Abstract
Rationale
With more frequent and intense precipitation events across the globe due to a changing climate, there is a need to understand the relationship between precipitation and respiratory health. Precipitation may trigger asthma exacerbations, but little is known about how precipitation affects lung function and airway inflammation in early adolescents.
Objectives
To determine if short-term precipitation exposure is associated with lung function and airway inflammation in early adolescents and if ever having a diagnosis of asthma modifies associations of precipitation with lung function and airway inflammation.
Methods
In a prospective prebirth cohort, Project Viva, that included 1,019 early adolescents born in the northeastern United States, we evaluated associations of 1-, 2-, 3-, and 7-day moving averages of precipitation in the preceding week and forced expiratory volume in 1 second, forced vital capacity, and fractional exhaled nitric oxide (FeNO) using linear regression. We used log-transformed FeNO with effect estimates presented as percentage change. We adjusted for maternal education and household income at enrollment; any smoking in the home in early adolescence; child sex, race/ethnicity, and ever asthma diagnosis; and age, height, weight, date, and season (as sine and cosine functions of visit date) at the early adolescent visit and moving averages for mean daily temperature (same time window as exposure).
Results
In fully adjusted linear models, 3- and 7-day moving averages for precipitation were positively associated with FeNO but not lung function. Every 2-mm increase in the 7-day moving average for precipitation was associated with a 4.0% (95% confidence interval, 1.1, 6.9) higher FeNO. There was evidence of effect modification by asthma status: Precipitation was associated with lower forced vital capacity and higher FeNO among adolescents with asthma. We also found that outdoor aeroallergen sensitization (immunoglobulin E against common ragweed, oak, ryegrass, or silver birch) modified associations of precipitation with FeNO, with higher FeNO in sensitized adolescents compared with nonsensitized adolescents. The associations of precipitation with FeNO were not explained by relative humidity or air pollution exposure.
Conclusions
We found that greater short-term precipitation may trigger airway inflammation in adolescents, particularly among those with asthma.
Keywords: lung function, airway inflammation, fractional exhaled nitric oxide, rain, weather
As human-induced climate change drives more frequent and intense precipitation events across the globe, there is a need to understand the relationship between precipitation and respiratory health (1). Yet, few studies have examined how exposure to precipitation affects lung function and airway inflammation in early adolescents with and without asthma.
Precipitation, which encompasses all water particles that fall from the atmosphere to earth, including rain and snow, may trigger asthma exacerbations, resulting in increased risk of emergency department visits and hospitalizations for children and adults (2, 3). Although the exact mechanism is unclear, rain can rapidly create conditions conducive to mold formation and cause pollen grain rupture and fungal spore release within hours to days, all of which promote airway inflammation (4–6).
We examined associations of short-term precipitation with lung function and airway inflammation in early adolescents living in the northeastern United States, a region projected to have the largest increase in annual precipitation relative to other parts of the country (7). We hypothesized that higher levels of precipitation would be associated with lower lung function and higher airway inflammation and, furthermore, that these associations would be greater among early adolescents with asthma. Portions of these results were presented in abstract form at the 2022 American Thoracic Society International Conference in San Francisco, California, in May 2022 (8) and at the 2022 International Society for Environmental Epidemiology Conference in Athens, Greece, in September 2022 (9).
Methods
Study Population
Study subjects were adolescents in a longitudinal prebirth cohort, Project Viva, based in eastern Massachusetts (10). Mothers were enrolled during pregnancy between 1999 and 2002 at Atrius Harvard Vanguard Medical Associates, and then, postpartum, children participated in up to six in-person study visits, including one during the early adolescent years (ages 11.9–16.6 yr), during which medical history was obtained on the basis of maternal report, including respiratory medication use, and measurements of spirometry and fractional exhaled nitric oxide (FeNO) were performed. The early adolescent study visit is the first one to measure both spirometry and FeNO, which is why it was chosen for this study. Children who moved away from the northeastern United States during the course of the study continued to be followed, and their exposure data were updated on the basis of their new address. We included in the present analyses children who participated in the early adolescent visit and had exposure data and either spirometry (n = 972) or FeNO (n = 923) measurements: 1,019 early adolescents had exposure data and either spirometry or FeNO or both. All mothers provided written informed consent for themselves and their children, and early adolescents provided verbal assent. The study was approved by the institutional review board of Beth Israel Deaconess Medical Center.
Demographic and Questionnaire Data
We obtained demographic and medical history data from questionnaires and interviews. Child asthma history was reported by mothers at the early adolescent study visit. We asked if the adolescent had ever been diagnosed with asthma by a doctor, had used asthma medications in the past year, or had wheezing symptoms in the past year. Current asthma was defined as having a prior diagnosis of asthma plus wheezing symptoms or use of asthma medications in the past year. For our analyses, we defined ever asthma as having either a prior diagnosis of asthma or a current diagnosis of asthma. Child race/ethnicity (Asian, Black, White, Hispanic, or other) was based on mother-reported responses during a study visit in early childhood. The inclusion of race and ethnicity, which are social constructs without biological meaning, as variables in analyses are meant to help identify inequities and disparities rather than attribute pathophysiological differences to racial groups. Measures of socioeconomic status included in our analyses were self-reported annual household income more than USD $70,000 and maternal college graduation (yes vs. no) at enrollment. Household tobacco use was defined as the presence or absence of anyone in the child’s home who smokes, based on questionnaire responses in early adolescence.
Exposure Assessment
Spatially and temporally resolved daily precipitation measurements are based on the PRISM (Parameter-elevation Relationships on Independent Slopes Model), an 800-m resolution climate dataset for the United States maintained by Oregon State University (11). Participant residential addresses were geocoded and linked to the exposure data. We define precipitation as daily precipitation measured in millimeters, with exposure periods defined as the day before the study visit and the 2-, 3-, and 7-day moving averages of precipitation preceding the study visit. Moving averages are defined as the total precipitation over the specified time period preceding the study visit divided by the specified time period (e.g., the 7-d moving average is the total amount of precipitation over the preceding 7 d, divided by 7), similar to prior published work in this cohort (12). For analyses incorporating air pollution, we used modeled daily air pollution data generated using machine learning algorithms developed at the Harvard School of Public Health on the basis of neural network, gradient boosting, and random forest with integrated satellite data, land-use data, and chemical transport model outputs for fine particulate matter with an aerodynamic diameter ⩽2.5 μm (PM2.5), nitrogen dioxide (NO2), and ground-level ozone (O3) on a 1 × 1–km resolution (13–15).
Lung Function Measurement in Early Adolescence
Trained research assistants obtained forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), measured in liters, using the EasyOne Spirometer (ndd Medical Technologies) in accordance with the American Thoracic Society guidelines for acceptability and reproducibility (16). Each child had to produce at least three acceptable spirograms, two of which had to meet criteria for reproducibility, defined as a ⩽0.150-L difference between the two largest FEV1 values and the two largest FVC values, for inclusion.
Exhaled Nitric Oxide Measurement in Early Adolescence
FeNO concentration, a marker of eosinophilic airway inflammation, was measured twice for each participant with a portable handheld electrochemical device (NIOX MINO) validated by the clinical standard chemiluminescence FeNO analyzer (17, 18). To avoid ambient nitric oxide (NO) measurement, participants were instructed to inhale through an NO scrubbing filter and exhale into room air on two efforts. On the third breath, participants exhaled into the FeNO analyzer. Only the last 3 seconds of exhalation were used to ensure lower rather than upper airway measurements of NO. Nose clips were not used. Mean FeNO was calculated as the mean of two efforts and logarithmically transformed because of nonnormality, consistent with prior published work by this group (19). Effect estimates for log-transformed FeNO are presented as percentage change, calculated as (eβ − 1) × 100.
Aeroallergen Sensitization Measurement in Early Adolescence
Allergen extract–specific immunoglobulin E (IgE) antibodies were measured by ImmunoCap (Thermo Fisher Scientific/Phadia) in a subset of participants at the early adolescent study visit (20, 21). We defined outdoor aeroallergen sensitization as having any IgE >0.35 IU/ml against Betula pendula (silver birch tree), Quercus (oak tree), Lolium (ryegrass), and Ambrosia artemisiifolia (common ragweed), similar to prior studies (19, 22). We defined indoor aeroallergen sensitization as having any IgE >0.35 IU/ml against Dermatophagoides farina (dust mite), cat dander, dog dander, Aspergillus fumigatus (a ubiquitous airborne fungus), and Alternaria alternata (a fungus found in plants and soil).
Statistical Analysis
Potential confounders and predictors of outcome were selected a priori on the basis of published and anticipated associations with our outcomes (lung function and FeNO) and our exposure of interest (precipitation). Primary models were adjusted for predictors of lung function, including sex; age; height; weight; and 1-, 2-, 3-, and 7-day moving averages of mean daily temperature matching the precipitation exposure time window. We also adjusted for maternal education and household income at enrollment, any smoking in the home in early adolescence, race/ethnicity, ever asthma diagnosis, date of visit, and season (as sine and cosine functions of visit date) at the early adolescent visit. We performed sensitivity analyses also adjusting for the 1-, 2-, 3-, and 7-day moving averages of the air pollutants PM2.5, NO2, and O3. We also performed sensitivity analyses also adjusting for 1-, 2-, 3-, and 7-day moving averages of relative humidity matching the precipitation exposure time window.
We analyzed associations of 1-, 2-, 3-, and 7-day moving averages of precipitation preceding the study visit with FEV1, FVC, and FeNO by linear regression. We evaluated the linearity of each association by plotting generalized additive mixed models with penalized splines. We tested if associations of precipitation with lung function and airway inflammation were modified by ever asthma diagnosis, season of testing, aeroallergen sensitization (indoor and outdoor aeroallergens), and sex using interaction terms.
All measures of association are reported as β-coefficients with a 95% confidence interval (CI). A two-sided P value less than 0.05 was used for statistical significance for main effects. A two-sided P value less than 0.10 was used to assess interaction in effect modification analyses. All analyses were performed using R (R Foundation for Statistical Computing) (23).
Results
Participant characteristics are summarized in Table 1. This cohort is evenly balanced by sex (49.7% female), with a slight majority White race/ethnicity (64.4%), and with high maternal education on average (71.7% with college degree) and low rates of household smoking (11.4%). A proportion of 26.1% of participants had a history of asthma. A subset of participants had aeroallergen IgE measurement (n = 638), and of those, 58.0% of participants had aeroallergen IgE >0.35 IU/ml, suggesting aeroallergen sensitization.
Table 1.
Characteristics of study participants (N = 1,019)
| Characteristic | Mean ± SD or % |
|---|---|
| Female sex | 49.7% |
| Age, yr | 13.2 ± 0.9 |
| Height, cm | 159.9 ± 9.0 |
| Weight, kg | 54.1 ± 14.8 |
| Race/ethnicity | |
| Asian | 2.8% |
| Black | 16.1% |
| Hispanic | 4.5% |
| White | 64.4% |
| Other | 12.1% |
| Ever asthma diagnosis | 26.1% |
| Medication use to treat breathing problems in previous 3 d | 4.1% |
| Aeroallergen sensitization* | 58.0% |
| Any smoking in the home in early adolescence | 11.4% |
| Maternal college degree at enrollment | 71.7% |
| Annual household income >$70,000 at enrollment | 64.3% |
| Season of early adolescent visit | |
| Winter | 22.7% |
| Spring | 23.2% |
| Summer | 28.0% |
| Autumn | 17.8% |
| Spirometry | |
| FEV1, L | 2.8 ± 0.6 |
| FVC, L | 3.3 ± 0.7 |
| FeNO, parts per billion | 25.9 ± 26.9 |
Definition of abbreviations: FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; SD = standard deviation.
Aeroallergen sensitization is defined as having immunoglobulin E against indoor and outdoor allergens using a cutoff of 0.35 IU/ml; 381 participants did not have aeroallergen data.
The median daily precipitation was 2.0 mm (interquartile range, 4.0 mm; minimum, 0 mm; maximum, 27.3 mm) for the 7 days preceding the study visit. The mean (standard deviation) FeNO was 25.9 (26.9) parts per billion (ppb), and 11.8% of the early adolescents with FeNO measurements had FeNO levels above the upper limits of normal (35 ppb for age younger than 12 and 50 ppb for ages 12 and older) (24).
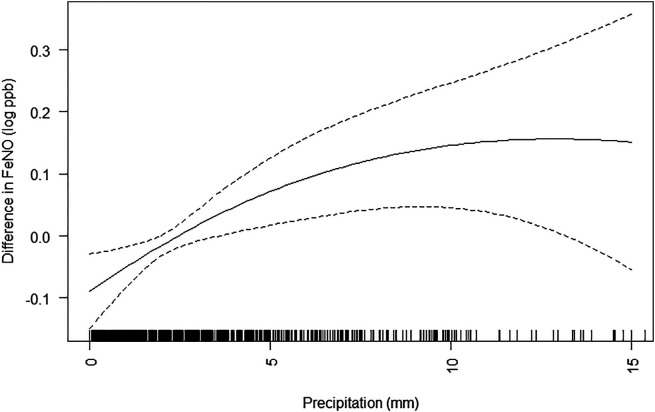
The main associations of precipitation with measurements of FeNO and lung function are shown in Table 2. The 3- and 7-day moving averages for precipitation were associated with a higher FeNO (Figure 1). Associations were largely unchanged with adjustment for air pollutants PM2.5, NO2, and O3 in sensitivity analyses (Table E1 in the data supplement). Associations were also unchanged when also adjusting for relative humidity.
Table 2.
Associations of precipitation (per 2 mm per day) at home address with fractional exhaled nitric oxide and lung function in early adolescence
| Precipitation Moving Averages | Difference in FeNO (95% CI) | Difference in FEV1 (ml) (95% CI) | Difference in FVC (ml) (95% CI) |
|---|---|---|---|
| 1 d | 0.1% (−1.1 to 1.4) | 1.7 (−4.7 to 8.0) | 0.7 (−6.0 to 7.4) |
| 2 d | 1.5% (−0.2 to 3.2) | 3.0 (−5.8 to 11.8) | 2.9 (−6.4 to 12.3) |
| 3 d | 2.1% (0.2 to 4.0)* | 3.6 (−6.5 to 13.6) | 2.3 (−8.3 to 12.9) |
| 7 d | 4.0% (1.1 to 6.9)* | 0.5 (−14.4 to 15.4) | 0.5 (−15.2 to 16.3) |
Definition of abbreviations: CI = confidence interval; FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity.
Linear regression models are adjusted for maternal education and household income at enrollment; any smoking in the home in early adolescence; child sex, race/ethnicity, and ever asthma diagnosis; age, height, weight, date, and season (as sine and cosine functions of visit date) at the early adolescent visit; and moving averages for mean daily temperature (same time window as exposure).
P < 0.05.
Figure 1.
Generalized additive model with 3 degrees of freedom for the association of 7-day moving average for precipitation (millimeters) and difference in log FeNO (parts per billion). Dotted lines represent 95% confidence interval. Black shading on x-axis reflects density of observations. Generalized additive model was adjusted for maternal education and household income at enrollment; any smoking in the home in early adolescence; child sex, race/ethnicity, and ever asthma diagnosis; and age, height, weight, date, and season (as sine and cosine functions of visit date) at the early adolescent visit and 7-day moving average for mean daily temperature (same time window as exposure). FeNO = fractional exhaled nitric oxide.
By tertile of precipitation, low (<5 mm), medium (5–10 mm), and high (>10 mm), we found that exposure to medium levels of precipitation relative to low levels of precipitation on the day preceding study visit was associated with a 153.2 ml lower FVC (95% CI, −252.9 to −53.5) among early adolescents. Exposure to high levels relative to low levels of precipitation on the day preceding study visit was associated with a 42.7-ml lower FVC (95% CI, −165.7 to 80.3) in early adolescents.
Associations of precipitation with lung function and FeNO differed by asthma status (Table 3). Associations between precipitation and FeNO were more positive in early adolescents with asthma compared with the overall study population. Early adolescents with asthma had a 5.8% (95% CI, 1.8–9.9) higher FeNO per 2-mm increment in 3-day moving average of precipitation as compared with early adolescents without asthma, who had a 1.0% (95% CI, −1.2 to 3.2) higher FeNO (Pinteraction = 0.04). We also found lower FVC in adolescents with asthma than in adolescents without asthma (Pinteraction < 0.1) in association with precipitation: Per 2-mm increment in 1-day moving average for precipitation, FVC was 15.6 ml lower (95% CI, −28.1 to −3.1) with similar direction in effect estimates for both FEV1 and the other moving averages for precipitation. In adolescents without asthma, there was a similar direction in the associations for FEV1 and FVC across the different moving averages.
Table 3.
Associations of precipitation (per 2 mm per day) at home address with airway inflammation measured by FeNO and lung function measured by forced expiratory volume in 1 s and forced vital capacity in early adolescence, by ever asthma diagnosis
| Precipitation Moving Averages | Difference in FeNO, FEV1, or FVC (95% CI) |
||
|---|---|---|---|
| With History of Asthma | Without History of Asthma | P interaction | |
| FeNO | |||
| 1 d | 0.1% (−2.1 to 2.2) | 0.2% (−1.3 to 1.7) | 0.93 |
| 2 d | 3.2% (0.0 to 6.6) | 0.8% (−1.1 to 2.8) | 0.22 |
| 3 d | 5.8% (1.8 to 9.9) | 1.0% (−1.2 to 3.2) | 0.04* |
| 7 d | 6.4% (1.4 to 11.6) | 2.7% (−0.7 to 6.3) | 0.24 |
| FEV1, ml | |||
| 1 d | −4.0 (−15.9 to 7.9) | 3.9 (−3.6 to 11.5) | 0.27 |
| 2 d | −12.2 (−29.4 to 4.9) | 8.4 (−1.8 to 18.6) | 0.04* |
| 3 d | −13.0 (−33.5 to 7.5) | 8.8 (−2.7 to 20.2) | 0.07* |
| 7 d | −6.2 (−33.6 to 21.3) | 3.3 (−14.4 to 21.0) | 0.57 |
| FVC, ml | |||
| 1 d | −15.6 (−28.1 to −3.1) | 7.2 (−0.7 to 15.1) | <0.01* |
| 2 d | −16.2 (−34.3 to 1.9) | 9.7 (−1.1 to 20.5) | 0.02* |
| 3 d | −16.6 (−38.3 to 5.0) | 8.2 (−4.0 to 20.3) | 0.05* |
| 7 d | −6.2 (−35.2 to 22.8) | 3.3 (−15.4 to 22.0) | 0.59 |
Definition of abbreviations: CI = confidence interval; FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity.
Linear regression models are adjusted for maternal education and household income at enrollment; any smoking in the home in early adolescence; child sex, race/ethnicity, and ever asthma diagnosis; age, height, weight, date, and season (as sine and cosine functions of visit date) at the early adolescent visit; and moving averages for mean daily temperature (same time window as exposure).
Pinteraction < 0.1.
We also found that outdoor aeroallergen sensitization (IgE against common ragweed, oak, ryegrass, or silver birch), but not indoor aeroallergen sensitization (IgE against dust mite, cat dander, dog dander, Aspergillus fumigatus, Alternaria alternata), modified associations of precipitation with FeNO: Per 2-mm increment in 3-day moving average for precipitation, there was a 5.1% (95% CI, 0.9–9.5) higher FeNO in sensitized adolescents compared with 0.7% (95% CI, −1.9 to 3.4) higher FeNO in nonsensitized adolescents (Pinteraction = 0.8) (Table E2). Outdoor and indoor aeroallergen sensitization did not modify associations of precipitation with lung function (Pinteraction > 0.1 for all moving averages). Associations did not differ by season of testing (Pinteraction > 0.1 for all moving averages).
Participant sex modified the association of precipitation on FEV1, FVC, and FeNO at different moving averages (Table E3). The association of 7-day moving average of precipitation with FeNO was greater among females (6.9% [95% CI, 2.7–11.3] higher FeNO per 2-mm precipitation) than among males (1.4% [95% CI, −2.5 to 5.3] higher FeNO per 2-mm precipitation).
Discussion
In this cohort of adolescents living in the northeastern United States unselected for the presence of asthma, an increase in precipitation over the preceding 7 days was associated with airway inflammation, as measured by exhaled NO, with larger associations seen in those with asthma. The associations were not explained by relative humidity or air pollution exposure.
Few studies have described associations of precipitation with lung function and airway inflammation, especially in healthy children, instead focusing on children and adults with asthma. Two studies in England offer conflicting results, with one study finding that preceding day rainfall was associated with hospitalization for asthma in children and another study finding no association between rainfall and asthma hospitalization in children and adults (5, 25). A U.S.-based study in Maryland found that extreme precipitation events, defined as levels above the 90th percentile relative to a historical baseline, were associated with increased risk of hospitalization for asthma, though in the age 5–17 years subgroup, this association was not significant (3). There is a growing body of literature describing exposure to thunderstorms and tropical cyclones, during which heavy precipitation can occur, and increased risk of hospitalization for respiratory disease (26–28). In particular, thunderstorm events such as the one that occurred in November 2016 in Melbourne, Australia, and led to thousands of emergency department visits for asthma and 10 deaths, have prompted investigations into what is now termed “thunderstorm asthma” (28, 29). Evaluating whether the presence of rain during a thunderstorm affects asthma, a study found thunderstorms with rain, and not those without rain, increased the risk of asthma emergency department visits (2).
Pollen is often implicated as a key mechanism in thunderstorm asthma. Heavy rain and lightning rupture pollen grains into small, easily inhaled particles and wind widely transports the particles (29). Supporting the hypothesis that pollen is a potential mediator in rain-associated health effects, we found that aeroallergen sensitization to different species of grass, tree, and weed plants amplified associations of precipitation with airway inflammation. However, results for pollen from prior studies have been mixed, with some finding higher risk of asthma exacerbation during thunderstorms with high pollen and others finding either lower risk or no difference in asthma exacerbations in relation to high pollen concentrations (30–33). Thus, if not pollen, these studies also suggest mold formation may be a risk factor for asthma exacerbation after a thunderstorm. In addition, exposure to fungal spores is associated with lower lung function in children with asthma (34). In the context of our findings, which demonstrate associations of precipitation with airway inflammation for the longer moving averages, mold or fungus exposure may be a more plausible mediator. Unlike pollen fragment concentrations, which spike immediately after rainfall and then dissipate, mold and fungus concentrations may peak after 3–7 days (35, 36). However, we did not find that indoor aeroallergen sensitization, which included IgE against Aspergillus mold, modified associations of precipitation with lung function or airway inflammation. Another potential mechanism by which precipitation may cause airway inflammation is through sulfuric acid, or “acid rain.” A controlled human exposure study exposed participants to sulfuric acid water droplets and found increases in lower respiratory symptoms in both healthy subjects and participants with asthma and decreases in lung function in participants with asthma after exposure to acidic water droplets (37). Whether the associations we find are related directly to precipitation exposure or through an associated exposure (e.g., aeroallergen) is unknown and warrants further investigation, including human controlled exposure experiments.
Medium levels (5–10 mm) of precipitation relative to low levels of precipitation (<5 mm) were associated with lower lung function with similar direction in the effect for high levels of precipitation relative to low levels. The lack of a clear dose response may be related to changes in behavior. (High precipitation periods may cause adolescents to spend more time inside.) Alternatively, if precipitation effects on lung health are mediated through bioaerosols, it is possible that medium levels of precipitation are more conducive for mold or fungus formation or pollen grain rupture, whereas high levels of precipitation either “drown” the source of the bioaerosol or “wash” it from the air.
We also found that asthma diagnosis modifies the association of precipitation with lung function. At shorter precipitation moving averages, adolescents with asthma had a lower FVC, and adolescents without asthma had a higher FVC in association with precipitation. These differences in the direction of association by asthma status were also seen for longer moving averages and with FEV1. Although it is somewhat surprising to find that precipitation was associated with worse lung function in early adolescents with asthma but higher lung function in early adolescents without asthma, this finding is plausible based on physiologic and controlled exposure studies on humidity. Humidified air has been shown to improve lung function in healthy young adults (38, 39), whereas in children and young adults with asthma, moist air can worsen lung function (40, 41). In a controlled human inhalation-exposure study in mostly young adults, asthma status modified responses to humidified air (41). Although young adults with asthma had an increase in airway resistance with hot humidified air, young adults without asthma had no significant increase in airway resistance, which the authors postulate may be explained by differences in activation of bronchopulmonary C-fiber sensory nerves and their relation to airway inflammation. If the differential susceptibility we describe is being driven not by precipitation but by an associated exposure (e.g., mold, pollen, or fungal spores), it is possible that adolescents with asthma develop airway hyperresponsiveness from these associated exposures without experiencing the benefits of higher air moisture content on lung function. Controlled exposure experiments are needed to verify the direct effects of humidity and precipitation on airway inflammation, especially in those with asthma, and to test for interactive effects of concurrent pollutant exposures.
We observed sex differences in associations between precipitation and lung function and airway inflammation. At shorter moving averages, exposure to precipitation was associated with lower lung function in females than in males, whereas at longer moving averages, the trend was reversed: Longer moving averages of precipitation were associated with lower lung function in males than in females. In females, the association of 7-day precipitation with higher FeNO was of greater magnitude than in males. It is unclear what is driving these sex differences. One potential explanation may relate to sex hormone differences, which have been shown to alter lung function in adolescents with asthma and may also alter the airway response to precipitation and associated exposures (42). Behavioral sex differences, such as time spent outdoors, may be another explanation because male children generally spend more time outdoors than female children (43). FeNO and spirometry measure different aspects of pulmonary physiology; thus, discrepancies in the spirometry and FeNO responses to precipitation among females compared with males are plausible (44).
Our study has a number of strengths. We included adolescents from a large, well-phenotyped cohort study. Our exposure estimates include high-resolution modeled precipitation on an 800 × 800–m scale. Unlike researchers in many studies that used measured air pollution concentrations from central site monitors, we adjusted for air pollution using validated spatially and temporally resolved models to estimate home address concentrations. We further adjusted for a comprehensive set of potential individual and family confounders and predictors of lung function and airway inflammation.
Our study has several limitations. Because of geographic differences in precipitation, our results may not be generalizable to other regions of the country. In addition, we assigned precipitation exposure to home addresses to estimate individual exposure, though this may not reflect actual exposure to precipitation. Our ability to detect differences in spirometry and FeNO in subgroup analyses of adolescents with asthma is limited by the smaller sample size (n = 292), and we were unable to assess for differences in asthma symptoms and medication use in this subgroup. Exposure to pollen and mold was not evaluated in this study and may mediate the associations between precipitation exposure and both lung function and FeNO that we identified. There remains uncertainty whether pollen and other bioaerosols, such as fungi and mold, are mechanistically involved in associations between precipitation and airway inflammation. This warrants further investigation, especially among those with asthma and aeroallergen sensitization.
In the Northeast, increases in annual total precipitation and extreme precipitation events are expected in the future as a result of climate change (45, 46). Determining if differences in exposure to precipitation are associated with acute changes in lung function and airway inflammation, especially in higher-risk groups such as asthma, will inform public health measures and may provide valuable information for counseling families about the risks of environmental exposures.
Conclusions
Greater short-term precipitation may trigger airway inflammation in adolescents, particularly among those with asthma.
Acknowledgments
Acknowledgment
The authors thank the mothers, children, and staff of Project Viva. The authors also thank the PRISM Climate Group at Oregon State University for their work on spatial climate analyses.
Footnotes
Supported by National Institutes of Health, National Institute of Environmental Health Sciences, grants UG3OD023286, P30-ES000002, and R01HD023286.
Author Contributions: N.J.N., S.L.R.-S., H.L.-G., E.O., D.R.G., and M.B.R. conceived of and designed the study. N.J.N., S.L.R.-S., H.L.-G., K.C., J.C.B., E.O., D.R.G., and M.B.R. contributed to the acquisition, analysis, and interpretation of the data. N.J.N., S.L.R.-S., H.L.-G., K.C., J.C.B., E.O., D.R.G., and M.B.R. participated in revising the manuscript and approved the final version submitted for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, et al. Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2021. [Google Scholar]
- 2. Grundstein A, Sarnat SE, Klein M, Shepherd M, Naeher L, Mote T, et al. Thunderstorm associated asthma in Atlanta, Georgia. Thorax . 2008;63:659–660. doi: 10.1136/thx.2007.092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soneja S, Jiang C, Fisher J, Upperman CR, Mitchell C, Sapkota A. Exposure to extreme heat and precipitation events associated with increased risk of hospitalization for asthma in Maryland, U.S.A. Environ Health . 2016;15:57. doi: 10.1186/s12940-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’Amato G, Vitale C, D’Amato M, Cecchi L, Liccardi G, Molino A, et al. Thunderstorm-related asthma: what happens and why. Clin Exp Allergy . 2016;46:390–396. doi: 10.1111/cea.12709. [DOI] [PubMed] [Google Scholar]
- 5. Khot A, Burn R, Evans N, Lenney W, Storr J. Biometeorological triggers in childhood asthma. Clin Allergy . 1988;18:351–358. doi: 10.1111/j.1365-2222.1988.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 6. Weinhold B. A spreading concern: inhalational health effects of mold. Environ Health Perspect . 2007;115:A300–A305. doi: 10.1289/ehp.115-a300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Kunkel KE, Stevens LE, Buddenberg A, Dobson JG, Easterling DR.2015.
- 8. Nassikas N, Rifas-Shiman S, Luttmann-Gibson H, Stanley Lee A, Chen K, Blossom JC, et al. Short-term exposure to precipitation and respiratory health in adolescents. ATS 2022 International Conference. Am J Respir Crit Care Med . 2022;205:A5165. [Google Scholar]
- 9. Nassikas NJ, Rifas-Shiman S, Luttmann-Gibson H, Chen K, Blossom JC, Oken E, et al. Precipitation and lung health in children with asthma. 2022 Annual International Society for Environmental Epidemiology Conference. Environ Health Perspect . 2022 [Google Scholar]
- 10. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol . 2015;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oregon State University, PRISM Climate Group. 2022. https://prism.oregonstate.edu
- 12. Zanobetti A, Coull BA, Luttmann-Gibson H, van Rossem L, Rifas-Shiman SL, Kloog I, et al. Ambient particle components and newborn blood pressure in Project Viva. J Am Heart Assoc . 2021;10:e016935. doi: 10.1161/JAHA.120.016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, et al. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol . 2020;54:11037–11047. doi: 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. Assessing NO2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol . 2020;54:1372–1384. doi: 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int . 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17. Khalili B, Boggs PB, Bahna SL. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy . 2007;62:1171–1174. doi: 10.1111/j.1398-9995.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 18. Boot JD, de Ridder L, de Kam ML, Calderon C, Mascelli MA, Diamant Z. Comparison of exhaled nitric oxide measurements between NIOX MINO electrochemical and Ecomedics chemiluminescence analyzer. Respir Med . 2008;102:1667–1671. doi: 10.1016/j.rmed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 19. Flashner BM, Rifas-Shiman SL, Oken E, Camargo CA, Jr, Platts-Mills TAE, Workman L, et al. Contributions of asthma, rhinitis and IgE to exhaled nitric oxide in adolescents. ERJ Open Res . 2021;7:00945-2020. doi: 10.1183/23120541.00945-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peng C, Cardenas A, Rifas-Shiman SL, Hivert MF, Gold DR, Platts-Mills TA, et al. Epigenome-wide association study of total serum immunoglobulin E in children: a life course approach. Clin Epigenetics . 2018;10:55. doi: 10.1186/s13148-018-0488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson JM, Workman L, Schuyler AJ, Rifas-Shiman SL, McGowan EC, Oken E, et al. Allergen sensitization in a birth cohort at midchildhood: focus on food component IgE and IgG4 responses. J Allergy Clin Immunol . 2018;141:419–423.e5. doi: 10.1016/j.jaci.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salo PM, Arbes SJ, Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol . 2014;134:350–359. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. 2021. https://www.r-project.org/
- 24. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med . 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson W, Prescott GJ, Packham S, Mullins J, Brookes M, Seaton A. Asthma admissions and thunderstorms: a study of pollen, fungal spores, rainfall, and ozone. QJM . 2001;94:429–433. doi: 10.1093/qjmed/94.8.429. [DOI] [PubMed] [Google Scholar]
- 26. Weinberger KR, Kulick ER, Boehme AK, Sun S, Dominici F, Wellenius GA. Association between Hurricane Sandy and emergency department visits in New York City by age and cause. Am J Epidemiol . 2021;190:2138–2147. doi: 10.1093/aje/kwab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parks RM, Anderson GB, Nethery RC, Navas-Acien A, Dominici F, Kioumourtzoglou M-A. Tropical cyclone exposure is associated with increased hospitalization rates in older adults. Nat Commun . 2021;12:1545. doi: 10.1038/s41467-021-21777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thien F, Beggs PJ, Csutoros D, Darvall J, Hew M, Davies JM, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health . 2018;2:e255–e263. doi: 10.1016/S2542-5196(18)30120-7. [DOI] [PubMed] [Google Scholar]
- 29. Grundstein A, Shepherd M, Miller P, Sarnat SE. The role of mesoscale-convective processes in explaining the 21 November 2016 epidemic thunderstorm asthma event in Melbourne, Australia. J Appl Meteorol Climatol . 2017;56:1337–1343. [Google Scholar]
- 30. Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol . 1995;95:955–961. doi: 10.1016/s0091-6749(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 31. Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol . 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 32. Newson R, Strachan D, Archibald E, Emberlin J, Hardaker P, Collier C. Effect of thunderstorms and airborne grass pollen on the incidence of acute asthma in England, 1990-94. Thorax . 1997;52:680–685. doi: 10.1136/thx.52.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith ML, MacLehose RF, Chandler JW, Berman JD. Thunderstorms, pollen, and severe asthma in a midwestern, USA, urban environment, 2007-2018. Epidemiology . 2022;33:624–632. doi: 10.1097/EDE.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 34. Tham R, Erbas B, Dharmage SC, Tang ML, Aldakheel F, Lodge CJ, et al. Outdoor fungal spores and acute respiratory effects in vulnerable individuals. Environ Res . 2019;178:108675. doi: 10.1016/j.envres.2019.108675. [DOI] [PubMed] [Google Scholar]
- 35. Matveeva L, Pakhtinov V, Tikhonov Y. Study of micromycete destructive power in gypsum and polymeric binding composite construction materials. IOP Conf Ser Mater Sci Eng . 2019;687:022031. [Google Scholar]
- 36.Institute of Medicine, Committee on Damp Indoor Spaces and Health. Human health effects associated with damp indoor environments. 2004. https://www.ncbi.nlm.nih.gov/books/NBK215639/?report=reader
- 37. Hackney JD, Linn WS, Avol EL. Acid fog: effects on respiratory function and symptoms in healthy and asthmatic volunteers. Environ Health Perspect . 1989;79:159–162. doi: 10.1289/ehp.8979159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim JH, Hyong IH. Analysis of changes in pulmonary functions at rest following humidity changes. J Phys Ther Sci . 2015;27:1063–1065. doi: 10.1589/jpts.27.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eschenbacher WL, Moore TB, Lorenzen TJ, Weg JG, Gross KB. Pulmonary responses of asthmatic and normal subjects to different temperature and humidity conditions in an environmental chamber. Lung . 1992;170:51–62. doi: 10.1007/BF00164755. [DOI] [PubMed] [Google Scholar]
- 40. Aitken ML, Marini JJ, Culver BH. Humid air increases airway resistance in asthmatic subjects. West J Med . 1988;149:289–293. [PMC free article] [PubMed] [Google Scholar]
- 41. Hayes D, Jr, Collins PB, Khosravi M, Lin R-L, Lee L-Y. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med . 2012;185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med . 2018;18:58. doi: 10.1186/s12890-018-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klinker CD, Schipperijn J, Kerr J, Ersbøll AK, Troelsen J. Context-specific outdoor time and physical activity among school-children across gender and age: using accelerometers and GPS to advance methods. Front Public Health . 2014;2:20. doi: 10.3389/fpubh.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spergel JM, Fogg MI, Bokszczanin-Knosala A. Correlation of exhaled nitric oxide, spirometry and asthma symptoms. J Asthma . 2005;42:879–883. doi: 10.1080/02770900500371344. [DOI] [PubMed] [Google Scholar]
- 45.Easterling DR, Kunkel KE, Arnold JR, Knutson T, LeGrande AN, Leung LR, et al. In: Climate science special report: Fourth National Climate Assessment, Volume I. Wuebbles DJ, Fahey DW, Hibbard KA, Dokken DJ, Stewart BC, Maycock TK, editors. Washington, DC: U.S. Global Change Research Program; 2017. Precipitation change in the United States; pp. 207–230. [Google Scholar]
- 46.Gonzalez P, Garfin GM, Breshears DD, Brooks KM, Brown HE, Elias EH, et al. In: Impacts, risks, and adaptation in the United States: Fourth National Climate Assessment, Volume II. Reidmiller DR, Avery CW, Easterling DR, Kunkel KE, Lewis KLM, Maycock TK, et al., editors. Washington, DC: U.S. Global Change Research Program; 2018. Southwest; pp. 1101–1184. [Google Scholar]