Abstract
Introduction
Prolonged use of antibiotics is closely related to antibiotic-associated infections, antimicrobial resistance and adverse drug events. The optimal duration of antibiotic treatment for Gram-negative bacteremia (GNB) with a urinary tract source of infection is poorly defined.
Methods and analysis
Investigator-initiated multicentre, non-blinded, non-inferiority randomised controlled trial with two parallel treatment arms. One arm will receive shortened antibiotic treatment of 5 days and the other arm will receive antibiotic treatment of 7 days or longer. Randomisation will occur in equal proportion (1:1) no later than day 5 of effective antibiotic treatment as determined by antibiogram. Immunosuppressed patients and those with GNB due to non-fermenting bacilli (Acinetobacter spp, Pseudomonas spp), Brucella spp, Fusobacterium spp or polymicrobial growth are ineligible.
The primary endpoint is 90-day survival without clinical or microbiological failure to treatment. Secondary endpoints include all-cause mortality, total duration of antibiotic treatment, hospital readmission and Clostridioides difficile infection. Interim safety analysis will be performed after the recruitment of every 100 patients. Given an event rate of 12%, a non-inferiority margin of 10%, and 90% power, the required sample size to determine non-inferiority is 380 patients. Analyses will be performed on both intention-to-treat and per-protocol populations.
Ethics and dissemination
The study is approved by the Danish Regional Committee on Health Research (H-19085920) and the Danish Medicines Agency (2019-003282-17). The results of the main trial and each of the secondary endpoints will be submitted for publication in a peer-reviewed journal.
Trial registration number
Keywords: Infection control, Clinical trials, INFECTIOUS DISEASES, Clinical Trial, Urinary tract infections
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The trial design—this is a multicentre, randomised, non-inferiority study—which will reduce the risk of confounding bias.
The design of the study strives to be as close to standard clinical practice as possible, which enables the findings of this study to be applicable in a routine clinical setting.
The strict eligibility criteria will possibly limit the generalisability of the results to all patient groups, as only patients with uncomplicated disease are included.
Introduction
Background
The incidence of Gram-negative bacteraemia continues to increase and remains a major cause of morbidity and mortality in both hospitalised and community-dwelling patients.1 From 1997 to 2002, the proportion of bacteraemia caused by Gram-negative bacteria was 43% in Europe,2 and a study from the European Antimicrobial Resistance Surveillance System reported an increase in bacteraemia due to Escherichia coli by 8.1% per year from 2002 to 2008.3 Overall, Gram-negative bacteria account for half of all cases of bacteraemia.4 In Denmark, in 2017 there were >6000 cases of bacteraemia due to the two most common Gram-negative bacteria, E. coli and Klebsiella pneumoniae.5
Prolonged use of antibiotics is closely related to antibiotic-associated infections, antimicrobial resistance and adverse drug events.6–9 The latter is particularly concerning for patient safety as it may result in sequelae and prolonged hospital stay. It has been shown that the risk of acute renal failure and the risk of C. difficile infection increase with each day of prophylactic antibiotic treatment prior to surgery,10 and that for every 10 days of additional antibiotic treatment, the risk of adverse drug events increases by 3%.9 Antibiotic stewardship and rationale use of antibiotics to treat infections are important strategies that may reduce the duration of antibiotic therapy and thereby reduce adverse events, reduce selective pressure on the bacterial microbiota and prevent the emergence of resistance.11 12
The optimal duration of therapy for bloodstream infections due to Gram-negative bacteraemia has been poorly defined. International and Danish national recommendations suggest 7–14 days of antibiotic therapy for Gram-negative bacteraemia and pyelonephritis according to disease severity.13–15 However, in the absence of guidelines, wide variability exists, and recommendations are based on individual expert opinions.16 An observational study found that patients receiving short course (6–10 days) compared with prolonged course (11–16 days) antibiotic therapy for Gram-negative bacteraemia had similar outcomes.17 Interestingly, there was a trend towards a protective effect of short-course antibiotic therapy on the subsequent emergence of multi-drug resistant Gram-negative bacteraemia (OR 0.59; 95% CI 0.32 to 1.09; p=0.09). A recent randomised non-inferiority trial found that a 7-day course of antibiotics was the preferential treatment for Gram-negative bacteraemia if the source was properly controlled.18 Another randomised controlled trial found that an antibiotic course of 7 days was non-inferior to 14 days in patients hospitalised with Gram-negative bacteraemia achieving clinical stability before day 7.19 Investigators in Switzerland found that 7 days of treatment were non-inferior to 14 days of treatment and that 5 days of treatment was safe and efficient in the group receiving an individualised duration of treatment determined by clinical response and 75% reduction in peak C reactive protein values.20
Other studies on the duration of treatment in Gram-negative bacteraemia also support the safety of shorter antibiotic treatment, but many of these studies have important limitations including small sample sizes, lack of comparator arms or confounding by indication.21–25 Randomised controlled trials evaluating the use of procalcitonin in the management of sepsis including those caused by Gram-negative bacteria also demonstrated the safety of shorter antibiotic courses.21 22
Given the high stakes of antibiotic overconsumption in an ageing population and that only a very few randomised controlled trials have investigated the optimal treatment length of Gram-negative bacteraemia, formal evaluation of the safety and efficacy of shortened antibiotic treatment is of immense clinical and public health importance. This study will be designed as a randomised controlled multicentre trial, that will determine whether 5 days of antibiotic therapy is non-inferior to 7 days or longer of therapy for Gram-negative bacteraemia. The inclusion of multiple centres, the study design and inclusion criteria allowing a representative cohort of eligible patients, make it highly likely that the outcomes of this trial will have a significant impact on clinical practice.
Objective
This study aims to assess the efficacy and safety of shortened antibiotic duration (5 days) in the treatment of Gram-negative bacteraemia with a urinary tract source of infection in hospitalised immunocompetent adults compared with 7 days or more of antibiotic treatment.
Methods
Trial design and randomisation
An investigator-initiated multicentre, non-blinded, non-inferiority randomised controlled trial with two parallel treatment arms. One arm will receive shortened antibiotic treatment guided by clinical stability criteria (intervention group) and the other arm will receive standard antibiotic treatment (control group). As the treatment duration relies on continuous evaluation of clinical stability criteria of the participants, blinding of study personnel and participants is not practicable. The design of the study also strives to be as close to standard clinical practice as possible. Therefore, study investigators, trial participants or treating physicians will not be blinded to treatment allocation.
Confirmation of study eligibility will be performed by entering key variables into a secure web-based programme (Research Electronic Data Capture, REDCap) with subsequent automatic patient randomisation in the two parallel arms (ratio 1:1) no later than day 5 after initiation of microbiologically effective empiric antibiotic treatment. The randomisation list will be generated centrally in random blocs and stratified according to hospital and aetiology. The randomisation key will be stored in a locked and secure environment at Copenhagen University Hospital—Hvidovre Hospital.
Study setting
The following 12 hospitals, representing all 5 regions of Denmark, will participate in the study: Copenhagen University Hospital—Amager and Hvidovre, Copenhagen University Hospital—Rigshospitalet, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen University Hospital—Herlev and Gentofte, Copenhagen University Hospital—North Zealand, Zealand University Hospital—Roskilde, Odense University Hospital, Kolding Hospital, Silkeborg Hospital, Herning Hospital, Aarhus University Hospital and Aalborg University Hospital.
Eligibility criteria
Inclusion and exclusion criteria are listed in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|
|
ATC, anatomical therapeutic chemical; HIV, human immunodeficiency virus.
Gram-negative bacteraemia is defined as the growth of a single Gram-negative micro-organism in one or more blood cultures associated with evidence of infection. Both community-acquired and hospital-acquired Gram-negative bacteraemia will be included.
Evidence of a urinary tract source of infection is defined as growth of the same species of Gram-negative micro-organism in blood and urine or at least one clinical symptom compatible with urinary tract infection (dysuria, polyuria, haematuria, pelvic pain, cloudy or strong-smelling urine).
Eligible participants must fulfil all the inclusion and none of the exclusion criteria.
Interventions
Intervention group: will receive antibiotic treatment for 5 days if clinically stable, that is, discontinuation of antibiotics at day 5 if the participant has a temperature of 37.8°C or less and fulfils all criteria of clinical stability at time of randomisation. Criteria of clinical stability are systolic blood pressure >90 mm Hg, heart rate <100 beats/min, respiratory rate <24/min and peripheral oxygen saturation >90% without supplemental oxygen.
Control group: will receive antibiotic treatment for a minimum of 7 days at the discretion of their treating physician.
Treatment
Participants will receive antibiotic treatment according to local and national guidelines as well as to antimicrobial susceptibility of the identified Gram-negative bacteria. Participation in the study will only affect treatment duration and will not influence the choice of treatment concerning the type, dose or route of administration of antibiotic treatment.
Antibiotics considered appropriate for empiric treatment of Gram-negative bacteraemia are listed in table 2.
Table 2.
Acceptable empirical antibiotic treatment of Gram-negative bacteria if susceptible by antibiogram
| Antibiotic | Administration* | Standard dose* | Frequency* | Dose adjustment* |
| Penicillins | ||||
| Piperacillin/tazobactam | IV | 4 g/0.5 g | Every 6 or 8 hours | Renal impairment and weight |
| Ampicillin† | IV | 1–2 g | Every 6 or 8 hours | Renal impairment and weight |
| Mecillinam | IV | 0.8–1 g | Every 8 hours | Renal impairment and weight |
| Cephalosporins | ||||
| Cefuroxime‡ | IV | 1.5 g | Every 6 hours | Renal impairment and weight |
| Cefotaxime‡ | IV | 1 g | Every 12 hours | Renal impairment and weight |
| Ceftazidime | IV | 1 g | Every 8 or 12 hours | Renal impairment and weight |
| Ceftriaxone | IV | 2 g | Every 24 hours | Renal and hepatic impairment and weight |
| Carbapenems | ||||
| Meropenem | IV | 1 g | Every 8 hours | Renal impairment and weight |
| Ertapenem | IV | 1 g | Every 24 hours | – |
| Aminoglycosides | ||||
| Gentamicin†‡/tobramycin | IV | 5–7 mg/kg | Every 24 hours | Renal impairment and weight |
| Fluoroquinolone | ||||
| Ciprofloxacin | IV | 400 mg | Every 12 hours | Renal impairment and weight |
*Standard recommendations.
†Combination therapy of ampicillin and gentamicin.
‡Monotherapy of cefuroxime/cefotaxime or combination therapy of cefuroxime/cefotaxime and gentamicin.
IV, intravenous.
Antibiotics considered appropriate for targeted treatment of Gram-negative bacteraemia are listed in table 3.
Table 3.
Acceptable targeted antibiotic treatment of Gram-negative bacteria if susceptible by antibiogram
| Antibiotic | Administration* | Standard dose* | Frequency* | Dose adjustment* |
| Penicillin | ||||
| Mecillinam | IV | 800–1000 mg | Every 8 hours | – |
| Pivmecillinam | PO | 800 mg | Every 8 hours | – |
| Amoxicillin/clavulanate | PO | 1000 mg/250 mg | Every 6 hours | Renal impairment and weight |
| Pipercillin/tazobactam | IV | 4 g/0.5 g | Every 6 or 8 hours | Renal impairment and weight |
| Ampicillin | IV | 2 g | Every 6 or 8 hours | Renal impairment and weight |
| Pivampicillin | PO | 500 mg | Every 6 or 8 hours | Renal impairment and weight |
| Amoxicillin | PO | 1 g | Every 6 or 8 hours | Renal impairment and weight |
| Cephalosporin | ||||
| Cefuroxime | IV | 1.5 g | Every 8 hours | Renal impairment and weight |
| Cefuroxime | PO | 500 mg | Every 12 hours | Renal impairment and weight |
| Cefotaxime | IV | 1 g | Every 8 hours | Renal impairment and weight |
| Ceftazidime | IV | 1 g | Every 8 or 12 hours | Renal impairment and weight |
| Ceftriaxone | IV | 2 g | Every 24 hours | Renal and hepatic impairment and weight |
| Carbapenem | ||||
| Meropenem | IV | 1 g | Every 8 hours | Renal impairment and weight |
| Ertapenem | IV | 1 g | Every 24 hours | – |
| Aminoglycoside | ||||
| Gentamicin/tobramycin | IV | 5–7 mg/kg | Every 24 hours | Renal impairment and weight |
| Fluoroquinolone | ||||
| Ciprofloxacin | IV | 400 mg | Every 12 hours | Renal impairment and weight |
| Ciprofloxacin | PO | 500–750 mg | Every 12 hours | Renal impairment and weight |
| Sulfamethizole | PO | 1 g | Every 12 hours | Renal impairment and weight |
| Nitrofurantoin | PO | 100 mg | Every 6 hours | Renal impairment and weight |
| Trimethoprim | PO | 200 mg | Every 12 hours | Renal impairment and weight |
| Sulfamethoxazole/trimethoprim | PO | 800 mg/160 mg | Every 12 hours | Renal impairment and weight |
*Standard recommendation.
IV, intravenous; PO, orally.
Antibiotics will be administered to participants by clinical staff as in usual clinical care during hospitalisation. Practical procedures related to antibiotic treatment, including labelling of applied drugs, will follow normal local instructions while the participant is hospitalised. If participants are discharged before the end of therapy, the exact amount of remaining antibiotics will be delivered from the hospital in the original packaging supplied with the additional label. Trained personnel will perform the additional labelling.
Treatment adherence is evaluated by checking medicine administration records of inpatients or by thoroughly interviewing outpatients about their consumption at the planned telephone interview on day 14. Outpatients will be instructed to document self-administrated antibiotic treatment at home to ensure a more accurate measurement of adherence following hospital discharge. Protocol violations will be reported if patients are assessed to be non-compliant (received <80% of scheduled doses).
Outcomes
Primary outcome
A 90-day survival without clinical or microbiological failure to treatment as defined:
All-cause mortality from the day of randomisation until day 90.
Microbiological failure: Recurrent bacteraemia due to the same micro-organism as verified by sequence analysis occurring after day 5 of antibiotic treatment and until day 90.
-
Clinical failure: Reinitiation of therapy against Gram-negative bacteraemia for more than 48 hours due to clinical worsening suspected to be due to the initial infecting organism and for which there is no alternate diagnosis/pathogen suspected from the day of randomisation and until day 90
Distant complications of initial infection, defined by the growth of the same bacteria as in the initial bacteraemia (eg, endocarditis, meningitis).
Local suppurative complication that was not present at infection onset (eg, renal abscess in pyelonephritis).
Secondary outcomes
All-cause mortality on days 14, 30 and 90.
Total duration of antibiotic treatment.
Duration and type of antibiotic treatment.
Total length of hospital stay.
Hospital readmission within 30 and 90 days.
Antibiotic adverse events.
Use of any type of antimicrobials after discharge.
Severe adverse events grade >3 as described elsewhere (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
Acute kidney injury.
C. difficile infection.
Infection with multidrug-resistance organism.
A single bacteraemic episode was defined as including all positive blood cultures with the same organism within a 5-day period, therefore, recurrence was defined as occurring after 5 days of antibiotic treatment and until day 90.
Participant timeline
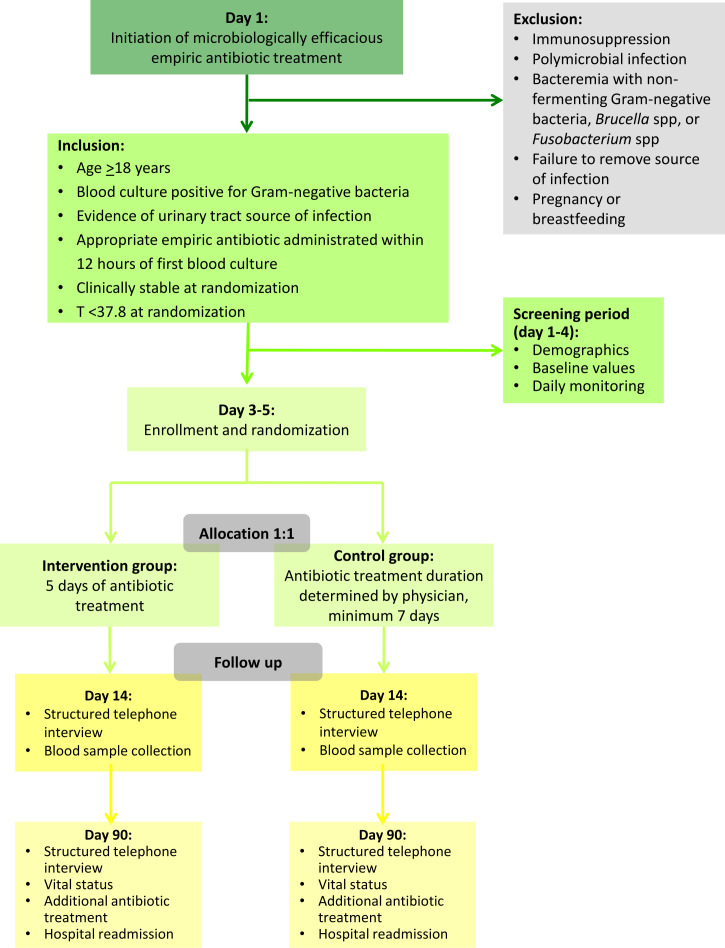
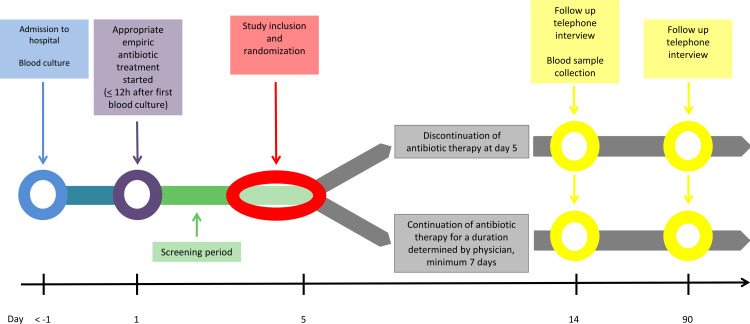
After an initial observation and information period, the participants will be included in the study and randomised no later than day 5 after the initiation of appropriate empiric antibiotic treatment. Participants will be followed for 90 days. On day 14 (12–16) of follow-up, participants will be scheduled for blood sample collection and standardised telephone interview. On day 90 (83–97) of follow-up, participants will be scheduled for a final standardised telephone interview. The study flow chart depicts the inclusion, randomisation, allocation, follow-up and analysis of participants throughout the study (see figure 1). The participant timeline is illustrated in figure 2.
Figure 1.
Flow chart on study level.
Figure 2.
Flow chart on participant level.
Sample size
Based on a similar previous study, short-term mortality is expected to be approximately 12% in both treatment arms. Failure is expected to be 8% in both study arms.19 Because individuals who are not stable by day 5, who have complicated infections, who have a polymicrobial infection or infection with Pseudomonas spp, Brucella spp and Fusobacterium spp are not eligible, we anticipate these rates to be lower at 8% and 4%, respectively. This corresponds to an estimated event rate for the primary outcome of approximately 12%, equivalent to a 90-day survival without clinical or microbiological failure to treatment or relapse of 88% in both treatment arms.
Non-inferiority is defined as an absolute risk difference or margin in the primary endpoint of up to 10%, as recommended by the European Medicines Agency.26 Given an α of 5% and a β of 90% then 362 randomised individuals are required to be sure that the lower limit of a one-sided 95% CI will exclude a difference in favour of the longer course of antibiotics of more than 10%. Allowing for a dropout rate of 5%, 380 individuals will be included.
A sample size re-estimation (SSR) will be considered at the first planned interim analysis. If the overall event rate falls outside the expected event rate of 12%, an SSR based on a blinded review of overall data (ie, without knowledge of the group-specific event rates) will be performed. If the overall event rate is lower than expected, then the final sample size will be reduced, using the original sample size formula and replacing the initial estimate with the observed rate. The non-inferiority margin may be reduced to ensure an appropriate margin relative to the event rate. If the overall event rate is higher than expected, the sample size will be increased correspondingly. Any sample size adjustment will be reported to the regulatory authorities as a protocol amendment.
Recruitment
Investigators and treating physicians at participating sites will identify patients eligible for the trial during days 1–4 after initiation of empiric antibiotic treatment. Cases are identified by participating Departments of Clinical Microbiology and Infectious Diseases at each centre. All participants are hospitalised at enrolment.
The principal investigator will handle questions concerning the recruitment or enrolment of participants, while the study is running.
Patient and public involvement
No patient involved.
Trial status
The first trial participant was enrolled in March 2020. Recruitment is expected to be completed in February 2026.
Data collection, management and analysis
Data collection
At inclusion, data will be obtained from the initial observation period, including baseline diagnostic values, daily vital signs, treatment adherence and microbiology test results. Furthermore, demographic characteristics will be obtained. Subsequently, participants are scheduled for standardised telephone consultation on days 12–16 after initiation of empiric antibiotic treatment. A follow-up on days 83–97 will include final standardised telephone consultation, registration of additional antibiotics, readmissions and vital status. Data collection during the observation and study period is specified in separate sections below and an overview is presented in table 4.
Table 4.
Data collection
| Observation period* | Study period† | |||
| Day 1 | Days 2–5 | Day 14 (12–16) | Day 90 (83–97) | |
| Informed consent | X | |||
| Inclusion | X | |||
| Randomisation | X | |||
| Demographics‡ | X | |||
| Comorbidity§ | X | |||
| Vital signs¶ | X | X | ||
| Urine culture | X | |||
| Blood test** | X | (X) | X | |
| Microbiology | X | X | ||
| CCI score†† | X | |||
| qSOFA score‡‡ | X | X | ||
| PBS§§ | X | X | ||
| Treatment adherence | X | X | ||
| Adverse events | X | X | ||
| Additional antibiotics | X | X | ||
| Readmission | X | |||
| Vital status | X | |||
*From day 1 of efficacious empiric antibiotic treatment to inclusion.
†From inclusion to the end of the trial on day 90.
‡Age, gender, tobacco use, alcohol consumption, medication, medical history, nursing home residency and activities of daily living.
§Liver disease, heart disease, congestive heart failure, cerebrovascular disease, renal disease, chronic obstructive pulmonary disorder, diabetes mellitus, neoplastic disease, haematological disease, peripheral vascular disease, dementia, connective tissue disease and ulcer.
¶BP, heart rate, temperature, respiratory rate, peripheral oxygen saturation.
**Haemoglobin, leucocytes (WCCs), platelet count, CRP, creatinine, urea, sodium, potassium, bilirubin, alanine aminotransferase, glucose.
††CCI: diabetes with diabetic complications, congestive heart failure, peripheral vascular disease, chronic pulmonary disease, mild and severe liver disease, hemiplegia, renal disease, leukaemia, lymphoma, metastatic tumour and AIDS.31
‡‡qSOFA score: Glasgow Coma Score <15, respiratory rate >22, systolic BP ≤100.
§§PBS: temperature, BP, mental status, respiratory status, cardiac status
BP, blood pressure; CCI, Charlson’s Comorbidity Index; CRP, C reactive protein; PBS, Pitt Bacteraemia Score; qSOFA, quick sequential organ failure assessment; WCC, white cell count.
Data management
All data on participants, including demographics, medical history, laboratory and investigational results, will be registered and kept in an electronic case report form (eCRF). The eCRFs will be stored in a secure web application for managing online databases (REDCap) designed for non-commercial clinical research. There will exist one CRF for each participant for the collection of trial data. Obtained data will be entered manually by investigators or appointed research nurses/assistants into the CRFs. Only personnel associated with the research project (sponsor, investigators, subinvestigators and research nurses/assistants) will have encoded access to the CRFs via personal user ID and password.
Sponsor and investigators are obliged to handle all data on trial participants confidentially by the Act on Processing of Personal Data. The primary investigator is responsible for completed CFRs for all trial participants. At the end of the study, the primary investigator will extract data from the electronic database to perform the planned analyses on primary and secondary outcomes. Study data will subsequently be published only in pseudonymous form.
Statistical methods
Descriptive statistics will be presented as frequency tables, means with SD, or medians with IQRs.
Both intention-to-treat (ITT) and per-protocol (PP) analyses will be performed. ITT analysis will comprise all participants including dropouts. Categorical variables will be analysed with χ2 test or Fisher’s exact test. Continuous variables will be subject to Student’s t-test or Wilcoxon rank sum test.
Subgroup-analyses are planned for disease severity, antibiotic group, resistant pathogens and investigating centre. Resistant pathogens are defined as extended-spectrum beta-lactamase producing or carbapenemase-producing Enterobacteriaceae, or pathogens with lack of susceptibility to minimum one agent in three or more classes of antibiotic.
Non-inferiority plots will be performed on the primary outcome for both ITT and PP analyses.
For all statistical analyses except for the non-inferiority analysis, a two-sided p<0.05 is considered statistically significant.
The statistical analysis plan is available in online supplemental material.
bmjopen-2022-068606supp001.pdf (361.1KB, pdf)
Data monitoring
External monitoring will be performed according to International Conference on Harmonisation-Good Clinical Practice (ICH-GCP). Following a monitoring plan and written standard operating procedures, monitors will verify that the clinical trial is conducted and generated, documented and reported in compliance with the protocol, GCP and applicable regulatory requirements.
The investigating team will provide direct access to all trial-related source data, documents, and reports for monitoring and auditing by the sponsor and inspection by local and regulatory authorities.
The primary endpoint will be evaluated and determined by an independent committee blinded to randomisation.
Interim analysis
We will perform interim analyses after the recruitment of every 100 participants. This serves to evaluate primary endpoints and potential adverse events by an independent data and safety monitoring board.
The Haybittle-Peto method is applied to demonstrate overwhelming differences between the two treatment groups that necessitate premature termination of the trial. A significant p value of 0.001 in the interim analyses will correspond to a p value of 0.05 in the final analysis.
Harms
Investigators and sponsor are obliged to follow the study protocol including reporting all adverse events, serious adverse events and suspected unexpected serious adverse reactions to the relevant authorities as outlined by the Danish Health and Medicine Authority and the European Commission.27 28
Participants will be thoroughly asked if they have experienced any adverse event at inclusion and the planned follow-up by phone on days 14 and 90. Adverse events will be registered in predefined CRFs. All adverse events will be followed until they have abated, or until a stable situation has been reached.
All adverse events must be evaluated by investigators and sponsor to determine possible causal association with the antibiotic treatment. At study termination, a final report of registered events in the CRFs will be sent to the Danish Medicines Agency and the Health Research Ethics Committee of the Capital Region of Denmark. Serious adverse reactions, suspected unexpected serious adverse reactions and information on the general safety of the participants will be listed in an annual safety report to the Danish Medicines Agency and the regional Health Research Ethics Committee.
Discussion
The results of this study may have major implications on antibiotic use in Gram-negative bacteraemia with urinary tract infection as source of infection. Regardless of the results, the study will provide valuable information about current treatment practices by either validating or revising them in light of the scientific evidence. To the best of our knowledge, this is the first study to assess the safety and efficacy of 5 days of treatment in Gram-negative bacteraemia guided by clinical stability criteria. We chose 90-day survival without microbiological and clinical failure as the primary endpoint, as we consider failure and mortality to be the most important measures for clinical safety.
As a potential disadvantage related to shortened antibiotic duration would be the risk of treatment failure, it is, therefore, crucial to carefully select study participants when considering both efficacy and safety. Accordingly, the inclusion and exclusion criteria of the study were thoroughly based on available data on risk factors related to clinical outcomes. Relying on clinical stability criteria in shortening antibiotic treatment is partly based on a large randomised controlled trial showing that shortened antibiotic treatment against Gram-negative bacteraemia is non-inferior to longer antibiotic treatment in patients that reached clinical stability within 7 days of treatment.19 Treatment failure in patients hospitalised with Gram-negative bacteraemia has been shown to correlate with initial disease severity, for example, by Pitt bacteraemia score, end-stage liver disease and immunosuppression.17 According to protocol, all participants are scheduled for a blood test on day 14 and a standardised telephone interview on day 14, and the last day of follow-up, on day 90, which will ensure early detection of potential treatment failure.
A limitation to this study is that our strict eligibility criteria will possibly limit the generalisability of the results to all patient groups, as only patients with uncomplicated disease are included.
If shortened antibiotic duration in patients hospitalised with Gram-negative bacteraemia can be proven to be non-inferior to standard antibiotic treatment, it would likely relieve antibiotic selective pressure and thereby lower the development of bacterial resistance.3 6 11 12 From the perspective of the participants, one might expect fewer side effects and better treatment adherence, as prolonged antibiotic use has been associated with an increased risk of side effects.9 On a community level, this change would lead to a reduction in overall healthcare costs, as shortened antibiotic duration would result in a decrease in total antibiotic consumption and thereby length of hospital stay.29–31 As Gram-negative bacteria are accountable for great proportions of bacteraemia and thereby antibiotic prescription, this decrease could be quite significant from a national perspective.
Ethics and dissemination
Research ethics approval
The study has been approved by Danish National Committee on Health Research (H-19085920), the Danish Medicines Agency (2019-003282-17), and the Danish Data Protection Registry (P-2020-42). The study will be conducted according to ICH-GCP and monitored by GCP units in Denmark.
Consent or assent
Eligible participants will be scheduled for consultation no later than on day 5 after initiation of appropriate empiric antibiotic treatment. They will receive both verbal and written information about the study, and subsequently, be offered participation.
Participants will need to sign the informed consent form to be randomised. Participants are asked regardless of initiating empirical treatment at inclusion. The informed consent process for women of childbearing age will include questions on possible pregnancy and will be registered in the eCRF. If there is a possibility of pregnancy, a pregnancy test will be performed with informed consent. As the intervention period is short (5–14 days) and occurs during hospital admission, it is not anticipated that contraceptive advice is relevant.
The consent form is available in online supplemental material.
Post-trial care
All areas of the Danish healthcare system and all authorised healthcare professionals are covered by a publicly funded compensation scheme. The scheme covers if the participants are injured in connection with treatment at a public hospital. The scheme also covers medicinal product injuries. At inclusion, the participants will be informed by the investigator of the compensation and complaint avenues in case a drug injury arises with the participant, according to ‘Lov om klage- og erstatningsadgang inden for sundhedsvæsenet’.32
Protocol amendments
All protocol amendments have been approved by Danish Regional Committee on Health Research and the Danish Medicines Agency. An overview of protocol changes is shown in table 5.
Table 5.
Summary of protocol changes
| Protocol version | Protocol changes |
| Version 3, 18 February 2020 | Approved for study start |
| Version 4, 22 September 2020 |
|
| Version 5, 12 November 2021 |
|
Access to data
The study is registered at www.clinicaltrials.gov before initiation (ClinicalTrials.gov: NCT04291768, registered on the 24 February 2020).
Anonymised trial data will be made available through relevant public databases when the trial ends. On request, anonymised patient-level data, the statistical code and other relevant supporting information will be made available by contact to the corresponding author.
Dissemination policy
The data obtained from all participating sites will be pooled and analysed together as soon as possible after trial completion. Individual researchers will not publish data from the trial until the main study publication has been released. A manuscript with the results of the primary study will be published in a peer-reviewed journal with the primary investigator as the first author, the sponsor as the senior author, and the participating investigators as coauthors according to their work and involvement in the study.
Supplementary Material
Acknowledgments
Haakon Sandholt for statistical support.
Footnotes
Twitter: @STingsgard, @israelsen_sb
Contributors: TB is the sponsor of the study. ST is the coordinating principal investigator. TB conceived the research question of the study. TB and ST obtained the funding for the study. TB, ST, SBI, LT-U and CØA participated in the design of the study. ST drafted the study protocol. TB, ST, SBI, LT-U, BL, ISJ, SL, CØA, PR, KFK and AK contributed to the implementation of the study and revised the protocol critically for important intellectual content. All authors read and approved the final manuscript.
Funding: The study is partly financially supported by a grant from Hvidovre Hospital Strategic Research Fund, Danish Regions Medicine Grant, grant number EMN-2019-01055, and Capital Region of Denmark Research Fund, grant number A6688. Additional federal funding will be sought. Study participants and the Research Ethics Committee of the Capital Region of Denmark will be informed if additional funding has been granted.
Disclaimer: Sponsor and investigators are independent of economic or competing interests. Participants will not be financially reimbursed. Results from the study are only for scientific and public use and have no commercial interest.
Competing interests: TB reports grants from Novo Nordisk Foundation, Lundbeck Foundation, Simonsen Foundation, GSK, Pfizer, Gilead, Kai Hansen Foundation and Erik and Susanna Olesen’s Charitable Fund; personal fees from GSK, Pfizer, Boehringer Ingelheim, Gilead, MSD, Pentabase ApS, Becton Dickinson, Janssen and Astra Zeneca; outside the submitted work. The remaining authors have no conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Kang C-I, Kim S-H, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 2005;49:760–6. 10.1128/AAC.49.2.760-766.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY antimicrobial surveillance program (1997-2002). Diagn Microbiol Infect Dis 2004;50:59–69. 10.1016/j.diagmicrobio.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 3.de Kraker MEA, Jarlier V, Monen JCM, et al. The changing epidemiology of bacteraemias in Europe: trends from the European antimicrobial resistance surveillance system. Clin Microbiol Infect 2013;19:860–8. 10.1111/1469-0691.12028 [DOI] [PubMed] [Google Scholar]
- 4.Søgaard M, Nørgaard M, Dethlefsen C, et al. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clin Infect Dis 2011;52:61–9. 10.1093/cid/ciq069 [DOI] [PubMed] [Google Scholar]
- 5.DANMAP 2017 . Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. 2017. [Google Scholar]
- 6.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. The Lancet 2005;365:579–87. 10.1016/S0140-6736(05)17907-0 [DOI] [PubMed] [Google Scholar]
- 7.Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:2369–70. 10.1056/NEJMc1505190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha BA. Antibiotic side effects. Med Clin North Am 2001;85:149–85. 10.1016/s0025-7125(05)70309-6 [DOI] [PubMed] [Google Scholar]
- 9.Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017;177:1308–15. 10.1001/jamainternmed.2017.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branch-Elliman W, O’Brien W, Strymish J, et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019;154:590. 10.1001/jamasurg.2019.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the centers for disease control and prevention. Clin Infect Dis 2014;59 Suppl 3:S97–100. 10.1093/cid/ciu542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice LB. The Maxwell Finland Lecture: for the duration-rational antibiotic administration in an era of antimicrobial resistance and Clostridium difficile. Clin Infect Dis 2008;46:491–6. 10.1086/526535 [DOI] [PubMed] [Google Scholar]
- 13.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases Society of America and the European Society for microbiology and infectious diseases. Clin Infect Dis 2011;52:e103–20. 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 14.Askjær N, Jarløv JO, Lindhardt BØ, et al. Antibiotikavejledning for primærsektoren. n.d. Available: https://www.sundhed.dk/content/cms/21/103521_20220510-antibiotikavejledning.pdf
- 15.G. Bonkat RP. EAU guidelines on urological infections. 2018. [Google Scholar]
- 16.Corona A, Bertolini G, Ricotta AM, et al. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother 2003;52:849–52. 10.1093/jac/dkg447 [DOI] [PubMed] [Google Scholar]
- 17.Chotiprasitsakul D, Han JH, Conley AT, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course vs prolonged-course antibiotic therapy. Open Forum Infect Dis 2017;4:S31. 10.1093/ofid/ofx162.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina J, Montero-Mateos E, Praena-Segovia J, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect 2022;28:550–7. 10.1016/j.cmi.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 19.Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clinical Infectious Diseases 2019;69:1091–8. 10.1093/cid/ciy1054 [DOI] [PubMed] [Google Scholar]
- 20.von Dach E, Albrich WC, Brunel A-S, et al. Effect of C-reactive protein–guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia. JAMA 2020;323:2160. 10.1001/jama.2020.6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT) -guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009;394:221–6. 10.1007/s00423-008-0432-1 [DOI] [PubMed] [Google Scholar]
- 22.Nobre V, Harbarth S, Graf J-D, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008;177:498–505. 10.1164/rccm.200708-1238OC [DOI] [PubMed] [Google Scholar]
- 23.Corey GR, Stryjewski ME, Everts RJ. Short-Course therapy for bloodstream infections in immunocompetent adults. Int J Antimicrob Agents 2009;34 Suppl 4:S47–51. 10.1016/S0924-8579(09)70567-9 [DOI] [PubMed] [Google Scholar]
- 24.Havey TC, Fowler RA, Pinto R, et al. Duration of antibiotic therapy for critically ill patients with bloodstream infections: a retrospective cohort study. Can J Infect Dis Med Microbiol 2013;24:129–37. 10.1155/2013/141989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corona A, Wilson APR, Grassi M, et al. Prospective audit of bacteraemia management in a university hospital ICU using a general strategy of short-course monotherapy. J Antimicrob Chemother 2004;54:809–17. 10.1093/jac/dkh416 [DOI] [PubMed] [Google Scholar]
- 26.CPMP . Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. 2022. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf [Accessed 12 Sep 2022].
- 27.Sundhedsstyrelsen . Bekendtgørelse om god klinisk praksis i forbindelse med kliniske forsøg med lægemidler på mennesker (GCP-bekendtgørelse). 2013. [Google Scholar]
- 28.European Commission . Detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from clinical trials on medicinal products for human use. 2011. [Google Scholar]
- 29.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006;42 Suppl 2:S82–9. 10.1086/499406 [DOI] [PubMed] [Google Scholar]
- 30.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther 2008;6:751–63. 10.1586/14787210.6.5.751 [DOI] [PubMed] [Google Scholar]
- 31.Evans HL, Lefrak SN, Lyman J, et al. Cost of gram-negative resistance. Crit Care Med 2007;35:89–95. 10.1097/01.CCM.0000251496.61520.75 [DOI] [PubMed] [Google Scholar]
- 32.Sundhedsministeriet . Bekendtgørelse af lov om klage- og erstatningsadgang inden for sundhedsvæsenet. 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068606supp001.pdf (361.1KB, pdf)