Objective:
To compare pancreaticoduodenectomy (PD) and total pancreatectomy (TP) with islet autotransplantation (IAT) in patients at high risk of postoperative pancreatic fistula (POPF).
Background:
Criteria to predict the risk of POPF occurrence after PD are available. However, even when a high risk of POPF is predicted, TP is not currently accepted as an alternative to PD, because of its severe consequences on glycaemic control. Combining IAT with TP may mitigate such consequences.
Methods:
Randomized, open-label, controlled, bicentric trial (NCT01346098). Candidates for PD at high-risk pancreatic anastomosis (ie, soft pancreas and duct diameter ≤3 mm) were randomly assigned (1:1) to undergo either PD or TP-IAT. The primary endpoint was the incidence of complications within 90 days after surgery.
Results:
Between 2010 and 2019, 61 patients were assigned to PD (n=31) or TP-IAT (n=30). In the intention-to-treat analysis, morbidity rate was 90·3% after PD and 60% after TP-IAT (P=0.008). According to complications’ severity, PD was associated with an increased risk of grade ≥2 [odds ratio (OR)=7.64 (95% CI: 1.35–43.3), P=0.022], while the OR for grade ≥3 complications was 2.82 (95% CI: 0.86–9.24, P=0.086). After TP-IAT, the postoperative stay was shorter [median: 10.5 vs 16.0 days; P<0.001). No differences were observed in disease-free survival, site of recurrence, disease-specific survival, and overall survival. TP-IAT was associated with a higher risk of diabetes [hazard ratio=9.1 (95% CI: 3.76–21.9), P<0.0001], but most patients maintained good metabolic control and showed sustained C-peptide production over time.
Conclusions:
TP-IAT may become the standard treatment in candidates for PD, when a high risk of POPF is predicted.
Keywords: islet autotransplantation, pancreatic cancer, pancreaticoduodenectomy, pancreoprivic diabetes, total pancreatectomy
Pancreatic leakage is the main cause of morbidity after pancreaticoduodenectomy (PD), with an incidence of clinically relevant postoperative pancreatic fistula (CR-POPF)1 ranging from 10% to 33%.2 Postoperative pancreatic fistula (POPF)-associated complications may impact both short-term and long-term outcomes: longer hospital stay, delayed recovery, hemorrhage, and sepsis, as well as worse oncologic outcome in patients with malignancy, has been associated with POPF.3 Total pancreatectomy (TP) eliminates any risk of POPF, but it is not currently considered an alternative to PD, due to the quality of life (QOL) deterioration associated with lifelong pancreatic exocrine and endocrine insufficiency (“brittle” diabetes).4,5 Due to the availability of long-acting basal insulin,6 modern enzyme preparations7 and to centralization of surgery at high-volume centers,8 perioperative outcomes, postoperative therapies, and QOL after TP have progressively improved during the time.8 On this basis, TP has been suggested as an alternative to PD in selected cases.9,10 The possibility to perform TP followed by islet autotransplantation (IAT) can further support this approach.11 Endocrine islets can be isolated with standardized procedures from the “healthy” pancreatic portion and transplanted without requiring immunosuppression, facilitating glucose metabolism control with a reduced need for exogenous insulin.12 TP-IAT is generally performed in patients with painful chronic pancreatitis, according to the so-called “Minnesota criteria.”13 Starting from 2008 we applied IAT to patients with other diseases of the pancreas, including patients undergoing resection for pancreatic or periampullary malignancy, according to the so-called “Milan Protocol.”14 The first results about the feasibility, efficacy, and safety of IAT for a broader spectrum of indications,12 allowed us to hypothesize that TP-IAT rather than PD may be used in selected cases. This study addresses for the first time the role for TP-IAT in patients undergoing PD at high risk for POPF.
METHODS
Study Design and Patients
This study was conducted to test the hypothesis that TP-IAT is associated with lower morbidity compared with PD and pancreaticojejunostomy in patients with both soft pancreas and small pancreatic duct (≤3 mm). We designed a prospective, parallel assignment, randomized study (NCT01346098) conducted at 2 Italian centers (San Raffaele Hospital, Milan; Humanitas Research Hospital, Rozzano), that was approved by the Ethics Committees of the 2 institutions. The study started as monocentric at San Raffaele Hospital but it became bicentric (from July 2013), as part of the original surgical team moved to Humanitas Research Hospital. From October 2010 to September 2019, patients scheduled for PD were screened for eligibility. Patients aged 18 years and above, with fasting glycemia <126 mg/dL without glucose-lowering medications and duct diameter ≤3 mm on computed tomography scan were considered eligible for the study. Patients were not eligible in case of multifocal neoplasia (including multifocal intraductal papillary-mucinous neoplasm and/or main duct dilation affecting the distal pancreas) or positive resection margin at frozen section examination. To better assess multifocality, all patients underwent endoscopic ultrasonography before surgery.
Study Treatment and Randomization
During PD, 1 of the 2 senior surgeons (G.B. and A.Z., both with large experience in pancreatic surgery) manually assessed the pancreas consistency and measured the main pancreatic duct size by inserting round-tip catheters of different sizes. After confirming soft pancreas consistency and duct diameter ≤3 mm, the patient was randomly assigned either to PD with pancreaticojejunostomy (group A) or TP-IAT (group B). The 2 participating centers competed for enrollment and randomization was managed by an independent randomization manager (C.M.). The treatment allocation was performed by minimization, aimed to balance the 2 groups for age, sex, malignant versus nonmalignant pancreatic disease, body mass index, and American Society of Anesthesiologists score. A protocol for enhanced recovery after surgery was applied.15 Surgery was accomplished as follows:
Group A (PD): an end-to-side 2-layer duct-to-mucosa pancreaticojejunostomy was performed. Two passive silicon drains were applied. Group B (TP-IAT): the surgeon completed TP aiming to preserve the spleen, along with splenic vessels. The pancreatic body-tail was sent to the islet isolation facility, where islets were obtained according to the method previously described.16,17 The purified islets were transplanted via infusion into the portal vein, either intraoperatively, or by percutaneous transhepatic portal vein infusion within 48 hours from surgery. In patients in whom portal infusion was contraindicated, islets were infused in the bone marrow at the level of the iliac crest, as previously described.18 Patients in both groups were discharged based on the following criteria: absence of fever, adequate pain control with oral analgesics, ability to take solid foods (at least 1000 kcal/d), the passage of stools, and adequate mobilization. Pancreatic enzyme supplementation was routinely prescribed in both groups. The dosage was modified according to the frequency of bowel movements, clinical steatorrhea, and weight loss.
Follow-up and Endpoints
All patients were followed for 12 months after surgery or until death. Patients were seen in the outpatient clinics at months 1, 3, 6, and 12. After 12 months, overall survival, disease-specific survival, disease-free survival, diabetes-free survival, and insulin-free survival were recorded. The primary endpoint of the study was the incidence of complications within 90 days after surgery. Complications were graded according to the Clavien-Dindo classification.19 The overall complication rate is reported as the number of patients with at least 1 complication. Secondary endpoints of the study were: (i) incidence of specific complications; (ii) length of hospital stay; (iii) unplanned readmission within 90 days; (iv) incidence of endocrine and exocrine pancreatic insufficiency; (v) overall survival, disease-specific survival, and disease-free survival. POPF was defined according to the International Study Group on Pancreatic Surgery (ISGPS).1 Delayed gastric emptying and postpancreatectomy hemorrhage were defined according to ISGPS.20,21 Endocrine function was assessed by fasting plasma glucose, fasting C-peptide, insulin requirement, and glycated hemoglobin (HbA1c). In a subgroup of patients, C-peptide values after intravenous arginine stimulation was also evaluated. Diabetes was diagnosed and treated according to the American Diabetes Association recommendations.22 Severe hypoglycaemic episodes were defined according to the American Diabetes Association Workgroup on Hypoglycemia23 and were recorded from day 75 to day 365. Pancreatic exocrine insufficiency was assessed by evaluating symptoms of fat malabsorption, such as steatorrhea, weight loss, abdominal pain, and frequency of bowel movements. Fat-soluble vitamins such as A, D, and E were measured in a subgroup. The data collectors, outcome assessors, and data analysts were blinded to patients’ allocation.
Sample Size and Statistical Analysis
The sample size was calculated a priori on the primary endpoint. There were no data available to estimate the effect size of TP-IAT on the morbidity rate of the study population. As the overall morbidity rate in prospective studies about PD was reported around 60%24–26 and assuming a 50% increase due to the high-risk pancreatic remnant,1,27 we expected a 90% overall morbidity rate in group A. With these assumptions, 30 patients for each group were planned to be included, to provide 80% power to detect a drop from 90% to 60% (α=0.05) in the overall morbidity rate. Data are presented as mean±SD or median (25th–75th percentiles), according to their distribution. The primary endpoint, as all the categorical variables, was compared using the χ2 test or Fisher exact test as appropriate. Variables with a normal distribution were compared with the unpaired Student t test. Variables with a non-normal distribution were compared with the Mann-Whitney U test. Survival was estimated according to Kaplan-Meier. Hazard ratio (HR) and odds ratio (OR) adjusted for age and sex were calculated using Cox proportional hazard and logistic regression, respectively. Two-tailed P values are reported, with P value <0.05 indicating statistical significance. All confidence intervals are 2-sided and not adjusted for multiple testing. Statistical analyses were performed with SPSS 24 (SPSS Inc./IBM) and GraphPad Prism, version 5.04.
RESULTS
Patient Disposition and Baseline Characteristics
Eighty-nine patients undergoing PD considered at high-risk for POPF were enrolled from October 2010 to September 2019. Twenty-eight patients (31.4%) were excluded at surgery: 18 did not meet the criteria for high-risk anastomosis (no soft pancreas: n=14, duct diameter >3 mm: n=4); 6 showed locally advanced or metastatic disease; 4 did not meet other protocol inclusion/exclusion criteria (prohibited medications n=2; disease involvement of the pancreatic remnant with indication to TP: n=2). Sixty-one patients underwent randomization and were included in the intention-to-treat (ITT) analysis: 31 were allocated to PD (group A), 30 were allocated to TP-IAT (group B). Fifty-seven patients out of the 61 randomized (93.4%) complied with the trial protocol and were included in the per-protocol (PP) analysis. Four subjects withdrew from the study: 3 subjects in group A underwent completion pancreatectomy for technical problems during fashioning of pancreatic anastomosis (2 of 3 received IAT as rescue therapy), and 1 patient in group B did not receive IAT due to postoperative liver hypoperfusion. The 18 patients intraoperatively excluded because they lack the high-risk criteria were, however, followed and included in post hoc analyses (group C). Patients’ inclusion in analysis sets is shown in Supplementary Figure 1 (Supplemental Digital Content 1, http://links.lww.com/SLA/E272). Patients’ characteristics are reported in Table 1. No difference was detected between treatment groups for any demographic and baseline values, apart from a higher age in group B (P=0.004). Fifty of 61 randomized patients (82%) completed day 365 visit after surgery (n=9 died before day 365, n=2 were lost to follow-up). The oncological follow-up was assessed for patients with pancreatic or periampullary adenocarcinoma (24 of 31 in group A and 21 of 30 in group B) (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E272).
TABLE 1.
Characteristics of Patients
| Mean±SD/Median (25th–75th percentile) | |||
|---|---|---|---|
| Group A: Pancreatoduodenectomy with high-risk pancreatic anastomosis | Group B: TP+IAT | Group C: Pancreatoduodenectomy with anastomosis at lower risk of mortality | |
| N | 31 | 30 | 18 |
| Center 1 [n (%)] | 13 (41.9) | 12 (40) | 15 (83.3) |
| Age (y) | 62±11 | 69±7.8 | 66±11 |
| Sex (male/female) | 20/11 | 19/11 | 13/5 |
| Weight (kg) | 74.7±13.3 | 72.6±12 | 72.8±23.8 |
| BMI (kg/m2) | 25.25±4 | 26.2±3.6 | 25.27±7.9 |
| ASA score: 1/2/3/4 (%) | 9.7/64.5/25.8/0 | 10/70/16.7/3.3 | 5.6/83.3/11.1/0 |
| EGFR (mL/min/1.73 m2) | 99.4 (84.1–115.1) | 89.5 (77.6–112.6) | 86 (76.9–112.8) |
| Glucose (mg/dL) | 98.7±18 | 97.4±15.2 | 103.11±32 |
| HbA1c (%) | 5.25±0.71 | 5.41±0.6 | 5.49±0.75 |
| Insulin (mU/mL) | 10.4 (5.6–16.2) | 10.1 (5.6–18.4) | 7.4 (5.5–10.8) |
| C-peptide (ng/mL) | 3.08 (1.9–5.45) | 3.4 (2.38–4.8) | 2.05 (1.66–4.33) |
| HOMA2-IR | 1.72 (1.04–2.96) | 1.9 (1.05–3.41) | 1.06 (0.96–1.41) |
| HOMA2-%B | 131 (108–189) | 156 (125–183) | 147 (85–234) |
| White blood cell (×109/L) | 6.46 (5.67–8) | 6.97 (5.88–7.83) | 8.4 (6.5–10.2) |
| Hemoglobin (g/dL) | 13 (11.9–13.9) | 12.75 (11.17–13.6) | 12.7 (11.15–13.7) |
| Total bilirubin | 1.3 (0.57–2.55) | 1.66 (0.6–8.48) | 1.53 (0.94–4.79) |
ASA indicates American Society of Anesthesiologists; BMI, body mass index; EGFR, estimated glomerular filtration rate; HOMA Homeostatic Model Assessment; IR, Insulin Resistance; %B, beta cell function.
Islet Transplantation
All islet preparations were considered adequate for transplantation (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E272). Of the 29 transplanted patients, 4 received fresh islet (13.8%) and 25 cultured islets (86.2%; median time of culture 15 hours). Patients received a median of 1.863 islet equivalent/kg (1.410–2.285). The volume of islet tissue infused was 1.5 mL (1–2.5) with a 45% (28.7–70) purification. The site for transplantation was the liver in 27 (93.1%) and bone marrow in 2 recipients (6.9%). Portal vein pressure change after the infusion was irrelevant [median: Δ portal vein pressure: 0 (0–1) cm H2O]. Procedure-related complications occurred in 5 patients (17.2%). Portal vein thrombosis occurred in 1 patient, successfully treated with anticoagulation therapy. Four subjects had bleeding related to the transhepatic access: in 3 cases only minor bleeding occurred, requiring no intervention (ultrasound-detected), while 1 case resulted in hemothorax, requiring transfusion and surgery.
Efficacy Outcomes
A total of 119 complications were observed in 61 patients (Table 2). In ITT analysis, 28 patients (90.3%) in group A and 18 (60%) in group B reported at least 1 complication [P=0.008; Fig. 1; OR=4.54 (1.07–19.3), P=0.04, Table 3]. Twenty-three patients in group A (74.2%) and 11 patients in group B (36.7%; P=0.0078) had >1 complication. Median number of complications per patient was 2 (1–4) in group A and 1 (0–2.25) in group B [P=0.004, Fig. 1; OR=4.32 (1.36–13.7), P=0.013, Table 3]. Details of complications, intraoperative, and postoperative data are shown in Table 3 and Supplementary Table 3 (Supplemental Digital Content 1, http://links.lww.com/SLA/E272). The risk of complications requiring at least medical (grade ≥2) or invasive treatment (grade ≥3) was higher in group A [OR=7.64 (1.35–43.3, P=0.022), and OR=2.82 (0.86–9.24, P=0.086), respectively] (Table 2, Fig. 1). Patients with intra-abdominal complications were more common in group A than in group B [90.3% vs 53.3%; P=0.0016, Fig. 1; OR=6.3 (1.49–26.7), P=0.012, Table 3] with a median of 2 (1–3) and 1 (0–1) complications per patient, respectively (P=0.002; Fig. 1). Intraoperative blood loss tended to be higher in group B [800 mL (450–950) vs 500 mL (350–750), P=0.086) with a higher rate intraoperative blood transfusion (56.7% vs 12.9%; P<0.001) and splenectomy (13.3% vs 0%; P=0.053). The median postoperative hospital stay was 10.5 (9–13.25) days in group B versus 16 (12–30) days in group A (P<0.001). All the results were confirmed in PP analysis (data not shown and Table 3). A post hoc analysis was conducted including patients of group C (patients intraoperatively excluded for not meeting high-risk criteria), to evaluate whether the study design did indeed identify a high-risk population for POPF. OR for CR-POPF in group A with respect to group C was 4.55 (1.23–16.9, P=0.023). A more extensive comparison of complications between groups A and C is reported in Supplementary Tables 4 and 5 (Supplemental Digital Content 1, http://links.lww.com/SLA/E272).
TABLE 2.
Comparison of Complications Between PD (Group A) and TP+IAT (Group B)
| n (%) | |||
|---|---|---|---|
| Group A: Pancreatoduodenectomy with high-risk pancreatic anastomosis | Group B: TP+IAT | P | |
| N | 31 (100) | 30 (100) | |
| Abdominal complications’ rate | 28 (90.3) | 16 (53.3) | 0.0016 |
| Pancreatic fistula | 23 (74.2) | 0 (0) | <0.0001 |
| Grade A | 4 (17.4) | — | |
| Grade B | 13 (56.5) | — | |
| Grade C | 6 (2.1) | — | |
| Delayed gastric emptying | 6 (19.4) | 3 (10) | 0.473 |
| Postpancreatectomy hemorrhage | 7 (22.6) | 4 (13.3) | 0.508 |
| Relaparotomy | 4 (12.9) | 3 (10) | 1 |
| Splenectomy | 0 (0) | 4 (13.3) | 0.053 |
| Duodenojejunal anastomosis leakage | 3 (9.7) | 0 (0) | 0.238 |
| Ileocolic anastomosis leakage | 2 (6.5) | 0 (0) | 0.492 |
| Biliary fistula | 5 (16.1) | 0 (0) | 0.053 |
| Lymphatic fistula | 0 | 2 (6.7) | 0.238 |
| Abdominal fluid collections | 4 (12.9) | 1 (3.3) | 0.354 |
| Liver ischemia | 1 (3.2) | 2 (6.7) | 0.612 |
| Acute pancreatitis | 3 (9.7) | 0 (0) | 0.238 |
| Cholangitis | 1 (3.2) | 0 (0) | 1 |
| Portal thrombosis | 0 (0) | 1 (3.3) | 0.492 |
| Bowel obstruction | 1 (3.2) | 0 (0) | 1 |
| Wound infection | 4 (12.9) | 4 (13.3) | 1 |
| Hepatic hematoma | 0 (0) | 1 (3.3) | 0.492 |
| Ascites | 1 (3.29) | 0 (0) | 1 |
| Total abdominal complications | 65 | 25 | |
| Other complications | 12 (38.7) | 10 (33.3) | 0.791 |
| Pneumonia | 2 (6.5) | 3 (10) | 0.671 |
| Sepsis | 6 (19.4) | 3 (10) | 0.473 |
| Arrhythmia | 1 (3.2) | 2 (6.7) | 0.612 |
| Pulmonary embolism | 1 (3.2) | 0 (0) | 1 |
| Urinary retention | 0 (0) | 1 (3.3) | 0.492 |
| Urinary infection | 0 (0) | 1 (3.3) | 0.492 |
| Pleural effusion | 2 (6.5) | 4 (13.3) | 0.425 |
| Pancytopenia | 1 (3.2) | 0 (0) | 1 |
| Acute peripheral arterial occlusion | 1 (3.2) | 0 (0) | 1 |
| Jugular vein thrombosis | 1 (3.2) | 0 (0) | 1 |
| Stroke | 1 (3.2) | 0 (0) | 1 |
| Total other complications | 16 | 14 | |
| Complication score | |||
| 0 | 2 (6.5) | 9 (30) | 0.130 |
| 1 | 0 (0) | 1 (3.3) | |
| 2 | 15 (48.4) | 13 (43.3) | |
| 3 | 9 (29) | 4 (13.3) | |
| 4 | 2 (6.5) | 2 (6.7) | |
| 5 | 3 (9.7) | 1 (3.3) | |
| Complications with score ≥2 | 29 (93.5) | 20 (66.7) | 0.011 |
| Complications with score ≥3 | 14 (45.2) | 7 (23.3) | 0.106 |
FIGURE 1.
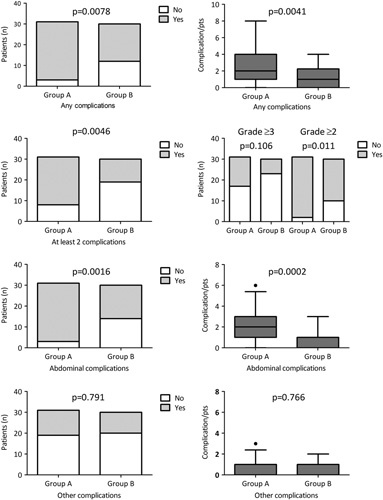
Complications after pancreatic surgery. All and abdominal complications graded according to the Clavien-Dindo classification were evaluated (time frame 90 days from discharge) in 61 subjects considered at high risk for pancreaticojejunostomy disruption (eg, small pancreatic duct, soft pancreas) and randomly assigned to PD with pancreatic anastomosis (group A, n=31) or TP-IAT (group B, n=30). Data are expressed as histogram or box (median and 25th–75th percentiles), whiskers (5th–95th percentiles), outliers as dots. Analysis were performed by a 2-sided Fisher exact test or Mann-Whitney test.
TABLE 3.
Group A Versus Group B Univariate Logistic Regression Analysis of Complications Adjusted for Age and Sex
| ITT | PP | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Any complications | 4.54 | 1.07–19.3 | 0.04 | 14.1 | 1.62–12.2 | 0.016 |
| Abdominal complications | 6.3 | 1.49–26.7 | 0.012 | 19.37 | 2.24–16.7 | 0.007 |
| Other complications | 1.24 | 0.40–3.83 | 0.706 | 1.26 | 0.39–4.01 | 0.7 |
| At least 2 complications | 4.32 | 1.36–13.7 | 0.013 | 6.23 | 1.77–22 | 0.004 |
| Complications with grade ≥2 | 7.64 | 1.347–43.34 | 0.022 | 17.603 | 1.733–17.8 | 0.015 |
| Complications with grade ≥3 | 2.82 | 0.86–9.24 | 0.086 | 3.056 | 0.888–10.512 | 0.076 |
| Relaparotomy | 1.681 | 0.328–8.624 | 0.533 | 1.37 | 0.24–7.75 | 0.719 |
| Readmission after surgery | 1.07 | 0.30–3.80 | 0.912 | 1.283 | 0.354–4.65 | 0.705 |
Metabolic Follow-up
In ITT analysis, group B was associated with a significantly higher risk of diabetes [13 of 31 (41.9%) patients in group A and 29 of 30 (96.7%) patients in group B; B vs A: HR=9.1 (3.76–21.9); P<0.0001] and insulin dependency [6 of 31 (19.4%) patients in group A and 28 of 30 (93.3%) patients in group B; B vs A HR=12.32 (4.2–36.2); P<0.0001] (Fig. 2). In group B, 4 of 29 (13.8%) patients undergoing IAT reached insulin independence, 22 patients (75.9%) had partial graft function, 3 patients (10.3%) had primary nonfunction (C-peptide level <0.3 ng/mL). At the last follow-up available, 2 of 22 patients who gained graft function, did not maintained C-peptide levels >0.3 ng/mL, and 2 of 4 subjects lost insulin independence while maintaining partial graft function. During the first year postsurgery, fasting glycemia, HbA1c, insulin requirement, fasting and stimulated C-peptide levels were constantly better in group A than in group B (Fig. 2). Despite this, patients in group B maintained a good metabolic control and showed sustained C-peptide production over time. At the last metabolic evaluation available [group A: day 504 (386–1490); group B: day 388 (235–1307)], median HbA1c was: 5.6% (5.3–6.3) and 7.4% (6.6–8.45), P<0.0001; median fasting glycemia 102 mg/dL (93–123) and 157 mg/dL (131–193), P<0.0001. No episodes of severe hypoglycemia were reported in both groups. All patients in both groups A and B showed pancreatic exocrine insufficiency at the week 52 visit, as documented by the need of pancreatic enzyme replacement therapy (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E272). The pancrelipase dosage was constantly higher in group B (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E272). The exocrine insufficiency was well compensated in both groups, with a similar prevalence of symptoms like steatorrhea, weight loss, abdominal pain, and frequency of fecal discharge. Levels of albumin and fat-soluble vitamins (A, D, E), available in a subgroup of patients, were similar in the 2 groups.
FIGURE 2.
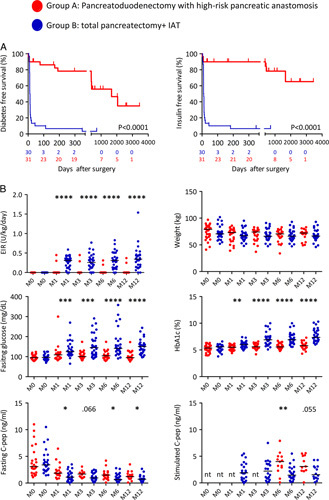
Glucose tolerance after pancreatic surgery. A, ITT analysis of the probability of diabetes-free survival and insulin-free survival according to Kaplan-Meier in ITT analysis. Group A consists of 31 patients assigned to PD with pancreatic anastomosis. Group B consists of 30 patients assigned to TP-IAT. B, ITT analysis of insulin requirement, weight, fasting glucose, HbA1c, fasting C-peptide and stimulated C-peptide (maximum value during arginine test) reported as dot plot before (group A, n=28; group B, n=29) and at month 1 (group A, n=26; group B, n=28), 3 (group A, n=25; group B, n=27), 6 (group A, n=23; group B, n=23), and 12 (group A, n=20; group B, n=17) after surgery. Analysis were performed by log-rank (Mantel-Cox) test or 2-sided Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Patient Survival and Oncologic Follow-up
Survival was calculated for each group for both overall patients and the subgroup with pancreatic or periampullary adenocarcinoma. At last follow-up [group A: median: 7.1 years (95% CI: 5.5–8.6); group B: 7.2 years (95% CI: 5.1–9.3)], 17 of 31 (54.8%) patients in group A and 17 of 30 (56.7%) patients in group B were alive (ITT analysis). In group A, there were 3 in-hospital deaths and 11 patients died for malignancy relapse. In group B, there was 1 in-hospital death, 9 died for malignancy relapse, and 3 for other causes (metachronous colon cancer, subarachnoid hemorrhage, and bowel obstruction). Overall survival did not significantly differ between the 2 groups (Table 4, Fig. 3), both in the ITT [risk for death A vs B: HR=1.36 (0.61–3.0), P=0.454] and PP analysis [A vs B: HR=1.48 (0.64–3.41), P=0.361]. The 1-year survival in ITT was 77.4% and 93.3% for groups A and B, respectively [P=0.147; risk for death A vs B: OR=4.1 (0.77–21.5), P=0.097]. The 2-year survival in ITT was 64.5% and 83.3% for groups A and B, respectively [P=0.146; risk for death A vs B: OR=2.7 (0.82–9.2), P=0.1]. When considering patients with adenocarcinoma, disease-free survival, site of recurrence, disease-specific survival, and overall survival did not significantly differ in the 2 groups (Table 4, Fig. 3). We also performed a post hoc analysis where groups A and C were merged as a single group including all patients undergoing PD (Supplemental Fig. 4, Supplemental Digital Content 1, http://links.lww.com/SLA/E272). In the ITT analysis the overall 1- and 2-year survival rate in group A+C was significantly poorer (73.5% and 63.3%, respectively) than that in group B [1 year, P=0.029, risk for death A+C vs B: OR=5.06 (1.05–24.2), P=0.043; 2 years, P=0.075, A+C vs B: OR=2.9 (0.94–8.9), P=0.063]. Concordantly, overall survival and disease-specific survival were quite similar between groups A and C, while a trend for better overall survival in group B versus group C was evident (Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/SLA/E272). In the subgroup of patients with adenocarcinoma, the post hoc analysis confirmed a significant (PP analysis) or close to the significance (ITT analysis) lower risk of mortality of TP-IAT than PD (Table 4, Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/SLA/E272).
TABLE 4.
Univariate Logistic Regression Analysis of Survival Adjusted for Age and Sex
| ITT | PP | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| All patients | Group A (n=31) vs group B (n=30) | Group A (n=28) vs group B (n=29) | ||||
| Death | 1.36 | 0.61–3.01 | 0.454 | 1.48 | 0.64–3.41 | 0.361 |
| Patients with periampullary carcinoma* | Group A (n=24) vs group B (n=21) | Group A (n=21) vs group B (n=20) | ||||
| Death | 1.67 | 0.69–4.03 | 0.251 | 1.88 | 0.74–4.78 | 0.182 |
| Disease-specific death | 1.81 | 0.73–4.49 | 0.203 | 2.06 | 0.78–5.4 | 0.144 |
| Disease recurrence | 1.28 | 0.51–3.24 | 0.60 | 1.54 | 0.59–4 | 0.38 |
| All patients | Group A+C (n=49) vs group B (n=30) | Group A+C (n=46) vs group B (n=29) | ||||
| Death | 1.52 | 0.76–3.04 | 0.239 | 1.63 | 0.79–3.34 | 0.185 |
| Patients with periampullary carcinoma* | Group A+C (n=39) vs group B (n=21) | Group A+C (n=36) vs group B (n=20) | ||||
| Death | 2.06 | 0.92–4.60 | 0.079 | 2.38 | 1.02–5.57 | 0.046 |
| Disease-specific death | 2.2 | 0.9–5.09 | 0.066 | 2.56 | 1.05–6.25 | 0.039 |
| Disease recurrence | 1.46 | 0.63–3.35 | 0.38 | 1.7 | 0.71–4.05 | 0.232 |
Adjusted for tumor.
FIGURE 3.
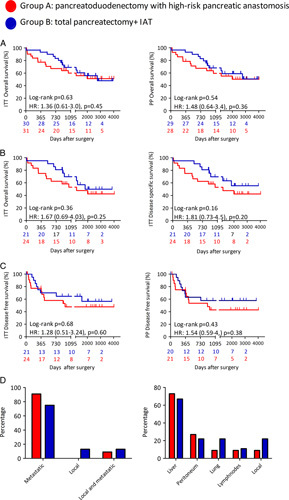
Patient survival and oncologic follow-up. A, ITT and PP probability of overall survival after surgery, according to Kaplan-Meier. B, ITT probability of overall survival and disease-specific survival in subjects with epithelial malignancy, according to Kaplan-Meier. C, Probability of disease-free survival in subjects with epithelial malignancy, according to Kaplan-Meier. D, Site of the first recurrence in subjects with malignancy (group A, n=11; group B, n=9). Analysis was performed by log-rank (Mantel-Cox) test or χ2 test as appropriated. The univariate HRs adjusted for age and sex (and tumor grade in patients with malignant neoplasm) were reported.
DISCUSSION
Our study addressed for the first time in a randomized prospective design the role for preemptive TP-IAT in selected patients undergoing PD at high risk for POPF (eg, small pancreatic duct, soft pancreas). At the time when the study was designed, no validated fistula risk score was available, so we adopted 2 simple and well-known criteria to select the pancreas at high-risk for POPF: soft pancreas and main duct diameter ≤3 mm, which were already suggested as risk factors for POPF by some authors.28 Such criteria are identical to the ones recently proposed and validated by the ISGPS to define patients undergoing PD at the highest risk of POPF.29 The results indicate that TP-IAT can be considered a valid alternative to PD in these patients, as it reduced complication number, severity, and length of hospital stay. Of note, a trend toward a reduction of mortality, even for patients with malignancy was also evident. As expected, TP-IAT was associated to a higher risk of diabetes, but IAT was able to preserve, at least in part, the endogenous insulin secretion, mitigating the impact of pancreoprivic diabetes and assuring good metabolic control without severe hypoglycemic episodes. Unlike what is normally done in patients with intractable chronic pancreatitis undergoing IAT, we chose to exclude patients with preoperative diabetes from the study. We have decided to be conservative to ensure the best chance of success in achieving postsurgical insulin independence. In fact, at the time the study was designed the sole predictor of insulin independence consistently reported in prior studies was higher islet yield.30 Since the condition of diabetes is associated with a reduction in beta cell mass,31 it was presumed that the number of isolated islets would be lower in the case of preoperative diabetes. This would have significantly affected the chance of success, considering that the amount of usable pancreatic tissue would have been lower than in the patient with chronic pancreatitis. Moreover, diabetes mellitus and its related factors such as hyperinsulinemia have been linked to pancreatic cancer outcomes32 and we would avoid a further confounding factor in a numerically limited series. This choice certainly slowed the recruitment of patients into the study considering that the prevalence of preoperative diabetes in our experience is higher than 60% in the case of subjects with pancreatic cancer.33
As regards exocrine insufficiency, enzyme replacement therapy was effective in treating exocrine insufficiency in both groups, although a higher dose was required in the TP-IAT group. Although conducted in a limited number of patients, this trial has generated valuable knowledge both in the field of pancreatic surgery and islet transplantation. In the field of pancreatic surgery, TP is still poorly accepted, due to the adverse effects of endocrine and exocrine insufficiency on QOL.34 More recently, some studies reported better result in terms of surgical outcome for TP than PD in patients at high risk for POPF,35–38 suggesting that this approach could be an alternative in a selected group of patients. Our results further support this possibility, since IAT was able to provide partial insulin secretion in around 90% of patients undergoing TP, with good metabolic control (HbA1c level of ≤7.5%39) in 62% of subjects at last follow-up, without severe hypoglycemic episode or diabetes-related mortality. TP-IAT should be further considered in light of the latest evidence of the impact of POPF on the long-term outcome.40,41 In fact, TP may prevent a long-lasting decline of patients’ general condition associated with CR-POPF. This is particularly relevant in patients with adenocarcinoma, where a complicated clinical course may delay the start of adjuvant therapy, impacting oncological outcome.40,42–44 This may have contributed to the observed better survival in TP-IAT. Even if our study was underpowered to detect a significant difference in survival (power level of 58% and 60% for 1- and 2-year survival), TP-IAT showed a strong trend to lower risk of mortality than PD. This hypothesis will be tested in further studies, such as the recently started TP-IAT-01 trial (NCT05116072), which hypothesize that TP-IAT rather than PD may improve the access to adjuvant chemotherapy.
In the field of islet transplantation, this study definitively confirmed IAT could be indicated for pancreas diseases other than chronic pancreatitis. The fear of infusing malignant cells inside the islet preparation has limited the use of this procedure for patients with malignancy. We previously reported the largest series of patients undergoing IAT after pancreatic resection for a wide spectrum of disease besides chronic pancreatitis,12,16,17 suggesting the possibility to extend IAT indications (Milan protocol14). For the first time in a randomized prospective design, we confirmed that IAT is feasible, safe, and effective in patients with periampullary cancer. We failed to perform IAT in only 1 of 30 (3.3%) of eligible patients, with a low rate of overall IAT-related complications (17.2%), or potentially severe complications (3.4%). This rate is, however, higher than observed in allotransplantation, probably due to the association with a complex surgery. As disease-free survival and the site of first recurrence were not different between TP-IAT and PD, an additional risk of disseminating tumor cells to the liver because in subjects with malignancy receiving IAT can be excluded. Finally, the study showed the feasibility and safety of a remote isolation facility. High-volume pancreatic surgery centers should collaborate with a remote islet isolation facility to give access to IAT to a greater number of patients.
Even if this study has overcome some of the limitations of previous ones (ie, short follow-up, retrospective design), some remain. The study population, even if made up mainly of subjects with periampullary carcinomas, is heterogenous in terms of indication for pancreatectomy, that is, a mix of malignant or benign tumors of the pancreas, duodenum, or ampulla. The number of patients was still relatively small. The definition of PD at high risk for POPF, even recently validated, was quite simple; a better risk stratification45 would make the results even more valuable. Major complications or mortality, more reliable and clinically relevant outcomes, were not chosen as the primary outcome because the sample size required for an adequate power was too high and would have significantly impacted on feasibility. The study developed over a long period during which some standard treatment changed, leading to potential time bias. Eighty-nine patients represented around 5% of PD treated in the study period. The enrollment was slow because of the introduction of some exclusion criteria such as the presence of preoperative diabetes, the complexity of the coordination of the surgical activity with the activity of the isolation facility, and the innovativeness of the approach not always easily accepted by patients. Last, QOL analysis, using a validated questionnaire, was not available.
However, taken together, our data demonstrated the feasibility, efficiency, and safety of TP-IAT as standard treatment in patient candidates for PD at high risk for POPF. The decision to perform TP-IAT requires assessing the risk-benefit ratio in each individual case and should be discussed with the candidate patient. We confirmed IAT as a possible choice in patients with pancreas diseases other than chronic pancreatitis. In this direction our data calls for the launching of a multicenter randomized controlled trial, comparing PD with TP-IAT in patients with a high risk of POPF, with a homogeneous and large study population of pancreatic ductal carcinoma, and with an oncological primary endpoint.
Supplementary Material
Footnotes
G.B. and A.Z. contributed equally.
L.P., G.B., and A.Z. contributed to conceptualization and study design; accessed and verified the underlying study data. P. Magistretti, P. Maffi, F.G., G.C., and F.A. contributed to the data collection. M.S., C.M., A.M., R.M., N.P., S.P., F.D.C., D.P., S.C., and R.N. contributed to the methods. L.P., P.B., and A.Z. had access to raw data. R.M. contributed to data curation. Funding was acquired by L.P; he analyzed the data; wrote the original draft of the report. L.P., G.B., A.Z., and M.F. contributed to the data interpretation. G.B., A.Z., M.F., and M.S. reviewed and edited the report. G.B. and L.P. are responsible for the final submission of the manuscript for publication and all authors approved the final version before submission.
Supported by Italian Ministry of Heath (Ricerca finalizzata RF-2009-1483387). The funders had no role in the study design and result analysis. They had no opportunity to review the manuscript and the authors are solely responsible for the final content and interpretation of the collected data.
L.P. declares honoraria for advisory roles from Dompè and Roche in the past 36 months. L.P. declares grants from Associazione Italiana per la Ricerca sul Cancro (AIRC), Juvenile Diabetes Research Foundation (JDRF), Italian Ministry of Research, EFSD/JDRF/Lilly European Programme in Type 1 Diabetes Research, European Commission Horizon 2020 outside of the submitted work. F.D.C. declares a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC). S.C. declares a grant from Italian Ministry of Health and Fondazione “Nadia Valsecchi.” C.M. declares personal fees from Roche Diabetes Care Italy in the past 36 months outside of the submitted work.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Gianpaolo Balzano, Email: balzano.gianpaolo@hsr.it.
Alessandro Zerbi, Email: alessandro.zerbi@hunimed.eu.
Francesca Aleotti, Email: aleotti.francesca@hsr.it.
Giovanni Capretti, Email: giovanni.capretti@hunimed.eu.
Raffella Melzi, Email: melzi.raffaella@hsr.it.
Nicolò Pecorelli, Email: pecorelli.nicolo@hsr.it.
Alessia Mercalli, Email: mercalli.alessia@hsr.it.
Rita Nano, Email: nano.rita@hsr.it.
Paola Magistretti, Email: magistretti.paola@hsr.it.
Francesca Gavazzi, Email: francesca.gavazzi@cancercenter.humanitas.it.
Francesco De Cobelli, Email: decobelli.francesco@hsr.it.
Dario Poretti, Email: dario.poretti@humanitas.it.
Marina Scavini, Email: scavini.marina@hsr.it.
Chiara Molinari, Email: molinari.chiara@hsr.it.
Stefano Partelli, Email: partelli.stefano@hsr.it.
Stefano Crippa, Email: crippa.stefano@hsr.it.
Paola Maffi, Email: maffi.paola@hsr.it.
Massimo Falconi, Email: falconi.massimo@hsr.it.
Lorenzo Piemonti, Email: piemonti.lorenzo@hsr.it.
REFERENCES
- 1. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 2. Reid-Lombardo KM, Farnell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451–1459. [DOI] [PubMed] [Google Scholar]
- 3. Kamphues C, Bova R, Schricke D, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19:856–863. [DOI] [PubMed] [Google Scholar]
- 4. Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–287. [DOI] [PubMed] [Google Scholar]
- 5. Parsaik AK, Murad MH, Sathananthan A, et al. Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta-analysis of the literature. Clin Endocrinol (Oxf). 2010;73:723–731. [DOI] [PubMed] [Google Scholar]
- 6. Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther. 2011;13:S-5–S-14. [DOI] [PubMed] [Google Scholar]
- 7. Hammer HF. Pancreatic exocrine insufficiency: diagnostic evaluation and replacement therapy with pancreatic enzymes. Dig Dis. 2010;28:339–343. [DOI] [PubMed] [Google Scholar]
- 8. Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. [DOI] [PubMed] [Google Scholar]
- 9. Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209–216. [DOI] [PubMed] [Google Scholar]
- 10. Casadei R, Monari F, Buscemi S, et al. Total pancreatectomy: indications, operative technique, and results. Updates Surg. 2010;62:41–46. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto S. Autologous islet cell transplantation to prevent surgical diabetes. J Diabetes. 2011;3:328–336. [DOI] [PubMed] [Google Scholar]
- 12. Balzano G, Maffi P, Nano R, et al. Extending indications for islet autotransplantation in pancreatic surgery. Ann Surg. 2013;258:210–218. [DOI] [PubMed] [Google Scholar]
- 13. Dudeja V, Beilman GJ, Vickers SM. Total Pancreatectomy with islet autotransplantation in patients with malignancy: are we there yet? Ann Surg. 2013;258:219–220. [DOI] [PubMed] [Google Scholar]
- 14. Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep. 2014;14:512. [DOI] [PubMed] [Google Scholar]
- 15. Braga M, Pecorelli N, Ariotti R, et al. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg. 2014;38:2960–2966. [DOI] [PubMed] [Google Scholar]
- 16. Balzano G, Nano R, Maffi P, et al. Salvage islet auto transplantation after relaparatomy. Transplantation. 2017;101:2492–2500. [DOI] [PubMed] [Google Scholar]
- 17. Balzano G, Maffi P, Nano R, et al. Autologous islet transplantation in patients requiring pancreatectomy: a broader spectrum of indications beyond chronic pancreatitis. Am J Transplant. 2016;16:1812–1826. [DOI] [PubMed] [Google Scholar]
- 18. Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes. 2013;62:3523–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)–an international study group of pancreatic surgery (ISGPS) definition. Surgery. 2007;142:20–25. [DOI] [PubMed] [Google Scholar]
- 21. Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–768. [DOI] [PubMed] [Google Scholar]
- 22. Care D. Care in Diabetes—2022. Diabetes Care. 2022;45:S17. [DOI] [PubMed] [Google Scholar]
- 23. Childs BP, Clark NG, Cox DJ, et al. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245. [DOI] [PubMed] [Google Scholar]
- 24. Seiler C, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection—long term results. J Br Surg. 2005;92:547–556. [DOI] [PubMed] [Google Scholar]
- 25. Kawai M, Yamaue H. Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today. 2010;40:1011–1017. [DOI] [PubMed] [Google Scholar]
- 26. Topal B, Aerts R, Hendrickx T, et al. Determinants of complications in pancreaticoduodenectomy. Eur J Surg Oncol. 2007;33:488–492. [DOI] [PubMed] [Google Scholar]
- 27. Poon RT, Fan ST, Lo CM, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;246:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pratt WB, Callery MP, Vollmer CM. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. [DOI] [PubMed] [Google Scholar]
- 29. Schuh F, Mihaljevic AL, Probst P, et al. A simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: a classification of the International Study Group of Pancreatic Surgery (ISGPS). Ann Surg. 2023;277:e597–e608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. [DOI] [PubMed] [Google Scholar]
- 31. Cho JH, Kim JW, Shin JA, et al. β-cell mass in people with type 2 diabetes. J Diabetes Investig. 2011;2:6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dugnani E, Balzano G, Pasquale V, et al. Insulin resistance is associated with the aggressiveness of pancreatic ductal carcinoma. Acta Diabetol. 2016;53:945–956. [DOI] [PubMed] [Google Scholar]
- 33. Dugnani E, Gandolfi A, Balzano G, et al. Diabetes associated with pancreatic ductal adenocarcinoma is just diabetes: results of a prospective observational study in surgical patients. Pancreatology. 2016;16:844–852. [DOI] [PubMed] [Google Scholar]
- 34. Dresler CM, Fortner JG, Mcdermott K, et al. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Capretti G, Donisi G, Gavazzi F, et al. Total pancreatectomy as alternative to pancreatico-jejunal anastomosis in patients with high fistula risk score: the choice of the fearful or of the wise? Langenbecks Arch Surg. 2021;406:713–719. [DOI] [PubMed] [Google Scholar]
- 36. Luu AM, Olchanetski B, Herzog T, et al. Is primary total pancreatectomy in patients with high-risk pancreatic remnant justified and preferable to pancreaticoduodenectomy?—A matched-pairs analysis of 200 patients. Gland Surg. 2021;10:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marchegiani G, Perri G, Burelli A, et al. High-risk pancreatic anastomosis vs. total pancreatectomy after pancreatoduodenectomy: postoperative outcomes and quality of life analysis. Ann Surg. 2021;275:663–672. [DOI] [PubMed] [Google Scholar]
- 38. Pulvirenti A, Pea A, Rezaee N, et al. Perioperative outcomes and long-term quality of life after total pancreatectomy. Br J Surg. 2019;106:1819–1828. [DOI] [PubMed] [Google Scholar]
- 39. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64:2609–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe Y, Nishihara K, Matsumoto S, et al. Effect of postoperative major complications on prognosis after pancreatectomy for pancreatic cancer: a retrospective review. Surg Today. 2017;47:555–567. [DOI] [PubMed] [Google Scholar]
- 41. Grego A, Friziero A, Serafini S, et al. Does pancreatic fistula affect long-term survival after resection for pancreatic cancer? a systematic review and meta-analysis. Cancers. 2021;13:5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dhayat SA, Tamim ANJ, Jacob M, et al. Postoperative pancreatic fistula affects recurrence-free survival of pancreatic cancer patients. PLoS One. 2021;16:e0252727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uchida Y, Masui T, Nagai K, et al. Postoperative pancreatic fistulas decrease the survival of pancreatic cancer patients treated with surgery after neoadjuvant chemoradiotherapy: a retrospective analysis. Surg Oncol. 2020;35:527–532. [DOI] [PubMed] [Google Scholar]
- 44. Mackay TM, Smits FJ, Roos D, et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB. 2020;22:233–240. [DOI] [PubMed] [Google Scholar]
- 45. Trudeau MT, Casciani F, Ecker BL, et al. The Fistula Risk Score Catalog: toward precision medicine for pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2022;275:e463–e472. [DOI] [PubMed] [Google Scholar]


