Abstract
This study examined the intestinal antibody response in 26 healthy volunteers challenged with Cryptosporidium parvum oocysts. Fecal extracts were assayed for total secretory immunoglobulin A (IgA) and C. parvum-specific IgA reactivity. Specific IgA reactivity was standardized to IgA concentration and expressed as a reactivity index (RI). Anti-C. parvum fecal IgA (fIgA) increased significantly in 17 of 26 (65.4%) following oocyst ingestion. Of those with detectable responses, 59, 76.5, and 94.1% were positive by days 7, 14, and 30, respectively. Volunteers receiving high challenge doses (>1,000 and 300 to 500 oocysts) had higher RIs (RI = 5.57 [P = 0.027] and RI = 1.68 [P = 0.039], respectively) than those ingesting low doses (30 to 100 oocysts; RI = 0.146). Subjects shedding oocysts and experiencing a diarrheal illness had the highest fIgA reactivity. When evaluated separately, oocyst excretion was associated with an increased fIgA response compared to nonshedders (RI = 1.679 versus 0.024, respectively; P = 0.003). However, in subjects experiencing diarrhea with or without oocyst shedding, a trend toward a higher RI (P = 0.065) was seen. Extracts positive for fecal IgA were further examined for IgA subclass. The majority of stools contained both IgA1 and IgA2, and the relative proportions did not change following challenge. Also, no C. parvum-specific IgM or IgG was detected in fecal extracts. Thus, fecal IgA to C. parvum antigens was highly associated with infection in subjects who had no evidence of previous exposure and may provide a useful tool in detecting recent infections.
Cryptosporidium parvum is a coccidial protozoan that infects the intestinal mucosa of humans and other mammalian species. The infection may be asymptomatic or display an illness characterized by profuse diarrhea accompanied by enteric symptoms. Illness is usually acute and self-limiting in immunocompetent individuals, but it can become persistent and potentially fatal in the immunocompromised.
The antibody response to C. parvum is not clearly understood, and the exact role it plays during infection is controversial. The mucosal response is especially intriguing since the parasite inhabits the bowel, is noninvasive, and is strictly limited to the apical region of enterocytes. Thus, one might expect that the most likely antibody involved in clearance or subsequent protection would be secretory immunoglobulin A (IgA). Several lines of evidence in animals and humans suggest that this may be the case.
In vitro studies have demonstrated that a number of anti-Cryptosporidium monoclonal antibodies can bind to the sporozoite surface and prevent attachment and infection (reviewed in reference 24). In a more recent study, investigators extended those observations by producing dimeric IgA monoclonals that significantly reduced the intensity of the infection in neonatal mice (7). Other studies with neonatal animals have shown that mucosal antibody responses to the pathogen accompany resolution of diarrhea and oocyst shedding and vary from species to species (12, 19–21, 25). In mice, IgA and IgG appeared in intestinal secretions at day 15 to 30 postinfection (p.i.) (23). In another study, mouse fecal IgA (fIgA) was first detected between days 11 and 37 p.i. and persisted until day 55 p.i. (26). In ovine studies, specific fecal IgA appeared on days 6 to 16 p.i. and peaked at day 13 p.i. (12, 19). Similar kinetics were observed in experimentally infected calves; however, in addition to IgA, IgG and IgM were found in the fecal extracts (20, 21). In both lambs and calves, reduction and resolution of oocyst shedding were related to increasing fIgA titers (12, 20, 21); this observation, however, was not made in the mouse model (26). However, when lambs were rechallenged with C. parvum oocysts, no significant changes in antibody titer were observed (19, 21).
Furthermore, individuals with congenital hypogammaglobulinemia develop chronic infections and are unable to clear C. parvum (14). In studies in which infected mice (9, 22), cows (8), and immunocompetent (16) and immunocompromised (15) humans were given hyperimmune bovine colostrum, diarrhea was lessened, fewer oocysts were shed, and infection was cleared more rapidly than in controls. However, other studies have shown that high levels of Cryptosporidium-specific IgA can be found in human immunodeficiency virus (HIV)-infected individuals with chronic cryptosporidiosis (3), suggesting that antibody alone may not be sufficient.
Salivary IgA to C. parvum has also been studied in immunocompetent and immunocompromised individuals (3, 10). HIV-positive individuals who cleared C. parvum infection had higher salivary IgA levels than HIV-positive individuals with chronic infections (10), and the latter group had a greater specific salivary IgA level than individuals (HIV positive or healthy) without C. parvum infection (3).
A Cryptosporidium volunteer study (6) conducted at the University of Texas Health Science Center has provided the opportunity to study mucosal antibody in healthy adults before and after challenge with known doses of C. parvum oocysts. Fecal extracts were assayed by enzyme-linked immunosorbent assay (ELISA) for total IgA concentration and anti-C. parvum IgA reactivity, and data were compared to clinical and infection parameters. Fecal extracts were also examined for IgA subclass and the presence of C. parvum-specific IgM and IgG.
MATERIALS AND METHODS
Volunteers and specimen collection.
The stool specimens used in this study were collected between March 1993 and January 1994 as part of the Cryptosporidium volunteer study conducted at the Texas Medical Center in Houston (6). The Cryptosporidium Volunteer Study was approved by the University of Texas (Houston) Health Science Center Committee for the Protection of Human Subjects. Briefly, 29 volunteers were enrolled into the study on the basis of their good general health and HIV-negative status and the absence of C. parvum serum antibodies. Volunteers were then challenged with known doses of C. parvum oocysts (Iowa strain), ranging from 30 to 106, and monitored for 45 to 60 days. Stool specimens were collected every day for the first 2 weeks and three times per week thereafter. Volunteers were instructed to collect each stool, store it at 4°C, and deliver the specimen to the study nurse by the next day. Samples were categorized for consistency by the volunteer and confirmed by the nursing staff. Each stool was then weighed and examined for Cryptosporidium, and the oocysts were quantitated by direct immunofluorescence assay using a commercial kit (Merifluor Cryptosporidium/Giardia kit; Meridian Diagnostics, Cincinnati, Ohio). Remaining specimens were stored at −90°C until use.
At the completion of the study, participants were categorized according to infection status and clinical outcome. The definitions used in this study have been described previously (2). Fifteen volunteers had a diarrheal illness; six others were shedding oocysts but asymptomatic, and the remaining eight were asymptomatic and negative for fecal oocysts. Of these 29 individuals, 26 had multiple pre- and postchallenge stool samples available for study.
Fecal antibody extraction.
Stool specimens were selected to represent 4-day intervals (where possible) over the course of the experiment. The extraction method was adapted from that of a volunteer challenge study of Norwalk virus (18). Briefly, 2 g of stool was diluted in 5 ml of 0.15 M phosphate-buffered saline (PBS) (pH 7.2) and thoroughly mixed. Following a 30-min incubation in a 56°C water bath, the sample was delipidated with 5 ml of 1,1,2-trichloro-trifluoroethane (Fisher Scientific, Pittsburgh, Pa.). The suspension was centrifuged at 1,500 × g for 25 min at 4°C. The supernatant was collected and further centrifuged at 10,000 × g for 60 min at 4°C. The supernatant was then removed and dialyzed against 4 liters of deionized, distilled water for 12 h followed by 4 liters of PBS for another 12 h. The dialysates were filtered in tandem (5- and 0.5-μm-pore-size filters), and filtrates were stored at −90°C until use.
ELISA.
ELISAs were performed to detect and quantify IgA in the fecal extracts. Unless otherwise stated, all incubation steps were performed for 1 h at 37°C and followed by three wash steps using 0.15 M PBS (pH 7.2) containing 0.1% polysorbate 20 (Tween 20) (ICI Americas, Inc., Wilmington, Del.). Total fecal IgA was quantified using a sandwich ELISA. Microtitration plates (Nunc, Roskilde, Denmark) were coated for 16 h at 4°C with anti-human IgA secretory component (Sigma Immuno Chemicals, St. Louis, Mo.). The wells were blocked with a 5% dry milk solution for 1 h. Fecal extracts (50 μl) were then added to duplicate wells and incubated. Each plate also contained duplicate wells of six known concentrations (15.6 to 0.49 ng) of purified human secretory IgA (Cappel, Durham, N.C.). Addition of peroxidase-conjugated anti-human secretory IgA (Cappel) diluted 1:1,000 in PBS was followed by the incubation of H2O2-activated 1 M 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] (ABTS; Boehringer Mannheim GmbH, Mannheim, Germany) at room temperature. Optical densities were read at 15 and 30 min with a Titertek Multiskan MCC/340 ELISA reader (Flow Laboratories, McLean, Va.) using a 414-nm-pore-size filter. For each plate, a standard curve was constructed from the known IgA protein and used to determine the IgA concentration of each fecal extract. The concentrations determined were expressed as nanograms of IgA per 50 μl of extract.
C. parvum-specific reactivity was detected essentially as described previously for serum antibodies (17). Reagent control wells consisted of horseradish peroxidase-conjugated anti-human secretory IgA (Cappel) and ABTS substrate without fecal extract. Wells were read spectrophotometrically (414 nm) after 30 min. Extracts from each subject (i.e., different time points) were always tested on the same plate. The mean optical density (OD) of specific IgA in postchallenge extracts was compared to the mean OD of the matched prechallenge specimen. Postchallenge extract values were considered significantly increased if the mean OD exceeded 2 standard deviations (SD) above the mean prechallenge OD. All specific secretory IgA absorbances were standardized using total IgA concentrations to control for stool consistency. A reactivity index (RI) for each extract was calculated as the ratio of the specific IgA absorbance to total secretory IgA (in nanograms) of that sample (same volume) multiplied by 100.
For each individual, four or five of the postchallenge, IgA-positive fecal extracts were pooled and examined for specific IgA subclass reactivity. ELISAs were performed in a manner similar to the specific fIgA ELISAs. After incubation with the pooled fecal extract, wells were reacted with mouse anti-human IgA1 or IgA2 monoclonal antibodies (Cappel) (1:1,000) followed by peroxidase-conjugated rabbit anti-mouse IgG (1:1,000; Zymed). Absorbances of >2 SD above the conjugate control were considered positive.
Dot blot assays for IgM and IgG.
Known concentrations of purified oocysts were subjected to freeze-thawing three times, homogenized, and diluted 1:2 in sodium dodecyl sulfate (SDS) solubilizing solution (0.5 M Tris [pH 6.8], 12.5% glycerol, 20% SDS, 0.05% 2-mercaptoethanol, 0.0025% bromphenol blue). After boiling for 7 min, the antigen preparation was aliquoted and stored at −20°C until use.
Dot blots were used to test for specific IgG and IgM in fecal extracts. Antigen from approximately 5 × 104 oocysts was applied to a nitrocellulose membrane and allowed to dry. Membranes were blocked for 1 h with a 5% dry milk solution at room temperature. Following two washes in 0.15 M PBS containing 0.1% Tween 20 (ICI Americas, Inc.), membranes were incubated for 24 h at 4°C in undiluted prechallenge or pooled postchallenge fecal extracts. The membranes were washed and probed with horseradish peroxidase-conjugated anti-human IgG (Zymed) or anti-human IgM (1:1,000) (Zymed) for 2 h at room temperature. After washing, binding was detected with 1 mM 3,3′,5,5′-tetramethylbenzidine (Pierce, Rockford, Ill.), and the reaction was terminated with distilled water. Reagent controls included antibody conjugate and substrate without fecal extract. Membranes were allowed to dry and then scanned using a GS-700 imaging densitometer (Bio-Rad). A positive reaction was defined visually as increased intensity over the reagent control.
Statistical analysis.
Comparison of the total or C. parvum-specific fIgA versus various clinical or parasitological parameters required several analytical methods. For all methods, a P value of <0.05 was considered significant. Examination of the data revealed non-Gaussian distributions; therefore, the median rather than the mean was a more appropriate measure of central tendency. Nonparametric tests, including chi-square, Fisher's exact test, and the Mann-Whitney U test, were used to compare antibody positivity to illness and infection measures. The change in antibody response (pre- versus postchallenge) in relation to a variety of illness and infection measures was analyzed using the Kruskal-Wallis test.
RESULTS
Twenty-six volunteers had stool samples collected before and at multiple time points following challenge. Diarrheic stools were subjected to microbiological workup as previously described (2), and no other commonly recognized bacterial, viral, or parasitic pathogens (other than Cryptosporidium) were identified in any of the individuals in question. Each volunteer served as his or her own control for determining the net change in fIgA. All samples from each subject were run on the same plate. Reagent control wells were included in each plate, and repeat wells showed a coefficient of variation of 20% or less. The reagent control had a median absorbance value of 0.129. Total fIgA was also determined for each stool sample and used to standardize specific fIgA values (RI).
Total fIgA concentration.
Total fIgA refers to all measurable fIgA regardless of antigenic specificity. Known concentrations of secretory IgA (0.49 to 15.60 ng) constituted the standard curve and had typical absorbance values in the range of 0.150 to 0.610 for the respective concentrations. fIgA concentrations in prechallenge specimens ranged from 0 to 215.0 ng/50 μl of fecal extract, with a mean of 22.9 ± 43.6 ng/50 μl, which is equivalent to 1.15 μg of IgA per g of stool. Of the 26 volunteers tested, 22 had measurable prechallenge levels of fIgA. The majority (80%) of subjects had levels of ≤31.3 ng/50 μl. Prechallenge fIgA was not significantly associated with challenge dose (P ≥ 0.245) or postchallenge clinical outcome (P ≥ 0.186), oocyst shedding (P = 0.493), and/or diarrhea (P = 0.836) (Table 1).
TABLE 1.
Total fIgA in 26 volunteers before and after challenge with Cryptosporidium oocystsa
| Categoryb | n | fIgA (ng/50 μl)
|
|||||
|---|---|---|---|---|---|---|---|
| Prechallenge
|
Postchallenge (net change)
|
||||||
| Median | Quartiles (Q1, Q3) | P | Median | Quartiles (Q1, Q3) | P | ||
| Dose (oocysts) | |||||||
| >1,000* | 5 | 3.09 | 0.70, 9.87 | 0.245 | 463.10 | 33.00, 537.00 | 0.527 |
| 300–500* | 9 | 8.00 | 2.00, 26.20 | 0.887 | 212.40 | 42.00, 555.00 | 0.943 |
| 30–100 | 12 | 12.64 | 1.20, 39.94 | 150.40 | 34.00, 2,765.00 | ||
| Clinical group | |||||||
| Diarrhea, oocysts** | 12 | 6.02 | 1.66, 26.43 | 0.447 | 214.72 | 53.00, 720.00 | 0.186 |
| Diarrhea, no oocysts** | 3 | 22.98 | 0.00, 70.5 | 0.493 | 50.72 | 27.70, 95.80 | 0.909 |
| Mild/no symptoms, oocysts** | 4 | 18.43 | 4.4, 167.20 | 0.186 | 588.54 | 109.00, 3,785.00 | 0.272 |
| No oocysts or symptoms | 7 | 2.87 | 0.82, 11.89 | 46.45 | 8.00, 600.00 | ||
| Infection | |||||||
| Presumed infected | 19 | 13.38 | 1.40, 29.00 | 0.272 | 212.41 | 52.00, 806.00 | 0.236 |
| Uninfected | 7 | 2.87 | 0.82, 11.89 | 46.45 | 8.00, 600.00 | ||
| Oocysts | |||||||
| Detected | 16 | 10.32 | 1.80, 27.60 | 0.493 | 214.73 | 71.00, 870.00 | 0.073 |
| Not detected | 10 | 5.44 | 0.62, 25.05 | 48.58 | 12.00, 311.00 | ||
| Illness | |||||||
| Diarrhea | 15 | 7.26 | 1.19, 29.00 | 0.836 | 206.10 | 47.00, 464.00 | 0.979 |
| No diarrhea | 11 | 8.00 | 1.40, 23.50 | 212.40 | 13.00, 966.00 | ||
Each value represents the amount of total IgA in fecal extracts. Net change for each subject was calculated by subtracting the prechallenge fIgA concentration from the postchallenge sample containing the highest antibody level.
Statistical significance determined by Kruskal-Wallis test. *, compared to the 30–100 challenge dose group. **, compared to the no oocysts or symptoms group.
Each subject had multiple postchallenge stool samples. The fIgA level was measured in each stool extract. In order to evaluate the overall IgA response, the peak postchallenge value was used in the calculations (Table 1). Postchallenge fIgA levels ranged from 0 to 4,725.3 ng/50 μl, with a median increase of 210.3 ng/50 μl. As with prechallenge levels, changes in postchallenge fIgA were not associated with challenge dose (P ≥ 0.527), outcome group (P ≥ 0.186), presumed infection (P = 0.236), oocyst shedding (P = 0.073), or diarrhea (P = 0.979) (Table 1). Of note, in most volunteers total fIgA levels varied little from day to day, fluctuating by 10.6 ng/50 μl of extract (median change).
fIgA subclass determination.
Thirteen prechallenge and 17 postchallenge stool samples containing measurable total fIgA were tested for relative concentrations of IgA1 and IgA2 subclasses (data not shown). Prior to challenge, IgA2 accounted for approximately 65.8% ± 33.1% of the relative absorbances. Analysis of postchallenge samples showed that both subclasses increased approximately equally (1.2- to 1.4-fold) and that IgA2 remained predominant (77.1% ± 24.2%). Thus, Cryptosporidium infection was associated with increases in both IgA subclasses but did not appear to significantly alter the relative subclass distribution.
To determine if any serum antibody was present in fecal extracts, pre- and postchallenge extracts from 19 volunteers were analyzed by dot blot for IgG and IgM (data not shown). All extracts were tested in duplicate with appropriate controls, and no IgG or IgM reactivity was observed. These results indicate that little or no serum leakage into the intestinal lumen occurred and that the fIgA detected in these assays was likely to be secreted locally.
Prechallenge anti-C. parvum fIgA.
Prechallenge specific fIgA absorbances (A414) ranged from 0.001 to 0.360, with a mean of 0.067 ± 0.106. Of the 26 volunteers tested, 17 had prechallenge specific fIgA absorbances below that of the reagent control and 9 had absorbances from 0.129 to 0.360. A retrospective analysis was done on these nine individuals to examine if the prechallenge specific fIgA absorbances were associated with any particular postchallenge outcome. For the three dosage groups, the median absorbance values for prechallenge specific fIgA were 0.037 ± 0.058 (30 to 100 oocysts), 0.115 ± 0.159 (100 to 300 oocysts), and 0.043 ± 0.044 (>1,000 oocysts), levels that were not significantly different. Likewise, no significant associations were found between prechallenge anti-Cryptosporidium fIgA and oocyst shedding or symptoms (data not shown). To control for stool consistency, samples were standardized to total fIgA concentration, and the RI was calculated for each extract. Prechallenge RIs ranged from 0.002 to 7.95, with a mean of 1.08 ± 2.12 and median of 0.100. As with the nonstandardized absorbance values, no associations with clinical or infection outcomes were observed.
Postchallenge anti-C. parvum fIgA and shedding patterns.
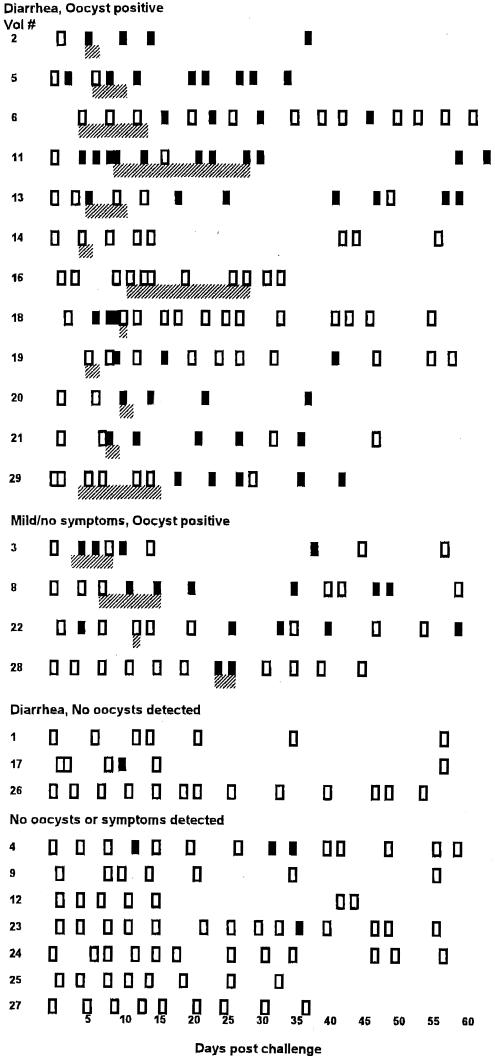
Multiple stool samples from each volunteer were evaluated for an increase in anti-C. parvum-specific fIgA following challenge (Fig. 1). Seventeen volunteers (65.3%) showed anti-C. parvum fIgA reactivity in one or more stool samples following challenge, and 15 of 17 subjects demonstrating anti-C. parvum fIgA had multiple positive stool samples. The earliest detectable positive stool was seen at day 2 postchallenge; however, most subjects (10 of 17, 59%) had an initial response after 7 days, and 13 of 17 (76.5%) volunteers were positive by 14 days postchallenge. Only one individual became positive after 30 days, and this subject had only one positive stool. Although stool samples from later time points were not always available, it should be noted that five individuals remained positive for anti-Cryptosporidium fIgA for more than 6 weeks. Day-to-day variability was noted in the results; that is, most volunteers having positive specific fIgA had days on which they were negative, indicating a variation in the amount of antibody secreted.
FIG. 1.
Cryptosporidium-specific fIgA in 26 experimentally challenged volunteers over the course of the study period. Each row represents a single volunteer, and each rectangle represents a stool sample that was tested for fIgA reactivity. Samples which yielded absorbance values of >2 SD above the prechallenge absorbance value were considered positive (solid rectangle); extracts that fell below the 2-SD cutoff were considered negative (open rectangle). Also shown are the days on which oocyst shedding occurred (hatched area). The volunteers are grouped by clinical outcome.
The appearance of specific fIgA in relationship to oocyst shedding patterns was evaluated in 14 volunteers positive for both parameters (Fig. 1). No common pattern was seen. Specific fIgA preceded oocyst detection in four cases, occurred concurrently in six, and followed oocyst shedding in four cases. It was noted that no specific fIgA was seen in volunteer 16, who excreted the greatest number of oocysts (109), or in volunteer 14, who experienced a more moderate infection (107).
fIgA reactivity and infection outcomes.
The specific fIgA data for infection outcomes were expressed as the RI (Table 2). To standardize for stool consistency, the postchallenge peak absorbance values for each volunteer were used to calculate RI. After challenge, there was an overall increase in RIs (1.08 ± 2.12 versus 7.19 ± 12.33, P = 0.01). Challenge dose, infection, and illness were evaluated for possible association with the postchallenge increases in fIgA reactivity. Since excretion of oocysts is influenced by the dose received, analysis of fIgA levels per challenge dose was performed. Challenge dosages were grouped as low dose (30 to 100 oocysts), moderate dose (300 to 500 oocysts), and high dose (≥1,000 oocysts). Individuals who received ≥300 oocysts had significantly higher RIs than those in the low-dose group (30 to 100 oocysts, P ≤ 0.039). These data suggest that greater numbers of replicating parasites are needed for maximal stimulation of specific fIgA.
TABLE 2.
Anti-Cryptosporidium fIgA and outcome parametersa
| Category | n | Median change in fIgA RI | Quartiles (Q1, Q3) | P |
|---|---|---|---|---|
| Challenge dose | ||||
| >1,000* | 5 | 5.57 | 4.24, 7.81 | 0.027 |
| 300–500* | 9 | 1.68 | 0.75, 20.62 | 0.039 |
| 30–100 | 12 | 0.15 | −0.03, 0.50 | |
| Clinical group | ||||
| Oocyst positive | ||||
| With diarrhea** | 12 | 4.24 | 1.35, 27.92 | 0.022 |
| Without diarrhea** | 4 | 0.82 | 0.34, 4.48 | 0.257 |
| Oocyst negative | ||||
| With diarrhea** | 3 | 0.07 | −0.38, 0.22 | 0.909 |
| No symptoms | 7 | 0.02 | −0.05, 6.71 | |
| Oocyst shedding | ||||
| Yes | 16 | 2.78 | 0.68, 7.96 | 0.003 |
| No | 10 | 0.03 | −0.05, 1.85 | |
| Infection | ||||
| Presumed infected | 19 | 1.68 | 0.43, 7.50 | 0.060 |
| Presumed uninfected | 7 | 0.02 | −0.05, 6.71 | |
| Diarrhea | ||||
| Yes | 15 | 2.41 | 0.50, 8.11 | 0.065 |
| No | 11 | 0.30 | −0.004, 5.56 |
fIgA is expressed as the median change in the RI. The RIs for each volunteer were calculated as the ratio of the net absorbance divided by the total fIgA concentration. Postchallenge indices used for comparisons in the table represent the RI of the peak postchallenge absorbance value for each volunteer.
Statistical significance determined by Kruskal-Wallis test. *, compared to the 30–100 challenge dose group. **, compared to the oocyst negative, no symptoms group.
Volunteers in the “oocyst positive with diarrhea” group had significantly higher RIs (P = 0.022) than any of the other outcome categories. To further explore the individual factors most influential in the specific fIgA response, volunteers were grouped according to their illness outcome. Those with diarrhea had RIs that did not differ significantly (P = 0.065) from the RIs of those without diarrhea. This lack of association was also apparent when the group was limited to oocyst shedders with and without diarrhea (P = 0.368; data not shown). In contrast, when oocyst shedders (with or without diarrhea) were compared to nonshedders, a high correlation (P = 0.003) with the presence of anti-C. parvum fIgA was observed.
This high correlation of specific fIgA and oocyst shedding was examined in greater detail in the 16 volunteers shedding oocysts. Only two volunteers in this group failed to show a specific fIgA increase. All 16 subjects were evaluated for onset of oocyst shedding, duration of shedding, and intensity of infection by comparing these parameters with RIs from each subject. None of the parameters showed a statistically significant association with an increase in specific fIgA. However, volunteers shedding fewer than 5 × 106 oocysts over the study period had slightly higher RIs (mean = 11.3) than those shedding greater than 5 × 106 oocysts (mean = 6.1). These findings, along with the lack of measurable specific fIgA in volunteers 14 and 16, suggest that a portion of the antibody may be binding to parasite developmental stages in the gut lumen.
In other studies (2, 17), some subjects experienced diarrhea in the absence of oocysts detectable by immunofluorescence. However, a more sensitive flow cytometric analysis of several samples indicated that low levels of oocysts could often be found. A classification of “presumed infection” was adopted for such individuals. In the present study, three individuals fell into this category. These presumed infected volunteers tended to have an increase in RI (P = 0.06) compared to the presumed uninfected group. Take together, data from this study suggest that the number of oocysts ingested (challenge dose) and the presence of replicating parasites are necessary and sufficient to stimulate a significant increase in anti-C. parvum fIgA. Furthermore, while diarrhea may contribute to this increase, it does not appear to be the principal factor.
DISCUSSION
The present study investigated the fIgA response in healthy individuals exposed to C. parvum under controlled conditions. Volunteers for this study were selected on the basis of their serological status; fIgA reactivity was unknown at the time of enrollment. A retrospective evaluation of prechallenge specific fIgA showed low absorbance values overall, suggesting that these volunteers had not been recently infected with C. parvum. Further analysis failed to identify any associations between prechallenge absorbances and the outcome of the subsequent challenge.
After oocyst challenge there was a significant increase overall in total and specific fIgA levels. Highest responses were associated with moderate-to-high challenge doses and/or oocyst shedding. Thus, antigen concentration at the initiation of infection (i.e., challenge dose) and the continuing stimulation of parasite replication appeared to have the strongest correlation with the specific response. The increased permeability of the intestinal epithelium in volunteers with diarrhea may also have contributed to enhanced antibody secretion, but diarrhea alone was not associated with an increase in specific fIgA, nor were any other antibody isotypes detected. Indeed, volunteers 1, 17, and 26, who experienced a diarrheal illness in the absence of detectable oocysts, had little or no increase in specific fIgA. It is possible that in these individuals, control mechanisms may have curtailed parasite replication before sufficient numbers were present to elicit a specific IgA response and before oocysts could be formed.
Furthermore, two volunteers who had diarrhea and were shedding oocysts failed to yield measurable specific fIgA. A dilutional effect from the diarrhea may have made antibody detection difficult; however, this was unlikely since several other volunteers had greater stool volumes and readily detected fecal antibodies. It should also be noted that all volunteers were screened for IgA deficiency prior to challenge, and volunteers 14 and 16 had normal serum IgA levels. Second, it is possible that parasite-antibody complexes may have formed with extracellular stages (i.e., merozoite, microgametocyte, or oocyst) of the parasite. However, most individuals had detectable fIgA levels, suggesting that if complexes were formed, fIgA levels exceeded the antigen concentration. Finally, it is possible that not all volunteers were equally compliant in collection and proper storage of stool samples. That is, low levels of specific fIgA and/or oocysts could have escaped detection due to a missed stool collection or degradation of a sample that was not kept under optimal conditions. Additionally, two volunteers had no evidence of infection but had an increase in specific fIgA. One of these individuals had only a single stool sample which barely met the definition of IgA positive. The other volunteer, however, had multiple positive samples, which suggests a possible asymptomatic infection with an oocyst concentration below the detectable limit.
Total fIgA in the prechallenge stool samples was about 20-fold lower than the concentrations reported for jejunal aspirates in other studies (1, 13). These lower IgA concentrations could be due, at least in part, to the differences in the character of the samples, i.e., aspirates versus stool, and stool preparation steps. Stool consistency can also affect the concentration of antibody in a fecal extract; therefore, in this study the absorbance data were standardized to total fIgA in each sample. Results obtained from absorbances versus RIs led to the same conclusions except in two instances where the RI altered the interpretation. Thus, it is important to present specific fIgA results in the context of IgA concentration, which provides a more accurate depiction of the mucosal response to C. parvum.
The relative proportions of IgA subclasses vary according to their location. In mucosal secretions, IgA2 typically accounts for 30 to 40% of the total secretory antibody, compared to 20% or less in serum (5). In the present study, the amount of antibody subclasses present was examined for the relative proportions of IgA1 and IgA2 in fecal extracts before and after oocyst challenge. Both subclasses were present in the majority of fecal extracts, and the relative proportions did not change significantly after C. parvum infection. It is possible that these results may have been influenced by the stability of these molecules, since IgA1 and certain allotypes of IgA2 are more susceptible to bacterial proteolytic cleavage than secretory IgA2 (11). However, if degradation did occur, it was present equally before and after infection. Also, IgA1 is associated with peptide epitopes, compared to IgA2, which favors polysaccharide epitopes (25). Our results suggest that both types of antigens are likely present in C. parvum infection. Indeed, neutralizing antibodies to both kinds of epitopes have been described (24).
Anti-C. parvum fIgA was found to be a strong and significant indicator of active or recent infection, and a trend toward a higher RI was seen in those who produced lower numbers of total oocysts. This result stands in direct contrast to the lack of specific serum IgG increases in these same volunteers (17). This disparity between the mucosal and systemic responses has been seen in other instances and supports the notion that, while the systems are not totally segregated, they are independently regulated. Cryptosporidium is limited to the apical region of the enterocyte and does not invade the lamina propria or beyond. As such, in the naive individual the parasite elicits a strong secretory antibody response but fails to provide adequate immune stimulation for serum antibody detectable by ELISA. From the present data, as well as previous serological studies in seronegative and seropositive volunteers (2, 17), we conclude that specific fIgA occurs during the primary infection, whereas the serum antibody response is seen in persons who have been exposed to the parasite more than once. The persistence of fIgA is unknown, but it appeared to remain at detectable levels for up to 6 weeks in some volunteers. This is consistent with observations of transient intestinal IgA in Cryptosporidium-infected calves (4). If secretory IgA is produced in the majority of infected individuals and if it is relatively transient, it would be of value in outbreak situations where those who were infected recently could be distinguished from those who had been exposed in the past. In addition, since specific secretory antibodies produced on one mucosal surface can also be found at other mucosal sites, anti-C. parvum antibodies may be detectable in saliva. If so, collection of saliva would be distinctly advantageous over blood samples in epidemiological surveys, especially those involving infants and young children. The studies needed to test this idea are now possible, since saliva samples were collected prior to and following experimental challenge of our volunteers.
In summary, the anti-Cryptosporidium fIgA response in the human gut was closely linked to the number of parasites in the challenge dose and to the establishment of infection. Other illness and infection parameters showed little to no influence on the fIgA level. Experiments to study the fecal antibody response in these same individuals who were rechallenged after 1 year are now in progress.
ACKNOWLEDGMENTS
This study was supported, in part, by a grant from the Environmental Protection Agency (CR 824759). The Clinical Research Center activities were supported by National Institutes of Health General Clinical Research Centers grant M01-RR-02558.
REFERENCES
- 1.Bull D M, Bienenstock J, Tomasi T B., Jr Studies on human intestinal immunoglobulin A. Gastroenterology. 1971;60:370–380. [PubMed] [Google Scholar]
- 2.Chappell C L, Okhuysen P C, Sterling C R, Wang C, Jakubowski W, DuPont H L. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum IgG. Am J Trop Med Hyg. 1998;60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 3.Cozon G, Biron F, Jeannin M, Cannella D, Revillard J P. Secretory IgA antibodies to Cryptosporidium parvum in AIDS patients with chronic cryptosporidiosis. J Infect Dis. 1994;169:696–699. doi: 10.1093/infdis/169.3.696. [DOI] [PubMed] [Google Scholar]
- 4.de Graaf D C, Peeters J E. Specific interferon-gamma, IgA and IgM responses after experimental infection of neonatal calves with Cryptosporidium parvum. Int J Parasitol. 1997;27:131–134. doi: 10.1016/s0020-7519(96)00167-1. [DOI] [PubMed] [Google Scholar]
- 5.Delacroix D L, Dive C, Rambaud J C, Vaerman J P. IgA subclasses in various secretions and in serum. Immunology. 1982;47:383–385. [PMC free article] [PubMed] [Google Scholar]
- 6.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 7.Enriquez F J, Riggs M W. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect Immun. 1998;66:4469–4473. doi: 10.1128/iai.66.9.4469-4473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayer R, Andrews C, Ungar B L, Blagburn B. Efficacy of hyperimmune bovine colostrum for prophylaxis of cryptosporidiosis in neonatal calves. J Parasitol. 1989;75:393–397. [PubMed] [Google Scholar]
- 9.Fayer R, Perryman L E, Riggs M W. Hyperimmune bovine colostrum neutralizes Cryptosporidium sporozoites and protects mice against oocyst challenge. J Parasitol. 1989;75:151–153. [PubMed] [Google Scholar]
- 10.Flanigan T. Human immunodeficiency virus infection and cryptosporidiosis: protective immune responses. Am J Trop Med Hyg. 1994;50(Suppl.):29–35. [PubMed] [Google Scholar]
- 11.Fujiyama Y, Kobayashi K, Senda S, Benno Y, Bamba T, Hosoda S. A novel IgA protease from Clostridium sp. capable of cleaving IgA1 and IgA2 A2m(1) but not IgA2 A2m(2) allotype paraproteins. J Immunol. 1985;134:573–576. [PubMed] [Google Scholar]
- 12.Hill B D, Blewett D A, Dawson A M, Wright S. Analysis of the kinetics, isotype and specificity of serum and coproantibody in lambs infected with Cryptosporidium parvum. Res Vet Sci. 1990;48:76–81. [PubMed] [Google Scholar]
- 13.Hjelt K, Sorensen C H, Nielsen O H, Krasilnikoff P A. Concentrations of IgA, IgM, secretory IgM, IgD, and IgG in the upper jejunum of children without gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 1988;7:867–871. doi: 10.1097/00005176-198811000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Lasser K H, Lewin K L, Ryning F W. Cryptosporidial diarrhea in a patient with congenital hypogammaglobulinemia. Hum Pathol. 1979;10:234–240. doi: 10.1016/s0046-8177(79)80012-x. [DOI] [PubMed] [Google Scholar]
- 15.Nord J, Ma P, DiJohn D, Tzipori S, Tacket C O. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS. 1990;4:581–584. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Okhuysen P C, Chappell C L, Crabb J, Valdez L M, Douglass E T, DuPont H L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26:1324–1329. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 17.Okhuysen P C, Chappell C L, Sterling C R, Jakubowski W, DuPont H L. Susceptibility and serological response of healthy adults to reinfection with Cryptosporidium parvum. Infect Immun. 1998;66:441–443. doi: 10.1128/iai.66.2.441-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okhuysen P C, Jiang X, Liming Y, Johnson P C, Estes M K. Viral shedding and fecal IgA response after Norwalk virus infection. J Infect Dis. 1995;171:566–569. doi: 10.1093/infdis/171.3.566. [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Mora L M, Wright S E. Age-related resistance in ovine cryptosporidiosis: patterns of infection and humoral immune response. Infect Immun. 1994;62:5003–5009. doi: 10.1128/iai.62.11.5003-5009.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters J E, Villacorta I, Naciri M, Vanopdenbosch E. Specific serum and local antibody responses against Cryptosporidium parvum during medication of calves with halofuginone lactate. Infect Immun. 1993;61:4440–4445. doi: 10.1128/iai.61.10.4440-4445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeters J E, Villacorta I, Vanopdenbosch E, Vandergheynst D, Naciri M, Ares-Mazas E, Yvore P. Cryptosporidium parvum in calves: kinetics and immunoblot analysis of specific serum and local antibody responses (immunoglobulin A [IgA], IgG, and IgM) after natural and experimental infections. Infect Immun. 1992;60:2309–2316. doi: 10.1128/iai.60.6.2309-2316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perryman L E, Riggs M W, Mason P H, Fayer R. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–259. doi: 10.1128/iai.58.1.257-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reperant J M, Naciri M, Chardes T, Bout D T. Immunological characterization of a 17-kDa antigen from Cryptosporidium parvum recognized early by mucosal IgA antibodies. FEMS Microbiol Lett. 1992;78:7–14. doi: 10.1016/0378-1097(92)90280-2. [DOI] [PubMed] [Google Scholar]
- 24.Riggs M R. Immunology: host response and development of passive immunotherapy and vaccines. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 133–135. [Google Scholar]
- 25.Russell M W, Lue C, van den Wall Bake A W, Moldoveanu Z, Mestecky J. Molecular heterogeneity of human IgA antibodies during an immune response. Clin Exp Immunol. 1992;87:1–6. doi: 10.1111/j.1365-2249.1992.tb06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarazona R, Lally N C, Dominguez-Carmona M, Blewett D A. Characterization of secretory IgA responses in mice infected with Cryptosporidium parvum. Int J Parasitol. 1997;274:417–423. doi: 10.1016/s0020-7519(96)00189-0. [DOI] [PubMed] [Google Scholar]