID-19 treatment for people with employer coverage [https://www.healthsystemtracker.org/brief/potential-costs-of-coronavirus-treatment-for-people-with-employer-coverage/]
- 30.Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, Silk BJ, Gundlapalli AV, Harris AM, Boehmer TK et al: Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥ 18 Years Who Completed a Primary COVID-19 Vaccination Series — 465 Health Care Facilities, United States, December 2020–October 2021. MMWR 2022, 71(1):19–25. [DOI] [PMC free article] [PubMed]
- 31.Li Y, Liang M, Gao L, Ayaz Ahmed M, Uy JP, Cheng C, Zhou Q, Sun C. Face masks to prevent transmission of COVID-19: a systematic review and meta-analysis. Am J Infect Control. 2021;49(7):900–906. doi: 10.1016/j.ajic.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer CB, Adrien N, Silguero JJ, Hopper JJ, Chowdhury AI, Werler MM. Mask adherence and rate of COVID-19 across the United States. PLoS One. 2021;16(4):e0249891. doi: 10.1371/journal.pone.0249891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hearne BN, Nino MD: Understanding how race, ethnicity, and gender shape mask-wearing adherence during the COVID-19 pandemic: evidence from the COVID Impact Survey. J Racial Ethn Health Disparities 2021. [DOI] [PMC free article] [PubMed]
- 34.Feehan DM, Mahmud AS. Quantifying population contact patterns in the United States during the COVID-19 pandemic. Nat Commun. 2021;12(1):893. doi: 10.1038/s41467-021-20990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palisade Corporation: @RISK 8.2.0: Risk Analysis Add-in for Microsoft Excel. In.
- 36.Kamlet MS: A framework for cost-utility analysis of government healthcare programs. In.: Office of Disease Prevention and Health Promotion, Public Health Service, U.S. Department of Health and Human Services; 1992.
- 37.Kim L: These 18 States are grappling with critical hospital worker shortages as Covid Hospitalizations Surge. In: Forbes. 2022.
- 38.Guidance on Quarantine and Isolation for Health Care Personnel (HCP) Exposed to SARS-CoV-2 and Return to work for HCP with COVID-19 [https://www.cdph.ca.gov/Programs/CHCQ/LCP/Pages/AFL-21-08.aspx]
- 39.Cano R: Hundreds of S.F. employees are under quarantine as omicron strains city services. In: San Francisco Chronicle San Francisco, CA; 2022.
- 40.Hookway J, Grove T: As Omicron spreads, governments race to ease staff shortages. In: The Wall Street Journal. 2021.
- 41.Gentry D: COVID-positive workers pressured to stay on the job, say OSHA complaints. In: Nevada Current 2022.
- 42.Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera R, Poon LLM, Nicholls JM, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pekosz A, Parvu V, Li M, Andrews JC, Manabe YC, Kodsi S, Gary DS, Roger-Dalbert C, Leitch J, Cooper CK. Antigen-Based testing but not real-time polymerase chain reaction correlates with severe Acute Respiratory Syndrome Coronavirus 2 viral culture. Clin Infect Dis. 2021;73(9):e2861-e2866. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article and its supplementary information files.