Abstract
Staphylococcus aureus and Salmonella spp. are common causes of bone diseases; however, the immune response during such infections is not well understood. Colony-stimulating factors (CSF) have a profound influence on osteoclastogenesis, as well as the development of immune responses following infection. Therefore, we questioned whether interaction of osteoblasts with two very different bacterial pathogens could affect CSF expression by these cells. Cultured mouse and human osteoblasts were exposed to various numbers of S. aureus or Salmonella dublin bacteria, and a comprehensive analysis of granulocyte-macrophage (GM)-CSF, granulocyte (G)-CSF, macrophage (M)-CSF, and interleukin-3 (IL-3) mRNA expression and cytokine secretion was performed. Expression of M-CSF and IL-3 mRNAs by mouse osteoblasts was constitutive and did not increase significantly following bacterial exposure. In contrast, GM-CSF and G-CSF mRNA expression by mouse osteoblasts was dramatically upregulated following interaction with either viable S. aureus or Salmonella. This increased mRNA expression also translated into high levels of GM-CSF and G-CSF secretion by mouse and human osteoblasts following bacterial exposure. Viable S. aureus and Salmonella induced maximal levels of CSF mRNA expression and cytokine secretion compared to UV-killed bacteria. Furthermore, GM-CSF and G-CSF mRNA expression could be induced in unexposed osteoblasts separated by a permeable Transwell membrane from bacterially exposed osteoblasts. M-CSF secretion was increased in cultures of exposed human osteoblasts but not in exposed mouse osteoblast cultures. Together, these studies are the first to define CSF expression and suggest that, following bacterial exposure, osteoblasts may influence osteoclastogenesis, as well as the development of an immune response, via the production of these cytokines.
Two principal cell populations are responsible for the continual process of bone remodeling. Osteoclasts derive from myeloid precursors (59) and drive the resorption of bone by acidification and release of lysosomal enzymes (73). Conversely, osteoblasts derive from a mesenchymal bone marrow precursor (3) and produce components of bone, principally type I collagen. Osteoblasts also catalyze the calcification process and produce soluble factors which serve to modulate the activity, or the formation, of osteoclasts. In this manner, osteoblasts function as a principal director of osteoclast function, and it is the communication between these two cell populations that constitutes one of the most important mechanisms of bone formation.
Among the osteoblast-derived factors which may have profound effects on osteoclastogenesis, and/or the activity of osteoclasts, are the colony-stimulating factors (CSF) macrophage (M)-CSF, granulocyte (G)-CSF, and granulocyte-macrophage (GM)-CSF. For example, op/op mice are deficient in a functional gene encoding M-CSF (83) and due to reduced M-CSF production (5, 20, 40) these mice have reduced numbers of osteoclasts and are osteopetrotic. Furthermore, M-CSF has been shown to facilitate osteoclastogenesis and also to activate osteoclast function (30, 37, 39, 72, 82). Since M-CSF augments osteoclast maturation and activity, the presence of this CSF has been closely linked with bone resorption (12, 42, 56). The fact that osteoblasts can upregulate their expression of M-CSF (22, 38, 62, 63, 79) suggests that osteoblast-derived M-CSF secretion may stimulate osteoclast development and activity (11).
Similarly, the presence of excess G-CSF stimulates bone resorption. When this CSF was used as an exogenous therapy (67, 71) or genetically overexpressed (70), increased bone resorption was observed. The mechanisms of G-CSF-induced bone resorption are not clear, although it has been suggested that osteoblast-derived G-CSF (21, 68) might affect bone remodeling.
GM-CSF, like M-CSF, facilitates the early differentiation of myeloid precursors into osteoclast precursors (29, 45, 46, 58, 69). Conversely, however, GM-CSF seems to limit the formation of the more mature, multinucleated osteoclasts (33, 65, 66, 76). Since osteoblasts can also be stimulated to secrete GM-CSF (21, 32, 51, 80), it is also possible that osteoblast-derived GM-CSF contributes to the regulation of osteoclastogenesis.
Bacterial infections and their products can be potent stimulators of osteoclastogenesis and bone resorption (55). Bacteria can destroy bone by several possible mechanisms, including the production of acids or proteases, or by indirectly stimulating osteoclastogenesis. However, it is unclear what mechanisms are the most important in the pathophysiology of bacterium-induced bone resorption. This question is further complicated by the diversity of bone and joint diseases (e.g., osteomyelitis, arthritis, or periodontitis) and by the diversity of bacterial species which can act as causative agents (e.g., Staphylococcus spp. and Salmonella spp.). Since osteoblasts dictate osteoclast function, it is logical to begin to question how bacterial infections might affect osteoblast activity. Surprisingly, few studies to date have addressed the response of osteoblasts following their interaction with viable bacteria capable of causing bone diseases (55).
Osteoblast-induced CSF production at the site of bacterial infection in the bone could have several consequences. First, excess production of M-CSF or G-CSF could increase osteoclastogenesis and promote bone resorption. Alternatively, induced production of GM-CSF would likely limit the formation of multinucleated osteoclasts. Each of these CSF could also have profound effects on hematopoiesis and augment the inflammatory response at the site of bone infection. However, such speculations have little meaning in the absence of an understanding of which CSF are produced in response to infection.
In the present study, we have addressed for the first time the ability of gram-positive (Staphylococcus aureus) and gram-negative (Salmonella dublin) bacteria to induce CSF expression in cultured mouse and human osteoblasts. Surprisingly, we found that viable Staphylococcus or Salmonella could induce significant levels of GM-CSF and G-CSF production in both mouse and human osteoblasts. Only human osteoblasts directly exposed to bacteria could be induced to increase their secretion of M-CSF over constitutive levels. These results strongly suggest that osteoblasts respond to bacterial challenge by the production of CSF that may contribute to the host response and modulate osteoclastogenesis.
MATERIALS AND METHODS
Isolation and culture of mouse osteoblasts.
Primary osteoblast cell cultures were prepared from mouse neonate calvariae by sequential collagenase-protease digestion as previously described by our laboratory (9). Cell isolates were pooled in osteoblast growth medium (OBGM) consisting of Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Equitech, Ingram, Tex.), 25 mM HEPES, 2 g of sodium bicarbonate per liter, 75 μg of glycine per ml, 100 μg of ascorbic acid per ml, 40 ng of vitamin B12 per ml, 2 μg of p-aminobenzoic acid per ml, 200 ng of biotin per ml, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml (Fungizone) (pH 7.4) (60). Cells were seeded at a density of 105 per well in 12-well cluster plates and incubated at 37°C in a 5% CO2 atmosphere until they reached confluence (6 to 7 days). Greater than 99% of these cells expressed markers of osteoblasts, including type I collagen, osteocalcin, and alkaline phosphatase when subjected to immunofluorimetric analyses as previously described (9).
Normal human osteoblast cultures.
Normal human osteoblasts (Clonetics, San Diego, Calif.) were purchased and propagated in accordance with the guidelines provided by the vendor and as previously described by our laboratory (9). Cells were seeded in 25-cm2 flasks and incubated at 37°C in a 5% CO2 atmosphere and medium supplied by the manufacturer, which contained 10% fetal calf serum, ascorbic acid, gentamicin, and amphotericin B. After the cells reached approximately 80% confluence (5 to 9 days), they were removed from the flasks using 0.025% trypsin–0.01% EDTA, washed in medium, and seeded into six-well plates. After the cells reached approximately 80% confluence (6 to 7 days), they were infected with bacteria as described below. These commercially available cells have been extensively characterized as osteoblasts (28).
Exposure of cultured mouse and human osteoblasts to bacteria.
S. aureus strain UAMS-1 (ATCC 49230) is a clinical osteomyelitis isolate and was grown overnight in 5 ml of tryptic soy broth in a shaking water bath at 37°C. S. dublin strain SL1363 is a wild-type strain found in mice which is pathogenic and potentially lethal when given to susceptible mice (50% lethal dose, 106 bacteria) and was grown overnight in 5 ml of Luria broth at 37°C. Bacteria were harvested by centrifugation for 10 min at 4,300 × g at 4°C and washed once in 5 ml of Hanks balanced salt solution. The pellet was then resuspended in 5 ml of OBGM without antibiotics. Confluent layers of osteoblasts were exposed to S. aureus or Salmonella suspensions at the indicated ratios of bacteria to osteoblasts in 4 ml of OBGM without antibiotics for 45 min at 37°C. After the bacteria had been washed off the osteoblasts, the cultures were incubated for 3 h in 4 ml of OBGM supplemented with 25 μg of gentamicin per ml to kill any remaining extracellular bacteria. Cultures were washed again and incubated in gentamicin-containing medium for the indicated periods of time for determination of CFU as previously described (19), for RNA isolation, or for collection of culture supernates.
In some experiments, S. aureus or Salmonella bacteria were exposed to short-wavelength (250-nm) UV light for 5 or 3 min, respectively, prior to addition to cultured osteoblasts. Times used for UV inactivation were empirically determined to reduce the percentage of viable bacteria to less than 0.01% as determined by colony counting (19).
RNA isolation, reverse transcription, and semiquantitative PCR.
At the indicated times, RNA was extracted from cultured mouse osteoblasts, reverse transcribed, and subjected to semiquantitative reverse transcription (RT)-PCR as previously described (6–9, 47). Briefly, total RNA was isolated using TRIZOL Reagent (Gibco-BRL, Gaithersburg, Md.) and 2 μg of total RNA was reverse transcribed in the presence of random hexamers using 200 U of RNase H-free Moloney leukemia virus reverse transcriptase (Superscript II; Gibco-BRL) in the buffer supplied by the manufacturer. PCR was performed on 5% of the total cDNA to quantify expression of the mRNAs encoding interleukin-6 (IL-6), IL-12p40, IL-12p35, and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) using denaturation at 95°C, annealing at 60°C, and extension at 72°C (Robocycler 40; Stratagene, La Jolla, Calif.), with the first of 27 total cycles having extended denaturation and annealing times. PCR primers were derived from the published sequences of IL-3 (10), GM-CSF (50), M-CSF (17), and G-CSF (75) and included CTGATGCTCTTCCACCTGGGACTCC and CATTCGCAGATGTAGGCAGGCAACA to amplify mouse IL-3, TTTACTTTTCCTGGGCATTGTGGTC and CCGCATAGGTGGTAACTTGTGTTTC to amplify mouse GM-CSF, GGCTGGCTTGGCTTGGGATGATTCT and GTCTGTCAGTCTCTGCCTGGATGCTG to amplify mouse M-CSF, CGAAGGCTTCCCTGAGTGGCTGCTCTA and GGACACCTCCTGCCCGGCGCTGG to amplify mouse G-CSF, and CCATCACCATCTTCCAGGAGCGAG and CACAGTCTTCTGGGTGGCAGTGAT to amplify G3PDH.
Following PCR, 15% of each amplified sample was electrophoresed on ethidium bromide-stained agarose gels and visualized under UV illumination. Densitometric analysis of the RT-PCR product bands was performed using NIH Image (obtained from the National Institutes of Health website [http://rsb.info.nih.gov/nih-image]). Each gel image was imported into NIH Image by Adobe Photoshop (Adobe Systems, San Jose, Calif.), a gel-plotting macro was used to outline the bands, and the intensity was calculated on the Uncalibrated OD setting.
To ensure that similar amounts of input RNA were reverse transcribed, RNA was quantified by DNA dipsticks prior to the cDNA reactions (InVitrogen, San Diego, Calif.). In addition, PCR amplification of the G3PDH housekeeping gene was performed on cDNA from each sample to ensure equal RNA input and RT similar efficiencies. The identity of the amplified fragments was verified by size comparison with DNA standards (Promega).
Quantification of IL-3, GM-CSF, M-CSF, and G-CSF secretion in culture supernatants.
Capture enzyme-linked immunosorbent assays (ELISAs) were performed to quantify mouse and human GM-CSF, G-CSF, M-CSF, and IL-3 secretion using a methodology which has been previously described (7, 8). The following pairs of capture and detection antibodies were used to quantify mouse CSF secretion, respectively: anti-mouse GM-CSF (clone MP1-22E9) and biotinylated anti-mouse GM-CSF (clone MP1-31G6; PharMingen, San Diego, Calif.), polyclonal anti-mouse G-CSF and biotinylated anti-mouse G-CSF (clone 67604.111; R&D Systems, Minneapolis, Minn.), polyclonal anti-mouse M-CSF and biotinylated polyclonal anti-mouse M-CSF (R&D Systems), and anti-mouse IL-3 (clone MP2-8F8) and biotinylated anti-mouse IL-3 (clone MP2-43D11; PharMingen).
The following pairs of capture and detection antibodies were used to quantify human CSF secretion, respectively: anti-human GM-CSF (clone BVD2-23B6) and biotinylated anti-human GM-CSF (clone BVD2-21C11; PharMingen), anti-human G-CSF (clone BVD-13-3A5) and biotinylated anti-human G-CSF (clone BVD11-37G10; PharMingen), polyclonal anti-human M-CSF and biotinylated anti-human M-CSF (clone 26730.11; R&D Systems), and anti-human IL-3 (clone BVD8-3G11) and biotinylated anti-human IL-3 (clone BVD3-1F9; PharMingen).
It was necessary to biotinylate the anti-mouse G-CSF (clone 67604.111), the polyclonal anti-mouse M-CSF, and the anti-human M-CSF (clone 26730.11) antibodies in our laboratory since these reagents were only available in purified form. This was accomplished using the EZ-Link Sulfo-NHS biotinylation kit (Pierce, Rockford, Ill.) in accordance with the directions supplied by the manufacturer.
Capture antibodies were used to coat high protein binding microtiter plates (Corning, Corning, N.Y.) at 15 μg/ml for 18 h. After washing, antibody-coated plates were blocked with 2% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) in phosphate-buffered saline for 2 h; this was followed by addition of supernatants taken from osteoblast cultures at the indicated times after bacterial exposure. After overnight incubation, unbound material was washed off and biotinylated detection antibodies were added at 10 μg/ml and the mixture was incubated for 2 h. After washing, bound antibody was detected by addition of streptavidin-horseradish peroxidase (Southern Biotechnology Associates, Birmingham, Ala.) for 45 min; the substrate tetramethylbenzidine (Promega, Madison, Wis.) was then added. Colorimetric reactions were stopped by the addition of 0.5 M H2SO4, and A450 was measured (model 550 microplate reader; Bio-Rad, Hercules, Calif.).
Cytokine levels in culture supernatants were quantified by extrapolation from standard curves generated by determining absorbances using limiting dilutions of recombinant cytokines purchased from PharMingen or R&D Systems. The sensitivities of the ELISAs used to quantify mouse or human CSF were determined to be 50 and 50 pg/ml for GM-CSF, 30 and 40 pg/ml for G-CSF, 90 and 60 pg/ml for M-CSF, and 30 and 50 pg/ml for IL-3. Results are presented as means of triplicate determinations ± standard deviations. Statistically significant differences in CSF secretion were determined using the Student t test (GraphPad, San Diego, Calif.).
Transwell cocultures of bacterium-exposed mouse osteoblasts and unexposed mouse osteoblasts.
Mouse osteoblasts were cultured in the bottom chamber of a 12-well Transwell (Corning) tissue culture plate and exposed to S. aureus or Salmonella at various ratios of bacteria to osteoblasts in medium containing no antibiotics. After 45 min, extracellular bacteria were washed off and culture medium containing gentamicin was added. Unexposed mouse osteoblasts were also cultured in the upper Transwell chamber and physically separated from the bacterium-exposed osteoblasts by a 0.45-μm-pore-size membrane. Cells were cocultured for 12 h, and then RNA was isolated from cells within the upper and lower chambers. RT-PCR was performed to detect CSF mRNA expression as described above.
RESULTS
Enumeration of viable intracellular bacteria following exposure of mouse osteoblasts.
The ability of S. aureus and Salmonella to enter mouse osteoblasts was demonstrated using a measurement of viable intracellular bacteria. Mouse osteoblasts were exposed to the indicated numbers of bacteria for 45 min in medium without antibiotics. After washing off of extracellular bacteria, medium containing gentamicin was added for 3 h of incubation to kill the remaining extracellular bacteria. This procedure was demonstrated to be completely effective in eliminating extracellular viable bacteria as determined by plating of culture supernatants onto tryptic soy agar to quantify the number of viable extracellular bacteria remaining. Following bacterial inoculation, osteoblasts were lysed and dilutions of the lysates were plated onto tryptic soy agar to quantify the number of viable bacteria present. At an initial exposure ratio of 250 S. aureus bacteria to one osteoblast, approximately 1 viable bacterium remained per osteoblast at 3 h postexposure (Table 1). A similar result was obtained for Salmonella at an initial exposure ratio of 10 bacteria to one osteoblast. Taken together, the results in Table 1 serve to illustrate the small number of viable bacteria which remained following the brief initial exposure of the cultured osteoblasts to S. aureus or Salmonella.
TABLE 1.
Enumeration of viable bacteria following infection of mouse osteoblasts
| Initial bacterial exposure | No. of osteoblasts (106) | Ratio of bacteria to osteoblasts | Mean no. of CFU ± SD (106) at:
|
|
|---|---|---|---|---|
| 3 h | 24 h | |||
| S. aureus | ||||
| 1.3 × 109 | 5 | 250:1 | 6.7 ± 0.4 | 4.0 ± 0.3 |
| 4.2 × 108 | 5 | 75:1 | 3.3 ± 1.1 | 1.8 ± 0.4 |
| 1.3 × 108 | 5 | 25:1 | 0.1 ± 0.05 | 0.1 ± 0.02 |
| Salmonella | ||||
| 5.0 × 107 | 5 | 10:1 | 3.5 ± 0.9 | 0.9 ± 0.2 |
| 1.7 × 107 | 5 | 3:1 | 0.2 ± 0.02 | 0.2 ± 0.06 |
| 5.0 × 106 | 5 | 1:1 | 0.1 ± 0.04 | 0.1 ± 0.02 |
S. aureus-induced CSF mRNA expression by mouse osteoblasts.
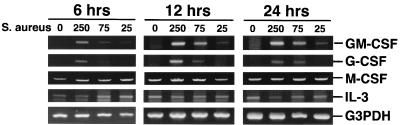
Mouse osteoblasts were cultured in the presence of various numbers of S. aureus bacteria for 45 min, and then viable extracellular bacteria were eliminated. At various times postexposure, RNA was isolated from these cells and a semiquantitative RT-PCR was performed to determine CSF mRNA expression. GM-CSF and G-CSF mRNAs were each induced more than 15-fold from minimal constitutive levels by 12 h postexposure at an initial exposure ratio of 250:1 (Fig. 1). Importantly, increases in mRNA levels occurred in a dose-dependent manner that paralleled the numbers of S. aureus bacteria initially added. In five different RT-PCR analyses, measurable levels of GM-CSF and G-CSF mRNAs were always induced (14.4 ± 3.4- and 12.6 ± 3.9-fold, respectively, by 12 h postexposure). This was in contrast to M-CSF and IL-3 mRNA expression, which was constitutive but did not increase significantly by 12 h postinfection. These time-dependent increases in GM-CSF and G-CSF message expression could not be ascribed to differences in input RNA or to differences in the efficiency of RT, as evidenced by RT-PCR amplification of the G3PDH housekeeping gene for each sample.
FIG. 1.
CSF mRNA expression following exposure of mouse osteoblasts to S. aureus. Murine osteoblasts were left unexposed (lanes 0) or exposed to S. aureus at the indicated ratio (250:1, 75:1, or 25:1 bacterium-to-osteoblast ratio) for 45 min, and then extracellular bacteria were removed. RNA was isolated 6, 12, and 24 h following exposure to bacteria, and semiquantitative RT-PCR was performed for each mRNA species. Results are presented as amplified products electrophoresed on ethidium bromide-stained agarose gels. RT-PCR amplification of the G3PDH housekeeping gene was performed to ensure that similar amounts of input RNA and similar RT efficiencies were being compared. These studies were performed three times with similar results.
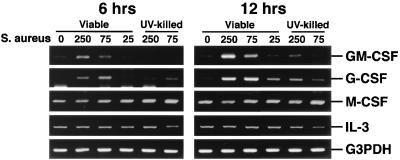
These results were observed in a second set of experiments (Fig. 2), which also included expression of CSF mRNA following interaction with UV-killed S. aureus. It was clear from these studies that UV-killed S. aureus had a reduced capacity to induce GM-CSF and G-CSF mRNA expression compared to viable bacteria (e.g., 5.0- and 2.4-fold reductions, respectively, at 12 h for an initial exposure ratio of 250:1).
FIG. 2.
CSF mRNA expression following exposure of mouse osteoblasts to viable or UV-killed S. aureus. Murine osteoblasts were left unexposed (lanes 0) or exposed to viable or UV-killed S. aureus at the indicated ratio (250:1, 75:1, or 25:1 bacterium-to-osteoblast ratio) for 45 min, and then extracellular bacteria were removed. RNA was isolated 6 and 12 h following exposure to bacteria, and a semiquantitative RT-PCR was performed for each mRNA species. Results are presented as amplified products electrophoresed on ethidium bromide-stained agarose gels. RT-PCR amplification of the G3PDH housekeeping gene was performed to ensure that similar amounts of input RNA and similar RT efficiencies were being compared. These studies were performed three times with similar results.
Salmonella-induced CSF mRNA expression by mouse osteoblasts.
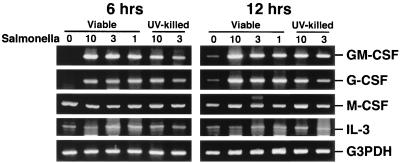
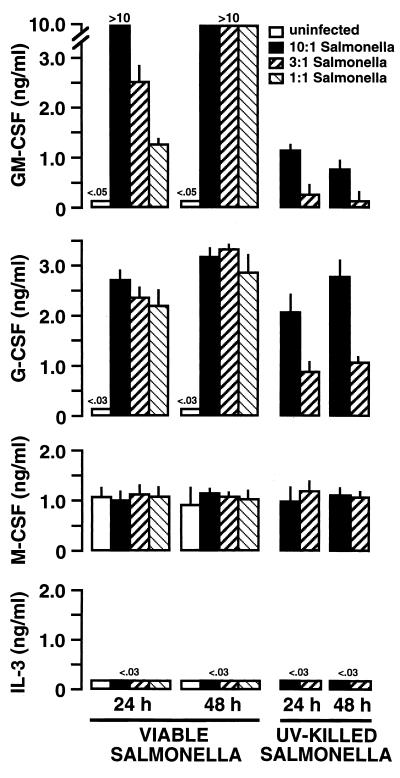
Similar experiments were performed to assess the ability of Salmonella to induce CSF mRNA expression by mouse osteoblasts. As shown in Fig. 3, Salmonella was a potent inducer of GM-CSF and G-CSF mRNA expression (e.g., each greater than 22-fold at 6 h for an initial exposure ratio of 10:1) but could not significantly increase expression of mRNA encoding M-CSF or IL-3 over constitutive levels (Fig. 3). Surprisingly, UV-killed Salmonella was also a potent stimulator of GM-CSF and G-CSF mRNA expression (e.g., each greater than 20-fold at 6 h for an initial exposure ratio of 10:1).
FIG. 3.
CSF mRNA expression following exposure of mouse osteoblasts to Salmonella. Murine osteoblasts were exposed to S. dublin at the indicated ratio (30:1, 10:1 or 3:1 bacterium-to-osteoblast ratio) for 45 min, and then extracellular bacteria were removed. RNA was isolated 6 and 12 h following exposure to bacteria, and a semiquantitative RT-PCR was performed for each mRNA species. Results are presented as amplified products electrophoresed on ethidium bromide-stained agarose gels. RT-PCR amplification of the G3PDH housekeeping gene was performed to ensure that similar amounts of input RNA and similar RT efficiencies were being compared. These studies were performed three times with similar results.
S. aureus-induced CSF secretion by mouse osteoblasts.
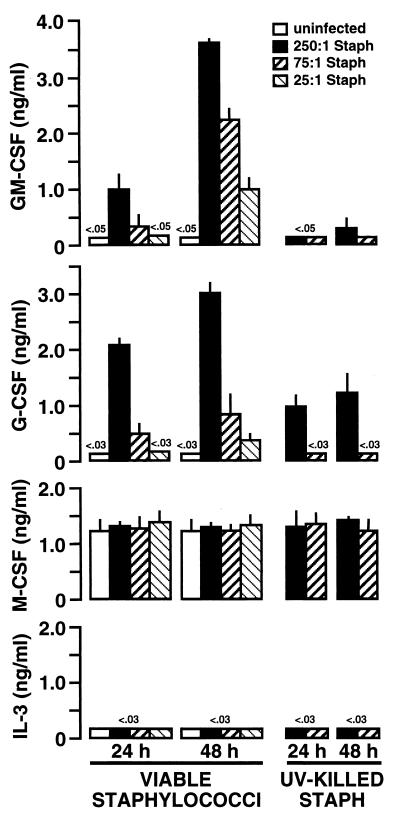
To determine whether the elevations in mRNA encoding GM-CSF in mouse osteoblasts exposed to S. aureus seen in the previous figures were mirrored by alterations in the translation and secretion of this cytokine, specific capture ELISAs were performed. Culture supernatants of untreated osteoblasts or osteoblasts exposed to various numbers of S. aureus bacteria for 24 and 48 h were assayed for the presence of CSF. Figure 4 shows the results of one such set of assays. Viable S. aureus induced GM-CSF and G-CSF secretion as early as 24 h postexposure, and this production continued to increase by 48 h. A dose-response relationship between cytokine secretion and the inoculum of S. aureus used was observed. Conversely, UV-killed S. aureus was much less potent an inducer of these two CSF. Production of M-CSF was observed, but the presence of viable or UV-killed S. aureus did not significantly alter the constitutive secretion of this cytokine (Fig. 4). IL-3 secretion by mouse osteoblasts was below the level of sensitivity of the ELISA (i.e., 30 pg/ml) under all of the experimental conditions used.
FIG. 4.
Dose-dependent S. aureus (Staph) induction of CSF secretion by cultured mouse osteoblasts. Osteoblasts (107) were exposed to various numbers of viable or UV-killed S. aureus bacteria (250:1, 75:1, and 25:1 bacterium-to-osteoblast ratios) for 45 min. Extracellular bacteria were then eliminated, and culture supernatants were taken 24 and 48 h postexposure. Specific capture ELISAs were performed to quantify CSF secretion. Results are presented as means of triplicate determinations ± standard deviations. These studies were performed three times with similar results.
Salmonella-induced CSF secretion by mouse osteoblasts.
Interaction with viable Salmonella was especially potent at inducing GM-CSF secretion, whereas interaction with UV-killed bacteria was approximately 20-fold less effective (Fig. 5). A similar trend was observed for Salmonella-induced G-CSF secretion; however, reductions in G-CSF secretion following interaction with UV-killed Salmonella were less than threefold compared to the levels obtained following infection with viable bacteria. Salmonella did not increase M-CSF secretion over constitutive levels, and no detectable IL-3 secretion was observed (Fig. 5).
FIG. 5.
Dose-dependent Salmonella induction of CSF secretion by cultured mouse osteoblasts. Osteoblasts (107) were exposed to various numbers of viable or UV-killed Salmonella bacteria (30:1, 10:1, and 3:1 bacterium-to-osteoblast ratios) for 45 min. Extracellular bacteria were then eliminated, and culture supernatants were taken 24 and 48 h postexposure. Specific capture ELISAs were performed to quantify CSF secretion. Results are presented as means of triplicate determinations ± standard deviations. These studies were performed three times with similar results.
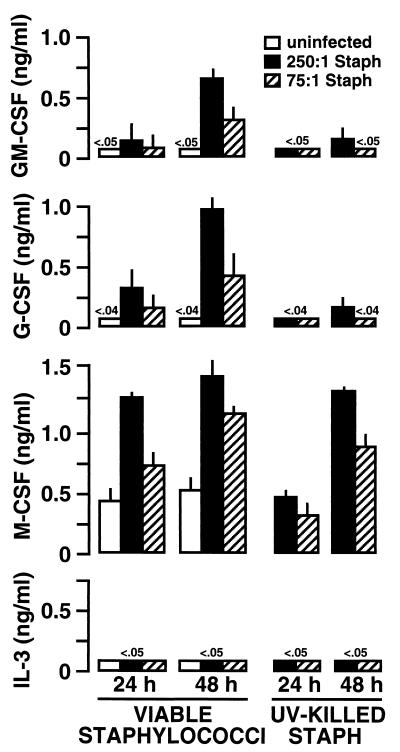
S. aureus-induced CSF secretion by human osteoblasts.
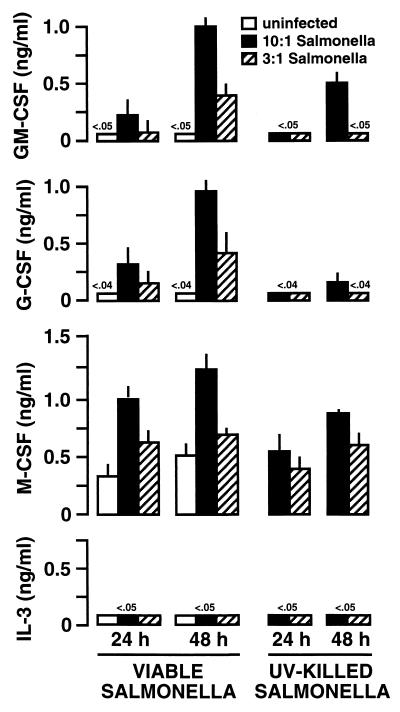
The abilities of bacteria to interact with mouse osteoblasts and to induce dramatic GM-CSF and G-CSF expression were surprising, since these cells are not known for the ability to mount a host response against infection. We next investigated whether a similar osteoblast-initiated host response is common to cultured human osteoblasts. Normal human osteoblasts were obtained (Clonetics) and subcultured in our laboratory as previously described (9). Following treatment with various inoculums of S. aureus or Salmonella, supernatants were taken from these cultures and ELISAs were performed to quantify CSF secretion. Exposure of osteoblasts to S. aureus (Fig. 6) or Salmonella (Fig. 7) induced substantial GM-CSF and G-CSF secretion in a dose-dependent manner. As with mouse osteoblasts, UV-inactivated bacteria were less effective in stimulating the secretion of these particular CSF. Furthermore, the cytokine quantities secreted by human osteoblasts were similar to those secreted by mouse osteoblasts when differences in cell culture density were taken into account. Thus, for GM-CSF and G-CSF secretion, human and mouse osteoblasts had similar responses to the presence of these bacteria. The fact that both mouse and human osteoblasts respond to bacterial exposure with CSF secretion suggests that this is a conserved response and adds further weight to the potential importance of this finding.
FIG. 6.
Dose-dependent S. aureus (Staph) induction of CSF secretion by cultured human osteoblasts. Osteoblasts (3 × 106) were exposed to various numbers of viable or UV-killed S. aureus bacteria (250:1, 75:1, and 25:1 bacterium-to-osteoblast ratios) for 45 min. Extracellular bacteria were then eliminated, and culture supernatants were taken 24 and 48 h postexposure. Specific capture ELISAs were performed to quantify CSF secretion. Results are presented as means of triplicate determinations ± standard deviations. These studies were performed twice with similar results.
FIG. 7.
Dose-dependent Salmonella induction of CSF secretion by cultured human osteoblasts. Human osteoblasts (3 × 106) were exposed to various numbers of viable or UV-killed Salmonella bacteria (30:1, 10:1, and 3:1 bacterium-to-osteoblast ratios) for 45 min. Extracellular bacteria were then eliminated, and culture supernatants were taken 24 and 48 h postexposure. Specific capture ELISAs were performed to quantify CSF secretion. Results are presented as means of triplicate determinations ± standard deviations. These studies were performed twice with similar results.
However, unlike mouse osteoblasts, human osteoblasts upregulated their expression of M-CSF in response to both S. aureus (Fig. 6) and Salmonella (Fig. 7). While there was constitutive expression of M-CSF by these cells, exposure to viable bacteria elicited further induction of this cytokine. These increases followed a dose response, and it was especially surprising to find that UV-killed bacteria were similar to viable bacteria in the ability to augment M-CSF secretion (Fig. 6 and 7). These results suggest that, while similar, the human osteoblast response to bacteria includes some increases in M-CSF secretion, a finding that was not observed using murine osteoblasts.
Finally, human osteoblasts did not secrete detectable levels of IL-3 in response to S. aureus or Salmonella exposure (Fig. 6 and 7). Taken together with the results obtained with mouse osteoblast cultures, these data suggest that osteoblasts are not a significant source of IL-3 during a host response to bacterial exposure.
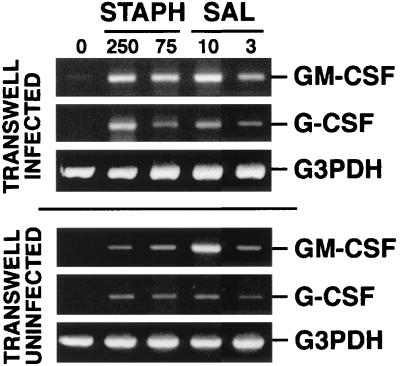
Soluble factors contribute to the induction of GM-CSF and G-CSF in infected mouse osteoblasts.
There are at least two possible mechanisms for bacterium-induced CSF secretion by osteoblasts. Direct interaction of bacteria with osteoblasts could provide signals which activate transcription of the appropriate genes, followed by translation and secretion. Alternatively, the interaction of bacteria with osteoblasts could induce these cells to secrete other soluble factors which could act in an autocrine or paracrine manner to induce GM-CSF or G-CSF expression indirectly. To address the latter possibility, a Transwell culture system was used. Mouse osteoblasts were grown in the bottom chamber of a Transwell plate, and these cells were exposed to various doses of viable S. aureus or Salmonella bacteria, followed by elimination of extracellular bacteria as in all of the previous experiments. Upper chambers were then placed in the Transwell plates containing uninfected osteoblasts. This created a coculture of infected osteoblasts in the lower chamber that were separated by a 0.2-μm membrane from the uninfected osteoblasts in the upper chamber. After 12 h of coculture, RNA was isolated from cells in the upper and lower chambers and assayed by RT-PCR for expression of mRNAs. As expected, S. aureus- or Salmonella-infected osteoblasts in the lower chamber demonstrated significant increases in GM-CSF and G-CSF mRNA expression (Fig. 8). However, we were surprised to find that uninfected osteoblasts in the upper chamber also had increased levels of GM-CSF and G-CSF mRNA expression, albeit to a lesser degree than that observed in infected osteoblasts. These dose-dependent increases in GM-CSF and G-CSF message expression could not be ascribed to differences in input RNA or to differences in the efficiency of RT, as evidenced by RT-PCR amplification of the G3PDH housekeeping gene for each sample (Fig. 8).
FIG. 8.
Induction of G-CSF and GM-CSF mRNA expression in cocultures of bacterially exposed and unexposed osteoblasts. Mouse osteoblasts were used to seed the upper and lower chambers of Transwell plates. Osteoblasts in the lower chamber were left unexposed (lane 0) or exposed to S. aureus or Salmonella at the indicated ratios (250:1 and 75:1 or 10:1 and 3:1 bacterium-to-osteoblast ratios, respectively) for 45 min, and then extracellular bacteria were removed. Following exposure of osteoblasts in the lower chamber, upper chambers containing unexposed osteoblasts were positioned in the Transwell plates. RNA was isolated from the upper and lower chambers 12 h following exposure to bacteria, and a semiquantitative RT-PCR was performed for each mRNA species. Results are presented as amplified products electrophoresed on ethidium bromide-stained agarose gels. RT-PCR amplification of the G3PDH housekeeping gene was performed to ensure that similar amounts of input RNA and similar RT efficiencies were being compared. These studies were performed twice with similar results.
Importantly, bacteria were not the source of this soluble factor, as determined in experiments in which viable S. aureus bacteria (top chamber) were separated from murine osteoblasts (lower chamber) within a Transwell plate. Viable S. aureus bacteria were added to the top chamber and incubated for 45 min, during which time osteoblasts were present in the bottom chamber but not in direct contact with the bacteria. After 45 min, gentamicin was added to each chamber. At 48 h later, supernatants were taken from the bottom chamber containing the osteoblasts and ELISAs were used to quantify GM-CSF or G-CSF secretion. Less than 100 pg of CSF secretion per ml was observed (data not shown). These studies demonstrate the necessity of S. aureus to directly interact with osteoblasts to induce a host response. Furthermore, addition of staphylococcal enterotoxin A at various concentrations (10, 1, and 0.1 ng/ml) induced no significant IL-12, IL-6, or GM-CSF secretion 48 h postaddition to murine osteoblasts compared to untreated cultures. These results further support the notion that S. aureus-induced effects on osteoblasts are not mediated via soluble factors of bacterial origin but require cellular contact.
Taken together, these results demonstrate that a soluble factor produced in cultures of osteoblasts directly exposed to bacteria can act in a paracrine manner to stimulate the expression of GM-CSF and G-CSF mRNAs in normal, uninfected osteoblasts.
DISCUSSION
These studies represent the first comprehensive analysis of bacterially induced CSF production by cultured mouse and human osteoblasts. The results presented here were surprising in that they demonstrated an unexpected ability of osteoblasts to respond to bacterial exposure by secreting high levels of GM-CSF and G-CSF. The amounts of CSF secreted by exposed osteoblasts were similar to that reported for activated macrophages (43, 64), supporting the relative biological importance of these findings. Furthermore, the conservation of osteoblast-derived GM-CSF and G-CSF secretion in response to bacterial exposure between humans and mice suggests the fundamental nature of this response. Taken together, the present results support the notion that osteoblasts can contribute to the host response during bone infection by acting as a significant source of cytokine secretion.
Bone resorption following infection is well documented; however, the mechanisms responsible for such clinical observations are not altogether clear (55). The present demonstration that bacterially exposed human osteoblasts can be induced to become potent secretors of GM-CSF, G-CSF, and M-CSF could have a significant impact on this process. These cytokines are believed to be important growth factors regulating the proliferation and differentiation of myeloid osteoclast precursors (2, 48, 54). The increased production of these growth factors mediated by S. aureus or Salmonella may significantly increase osteoclast numbers. Furthermore, we have previously reported (9) that S. aureus infection of osteoblasts induces substantial IL-6 secretion. It is well documented that osteoblast-derived IL-6 can directly or indirectly modulate the activity of bone-resorptive osteoclasts (18, 27, 31, 35), resulting in induction of osteoclast differentiation or osteoclast-mediated bone demineralization. The fact that viable bacteria can induce IL-6, GM-CSF, G-CSF, and M-CSF secretion from human osteoblasts points to the induction of a cytokine environment which would favor osteoclastogenesis during infection. Since bone diseases commonly result in aberrant bone remodeling, it is tempting to speculate that such a perturbation in the regulation of bone formation may result, in large part, from cytokine-mediated increases in bone resorption in infected tissue.
However, the process of osteoclastogenesis is a complex one, as demonstrated by the multiple effects of GM-CSF on this process. GM-CSF production or administration has been shown to limit the formation of more mature, multinucleated osteoclasts (33, 65, 66, 76). Therefore, excess GM-CSF production may also limit the latter stages of osteoclast formation. It is important to consider the net effect of this cytokine on osteoclastogenesis before definitive conclusions can be drawn as to the importance of increased GM-CSF production by osteoblasts during bone remodeling.
S. aureus and Salmonella are two common causative agents of bone diseases (13, 44, 57, 81) and were chosen for use in these investigations based on this fact. While common to bone diseases, these two pathogens have very dissimilar characteristics and modes of invasion. S. aureus is a gram-positive pathogen whose adherence to bone is an important component in its infectivity (14, 24) and possibly its formation of biofilms (13). Conversely, Salmonella is a gram-negative bacterium known for its ability to invade cells (23, 41). Therefore, the studies presented here also allow the first general comparisons concerning the types of responses elicited from osteoblasts following exposure to two very different pathogens. It was surprising to find that gram-positive and gram-negative bacteria were able to induce similar patterns of CSF secretion following interaction with osteoblasts. It is not clear why the profiles are similar, but this similarity may be suggestive of a common mechanism of activation. While staphylococci are typically regarded as noninvasive, extracellular pathogens that damage host cells after adhering to the extracellular matrix (14, 24), several recent studies have shown that S. aureus can be internalized by a number of cell types that are not generally considered to be phagocytic (1, 4, 53, 77). Indeed, several recent reports (19, 34, 36, 61) have demonstrated that S. aureus can be internalized by osteoblasts and persist intracellularly. Based on these observations, it is tempting to speculate that one component of S. aureus-mediated bone disease would be cell invasion and persistence, which allows these bacteria to evade humoral or neutrophil-mediated destruction. While the present study does not directly address the importance of viable intracellular bacteria in augmenting CSF production, it is clear that nonviable (and presumably noninvasive) bacteria induced less cytokine secretion. Thus, it is tempting to speculate that signals generated following intracellular invasion by viable bacteria are an important component of the induction of optimal CSF secretion; however, additional studies are required to define such mechanisms.
The major functions of osteoblasts include the formation of new bone and the modulation of osteoclast activity; however, evidence is increasing that osteoblasts have a surprising ability to respond to pathogens in a manner normally attributed to cells of the immune system. Recent studies in our laboratory have shown the ability of cultured mouse and human osteoblasts exposed to S. aureus (9) or Salmonella (unpublished data) to express high levels of IL-6 and IL-12p75. The ability of IL-6 (78), IL-12 (74), GM-CSF (26), and G-CSF (15) to augment immune responses against bacterial infections is well documented. In fact, GM-CSF has been found to augment the immune response against both S. aureus and Salmonella infections (16, 25, 26, 49, 52). The observation that both human and mouse osteoblasts can secrete significant levels of GM-CSF following exposure to either bacterium supports the notion that osteoblast-derived cytokines might significantly contribute to the development of a protective host response. Taken together, the present and previous (9) findings suggest that a third significant function of osteoblasts is an ability to contribute to the initiation of a host immune response following bone infection.
In addition to the potential protective effects of increased cytokine production by bacterially challenged osteoblasts, it should also be noted that such secretion might contribute to the destructive inflammation associated with bone infection. It is clear that cultured osteoblasts can secrete substantial amounts of IL-6 (9), IL-12 (9), GM-CSF, and G-CSF in response to bacterial exposure. Furthermore, our results indicate that maximal amounts of these cytokines occur with the presence of viable intracellular bacteria. This finding suggests that maximal cytokine secretion would accompany an active or chronic infection. In such a scenario, increased local cytokine production by osteoblasts responding to viable bacteria could serve to sustain the inflammatory immune response by maintaining the activated status of infiltrating macrophages, neutrophils, and/or lymphocytes at the site of infection.
ACKNOWLEDGMENTS
This work was supported by the North Carolina Biotechnology Center, the University of North Carolina at Charlotte Foundation, and the Foundation for the Carolinas.
REFERENCES
- 1.Almeida R A, Mathews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Al-Saffar N, Khwaja H A, Kadoya Y, Revell P A. Assessment of the role of GM-CSF in the cellular transformation and the development of erosive lesions around orthopaedic implants. Am J Clin Pathol. 1996;105:628–639. doi: 10.1093/ajcp/105.5.628. [DOI] [PubMed] [Google Scholar]
- 3.Aubin J E. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. [PubMed] [Google Scholar]
- 4.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begg S K, Radley J M, Pollard J W, Chisholm O T, Stanley E R, Bertoncello I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bost K L, Clements J D. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1995;63:1076–1083. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bost K L, Clements J D. Intracellular Salmonella dublin induces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–3192. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bost K L, Mason M J. Thapsigargin and cyclopiazonic acid initiate rapid and dramatic increases of IL-6 mRNA expression and IL-6 secretion in murine peritoneal macrophages. J Immunol. 1995;155:285–296. [PubMed] [Google Scholar]
- 9.Bost K L, Ramp W K, Nicholson N C, Bento J L, Marriott I, Hudson C M. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 10.Campbell H D, Ymer S, Fung M C, Young I G. Cloning and nucleotide sequence of the murine interleukin-3 gene. Eur J Biochem. 1985;150:297–304. doi: 10.1111/j.1432-1033.1985.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 11.Cecchini M G, Hofstetter W, Halasy J, Wetterwald A, Felix R. Role of CSF-1 in bone and bone marrow development. Mol Reprod Dev. 1997;46:75–83. doi: 10.1002/(SICI)1098-2795(199701)46:1<75::AID-MRD12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Corboz V A, Cecchini M G, Felix R, Fleisch H, van der Pluijm G, Lowik C W. Effect of macrophage colony-stimulating factor on in vitro osteoclast generation and bone resorption. Endocrinology. 1992;130:437–442. doi: 10.1210/endo.130.1.1727717. [DOI] [PubMed] [Google Scholar]
- 13.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham R, Cockayne A, Humphreys H. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol. 1996;44:157–164. doi: 10.1099/00222615-44-3-157. [DOI] [PubMed] [Google Scholar]
- 15.Dale D C, Liles W C, Summer W R, Nelson S. Review: granulocyte colony-stimulating factor—role and relationships in infectious diseases. J Infect Dis. 1995;172:1061–1075. doi: 10.1093/infdis/172.4.1061. [DOI] [PubMed] [Google Scholar]
- 16.Daley M, Williams T, Coyle P, Furda G, Dougherty R, Hayes P. Prevention and treatment of Staphylococcus aureus infections with recombinant cytokines. Cytokine. 1993;5:276–284. doi: 10.1016/1043-4666(93)90015-w. [DOI] [PubMed] [Google Scholar]
- 17.DeLamarter J F, Hession C, Semon D, Gough N M, Rothenbuhler R, Mermod J J. Nucleotide sequence of a cDNA encoding murine CSF-1 (macrophage-CSF) Nucleic Acids Res. 1987;15:2389–2390. doi: 10.1093/nar/15.5.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Mata J, Uy H L, Guise T A, Story B, Boyce B F, Mundy G R, Roodman G D. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Investig. 1995;95:2846–2852. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellington J K, Reilly S S, Ramp W K, Smeltzer M S, Kellam J F, Hudson M C. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 20.Felix R, Cecchini M G, Hofstetter W, Elford P R, Stutzer A, Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990;5:781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- 21.Felix R M, Cecchini G, Hofstetter W, Guenther H L, Fleisch H. Production of granulocyte-macrophage (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) by rat clonal osteoblastic cell population CRP 10/30 and the immortalized cell line IRC10/30-myc1 stimulated by tumor necrosis factor alpha. Endocrinology. 1991;128:661–667. doi: 10.1210/endo-128-2-661. [DOI] [PubMed] [Google Scholar]
- 22.Felix R, Halasy-Nagy J, Wetterwald A M, Cecchini G, Fleisch H, Hofstetter W. Synthesis of membrane- and matrix-bound colony-stimulating factor-1 by cultured osteoblasts. J Cell Physiol. 1996;166:311–322. doi: 10.1002/(SICI)1097-4652(199602)166:2<311::AID-JCP9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Finlay B B. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr Top Microbiol Immunol. 1994;192:163–185. doi: 10.1007/978-3-642-78624-2_8. [DOI] [PubMed] [Google Scholar]
- 24.Foster T J, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 25.Frenck R W, Sarman G, Harper T E, Buescher E S. The ability of recombinant murine granulocyte-macrophage colony-stimulating factor to protect neonatal rats from septic death due to Staphylococcus aureus. J Infect Dis. 1990;162:109–114. doi: 10.1093/infdis/162.1.109. [DOI] [PubMed] [Google Scholar]
- 26.Freund M, Kleine H D. The role of GM-CSF in infection. Infection. 1992;20:S84–S92. doi: 10.1007/BF01705024. [DOI] [PubMed] [Google Scholar]
- 27.Greenfield E M, Shaw S M, Gornik S A, Banks M A. Adenyl cyclase and interleukin 6 are downstream effectors of parathyroid hormone resulting in stimulation of bone resorption. J Clin Investig. 1995;96:1238–1244. doi: 10.1172/JCI118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gundle R, Beresford J N. The isolation and culture of cells from explants of human trabecular bone. Calcif Tissue Int. 1995;56:S8–S10. [Google Scholar]
- 29.Hattersley G, Chambers T J. Effects of interleukin 3 and of granulocyte-macrophage and macrophage colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol. 1990;142:201–209. doi: 10.1002/jcp.1041420125. [DOI] [PubMed] [Google Scholar]
- 30.Hattersley G, Owens J, Flanagan A M, Chambers T J. Macrophage colony stimulating factor (M-CSF) is essential for osteoclast formation in vitro. Biochem Biophys Res Commun. 1991;177:526–531. doi: 10.1016/0006-291x(91)92015-c. [DOI] [PubMed] [Google Scholar]
- 31.Hofbauer L C, Heufelder A E. Intercellular chatter: osteoblasts, osteoclast and interleukin 6. Eur J Endocrinol. 1996;134:425–426. doi: 10.1530/eje.0.1340425. [DOI] [PubMed] [Google Scholar]
- 32.Horowitz M C, Coleman D L, Flood P M, Kupper T S, Jilka R L. Parathyroid hormone and lipopolysaccharide induce murine osteoblast-like cells to secrete a cytokine indistinguishable from granulocyte-macrophage colony-stimulating factor. J Clin Investig. 1989;83:149–157. doi: 10.1172/JCI113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwood N J, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, Dunn A R, Martin T, Gillespie M T. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Investig. 1998;101:595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 35.Ishimi Y, Miyaura C, Jin C H, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 36.Jevon M, Guo C, Ma B, Mordan N, Nair S P, Harris M, Henderson B, Bentley G, Mehiji S. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun. 1999;67:2677–2681. doi: 10.1128/iai.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimi E, Shuto T, Koga T. Macrophage colony-stimulating factor and interleukin-1 alpha maintain the survival of osteoclast-like cells. Endocrinology. 1995;136:808–811. doi: 10.1210/endo.136.2.7835314. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan D L, Eielson C M, Horowitz M C, Insogna K L, Weir E C. Tumor necrosis factor-alpha induces transcription of the colony-stimulating factor-1 gene in murine osteoblasts. J Cell Physiol. 1996;168:199–208. doi: 10.1002/(SICI)1097-4652(199607)168:1<199::AID-JCP24>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Kodama H, Nose M, Niida S, Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991;173:1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodama H, Yamasaki A, Nose M, Niida S, Ohgame Y, Abe M, Kumegawa M, Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 42.Lees R L, Heersche J N. Macrophage colony stimulating factor increases bone resorption in dispersed osteoclast cultures by increasing osteoclast size. J Bone Miner Res. 1999;14:937–945. doi: 10.1359/jbmr.1999.14.6.937. [DOI] [PubMed] [Google Scholar]
- 43.Lenhoff S, Sallerfors B, Olofsson T. IL-10 as an autocrine regulator of CSF secretion by monocytes: disparate effects on GM-CSF and G-CSF secretion. Exp Hematol. 1998;26:299–304. [PubMed] [Google Scholar]
- 44.Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 45.Liggett W, Jr, Shevde N, Anklesaria P, Sohoni S, Greenberger J, Glowacki J. Effects of macrophage colony stimulating factor and granulocyte-macrophage colony stimulating factor on osteoclastic differentiation of hematopoietic progenitor cells. Stem Cells. 1993;11:398–411. doi: 10.1002/stem.5530110507. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzo J A, Sousa S L, Fonseca J M, Hock J M, Medlock E S. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Investig. 1987;80:160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marriott I, Bost K L, Mason M J. Differential kinetics for induction of interleukin-6 mRNA expression in murine peritoneal macrophages: evidence for calcium-dependent and independent-signalling pathways. J Cell Physiol. 1998;177:232–240. doi: 10.1002/(SICI)1097-4652(199811)177:2<232::AID-JCP5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Martin T J, Romas E, Gillespie M T. Interleukins in the control of osteoclast differentiation. Crit Rev Eukaryot Gene Expr. 1998;8:107–123. doi: 10.1615/critreveukargeneexpr.v8.i2.10. [DOI] [PubMed] [Google Scholar]
- 49.Mayer P, Schutze E, Lam C, Kricek F, Liehl E. Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice. J Infect Dis. 1991;163:584–590. doi: 10.1093/infdis/163.3.584. [DOI] [PubMed] [Google Scholar]
- 50.Miyatake S, Otsuka T, Yokota T, Lee F, Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985;4:2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modrowski D, Lomri A, Marie P J. Endogenous GM-CSF is involved as an autocrine growth factor for human osteoblastic cells. J Cell Physiol. 1997;170:35–46. doi: 10.1002/(SICI)1097-4652(199701)170:1<35::AID-JCP5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey P J, Charrier K. GM-CSF administration augments the survival of ity-resistant A/J mice, but not ity-susceptible C57BL/6 mice, to a lethal challenge with Salmonella typhimurium. J Immunol. 1990;144:557–561. [PubMed] [Google Scholar]
- 53.Murai M, Usui A, Seki K, Sakurada J, Masuda S. Intracellular localization of Staphylococcus aureus within primary cultured mouse kidney cells. Microbiol Immunol. 1992;36:431–443. doi: 10.1111/j.1348-0421.1992.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 54.Myint Y Y, Miyakawa K, Naito M, Shultz L D, Oike Y, Yamamura K, Takahashi K. Granulocyte/macrophage colony-stimulating factor and interleukin-3 correct osteopetrosis in mice with osteopetrosis mutation. Am J Pathol. 1999;154:553–566. doi: 10.1016/s0002-9440(10)65301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair S P, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neale S D, Sabokbar A, Howie D W, Murray D W, Athanasou N A. Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J Orthop Res. 1999;17:686–694. doi: 10.1002/jor.1100170510. [DOI] [PubMed] [Google Scholar]
- 57.Overturf G D. Infections and immunizations of children with sickle cell disease. Adv Pediatr Infect Dis. 1999;14:191–218. [PubMed] [Google Scholar]
- 58.Povolny B T, Lee M Y. The role of recombinant human M-CSF, IL-3, GM-CSF and calcitriol in clonal development of osteoclast precursors in primate bone marrow. Exp Hematol. 1993;21:532–537. [PubMed] [Google Scholar]
- 59.Quinn J M, Neale S, Fujikawa Y, McGee J O, Athanasou N A. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- 60.Ramp W K, Dillaman R M, Lenz L G, Gay D M, Roer R D, Ballard T A. A serum substitute promotes osteoblast-like phenotypic expression in cultured cells from chick calvariae. Bone Miner. 1991;15:1–17. doi: 10.1016/0169-6009(91)90107-b. [DOI] [PubMed] [Google Scholar]
- 61.Reilly S S, Hudson M C, Kellam J F, Ramp W K. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 62.Rubin J, Biskobing D M, Jadhay L, Fan D, Nanes M S, Perkins S, Fan X. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology. 1998;139:1006–1012. doi: 10.1210/endo.139.3.5778. [DOI] [PubMed] [Google Scholar]
- 63.Rubin J, Fan X, Thornton D, Bryant R, Biskobing D. Regulation of murine osteoblast macrophage colony-stimulating factor production by 1,25(OH)2D3. Calcif Tissue Int. 1996;59:291–296. doi: 10.1007/s002239900125. [DOI] [PubMed] [Google Scholar]
- 64.Sallerfors B, Olofsson T. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) secretion by adherent monocytes measured by quantitative immunoassays. Eur J Haematol. 1992;49:199–207. doi: 10.1111/j.1600-0609.1992.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 65.Shuto T, Jimi E, Kukita T, Hirata M, Koga T. Granulocyte-macrophage colony stimulating factor suppresses lipopolysaccharide-induced osteoclast-like cell formation in mouse bone marrow cultures. Endocrinology. 1994;134:831–837. doi: 10.1210/endo.134.2.8299579. [DOI] [PubMed] [Google Scholar]
- 66.Shuto T, Kukita T, Hirata M, Jimi E, Koga T. Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte-macrophage colony-stimulating factor production in mouse bone marrow cultures. Endocrinology. 1994;134:1121–1126. doi: 10.1210/endo.134.3.8119150. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki M, Sakamaki Y, Miyoshi A, Adachi K, Usami M, Nakayama H, Doi K. Histopathological study on bone changes induced by recombinant granulocyte colony-stimulating factor in rats. Exp Toxicol Pathol. 1997;49:253–259. doi: 10.1016/S0940-2993(97)80024-3. [DOI] [PubMed] [Google Scholar]
- 68.Taichman R S, Emerson S G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991;6:977–985. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi T, Wada T, Mori M, Kokai Y, Ishii S. Overexpression of the granulocyte colony-stimulating factor gene leads to osteoporosis in mice. Lab Investig. 1996;74:827–834. [PubMed] [Google Scholar]
- 71.Takamatsu Y, Simmons P J, Moore R J, Morris H A, To L B, Levesque J P. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465–3473. [PubMed] [Google Scholar]
- 72.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley E R, Kurokawa T, Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Investig. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teitelbaum S L, Tondravi M M, Ross F P. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukoc Biol. 1997;61:381–388. doi: 10.1002/jlb.61.4.381. [DOI] [PubMed] [Google Scholar]
- 74.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchiya M, Asano S, Kaziro Y, Nagata S. Isolation and characterization of the cDNA for murine granulocyte colony-stimulating factor. Proc Natl Acad Sci USA. 1986;83:7633–7637. doi: 10.1073/pnas.83.20.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Udagawa N, Horwood N J, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers T J, Martin T J, Gillespie M T. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usui A, Murai M, Seki K, Sakurada J, Masuda S. Conspicuous ingestion of Staphylococcus aureus organisms by murine fibroblasts in vitro. Microbiol Immunol. 1992;36:545–550. doi: 10.1111/j.1348-0421.1992.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 78.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 79.Weir E C, Horowitz M C, Baron R, Centrella M, Kacinski B M, Insogna K L. Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res. 1993;8:1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- 80.Weir E C, Insogna K L, Horowitz M C. Osteoblast-like cells secrete granulocyte-macrophage colony-stimulating factor in response to parathyroid hormone and lipopolysaccharide. Endocrinology. 1989;124:899–904. doi: 10.1210/endo-124-2-899. [DOI] [PubMed] [Google Scholar]
- 81.Workman M R, Philpott-Howard J, Bragman S, Brito-Babapulle F, Bellingham A J. Emergence of ciprofloxacin resistance during treatment of Salmonella osteomyelitis in three patients with sickle cell disease. J Infect. 1996;32:27–32. doi: 10.1016/s0163-4453(96)80006-5. [DOI] [PubMed] [Google Scholar]
- 82.Yao G Q, Sun B, Hammond E E, Spencer E N, Horowitz M C, Insogna K L, Weir E C. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J Biol Chem. 1998;273:4119–4128. doi: 10.1074/jbc.273.7.4119. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida H, Hayahsi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz L D. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]