Mesenchymal signaling plays a critical role in regulating epithelial proliferation during lung development. Hedgehog (HH) and platelet-derived growth factor (PDGF) pathways are central to lung morphogenesis, but the cross-talk between these signaling pathways is poorly characterized. Understanding this cross-talk represents a fundamental first step toward the discovery of therapeutic modalities for neonatal diseases of lung maldevelopment and injury. In this issue of the Journal, Yie and colleagues (pp. 523–536) increase our understanding of cellular proliferation cascades in that HH and PDGF pathways seem to converge during the highly complex process of pulmonary development in the postnatal period (1).
Lung morphogenesis is a highly complex process in which lung mesenchymal progenitors regulate epithelial proliferation and differentiation, forming a three-dimensional scaffold of the respiratory system. From the formation of the conducting airways to the alveolar septation and maturation, this dynamic process is characterized by distinct macroscopic steps. Pulmonary development does not conclude with birth but continues well into childhood. Growth factors are the signal conductors for this process, with multiple signaling pathways seemingly working in parallel or sequentially as their outputs peak and drop during different steps of development (2). Although multiple growth factors critical for lung morphogenesis have been identified, including HH and PDGF, little is known about the cross-talk between HH and PDGF pathways. These signaling conductors differentially affect cell populations in a temporal and spatial manner. Focusing on the mesenchymal cell populations, Yie and colleagues provide insight into the possible shared molecular cascade between HH and PDGF pathways during postnatal lung development.
The mesenchymal progenitor cells proliferate and differentiate into distinct mature cell populations, including fibroblasts. Recent single-cell RNA sequencing of mouse and human lung tissues identified several distinct fibroblast populations based on cellular markers, including myofibroblasts, lipofibroblasts, and matrix fibroblasts that support the developing pulmonary structures (2, 3). Published studies have implicated HH and PDGF signaling pathways in the regulation of fibroblast proliferation. Although both of these pathways play a fundamental role in postnatal alveolarization and lung maturation (2–4), their interactions remain poorly understood. In their paper, Yie and colleagues carefully document the phenotypes that arise from HH and PDGF signaling induction or inhibition at different stages of lung development, focusing primarily on postnatal sampling. The team found that early perinatal inhibition of HH signaling is followed by arrested alveolarization closely resembling lung phenotypes that resulted from PDGF murine knockout models. The emerging phenotypes included enlarged alveoli, decreased tissue-to-alveolar area ratios, and decreased HH transcriptional targets such as the glioma-associated zinc finger transcription factors (GLI). These findings were reinforced by previous studies (5–7), revealing decreased myofibroblast populations in lung development and supporting the authors’ hypothesis that HH and PDGF pathways converge at a critical point of alveolarization.
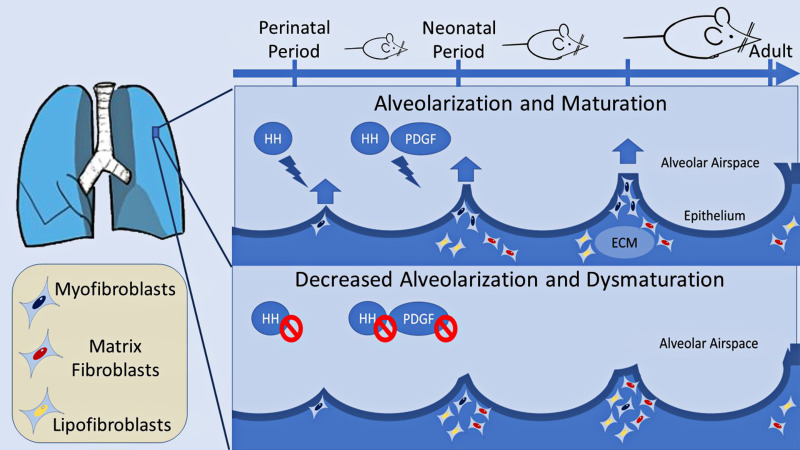
Researchers commonly use murine models to study lung development and phenotypes of human neonatal diseases. Newborn rodents exhibit lung phenotypes resembling the preterm human lung at the canalicular-saccular stage (8). Using HH and PDGF reporter mice, Yie and colleagues identified a cell population that seems to be sensitive to both HH and PDGF signaling and constitutes up to one-fifth of all alveolar cells. This population is more sensitive to HH and PDGF inhibition at the early perinatal period as opposed to the early neonatal period. Complex transcriptomic analysis reinforces the aforementioned findings of a common pathway because HH inhibition alters the expression of genes involved in the PDGF signaling cascade (such as Klf2, Klf9, Txnip, and Cdh11) that have previously been identified and recognized as targets of PDGF regulation (9). These gene expression changes were more pronounced in the myofibroblast population, and the findings were mirrored during PDGF conditional ablation. HH inhibition seemed to alter the lipofibroblast and matrix fibroblast populations at the expense of the myofibroblasts (Figure 1). The use of single-cell RNA sequencing was important to identify distinct fibroblast populations based on gene expression and in support of the authors’ findings.
Figure 1.
Hedgehog (HH) and platelet-derived growth factor (PDGF) control alveolar development and maturation by interacting with the mesenchyme. In HH and PDGF coreporter mice employed by Yie and colleagues (1), inhibition of either pathway leads to decreased alveolarization with abnormally enlarged alveoli and inhibition of secondary septation. The myofibroblast population is reduced after the pathway inhibition at a critical point of alveolar development in favor of lipofibroblasts and matrix fibroblasts. HH signaling is found to control downstream PDGF gene expression, indicative of cross-talk between the pathways. ECM = extracellular matrix.
The studies by Yie and colleagues provide convincing evidence of a common signaling cascade downstream of HH and PDGF, expanding our understanding of fibroblast proliferation during postnatal lung development using multiple signaling modalities. Understanding lung morphogenesis cascades is fundamental in our effort to treat lung diseases of the neonatal period that are the result of abnormal lung development or neonatal lung injury such as bronchopulmonary dysplasia (BPD). BPD is linked to prematurity and is characterized by lung developmental arrest caused by stressors such as hyperoxic exposure that exert differential involvement of the HH and PDGF pathways (10–12). The mesenchyme plays a critical role in BPD pathogenesis characterized by alveolar simplification, vascular rarefaction, and a variable degree of fibrosis similar to the phenotypic model seen in the studies by Yie and colleagues. Future research is needed to expand their findings using in vitro and organoid models as seen by Chapman and colleagues (13), where progenitor interaction of different origins may reveal additional molecular targets critical for HH and PDGF cross-talk. It is also beneficial to continue these studies with models of phenotypic rescue, especially in clinically relevant neonatal models of lung disease. In addition, exploration of other cell-to-cell interactions is essential. During alveolarization, HH signaling regulates various mesenchyme-derived cells, such as fibroblasts, pericytes, and endothelial cells, through activation of GLI and FOXF1 transcription factors, the latter of which plays a critical role in endothelial proliferation during lung development and lung injury (2, 14, 15). Therefore, the study by Yie and colleagues could be the starting point for examining the potential cross-talk between HH and PDGF in other cell populations, such as the lung endothelium and pericytes. Use of transgenic animal models is a critical step in expanding our understanding of processes that may be relevant to human physiology and pathophysiology; ultimately, it will be imperative to correlate these findings with human sequencing data and biomarkers from patients with BPD and other neonatal lung diseases.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2023-0031ED on February 16, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Yie TA, Loomis CA, Nowatzky J, Khodadadi-Jamayran A, Lin Z, Cammer M, et al. Hedgehog and platelet-derived growth factor signaling intersect during postnatal lung development. Am J Respir Cell Mol Biol . 2023;68:523–536. doi: 10.1165/rcmb.2022-0269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev . 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun X, Perl AK, Li R, Bell SM, Sajti E, Kalinichenko VV, et al. NHLBI LungMAP Consortium A census of the lung: CellCards from LungMAP. Dev Cell . 2022;57:112–145.e2. doi: 10.1016/j.devcel.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li R, Bernau K, Sandbo N, Gu J, Preissl S, Sun X. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife . 2018;7:e36865. doi: 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kugler MC, Loomis CA, Zhao Z, Cushman JC, Liu L, Munger JS. Sonic hedgehog signaling regulates myofibroblast function during alveolar septum formation in murine postnatal lung. Am J Respir Cell Mol Biol . 2017;57:280–293. doi: 10.1165/rcmb.2016-0268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao F, Li C, Danopoulos S, Al Alam D, Peinado N, Webster S, et al. Hedgehog-responsive PDGFRa+ fibroblasts maintain a unique pool of alveolar epithelial progenitor cells during alveologenesis. Cell Rep . 2022;39:110608. doi: 10.1016/j.celrep.2022.110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol . 2013;305:L229–L239. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 8. Damianos A, Kulandavelu S, Chen P, Nwajei P, Batlahally S, Sharma M, et al. Neonatal intermittent hypoxia persistently impairs lung vascular development and induces long-term lung mitochondrial DNA damage. J Appl Physiol (1985) . 2022;133:1031–1041. doi: 10.1152/japplphysiol.00708.2021. [DOI] [PubMed] [Google Scholar]
- 9. Chen WV, Delrow J, Corrin PD, Frazier JP, Soriano P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat Genet . 2004;36:304–312. doi: 10.1038/ng1306. [DOI] [PubMed] [Google Scholar]
- 10. Ahlfeld SK, Conway SJ. Aberrant signaling pathways of the lung mesenchyme and their contributions to the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol . 2012;94:3–15. doi: 10.1002/bdra.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dang H, Wang S, Yang L, Fang F, Xu F. Upregulation of Shh and Ptc1 in hyperoxia-induced acute lung injury in neonatal rats. Mol Med Rep . 2012;6:297–302. doi: 10.3892/mmr.2012.929. [DOI] [PubMed] [Google Scholar]
- 12. Oak P, Pritzke T, Thiel I, Koschlig M, Mous DS, Windhorst A, et al. Attenuated PDGF signaling drives alveolar and microvascular defects in neonatal chronic lung disease. EMBO Mol Med . 2017;9:1504–1520. doi: 10.15252/emmm.201607308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest . 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang G, Wen B, Deng Z, Zhang Y, Kolesnichenko OA, Ustiyan V, et al. Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling. Nat Commun . 2022;13:2080. doi: 10.1038/s41467-022-29746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ustiyan V, Bolte C, Zhang Y, Han L, Xu Y, Yutzey KE, et al. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev Biol . 2018;443:50–63. doi: 10.1016/j.ydbio.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]