Background:
The association between pretreatment skeletal muscle index (SMI) and long-term survival of pancreatic carcinoma patients remains unclear up to now.
Methods:
The PubMed, Web of Science and EMBASE databases were searched up to March 1, 2022 for relevant studies. The primary and secondary outcomes were overall survival and progression-free survival, respectively. The hazard ratios (HRs) and 95% confidence intervals (CIs) were combined to assess the relationship between pretreatment SMI and prognosis of pancreatic carcinoma patients. All statistical analysis was conducted by STATA 15.0 software.
Results:
Twenty retrospective studies involving 3765 patients were included. The pooled results demonstrated that lower pretreatment SMI was significantly related to poorer overall survival (HR = 1.42, 95% CI: 1.25–1.62, P < .001) and progression-free survival (HR = 1.41, 95% CI: 1.08–1.84, P = .012). Besides subgroup analysis based on the treatment (non-surgery vs surgery) and tumor stage (advanced vs early stage) showed similar results.
Conclusion:
Pretreatment SMI could serve as a promising and reliable prognostic factor for pancreatic carcinoma patients and lower pretreatment SMI predicted worse prognosis.
Keywords: meta-analysis, pancreatic carcinoma, prognosis, skeletal muscle mass index
1. Introduction
Pancreatic carcinoma remains the fourth leading cause of cancer-related death over the world and a considerable number of patients are diagnosed with advanced stage.[1,2] Despite great efforts in improving survival by developing more advanced treatment techniques in the systemic chemotherapy and operation, the prognosis of pancreatic carcinoma patients remains very poor with the 5-year survival rate of 9% at all tumor stages and 3% at advanced stage.[2,3] The reasons for high mortality are that patients are frequently diagnosed with unresectable cancer and pancreatic carcinoma has a high risk of metastasis and recurrence.[4,5]
Thus, it is a little hard to predict the survival of pancreatic carcinoma patients according to the tumor-node-metastasis stage. In recent years, a number of indexes which are easily obtained in clinics have been introduced and showed relatively high prognostic value in pancreatic carcinoma patients such as the controlling systemic immune-inflammation index, modified Glasgow prognostic score (mGPS), lymphocyte to monocyte ratio, platelet to lymphocyte ratio, and C-reactive protein to albumin ratio.[6–10] Unfortunately, these prognostic factors are limited in clinics due to the instability. On the other hand, growing evidence has indicated the close association between body composition and prognosis of cancer patients. Pancreatic carcinoma patients are frequently observed to experience body weight loss, especially skeletal muscle loss, which is related to the cancer progression.[11–13] The loss of skeletal muscle mass is relatively objective and accurate. Meanwhile, a lot of studies have demonstrated that the nutritional status of the body is closely associated with disease progression and long-term survival of cancer patients.[14–16] Besides, some studies revealed that pretreatment skeletal muscle index (SMI) is significantly related to poor prognosis of cancer patients.[17–21] However, the association between pretreatment SMI and survival of pancreatic cancer patients remains unclear up to now.
Thus, the aim of this meta-analysis was to further identify the prognostic role of pretreatment SMI in pancreatic carcinoma, which might help with the prediction of survival and formulation of treatment strategies for pancreatic cancer patients.
2. Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (2009) checklist.
2.1. Literature search
The PubMed, Web of Science and EMBASE databases were searched up to March 1, 2022 for studies which explored the prognostic value of pretreatment SMI in pancreatic carcinoma. The following key words were used during the literature search: pancreatic carcinoma, pancreatic cancer, skeletal muscle index, SMI, prognostic, survival and prognostic. The detailed search strategy was as follows: (pancreatic carcinoma OR pancreatic cancer) AND (skeletal muscle index OR SMI) AND (prognostic OR survival OR prognostic). Furthermore, the references of included studies were also reviewed for availability.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: patients were pathologically diagnosed with primary pancreatic carcinoma; the SMI was calculated through the computed tomography images before any anti-tumor treatment and association between pretreatment SMI and prognosis of pancreatic cancer patients was explored; the overall survival (OS) or progression-free survival (PFS) was defined as the clinical outcome and corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) were directly reported.
The exclusion criteria were as follows: reviews, letters, editorials, meeting abstracts, or case reports; overlapped or duplicated data; the HRs with 95% CIs were not directly provided in the articles; low quality studies with the Newcastle Ottawa Scale (NOS) score of 5 or lower.[22]
2.3. Data collection
The following data were collected from the included studies in this meta-analysis: the name of first author, publication year, country, tumor-node-metastasis stage, sample size, treatment (non-surgery vs surgery), cutoff value of SMI, endpoint (OS or PFS), HR and 95% CI.
2.4. Methodological quality assessment
As mentioned above, the methodological quality of included studies were evaluated according to the NOS score and only high-quality studies with a NOS score of 6 or higher were included in this meta-analysis.[22]
The literature search, selection, data collection and quality assessment were all conducted by 2 authors independently. Any disagreement was resolved by team discussion.
2.5. Statistical analysis
The HRs with 95% CIs were combined to identify the association between pretreatment SMI and prognosis of pancreatic carcinoma patients. The heterogeneity among studies was evaluated by Cochran’s Q test and Higgins I2 statistic. The P < .10 and/or I2 > 50% was defined as significant heterogeneity and the random-effects model was applied for the pooled effect estimates; otherwise, the fixed-effects model was applied.[23] Subgroup analyses stratified by the treatment (non-surgery vs surgery) and tumor stage (advanced vs early stage) were further conducted. Sensitivity analysis for OS was performed by excluding individual study from the meta-analysis each time. Begg’s funnel plot and Egger’s test were conducted to evaluate publication bias. Significant publication bias was defined as the P < .05, and then the trim-and-fill method was applied to assess the influence of potentially unpublished papers on the stability of the pooled results.[24] All statistical analysis was conducted by STATA 15.0 software (College Station, TX).
3. Results
3.1. Literature search and selection
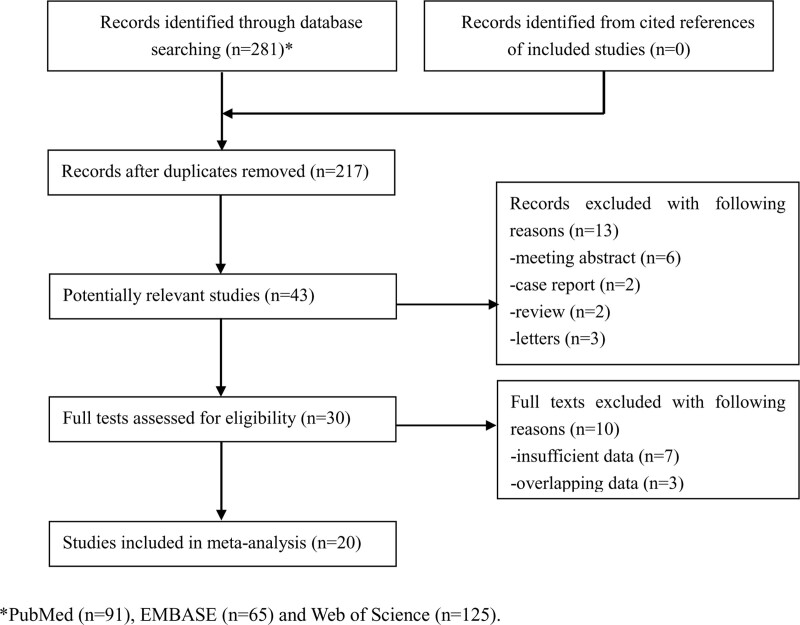
A total of 281 records were identified from 3 databases and 64 duplicated records were removed. Then 174 publications were removed after reading the tiles. After screening the abstracts and full texts of remaining 43 publications, 23 records were excluded. Eventually, a total of 20 retrospective studies involving 3765 participants were enrolled in the current meta-analysis.[25–44] The detailed literature searching and selection process is presented in Figure 1.
Figure 1.
The flow diagram of this meta-analysis.
3.2. Basic characteristics of included studies
Among included studies, most studies were from Asian countries and the sample size ranged from 55 to 484. Half of included studies focused on advanced stage patients. The other detailed information is shown in Table 1.
Table 1.
Basic characteristics of included studies.
| Author | Year | Country | Sample size | TNM stage | Treatment | Cutoff of SMI | Endpoint | NOS |
|---|---|---|---|---|---|---|---|---|
| Choi[25] | 2015 | Korea | 484 | Advanced | Non-surgery | Male: <42.2 cm2/m2, female: <33.9 cm2/m2 | OS | 7 |
| Park[26] | 2016 | Korea | 88 | Advanced | Non-surgery | Male: <49.18 cm2/m2, female: <31.09 cm2/m2 | OS | 7 |
| Ninomiya[27] | 2017 | Japan | 112 | I–IV | Surgery | Male: <43.75 cm2/m2, female: <38.5 cm2/m2 | OS | 7 |
| Okumura[28] | 2017 | Japan | 301 | I-II | Surgery | Male: <47.1 cm2/m2, female: <36.6 cm2/m2 | OS, PFS | 6 |
| Bian[29] | 2018 | China | 203 | III-IV | Non-surgery | Male: <42.0 2c m2/m2, female: <36.55 cm2/m2 | OS | 6 |
| EI Amrani[30] | 2018 | France | 107 | NR | Surgery | Male: <52.4 cm2/m2, female: <38.5 cm2/m2 | OS | 7 |
| Sugimoto[31] | 2018 | USA | 323 | NR | Surgery | Male: <55.4 cm2/m2, female: < 38.9 cm2/m2 | OS, PFS | 6 |
| Basile[32] | 2019 | Italy | 162 | Advanced | Non-surgery | Male: <53/43 cm2/m2, female: <41 cm2/m2 | OS | 6 |
| Gruber[33] | 2019 | Austria | 133 | I-IV | Surgery | Male: <52.4 cm2/m2, female: <38.5 cm2/m2 | OS | 8 |
| Kurita[34] | 2019 | Japan | 82 | Advanced | Non-surgery | Male: <45.3 cm2/m2, female: <37.1 cm2/m2 | OS | 8 |
| Lee[35] | 2019 | Korea | 57 | Advanced | Non-surgery | NR | OS, PFS | 6 |
| Naumann[36] | 2019 | Germany | 147 | I–IV | Mixed | Male: <52.4 cm2/m2, female: <38.5 cm2/m2 | OS | 7 |
| Wu[37] | 2019 | China | 146 | I–IV | Mixed | Male: <36.2 cm2/m2, female: <29.6 cm2/m2 | OS | 6 |
| Cho[38] | 2021 | Korea | 299 | Advanced | Mixed | Male:< 36.2 cm2/m2, female: <29.6 cm2/m2 | OS, PFS | 8 |
| Hsu[39] | 2021 | USA | 136 | I-IV | NR | Male: <43.75 cm2/m2, female: <38.5 cm2/m2 | OS | 8 |
| Kim[40] | 2021 | South Korea | 330 | Advanced | Non-surgery | Male: <53/43 cm2/m2, female: <41 cm2/m2 | OS | 7 |
| Nakano[41] | 2021 | Japan | 55 | Advanced | Non-surgery | Male: <42.2 cm2/m2, female: <33.9 cm2/m2 | OS | 7 |
| Peng[42] | 2021 | China | 116 | I-IV | Surgery | Male: <42.2 cm2/m2, female: <33.9 cm2/m2 | OS, PFS | 8 |
| Uemura[43] | 2021 | Japan | 69 | Advanced | Non-surgery | Male: <42 cm2/m2, female: <38 cm2/m2 | OS | 8 |
| Aziz[44] | 2022 | Netherlands | 415 | NR | Surgery | Male: <54.3/52.3 cm2/m2, female: <46.6/38.6 cm2/m2 | PFS | 6 |
NOS = Newcastle-Ottawa Scale, NR = not reported, OS = overall survival, PFS = progression-free survival, SMI = skeletal muscle index, TNM = tumor-node-metastasis.
3.3. The association between pretreatment SMI and OS of pancreatic cancer patients
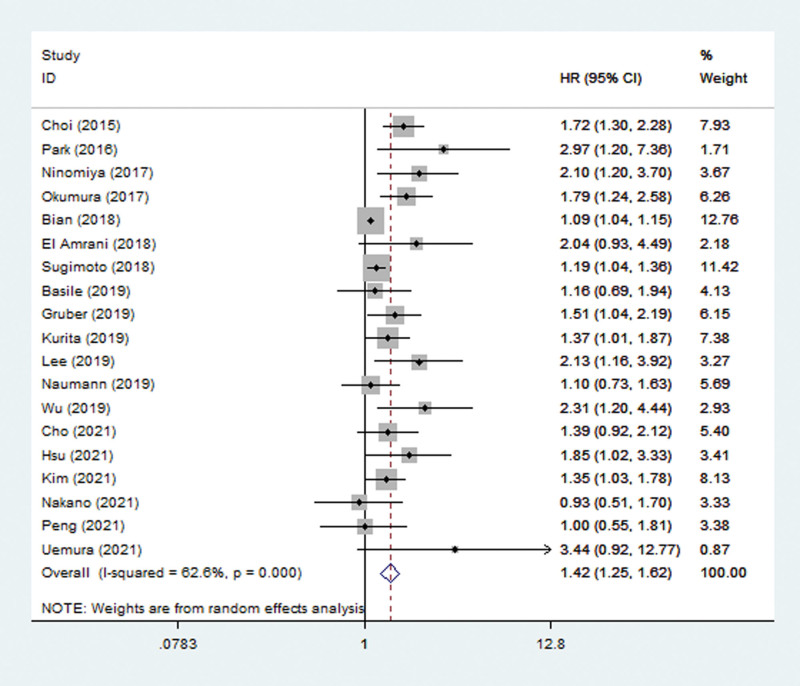
Nineteen studies explored the predictive role of pretreatment SMI for OS.[25–43] The pooled results indicated that pretreatment SMI was significantly related to OS (HR = 1.42, 95% CI: 1.25–1.62, P < .001; I2 = 62.6%, P < .001) (Fig. 2). Subgroup analysis based on the treatment showed that lower pretreatment SMI was a prognostic risk factor in both operated (HR = 1.46, 95% CI: 1.16–1.84, P = .001; I2 = 50.8%, P = .071) and non-operated (HR = 1.41, 95% CI: 1.14–1.73, P = .001; I2 = 68.4%, P = .001) patients. Besides, subgroup analysis stratified by the tumor stage also indicated that lower pretreatment SMI was associated with poorer OS in both advanced stage (HR = 1.40, 95% CI: 1.16–1.69, P = .001; I2 = 65.8%, P = .002) and early stage (HR = 1.79, 95% CI: 1.24–2.58, P = .002) patients (Table 2).
Figure 2.
The association between pretreatment SMI and overall survival of pancreatic carcinoma patients. CI = confidence interval, HR = hazard ratio, SMI = skeletal muscle index.
Table 2.
Results of meta-analysis.
| No. studies | HR | 95% CI | P value | I2 (%) | P value | |
|---|---|---|---|---|---|---|
| Overall survival | 19 | 1.42 | 1.25–1.62 | <.001 | 62.6 | <.001 |
| Treatment | ||||||
| Non-surgery | 9 | 1.41 | 1.14–1.73 | .001 | 68.4 | .001 |
| Surgery | 6 | 1.46 | 1.16–1.84 | .001 | 50.8 | .071 |
| Tumor stage | ||||||
| Advanced | 10 | 1.40 | 1.16–1.69 | .001 | 65.8 | .002 |
| Early | 1 | 1.79 | 1.24–2.58 | .002 | – | – |
| Progression-free survival | 5 | 1.41 | 1.08–1.84 | .012 | 67.1 | .016 |
CI = confidence interval, HR = hazard ratio.
3.4. The association between pretreatment SMI and PFS of pancreatic cancer patients
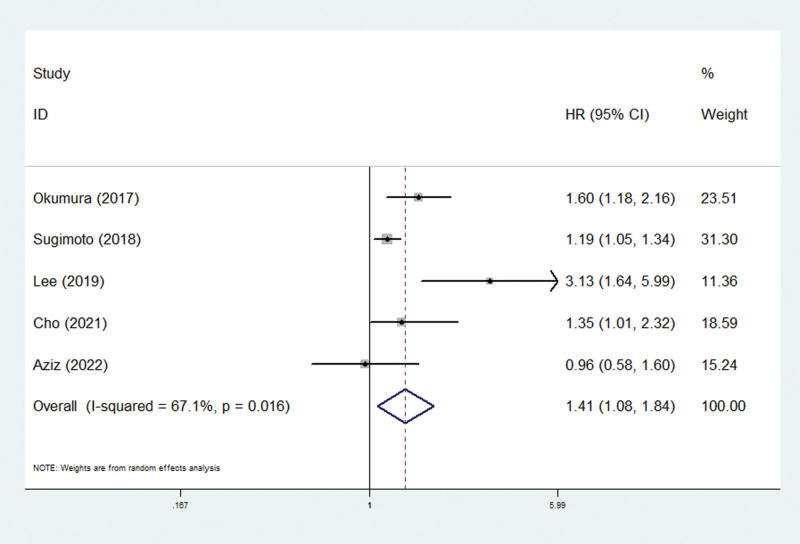
Only 5 studies explored the relationship between pretreatment SMI and PFS.[28,31,35,38,44] The pooled demonstrated that lower pretreatment SMI was a risk factor of worse PFS in pancreatic carcinoma patients (HR = 1.41, 95% CI: 1.08–1.84, P = .012; I2 = 67.1%, P = .016) (Fig. 3; Table 2).
Figure 3.
The association between pretreatment SMI and progression-free survival of pancreatic carcinoma patients. CI = confidence interval, HR = hazard ratio, SMI = skeletal muscle index.
3.5. Sensitivity analysis
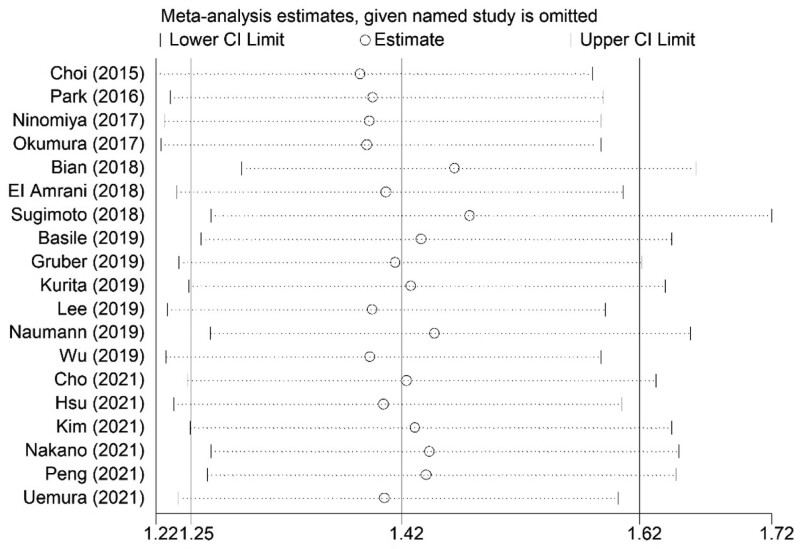
The sensitivity analysis showed that the results of the current meta-analysis were stable and reliable and none of included studies caused a significant impact on the overall results (Fig. 4).
Figure 4.
Sensitivity analysis about the association between pretreatment SMI and overall survival of pancreatic carcinoma patients. CI = confidence interval, SMI = skeletal muscle index.
3.6. Publication bias
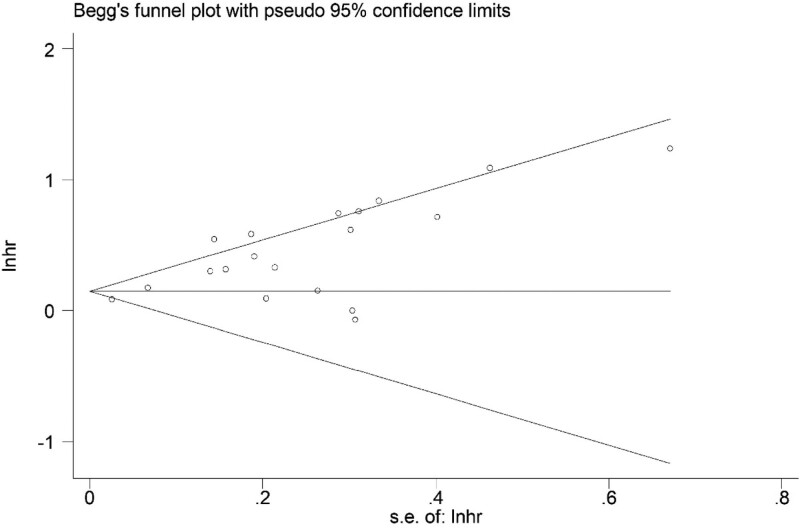
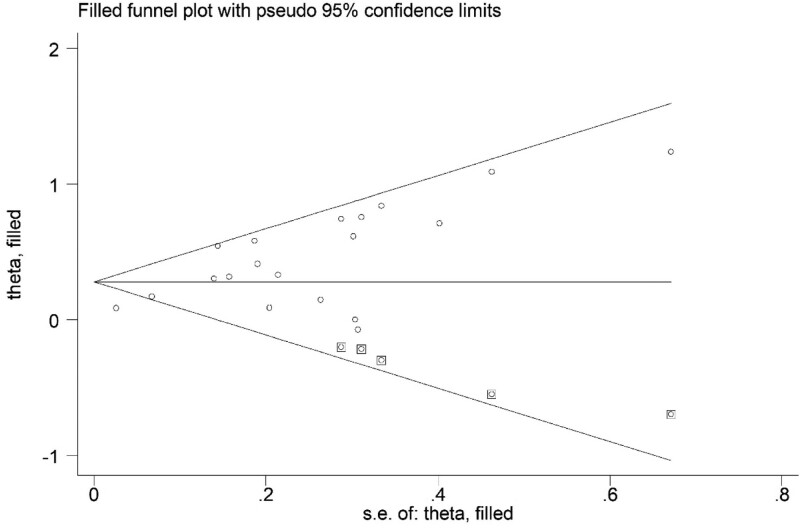
Based on the asymmetric Begg’s funnel plot (Fig. 5) and P < .001 of Egger’s test, significant publication bias was detected. Thus, the trim-and-fill method was applied. Five potentially unpublished articles were revealed (Fig. 6) and the pooled HRs in fixed-effects model and random-effects model were 1.149 (95% CI: 1.101–1.199, P < .001) and 1.322 (95% CI: 1.171–1.493, P < .001) separately, which indicated that these 5 potentially unpublished studies did not have a significant impact on the overall results and our conclusions were still reliable.
Figure 5.
Begg’s funnel plot.
Figure 6.
Trim Begg’s funnel plot.
4. Discussion
The current meta-analysis demonstrated that low pretreatment SMI was significantly associated with poor OS and PFS of pancreatic carcinoma patients after including 20 studies involving 3765 participates. Besides, the subgroup analysis based on the treatment and tumor stage showed similar findings. Thus, our study has indicated the high prognostic value of pretreatment SMI in pancreatic carcinoma patients. However, more prospective high-quality research is still needed to verify our findings.
SMI is usually applied to define the sarcopenia in clinics. Previous literatures have revealed that sarcopenia is a prognostic risk factor in several cancers including esophageal cancer, biliary tract cancer, head and neck cancer and gastric cancer.[45–48] Actually, sarcopenia is not only a decrease in muscle quantity or quality, but also a condition reflecting a disturbance of immunonutritional status, although its relationship with oncological microenvironment remains unclear up to now.[49] A lot of studies have indicated that skeletal muscle plays an important role in the systematic inflammation.[44,50] Besides, several inflammatory parameters such as the neutrophil to lymphocyte ratio, white blood cell count, C-reactive protein levels, erythrocyte sedimentation rate and systemic immune-inflammation index were found to be higher in sarcopenic patients.[44,51] As mentioned above, most of these inflammatory indexes have a high prognostic value in cancer patients. Thus, overall, it is believed that SMI could show a high prognostic value in pancreatic carcinoma and our results have well certified this conjecture.
Furthermore, there are several meta-analyses which revealed the clinical role of SMI in cancer patients.[52,53] Tranoulis et al included 21 studies and demonstrated that low SMI trended towards shorter OS (HR = 1.37, 95% CI: 0.99–1.90, P = .05) and was related to higher risk of postoperative complications [OR = 1.56, 95% CI: 1.16–2.11, P = .004] in women with epithelial ovarian malignancy.[53] Besides, Di Giorgio et al indicated that low SMI was significantly associated with peritoneal metastases in colorectal cancer (OR = 1.45, 95% CI: 1.04–2.03, P = .03) after including 4 relevant studies involving 582 patients.[52]
Although our meta-analysis demonstrated the significant association of pretreatment SMI with survival of pancreatic carcinoma patients, there are still some controversial fields worthy of more investigations. Only the prognostic role of pretreatment SMI in pancreatic carcinoma was identified in this meta-analysis. It is unclear that whether the change of SMI during the anti-tumor treatment could predict survival rates of pancreatic cancer patients. Besides, it is necessary to explore whether it’s possible to improve clinical outcomes of patients by increasing SMI values. In most included studies, sex-specific cutoff values of pretreatment SMI were applied. However, the baseline level of SMI could be affected by some parameters such as the disease stage and age. Thus, more specific thresholds of SMI should be determined, or subgroup analysis based on these parameters should be conducted in future research.
There are several limitations in this meta-analysis. First, all included studies were retrospectively conducted with relatively small sample sizes, which might cause some bias. Second, because of the lack of original data about the age, sex and other important parameters, we were unable to perform more subgroup analyses. Third, in this meta-analysis, we were unable to determine the optimal cutoff value of pretreatment SMI.
5. Conclusion
Pretreatment SMI could serve as a promising and reliable prognostic factor for pancreatic carcinoma patients and lower pretreatment SMI predicted worse prognosis. However, more prospective high-quality studies are still needed to further verify our findings.
Author contributions
Conceptualization: Li Yang, Haiwen Li.
Data curation: Xianghui Liao, Zhong Xie.
Formal analysis: Li Yang, Xianghui Liao.
Investigation: Xianghui Liao.
Methodology: Li Yang, Xianghui Liao.
Resources: Zhong Xie.
Software: Xianghui Liao.
Supervision: Haiwen Li.
Validation: Zhong Xie.
Writing – original draft: Li Yang, Zhong Xie.
Writing – review & editing: Haiwen Li.
Abbreviations:
- CI
- confidence interval
- HR
- hazard ratio
- NOS
- Newcastle Ottawa Scale
- OS
- overall survival
- PFS
- progression-free survival
- SMI
- skeletal muscle index
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Yang L, Liao X, Xie Z, Li H. Prognostic value of pretreatment skeletal muscle index in pancreatic carcinoma patients: A meta-analysis. Medicine 2023;102:19(e33663).
Contributor Information
Li Yang, Email: yangli2022lhw@163.com.
Xianghui Liao, Email: Liao.xianghui@163.com.
Zhong Xie, Email: xiexieg@126.com.
References
- [1].Mathur P, Sathishkumar K, Chaturvedi M, et al.; ICMR-NCDIR-NCRP Investigator Group. Cancer statistics, 2020: report from National Cancer Registry Programme, India. JCO Glob Oncol. 2020;6:1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- [5].Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li M, Li Z, Wang Z, et al. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med. 2022;22:637–46. [DOI] [PubMed] [Google Scholar]
- [7].Lin S, Fang Y, Mo Z, et al. Prognostic value of lymphocyte to monocyte ratio in pancreatic cancer: a systematic review and meta-analysis including 3338 patients. World J Surg Oncol. 2020;18:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Riauka R, Ignatavicius P, Barauskas G. Preoperative platelet to lymphocyte ratio as a prognostic factor for resectable pancreatic cancer: a systematic review and meta-analysis. Dig Surg. 2020;37:447–55. [DOI] [PubMed] [Google Scholar]
- [9].Wu D, Wang X, Shi G, et al. Prognostic and clinical significance of modified glasgow prognostic score in pancreatic cancer: a meta-analysis of 4,629 patients. Aging (Albany NY). 2021;13:1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie Q, Wang L, Zheng S. Prognostic and clinicopathological significance of C-reactive protein to albumin ratio in patients with pancreatic cancer: a meta-analysis. Dose Response. 2020;18:1559325820931290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Griffin OM, Duggan SN, Ryan R, et al. Characterising the impact of body composition change during neoadjuvant chemotherapy for pancreatic cancer. Pancreatology. 2019;19:850–7. [DOI] [PubMed] [Google Scholar]
- [12].Wochner R, Clauss D, Nattenmueller J, et al. Impact of progressive resistance training on CT quantified muscle and adipose tissue compartments in pancreatic cancer patients. PLoS One. 2020;15:e0242785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takeda T, Sasaki T, Suzumori C, et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol. 2021;26:1293–303. [DOI] [PubMed] [Google Scholar]
- [14].Ma X, Zou W, Sun Y. Prognostic value of pretreatment controlling nutritional status score for patients with pancreatic cancer: a meta-analysis. Front Oncol. 2021;11:770894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Capurso G, Pecorelli N, Burini A, et al. The impact of nutritional status on pancreatic cancer therapy. Expert Rev Anticancer Ther. 2022;22:155–67. [DOI] [PubMed] [Google Scholar]
- [16].Uemura S, Iwashita T, Ichikawa H, et al. Impact of Controlling nutritional status (CONUT) in patients with unresectable advanced pancreatic cancer receiving multi-agent chemotherapy: a single center, retrospective cohort study. Pancreatology. 2022;22:304–10. [DOI] [PubMed] [Google Scholar]
- [17].Kang Z, Cheng L, Li K, et al. Correlation between L3 skeletal muscle index and prognosis of patients with stage IV gastric cancer. J Gastrointest Oncol. 2021;12:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu X, Ji W, Zheng K, et al. The correlation between skeletal muscle index of the L3 vertebral body and malnutrition in patients with advanced lung cancer. BMC Cancer. 1148;21:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gadducci A, Simonetti E, Mezzapesa F, et al. Computed tomography-assessed skeletal muscle index and skeletal muscle radiation attenuation in patients with ovarian cancer treated with primary surgery followed by platinum-based chemotherapy: a single-center Italian study. Anticancer Res. 2022;42:947–54. [DOI] [PubMed] [Google Scholar]
- [20].Mascarella MA, Gardiner L, Patel T, et al. Cervical paraspinal skeletal muscle index outperforms frailty indices to predict postoperative adverse events in operable head and neck cancer with microvascular reconstruction. Microsurgery. 2022;42:209–16. [DOI] [PubMed] [Google Scholar]
- [21].Tang R, Deng JP, Zhang L, et al. Prognostic significance of the skeletal muscle index and systemic inflammatory index in patients with lymph node-positive breast cancer after radical mastectomy. BMC Cancer. 2022;22:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5:80–4. [Google Scholar]
- [23].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Li J, Chang S, et al. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: a systematic review and two meta-analyses based on four million cases. J Thorac Oncol. 2021;16:1893–908. [DOI] [PubMed] [Google Scholar]
- [25].Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10:e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park I, Choi SJ, Kim YS, et al. Prognostic factors for risk stratification of patients with recurrent or metastatic pancreatic adenocarcinoma who were treated with gemcitabine-based chemotherapy. Cancer Res Treat. 2016;48:1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ninomiya G, Fujii T, Yamada S, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017;39:45–51. [DOI] [PubMed] [Google Scholar]
- [28].Okumura S, Kaido T, Hamaguchi Y, et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol. 2017;24:3732–40. [DOI] [PubMed] [Google Scholar]
- [29].Bian X, Dai H, Feng J, et al. Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine (Baltimore). 2018;97:e10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].El Amrani M, Vermersch M, Fulbert M, et al. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: a retrospective analysis of 107 patients. Medicine (Baltimore). 2018;97:e12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sugimoto M, Farnell MB, Nagorney DM, et al. Decreased skeletal muscle volume is a predictive factor for poorer survival in patients undergoing surgical resection for pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2018;22:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Basile D, Parnofiello A, Vitale MG, et al. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2019;10:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gruber ES, Jomrich G, Tamandl D, et al. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14:e0215915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kurita Y, Kobayashi N, Tokuhisa M, et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology. 2019;19:127–35. [DOI] [PubMed] [Google Scholar]
- [35].Lee HS, Kim SY, Chung MJ, et al. Skeletal muscle mass predicts poor prognosis in patients with advanced pancreatic cancer undergoing second-line FOLFIRINOX chemotherapy. Nutr Cancer. 2019;71:1100–7. [DOI] [PubMed] [Google Scholar]
- [36].Naumann P, Eberlein J, Farnia B, et al. Continued weight loss and sarcopenia predict poor outcomes in locally advanced pancreatic cancer treated with chemoradiation. Cancers. 2019;11:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu CH, Chang MC, Lyadov VK, et al. Comparing Western and Eastern criteria for sarcopenia and their association with survival in patients with pancreatic cancer. Clin Nutr. 2019;38:862–9. [DOI] [PubMed] [Google Scholar]
- [38].Cho WK, Yu JI, Park HC, et al. Impact of sarcopenia on survival of pancreatic cancer patients treated with concurrent chemoradiotherapy. Tumori. 2021;107:247–53. [DOI] [PubMed] [Google Scholar]
- [39].Hsu TH, Schawkat K, Berkowitz SJ, et al. Artificial intelligence to assess body composition on routine abdominal CT scans and predict mortality in pancreatic cancer- a recipe for your local application. Eur J Radiol. 2021;142:109834. [DOI] [PubMed] [Google Scholar]
- [40].Kim IH, Choi MH, Lee IS, et al. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: a retrospective observational study. BMC Cancer. 2021;21:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nakano O, Kawai H, Kobayashi T, et al. Rapid decline in visceral adipose tissue over 1 month is associated with poor prognosis in patients with unresectable pancreatic cancer. Cancer Med. 2021;10:4291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Peng YC, Wu CH, Tien YW, et al. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. 2021;31:2472–81. [DOI] [PubMed] [Google Scholar]
- [43].Uemura S, Iwashita T, Ichikawa H, et al. The impact of sarcopenia and decrease in skeletal muscle mass in patients with advanced pancreatic cancer during FOLFIRINOX therapy. Br J Nutr. 2021;125:1140–7. [DOI] [PubMed] [Google Scholar]
- [44].Aziz MH, van Dongen JC, Saida L, et al. High systemic immune inflammation index is associated with low skeletal muscle quantity in resectable pancreatic ductal adenocarcinoma. Front Oncol. 2022;12:827755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takenaka Y, Takemoto N, Oya R, et al. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: a meta-analysis. PLoS One. 2021;16:e0259288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen F, Chi J, Liu Y, et al. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: a meta-analysis of cohort studies. Arch Gerontol Geriatr. 2022;98:104534. [DOI] [PubMed] [Google Scholar]
- [47].Chen F, Chi J, Zhao B, et al. Impact of preoperative sarcopenia on postoperative complications and survival outcomes of patients with esophageal cancer: a meta-analysis of cohort studies. Dis Esophagus. 2022;35:doab100. [DOI] [PubMed] [Google Scholar]
- [48].Watanabe J, Matsui R, Sasanuma H, et al. Body composition assessment and sarcopenia in patients with biliary tract cancer: a systematic review and meta-analysis. Clin Nutr. 2022;41:321–8. [DOI] [PubMed] [Google Scholar]
- [49].Hilmi M, Jouinot A, Burns R, et al. Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther. 2019;196:135–59. [DOI] [PubMed] [Google Scholar]
- [50].Tian J, Chung HK, Moon JS, et al. Skeletal muscle mitoribosomal defects are linked to low bone mass caused by bone marrow inflammation in male mice. J Cachexia Sarcopenia Muscle. 2022;15:1785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Öztürk ZA, Kul S, Türkbeyler İ H, et al. Is increased neutrophil lymphocyte ratio remarking the inflammation in sarcopenia? Exp Gerontol. 2018;110:223–9. [DOI] [PubMed] [Google Scholar]
- [52].Di Giorgio A, Rotolo S, Cintoni M, et al. The prognostic value of skeletal muscle index on clinical and survival outcomes after cytoreduction and HIPEC for peritoneal metastases from colorectal cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:649–56. [DOI] [PubMed] [Google Scholar]
- [53].Tranoulis A, Kwong FLA, Lakhiani A, et al. Prevalence of computed tomography-based sarcopenia and the prognostic value of skeletal muscle index and muscle attenuation amongst women with epithelial ovarian malignancy: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:1441–54. [DOI] [PubMed] [Google Scholar]