BACKGROUND:
Draining the chest cavity with 2 tubes is a common practice among thoracic surgeons. This research was conducted in Addis Ababa from March 2021 to May 2022. A total of 62 patients were included.
STUDY DESIGN:
This study was conducted to investigate the superiority of either single or double tube insertion after decortication. Patients were randomized in a ratio of 1:1. In group A, 2 tubes were inserted; in group B, single 32F tubes were inserted. Statistical analyses were performed using Statistical Package for Social Sciences version 27.0, Student’s t test and Pearson chi-square test.
RESULTS:
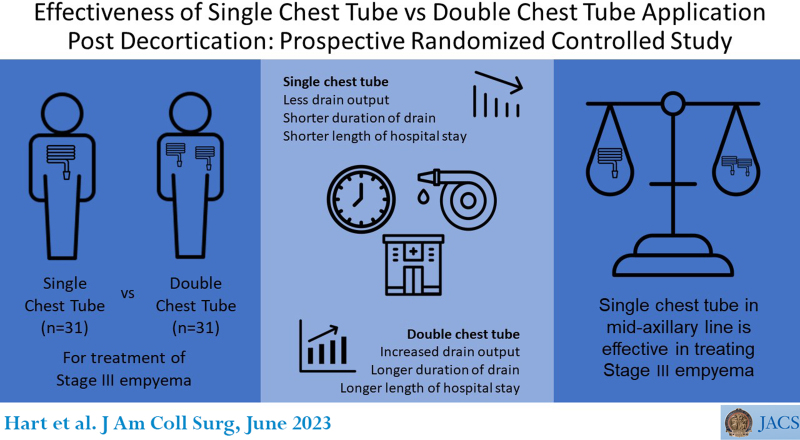
The age range of patients was 18 to 70 years, with a mean of 44 ± 14.4434 years; the male to female ratio was 2.9:1. The dominant underlying pathologies were tuberculosis and trauma (45.2% vs 35.5%); the right side was more involved (62.3%). Drain output was 1,465 ± 1,887.9751 mL in group A vs 1,018 ± 802.5662 mL in group B (p value = 0.00001); the duration of drains was 7.5498 ± 11.3137 days in group A vs 3.8730 ± 1.4142 days in group B (p value = 0.000042). The degree of pain was 2.6458 ± 4.2426 vs 2.000 ± 2.1213 in group A and group B, respectively (p value = 0.326757). The length of hospital stay was 21.5818 ± 11.9791 days in group A vs 13.6091 ± 6.2048 days in group B (p value = 0.00001). Group A had air leak of 90.3% vs 74.2% in group B; subcutaneous emphysema was 9.7% in group A and 12.9% in group B. There was no fluid recollection, and no patients required tube reinsertion.
CONCLUSIONS:
The placement of a single tube after decortication is effective in reducing drain output, time of drain, and hospital stay. There was no association with pain, and there was no effect on other endpoints.
We conducted a multicenter randomized controlled study to investigate the effectiveness of single or double chest tube insertion after decortication. We concluded that the placement of a single chest tube in the midaxillary line is as effective as double chest tube drainage in the management of empyema thoracis.
CONTINUING MEDICAL EDUCATION CREDIT INFORMATION.
Accreditation: The American College of Surgeons is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
AMA PRA Category 1 CreditsTM: The American College of Surgeons designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 CreditTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Of the AMA PRA Category 1 CreditsTM listed above, a maximum of 1 credits meet the requirement for Self-Assessment.
Draining of the chest after pulmonary resection and other thoracic surgery procedures that would otherwise lead to breach in the pleural cavity via tube drainage is a standard of care for patients undergoing thoracic surgery. Overwhelming evidence from research strongly suggests that the use of a single chest tube with smaller caliber chest tube size is as effective as double chest tube in the management of patients after pulmonary resection.1-7 The guidelines for enhanced recovery after lung surgery in the recommendations of Enhanced Recovery After Surgery (ERAS) Society and the European Society of Thoracic Surgeons strongly recommend the use of a single chest tube for the treatment of patients after lung resection.
In contrast, empyema thoracis is a commonly occurring pathology in developing countries with increased morbidity, and draining of the chest cavity with 2 chest tubes after decortication for an indication of empyema thoracis is a common practice among thoracic surgeons in sub-Saharan countries—a practice that is surgeon specific rather than evidence based: 1 is placed into the posterior and basilar region to drain fluid, while the other is directed towards the apex for removing the air from the chest. It is an assumption that complete control of fluid and air in the pleural space could be maintained by inserting 2 chest tubes.8
However, the placement of 2 chests tubes after pulmonary resection is associated with increased postoperative pain, which may have significant effect on postoperative breathing and chest wall compliance with subsequent postoperative complication such as collapsed lung and atelectasis.1-4 Less pain in the postoperative period, on the other hand, may improve the patient’s capability to perform respiratory exercises and thereby facilitates better lung expansion and decreases the rate of postoperative complication.1-7
In recent years, many thoracic surgeons have adopted thoracic drainage using a single chest tube in the midaxillary line and directed inferoposterior and extended upwards toward the apex. The first use of a single drain after a lobectomy was reported by Alex and colleagues4 in a nonrandomized study. They found no difference regarding the postoperative outcomes between the groups; however, less pain was observed in the single-tube group.5
A meta-analysis of 5 randomized studies searched from the Cochrane Central Register of Controlled Trials in the Cochrane Library, PubMed, Embase, the Indian Statistical Institute database, and the Chinese Biomedical Database comparing the use of single chest tube with double chest tube application after pulmonary lobectomy was conducted in 2016. Compared with double chest tubes, the single chest tube was found to significantly decrease the amount of drainage, the duration of chest tube drainage, the pain score, the number of patients who need thoracentesis, and the cost. However, there were no statistically significant differences between the 2 groups regarding the number of new drain insertions after operation.9-12
Pleural empyema is an infectious condition of the pleural cavity with resultant pus accumulation. The most frequent cause of pleural empyema is pneumonia, when a pre-existing parapneumonic effusion gets superinfected by bacteria, often due to perforation of a subpleural pulmonary abscess. Other common causes are tuberculosis, previous thoracic surgery or other invasive thoracic intervention, esophageal disease, trauma, subdiaphragmatic infection, and sepsis. Per the American Thoracic Society, pleural empyema is classified into 3 phases: the early exudative phase (stage I), the intermediate fibroproliferative phase (stage II), and the late organized phase (stage III). If stage II pleural fluid is not drained in conjunction with effective antibiotic therapy, the effusion may progress to stage III, the organizing stage. This final stage is characterized by fibroblasts that proliferate and invade the pleural fluid from both the visceral and parietal pleura, forming a thick pleural peel. Fibrin membranes are transformed by fibroblast into a web of thick nonelastic pleura. This can occasionally encase the lung, preventing expansion and resulting in “trapped lung.” This can functionally result in impaired gas exchange and produces a persistent pleural space, increasing the risk for continued infection.12-17
The treatment of choice, which is decortication, aims to increase the lung volume by freeing the trapped lung with surgical removal of the thickened pleura. There are several reports in the literature about the benefits of lung decortication; focusing mostly on the improvement of lung volume, perfusion, and diffusion capacities.18-22
Decortication, the removal of fibrous tissues constricting the lung, is becoming common in developing countries, and it is 1 of the procedures that requires chest tube drainage as part of the postoperative management. A thick fibrous membrane, which restricts lung expansion, may form on the lung surface after purulent fluid accumulates in the pleural space (pleural empyema) with resultant lung collapse and entrapment. Surgical removal of this fibrous tissue is therefore indicated to allow lung re-expansion. Lung decortication is a challenging and time-consuming procedure. Meticulous dissection is mandatory to peel the thickened fibrotic cortex off the visceral pleura without causing too many lesions that may consequently lead to prolonged air leak.13-17
Decortication is performed under general anesthesia with double-lumen endotracheal tube (preferably). Thoracotomy is performed, and the chest cavity is entered via the 5th or 6th intercostal space. Abscesses are evacuated, if any, and fibrotic and/or calcified tissues are dissected off the visceral pleural of the lung and sometimes the chest wall. Postoperative pain management and early chest and respiratory physiotherapy are initiated. However, due to the reduced chest-wall compliance and the nature/effects of the disease process on the lung, adequate re-expansion of the lung is oftentimes difficult to achieve. This is one of the major causes of morbidity and may instigate postoperative fluid collection, atelectasis, or persistent air leak, which may necessitate a second surgical intervention.8,13-16 In our setting, decortication via open thoracotomy, with the insertion of either a single chest tube or double chest tubes, is the surgical procedure most often employed.15,16
Statement of the problem
A review of the recent literature on treatment modalities of adult thoracic empyema was conducted to expose the controversies and verify where consensus exists. The roles of surgical drainage, lavage techniques, and debridement via video-assisted thoracoscopic surgery, decortication, thoracoplasty, and open window thoracostomy were considered using the Oxford Center of Evidence Based Medicine criteria. The lack of a single ideal treatment modality or policy reflects the complexity of the diagnosis and staging of this heterogeneous disease. The basic elements of intervention—drainage, different evacuation techniques, decortication, thoracoplasty, and open window thoracostomy—are well established technical modalities; however, neither a universally acceptable primary modality nor the gold standard of their sequence is available.23
Additionally, there is no established standard of practice guideline, as provided by research and meta-analysis or expert opinion, for the use of single chest tube vs double chest tube insertion after decortication. Thoracic centers in Ethiopia employed either single or double chest tubes based on the surgeon’s preference and sometimes on the intraoperative findings or the success of the decortication.
As a result of the lack of a guideline or consensus agreement on the use of chest tubes after decortication, there is a knowledge gap in the understanding of the effectiveness of draining the chest using either single or double chest tubes after decortication. Hence, this research was conducted to address such gap and set a platform for further large-scale study that would influence recommendations on standard of practice using chest tube drainage to facilitate adequate re-expansion of the lung and improve postoperative complication after decortication.
Objectives of the research
The objectives of this study were to compare the early postoperative advantages and disadvantages using single and double chest tube drainage after decortication for an indication of stage III empyema thoracis and to influence a decision on the best treatment options in terms of tube insertion, as well as to stimulate further research.
Scope of the research
This research involved all adult patients who were admitted to the thoracic surgery units with the diagnosis of empyema thoracis in 3 hospitals in Addis Ababa. However, only patients with documented American Thoracic Society stage III pleural empyema with respect to clinical and diagnostic evaluation and met the inclusion criteria were enrolled into this study. The research covered a period of 15 consecutive months beginning March 2021 and ending May 2022.
METHODS
Description of research area
This prospective randomized controlled study was conducted at 3 major hospitals in Addis Ababa: Tikur Anbessa Specialized Hospital, Menelik II Referral Hospital, and St Peter’s Specialized Hospital, with Tikur Anbessa Specialized Hospital being the primary investigation center. The research employed these hospitals to facilitate the generation of data sufficient to produce accurate and meaningful results that would affect consensus agreement and decision on the treatment protocol regarding single vs double chest tube placement postdecortication. These facilities are situated in Addis Ababa, the capital city of Ethiopia, and are specialized hospitals.
Tikur Anbessa Specialized Hospital, established in 1972, is the teaching hospital of the Addis Ababa University, College of Health Sciences. It is the largest referral hospital in the country, with over 700 in-patient beds. The thoracic surgery unit has a total of 21 in-patient beds and performs an average of 2 elective cases per day.
Menelik II Referral Hospital, in contrast, has a capacity of 54 in-patient beds in the surgical ward, which also hosts the thoracic unit. The unit performs, on an average, 4 operations per week.
St Peter’s Specialized Hospital was established in 1953 as a center of excellence, as a model of tuberculosis (TB) treatment, and as a general specialized hospital in East Africa. The surgical ward, which houses the thoracic unit, has a capacity of 44 beds distributed among all specialties and subspecialties of surgery, with 12 beds allocated to thoracic surgery.
Research design
The research is a multicenter, prospective randomized controlled study, an investigator-initiated study of the intervention type, conducted at 3 specialized hospitals in Ethiopia comparing the effectiveness of single chest tube vs double chest tube application after decortication. The research employed on-site interview-based questionnaires to generate the preoperative data. Intraoperative and postoperative data were collected using predesigned data collection forms.
In addition, the study was designed as a “blind pattern.” Even though the surgical teams that performed the operations were the same people who managed the patients preoperatively and postoperatively, the research was designed in such a way that it was unlikely that either of these groups would have preknowledge of the selection process. Either single or double chest tubes were placed randomly in a 1:1 ratio.
This prospective randomized controlled study included all registered patients admitted to the thoracic surgery units in the study areas with the diagnosis of empyema thoracis between Marcy 1, 2021, and May 31, 2022, but only those with the diagnosis of stage III empyema thoracic who met the inclusion criteria were enrolled into the study.
The sample size was calculated using the modified Cochran formula. Potential participants were identified and recruited into the study by surgeons, residents, and interns who worked in the aforementioned thoracic units. Patients were enrolled if they met the inclusion criteria specified and signed the informed consent forms. An average of 1.03 patients underwent operation per week for 60 weeks, which summed up to a total of 62 participants.
Inclusion criteria
The inclusion criteria included symptoms lasting for 6 weeks or longer; chest CT scan confirming the presence of an effusion with a pleural peel thickening; patients presenting with no rapidly fatal underlying illness; ability of the patients to undergo general anesthesia; patients presenting no allergies to anesthetic agents; patients having the ability to tolerate single-lung ventilation; intraoperative findings such as thickened or calcified pleura; and the surgeons’ ability to achieve complete decortication.
Exclusion criteria
The research excluded patients younger than age 18 years; pregnant patients; patients exhibiting signs of shock (hypotension, altered mental state, etc); patients participating in any other clinical trials during the trial period; patients with 2 or more comorbidities; and patients in whom concomitant lung resection was performed. Participants were screened by the interns, residents, fellows, and surgeons before the operation, during which time informed written consent forms were signed by either the participants or their legal representatives; a full medical history and physical examination was performed; blood samples were collected and sent for hematology, coagulation profile, blood chemistry, renal and liver function tests, and measurement of electrolytes (obtained within 14 days before the operation); 12-lead electrocardiograms were performed when indicated; and chest x-ray and CT scan were secured; finally, preanesthesiology evaluations were performed.
Randomization of patients
The surgical staff, who were not involved with either enrollment assessment or screening of participants, generated the allocation sequence. Individual randomization, stratified for centers with blocking to reduce variability, were performed at a 1:1 allocation to the intervention and controlled group. These patients were randomized into 2 groups: group A (the controlled group), in which double chest tubes were used, and group B (the intervention group), in which single chest tube was used. Either single or double chest tubes were placed at random, that is, if a single chest tube was placed in 1 patient, 2 chest tubes were inserted in the next patient. In most instances, the surgeons who did the preoperative preparation and the surgeons who performed the surgery, as well as the patient, were not aware of the selection process until completion of the decortication and maintenance of hemostasis were achieved. The research questionnaire forms were prenumbered, and the patients’ initials were indicated on the forms. The intraoperative data collection forms had spaces for numbering and placement of initials of the patients but were not filled out until the end of the operation.
These forms were kept private and locked up. When the surgeons completed the operation, and maintained hemostasis, a member of the research team selected 1 of the intraoperative data collection forms and recorded the patient’s initial and intraoperative parameters on the form, identified the research questionnaire form that matched the patient’s information, and transferred the number from the research questioner’s form to the data collection form. The staff reviewed the previous operative records of the last procedure and group involved and decided the next group.
Data collection methods
The patient parameters were measured and recorded preoperatively, intraoperatively, and postoperatively including the patients’ biodata, extent of the disease, intraoperative events, and postoperative complication. Preoperative data were recorded upon admission of the patients to the wards and subsequent enrollment into the program. The parameters measured included age, sex, educational status, marital status, occupation, monthly income, area of residence, number of persons in household, pathology, performance status, extent of disease, and investigative results. Intraoperative events were recorded during and immediately after the surgical procedure. These endpoints are related to the site of thoracotomy, pleural adhesion and levels of calcification, type of decortication achieved, intraoperative lung re-expansion achieved, and hemorrhage. The postoperative endpoints were recorded after extubating and transferred to the postanesthesia care unit (PACU) and wards up to the second follow-up visit. These include the primary and secondary endpoints. The primary and secondary endpoints were collected at morning and evening (night) hours by the focus person at each center and recorded on the data collection forms and kept private. These findings were also communicated, via text message, to the primary investigator as they were being recorded on each occasion and were entered in to the SPSS application contained on a laptop. The parameters collected and recorded include preoperative, intraoperative, and postoperative data including the primary and secondary endpoints using predesigned forms.
The research employed predesigned research questionnaire forms for on-site interview with the patients, review of patient’s medical records for recording of patient’s history, physical examination findings, and investigation for preoperative parameters. The research employed predesigned intraoperative data collection forms for recording of intraoperative data as observed during the operation.
Primary endpoints
The primary endpoints recorded postoperatively were the amount of drain per day; the duration of drain in days; and the pain score per day.
Secondary endpoints
The secondary endpoints recorded postoperatively were the presence and magnitude of air leak (mild, moderate, or severe); the need for reinsertion of the chest tube(s); the length of hospital stay; and subcutaneous emphysema. Mild air leak was defined as air bubbles during coughing; moderate air leak was air bubbles on moderate respiratory effort such as talking, and severe air leak was defined as spontaneous air bubbles or air bubbles at rest and without any respiratory effort.
Intervention
Before the start of operation, IV lines were established, and an epidural catheter was secured. If the epidural catheter placement failed, paravertebral/intercostal blocks were performed at the end of the operation. After induction of anesthesia, a double-lumen endotracheal tube was used for selective ventilation of the lungs. During the procedure, patients were monitored by electrocardiogram, arterial line, pulse oximeter, and urine output. The patients were placed in a lateral decubitus position and underwent decortication via posterolateral thoracotomy. During surgery, the patient received fluid maintaining adequate blood pressure and urine volume. After decortication, the presence of air leak was tested by a water submersion test using normal saline and inflating the lung at 30 to 40 mmHg positive pressure. In the case of significant air leak, sutures were applied. At the end of the procedure, the patients were then randomized 1:1 to receive either of 2 different types of chest drainage management: single chest tube or traditional 2 chest tubes, connected to the water-seal drain system already in use in each center. After surgery, the patients were transferred to the PACU/ICU, after which they were transferred to the wards.
The following activities and/or assessments were performed after operation: daily assessment of the cardiopulmonary parameters (blood pressure, oxygen saturation) throughout the hospitalization period; complete blood counts the first day after operation and then depending on the clinical condition of the patient or medical judgment; and chest x-ray immediately after return to the ward and afterward depending on clinical condition of the patient or medical judgment. After the drains were removed, another chest x-ray was taken before discharge.
Postoperative treatment included respiratory rehabilitation and mobilization and antibiotic prophylaxis. Pain was controlled by means of analgesic drugs per the research analgesic protocol.
The chest tube(s) were removed when physical findings and chest x-ray showed a complete lung expansion, and there was no detectable air leak with clinical evidence. During morning and evening rounds, the presence of air leak and the pleural effusion volume were checked for both types of drain systems. Air leak duration was calculated from the day of operation until the day air leak was no longer detectable. Duration of air leak for more than 7 days was considered prolonged air leak and was managed per each center’s routine. However, in these cases, the air leak duration was measured; data were collected until the leaks stopped, and no discharge from the hospital was considered before that day.
The operative techniques and approaches were driven by surgeon preference. However, the operative procedure of choice employed was posterolateral thoracotomy with both muscle sparing and muscle cutting techniques and the pleural cavity entered via the 5th or 6th intercostal space. The lung was mobilized, and decortication proceeded using both blunt and sharp dissections. Hemostasis was maintained, and air leaks were checked using warm saline and inflating the lung at 30 to 40 mmHg positive pressure. For the control group, two 32F chest tubes were inserted: 1 placed through the anterior axillary line towards the apex (upper chest tube) and the second placed in the midaxillary line in the dependent side (lower chest tube) of the hemithorax using two separate underwater seal bottles. In the single-tube group, a 32F chest tube was inserted in the midaxillary line at the dependent side of the hemithorax and directed towards the apex. For this study, single underwater seal drainage system or normal saline bottles converted to underwater seal were used. These patients were followed for a period of 2 months before exiting the research.
All patients spent about 1 to 24 hours in the postoperative anesthesia care unit/ICU and returned to the general wards if there were no contraindications. In our study, no patients had medical and/or other indications to be admitted to permanent ICU care. The patients were encouraged to start coughing and exercise on the first postoperative day. Drainage from the tubes and the presence and magnitude of air leaks were recorded twice daily, and x-rays were taken thrice: first on postoperative day 1, second at midway in the postoperative period before removal of the tubes, and third after removal of the tube. The indications for removal of the tubes in all groups were improved clinical parameters, air leaks have stopped, amount of pleural drainage decreased to less than 0.09 mL/kg/24 hours (150 mL/24 hours) of serous fluid for 48 hours or 0.0 mL/24 hours of blood or pus for 48 consecutive hours, and x-ray findings consistent with expanded lung. Patients without complications were discharged one day after removal of the tube(s).
Pain management
Surgery on the chest is one of the most painful of surgical procedures for both open and minimally invasive approaches. The key to enabling early recovery and ambulation is ensuring that postoperative pain is well controlled. Pain after thoracic surgery is generated from multiple structures and is transmitted via several afferent pathways. Factors that affect pain postoperatively can be divided into patient factors, analgesic technique, and operative approach. Effective pain control can facilitate a reduction in postoperative complication, particularly postoperative pulmonary complication. Thoracic epidurals were introduced into clinical practice for post-thoracotomy analgesia in the mid-1970s and have become the gold standard of post-thoracotomy analgesia since the mid-1990s.24,25
The pathogenesis of post-thoracotomy pain is complex. Nociceptive receptors are stimulated by the skin incision, division and retraction of the muscles, and retraction and sometimes fracture of ribs. In addition, ligaments may be stretched, costochondral joints dislocated, and intercostal nerves injured, causing further pain. The incised pleura is frequently irritated by partial surgical stripping, chest drains, and residual pleural blood; the resulting inflammatory responses activate further nociceptors. The central transmission of these multiple nociceptive signals amplifies pain transmission and increases pain perception through central sensitization.24,25
Among various approaches to analgesia, a multimodal approach is the most effective one in preventing acute and chronic pain, as well as in preventing postoperative complications. Multimodal analgesia comprises regional analgesia along with systemic analgesics. Among the regional; thoracic epidural is the gold standard for post-thoracotomy patients. Systemic analgesics were given in the form of systemic opioids, NSAIDS, paracetamol, and ketamine.24,25
All patients received multimodal analgesia throughout the procedure and postoperatively. The epidural catheter was placed before induction of anaesthesia for all study participants and stayed 48 to 72 hours postoperatively, depending on patients’ requirement. Bupivacaine (0.125%) and 2 mL per dermatome to be covered was given every 4 hours and based on patients’ requirement after assessing for pain. Systemic analgesia was given; which included morphine (0.1 mg/kg) IV Q every 4 to 6 hours and paracetamol (1 g) QID po once patients start sips. For patients who were at high risk for postoperative nausea and vomiting, diclofenac (75 mg) IM Q was given every 8 hours for 48 hours. The epidural catheter was removed once the patient was able to cough freely and ambulate without pain after 48 to 72 hours. Failed epidural catheterization was replaced with single shot paravertebral block with 2 mL/dermatome of bupivacaine (0.125%), either unilaterally or bilaterally based on chest tube placement and intercostal block. For this study, the visual analogue scale was used to assess the degree of pain.
Postoperative follow-up consisted of 2 scheduled visits with chest x-ray 30 days after discharge, with the last being 60 days postdischarge. All patients had greatly improved preprocedure symptoms and function by their last visit. Two of the patients with partially expanded lungs on discharge had attained full lung re-expansion on their last visit, while 1 patient showed no change on x-ray film at the last visit; 2 patients missed their first follow-up, and 6 missed their second follow-up.
Data validation/statistical analysis
Periodic review of the input data was performed in order to verify the completeness and consistency of the database. Checks on the consistency and plausibility of the reported data were carried out before data analysis, and a second review of the data entry process was also conducted by a second research team. The study is powered based on its primary endpoints: the amount of drain and duration of chest tube placement and the pain score, as well as the secondary endpoint: the length of hospital stay.
Statistical analyses were performed using the computer-based, Statistical Package for Social Sciences version 27.0 (SPSS Inc, Chicago, IL) program. The patients’ characteristics and results are represented as mean ± standard deviation. Student’s t-test for 2 independent means was used for numerical variables, and the Pearson chi-square test was used for categorical variables, where appropriate. A p value of less than 0.05 was considered significant.
Ethical considerations
The researchers obtained approval from the Department of Surgery Research Ethics Committee and Institutional Review Board of Addis Ababa University College of Health Sciences. The patients were educated, in language appropriate for their full understanding, on the nature and consequences of their surgical condition, surgical procedures, and research to be conducted and given the opportunity to reach an informed consent and sign a consent form. No patient was compelled to partake in the research, and every patient had the right to withdraw from the research at any time if he/she deemed it necessary. Great care was given to patient confidentiality and value for cultural practices and religious beliefs.
The patients were given the right to terminate the research and evaluation at any time point, without sacrificing further medical treatment. There were methods for the replacement of patients who might have withdrawn from the study or who may interrupt prematurely. However, no patients terminated or interrupted the study. The patient’s participation in the study did not involve additional risks other than those related to normal clinical and surgical practice for decortication as no new intervention was introduced.
RESULTS
The results are presented as tables to reflect the heterogeneity of the various findings in simple form and to facilitate easy navigation for our readers. Table 1 provides an overview of the distribution of the patients’ demographic data; Table 2 summarizes the patients’ signs/symptoms and time interval of presentation, as well as the underlying conditions that triggered the onset of the development of the surgical pathology under review. Table 3 provides insight on the events occurring during the operative procedures, while Table 4 contains the primary endpoints, and Table 5 provides an overview on the secondary endpoints. Table 6 outlines the determination of regression in both the primary and secondary endpoints.
Table 1.
Patient Demography
| Variable | Frequency | % |
|---|---|---|
| Age | ||
| 18 to 29 y | 33 | 53.2 |
| 30 to 39 y | 10 | 16.1 |
| 40 to 49 y | 3 | 4.8 |
| 50 to 59 y | 7 | 11.3 |
| 60 to 70 y | 9 | 16.5 |
| Total | 62 | 100 |
| Sex | ||
| Male | 64 | 74.2 |
| Female | 16 | 25.8 |
| Total | 62 | 100 |
| Marital status | ||
| Single | 30 | 48.4 |
| Married | 29 | 46.8 |
| Divorced | 1 | 1.6 |
| Widow(er) | 2 | 3.2 |
| Total | 62 | 100 |
| Educational status | ||
| Elementary | 12 | 19.4 |
| Junior high | 12 | 19.4 |
| High school diploma | 20 | 32.3 |
| Collage graduate | 11 | 17.7 |
| High school dropout | 4 | 6.5 |
| College dropout | 1 | 1.6 |
| No education | 2 | 3.2 |
| Total | 62 | 100 |
| Family size | ||
| <5 | 19 | 30.6 |
| 5 to 10 | 35 | 56.5 |
| >10 | 1 | 1.6 |
| Cannot estimate | 7 | 11.3 |
| Total | 62 | 100 |
Table 2.
Signs/Symptoms
| Variable | Frequency | % |
|---|---|---|
| History of TB | 28 | 45.2 |
| History of trauma | 22 | 35.5 |
| Dyspnea | 58 | 93.5 |
| History of cough | 48 | 77.4 |
| Characteristic of cough | ||
| Dry cough | 35 | 72.9 |
| Productive cough | 13 | 27.1 |
| Total | 48 | 100 |
| Onset of symptoms to presentation | ||
| 6 to 12 wk | 11 | 17.7 |
| 12 to 24 wk | 25 | 40.3 |
| 24 to 36 wk | 15 | 24.2 |
| 36 to 52 wk | 2 | 3.2 |
| 52 to 104 wk | 9 | 14.5 |
| Total | 62 | 100 |
| Blood-stained sputum | 3 | 6.3 |
| Chest pain | 59 | 95.2 |
| Fever | 15 | 24.2 |
| Weight loss | 29 | 46.8 |
| ECOG performance status | ||
| 0 | 43 | 69.3 |
| 1 | 15 | 24.2 |
| 2 | 4 | 6.5 |
| Total | 62 | 100 |
| Primary pathology | ||
| TB | 28 | 45.2 |
| Non-bullet-related trauma | 14 | 22.6 |
| Bullet trauma | 8 | 12.9 |
| Pneumonia | 5 | 8.1 |
| Hydatid cyst | 3 | 4.8 |
| Pneumothorax | 2 | 3.2 |
| COVID-19 | 1 | 1.6 |
| NHL | 1 | 1.6 |
| Total | 62 | 100 |
ECOG, Eastern Cooperative Oncology Group; NHL, non-Hodgkin lymphoma; TB, tuberculosis.
Table 3.
Intraoperative Events
| Variable | Frequency | % |
|---|---|---|
| Epidural catheter | 43 | 69.4 |
| Pleural adhesion | 50 | 80.6 |
| Degree of pleural calcification | ||
| None | 5 | 8.1 |
| Mild | 33 | 53.2 |
| Moderate | 24 | 38.7 |
| Total | 62 | 100 |
| Concomitant procedure | ||
| None | 44 | 71.0 |
| Cystectomy + bronchial hole closure | 3 | 4.8 |
| Pleurectomy | 15 | 24.2 |
| Total | 62 | 100 |
| Intraoperative blood transfusion | 7 | 11.3 |
| End of operation to extubation | ||
| 3 to 5 min | 30 | 48.4 |
| 5 to 10 min | 29 | 46.8 |
| 10 to 20 min | 3 | 4.8 |
| Total | 62 | 100 |
| Intraoperative lung re-expansion | ||
| Full | 48 | 77.4 |
| Partial | 13 | 21.0 |
| None | 1 | 1.6 |
| Total | 62 | 100 |
| Intraoperative air leak | ||
| Absent | 8 | 12.9 |
| Mild | 35 | 56.5 |
| Moderate | 18 | 29.0 |
| Severe | 1 | 1.6 |
| Total | 62 | 100 |
| Intraoperative blood loss | ||
| <500 mL | 30 | 48.4 |
| 500 mL | 21 | 33.9 |
| >500 to 800 mL | 11 | 17.7 |
| Total | 62 | 100 |
| Operative duration | ||
| 2 to 3 h | 19 | 30.6 |
| 3 to 4 h | 39 | 62.9 |
| 4 to 5 h | 4 | 6.5 |
| Total | 62 | 100 |
| Time spent in PACU/ICU | ||
| 1 to 2 h | 47 | 75.8 |
| 2 to 3 h | 5 | 8.1 |
| 3 to 4 h | 1 | 1.6 |
| 4 to 24 h | 9 | 14.5 |
| Total | 62 | 100 |
PACU, postanaesthesia care unit.
Table 4.
Primary Endpoints
| Group | N | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Group A | |||||
| Upper drain | |||||
| Total output, mL | 31 | 40 | 900 | 470.0000 | 608.1118 |
| Duration, d | 31 | 3 | 19 | 7.5498 | 11.3137 |
| Lower drain | |||||
| Total output, mL | 31 | 90 | 1900 | 995.0000 | 1,279.8633 |
| Duration, d | 31 | 3 | 5 | 3.8730 | 1.4142 |
| Pain score | 31 | 1 | 7 | 2.6458 | 4.2426 |
| Group B | |||||
| Total output, mL | 31 | 450 | 1585 | 1,018.0000 | 802.5662 |
| Duration, d | 31 | 3 | 5 | 3.8730 | 1.4142 |
| Pain score | 31 | 1 | 4 | 2.0000 | 2.1213 |
SD, standard deviation.
Table 5.
Secondary Endpoints
| Indicator | Group A | Group B | ||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Postoperative air leak | ||||
| None | 3 | 9.7 | 8 | 25.8 |
| Mild | 23 | 74.2 | 19 | 61.3 |
| Moderate | 5 | 16.1 | 4 | 12.9 |
| Total | 31 | 100 | 31 | 100 |
| Duration of air leak | ||||
| 24 h | 18 | 64.3 | 14 | 60.9 |
| 48 h | 5 | 17.9 | 6 | 26.1 |
| 72 h | 3 | 10.7 | 3 | 13.0 |
| 432 h | 2 | 7.1 | 0 | 00.0 |
| Total | 28 | 100 | 23 | 100 |
| Subcutaneous emphysema | ||||
| Yes | 3 | 9.7 | 4 | 12.9 |
| No | 28 | 90.3 | 27 | 87.1 |
| Total | 31 | 100 | 31 | 100 |
| Duration of subcutaneous emphysema | ||||
| 48 h | 2 | 66.7 | 1 | 25.0 |
| 72 h | 1 | 33.3 | 3 | 75.0 |
| Total | 3 | 100 | 4 | 100 |
| Lung re-expansion on first x-ray | ||||
| No x-ray taken | 0 | 00.0 | 1 | 3.2 |
| 25% to 50% | 1 | 3.2 | 0 | 00.0 |
| 50% to 75% | 10 | 32.3 | 10 | 32.3 |
| 75% to 100% | 20 | 64.5 | 20 | 64.5 |
| Total | 31 | 100 | 31 | 100 |
| Lung re-expansion on discharge | ||||
| 73% to 95% | 3 | 9.7 | 3 | 9.7 |
| 100% | 28 | 90.5 | 28 | 90.3 |
| Total | 31 | 100 | 31 | 100 |
| Discharge fluid recollection | ||||
| Yes | 0 | 00.0 | 0 | 00.0 |
| No | 31 | 100 | 31 | 100 |
| Length of hospital stay | ||||
| 5 to 10 d | 24 | 77.5 | 21 | 67.8 |
| 10 to 15 d | 3 | 9.7 | 7 | 22.6 |
| 15 to 25 d | 2 | 6.5 | 3 | 9.7 |
| 25 to 30 d | 1 | 3.2 | 0 | 00.0 |
| 30 to 45 d | 1 | 3.2 | 0 | 00.0 |
| Total | 31 | 100 | 31 | 100 |
| Reinsertion chest tube | ||||
| Yes | 0 | 0 | 0 | 0 |
| No | 31 | 100 | 31 | 100 |
| Total | 31 | 100 | 31 | 100 |
Table 6.
Significance Levels
| Indicator | Degree of freedom | p Value |
|---|---|---|
| Drain output | 4 | 0.00001 |
| Duration of drain | 4 | 0.000042 |
| Pain score | 1 | 0.326757 |
| Length of hospital stay | 4 | 0.00001 |
Of the study’s 62 patients, 53.2% (33) of the population were in the age range of 18 to 29 years; 16.1% (10) were in the range of 30 to 39 years; and 14.5% (9) were in the age group of 60 to 70 years. Another 11.3% (7) were in the age group of 50 to 60 years, while the ages of the remaining 4.8% (3) were 40 to 49 years.
In our sample, 74.2% (46) of the population were men, while 25.8% (16) were women (Table 1). The unmarried (single) population accounted for 48.4% (30), while the married group comprised 46.8% (29). Also, 3.2% (2) of the population were widow(er)s, while 1.6% (1) represented the divorced population.
In terms of education, 32.3% (20) of our sample completed high school. A total of 19.4% (12) completed elementary, and the same number (12) completed junior high school. 17.7% (11) completed college, while 6.5% (4) were high school dropouts. Finally, 3.2% (2) of the study population had no formal education, and 1.6% (1) were college dropouts.
A total of 56.5% (35) of our population had a family size of 5 to 10 people per household; 30.6% (19) had a family size of less than 5. Another 11.3% (7) could not estimate their family size, while the reminder 1.6% (1) had a family size of greater than 10 people per household (Table 1).
We found that 45.2% (28) of patients presented with a history of treatment for TB while 35.5% (22) had a history of trauma. A total of 95.2% (59) had chest pain; 93.5% (58) presented with dyspnea; 72.9% (53) had cough; 46.8% (29) experienced some form of weight loss; and 24.2% (26) had fever. Of the patients presenting with cough, 72.9 (35) had dry cough, while 27.1% (13) had productive cough, and 6.2% (3) of patients presented history of blood-stained sputum.
For the Eastern Cooperative Oncology Group (ECOG) performance status, 60.3% (43) had status of 0, 24.2% (15) had status of 1, and 6.5% (4) had status of 2.
A total of 40.3% (25) of patients presented within 12 to 24 weeks of unset of symptoms; 24.2% (15) presented within 24 to 36 weeks of the unset of symptoms; 17.7% (11) reported to the facility within 6 to 12 weeks of the onset of symptoms; 14.5% (9) presented between 52 and 104 weeks of the onset of symptoms; and 3.2% (2) of patients presented within 36 to 52 weeks of the onset of symptoms.
In our findings, 61.3% (38) of patients had right-sided empyema thoracis, while 38.7% (24) patients had left-sided empyema thoracis. The secondary pathologies included TB, which accounted for 45.2% (28) of patients, and non-bullet-related trauma, which accounted for 22.6% (14) of patients; another 12.9% (8) had bullet-related trauma; 4.8% (3) of patients had complicated hydatid cyst with pneumonia accounting for 8.0% (5) of cases, with pneumothorax making up 3.2% (2). The remaining patients presented with COVID-19 and non-Hodgkin lymphoma, with each accounting for 1.6% (1) (Table 2).
We found that 69.4% (43) of patients had an epidural catheter inserted, while 30.6% (19) had no epidural catheter. A total of 53.2% (33) of patients had mild pleural calcification, while 38.7% (24) had moderation calcification, and the remaining 8.1% (5) had no form of calcification.
Our results show that 71.0% (44) of our patients had no concomitant surgical procedure, while 24.2% (15) of patients had pleurectomy. The remaining 4.8% (3) had cystectomy plus bronchial hole closure.
Of the total population, 77.5% (48) had their lungs fully re-expanded at the end of decortication, and 21.0% (13) of patients attained partial re-expansion, while 1.6% (1) had no re-expansion at the end of decortication.
Before closure of the chest, 56.5% (35) of patients had mild air leak, 29.0% (21) of patients had moderate air leak, 12.9% (8) had no air leak, and 1.6% (1) had severe air leak.
A total of 48.4% (30) of patients had an estimated blood loss of less than 500 mL; 33.9% (21) had estimated blood loss of 500 mL; 17.7% (11) had an estimated blood loss of 600 to 800 mL; and 11.3% (7) of the patients received intraoperative blood transfusion.
In 62.9% (39) of our patients, surgery lasted for 3 to 4 hours, and in another 30.6% (19) of patients, surgery lasted 2 to 3 hours. In the remaining 6.5% (4) of patients, surgery lasted for more than 4 hours. The time from the end of surgery to extubation was recorded to be 3 to 5 minutes in 48.4% (30) of patients, 5 to 10 minutes in 46.8% (29), and 10 to 20 minutes in 4.6.% (4) of patients.
In terms of PACU/ICU stay, 75.8% (47) of patients spent 1 to 2 hours, and 14.5% (9) spent 4 to 24 hours. Another 8.1% (5) of patients spent 2 to 3 hours, while 1.6% (1) of patients spent 3 to 4 hours (Table 3).
Patients in group A had a total minimal drain output from the upper drain of 40 mL and a total maximum output of 900 mL. The minimal duration of the drain of the upper tube was 3 days, while the maximum duration of drain was 19 days for patients in group A. The minimal output from the lower drain was 90 mL, and the maximal output was 1,900 ml with the minimal duration of 3 days, while the maximum duration was 5 days in patients in group A. The minimal pain score was 1, while the maximal pain score was 7 in group A patients.
In patients in group B, the total minimal drain output was 450 mL, and the maximum output was 1,585 mL. The minimal duration of drain was 3 days, while the maximal duration was 5 days, with a minimal pain score of 1 and a maximum pain score of 4 (Table 4).
In group A, 74.2% (23) of patients had mild air leak, 16.1% (5) had moderate air leak, and 9.7% (3) had no air leak. Of those with air leak, 64.3% (18) had leak lasting for 24 hours, and 17.9% (5) had air leak lasting for 48 hours, while 10.7% (3) had air leak lasting for 72 hours, and the remaining 7.2% (2) had air leak lasting for 432 hours. In addition, 9.7% (3) of our patients had subcutaneous emphysema with subcutaneous emphysema lasting for 48 hours in 66.9% (2) and 72 hours in the remining 33.3% (1) of patients.
On postoperative day (POD) 1, 64.5% (20) of patients in group A had their lungs re-expanded to 75 to 100%; 32.3% (10) had 50 to 75% re-expansion, and 3.2% (1) had 25 to 50% re-expansion. On discharge, 90.3% (28) of patients had their lunge expanded to 100%, while 9.7% (3) had their lungs expanded to 75 to 95%. In all patients in group A, there was no fluid recollection, and none required reinsertion of the chest tube.
Of patients in group A, 77.5% (24) spent 5 to 10 days on admission; 9.7% (3) spent 10 to 15 days; 6.55 (2) spent 15 to 25 days; 3.2% (1) spent 25 to 30 days; and 3.2% (1) spent 30 to 45 days on admission.
In group B, 61.3% (19) of patients had mild air leak, 12.9% (4) had moderate air leak, and 25.8% (8) had no air leak. In 60.9% (14) of patients with air leak, the duration of leak was 24 hours, while 26.2% (6) of patients had leak for 48 hours, and 13.0% (3) had leak for 72.
Of patients in Group B, 12.9% (4) of patients developed subcutaneous emphysema lasting for 72 hours in 75.0% (3) and 48 hours 25.0% (1).
On POD 1, 66.7% (20) of patients in group B had their lungs expanded to 75 to 100%, and 32.3% (10) had 50 to 75% re-expansion, while on the day of discharge, 90.3% (28) of patients had their lungs expanded to 100%, and the remaining 9.7% (3) had their lungs re-expanded to 75 to 95% in patients.
In all patients in group B, there was no fluid recollection, and none required reinsertion of the chest tube. In group B patients, 67.8% (21) spent 5 to 10 days on admission, 22.6% (7) spent 10 to 15 days on admission, and 9.7% (3) spent 15 to 25 days on admission (Table 5).
The p value for the drain output was recorded as 0.00001 with a degree of freedom of 4, and that of the duration of drain was recorded as 0.000042 with a degree of freedom of 4, while the p value of the pain score was 0.326757 with a degree of freedom of 1. The p value of the length of hospital stay was 0.00001 with a degree of freedom of 4 (Table 6).
DISCUSSION
Adequate re-expansion of the lungs is essential in preventing postoperative complication after all forms of surgery that breached the chest cavity and pleural layers. The conventional and widely used practice of draining the chest with 2 tube placement is still being performed around the world. However, many surgeons use single tube placement, while a very few use double tube placement to drain the chest after decortication in Ethiopia based on the intraoperative findings. We conducted a randomized controlled study to investigate the associated benefits of double chest tube vs single chest tube drains with the immediate postoperative outcome after decortication. A total of 138 patients with the diagnosis of stage III empyema thoracis were registered during the period of the research. The contributions of each center were as follows: Tikur Anbessa Specialized Hospital, 54 patients (30 enrolled, 10 excluded, and 14 on waiting list); Menelik Specialized Hospital, 40 patients (19 enrolled, 16 excluded, and 5 on waiting list); and St Peter’s Specialized Hospital, 44 patients (13 enrolled and 31 excluded). However, only 62 patients met the inclusion criteria and were included in the study. The rest of the 76 patients did not meet the inclusion criteria and were excluded from the study. Of this number, 12 patients were excluded from the study due to inadequate and/or missing data; 36 patients did not meet the inclusion criteria and were treated with chest tube thoracostomy; and 9 patients were excluded due to concomitant lung resections, while the remaining 19 patients were still on the waiting lists. In all patients, a single underwater drainage system or normal saline bottles converted to underwater seal drainage systems were used. The drains were removed once the patient(s) met the protocol for removal of drains.
The complications occurring after operation until discharge or within 30 days postoperatively in discharged patients were considered postoperative complications; death during the same period was defined as perioperative death. However, there was no death recoded during and after these periods among our patients. Complication associated with decortication, as well as other general surgical complication, was managed by the surgical teams in accordance with the usual standards of management of postdecortication complication as is done for all other patients.
The age range between 18 to 70 years and the median age was 44 ± 14.4434. There was no association between the age and postoperative outcome. The male to female ratio was 2.9:1. In our study, 96.5% of the population had some form of formal education, and the average family size was between 1 and 10 people/household. TB and trauma were the most frequently occurring underlying pathologies (45.2% vs 35.5%). It was observed that 95% of all trauma patients had previous chest tube thoracostomy as the initial management but did develop complication later. The right side was the most affected (62.3%), and the common presenting symptoms were chest pain, dyspnea, cough, and weight loss in 95.2%, 93.5%, 72.9%, and 46.8% of these patients, respectively. The earliest presentation from onset of symptoms to the facilities was within 12 to 36 weeks with 64.5% (40) of patients presenting at within this time period. A total of 69.3% (43) of the participants had ECOG performance status of 0, while only 6.5% (4) had ECOG performance status of 2. There was no patient who had a score greater than 2.
As per the American Association for Thoracic Surgery consensus guidelines for the management of empyema, stage III should be managed either by video-assisted thoracoscopic surgery or open thoracotomy. For our study, the procedure of choice was open posterolateral thoracotomy through the 5th/6th intercostal space, and 61.3% of patients had right-sided thoracotomy. The sex/age distribution and disease/age correlation among the 2 groups were relatively dissimilar, with 13 patients in group A presenting with a history of trauma as compared with 10 patients in group B. In group A, 12 patients had a history of TB treatment as opposed to 15 in group B.
Epidural catheters were placed in 69.4% of the patients before general anesthesia, while the remainder had either paravertebral/intercostal blocks. On a few occasions (12.9% of patients), we had failures of epidural catheter placement, while in other instances (17.7% of patients), there was no catheter (market shortage). Patients with epidural catheter achieved better pain control compared with those without epidural (maximum pain score was 3 vs 7, respectively). Patients with epidural catheter in group B attained early pain improvement, with the removal of catheter beginning on POD 3, while day 4 was the earliest day of removal in those in group A. By POD 2, all patients in group B had a maximum pain score of 2, while only patients with epidural catheters in group A had similar scores on day 2. Also, by day 3, the maximum score in group B patients was 1, while in group A patients, a similar score of 1 was achieved on day 4. There was no recorded epidural abscess in all patients with epidural catheter. From these findings, it can be hypothesized that the use of epidural catheter after decortication has some benefits on patient outcomes. Pleural adhesion and pleural calcification were observed in 80.0% and 91.9% of patients, respectively. Intraoperative air leak was recorded in 87% of patients, and the average estimated blood loss was up to 500 mL (82.3%) with the maximum being 600 to 800 mL (17.7%). Intraoperative blood transfusion was administered to 11.3% of the patients. In 93.5% of patients, surgery lasted between 2 and 4 hours, while the average time from end of surgery to extubation was 5 to 10 minutes (95.2%). The average duration of time spent in the PACU/ICU was 1 to 2 hours as observed in 75.8% of patients. In 1 center, however, 14.5% of patients spent up to 24 hours in the ICU due to the center protocol. There was no significant intraoperative event recorded in any of the 62 patients. The number of chest tube insertions was randomly decided in a ratio of 1:1 with the decision regarding the next pattern of drainage system to be used based on the preceding chest tube pattern used; and the surgeons had no knowledge of the pattern of chest tube to be used until after completion of decortication and after maintenance of hemostasis was secured.
In our study, we measured the association between the number of chest tube insertions and the immediate postoperative endpoints related to drain output, duration of drain, and pain score as our primary endpoints, and the presence of air leak, the need for reinsertion chest tube, subcutaneous emphysema, lung re-expansion, and length of hospital stay as our secondary endpoints. The number of tubes to insert was selected randomly, and the tube(s) were connected to an underwater seal bottle without application of suction of any kind. Patients were subjected to x-ray evaluation on POD 1 and subsequent days as determined by drain output and the presence of air leak. However, 1 patient did not get a chest x-ray on POD 1 due to a technical fault in the radiology department. Primary and secondary endpoints were recoded every 12 hours, and the tube(s) were removed based on the protocol for removal of tubes as set for this study. All patients were placed under the same pain protocol as contained in the research. No patient required extra pain management intervention except those recommended by the research. There was a relative difference in primary endpoints comparing group A and group B, which signifies association of the number of drains with the observed immediate postoperative primary endpoints. The means output was 1,465 ± 1,887.9751 mL in group A vs 1,018 ± 802.5662 mL in group B (df = 4 and p value = 0.00001), which was significant at p < 0.05, and the median duration of drains recorded was 7.5498 ± 11.3137 days for those in group A vs 3.8730 ± 1.4142 days for those in group B (df = 4 and p value = 0.000042), which was also significant at p < 0.05. The median degree of pain was recorded as 2.6458 ± 4.2426 vs 2.000 ± 2.1213 in groups A and B, respectively (df = 1 and p value = 0.326757), which was not significant at p < 0.05.
There was no significant difference in secondary endpoints observed in both groups with the exception of length of hospital stay. Patients in group A had a relatively higher rate of air leak of 90.3% vs 74.2% in those in group B and lasted for a maximum of 72 hours in group B, while 2 patients in group A had air leak for up to 19 days. The presence of air leak, as observed in our study, had no association with the number of tubes used, and the presence of chest tube(s) did not influence the duration of air leak, as patients in group A had leaks for up to 19 days compared with those in group B, who had leaks for only 72 hours. The rate of subcutaneous emphysema in our study was relatively higher in those in group B, with 12.9% of patients presenting with subcutaneous emphysema postoperatively, while only 9.7% of patients in group A had postoperative subcutaneous emphysema. However, in both groups, subcutaneous emphysema lasted for 72 hours. It was observed that the rate of developing subcutaneous emphysema had no association with the insertion of chest tubes but to other pre- and intraoperative factors.
In our study, only 83.3% of patients with a history of trauma and 92% with history of TB in group A attained 100% re-expansion at discharge, while in group B, all patients with a history of trauma and those with a history of TB had 100% re-expansion at discharge. However, the overall full re-expansion of the lungs on discharge was the same in both groups, accounting for 90.3% with 100% re-expansion each. The remaining 9.7% had their lungs re-expanded to greater than 75% on discharge.
There was no fluid recollection, and no patients required reinsertion of chest tube(s). The median length of hospital stay was recorded as 21.5818 ± 11.9791 days in group A vs 13.6091 ± 6.2048 days in group B (df = 4 and p value = 0.00001), which was significant at p < 0.05. However, the time from end of operation to discharge of patients was 6 days maximum in both groups, with the exception of 2 patients in group A with persistent air leak. Other factors leading to delayed discharge from hospital were preoperative evaluation/preparation and factors relating to operating room readiness as observed in 8.1% of patients.
All patients had greatly improved preprocedural symptoms and function by their last visit. Of the patients with partially expanded lungs on discharge, 5 attained full lung re-expansion by their last visit, while 1 patient had no change on x-ray films at the last visit. However, 2 patients missed their first follow-up, and 6 missed their second follow-up.
Limitations of the Research
One of the major challenges to this research was the availability of chest tubes, 3-chamber underwater seal drainage system, epidural catheter, and the high cost associated. These materials were bought, but not all attempts to import them materialized. As a result, the team had to engage the local market to secure locally made chest drainage systems, and even those were difficult to find. In addition, 20 patients had to buy the chest tubes at their own expense as the funding for the project went low. Another major challenge was coordinating the admission and data collection processes across the 3 hospitals as seniors, fellows, and residents are rotated on monthly intervals. As a result of this, some patients were excluded due to incomplete or inappropriate data collection. Also, the research was limited in the preoperative laboratory evaluation of patients in term of thoracentesis, pleural fluid analysis, and pulmonary function test as there was a constant unstable supply stream of laboratory tests and reagents in the facilities and because of the high cost associated with getting these tests in the private sector. However, these factors did not have any impact on the outcomes of the study as they are not directly related to the evaluation of the primary and secondary endpoints being studied.
CONCLUSIONS
Empyema thoracis is a commonly occurring pathology in developing countries with increased morbidity owing to the high rate of TB infections and trauma occurrence and low access to early healthcare. The recommended treatment option for state III empyema thoracis is either video-assisted thoracoscopic surgery or open decortication followed by the placement of chest tube(s) for drainage. The researchers concluded that the commonest causes of empyema thoracis in our setting are TB and trauma and that more than 95% of patients with trauma developed empyema thoracis months after treatment with tube thoracostomy. The study also concluded that the placement of a single chest tube in the midaxillary line is effective in minimizing drain output, providing shorter time of drainage and relatively shorter length of hospital stay. However, there was no association with pain and the number of drains as observed in this study. The researchers recorded that the status of the lung parenchyma at presentation, degree of calcification, and lung parenchyma injury during decortication, among others factors, have a greater influence on the development of postoperative air leak and subcutaneous than the number of chest tubes, and the presence of a chest tube did not influence the duration of air leak as patients in group A had leak for up to 18 days compared to those in group B, who had leak for only 3 days. Otherwise, the need for reinsertion chest tube, fluid recollection, and the overall lung re-expansion were relatively the same in both groups.
In addition, the use of single underwater seal drains or the use of normal silane bottles converted to underwater seal drains are effective as the standard 3-chambers underwater seal drainage system. The use of epidural catheter seemed to be effective and safe in pain control after decortication, but the researchers could not reach an evidence-based conclusion, as the research did not consider the various predicting and/or influencing factors involved.
Recommendations
The researchers emphasized the need for further study, to include a larger population, investigating the effectiveness of either system of chest drain and long-term outcomes and the factors influencing the development of postoperative complication after decortication. Also, the researchers recommend a study to determine the advantages and/or disadvantages of the use of epidural catheter in the postoperative pain management of patients undergoing decortication. Finally, the researchers recommend a need to review the process of post-traumatic chest tube insertion and management so as to reduce the complication rate after management of chest trauma with tube thoracostomy.
Author Contributions
Conceptualization: Hart
Data curation: Hart, Mohammed, Tesfaye, Nadamo, Fekadu, Feyissa, Tadessa, Gebeyehu
Formal analysis: Hart
Funding acquisition: Hart
Investigation: Hart
Methodology: Hart
Project administration: Hart
Resources: Hart
Software: Hart
Supervision: Hart, Feyissa
Validation: Hart, Mohammed, Tesfaye, Nadamo, Fekadu, Feyissa, Tadessa, Gebeyehu
Visualization: Hart
Writing – original draft: Hart
Writing – review & editing: Hart, Mohammed, Tesfaye, Nadamo, Fekadu, Feyissa, Tadessa, Gebeyehu
Patient screening: Mohammed, Tesfaye, Nadamo, Fekadu, Feyissa, Tadessa, Gebeyehu
Acknowledgment:
We extend sincere thanks and appreciation to Addis Ababa University, through the College of Health Sciences, Tikur Anbessa Specialized Hospital, Menelik II Specialized Hospital, and St Peter’s Specialized Hospital for providing the environment to conduct this research. We acknowledge the contributions of Abebe Bezabih Tessema for his guidance and supervision throughout the course of this research. We also recognize the contributions of Ephraim Teffera Yiheyis, Patrick Okao, Dorothy Kezembe, and Annitha Nshemereirwe in proofreading and editing this research paper and also, Selamawit Jisso for working out our pain protocol. I acknowledge Robert and Mrs Louise Kpoto and J Fonati Koffa for their financial contributions toward my training process. Finally, we appreciate the patients, family members, and support staff of the hospitals for their cooperation and support during this research.
Abbreviations and Acronyms
- ECOG
- Eastern Cooperative Oncology Group
- PACU
- postanesthesia care unit
- POD
- postoperative day
- TB
- tuberculosis
CME questions for this article available at http://jacscme.facs.org
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose. Ronald J Weigel, CME Editor, has nothing to disclose.
REFERENCES
- 1.Fell SC, Kirby TJ, Shields TW, et al. Technical aspects of lobectomy. General Thoracic Surgery. Philadelphia, Lippincot Williams & Wilkins; 2006;433–457. [Google Scholar]
- 2.Khan IH, Vaughan R. A national survey of thoracic surgical practice in the UK. Int J Clin Pract 1999;53:252–256. [PubMed] [Google Scholar]
- 3.Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin 2005;15:105–121. [DOI] [PubMed] [Google Scholar]
- 4.Alex J, Ansari J, Bahalkar P, et al. Comparison of the immediate postoperative outcome of using the conventional two drains versus a single drain after lobectomy. Ann Thorac Surg 2003;76:1046–1049. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Caro A, Roca MJ, Torres J, et al. Successful use of a single chest drain postlobectomy instead of two classical drains: a randomized study. Eur J Cardiothorac Surg 2006;29:562–566. [DOI] [PubMed] [Google Scholar]
- 6.Zhou D, Deng XF, Liu QX, et al. Single chest tube drainage is superior to double chest tube drainage after lobectomy: a meta-analysis. J Cardiothorac Surg 2016;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil T, Grochowski Z, Warmus J, et al. Comparison of single chest tube versus double chest tube drainage after lung resection for the treatment of non-small cell lung cancer. J Thorac Oncol 2017;12:S2198–S2199. [Google Scholar]
- 8.Andrade-Alegre R, Garisto JD, Zebede S. Open thoracotomy and decortication for chronic empyema. Clinics 2008;63:789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Lv D, Li M, et al. The single chest tube versus the double chest tube application after pulmonary lobectomy: a systematic review and meta-analysis. J Can Res Ther 2016;12:C309–C312. [DOI] [PubMed] [Google Scholar]
- 10.Okur E, Baysungur V, Tezel C, et al. Comparison of the single or double chest tube applications after pulmonary lobectomies. Eur J Cardiothorac Surg 2009;35:32–36. [DOI] [PubMed] [Google Scholar]
- 11.Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am Med 1980;69:507–512. [DOI] [PubMed] [Google Scholar]
- 12.Ahmet FA, Harrison CV. The effect of prolonged pulmonary collapse on the pulmonary arteries. J Pathol Bacteriol 1963;85:357–360. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay M, Nathan D. Pneumonia and empyema: causal, casual or unknown. J Thorac Dis 2015;7:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witold R, Jan S, Grzegorz R, et al. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002;21:502–507. [DOI] [PubMed] [Google Scholar]
- 15.Tizazu A, Nega B. A 3-year review of patients with chronic empyema treated surgically at Tikur Anbessa Specialized Referral Hospital, Addis Ababa, Ethiopia. East Cent Afr J Surg 2016;21:22–27. [Google Scholar]
- 16.Amare A, Ayele B, Mekonned D. Thoracic empyema: cause and treatment outcome at Gondar University Teaching Hospital, Northwest Ethiopia. East Cent Afr J Surg 2010;15:119–123. [Google Scholar]
- 17.Nieminen MM, Antila P, Markkula H, Karvonen J. Effect of decortication in fibrothorax on pulmonary function. Respiration 1985;48:94–96. [DOI] [PubMed] [Google Scholar]
- 18.Mertol G, Erdal O, Volkan B, et al. Lung decortication for chronic empyema: effects on pulmonary function and thoracic asymmetry in the late period. Eur J Cardiothorac Surg 2009;36:754–758. [DOI] [PubMed] [Google Scholar]
- 19.Goshal AG, Saha AK, Roy DJ, Ghosh S. Fibrothorax-problem, profile and prevention. J Indian Med Assoc 1997;95:610–612. [PubMed] [Google Scholar]
- 20.Swoboda L, Laule K, Blatmann H, Hasse J. Decortication in chronic pleural empyema: investigation of lung function based on perfusion scintigraphy. Thorac Cardiovasc Surg 1990;38:359–361. [DOI] [PubMed] [Google Scholar]
- 21.Ryzman W, Skokowski J, Romanowicz G, et al. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002;2:502–507. [DOI] [PubMed] [Google Scholar]
- 22.Webb RW, Burford TH. The effects of atelectasis on pulmonary blood flow in the dog. AMA Arch Surg 1953;66:801–809. [DOI] [PubMed] [Google Scholar]
- 23.Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007;32:422–430. [DOI] [PubMed] [Google Scholar]
- 24.Slinger P. ed. Principles and Practice of Anesthesia for Thoracic Surgery, Nairobi, Springer Science+Business Media, LLC; 2011:pg675 [Google Scholar]
- 25.Attri JP, Kaur R, Kaur H, Makhni R. Post thoracotomy pain management: a review of current available modalities. North J ISA 2016;1:7–10. [Google Scholar]