Abstract
It has previously been reported that inhibition of delayed-type hypersensitivity-mediating functions of T cells during mycobacterial infection in mice is haplotype dependent. In the present study, we show that Mycobacterium bovis BCG infection induced, in susceptible C57BL/6 and BALB/c mice but not in resistant C3H/HeJ and DBA/2 mice, an important splenomegaly. An in vitro defect in T-cell proliferation in response to T-cell receptor (TCR) stimulation with mitogens or anti-CD3 antibodies was associated with enhanced levels of CD4+ and CD8+ T-cell apoptosis in susceptible but not in resistant mice 2 weeks after infection. Further investigations of C57BL/6 and C3H/HeJ mice revealed that in vivo splenomegaly was associated with destruction of the lymphoid tissue architecture, liver cellular infiltrates, and increased numbers of apoptotic cells in both spleen and liver tissue sections. Infection of C57BL/6 mice but not of C3H/HeJ mice induced massive production of tumor necrosis factor alpha (TNF-α) in serum, as well as an increase in Fas and Fas ligand (FasL) expression in T cells. In vitro addition of neutralizing anti-TNF-α antibodies led to a significant reduction in CD3-induced T-cell apoptosis of both CD4+ and CD8+ T cells of C57BL/6 mice, while the blockade of Fas-FasL interactions reduced apoptosis only in CD4+ but not in CD8+ T cells. Together, these results suggest that TNF-α and Fas-FasL interactions play a role in the activation-induced cell death (AICD) process associated with a defect in T-cell proliferation of the susceptible C57BL/6 mice. T-cell death by apoptosis may represent one of the important components of the ineffective immune response against mycobacterium-induced immunopathology in susceptible hosts.
Development of tuberculosis is often associated with a depression of cellular immunity, as shown by a loss of the tuberculin skin test reaction (17), a decrease in interleukin-2 (IL-2) secretion and in IL-2 receptor (IL-2R) expression (48), and a reduction in cell proliferation (32). In pulmonary tuberculosis, the predominant form of the disease, 17 to 25% of the patients are unresponsive to purified protein derivative (PPD) skin testing (7) and 40 to 60% of the patients have low blastogenic responses to PPD (24).
Immunosuppressive features are also manifest in some but not all strains of mycobacterium-infected mice (36). Strain variation in resistance to mycobacterial infections among mice has been noted for several decades. Some mouse strains are susceptible (bcgs) and others are resistant (bcgr) to various mycobacterial infections including those with Mycobacterium bovis BCG. Resistance or susceptibility to infection with intracellular pathogens such as Salmonella and Mycobacterium is controlled by the natural resistance-associated macrophage protein (Nramp1) gene on chromosome 1, which influences the rate of intracellular replication of these parasites in macrophages (15, 51). Kaledin et al. (18) observed that 4 weeks after intravenous inoculation of BCG, the number of viable bacilli recovered from the spleens of C57BL/6 mice was more than 20 times greater than the number recovered from a resistant mouse strain. Lagrange and Hurtrel (27) showed that the BCG, when injected intravenously, multiplied markedly in the spleens of C57BL/6 mice. No multiplication occurred in resistant mice, and the bacilli were steadily eliminated from the spleen. The innate susceptibility of mice to mycobacterial infection seemed to be expressed very early in the course of the host-parasite interaction. Resistance appears to involve macrophages able to limit the growth of the bacteria and subsequently eliminate them (44). Thus, it has been proposed that resistant mice are able to prevent bacterial growth without the need for a cellular response whereas susceptible mice will eventually control bacterial growth by the acquisition of cellular immunity (38). More recently, however, it has been shown that there is a selective regulation of costimulatory molecules on the surface of infected macrophages (42). It was found that B7 was down-regulated while ICAM-1 was up-regulated in susceptible BALB/c mice but not in resistant C3H/HeJ mice and that these changes resulted in the inhibition of delayed-type hypersensitivity-mediating functions of T helper cells from BALB/c mice. This depressed T-helper-cell function concerns not only mycobacterial antigens (42) but also recall antigens like keyhole limpet hemocyanin (42). In addition, a contribution of CD4+ and CD8+ T cells to acquired resistance to M. bovis has been suggested to occur in knockout mice deficient for major histocompatibility complex molecules (26).
Resistance or susceptibility to mycobacterial infection could be related to an inappropriate induction of T-cell tolerance caused by the dysregulation of physiological cell death programs (1, 47). Apoptosis may play a major role in the suppression of Th-1-dependent effector functions occurring during various infectious diseases (1, 14). Activation-induced cell death (AICD) has been reported to be dependent on the interactions between ligands and receptors belonging to the tumor necrosis factor (TNF) family, which includes TNF and Fas (35), and on the susceptibility of the cells to receptor-mediated death signal transduction (23). These death-triggering pathways are involved in the elimination of antigen-stimulated peripheral T cells to terminate an immune response and to limit inflammation (31, 45, 56). It has also been reported that such molecules may play a major role during human immunodeficiency virus (HIV) infection (12, 13, 19) and participate in Peyer's patch T-cell death in mice infected with Toxoplasma gondii (29).
We report here that a defect of T-cell proliferation in response to mitogen or anti-CD3 antibody in C57BL/6 and BALB/c (bcgs) mice but not in DBA/2 and C3H/HeJ (bcgr) mice after infection with M. bovis BCG is associated with the induction of both CD4+ and CD8+ T-cell apoptosis.
MATERIALS AND METHODS
Mouse strains.
C57BL/6 (Nramp1−), BALB/c (Nramp1−), C3H/HeJ (Nramp1+), and DBA/2 (Nramp1+) mice were purchased from Janvier (Le Genest, St Isle, France); 6-week-old animals were used for primary infection.
BCG infection.
Mice were inoculated in the retroorbital vein with 5 × 106 CFU of M. bovis BCG (vaccine strain 1173P2; World Health Organization, Stockholm, Sweden) freshly grown on Sauton medium (43). The mice were sacrificed 2 to 3 weeks later.
Antibodies and reagents.
Rat anti-mouse phycoerythrin-conjugated Thy-1.2 (53-2.1), rat anti-mouse Cy-chrome-conjugated B220 (RA3-6B2), rat anti-mouse Cy-chrome-conjugated CD4 (RM4-5), rat anti-mouse Cy-chrome-conjugated CD8 (53-6.7), fluorescein isothiocyanate (FITC)-conjugated hamster anti-mouse Fas (Jo2), rat anti-mouse TNF-α (MP6-XT3), rat immunoglobulin G1 (IgG1) isotype control (R3-34), and hamster anti-mouse CD3 (145-2C11) were purchased from Pharmingen (San Diego, Calif.). The hybrid protein containing the Fc portion of mouse IgG1 and the recombinant Fas antigen (Fas-Fc) was purchased from Alexis Corp. (San Diego, Calif.). Other reagents were staphylococcal enterotoxin B (Toxin Technology Inc., Madison, Wis.), concanavalin A (ConA), pokeweed M, and acridine orange dye (Immunotech, Marseille, France).
Cell culture conditions.
T cells from uninfected and infected mice were prepared from spleen using Lympholyte-M (Cedar Lane, Hornby, Ontario, Canada) density gradient centrifugation. B cells were depleted by incubating the cell suspensions at 4°C for 45 min with magnetic beads coated with goat anti-mouse IgG. Ig-positive cells were then removed using a magnetic concentrator (Immunotech). For C57BL/6, BALB/c, C3H/HeJ, and DBA/2, the T-cell purity was 92, 90, 80, and 92%, respectively. Cells were cultured in RPMI 1640 (Gibco, Courbevoie, France) supplemented with 10% heat-inactivated fetal calf serum (Boehringer Mannheim, Meylan, France), 100 U of penicillin (Gibco) per ml, 20 μg of streptomycin (Gibco) per ml, 2 mM l-glutamine, 5 × 10−5 M β-mercaptoethanol (Merck, Darmstadt, Germany), and 1 mM sodium pyruvate (Gibco).
T-cell proliferation.
T cells were cultured at a concentration of 5 × 104 cells/well in 96-well flat-bottom culture plates (Falcon, Becton Dickinson, Mountain View, Calif.) in the absence or presence of one of the following stimuli: 1 μg of ConA per ml, or 10 μg of anti-CD3 per ml. Each proliferation test was performed in triplicate. Cultures were incubated at 37°C in a humidified CO2 incubator for 3 days and then pulse-labeled with 1 μCi of [3H]thymidine (Amersham, Les Ulis, France). Cells were then harvested onto fiber filter strips using a multiharvester (Skatron, Lierbyen, Norway), and the incorporated radioactivity was determined by liquid scintillation counting (LKB, Wallac, Turku, Finland).
Apoptosis measurement.
Apoptosis was measured in vitro by three different methods. (i) Using the light microscope, the cells counted as apoptotic included cells with characteristic nuclear chromatin condensation and fragmentation, as well as already dead cells that had lost the trypan blue exclusion capacity, as previously described (11, 25). (ii) Using flow cytometry (FCM) analysis (FACScan; Becton Dickinson) after incubation of the cells with acridine orange nuclear dye (0.1 μg/ml) for 2 min, as described previously (11), apoptosis was detected by observing a distinct peak of reduced fluorescence intensity. (iii) Cells undergoing apoptosis were also identified by FCM using FITC-conjugated annexin V (R&D Systems, Abingdon, United Kingdom), a phospholipid-binding protein (50). This method was used to detect apoptotic T-cell subpopulations. T cells were first stained by being incubated with rat anti-mouse Cy-chrome-conjugated CD4 or rat anti-mouse Cy-chrome-conjugated CD8 antibodies, washed with phosphate-buffered saline, and then incubated in binding buffer with FITC-annexin V for 20 min at 4°C, as specified by the manufacturer (Immunotech). The cells were analyzed by FCM analysis.
Apoptosis was assessed in tissue sections by the terminal deoxytransferase (TdT)-mediated dUTP nick end labeling (TUNEL) method. Briefly, microscopy analysis of in situ DNA fragmentation was assessed on 5-μm paraffin sections of formalin-fixed spleens and livers. Tissue sections were washed once with TdT buffer (Gibco) and then incubated with 0.5 μM digoxigenin-dUTP and 5 U of TdT (Boehringer Mannheim), in 30 to 50 μl of TdT buffer. The sections were incubated for 1 h at 37°C and then washed in PBS. A sheep anti-digoxigenin-alkaline phosphatase-conjugated antibody (Boehringer Mannheim) was next added for 40 min at 37°C and revealed with naphthol AS-MX, Fast Red, and levamisol. The sections were counterstained with Harris hematoxylin and mounted with gelatin.
Fas expression.
Fas expression was assessed on T cells by double labeling using anti-mouse Cy-chrome-conjugated CD4 and anti-mouse Cy-chrome-conjugated CD8 antibodies, as well as the anti-mouse FITC-conjugated Fas antibodies. The cells were incubated for 30 min at 4°C, washed twice, and then analyzed by FCM.
RNA extraction and RT-PCR analysis.
Total RNA was extracted from T cells using RNAzol (Bioprobe, Montreuil, France) as recommended by the manufacturer. For each sample, equal amounts of total RNA (1 μg) were reverse transcribed with 200 U of Moloney murine leukemia virus reverse transcriptase (RT; Gibco BRL, Eragny, France), 4 U of RNasin (Promega, Lyon, France), 50 ng of oligo(dT), 2 mM each deoxynucleoside triphosphate, and 4 mM dithiothreitol in a final volume of 27 μl. The products were then denatured by heating at 95°C before being stored at −20°C. PCR amplification was performed using primers for β-actin (5′-GTG GGG CGC CCC AGG CAC CA-3′ and 5′-CTT TAG CAC GCA CTG TAA TTC CTC-3′), and FasL (5′-CAG CTC TTC CAC CTG CAG AAG G-3′ and 5′-AGA TTC CTC AAA ATT GAT CAG AGA GAG-3′). The cDNA samples were amplified using a DNA thermal cycler (Perkin Elmer Cetus, Saint-Quentin, France) for 35 cycles for β-actin and FasL at an annealing temperature of 55°C for FasL and of 60°C for β-actin. For each cDNA preparation, a control reaction was performed without RT to ensure that there was no contaminating genomic DNA. The PCR products were analyzed by agarose gel electrophoresis (1.5% agarose) in TBE (0.09 M Tris borate, 0.002 M EDTA [pH 8.0]) containing ethidium bromide (25 μg/50 ml of gel).
TNF-α assay.
Mouse TNF-α activity present in the sera collected by retroorbital puncture was determined at several time points after BCG infection from individual mice, using the Factor-test mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Genzyme, Cambridge, Mass.). Results are expressed as picograms per milliliter and are means of duplicate assays.
Statistical analysis.
The statistical significance (P) was assessed using Student's t test.
RESULTS
BCG-induced splenomegaly.
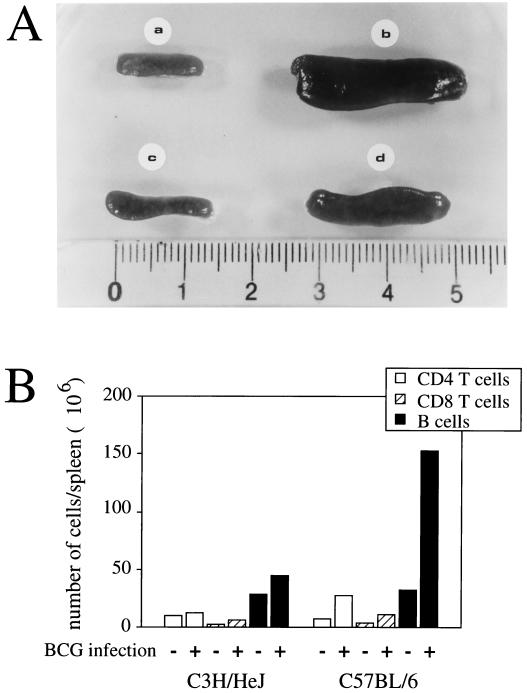
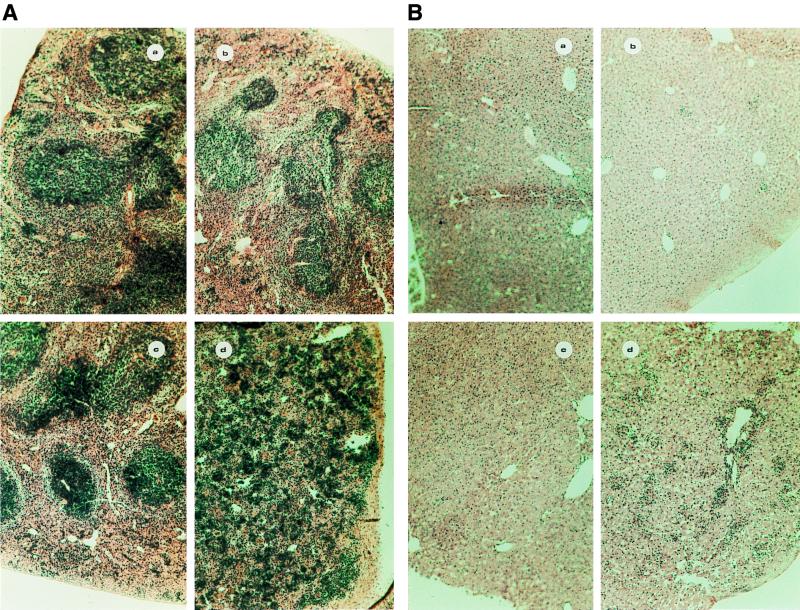
As shown in Fig. 1, infection of C57BL/6 and C3H/HeJ mice with BCG resulted in strong splenomegaly, which was more pronounced in C57BL/6 mice than in C3H/HeJ mice, as judged by macroscopic examination of the organs (Fig. 1A). This was confirmed by weighing the spleens of infected and uninfected animals. Although the spleens of the two mouse strains had similar weights when the animals were not infected (about 80 to 100 mg), infection with BCG resulted in an approximately threefold increase in the weight of C3H/HeJ spleens and in an eightfold increase in the weight of C57BL/6 spleens (data not shown). Similarly, in two additional mouse strains, BALB/c (bcgs) and DBA/2 (bcgr), BCG infection induced a splenomegaly more pronounced in BALB/c than in DBA/2 mice (data not shown). Subpopulation analysis indicated that BCG infection induced a major B-cell proliferation in the C57BL/6 mice, in contrast to C3H/HeJ mice (Fig. 1B). Moreover, the splenic architecture was largely disorganized in infected C57BL/6 mice, as assessed by histological analysis (Fig. 2A). Microscopic examination of the liver also revealed that infected C57BL/6 mice had a more pronounced cellular infiltration than did infected C3H/HeJ mice (Fig. 2B). These results suggest a more general immune activation associated with strong inflammation in infected susceptible mice than in infected resistant mice.
FIG. 1.
Splenomegaly and histological changes in spleens and livers of mice infected with BCG. Spleens were removed 2 weeks after intravenous infection with 5 × 106 CFU of BCG. (A) Spleens from uninfected C57BL/6 mice (a), BCG-infected C57BL/6 mice (b), uninfected C3H/HeJ mice (c), and BCG-infected C3H/HeJ mice (d). (B) Splenocyte subpopulations. Cell subpopulations were determined by combining the counting of absolute numbers of cells under the light microscope and the percentages of the subpopulations using FCM analysis. Results are from one of three independent experiments with similar results.
FIG. 2.
Histological analysis of the spleen (A) and liver (B) architecture in uninfected C3H/HeJ (a), BCG-infected C3H/HeJ (b), uninfected C57BL/6 (c), and BCG-infected C57BL/6 (d) mice. Transverse sections through the spleens and the livers were stained with hematoxylin. Magnification, ×100.
T-cell apoptosis related to susceptibility.
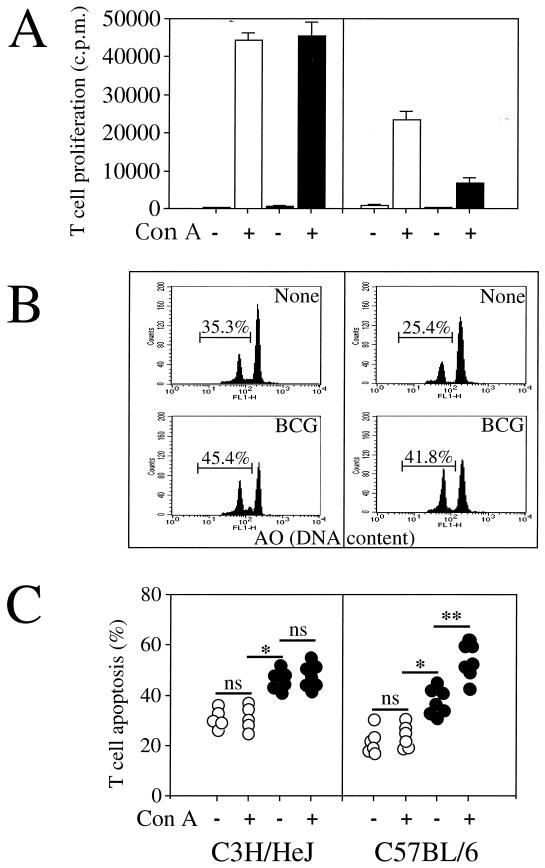
Defective T-cell proliferation during mycobacterium infection has been previously reported (42). The T-cell proliferation in the two mouse strains was therefore assessed. T cells were isolated by negative selection from the spleens of C57BL/6 and C3H/HeJ mice infected or not infected with 5 × 106 CFU of viable BCG. As shown in Fig. 3A, T cells from infected C3H/HeJ mice displayed similar polyclonal activation to those isolated from the uninfected mice. In contrast, the T-cell proliferation of C57BL/6 mice was considerably depressed after BCG infection.
FIG. 3.
The defect of T-cell proliferation in BCG-infected C57BL/6 mice is related to T-cell apoptosis. T cells were purified from spleens of uninfected (white bars) or BCG-infected (black bars) C3H/HeJ and C57BL/6 mice 2 weeks after intravenous infection with 5 × 106 CFU of BCG. The cells were cultured and restimulated in vitro in the absence (−) or presence (+) of ConA. (A) Proliferative T-cell responses were measured by [3H]thymidine incorporation. The data represent the means of values from pools of three mice assayed in triplicate. Results are from one of two independent experiments with similar results. (B) The percentage of spontaneous T-cell apoptosis from individual mice was measured by FCM analysis using acridine orange (AO) nuclear dye. T-cell apoptosis was also determined by light microscopy analysis (data not shown). Both determinations gave similar results, with less than a 5% difference between the two methods, as previously described (11). (C) T-cell apoptosis of uninfected animals is represented by the open circles, and T-cell apoptosis of BCG-infected animals is represented by the solid circles. Each symbol represents the result from an individual animal. Significant difference in spontaneous T-cell death between uninfected and infected mice is represented by ∗ (P < 0.05); significant difference in T-cell death between nonstimulated and activated T cells is represented by ∗∗ (P < 0.05). ns, not significant.
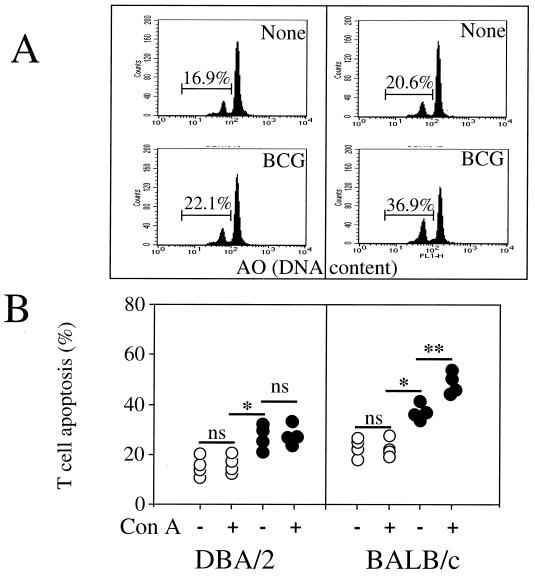
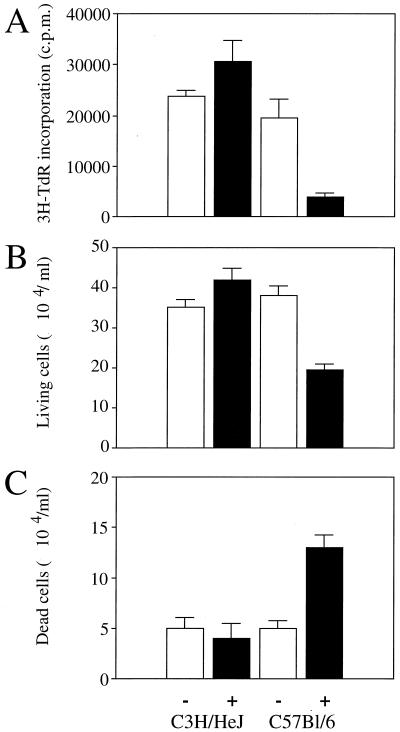
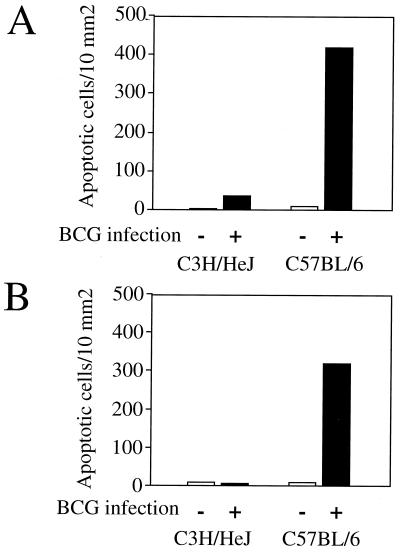
Since a defect of T-cell proliferation after infection with other pathogens has been reported to be related to T-cell death by apoptosis (1, 21, 29, 30), we investigated whether infection with BCG results in a more pronounced T-cell apoptosis in C57BL/6 mice than in C3H/HeJ mice. The percentage of apoptotic T cells from splenocytes of uninfected and BCG-infected mice was measured in the absence or presence of ConA for 18 h. T-cell death had the characteristic features of apoptosis, including typical nuclear chromatin condensation as visualised by light microscopy analysis (data not shown), and reduction in nuclear contents was assessed using acridine orange nuclear dye in an FCM analysis (Fig. 3B). For both mouse strains, BCG infection resulted in an increase in spontaneous T-cell apoptosis. In infected C3H/HeJ mice, the percentage of apoptotic T cells did not further increase after in vitro restimulation with ConA (Fig. 3C). In contrast, in BCG-infected C57BL/6 mice, stimulation with mitogen resulted in a substantial further increase in the number of T cells undergoing apoptosis. We further investigated two additional mouse strains, BALB/c (bcgs, Nramp1−) and DBA/2 (bcgr, Nramp1+), and in vitro analysis indicated that although BCG infection enhances spontaneous T-cell death (Fig. 4A), only T cells from BALB/c undergo apoptosis after T-cell stimulation (Fig. 4B). Moreover, stimulation of T cells using monoclonal antibody to the T-cell receptor–CD3 complex induced a decrease in thymidine incorporation (cell proliferation) (Fig. 5A), a decrease in T-cell numbers (viable cells) (Fig. 5B), and an increased incidence of cell death (Fig. 5C) after 3 days of culture in infected C57BL/6 mice in comparison to uninfected C57BL/6 mice and to both infected and uninfected C3H/HeJ mice. Together, these results suggest that the depressed T-cell proliferation observed above for BCG-infected C57BL/6 mice may be related to an increased susceptibility to apoptosis rather than to a process of anergy only. At 2 weeks after BCG infection, priming of a large fraction of splenic T cells for AICD was associated in vivo with numerous cells undergoing apoptosis in the spleen (Fig. 6A) and in liver infiltrates (Fig. 6B and C) in C57BL/6 mice in comparison to C3H/HeJ mice, as detected by in situ analysis of fragmented DNA using the TUNEL method.
FIG. 4.
T-cell apoptosis in BALB/c and DBA/2 mice. (A) The percentage of spontaneous T-cell apoptosis from individual mice was measured by FCM analysis using acridine orange nuclear dye. (B) T-cell apoptosis of uninfected animals is represented by the open circles, and T-cell apoptosis of BCG-infected animals is represented by the solid circles. Each symbol represents the result from an individual animal. Significant difference in spontaneous T-cell death between uninfected and infected mice is represented by ∗ (P < 0.05); significant difference in T-cell death between nonstimulated and activated T cells is represented by ∗∗ (P < 0.05). ns, not significant.
FIG. 5.
T cells from both uninfected (−) and BCG-infected (+) C3H/HeJ and C57BL/6 mice were cultured in the presence of anti-CD3 antibodies. (A) Lymphoproliferation was assessed after 3 days of activation with anti-CD3 antibodies, by measuring the incorporation of [3H]thymidine (3H-TdR). (B and C) Viable (B) and dead (C) T cells were counted under a light microscope after 72 h of in vitro activation. The data represent the means of values from pools of three animals and are representative of three independent experiments with similar results.
FIG. 6.
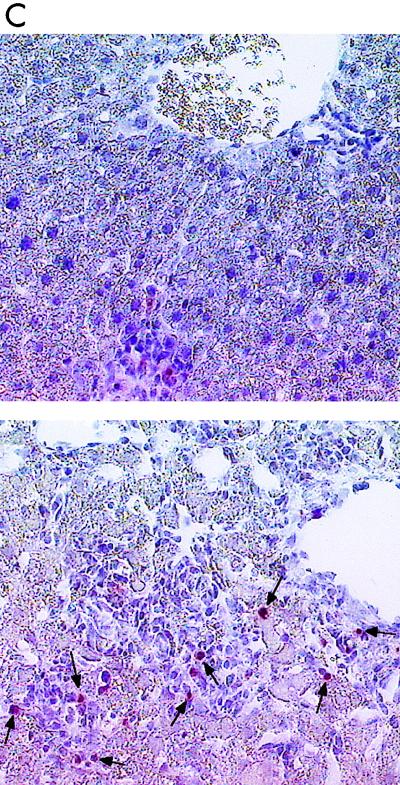
In vivo apoptosis. (A and B) Numbers of apoptotic cells in a paraffin sections of formalin-fixed spleens (A) and livers (B) from uninfected (−) mice and BCG-infected (+) mice. Tissue sections were examined by light microscope analysis after use of the TUNEL method. (C) Typical DNA fragmentation in the liver of infected C3H/HeJ mice (top) and C57BL/6 mice (bottom). Apoptotic cells are indicated by arrows. Magnification, ×200.
TNF-α production.
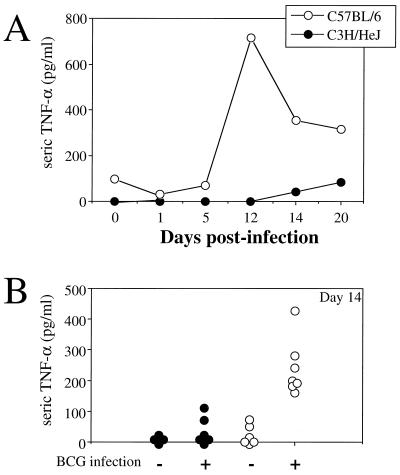
Next, we investigated whether the C3H/HeJ and C57BL/6 mouse strains differ in TNF-α production after BCG infection. The kinetics of TNF-α secretion from serum were assessed by ELISA from a pool of four sera (Fig. 7A) and from individual mice (Fig. 7B). High levels of TNF-α were detected only in the serum of C57BL/6 mice infected with BCG (Fig. 7). TNF-α production increased progressively, peaked 12 days after infection, and then started to decline. Only small amounts of TNF-α were detected in C3H/HeJ mice infected with BCG, and no TNF-α was detected in uninfected animals of either strain.
FIG. 7.
Kinetics of TNF-α production during BCG infection. (A) Pools of sera from four C3H/HeJ or C57BL/6 mice collected at several time points during the course of BCG infection were measured by ELISA. (B) Sera from individual mice were analyzed on day 14 postinfection. Each symbol represents the result from an individual animal.
Fas-FasL expression.
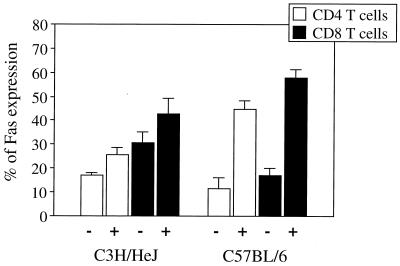
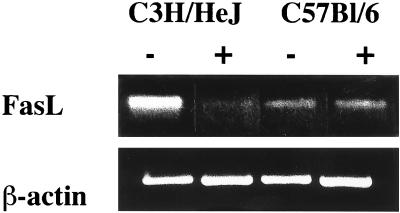
Fas expression was assessed by FCM on splenocytes isolated from infected and uninfected C3H/HeJ and C57BL/6 mice. Total splenocytes from BCG-infected C3H/HeJ mice expressed slightly higher levels of Fas than did splenocytes of uninfected C3H/HeJ mice, whereas BCG infection of C57BL/6 mice enhanced Fas expression much more strongly (data not shown). Analysis of T-cell subpopulations revealed that increased Fas expression was detected in both CD4+ and CD8+ T-cell populations 2 weeks after infection with BCG (Fig. 8). Again, the difference between infected and uninfected mice was much more pronounced for the C57BL/6 strain than for the C3H/HeJ strain. To investigate whether the controlling events of apoptosis involve changes in FasL expression, RT-PCR was performed on total RNA from T cells of both infected and uninfected mice. Figure 9 shows that FasL mRNA was similar in uninfected and in BCG-infected C57BL/6 mice. However, FasL expression, which was high in uninfected C3H/HeJ mice, decreased upon infection.
FIG. 8.
Analysis of Fas expression. Fas expression on CD4+ T cells and CD8+ T cells from spleens of C3H/HeJ and C57BL/6 mice was assessed by FCM using Cy-chrome-conjugated anti-CD4 and anti-CD8 and FITC-labelled anti-Fas antibodies prior to (−) or 2 weeks after (+) intravenous infection with 5 × 106 CFU of BCG. Data are expressed as the means and standard deviations from three individual animals per group. Results are from one of three independent experiments with similar results.
FIG. 9.
FasL mRNA expression. Total RNA was extracted from purified T cells of C3H/HeJ and C57BL/6 mice prior to (−) or 2 weeks after (+) intravenous infection with 5 × 106 CFU of BCG. RT-PCR was performed on pools of mRNA from three animals for each group, using specific primers for FasL, and β-actin was used as a control. Results are from one of two independent experiments with similar results.
Involvement of Fas and TNF-α in activation-induced T-cell death in BCG-infected C57BL/6 mice.
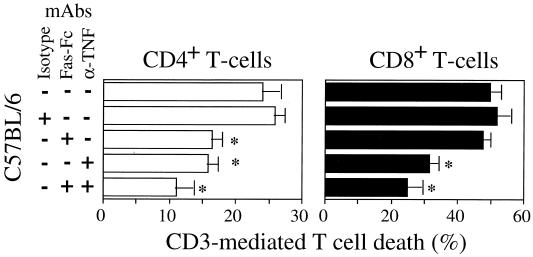
Since both TNF-α and Fas were expressed at higher levels in infected C57BL/6 mice than in infected C3H/HeJ mice, we examined whether these factors participate in the regulation of activation-induced T-cell death. The contribution of TNF-α and FasL to AICD following BCG infection was determined by using purified T cells incubated for 18 h in the presence or absence of neutralizing anti-TNF-α antibodies and of antagonistic Fas antibodies (Fas-Fc protein). T-cell depletion was assessed by FCM using FITC-conjugated annexin V labeling that detects apoptotic cells in each of the T-cell subpopulations studied. The addition of Fas-Fc prevented the death of CD4+ T cells from infected C57BL/6 mice, whereas apoptosis of the CD8+ T cells from these mice remained unaffected (Fig. 10). The anti-TNF-α antibodies inhibited apoptosis of both CD8+ T cells and CD4+ T cells from the infected C57BL/6 mice. Together, these results suggest that TNF-α and FasL participate in the control of T-cell apoptosis.
FIG. 10.
Inhibition of AICD in BCG-infected C57BL/6 mice. T cells from BCG-infected C57BL/6 mice were incubated in the presence of anti-CD3 antibodies and treated in the absence (−) or presence (+) of neutralizing anti-TNF-α antibody and Fas-Fc hybrid protein, either alone or combined, and of isotype antibody control. After 18 h of in vitro activation, CD4+ and CD8+ T-cell depletions were determined by FCM using FITC-conjugated annexin V in comparison to unstimulated T cells (specific spontaneous CD4+ and CD8+ T-cell death was 9.5 and 18%, respectively). Significance differences are marked by ∗ (P < 0.05). The data represent the means of values from three independent experiments. mAbs, monoclonal antibodies.
DISCUSSION
In this study, we show that BCG susceptibility is associated with T-cell apoptosis. Our data suggest that in susceptible mouse strains (C57BL/6 and BALB/c) a defect in in vitro T-cell proliferation after mitogen or TCR restimulation involves apoptosis and that this depressed cell-mediated immunity (CMI) was not observed in BCG-resistant mouse strains (C3H/HeJ and DBA/2). In addition, further investigations with C57BL/6 and C3H/HeJ mice suggest that activation-mediated T-cell apoptosis in infected C57BL/6 mice involved mainly TNF-α and Fas-FasL.
T-cell apoptosis is considered to be an important regulatory mechanism of the immune response and is involved in the loss of effector functions during infectious diseases. Recent studies reported that infection with the protozoan parasites Trypanosoma cruzi and Toxoplasma gondii leads to a downregulation of the CMI associated with the induction of CD4+ T-cell apoptosis (21, 30). In Schistosoma mansoni-infected mice, the immunopathologic granulomatous response to parasite eggs, which is associated with a global functional defect in CMI, is also related to a CD4+ and CD8+ T-cell apoptotic process (1, 14). Xu et al. (54) have reported a drastic depletion of CD3+ T cells in susceptible but not in resistant mice at the final stage of infection with Mycobacterium avium. During the preparation of our manuscript, Das et al. (8) reported that M. tuberculosis infection of a susceptible host results in the abnormal death of CD4+ T cells after in vitro stimulation. Together with our observations, these results suggest that mycobacteria are able to trigger death by apoptosis. Nevertheless, no apparent net depletion in T lymphocytes was observed in susceptible mice, suggesting a compensatory reconstitution of dying cells, with a T-cell turnover. Such turnover of T cells in the absence of depletion has been reported to occur in CD8+ T cells during the asymptomatic phase of HIV infection in humans and in nonhuman primates (39, 41, 53). The Nramp1 gene product controls the innate resistance and susceptibility of macrophages to microorganisms (15, 51). Although Nramp1 has been clearly associated in the control of M. bovis, less evidence for a role in the control of M. tuberculosis and M. avium has been demonstrated (33). Our observation suggest that at least in two resistant and two susceptible mouse strains, a segregation between the Nramp1 gene and apoptosis is observed.
The expression of a family of ligands (TNF-α and FasL) and receptors (TNF receptor and Fas), as well as the induction of susceptibility of these cells to receptor-initiated signals, has been involved in the process of T-cell apoptosis (35). We suggested a pivotal role for TNF-α during BCG infection of C57BL/6 mice. First, our observation indicates an important TNF-α production in the sera of infected C57BL/6 mice but not of C3H/HeJ mice; second, neutralizing antibodies to TNF-α prevent CD3-mediated CD4+ and CD8+ T-cell apoptosis. Moreover, this TNF-α production coincides with an important inflammatory response characterized by intense cellular infiltrates in the livers of these mice and destruction of the architecture of the spleen. The TUNEL method revealed the presence of a large number of apoptotic cells in the liver infiltrates in C57BL/6 mice compared to the number in C3H/HeJ mice. Kindler et al. (22) have shown that injection of anti-TNF-α antibodies into mice infected with BCG strongly interferes with the development of granulomas and that TNF-α is central in the formation of BCG-induced granulomas. Doherty and Sher (9) have also recently shown that 2 weeks after infection with M. avium the mitogen response of C57BL/6 mice was clearly suppressed compared to that of uninfected mice. Using TNF-α receptor-deficient mice, they showed the importance of TNF-α in this immunosuppression. Additionally, the splenomegaly of these knockout mice was greatly diminished. Although immunopathology was strongly reduced in these mice, no increase in the control of M. avium bacterial growth was observed (9). Nevertheless, a recent report by Ehlers et al. (10) indicated, in contrast, that TNF receptor p55 gene-deficient mice develop granulomas that become necrotic and cause tissue damage following M. avium infection. Thus, apoptosis would be involved in restricting rather than exacerbating the inflammatory response. Therefore, whether TNF-α-mediated apoptosis during BCG or other mycobacterial infections in susceptible hosts only participates in immunopathology or alters the effectiveness of immune-mediated control of the bacterial load remains to be assessed. In tuberculosis patients, blood monocytes (46) and alveolar macrophages (40) release TNF-α in large quantities, and this cytokine is also present in tuberculous lesions (5). Recent data indicate that enhanced levels of apoptosis occur during active M. tuberculosis infection in human (16). Thus, spontaneous apoptosis and AICD are increased in both CD4+ and CD8+ T cells of newly diagnosed tuberculosis-infected persons over those in healthy subjects. Culture supernatants show the presence of abnormal levels of TNF-α and soluble Fas molecules in these patients.
Our results demonstrate that BCG infection increases the proportions of both CD4+ and CD8+ T cells expressing Fas in both C57BL/6 and C3H/HeJ mouse strains, compared to uninfected mice. However, the percentage of T cells, and in particular of CD4+ T cells, expressing Fas was significantly higher in C57BL/6 than in C3H/HeJ mice. We also found that level of FasL mRNA was not affected upon BCG infection in C57BL/6 mice whereas the level of FasL mRNA, which was more abundant in the uninfected C3H/HeJ mice than in the uninfected C57BL/6 mice, decreased drastically upon BCG infection in this mouse strain. However, our data indicate that Fas-FasL participates in AICD of CD4+ T cells but not in that of CD8+ T cells in C57BL/6 mice. At this time, we cannot generalize those mechanisms to all mouse strains. Although we observed that C57BL/6 and BALB/c mice are equaly susceptible to AICD when infected with BCG, it should be noted that these two strains of mice differ in their resistance and susceptibility to leishmania, being considered Th1 and Th2 models of the immune response to infection, respectively (28). Therefore, it is possible that in the context of BCG infection, several mechanisms in addition to TNF-α and FasL may also operate in different mouse strains involving certain cytokines like TGF-β and IL-10, which have been reported to participate in AICD in other models of infection (12–14). Moreover, although both TNF-α- and Fas-mediated apoptosis contribute to the death of T lymphocytes in C57BL/6 mice, additional mechanisms could also participate in T-cell apoptosis. The in vitro prevention of T-cell apoptosis using neutralizing antibodies to TNF-α and Fas was not complete, suggesting the potential involvement of other pathways of T-cell depletion. Other recently described ligands, like the TNF-related apoptosis-inducing ligand (TRAIL), a new member of the TNF family which has been recently shown to trigger AICD in HIV-infected individuals, could play a role (20). However, whether TRAIL or any other death factor is involved in T-cell death during mycobacterial infections requires further investigation.
The precise mechanisms by which mycobacteria induce T-cell apoptosis and the bacterial components responsible remain to be elucidated. Ozeki et al. (37) have demonstrated that the mycobacterial cord factor induces apoptosis in the mouse thymus in vivo. However, nothing is known about the potential involvement of cord factor in the induction of apoptosis in mature T cells. Evidence has also emerged suggesting that monocyte-derived macrophages acquire the ability to selectively induce apoptosis of T cells in an activation-specific fashion (34). Badley et al. (2, 3) have reported that upregulation of FasL expression by HIV in human macrophages mediates apoptosis of uninfected T lymphocytes and may therefore participate in T-cell depletion in HIV-infected individuals. Since mycobacteria are able to invade phagocytic cells but not lymphocytes, it is tempting to postulate a similar scenario for mycobacterial infections. In this regard, it has been previously reported that both whole organisms and antigens derived from M. bovis and M. tuberculosis induced the production of TNF-α from monocytes (4, 49, 52, 55). Thus, activated macrophages could then provide an important source of TNF-α and/or FasL, which may participate in T-cell depletion and therefore in immunosuppression. However, the role of Fas-FasL in host-pathogen interactions may be complex, since recent data have shown that resolution of lesions induced by Leishmania major in mice was dependent on a functional Fas pathway that was responsible for the apoptosis of infected macrophages (6).
Finally, taken together, our results suggest that induction of apoptosis in both CD4+ and CD8+ T-cell subsets may contribute to mycobacterium-mediated immune dysregulation in susceptible hosts.
ACKNOWLEDGMENTS
This work was supported by Institut Pasteur de Lille, INSERM, Région Nord-Pas de Calais, and the EC Biotech program. J.E. was supported by the Human Science Frontier Program and ANRS.
L.K. and J.E. contributed equally to this work.
REFERENCES
- 1.Ameisen J-C, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. doi: 10.1111/j.1600-065x.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 2.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes P, Chatterjee D, Abrams J, Lu S, Wang E, Yamamura M, Brennan P, Modlin R. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 5.Barnes P F, Fong S J, Brennan P J, Twomey P E, Mazumder A, Modlin R L. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 6.Conceicao-Silva F, Hahne M, Schröter M, Louis L, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol. 1998;28:237–245. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Daniel T M, Oxtoby M J, Pinto E, Moreino S. The immune spectrum in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1981;123:556–559. doi: 10.1164/arrd.1981.123.5.556. [DOI] [PubMed] [Google Scholar]
- 8.Das G, Vohra H, Saha B, Agrewala J N, Mishra G C. Apoptosis of Th1-like cells in experimental tuberculosis (TB) Clin Exp Immunol. 1999;115:324–328. doi: 10.1046/j.1365-2249.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 10.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel E, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estaquier J, Idziorek T, De Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen J-C. Programmed cell death and AIDS: the significance of T-cell apoptosis in pathogenic and non pathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estaquier J, Idziorek T, Zou W, Emilie D, Farber C M, Bourez J M, Ameisen J-C. T helper type 1/T helper type 2 cytokines and T-cell death: preventive effect of IL-12 on activation-induced and CD95 (Fas/Apo-1)-mediated apoptosis of CD4+ T cells from HIV-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen J-C. Fas-mediated apoptosis of CD4+ and CD8+ T cells from HIV-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- 14.Estaquier J, Marguerite M, Sahuc F, Bessis N, Auriault C, Ameisen J-C. Interleukin-10-mediated T cell apoptosis during the T helper type 2 cytokine response in murine Schistosoma mansoni parasite infection. Eur Cytokine Netw. 1997;8:153–160. [PubMed] [Google Scholar]
- 15.Govoni G, Gros P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch C S, Toossi Z, Vanham G, Johnson J L, Peters P, Okwera A, Mugerwa R, Mugyenyi P, Ellner J J. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–953. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 17.Holden M, Dubin M R, Diamond P H. Frequency of negative intermediate-strength tuberculin sensitivity in patients with active tuberculosis. N Engl J Med. 1971;285:1506–1509. doi: 10.1056/NEJM197112302852704. [DOI] [PubMed] [Google Scholar]
- 18.Kaledin V I, Kurunov Y N, Serova I A. Inhibition and stimulation of the growth of Krebs-2 carcinoma by BCG vaccine. J Natl Cancer Inst. 1977;58:1271–1277. doi: 10.1093/jnci/58.5.1271. [DOI] [PubMed] [Google Scholar]
- 19.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in HIV-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A. Interleukin-1β converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan I A, Matsura T, Kasper L H. Activation-mediated CD4+ T-cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 22.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassali P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 23.Klas C, Debatin K M, Jonker R R, Krammer P H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 24.Kleinhenz M E, Ellner J J. Antigen responsiveness during tuberculosis: regulatory interaction of T-cell subpopulations and adherent cells. J Lab Clin Med. 1987;110:31–40. [PubMed] [Google Scholar]
- 25.Kremer L, Estaquier J, Brandt E, Ameisen J-C, Locht C. Mycobacterium bovis bacillus Calmette Guérin infection prevents apoptosis of resting human monocytes. Eur J Immunol. 1997;27:2450–2456. doi: 10.1002/eji.1830270945. [DOI] [PubMed] [Google Scholar]
- 26.Ladel C H, Daugelat S, Kaufmann S H E. Immune response to Mycobacterium bovis Calmette Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 27.Lagrange P H, Hurtrel B. The influence of BCG vaccination on murine leprosy in C57BL/6 and C3H mice. Ann Immunol (Paris) 1979;130C:687–709. [PubMed] [Google Scholar]
- 28.Launois P, Tacchini-Cottier F, Parra-Lopez C, Louis J A. Cytokines in parasitic diseases: the example of cutaneous leishmaniasis. Int Rev Immunol. 1998;17:157–180. doi: 10.3109/08830189809084491. [DOI] [PubMed] [Google Scholar]
- 29.Liesenfeld O, Kosek J C, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun. 1997;65:4682–4689. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes M F, da Veiga V F, Santos A R, Fonseca M E, Dos-Reis G A. Activation-induced CD4+ T-cell death by apoptosis in experimental Chagas' disease. J Immunol. 1995;154:744–752. [PubMed] [Google Scholar]
- 31.Lynch D, Ramsdell H F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;12:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 32.Masen U G, III, Greenberg L E, Yen S S, Kirkpatrick C H. Indomethacin-responsive mononuclear cell dysfunction in “atypical” mycobacteriosis. Cell Immunol. 1982;71:54–65. doi: 10.1016/0008-8749(82)90495-6. [DOI] [PubMed] [Google Scholar]
- 33.Medina E, North R. Evidence inconsistent with a role for the BCG gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J Exp Med. 1996;183:1045–1051. doi: 10.1084/jem.183.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munn D H, Pressey J, Beall A C, Hudes R, Alderson M R. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. J Immunol. 1996;156:523–532. [PubMed] [Google Scholar]
- 35.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 36.Orme I M, Andersen P, Boon W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 37.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose-6,6′-dimycolate) Infect Immun. 1997;65:1793–1799. doi: 10.1128/iai.65.5.1793-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier M, Forget A, Bourassa D, Gros P, Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982;129:2179–2185. [PubMed] [Google Scholar]
- 39.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 40.Rook G A W, Al Attiyah R. Cytokines and the Koch phenomenon. Tubercle. 1991;72:13–20. doi: 10.1016/0041-3879(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 41.Sachsenberg N, Perelson A S, Yerly S, Schockmel G A, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha B, Das G, Vohra H, Ganguly N K, Mishra G C. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–2624. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 43.Sauton M B. Sur la nutrition minérale du bacille tuberculeux. C R Acad Sci. 1912;155:860–861. [Google Scholar]
- 44.Skamene E, Gros P, Forget A, Kongshavn P A, St. Charles C, Taylor B A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982;297:506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- 45.Sytwu H-K, Liblau R S, McDewitt H O. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 46.Takashima T, Ueta C, Tsuyuguchi I, Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 48.Toossi Z, Kleinhenz M E, Ellner J J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valone S, Rich E, Wallis R, Ellner J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988;56:3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 51.Vidal S, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: identification of a candidate gene for BCG. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 52.Wallis R, Paranjape R, Philips M. Identification by two dimensional gel electrophoresis of a 58-kilodalton tumor necrosis factor-inducing protein of Mycobacterium tuberculosis. Infect Immun. 1993;61:627–633. doi: 10.1128/iai.61.2.627-632.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolthers K C, Bea G, Wisman A, Otto S A, de Roda Husman A M, Schaft N, de Wolf F, Goudsmit J, Coutinho R A, van der Zee A G, Meyaard L, Miedema F. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 1996;274:1543–1547. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 54.Xu D L, Goto Y, Amoako K K, Nagatomo T, Uchida K, Shinjo T. Immune responsiveness in Mycobacterium avium-infected mice: changes in the proportion of T cell subsets and antibody production during the course of infection. Clin Exp Immunol. 1995;102:523–528. doi: 10.1111/j.1365-2249.1995.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Doerfler M, Theodore C, Guillemein B, Rom W. Mechanisms of stimulation of interleukin-1β and tumor necrosis factor-α by mycobacterium tuberculosis components. J Clin Investig. 1993;91:2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]