Abstract
Objectives
Hepatectomy is the best treatment for patients with intrahepatic cholangiocarcinoma (ICC) at present, but there has been controversy about the width of surgical margins. In this study, we systematically investigated the effects of different surgical margin widths on the prognosis of patients with ICC undergoing hepatectomy.
Design
Systematic review and meta-analysis.
Data sources
PubMed, Embase and Web of Science databases were systematically searched from inception to June 2022.
Eligibility criteria
Cohort studies reported in English with patients who underwent negative marginal (R0) resection were included. The effects of surgical margin width on overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS) in patients with ICC were assessed.
Data extraction and synthesis
Two investigators independently conducted literature screening and data extraction. Risk of bias was assessed using funnel plots and quality was assessed by the Newcastle–Ottawa Scale. Forest plots of HRs and their 95% CIs for outcome indicators were plotted. Heterogeneity was assessed and determined quantitatively using I2, and the stability of the study results was evaluated using sensitivity analysis. Analyses were performed using Stata software.
Results
Nine studies were included. With the wide margin group (≥10 mm) as the control, pooled HR of OS in the narrow margin group (<10 mm) was 1.54 (95% CI 1.34 to 1.77). HRs of OS in three subgroups where the margin was less than 5 mm ranged from 5 mm to 9 mm, or was less than 10 mm in length were 1.88 (1.45 to 2.42), 1.33 (1.03 to 1.72) and 1.49 (1.20 to 1.84), respectively. Pooled HR of DFS in the narrow margin group (<10 mm) was 1.51 (1.14 to 2.00). Pooled HR of RFS in the narrow margin group (<10 mm) was 1.35 (1.19 to 1.54). HRs of RFS in three subgroups where the margin was less than 5 mm ranged from 5 mm to 9 mm, or was less than 10 mm in length were 1.38 (1.07 to 1.78), 1.39 (1.11 to 1.74) and 1.30 (1.06 to 1.60), respectively. Neither lymph node lesions (HR 1.44, 95% CI 1.22 to 1.70) nor lymph node invasion (2.14, 1.39 to 3.28) was favourable for postoperative OS in patients with ICC. Lymph node metastasis (1.31, 1.09 to 1.57) was unfavourable for RFS in patients with ICC.
Conclusion
Patients with ICC who underwent curative hepatectomy with a negative margin ≥10 mm may have a long-term survival advantage, but lymph node dissection also needs to be considered. In addition, tumour-related pathological features need to be explored to see if they affect the surgical outcome of R0 margins.
Keywords: Hepatobiliary disease, Hepatology, Hepatobiliary tumours
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The Newcastle–Ottawa Scale was used to evaluate the quality of the included studies.
Publication bias analysis was performed using Egger’s test and Begg’s funnel plot.
Sensitivity analysis was used to determine the stability and strength of the combined results.
Differences based on the type of study (eg, single-centre and multicentre studies) and the limited number of studies may have impacted the results.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignancy originating in the liver, which makes up 10%–15% of primary liver cancers. However, without distinct pathogenic factors or clinicopathological features, the diagnosis of ICC tends to be hard.1 2 Most patients have to receive hepatobiliary resection since the disease has progressed to the advanced stage of ICC.3 Limited knowledge about its pathological features also adds difficulty to the prognosis of patients with ICC. Even after the radical surgery, the recurrence rate remains high and the 5-year survival rate ranges from only 30% to 35%. The past three decades have seen the incidence and mortality rate of ICC keep elevating with a rather poor prognosis.4
Up to now, radical hepatectomy remains the best option for potentially curative treatment of patients with ICC, mainly to achieve negative marginal (R0) resection.5 6 But high local recurrence rate after R0 resection may be related to the location and extent of the primary lesion, lymph node involvement and surgical margin status, leading to a poor prognosis.5 7 Additionally, surgical margin width is also of prognostic essence after ICC resection, but the definition of the width remains controversial. A recent multicentre study reported that patients with a margin width ≥10 mm have better long-term prognostic outcomes relative to patients with a surgical margin width <10 mm8; however, another study stated that wide margin hepatectomy does not produce a survival benefit in all patients with ICC and is more beneficial for patients without lymph node metastases.9 Hence, it is necessary to evaluate the margin width in patients with ICC undergoing R0 resection.
Li et al10 evaluated the relationship between surgical margin status and survival benefit in ICC by meta-analysis and found that negative surgical margins are more beneficial for overall survival (OS) and disease-free survival (DFS) after surgical resection of patients with ICC, thus emphasising the importance of R0 resection. In a recent meta-analysis of the effect of surgical margin width on OS in patients with ICC, it is similar that patients with ICC with R0 ≥10 mm have a longer survival benefit than those with <10 mm.11 But this analysis did not provide statistical analysis of DFS, recurrence-free survival (RFS) or a more refined stratification of the range of R0 margin width, making the findings lacking reference value for clinical treatment at the present stage. Therefore, this study was updated from the above meta-analysis to investigate the effect of margin width on OS, DFS and RFS in patients with ICC who underwent R0 surgical resection in recent years, as well as a stratification study of margin width (<5 mm, 5–9 mm, <10 mm and ≥10 mm), to provide more evidence-based medical evidence for the determination of surgical margin width in patients with ICC.
Methods
Search strategy
Systematic searches were done of PubMed, Embase and Web of Science to collect relevant studies available by June 2022. The literature search took the form of a combination of medical subject headings and free words, mainly including (((((Cholangiocarcinomas) OR (Cholangiocellular Carcinoma)) OR (Intrahepatic Cholangiocarcinoma)) OR (Cholangiocarcinomas, Intrahepatic)) AND (Surgical margin width)) OR (Length of surgical margin) (online supplemental file). The reference lists of included studies were manually screened for relevant studies that may meet the inclusion requirements.
bmjopen-2022-067222supp001.pdf (59.4KB, pdf)
Inclusion criteria
(1) Patients with ICC (confirmed by pathological examination) received potentially curative hepatectomy; (2) patients underwent R0 resection (which was defined as the distance between the non-tumorous tissue and cancer cells >1 mm)2 with clear surgical margin edge; (3) patients were classified according to the width of the resection margin, defined as the shortest distance from the edge of the tumour to the line of resection12; patients with margin widths shorter than 4 mm or ranging from 5 to 10 mm were included in the narrow margin group (<10 mm), and those with margin widths equal to 10 mm or above were included in the wide margin group (≥10 mm); (4) the correlations of surgical margin width with OS, DFS and RFS were presented in the included studies; the HR and 95% CI could be obtained directly from the literature or could be calculated indirectly.
Exclusion criteria
(1) Abstract, literature reviews, pathological reports, editorials and expert reviews; (2) studies published repeatedly; (3) study results reached not through calculation; (4) animal studies; (5) studies that group patients with different cut-off points instead of 5 mm and 10 mm; (6) repeat resection for recurrence; (7) patients with extrahepatic metastases.
Study selection
We selected studies by (1) basic information like title, first author, publication year, nation and the time of research; (2) baseline characteristics like sample size, disease, average age and sex; (3) key factors that bias HR evaluation; and (4) outcome indicators and measured data.
Data extraction
To minimise bias, we had two investigators select studies and extract data in duplicate independently and then adopted cross-validation to measure their accuracy. Disagreement was settled by further discussion or judged by the third investigator. Subsequently, a data extraction sheet designed for this study was used to abstract the following information: (1) basic information about included studies like the name of the first author, publication year, nation, type of article and research period; (2) baseline characteristics of included cohort like the number of people receiving R0 resection, sex, age, subgroup threshold, lymph node metastasis, number of people in the narrow margin group of <10 mm and the wide margin group of ≥10 mm, the longest follow-up time, liver parenchymal dissection techniques and instrumentation, tumour subtypes, and adjuvant chemotherapy and radiotherapy; (3) primary outcome indicator: HR and 95% CI for prognostic OS and DFS for patients in each group; secondary outcome indicator: HR and 95% CI for RFS and lymph node status. OS was defined as the interval from the date of surgery to the patient’s death or last follow-up. DFS was defined as the interval from the date of surgery to the date of first recurrence, secondary malignancy or death of any disease course. RFS was defined as the interval from the date of surgery to the date of first tumour recurrence, secondary malignancy or death with evidence of recurrence. Tumour morphology was typologically defined based on preoperative imaging and case reports, and ICC was classified into three categories based on the macroscopic types proposed by the Japanese Liver Cancer Study Group: mass-forming (MF) type, periductal infiltrating type and intraductal growth type.9 For HR and 95% CI of DFS, RFS and OS, if not directly available from the literature, data such as survival rate can also be intercepted from survival graphs and entered into Excel with information such as follow-up time, and finally combined effect sizes by meta-analysis using RevMan software.13
Quality assessment of included studies
Included studies were evaluated by two investigators using the Newcastle–Ottawa Scale (NOS), an assessment scale covering eight items including the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case–control or cohort studies, respectively. Any disagreement in assessment was resolved by a third investigator.
Statistical analysis
We used Stata V.MP16 software to conduct statistical analysis. Between-study heterogeneity was tested by χ2 test (α=0.1) and further evaluated using I2. When I2 ≤50%, a fixed-effects model of the meta-analysis was employed; when I2 >50%, a random-effects model was used to analyse possible reasons, together with subgroup and descriptive analysis. If heterogeneity arises from poor research quality, sensitivity analysis ensued to evaluate the stability and certainty of meta-analysis. Publication bias analysis used Egger’s test and Begg’s funnel plot. If the funnel plot was symmetrical, it indicated a lack of publication bias. The inspection level of meta-analysis was set as α=0.05.
Patient and public involvement
None.
Results
Selected studies and quality assessment
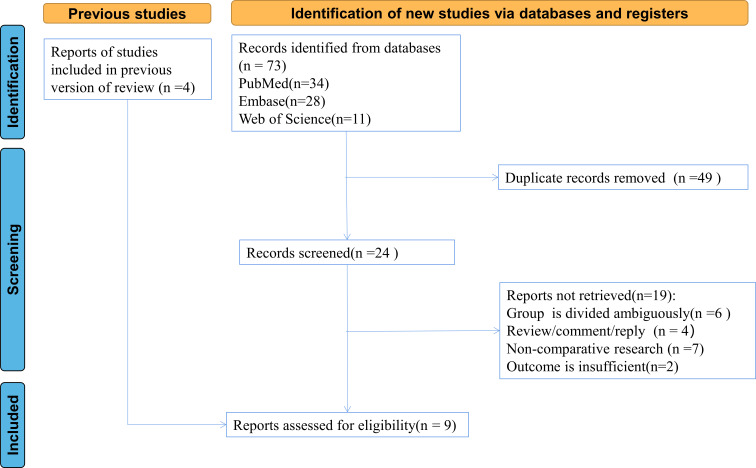
Initial searches returned 73 relevant studies (34 from PubMed; 28 from Embase; 11 from Web of Science). After screening the title and abstract of these entries identified in the search, 24 studies were retained. Eventually, nine studies were included after reading their full-text publications.1–4 8 9 12 14 15 Among these included studies published from 2008 to 2021, three were conducted in China, one in Austria, one in Korea, one in France, two in the USA and one in Japan. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for included studies was presented in figure 1. NOS scores of included studies in table 1 showed that top-rated retrospective cohort studies were of high quality.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the included studies.
Table 1.
Quality assessment of included studies
| Study | Selection | Comparability | Outcome | Score | |||||
| A | B | C | D | E | F | G | H | ||
| Tamandl et al3 | * | * | * | * | * | * | * | * | 8 |
| Cho et al1 | * | * | * | * | * | * | * | 7 | |
| Farges et al4 | * | * | * | * | * | * | * | * | 8 |
| Spolverato et al12 | * | * | * | * | * | * | * | * | 8 |
| Ma et al2 | * | * | * | * | * | * | * | * | 8 |
| Watanabe et al9 | * | * | * | * | ** | * | * | ** | 10 |
| Bartsch et al14 | * | * | * | * | * | * | * | 7 | |
| Zhu et al15 | * | * | * | * | ** | * | 6 | ||
| Liu et al8 | * | * | * | * | * | * | * | * | 8 |
Each study can have up to one ‘*’ for each item on ‘Selection’, and up to two ‘**’ for each item on ‘Comparability’ and ‘Outcome’.
A: representativeness of the exposed cohort; B: selection of the non-exposed cohort; C: ascertainment of exposure; D: demonstration that outcome of interest was not present at the start of the study; E: comparability of cohorts based on the design or analysis; F: assessment of outcome; G: was follow-up long enough for outcomes to occur; H: adequacy of follow-up of cohorts.
Characteristics of included studies
As presented in table 2, most involved patients aged around 60 years. The majority of resections were done with modern technology or dissection devices, such as Cavitron ultrasonic surgical attractor or ultrasonic dissector. The baseline characteristics of narrow (<10 mm) and wide (≥10 mm) margin groups were similar across the nine included studies. The follow-up length ranges from 1 to 84 months. In four studies, several patients were treated with neoadjuvant or adjuvant treatment. Six studies analysed the tumour morphology, with a predominance of MF type. Only three reported lymph node metastasis (23.94%–70.5%). Besides, the number of people in the study by Ma et al2 could not be clearly extracted for the narrow margin group (<10 mm) and the wide margin group (≥10 mm), so the exact number of people in both groups could not be clarified. But some survival data could be extracted from that study, and therefore were also included in our study for survival analysis.
Table 2.
Patients’ characteristics by study
| Author | Year | Nation | No of patient | Age, M (Q25~Q75) | Sex, male (%) | Follow-up, month |
Resection width | Lymph node metastasis, n | Adjuvant radiotherapy/chemotherapy | Morphological type | |
| ≥10 mm | <10 mm | ||||||||||
| Tamandl et al3 | 2008 | Austria | 53 | 63.2 (33.3~85.8) | 29 (39) | 1–64 | 15 | 38 | NR | NR | NR |
| Cho et al1 | 2010 | Korea | 63 | 61.4 (27~82) | 41 (65) | NR | 23 | 40 | 13 | NR | MF: 45 Others: 18 |
| Farges et al4 | 2011 | France | 161 | NR | 108 (51) | NR | 45 | 116 | 47 | NC:12 AC: 51 |
MF: 212 |
| Spolverato et al12 | 2015 | USA | 440 | NR | 302 (52) | NR | 174 | 266 | NR | AC: 212 AR: 44 |
MF: 326 Others: 114 |
| Ma et al2 | 2016 | China | 95 | 61 (25~79) |
58 (54) | 1–60 | NR | NR | NR | NR | NR |
| Zhu | 2020 | China | 109 | NR | 66 (52) | 1–54 | 17 | 92 | NR | NC: 1 | MF: 118 Others: 8 |
| Watanabe et al9 | 2020 | Japan | 635 | 64.2 (32.3~84.4) | 388 (61) | 1–84 | 237 | 398 | 152 | NR | MF: 401 Others: 234 |
| Bartsch et al14 | 2020 | USA | 131 | NR | NR | 0–24 | 22 | 109 | NR | PC: 3 | NR |
| Liu et al8 | 2021 | China | 478 | 58 (49~64) | 287 (60) | NR | 195 | 283 | NR | NR | MF: 154 Others: 324 |
AC, adjuvant chemotherapy; AR, adjuvant radiotherapy; MF, mass-forming; NC, neoadjuvant chemotherapy; NR, not reported; PC, preoperative chemotherapy.
Meta-analysis results
Overall survival
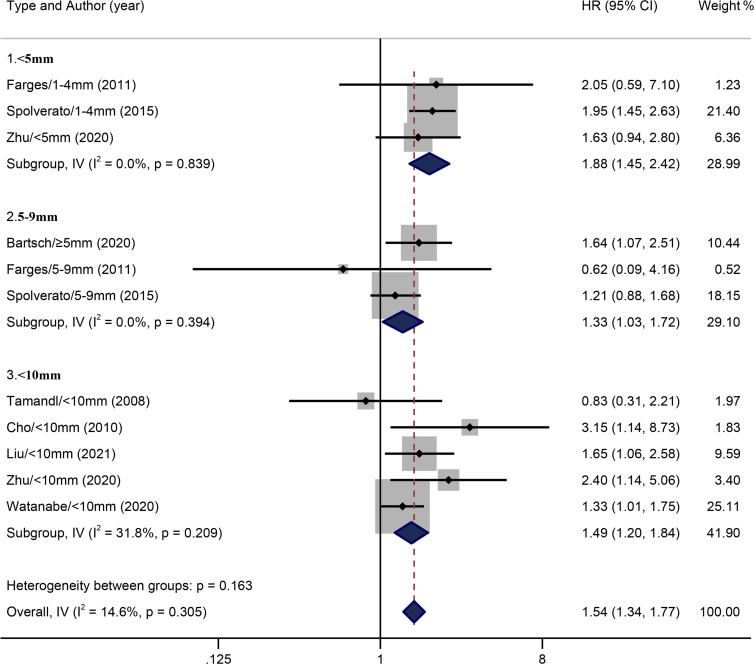
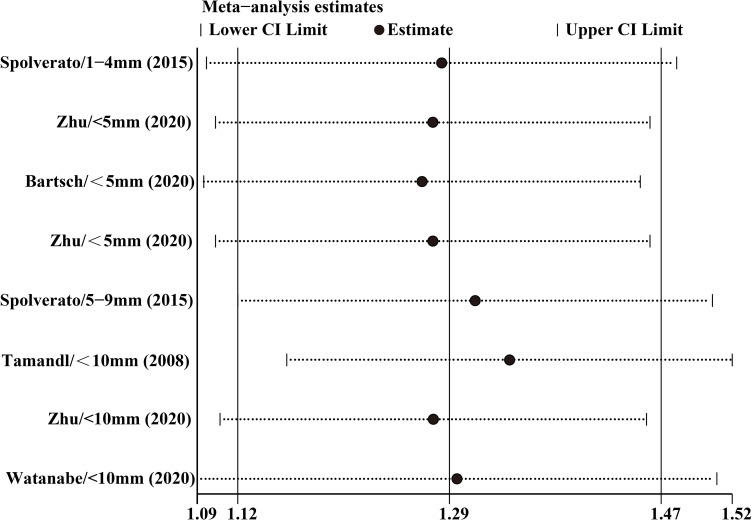
Nine included studies all related to the influence of surgical margin width on the OS of patients with ICC. This meta-analysis synthesised relevant data by categorising margin width into <10 mm and ≥10 mm groups, and the former was further categorised into three subgroups: <5 mm (1–4 mm, three studies) 5–9 mm (≥5 mm, three studies) and <10 mm (five studies). There was no overall heterogeneity in the included studies (I2=14.6%, p=0.305). The fixed-effects model meta-analysis indicated that, compared with the wide margin group (≥10 mm), pooled HR of the narrow margin group (<10 mm) stood at 1.54 (95% CI: 1.34 to 1.77). No significant heterogeneity was found across three subgroups: <5 mm (I2=0.0%, p=0.839), 5–9 mm (I2=0.0%, p=0.394) and <10 mm (I2=31.8%, p=0.209) groups. Compared with the wide margin group, pooled HRs of these three subgroups (<5 mm, 5–9 mm and <10 mm groups) were 1.88 (95% CI: 1.45 to 2.42), 1.33 (95% CI: 1.03 to 1.72) and 1.49 (95% CI: 1.20 to 1.84), respectively, as shown in figure 2.
Figure 2.
Results of HR pooled analysis of the overall survival rate of the included studies (with the wide margin group ≥10 mm as the control).
Disease-free survival
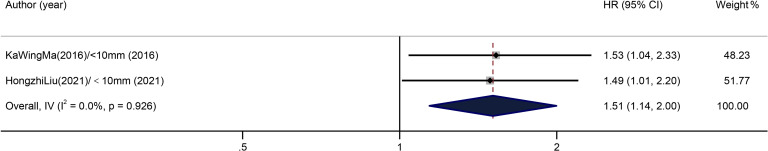
Two included studies relating to the influence of margin width on DFS of patients with ICC showed no overall heterogeneity (I2=0.0%, p=0.926). According to the result of the fixed-effects model in figure 3, with the wide margin group (≥10 mm) as the control, the overall pooled HR of the narrow margin group (<10 mm) was 1.51 (95% CI: 1.14 to 2.00) (figure 3).
Figure 3.
Results of HR pooled analysis of disease-free survival in the included studies (with the wide margin group ≥10 mm as the control).
Recurrence-free survival
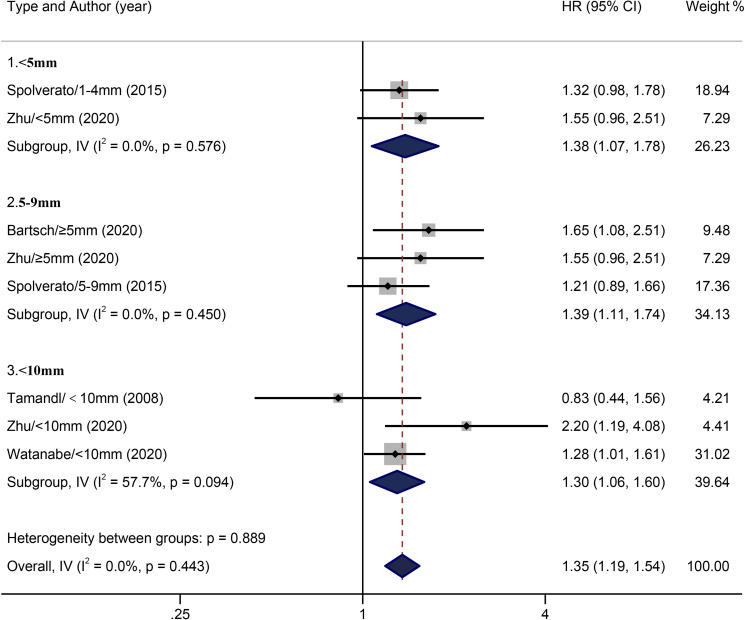
With five included studies concerning the influence of margin width on RFS of patients with ICC, we categorised the outcome variable in the same way as we conducted study on OS. With no heterogeneity across the included studies (I2=0.0%, p=0.443), the pooled HR was 1.35 (95% CI: 1.19 to 1.54). The fixed-effects model of subgroup analysis found no heterogeneity across <5 mm (I2=0.0%, p=0.576) and 5–9 mm (I2=0.0%, p=0.450) groups. Compared with the wide margin group (≥10 mm), pooled HRs of <5 mm and 5–9 mm groups were 1.38 (95% CI: 1.07 to 1.78) and 1.39 (95% CI: 1.11 to 1.74), respectively. With heterogeneity (I2=57.7%, p=0.094) in the narrow margin (<10 mm) group, compared with the wide margin (≥10 mm) group, the pooled HR of the narrow margin group was found to be 1.30 (95% CI: 1.06 to 1.60) in comparison with the wide margin group (≥10 mm) (figure 4).
Figure 4.
Results of HR pooled analysis of recurrence-free survival in the included studies (with the wide margin group ≥10 mm as the control).
Correlation between lymph node status and prognosis
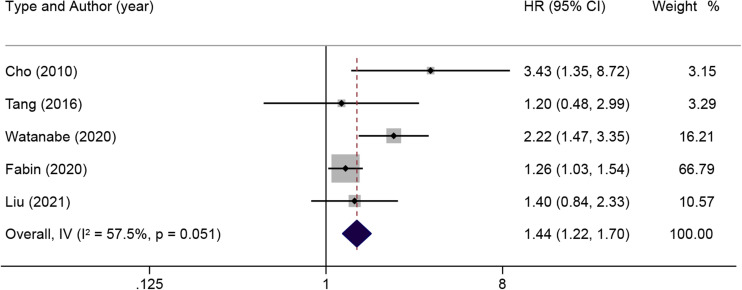
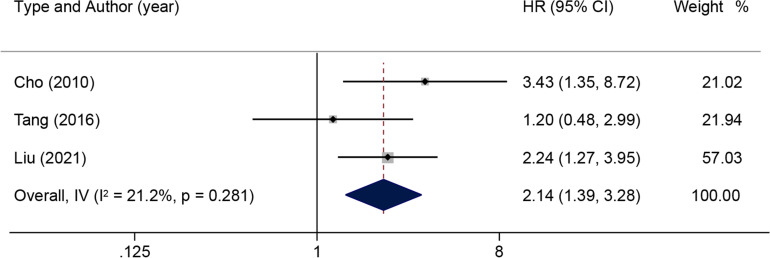
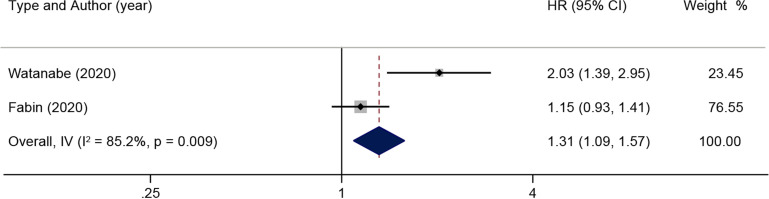
Subsequently, a subgroup analysis was done on the prognostic impact related to lymph node status. When there was moderate heterogeneity in the effect of lymph node lesions on OS (I2=57.5%, p=0.051) according to the pooled HR and 95% CI of the multiple analyses of five positive lymph nodes, a random-effects model was used for subsequent analysis (figure 5). The results illustrated that lymph node lesions were detrimental to OS in patients with ICC (HR: 1.44; 95% CI: 1.22 to 1.70). When there was no significant heterogeneity in the effect of lymph node invasion on OS (I2=21.2%, p=0.281), a fixed-effects model was used (figure 6). The results reported that patients with ICC in the presence of lymph node invasion had markedly shorter OS (HR: 2.14; 95% CI: 1.39 to 3.28). In addition, the pooled HR of RFS associated with lymph node metastasis was analysed, and the results showed notable heterogeneity (I2=85.2%, p=0.009) (figure 7). The results of the random-effects model demonstrated that lymph node metastasis was detrimental to RFS in patients with ICC (HR: 1.31, 95% CI: 1.09 to 1.57).
Figure 5.
Results of HR pooled analysis of lymph node lesions on overall survival in patients with intrahepatic cholangiocarcinoma (with the wide margin group ≥10 mm as the control).
Figure 6.
Results of HR pooled analysis of lymph node invasion on overall survival in patients with intrahepatic cholangiocarcinoma (with the wide margin group ≥10 mm as the control).
Figure 7.
Results of HR pooled analysis of lymph node metastasis on recurrence-free survival in patients with intrahepatic cholangiocarcinoma (with the wide margin group ≥10 mm as the control).
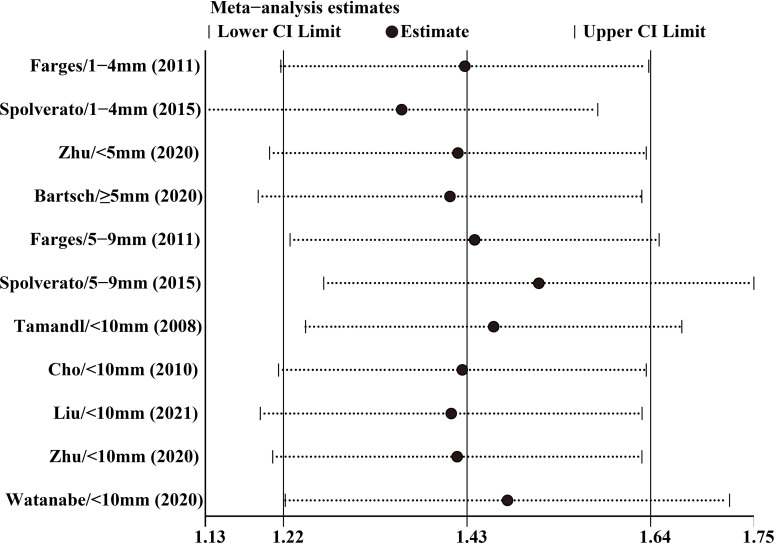
Sensitivity analysis
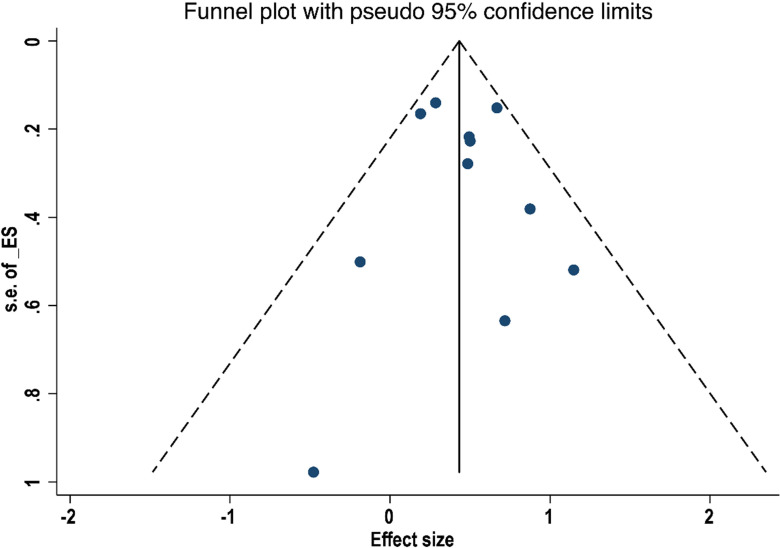
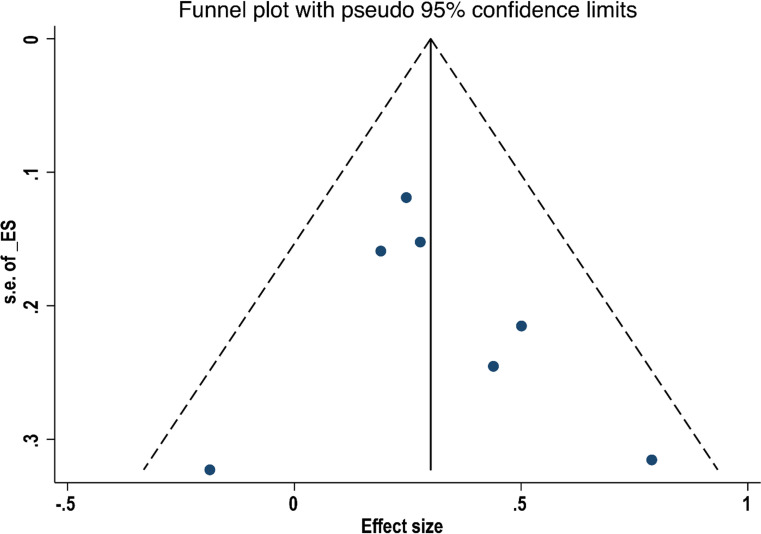
By excluding one study at a time, a sensitivity analysis of OS and RFS was conducted. Results in figures 8 and 9 showed no significant difference between the effect size and the total effect size of OS and RFS, implying that the result reached in this study was relatively stable. Egger’s test did not detect substantial publication bias in both OS (p=0.508) and RFS (p=0.523), and the Begg’s funnel plot was symmetrical (figures 10 and 11). However, differences based on the type of study (single-centre and multicentre studies) and the limited number of studies may affect the above statistical results.
Figure 8.
Sensitivity analysis of overall survival after leave-one-out analyses.
Figure 9.
Sensitivity analysis of recurrence-free survival after leave-one-out analyses.
Figure 10.
Funnel plot of the relationship between surgical margin width and overall survival in patients with intrahepatic cholangiocarcinoma.
Figure 11.
Funnel plot of the relationship between surgical margin width and recurrence-free survival in patients with intrahepatic cholangiocarcinoma.
Discussion
Current status of surgery for ICC
The incidence and mortality rates of ICC keep climbing across the world; most patients are not diagnosed until ICC reached an advanced stage.1
Currently, complete surgical resection with negative histological margins (R0) remains the only curative treatment modality favouring long-term survival outcomes in patients with ICC, but only a minority of patients have resectable lesions, resulting in poor postoperative survival.5 16 A few studies have shown a better survival benefit for patients with ICC undergoing R0 resection compared with R1 resection.2 12 However, margin status, lymph node status and the presence of vascular invasion all contribute to the poor prognosis of patients with ICC after resection.17–19 Most patients with ICC usually require adjuvant therapy.20 In addition, investigators are concerned that in patients with ICC undergoing R0 resection, the margin width also affects long-term survival after surgery.8 14 However, there has been controversy regarding the effect of R0 margin width on the prognostic survival of patients with ICC. Thus, this meta-analysis was done to investigate the effect of margin width on survival outcomes after ICC resection.
The impact of margin width on outcomes of patients with ICC
In 2016, Tang et al11 published the first meta-analysis of the effect of margin width on prognostic survival in patients with ICC. This study indicated that patients with wide margin (≥10 mm) have a survival advantage over those with narrow margin (<10 mm) (HR: 1.59, 95% CI: 1.09 to 2.32). Based on these investigations, we updated the study related to the effect of surgical margin width on the prognosis survival of patients with ICC. Two irrelevant studies with a limited sample size in the 2016 meta-analysis were excluded and five eligible retrospective cohort studies published after 2016 were included. Besides, this study also filled the void of RFS (four included studies) and DFS (two included studies).
Nine included studies all focused on the MF type of ICC (as it accounts for over 66% of ICCs1) and categorised the outcome variable into five groups: <1 mm, 1–4 mm, 5–9 mm, <10 mm and ≥10 mm. One included study went beyond our research scope as it further categorised wide margin into ≥15 mm group, and relevant data were excluded from the meta-analysis. Pooled HR results indicated that with the wide margin (≥10 mm) group as the control, patients with a margin shorter than 10 mm were prone to poor prognosis (pooled HR of OS: 1.54, 95% CI: 1.34 to 1.77). It was demonstrated that ICC tumour cells could metastasise by directly infiltrating the adjacent liver parenchyma, accompanied by vascular infiltration and perineural infiltration, and then cause pathological changes in intrahepatic epithelial cells and surrounding tissues.21 For most, metastasis is limited within 10 mm around the primary lesion; a 10 mm or more resection is expected to cure these patients with ICC. Ma et al2 suggested that margin width significantly impacts the OS of patients with ICC-MF after resection. With a margin width greater than 9 mm, OS increased from 35.7 months to 184.6 months. With a margin width of or greater than 10 mm, DFS increased from 14.1 months to 86 months. In a single-centre study, patients with a margin width ≥10 mm have longer OS (HR: 0.403, 95% CI: 0.191 to 0.854, p=0.018) and RFS (HR: 0.470, 95% CI: 0.242 to 0.914, p=0.026).15 Similarly, in the present study, analysis of the OS subgroup presented that the prognostic risk was substantially lower in patients in the 5–9 mm group (HR: 1.33, 95% CI: 1.03 to 1.72) than in the group with margin width <5 mm (HR: 1.88, 95% CI: 1.45 to 2.42). However, the difference was not noticeable in the RFS subgroup analysis. Therefore, margin width ≥10 mm could be the optimal margin width for prognosis.
Correlation between lymph node status and prognosis
A subgroup analysis of lymph node status was done. Several studies have reported that lymph node status, in addition to the margin width, is a pivotal prognostic risk factor affecting patients with ICC after surgery.1 22 Lymph node lesions, invasion and metastasis were factors for poor prognosis after undergoing R0 resection. In a national survey by the Japanese Liver Cancer Study Group, surgical margin width has a small impact on the postoperative prognosis of patients with ICC, but in patients without lymph node metastasis, wider surgical margins favoured postoperative survival outcomes.9 Additionally, by comparing the basic information of patients in the wide and narrow margin groups in this study, it was found that the wide margin group had a higher proportion of patients with single tumours and smaller tumour diameters; in contrast, patients in the narrow margin group had larger tumour diameters and invasion and a higher proportion of vascular invasion and advanced tumours, which may directly confound the comparison of prognostic survival times between the two groups. Liu et al8 conducted a statistical analysis of the clinical data of 478 patients with ICC from 13 hepatobiliary and pancreatic centres and used the propensity score matching method for pairwise inclusion at 1:1, matched other factors that may affect prognostic survival such as age, tumour type and lymph node metastasis without statistical differences, retaining only the difference in margin width (wide vs narrow margins with a 10 mm threshold) for comparison. The results showed that patients with wide margins had substantially improved OS and DFS compared with patients with narrow margin. But in an unpaired subgroup analysis, wide margins only improved the American Joint Committee on Cancer clinical stage I patients, and patients with lymphatic metastases did not benefit from wide margins. Therefore, we believed that setting the margin width at ≥10 mm may require reference to the patient’s tumour type and lymphatic involvement, and 10 mm or larger margins can be achieved as much as possible in patients with ICC without lymph node metastasis and single MF type to improve the patient’s prognosis for long-term survival benefit.
Adjuvant treatments such as chemotherapy, arterial chemoembolisation and chemoradiotherapy may be beneficial for the survival of postoperative patients with ICC with margins as well as positive lymph nodes.20 Recent meta-analysis results have suggested that lymph node dissection may not have a marked prognostic impact on patients with resectable ICC, but it is associated with postoperative recurrence.23 24 Hence, we speculated that adjuvant therapy for patients with ICC may also influence the choice of margin width, but in our study, a subgroup analysis of adjuvant therapy was not conducted as an ongoing study object.
Sensitivity analysis
Although the results of the sensitivity analysis did not show substantial differences between studies, the Spolverato et al 5–9 mm group in the OS sensitivity analysis12 and the Tamandl et al3 <10 mm group in the RFS sensitivity analysis were slightly prominent.3 The study by Spolverato et al12 reported that the 1-year OS rate of 100 patients who completed the 5–9 mm R0 margin (83.9%) was higher than the OS of 147 patients who completed the ≥10 mm R0 margin (79.8%), which may be a factor influencing the OS comparison.
Limitations
This study has some limitations. First, all the studies included in this study were published in English, and the exclusion of non-English literature may lead to selection bias. Second, the presence of single-centre and multicentre studies in this study may have contributed to some bias in the results. Third, factors such as type of liver resection, surgical instrumentation and adjuvant treatment were not analysed in subgroups, and the prognosis of patients with ICC by the above factors was inconclusive and warranted further study. Fourth, the size, number and location of preoperative tumours and their staging also varied, and evaluation of the impact of surgical margins on postoperative survival of patients with ICC in terms of tumour pathological characteristics may be required. Finally, since survival data were mostly obtained indirectly through calculations, the conclusions may differ somewhat from clinical trials.
Conclusion
In conclusion, the meta-analysis revealed that patients undergoing curative hepatectomy for ICC had a survival advantage for a wide margin of ≥10 mm compared with a narrow margin of <10 mm under certain conditions. However, surgeons should determine the margin width concerning the patient’s condition and should not consider <10 mm as a contraindication to surgery; in addition, lymph node status should be considered during clinical procedures, as it is also an important factor affecting the patient’s postoperative survival outcome. In summary, surgical margins of ≥10 mm should be achieved as much as possible for patients with ICC with negative lymph nodes, but further multicentre study results are still warranted to support this view.
Supplementary Material
Footnotes
Contributors: J-HJ drafted and revised the article. D-ZF analysed data. Y-TH gave final approval of the version to be published. All authors agreed to be accountable for all aspects of the work. J-HJ accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Cho SY, Park S-J, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol 2010;17:1823–30. 10.1245/s10434-010-0938-y [DOI] [PubMed] [Google Scholar]
- 2.Ma KW, Cheung TT, She WH, et al. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: a single-center experience. Medicine (Baltimore) 2016;95:e4133. 10.1097/MD.0000000000004133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787–94. 10.1245/s10434-008-0081-1 [DOI] [PubMed] [Google Scholar]
- 4.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 Study Group. Ann Surg 2011;254:824–9. 10.1097/SLA.0b013e318236c21d [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198–205. 10.1016/j.canlet.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Rahnemai-Azar AA, Weisbrod AB, Dillhoff M, et al. Intrahepatic cholangiocarcinoma: current management and emerging therapies. Expert Rev Gastroenterol Hepatol 2017;11:439–49. 10.1080/17474124.2017.1309290 [DOI] [PubMed] [Google Scholar]
- 7.Zhu AX, Knox JJ. Adjuvant therapy for intrahepatic cholangiocarcinoma: the debate continues. Oncologist 2012;17:1504–7. 10.1634/theoncologist.2012-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Lin L, Lin Z, et al. Impact of surgical margin width on long-term outcomes for intrahepatic cholangiocarcinoma: a multicenter study. BMC Cancer 2021;21:840. 10.1186/s12885-021-08560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe Y, Matsuyama Y, Izumi N, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: a nationwide survey of the liver cancer Study group of Japan. Surgery 2020;167:793–802. 10.1016/j.surg.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 10.Li M-X, Bi X-Y, Li Z-Y, et al. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res 2016;203:163–73. 10.1016/j.jss.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Lu W, Li B, et al. Influence of surgical margins on overall survival after resection of intrahepatic cholangiocarcinoma. Medicine (Baltimore) 2016;95:e4621. 10.1097/MD.0000000000004621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spolverato G, Yakoob MY, Kim Y, et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2015;22:4020–8. 10.1245/s10434-015-4472-9 [DOI] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartsch F, Baumgart J, Hoppe-Lotichius M, et al. Intrahepatic cholangiocarcinoma-influence of resection margin and tumor distance to the liver capsule on survival. BMC Surg 2020;20:61. 10.1186/s12893-020-00718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H, Wang L, Wang M, et al. Prognostic value of resection margin length after surgical resection for intrahepatic cholangiocarcinoma. The American Journal of Surgery 2021;222:383–9. 10.1016/j.amjsurg.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Hewitt DB, Brown ZJ, Pawlik TM. Surgical management of intrahepatic cholangiocarcinoma. Expert Review of Anticancer Therapy 2022;22:27–38. 10.1080/14737140.2022.1999809 [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Yuan L, Wang Y, et al. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg 2014;18:562–72. 10.1007/s11605-013-2447-3 [DOI] [PubMed] [Google Scholar]
- 18.Hammad AY, Berger NG, Eastwood D, et al. Is radiotherapy warranted following intrahepatic cholangiocarcinoma resection? the impact of surgical margins and lymph node status on survival. Ann Surg Oncol 2016;23(Suppl 5):912–20. 10.1245/s10434-016-5560-1 [DOI] [PubMed] [Google Scholar]
- 19.Wirasorn K, Ngamprasertchai T, Chindaprasirt J, et al. Prognostic factors in resectable cholangiocarcinoma patients: carcinoembryonic antigen, lymph node, surgical margin and chemotherapy. World J Gastrointest Oncol 2013;5:81–7. 10.4251/wjgo.v5.i4.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke Q, Lin N, Deng M, et al. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: a systematic review and meta-analysis. PLoS One 2020;15:e0229292. 10.1371/journal.pone.0229292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2019;28:587–99. 10.1016/j.soc.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 22.Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: the Mayo clinic experience. HPB (Oxford) 2015;17:244–50. 10.1111/hpb.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Jiang Y, Jiang L, et al. Effect of lymph node resection on prognosis of resectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Front Oncol 2022;12:957792. 10.3389/fonc.2022.957792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou R, Lu D, Li W, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford) 2019;21:784–92. 10.1016/j.hpb.2018.12.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067222supp001.pdf (59.4KB, pdf)
Data Availability Statement
No data are available.