Abstract
Long COVID is a public health emergency affecting millions of people worldwide, characterized by heterogeneous symptoms across multiple organ systems. Here, we discuss the current evidence linking thromboinflammation to postacute sequelae of COVID-19. Studies have found persistence of vascular damage with increased circulating markers of endothelial dysfunction, coagulation abnormalities with heightened thrombin generation capacity, and abnormalities in platelet counts in postacute sequelae of COVID-19. Neutrophil phenotype resembles acute COVID-19 with an increase in activation and Neutrophil Extracellular Trap formation. These insights are potentially linked by elevated platelet-neutrophil aggregate formation. This hypercoagulable state in turn can lead to microvascular thrombosis, evidenced by microclots and elevated D-dimer in the circulation as well as perfusion abnormalities in the lungs and brains of patients with long COVID. Also, COVID-19 survivors experience an increased rate of arterial and venous thrombotic events. We discuss 3 important, potentially intertwined hypotheses that might contribute to thromboinflammation in long COVID: lasting structural changes, most prominently endothelial damage, caused during initial infection; a persistent viral reservoir; and immunopathology driven by a misguided immune system. Finally, we outline the necessity for large, well-characterized clinical cohorts and mechanistic studies to clarify the contribution of thromboinflammation to long COVID.
Keywords: long COVID, PASC, platelets, thromboinflammation, thrombosis
1. Acute COVID-19—A Thromboinflammatory Disease
Since its outbreak in Wuhan, China, at the end of 2019, the pandemic caused by sarbecovirus SARS-CoV-2 has killed millions and inflicted immeasurable damage to societies, health care systems, and the economy. In a frantic effort to understand the pathophysiology of this disease, unprecedented global efforts were made to study the disease from multiple angles. Early on, it became evident that patients developing COVID-19 were prone to macrovascular thrombotic complications ranging from venous thromboembolism to stroke and myocardial infarction [[1], [2], [3], [4]]. Moreover, particularly in severe cases, COVID-19 presented itself as a systemic disease not only restricted to the lungs but also affecting the heart, kidneys, and gastrointestinal tract as well as the central and peripheral nervous system [5]. The reason for this is still not fully elucidated, but one proposed mechanism is that COVID-19 also constitutes a vascular disease. It has been shown to cause distinct immunopathology, with activation of monocytes, platelets, and neutrophils, which in turn leads to intravascular clotting and tissue hypoxia [6]. This element of COVID-19 pathophysiology has been termed COVID-19–associated coagulopathy [7].
Mechanistically, vascular endothelial cell dysfunction caused by indirect effects and, potentially, direct infection by SARS-CoV-2—the latter remaining subject of debate—lead to a prothrombogenic vascular phenotype and trigger platelet activation [[8], [9], [10]]. A hyperinflammatory innate immune response leads to upregulation of pathways linking immunity and thrombosis, including heightened neutrophil extracellular trap release, complement deposition, and platelet-leukocyte aggregate formation [[11], [12], [13], [14], [15], [16], [17]]. Lastly, hypercoagulability, characterized by enhanced fibrin formation and decreased fibrinolysis, reflects a systemic procoagulant state [18]. Interestingly, markers of thromboinflammation correlate with pulmonary dysfunction and mortality, highlighting a potential direct link to disease progression [12,19]. In summary, COVID-19–associated coagulopathy is a central element of COVID-10 pathophysiology by causing microvascular thrombosis, resulting in tissue ischemia and organ failure.
2. Long COVID-19—Heterogeneous Sequelae of SARS-CoV-2 Infection
With far-reaching vaccination efforts and the emergence of less lethal SARS-CoV-2 variants, the burden of severe COVID-19 has declined. In the wake of more than 600 million confirmed cases, a new public health issue emerged [20]: a significant proportion of patients develop debilitating symptoms after acute infection, a postinfectious state termed long COVID. This novel disease consists of 2 phases, one that is characterized by persistent symptoms (4–12 weeks after primary infection) and the actual post–acute sequelae of COVID-19 (PASC, more than 12 weeks after primary infection) [21]. The incidence estimates vary depending on sampling time point, methodology, and cohort, but the pooled prevalence of post–COVID-19 condition was reported to be up to 43% [22].
The clinical presentation of this syndrome spans multiple organ systems [23]. Close to 14% of patients with PASC report symptoms involving more than 1 organ system after the acute phase [24]. Symptoms include fatigue; neurologic symptoms like “brain fog”; cardiovascular problems like palpitations, chest pain, and dyspnea [25]. In addition to these self-reported symptoms, cohort studies have identified objective correlates of organ dysfunction, including troponin elevations reflecting cardiac injury, signs of myocarditis, pancreatitis, cutaneous manifestations, and changes in lung and brain functions [26]. Worryingly, this is also reflected in elevated mortality rates beyond the acute phase of SARS-CoV-2 infection: in a large cohort study including more than 70 000 patients after acute COVID-19, the hazard ratio (HR) for death from 30 days onwards postinfection was 1.59 compared to noninfected controls [27].
Risk factors for long COVID overlap with the ones identified for acute severe COVID-19 and include age, obesity, diabetes mellitus, poor health, and socioeconomic status prior to infection [28]. Interestingly though, female sex is a risk factor for PASC but protective in acute COVID-19, suggesting distinct mechanisms of disease [23]. Incidence of long COVID is hardly affected by disease severity, although severity of symptoms is influenced by the initial disease course [[29], [30], [31]].
Given the multiorgan involvement and observed cardiovascular complications, it is tempting to speculate that vascular thromboinflammation also plays a role in initiation and propagation of long COVID [32] (Table 1 ) [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]]. In the following paragraphs, we will summarize the evidence to date on this topic (Figure 1 ).
Table 1.
Comparison of thromboinflammation in acute COVID-19 vs. long COVID and postviral syndrome.
| Acute COVID-19 | Long COVID | Postviral syndrome (ME/CFS) | References | |
|---|---|---|---|---|
| Endothelium | Endotheliopathy, endothelitis. Direct infection of endothelial cells by SARS-CoV-2 debated | Elevated markers of endothelial injury (FVIII and VWF) and ADAMTS-13 decrease. Evidence of microvascular dysfunction | Evidence of peripheral/microvascular endothelial dysfunction and endothelin-1 upregulation | [18,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]] |
| Platelets | Platelet hyperreactivity, increased P-selectin expression, and release of CCL5/PF4 | Platelet hyperreactivity, thrombocytosis, elevated P-selectin expression, and circulating microclots | Conflicting evidence for heightened activation | [19,[45], [46], [47], [48], [49], [50]] |
| Neutrophils | CD10 low-density hyperreactive phenotype, increased NETosis, enhanced secretion, and increased PLA formation | CD10 low-density hyperreactive phenotype and increased PLA formation with consequent pulmonary dysfunction | Increase in neutrophil apoptosis | [11,13,14,[51], [52], [53], [54], [55]] |
| Coagulation system | Upregulation of procoagulant and downregulation of antithrombotic pathways, decreased fibrinolysis, and high fibrinogen | Increased thrombin-generating capacity and hypofibrinolysis | Very limited evidence indicating increased coagulation in some patients | [16,56,50,57] |
| Complement system | Increased complement activation and deposition | Limited evidence for complement activation in a subset of patients | Weak evidence of complement upregulation | [[58], [59], [60], [61]] |
| Monocytes/macrophages | Dysfunctional HLA-DRlow monocytes, increased PLA formation, and TF upregulation | Evidence of innate immune cell activation | Evidence of innate immune cell activation | [5,[62], [63], [64], [65], [66], [67]] |
| Microvascular thrombosis | Elevated D-dimer in severe disease, evidence of microvascular clots in multiple organs with neutrophil recruitment, and NET formation | Elevated D-dimer in some patients. Evidence of microvascular thrombosis scarce and brain, heart, and lung imaging studies show possible microthrombotic injury patterns | No evidence of microvascular clotting | [19,42,[68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83]] |
| Macrovascular thrombosis | Increased rates of arterial and venous thrombosis, including stroke, DVT, pulmonary embolism, and myocardial infarction | Increased incidence of thromboembolism and adverse cardiovascular events | No evidence for increased macrovascular thrombosis risk | [2,24,27,84] |
CCL5, Chemokine (C-C motif) ligand 5; CFS, chronic fatigue syndrome; DVT, deep vein thrombosis; HLA-DR, human leukocyte antigen DR; ME, myalgic encephalopathy; NET, neutrophil extracellular trap; NETosis, Neutrophil Extracellular Trap formation; PF4, platelet factor 4; PLA, platelet-leukocyte aggregate; TF, tissue factor; VWF, von Willebrand factor.
Figure 1.
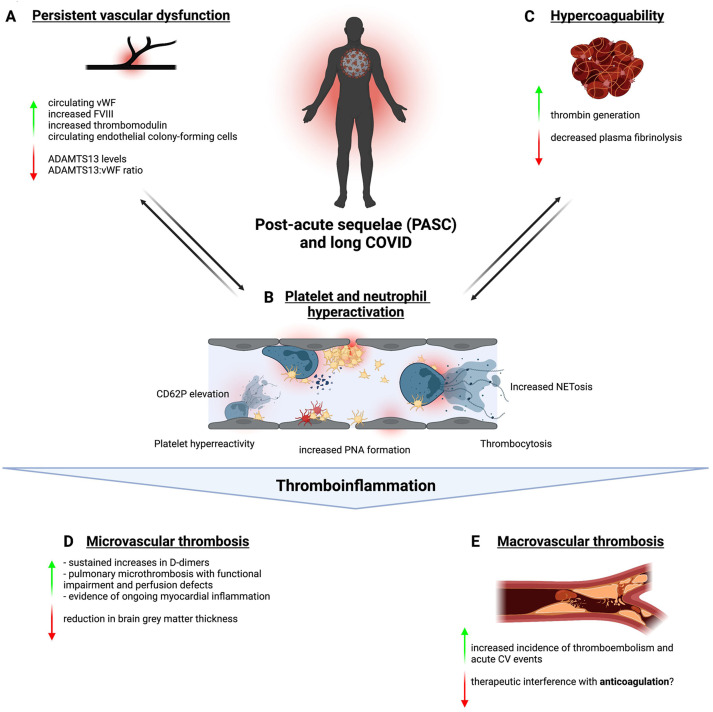
Clinical and experimental evidence for thromboinflammation in patients with long COVID. Persistent vascular dysfunction (A) with an imbalance of pro- and antithrombotic mediators, an overall neutrophil and platelet state skewed toward hyperactivation (B) as well as plasmatic upregulation of thrombogenic and downregulation of fibrinolytic mediators (C) contribute to PASC- and long COVID–associated thromboinflammation. Together, these factors sustain a prothrombotic state that leads to micro- (D) and macrovascular thrombosis (E). Created with BioRender (www.biorender.com). IFN, interferon; NET, neutrophil extracellular trap; PASC, post–acute sequelae of COVID-19.
3. Evidence Linking Thromboinflammation to Long Covid
3.1. Persistent vascular dysfunction
One critical aspect of thromboinflammation is endothelial dysfunction, which is prominently evident in multiple inflammatory diseases, including acute COVID-19 [18]. Studies involving patients after COVID-19 persistently identified increased levels of von Willebrand factor (VWF), factor VIII, and thrombomodulin, indicating endothelial dysfunction/activation [[33], [34], [35]]. Concomitantly, von Willebrand factor-cleaving protease - known as ADAMTS-13- levels were reduced, pointing toward a prothrombotic disbalance between VWF:ADAMTS-13 function [[36], [37], [38]]. In addition, endothelial colony-forming cells were elevated in patients after COVID-19, indicating endothelial injury [39,40]. Noninvasive measurements of microcirculatory function using postocclusion reactive hyperemia confirmed microvascular dysfunction [41]. While the association to symptom burden and organ dysfunction remained mostly unstudied, one group reported that markers of endothelial injury correlated with physical performance [33].
3.2. Coagulation abnormalities in PASC patients
In addition to endothelial dysfunction, there is limited evidence for hypercoagulability after COVID-19. von Meijenfeldt et al. [45] reported enhanced thrombin-generating capacity (assessed using thrombomodulin-modified calibrated automated thrombinography) and a plasma hypofibrinolytic state, while fibrinogen levels and prothrombin time had returned to normal values 4 months after hospitalization for COVID-19.
3.3. Platelet–neutrophil phenotype in PASC
Platelet–neutrophil interplay is a key mechanism of immunothrombosis in animal models and has been proposed as an important mechanism in acute COVID-19 coagulopathy [12,85,86]. Al-Aly et al. [27] found a HR of >3 for thrombocytosis, ie, platelet counts >400.000/μL in subjects after COVID. This could reflect reactive increases in platelet counts observed after other infections and could also predispose to thrombotic sequelae [45]. A small study reported hyperactive platelets, forming microclots, as the predominant finding in patients with PASC, and Martins-Gonçalves et al. [47] reported persistent platelet hyperreactivity in survivors of COVID-19 [46,48]. In a cohort of 21 patients with PASC, microfluidic assays revealed a significant increase in platelet binding to VWF and collagen, which correlated with increases in circulating VWF antigen levels [87].
Similarly, neutrophils in PASC show a distinct profile: the number of low-density granulocytes is increased, CD10 is downregulated, and neutrophils from patients with PASC are more prone to neutrophil extracellular trap formation than controls with no residual symptoms after COVID-19 [51]. In addition, platelet-neutrophil aggregate formation is also increased in individuals with PASC [51,52]. In a subset of patients with severe COVID-19, persistent interstitial lung changes correlated with elevated neutrophil counts and plasma proteome changes, indicating neutrophil activation and increased Neutrophil Extracellular Trap formation [53]. These limited studies outline an immunothrombotic platelet-neutrophil phenotype very similar to that observed in acute SARS-CoV-2 infection [11,13,14,54].
3.4. Microvascular thrombosis as a potential mechanism of PASC
Given these markers of thromboinflammation, is there evidence for microvascular thrombosis as a driver of PASC pathophysiology? Over an extended study period, assessing patients at up to 16 months postinfection, one study confirmed these abnormalities and identified elevated D-dimer as a marker of ongoing fibrin formation as well as elevated interleukin (IL)-6 indicating systemic inflammation in some patients [68]. Townsend et al. [69] confirmed sustained D-dimer increases in 150 patients at a mean of 80.5 days after acute disease, which was also observed in 29% of subjects who did not require hospitalization for COVID-19. In a meta-analysis of 15 publications describing 47 910 adults, fibrin degradation product D-dimer was elevated in 20% of patients (95% CI, 6–39), which was more pronounced than inflammatory markers (CRP in 8%; 95% CI, 5–12; IL-6 in 3%; 95% CI%, 1–7). This points toward persistent coagulation activation in a substantial amount of patients [88]. D-dimer elevation could be linked to symptomatic PASC in some studies [70,71]. However, diagnosis, management, and pathophysiology of microvascular thrombosis remain poorly defined [72]. Nevertheless, some of the features observed in PASC could be linked to small-vessel occlusion. For example, in patients with PASC having dyspnea, signs of air trapping and ground-glass opacities, indicating ongoing inflammation was prevalent on CT scans, independent of initial COVID-19 severity [73]. Air trapping in the small airways can be caused by bronchiolitis obliterans and fibrosis, which might reflect vessel occlusion [73]. In support of this hypothesis, case reports have highlighted individuals with severe dyspnea after COVID-19, in whom ventilation/perfusion imaging by single-photon emission computed tomography showed clear peripheral perfusion defects despite no structural correlate [[74], [75], [76]]. The authors argue that small-vessel clots could explain these findings. In line with this hypothesis, Prasannan et al. [77] were able to show that patients with PASC and impaired, objectively assessed exercise capacity were 4 times more likely to present with an elevated VWF Antigen/ADAMTS-13 ratio than patients who were not affected, linking persistent lung dysfunction to a prothrombotic phenotype. Long-term evidence of myocardial inflammation on magnetic resonance imaging (MRI) scans of patients with long COVID could also indicate microvascular injury and/or thrombosis [42,26].
As neurologic symptoms dominate the clinical picture of PASC, researchers have focused on identifying measurable correlates in the brain. Qin et al. [78] identified cerebral blood flow and white matter microstructural changes assessed using MRI scans 3 months after disease onset, which correlated with acute COVID-19 severity and markers of inflammation. Along these lines, a pre/post comparison of brain MRI in nonhospitalized patients confirmed a reduction in gray matter thickness compared with controls [79]. Mechanistically, microvascular thrombosis and complement activation have been described in acute COVID-19 and might extend to PASC pathophysiology [[82], [83]].
3.5. Macrovascular thrombosis risk
In addition to microvascular dysfunction, does PASC cause macrovascular thrombotic events? Interestingly, arterial and venous vascular events are not a leading manifestation of PASC [89]. Nevertheless, large cohort studies have highlighted an increased HR for thromboembolism and adverse cardiovascular events after acute COVID-19, which correlates with initial disease severity [24,27,84]. Xie et al. [90] report that individuals with COVID-19 are at increased risk of cerebrovascular disorders, arrhythmias, ischemic and nonischemic heart diseases, pericarditis, myocarditis, heart failure, and thromboembolic disease beyond the first 30 days after infection. A smaller study confirmed high 90-day postdischarge rates of arterial and venous thrombotic events, ameliorated in patients discharged with anticoagulation regimens after COVID-19 hospitalization [91]. In a population-based study, the HR for arterial thrombosis and venous thrombotic events was 1.34 (95% CI, 1.21–1.48) and 1.80 (95% CI, 1.50–2.17) during weeks 27 to 49, respectively, after COVID-19 infection [92]. The role of vaccination in preventing cardiovascular sequalae is also insufficiently addressed. In summary, the link to macrovascular thrombosis is incompletely understood, specifically how it relates to the abovementioned prothrombotic phenotype and PASC symptom burden.
4. Potential Mechanisms That Drive Thromboinflammation in Long Covid
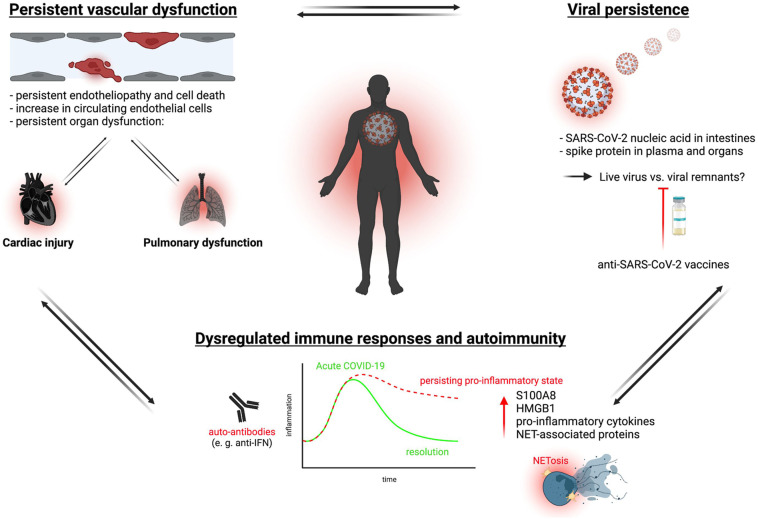
Collectively, there is ample evidence for thromboinflammatory dysregulation in a subset of patients with long COVID, which prompts questioning of the underlying mechanisms. Three important, potentially intertwined hypotheses that might contribute to a hypercoagulable state have been identified: (1) lasting structural changes, most prominently endothelial damage, caused during initial infection, (2) a persistent viral reservoir, and (3) immunopathology driven by a misguided immune system (Figure 2 ).
Figure 2.
Potential mechanisms driving thromboinflammation in long COVID. Three main mechanisms are thought to contribute as constant triggers sustaining long COVID–associated thromboinflammation: persistent endotheliopathy and irreparable damage after acute SARS-CoV-2 infection causing organ dysfunction; a proinflammatory, dysfunctional state of the immune system with long-lasting hyperactivation; and persistence of (possibly live) virus across various organs. Created with BioRender (www.biorender.com). PNA, platelet–neutrophil aggregate; VWF, von Willebrand factor.
4.1. Lasting structural changes
Multiple findings support the notion that lasting structural changes in the vasculature, including endotheliopathy, have some role in PASC. For example, multiple sequelae, including lung function defects and cardiac injury, correlate with initial disease severity and ameliorate over time in most patients [25,29,53]. In this scenario, the vasculopathy maintains a procoagulant state through multiple mechanisms implicated in acute COVID-19, which lags behind viral clearance [93,94]. This could explain persistent changes in the vasculature and related platelet hyperreactivity in the absence of viral persistence in the endothelium. Along these lines, the SARS-CoV-2 omicron sublineage that now dominates infection incidence is known to cause less severe disease and lower respiratory tract involvement, which correlates with decreases in long COVID [95].
4.2. Viral persistence
Another important potential mechanism is viral persistence in the body [96]. In a landmark study, SARS-CoV-2 nucleic acids and immunoreactivity were detected in the small bowel of 7 of 14 asymptomatic individuals 4 months after infection [97]. In routine colonoscopies in patients with inflammatory bowel disease, 24 of 46 patients showed SARS-CoV-2 RNA persistence after mild COVID in gut mucosa biopsies, and this correlated with reported long COVID symptoms [98]. In an autopsy study of subjects with COVID-19 infection, SARS-CoV-2 was detected in multiple organs, even in mild cases, up to 230 days after infection, further underlining the notion of viral persistence [99]. Another indicator that a direct viral effect might play a role in thromboinflammatory PASC is that vaccinations lower incidence of long COVID at least to some extent [100,101]. If this is the major mechanism, one would expect similar drivers of thromboinflammation in PASC as observed in acute COVID-19 [18]. While some similarities have been detected, most notably endothelial dysfunction (see above), others—like changes in platelet and neutrophil phenotypes—show distinct changes or remain understudied. Also, it is worth noting that the studies reporting SARS-CoV-2 RNA persistence were not able to demonstrate viable virus, but rather detected either spike protein or viral nucleic acids [98]. Therefore, it is possible that they describe noninfectious traces of the virus throughout the body.
4.3. Dysregulated immune response
A third potential driver could be a dysregulated immune response caused by acute infection, which then fails to return to baseline due to unknown mechanisms—potentially in conjunction with viral persistence or structural changes as reported above. Immunophenotyping of peripheral blood mononuclear cells in recovering patients revealed ongoing perturbations on a transcriptional level for up to 6 months after infection, with patients with long COVID showing a distinct profile. Observed changes included upregulation of the alarmins S100A8 and HMGB1, which are mediators and markers of innate immune activation [102]. Another study also found increased expression of acute inflammatory markers 9 weeks after acute infection, which correlated positively with initial COVID-19 severity [103]. A large study that performed longitudinal, multiomic profiling in 209 subjects revealed risk factors for PASC, which included latent Epstein-Barr virus reactivation and SARS-CoV-2 RNAemia as well as autoantibodies [62,104]. Interestingly, they found multiple factors known to be enriched in severe COVID-19, like cytotoxic CD4+ T cells, exhausted T cells, and myeloid-derived suppressor cells associated with PASC. A biomarker study identified persistent elevation of inflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor, potentially released by activated myeloid cells up to 24 months after acute illness [63]. This points toward ongoing immunopathology, and another study confirmed changes in circulating myeloid/lymphoid cells and elevated humoral responses in >200 individuals with long COVID [62]. Phetsouphanh et al. [64] found evidence of chronic inflammation with signs of increased monocyte activation and circulating interferons correlating with PASC occurrence, which represents an immunophenotype reminiscent of acute COVID-19. The observed immune dysregulation might also contribute to emergence of viral coinfections, which in turn associates with persistent symptoms after SARS-CoV-2 infection. In particular, reactivation of latent Epstein-Barr virus infection or pre-existing HIV infection increases the likeliness of long COVID symptoms [105].
5. Management of Thrombosis Risk in and Preventive Measures of PASC
How should thrombosis risk in PASC be managed clinically? Expert guidelines do not recommend anticoagulation after discharge unless prior venous thromboembolism occurred or other indications for anticoagulation exist [106,107]. Besides primary prevention of infection by nonpharmacologic interventions, vaccination seems to lower PASC incidence and remains a crucial component to fight COVID-19 and its consequences [108]. In addition, while the existing data are somewhat contradictory, there is some evidence that vaccination could also be efficacious in patients who already experience long COVID [109]. In a study involving 28 356 participants, receiving vaccination after SARS-CoV-2 infection decreased PASC incidence [101,110]. These data support the notion that viral persistence plays a role in PASC. Potentially, this could also implicate the use of antiviral drugs like nirmatrelvir and ritonavir (paxlovid) in long COVID, which has shown promise in case series as well as in primary prevention [[111], [112], [113]]. Paxlovid will now be evaluated for long COVID in an interventional trial (NCT05595369). Another large interventional randomized trial now seeks to identify treatment strategies for PASC: STIMULATE ICP (ISRCTN10665760) will recruit 4500 patients with long COVID and evaluate effects of the direct oral anticoagulant rivaroxaban targeting factor Xa, the anti-inflammatory drug colchicine, and antihistamines on disease course, potentially generating important pathophysiological insights [114]. It is important to note that even if a crucial role of thromboinflammation is confirmed in long COVID pathophysiology, established antithrombotic therapy might not be efficacious. For example, in acute severe COVID-19, there is ample evidence of coagulation activation, but anticoagulation did not show beneficial effects and was rather associated with harm [115]. The reasons for this remain elusive—it will potentially be necessary to develop new pharmacologic tools to target immune-triggered thrombosis in a specific manner [19].
6. Open Questions and future Direction of Research
In summary, there is some evidence that macrovascular thrombosis risk is elevated in PASC and that microvascular thromboinflammation might be a driver of long COVID pathophysiology. This warrants further research as the existing data are insufficient to inform diagnosis and preventive measures of this heterogenous syndrome (Table 2 ). This is due to the fact that most of these studies do not have a control arm and represent a high selection bias. In particular, large clinical cohorts with well-characterized participants and clear assessment of symptomatic burden are needed. Many cohorts assessed so far lack a clear correlation of PASC symptoms and vascular and/or immunologic changes. One confounder in long COVID research is that the definition of this disease potentially unites several distinct pathophysiological entities, including long-term consequences of initial organ damage, worsening of pre-existing conditions, and sequelae caused by virus persistence or immune dysregulation as outlined above [116]. Again, fine-grained longitudinal studies of patients with immune phenotyping, assessment of coagulation, vascular function and platelet/neutrophil parameters in conjunction with detailed information on pre-existing diseases, COVID-19 disease course, and findings of imaging/functional studies in the follow-up period are crucial. The United Kingdom–based Predictors of COVID-19 Outcomes (PRECIOUS) initiative, which seeks to unite and unify available data sets to create an anonymous resource to empower outcome, as well as hypothesis-generating analyses could serve as a starting point [117]. It is important to bear in mind that platelet and neutrophil functional analyses require direct execution upon sampling as these cell types are not viable upon freezing. This complicates large-scale studies and limits information that can be drawn from ongoing or completed studies that did not focus on these cell types. Therefore, specialized studies that investigate components of thromboinflammation in well-defined individuals with PASC are critical.
Table 2.
Open questions and directions for future research.
| Clinical management |
|---|
| Could antithrombotic therapy regimens show efficacy in ameliorating long COVID symptoms? |
| Which subsets of patients with long COVID are at high(est) risk of thrombotic complications? |
| Should patients with long COVID receive anti-platelet therapy or prophylactic/therapeutic anticoagulation to prevent thrombotic complications? |
| Diagnostics |
| How do markers of thromboinflammation correlate with long COVID symptoms qualitatively and quantitatively? |
| Are there specific thromboinflammatory phenotypes associated with PASC subtypes (ie, pulmonary/neurologic symptoms)? |
| Can clinical markers of endothelial cell damage (ie, circulating VWF) or coagulation activation (ie, D-dimer) serve to predict the risk of macrovascular complications or trajectories of PASC? |
| Pathophysiology |
| What is the relative contribution of complement, coagulation abnormalities, platelet phenotype and neutrophils to long COVID? |
| Does modification of thromboinflammation ameliorate long COVID outcomes in animal models? |
| How are ongoing vascular damage, virus persistence, and disturbances in immunity related to thromboinflammation? |
| How are microvascular thrombosis and macrovascular thrombosis linked mechanistically? |
PASC, post–acute sequelae of COVID-19; VWF, von Willebrand factor.
The incidence of postviral syndrome is not new: prior to the COVID-19 pandemic, some patients presented with long-lasting health issues after viral infections, a disease condition that was named myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). While insights into disease pathophysiology remain limited, there are some parallels to PASC (Table 1). For example, individuals with ME/CFS also exhibit evidence of endothelial dysfunction [118]. However, the links to platelet activation and coagulation abnormalities are weak in ME/CFS, and there are no reports on microvascular and macrovascular clotting. In summary, further studies directly comparing these 2 disease entities are needed to outline potentially shared disease mechanisms. Importantly, with the emergence of novel variants, it is most likely necessary to constantly update our understanding of PASC incidence and pathophysiology. To further confirm generated hypotheses mechanistically, animal models of PASC are necessary. Although promising findings were reported in a hamster model that shows chronic changes after SARS-CoV-2 infection, further data are needed to clarify to what extent this reflects the sequelae observed in humans and to investigate if immunothrombotic changes can be observed [119,120]. After successfully developing diagnostic, preventive, and therapeutic tools in acute COVID-19, it is once again necessary for clinicians, scientists, and communities globally to join forces and decipher this ongoing health crisis in the wake of the pandemic.
Acknowledgments
This study was supported by the Deutsche Herzstiftung e.V., Frankfurt a.M. (individual grants for R.K. and L.N.), Deutsche Forschungsgemeinschaft (DFG, SFB 1123 (L.N. [B06] and K.S. [A07]), and 512460086 [L.N.]), the German Center for Cardiovascular Research (DZHK) (Clinician Scientist Program [L.N.]), and the DFG Clinician Scientist Program PRIME (413635475, R.K.). The work was also supported by the European Research Council (ERC-“T-MEMORE” to K.S.), and the Society for Thrombosis and Haemostasis Research (individual grant for L.N.).
Author contributions
Initiation: L.N., initial draft: L.N., writing and editing: K.S., L.N., and R.K., figure design: R.K.
Declaration of competing interests
There are no competing interests to disclose.
Footnotes
Funding information This study was supported by the Deutsche Herzstiftung e.V., Frankfurt a.M. (individual grants for R.K. and L.N.), Deutsche Forschungsgemeinschaft (DFG), the DFG SFB 1123 (L.N. [B06] and K.S. [A07]), the German Center for Cardiovascular Research (Clinician Scientist Program [L.N.]), the DFG Clinician Scientist Program PRIME (413635475, R.K.), and the European Research Council (ERC-“T-MEMORE” to K.S.).
Manuscript handled by: Patricia Liaw
Final decision: Patricia Liaw, 10 April 2023
References
- 1.Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., Fralick M., Schou M., Lamberts M., Gerds T., Fosbøl E.L., Phelps M., Kragholm K.H., Andersen M.P., Køber L., Torp-Pedersen C., Solomon S.D., Gislason G., Biering-Sørensen T. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dybowska M., Wyrostkiewicz D., Opoka L., Lewandowska K., Sobiecka M., Tomkowski W., Szturmowicz M. Venous thromboembolic disease in COVID-19, pathophysiology, therapy and prophylaxis. Int J Mol Sci. 2022;23 doi: 10.3390/ijms231810372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortega-Paz L., Talasaz A.H., Sadeghipour P., Potpara T.S., Aronow H.D., Jara-Palomares L., Sholzberg M., Angiolillo D.J., Lip G.Y.H., Bikdeli B. COVID-19-associated pulmonary embolism: review of the pathophysiology, epidemiology, prevention, diagnosis, and treatment. Semin Thromb Hemost. 2022 doi: 10.1055/s-0042-1757634. [DOI] [PubMed] [Google Scholar]
- 5.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 6.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T., Warkentin T.E., Thachil J., Levi M., Levy J.H. Proposal of the definition for COVID-19-associated coagulopathy. J Clin Med. 2021:10. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M., Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdanov V.Y., Khirmanov V.N. SARS-CoV-2, platelets, and endothelium: coexistence in space and time, or a pernicious ménage à trois? Vasc Biol. 2022;4:R35–R43. doi: 10.1530/VB-22-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser R., Leunig A., Pekayvaz K., Popp O., Joppich M., Polewka V., Escaig R., Anjum A., Hoffknecht M.-L., Gold C., Brambs S., Engel A., Stockhausen S., Knottenberg V., Titova A., Haji M., Scherer C., Muenchhoff M., Hellmuth J.C., Saar K., et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., Scherer C., Rudelius M., Zoller M., Höchter D., Keppler O., Teupser D., Zwißler B., von Bergwelt-Baildon M., Kääb S., Massberg S., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolai L., Leunig A., Brambs S., Kaiser R., Joppich M., Hoffknecht M., Gold C., Engel A., Polewka V., Muenchhoff M., Hellmuth J.C., Ruhle A., Ledderose S., Weinberger T., Schulz H., Scherer C., Rudelius M., Zoller M., Keppler O.T., Zwißler B., et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemost. 2021;19:574–581. doi: 10.1111/jth.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M., Chang C.H., Zhang H., Shallow M., Bahel P., Owusu K., Yamamoto Y., Arora T., Atri D.S., Patel A., Gbyli R., Kwan J., Won C.H., Dela Cruz C., Price C., et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5:1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afzali B., Noris M., Lambrecht B.N., Kemper C. The state of complement in COVID-19. Nat Rev Immunol. 2022;22:77–84. doi: 10.1038/s41577-021-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvelli J., Demaria O., Vély F., Batista L., Chouaki B.N., Fares J., Carpentier S., Thibult M.-L., Morel A., Remark R., André P., Represa A., Piperoglou C. the Explore COVID-19 IPH group, the Explore COVID-19 Marseille Immunopole group, Cordier PY, Le Dault E, Guervilly C, Simeone P, Gainnier M, et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway E.M., Mackman N., Warren R.Q., Wolberg A.S., Mosnier L.O., Campbell R.A., Gralinski L.E., Rondina M.T., van de Veerdonk F.L., Hoffmeister K.M., Griffin J.H., Nugent D., Moon K., Morrissey J.H. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22:639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark K., Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18:666–682. doi: 10.1038/s41569-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ 2020 [accessed May 28, 2023].
- 21.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 24.Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., Lipsitch M., Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puntmann V.O., Martin S., Shchendrygina A., Hoffmann J., Ka M.M., Giokoglu E., Vanchin B., Holm N., Karyou A., Laux G.S., Arendt C., De Leuw P., Zacharowski K., Khodamoradi Y., Vehreschild M.J.G.T., Rohde G., Zeiher A.M., Vogl T.J., Schwenke C., Nagel E. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat Med. 2022;28:2117–2123. doi: 10.1038/s41591-022-02000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 28.Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., Huggins C.F., Kwong A.S.F., Silverwood R.J., Di Gessa G., Bowyer R.C.E., Northstone K., Hou B., Green M.J., Dodgeon B., Doores K.J., Duncan E.L., Williams F.M.K., OpenSAFELY Collaborative, Steptoe A., et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13:3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-Pérez O., Merino E., Leon-Ramirez J.-M., Andres M., Ramos J.M., Arenas-Jiménez J., Asensio S., Sanchez R., Ruiz-Torregrosa P., Galan I., Scholz A., Amo A., González-delaAleja P., Boix V., Gil J., COVID19-ALC research group Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willyard C. Could tiny blood clots cause long COVID’s puzzling symptoms? Nature. 2022;608:662–664. doi: 10.1038/d41586-022-02286-7. [DOI] [PubMed] [Google Scholar]
- 33.Fogarty H., Townsend L., Morrin H., Ahmad A., Comerford C., Karampini E., Englert H., Byrne M., Bergin C., O’Sullivan J.M., Martin-Loeches I., Nadarajan P., Bannan C., Mallon P.W., Curley G.F., Preston R.J.S., Rehill A.M., McGonagle D., Ni Cheallaigh C., Baker R.I., et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pretorius E., Vlok M., Venter C., Bezuidenhout J.A., Laubscher G.J., Steenkamp J., Kell D.B. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20:172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahamed J., Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest. 2022:132. doi: 10.1172/JCI161167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fogarty H., Ward S.E., Townsend L., Karampini E., Elliott S., Conlon N., Dunne J., Kiersey R., Naughton A., Gardiner M., Byrne M., Bergin C., O’Sullivan J.M., Martin-Loeches I., Nadarajan P., Bannan C., Mallon P.W., Curley G.F., Preston R.J.S., Rehill A.M., et al. Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction. J Thromb Haemost. 2022;20:2429–2438. doi: 10.1111/jth.15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masias C., Cataland S.R. The role of ADAMTS13 testing in the diagnosis and management of thrombotic microangiopathies and thrombosis. Blood, The Journal of the American Society of Hematology. 2018;132:903–910. doi: 10.1182/blood-2018-02-791533. [DOI] [PubMed] [Google Scholar]
- 38.Favaloro E.J., Henry B.M., Lippi G. 4th ed. Thieme Medical Publishers Inc; The Journal of the American Society of Hematology; Washington: 2018. Increased VWF and decreased ADAMTS-13 in COVID-19: creating a milieu for (micro) thrombosis; pp. 400–418. [DOI] [PubMed] [Google Scholar]
- 39.Alvarado-Moreno J.A., Davila-Moreno J., Dominguez-Reyes V., Arreola-Diaz R., Isordia-Salas I., Chavez-Gonzalez A., Majluf-Cruz A. Morphological and functional alterations in endothelial colony-forming cells from recovered COVID-19 patients. Thromb Res. 2021;206:55–59. doi: 10.1016/j.thromres.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poyatos P., Luque N., Eizaguirre S., Sabater G., Sebastián L., Í Francisco-Albesa, Peracaula M., Boixadé M., Orriols R., Tura-Ceide O. Post-COVID-19 patients show an increased endothelial progenitor cell production. Transl Res. 2022;243:14–20. doi: 10.1016/j.trsl.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charfeddine S., Ibn Hadj Amor H., Jdidi J., Torjmen S., Kraiem S., Hammami R., Bahloul A., Kallel N., Moussa N., Touil I., Ghrab A., Elghoul J., Meddeb Z., Thabet Y., Kammoun S., Bouslama K., Milouchi S., Abdessalem S., Abid L. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc Med. 2021;8:1702. doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrow A.J., Sykes R., McIntosh A., Kamdar A., Bagot C., Bayes H.K., Blyth K.G., Briscoe M., Bulluck H., Carrick D., Church C., Corcoran D., Findlay I., Gibson V.B., Gillespie L., Grieve D., Hall Barrientos P., Ho A., Lang N.N., Lennie V., et al. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat Med. 2022;28:1303–1313. doi: 10.1038/s41591-022-01837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherbakov N., Szklarski M., Hartwig J., Sotzny F., Lorenz S., Meyer A., Grabowski P., Doehner W., Scheibenbogen C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020;7:1064–1071. doi: 10.1002/ehf2.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newton D.J., Kennedy G., Chan K.K.F., Lang C.C., Belch J.J.F., Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. 2012;154:335–336. doi: 10.1016/j.ijcard.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Zheng S.-Y., Xiao Q.-Y., Xie X.-H., Deng Y., Ren L., Tian D.-Y., Luo Z.-X., Luo J., Fu Z., Huang A.-L., Liu E.M. Association between secondary thrombocytosis and viral respiratory tract infections in children. Sci Rep. 2016;6 doi: 10.1038/srep22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pretorius E., Venter C., Laubscher G.J., Kotze M.J., Oladejo S.O., Watson L.R., Rajaratnam K., Watson B.W., Kell D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins-Gonçalves R., Campos M.M., Palhinha L., Azevedo-Quintanilha I.G., Abud Mendes M., Ramos Temerozo J., Toledo-Mendes J., Rosado-de-Castro P.H., Bozza F.A., Souza Rodrigues R., Hottz E.D., Bozza P.T. Persisting platelet activation and hyperactivity in COVID-19 survivors. Circ Res. 2022;131:944–947. doi: 10.1161/CIRCRESAHA.122.321659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner S, Naidoo CA, Usher TJ, Kruger A, Venter C, Laubscher GJ, Khan MA, Kell DB, Pretorius E. Increased levels of inflammatory molecules in blood of long COVID patients point to thrombotic endotheliitis. medRxiv 2022:2022.10.13.22281055. [DOI] [PubMed]
- 49.Kennedy G., Norris G., Spence V., McLaren M., Belch J.J.F. Is chronic fatigue syndrome associated with platelet activation? Blood Coagul Fibrinolysis. 2006;17:89–92. doi: 10.1097/01.mbc.0000214705.80997.73. [DOI] [PubMed] [Google Scholar]
- 50.Berg D., Berg L.H., Couvaras J., Harrison H. Chronic fatigue syndrome and/or fibromyalgia as a variation of antiphospholipid antibody syndrome: an explanatory model and approach to laboratory diagnosis. Blood Coagul Fibrinolysis. 1999;10:435–438. doi: 10.1097/00001721-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Dean L.S., Devendra G., Jiyarom B., Subia N., Tallquist M.D., Nerurkar V.R., Chang S.P., Chow D.C., Shikuma C.M., Park J. Phenotypic alteration of low-density granulocytes in people with pulmonary post-acute sequalae of SARS-CoV-2 infection. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1076724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pisareva E., Badiou S., Mihalovičová L., Mirandola A., Pastor B., Kudriavtsev A., Berger M., Roubille C., Fesler P., Klouche K., Cristol J.P., Thierry A.R. Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients. J Med Virol. 2023;95 doi: 10.1002/jmv.28209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George P.M., Reed A., Desai S.R., Devaraj A., Faiez T.S., Laverty S., Kanwal A., Esneau C., Liu M.K.C., Kamal F., Man W.D.C., Kaul S., Singh S., Lamb G., Faizi F.K., Schuliga M., Read J., Burgoyne T., Pinto A.L., Micallef J., et al. A persistent neutrophil-associated immune signature characterizes post–COVID-19 pulmonary sequelae. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abo5795. [DOI] [PubMed] [Google Scholar]
- 54.Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L., De Domenico E., Wendisch D., Grasshoff M., Kapellos T.S., Beckstette M., Pecht T., Saglam A., Dietrich O., Mei H.E., Schulz A.R., et al. evere COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy G., Spence V., Underwood C., Belch J.J.F. Increased neutrophil apoptosis in chronic fatigue syndrome. J Clin Pathol. 2004;57:891–893. doi: 10.1136/jcp.2003.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Magnusson M., Mackman N., Thalin C., Lisman T. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5:756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hannan K.L., Berg D.E., Baumzweiger W., Harrison H.H., Berg L.H., Ramirez R., Nichols D. Activation of the coagulation system in Gulf War Illness: a potential pathophysiologic link with chronic fatigue syndrome. A laboratory approach to diagnosis. Blood Coagul Fibrinolysis. 2000;11:673–678. doi: 10.1097/00001721-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 58.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., Tonby K., Barratt-Due A., Sokolova M., Schjalm C., Chaban V., Kolderup A., Tran T., Tollefsrud Gjølberg T., Skeie L.G., Hesstvedt L., Ormåsen V., Fevang B., Austad C., Müller K.E., et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117:25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colarusso C., Maglio A., Terlizzi M., Vitale C., Molino A., Pinto A., Vatrella A., Sorrentino R. Post-COVID-19 patients who develop lung fibrotic-like changes have lower circulating levels of IFN-β but higher levels of IL-1α and TGF-β. Biomedicines. 2021;9 doi: 10.3390/biomedicines9121931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sorensen B., Streib J.E., Strand M., Make B., Giclas P.C., Fleshner M., Jones J.F. Complement activation in a model of chronic fatigue syndrome. J Allergy Clin Immunol. 2003;112:397–403. doi: 10.1067/mai.2003.1615. [DOI] [PubMed] [Google Scholar]
- 61.Castro-Marrero J., Zacares M., Almenar-Pérez E., Alegre-Martín J., Oltra E. Complement component C1q as a potential diagnostic tool for myalgic encephalomyelitis/chronic fatigue syndrome subtyping. J Clin Med. 2021;10:4171. doi: 10.3390/jcm10184171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein J., Wood J., Jaycox J., Lu P., Dhodapkar R.M., Gehlhausen J.R., Tabachnikova A., Tabacof L., Malik A.A., Kamath K., Greene K., Monteiro V.S., Peña-Hernandez M., Mao T., Bhattacharjee B., Takahashi T., Lucas C., Silva J., Mccarthy D., Breyman E., et al. Distinguishing features of long COVID identified through immune profiling. medRxiv. 2022 doi: 10.1038/s41586-023-06651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultheiß C., Willscher E., Paschold L., Gottschick C., Klee B., Henkes S.-S., Bosurgi L., Dutzmann J., Sedding D., Frese T., Girndt M., Höll J.I., Gekle M., Mikolajczyk R., Binder M. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., Kelleher A.D., Matthews G.V. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 65.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184:1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed F., Vu L.T., Zhu H., Iu D.S.H., Fogarty E.A., Kwak Y., Chen W., Franconi C.J., Munn P.R., Levine S.M., Stevens J., Mao X., Shungu D.C., Moore G.E., Keller B.A., Hanson M.R., Grenier J.K., Grimson A. Single-cell transcriptomics of the immune system in ME/CFS at baseline and following symptom provocation. bioRxiv. 2022 doi: 10.1016/j.xcrm.2023.101373. 10.13.512091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitami T., Fukuda S., Kato T., Yamaguti K., Nakatomi Y., Yamano E., Kataoka Y., Mizuno K., Tsuboi Y., Kogo Y., Suzuki H., Itoh M., Morioka M.S., Kawaji H., Koseki H., Kikuchi J., Hayashizaki Y., Ohno H., Kuratsune H., Watanabe Y. Deep phenotyping of myalgic encephalomyelitis/chronic fatigue syndrome in Japanese population. Sci Rep. 2020;10 doi: 10.1038/s41598-020-77105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan B.E., Wong S.W., Sum C.L.L., Lim G.H., Leung B.P., Tan C.W., Ramanathan K., Dalan R., Cheung C., Lim X.R., Sadasiv M.S., Lye D.C., Young B.E., Yap E.S., Chia Y.W. COVID-19 Clotting and Bleeding Investigators. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: assessing the long-term outcomes in COVID-19 patients. Am J Hematol. 2022;97:915–923. doi: 10.1002/ajh.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Townsend L., Fogarty H., Dyer A., Martin-Loeches I., Bannan C., Nadarajan P., Bergin C., O’Farrelly C., Conlon N., Bourke N.M., Ward S.E., Byrne M., Ryan K., O’Connell N., O’Sullivan J.M., Ni Cheallaigh C., O’Donnell J.S. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19:1064–1070. doi: 10.1111/jth.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pasini E., Corsetti G., Romano C., Scarabelli T.M., Chen-Scarabelli C., Saravolatz L., Dioguardi F.S. Serum metabolic profile in patients with long-covid (PASC) syndrome: clinical implications. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalaivani M.K., Dinakar S. Association between D-dimer levels and post-acute sequelae of SARS-CoV-2 in patients from a tertiary care center. Biomark Med. 2022;16:833–838. doi: 10.2217/bmm-2022-0050. [DOI] [PubMed] [Google Scholar]
- 72.Bray M.A., Sartain S.E., Gollamudi J., Rumbaut R.E. Microvascular thrombosis: experimental and clinical implications. Transl Res. 2020;225:105–130. doi: 10.1016/j.trsl.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho J.L., Villacreses R., Nagpal P., Guo J., Pezzulo A.A., Thurman A.L., Hamzeh N.Y., Blount R.J., Fortis S., Hoffman E.A., Zabner J., Comellas A.P. Quantitative chest CT assessment of small airways disease in post-acute SARS-CoV-2 infection. Radiology. 2022;304:185–192. doi: 10.1148/radiol.212170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buonsenso D., Di Giuda D., Sigfrid L., Pizzuto D.A., Di Sante G., De Rose C., Lazzareschi I., Sali M., Baldi F., Chieffo D.P.R., Munblit D., Valentini P. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health. 2021;5:677–680. doi: 10.1016/S2352-4642(21)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heiss R, Wagner AL, Tan L, Regensburger AP, Schmidt S, Ewert F, Mammadova D, Bühler A, Vogel-Claussen J, Voskrebenzev A, Rauh M, Rompel O, Nagel AM, Lévy S, Bickelhaupt S, May MS, Uder M, Metzler M, Trollmann R, Woelfle J, et al. Persisting pulmonary dysfunction in pediatric post-acute covid-19. SSRN Journal.
- 76.Heiss R., Tan L., Schmidt S., Regensburger A.P., Ewert F., Mammadova D., Buehler A., Vogel-Claussen J., Voskrebenzev A., Rauh M., Rompel O., Nagel A.M., Lévy S., Bickelhaupt S., May M.S., Uder M., Metzler M., Trollmann R., Woelfle J., Wagner A.L., Knieling F. Pulmonary dysfunction after pediatric COVID-19. Radiology. 2022;306 doi: 10.1148/radiol.221250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasannan N., Heightman M., Hillman T., Wall E., Bell R., Kessler A., Neave L., Doyle A., Devaraj A., Singh D., Dehbi H.M., Scully M. Impaired exercise capacity in post-COVID-19 syndrome: the role of VWF-ADAMTS13 axis. Blood Adv. 2022;6:4041–4048. doi: 10.1182/bloodadvances.2021006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin Y., Wu J., Chen T., Li J., Zhang G., Wu D., Zhou Y., Zheng N., Cai A., Ning Q., Manyande A., Xu F., Wang J., Zhu W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021:131. doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee M.H., Perl D.P., Steiner J., Pasternack N., Li W., Maric D., Safavi F., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J.T., Hefti M., Folkerth R.D., Nath A. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145:2555–2568. doi: 10.1093/brain/awac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., Masliah E., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J., Hefti M., Folkerth R.D., Nath A. Microvascular injury in the brains of patients with covid-19. N Engl J Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.-H., Wood J., O’Dea M.R., Dutton S., Shamardani K., Nwangwu K., Mancusi R., Yalçın B., Taylor K.R., Acosta-Alvarez L., Malacon K., Keough M.B., Ni L., Woo P.J., Contreras-Esquivel D., Toland A.M.S., et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 84.Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., Banerjee A. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicolai L., Massberg S. Platelets as key players in inflammation and infection. Curr Opin Hematol. 2020;27:34–40. doi: 10.1097/MOH.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 86.Nicolai L., Gaertner F., Massberg S. Platelets in host defense: experimental and clinical insights. Trends Immunol. 2019;40:922–938. doi: 10.1016/j.it.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Constantinescu-Bercu A., Kessler A., de Groot R., Dragunaite B., Heightman M., Hillman T., Price L.C., Brennan E., Sivera R., Vanhoorelbeke K., Singh D., Scully M. Analysis of thrombogenicity under flow reveals new insights into the prothrombotic state of patients with post-COVID syndrome. J Thromb Haemost. 2023;21:94–100. doi: 10.1016/j.jtha.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giannis D., Allen S.L., Tsang J., Flint S., Pinhasov T., Williams S., Tan G., Thakur R., Leung C., Snyder M., Bhatia C., Garrett D., Cotte C., Isaacs S., Gugerty E., Davidson A., Marder G.S., Schnitzer A., Goldberg B., McGinn T., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knight R., Walker V., Ip S., Cooper J.A., Bolton T., Keene S., Denholm R., Akbari A., Abbasizanjani H., Torabi F., Omigie E., Hollings S., North T.L., Toms R., Jiang X., Angelantonio E.D., Denaxas S., Thygesen J.H., Tomlinson C., Bray B., et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146:892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thachil J. Hypoxia—an overlooked trigger for thrombosis in COVID-19 and other critically ill patients. J Thromb Haemost. 2020;18:3109–3110. doi: 10.1111/jth.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antonelli M., Pujol J.C., Spector T.D., Ourselin S., Steves C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399:2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swank Z., Senussi Y., Manickas-Hill Z., Yu X.G., Li J.Z., Alter G., Walt D.R. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76:e487–e490. doi: 10.1093/cid/ciac722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., Cipolla M., Viant C., Barnes C.O., Bram Y., Breton G., Hägglöf T., Mendoza P., Hurley A., Turroja M., Gordon K., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zollner A., Koch R., Jukic A., Pfister A., Meyer M., Rössler A., Kimpel J., Adolph T.E., Tilg H. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;163:495–506.e8. doi: 10.1053/j.gastro.2022.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stein S.R., Ramelli S.C., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., Winkler C.W., Sun J., Dickey J.M., Ylaya K., Ko S.H., Platt A.P., Burbelo P.D., Quezado M., Pittaluga S., Purcell M., Munster V.J., Belinky F., Ramos-Benitez M.J., Boritz E.A., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., Peligro P.J., Casimiro M., Guerrero J.J., Gellaco M.M.L., Lippi G., Henry B.M., Fernández-de-Las-Peñas C. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tannous J., Pan A.P., Potter T., Bako A., Dlouhy K., Drews A., Sostman H.D., Vahidy F.S. Real world evidence of effectiveness of COVID-19 vaccines and anti SARS-CoV-2 monoclonal antibodies against post-acute sequelae of SARS-CoV-2 infection. medRxiv. 2022:2022. doi: 10.1136/bmjopen-2022-067611. 06.30.22277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., Garcia-Valtanen P., Al-Delfi Z., Gummow J., Ferguson C., O’Connor S., Reddi B.A.J., Hissaria P., Shaw D., Kok-Lim C., Gleadle J.M., Beard M.R., Barry S.C., Grubor-Bauk B., Lynn D.J. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chun H.J., Coutavas E., Pine A.B., Lee A.I., Yu V.L., Shallow M.K., Giovacchini C.X., Mathews A.M., Stephenson B., Que L.G., Lee P.J., Kraft B.D. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. 2021:6. doi: 10.1172/jci.insight.148476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., Kornilov S.A., Scherler K., Pavlovitch-Bedzyk A.J., Dong S., Lausted C., Lee I., Fallen S., Dai C.L., Baloni P., Smith B., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peluso M.J., Deveau T.-M., Munter S.E., Ryder D., Buck A., Beck-Engeser G., Chan F., Lu S., Goldberg S.A., Hoh R., Tai V., Torres L., Iyer N.S., Deswal M., Ngo L.H., Buitrago M., Rodriguez A., Chen J.Y., Yee B.C., Chenna A., et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023:133. doi: 10.1172/JCI163669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., Davila J., DeSancho M.T., Diuguid D., Griffin D.O., Kahn S.R., Klok F.A., Lee A.I., Neumann I., Pai A., Righini M., Sanfilippo K.M., Siegal D., Skara M., Terrell D.R., et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv. 2022;6:664–671. doi: 10.1182/bloodadvances.2021005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wynberg E., Han A.X., Boyd A., van Willigen H.D.G., Verveen A., Lebbink R., van der Straten K., Kootstra N., van Gils M.J., Russell C., Leenstra T., de Jong M.D., de Bree G.J., Prins M. RECoVERED Study Group. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): a prospective cohort study. Vaccine. 2022;40:4424–4431. doi: 10.1016/j.vaccine.2022.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ayoubkhani D., Bermingham C., Pouwels K.B., Glickman M., Nafilyan V., Zaccardi F., Khunti K., Alwan N.A., Walker A.S. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., Pypstra R., Rusnak J.M. EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peluso M.J., Anglin K., Durstenfeld M.S., Martin J.N., Kelly J.D., Hsue P.Y., Henrich T.J., Deeks S.G. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7:95–103. doi: 10.20411/pai.v7i1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie Y., Choi T., Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv. 2022:2022. 11.03.22281783. [Google Scholar]
- 114.Forshaw D., Wall E.C., Prescott G., Dehbi H.-M., Green A., Attree E., Hismeh L., Strain W.D., Crooks M.G., Watkins C. STIMULATE-ICP: A pragmatic, multi-centre, cluster randomised trial of an integrated care pathway with a nested, Phase III, open label, adaptive platform randomised drug trial in individuals with long COVID: a structured protocol. medRxiv. 2022 doi: 10.1371/journal.pone.0272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reis S., Popp M., Schießer S., Metzendorf M.-I., Kranke P., Meybohm P., Weibel S. Anticoagulation in COVID-19 patients– an updated systematic review and meta-analysis. Thromb Res. 2022;219:40–48. doi: 10.1016/j.thromres.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruffieux H., Hanson A.L., Lodge S., Lawler N.G., Whiley L., Gray N., Nolan T.H., Bergamaschi L., Mescia F., Turner L., de Sa A., Pelly V.S., Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) BioResource COVID-19 Collaboration. Kotagiri P., Kingston N., Bradley J.R., Holmes E., Wist J., Nicholson J.K., Lyons P.A., et al. A patient-centric modeling framework captures recovery from SARS-CoV-2 infection. Nat Immunol. 2023;24:349–358. doi: 10.1038/s41590-022-01380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.National Institute of Health Research. The PRECIOUS study, https://www.preciouscovidoutcomes.org; 2023 [accessed May 28, 2023].
- 118.Haffke M., Freitag H., Rudolf G., Seifert M., Doehner W., Scherbakov N., Hanitsch L., Wittke K., Bauer S., Konietschke F., Paul F., Bellmann-Strobl J., Kedor C., Scheibenbogen C., Sotzny F. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20:138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frere J.J., Serafini R.A., Pryce K.D., Zazhytska M., Oishi K., Golynker I., Panis M., Zimering J., Horiuchi S., Hoagland D.A., Møller R., Ruiz A., Kodra A., Overdevest J.B., Canoll P.D., Borczuk A.C., Chandar V., Bram Y., Schwartz R., Lomvardas S., et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jansen E.B., Orvold S.N., Swan C.L., Yourkowski A., Thivierge B.M., Francis M.E., Ge A., Rioux M., Darbellay J., Howland J.G., Kelvin A.A. After the virus has cleared—can preclinical models be employed for long COVID research? PLOS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010741. [DOI] [PMC free article] [PubMed] [Google Scholar]