Abstract
Five reciprocal cycles of subtractive hybridization using cDNA generated from fibroblasts with normal lipopolysaccharide (LPS) responsiveness (lpsn) and from hyporesponsive (lpsd) fibroblasts have led to the finding that caveolin-1 is expressed at markedly higher levels of mRNA in lpsd than in lpsn fibroblasts. Caveolin-1 message can also be readily detected via reverse transcription-PCR in the RAW264.7 and J774.1 macrophage-like cell lines as well as in primary thioglycolate (TG)-elicited mouse peritoneal macrophages. In RAW264.7 cells, both caveolin-1 mRNA and protein levels are down-regulated by LPS. In TG-elicited C3HeB/FeJ peritoneal macrophages, in contrast, expression of both caveolin-1 protein and mRNA is up-regulated in vitro in response to LPS stimulation. The up-regulation of caveolin-1 protein expression in C3HeB/FeJ peritoneal macrophages can be demonstrated at concentrations as low as 1.0 pg of LPS/ml. However, LPS concentrations approximately 4 orders of magnitude higher (104 pg/ml) were required to stimulate the LPS-hyporesponsive C3H/HeJ mice peritoneal macrophages such that significant caveolin-1 protein up-regulation was detected. Caveolin-1, a principal component of plasmalemmal caveolae, has been reported as a potentially important regulator for signal transduction during cellular stimulation. The results described in this report suggest that caveolin-1 expression may be associated with LPS signaling/internalization.
The mutant inbred mouse strain C3H/HeJ was first reported in 1965 (4) to manifest an altered immunologic responsiveness to bacterial products and in 1968 (28) to be unique in its responsiveness to lipopolysaccharide (LPS) (24). The latter studies phenotypically defined the lps gene, which controls responsiveness to LPS. This gene has been mapped to chromosome 4 between the ps (polysyndacty) and mup-1 (major urinary protein-1) loci (34). Genotypically, the lps gene can therefore be defined as a “normal” lpsn allele and a “defective” lpsd allele. In vitro studies in a variety of host immune-inflammatory cells have clearly established C3H/HeJ mice to be hyporesponsive to LPS.
To better understand the mechanism(s) of innate LPS recognition and to provide insight for potential clinical therapeutic intervention in gram-negative sepsis, many laboratories, including our own, have intensively pursued studies in efforts to define the C3H/HeJ molecular genetic defect. Using transfection of C3H/HeJ splenic B cells with cDNA produced from lpsn C3H/HeOuJ mice, and differential functional screening approaches, Kang et al. (15) were able to isolate a cDNA that has sequence homology to the gene encoding Ran/TC4 GTPase. This gene has been localized to chromosome 4 and has recently been reported to be mutated in C3H/HeJ mice, which may account for their resistance to endotoxin-induced lethality (33). In another recent study, by applying differential display analysis of macrophage cell lines derived from C3H/HeJ and C3H/HeN mice, Jin et al. (13, 14) have reported that secretory leukocyte protease inhibitor (SLPI) and matrix metalloprotease-9 (MMP-9) were constitutively overexpressed in lpsd but not in lpsn macrophages. SLPI functions as an LPS-induced LPS response inhibitor, whereas the role of MMP-9 in the LPS response is not known. However, the genes for SLPI and MMP-9 were not mapped to chromosome 4.
More recently, Poltorak et al. (22) and Qureshi et al. (23), using high-resolution genetic and physical mapping of the lps allele, came to the same important conclusion: that C3H/HeJ mice manifest a missense mutation in the coding region of the Toll-like receptor-4 gene (tlr4). Although there are now reports that would strongly implicate tlr4 as the functional LPS-responsive gene (1, 5, 12, 20), the actual overall significance of this gene of the lps allele (tlr4) for LPS responsiveness remains clouded following the recently published comprehensive genetic backcross studies of Vogel and colleagues (31). These investigators clearly demonstrated that the expression of a normal lpsn allele (tlr4) molecule is apparently not a prerequisite for LPS sensitivity when the lpsd allele is present in the hemizygous state. More studies need to be done in order to fully understand the involvement of Toll-like receptor 4 (TLR4) in LPS signaling. Nevertheless, this finding raises the challenging question of precisely which genes whose products contribute to LPS signaling in concert with TLR4 might be located in regions of the mouse chromosome other than the long-recognized allele in chromosome 4 but still would be important to the LPS-responsive phenotype.
C3HeB/FeJ (lpsn) and C3H/HeJ (lpsd) mice are well recognized to be fully histocompatible strains (9). We have, therefore, hypothesized that a subtractive cDNA library constructed from the lpsn and lpsd cells would be expected to contain clones carrying relevant genes contributing to LPS responsiveness in cellular signaling. As will be summarized in this report, we have employed such a subtractive hybridization approach to identify differentially expressed genes present in two fibroblast cell lines developed from lpsn and lpsd mouse strains. These cell lines manifest the appropriate LPS phenotype, as assayed by interleukin-6 (IL-6) secretion in response to LPS stimulation. The results of those studies reported here focus on the caveolin-1 gene, one of the major genes identified through our extensive molecular analysis of these subtractive cDNA libraries.
Earlier published studies identified caveolin-1 as a structural marker protein of the membrane coats of caveolae (8, 25). Caveolae have been generally defined as non-clathrin-coated plasmalemmal microdomains found in many mammalian cells, and these membrane domains have been found to be highly enriched in glycosphingolipids, cholesterol, sphingomyelin, and lipid-anchored membrane proteins. Recently, an increasing number of signal transduction molecules have been discovered to be caveola associated (reviewed in references 21 and 27). For example, caveolin-1 has been reported to interact with regulatory G-protein α subunits, Ha-Ras, Src family tyrosine kinases, endothelial nitric oxide synthase (eNOS), epidermal growth factor receptor (EGF-R) (and related receptor tyrosine kinases), and protein kinase C isoforms. Interestingly, the activities of eNOS and G-protein α subunits, and the autoactivation of the Src family tyrosine kinases, have been reported to be suppressed by caveolin-1 (6).
The caveolin-1 gene is localized to murine chromosome 6, specifically, in chromosome region 6-A2 (7). Remarkably, much like the Toll-like family of receptors, which is involved in innate immunity (11), the caveolin gene family is also structurally and functionally conserved from Caenorhabditis elegans to humans (29), suggesting an essential and/or critical role of caveolins in organizing and concentrating signaling molecules within caveolae. In this report, we describe results that demonstrate, both at the level of mRNA and at the level of cellular protein, that caveolin-1 expression in mouse macrophages is significantly affected in vitro by LPS. However, depending on the source of macrophages, this expression is either up- or down-regulated by LPS, most likely reflective of the diversity of responses of macrophages to LPS stimulation in vitro. Furthermore, regulation of cellular caveolin-1 protein expression required LPS concentrations at least 4 orders of magnitude higher in cells from LPS-hyporesponsive inbred mutant C3H/HeJ mice than those in cells of LPS-responsive C3HeB/FeJ mice.
We suggest from these studies that the caveolin-1 gene product is a potentially important cellular regulatory protein that may contribute to cellular signaling triggered by the addition of LPS, to which the C3H/HeJ mouse is hyporesponsive.
MATERIALS AND METHODS
C3H fibroblast cell lines.
The mouse embryonic fibroblast cell lines SVC3H and 776-eB/FeJ, developed from the LPS-hyporesponsive C3H/HeJ strain and the LPS-responsive C3HeB/FeJ strain, respectively, were the generous gift of Linda R. Gooding (Department of Microbiology and Immunology, Emory University School of Medicine, Atlanta, Ga.). Cells were cultured with RPMI 1640 medium plus 10% fetal calf serum (FCS), penicillin and streptomycin (each at 100 U/ml), and 2 mM l-Glu. Upon stimulation with Escherichia coli O111:B4 LPS (10 ng/ml), 776-eB/FeJ fibroblasts produce readily detectable levels of IL-6 (6 ng/ml, quantitated by enzyme-linked immunosorbent assay [ELISA] analysis of the culture supernatant using recombinant mouse IL-6 as the standard) but, as expected, no detectable nitric oxide. Under otherwise identical conditions (even at significantly higher LPS concentrations), SVC3H fibroblasts produce neither detectable IL-6 nor nitric oxide after LPS stimulation. These results confirm that 776-eB/FeJ cells (lpsn) and SVC3H cells (lpsd) manifest the appropriate LPS phenotypes. E. coli O111:B4 LPS was purchased from List Laboratories (Campbell, Calif.). Antibodies specific to mouse IL-6 and recombinant mouse IL-6 were purchased from PharMingen (San Diego, Calif.).
Macrophage cell lines and TG-elicited peritoneal macrophages.
RAW264.7 and J774.1 murine macrophage-like cells (American Type Culture Collection, Manassas, Va.) were cultured with RPMI 1640 medium plus 10% FCS, penicillin and streptomycin (each at 100 U/ml), and 2 mM l-Glu. Primary cultures of C3H/HeJ (lpsd) and C3HeB/FeJ (lpsn) thioglycolate (TG)-elicited macrophages (2) were prepared as the adherent cell population from peritoneal exudate lavage fluid 5 days after intraperitoneal injection of 1.5 ml of 4% Brewer TG (Difco Laboratories).
PCR-based subtractive cDNA and isolation of differentially expressed genes.
Double-stranded cDNAs were synthesized with the mRNAs isolated from 776-eB/FeJ (lpsn) and SVC3H (lpsd) fibroblast cells, which were used as “tracer” and “driver” preparations or vice versa, respectively, as defined in the protocol (3). Two sets of subtraction, [lpsn]0 − [lpsd]0 and [lpsd]0 − [lpsn]0, were performed, thus yielding [lpsn]1 and [lpsd]1 preparations, respectively. Five sequential reciprocal subtraction cycles were performed from these two cell populations, basically by using the same protocol as that used to prepare the first-generation subtractive cDNA, to produce two subtractive cDNAs, [lpsn]5 and [lpsd]5. Thus, genes preferentially expressed in lpsn cells to a much greater extent than in lpsd cells were isolated as [lpsn]5, and genes expressed preferentially in lpsd cells to a greater extent than in lpsn cells were isolated as [lpsd]5. In each case the “driver” was labeled with bio-11-dUTP (biotin-conjugated dUTP; Enzo Diagnostics Inc.) during the PCR amplification steps. “Tracer” and “driver” cDNAs were mixed together at a weight ratio of 1:20, denatured, and allowed to reanneal. Driver-driver and tracer-driver hybrids were removed by the addition of streptavidin followed by phenol-chloroform extraction. The subtractive DNAs that were selectively enriched by this process were then amplified by PCR, and the process was essentially repeated until a total of 5 reciprocal subtraction cycles were performed to obtain the enriched genes [lpsn]5 and [lpsd]5, which were differentially expressed between the two cell lines. The progress of the subtractive enrichment was evaluated by slot blot hybridization.
Our results showed that, as expected, [lpsn]5 hybridized strongly to itself but only very weakly to [lpsd]5, and vice versa. The [lpsn]5 subtractive cDNAs were cloned in the pBluescript vector in the EcoRI cloning site, and the [lpsd]5 subtractive cDNAs were cloned in the pGEM-T Easy vector (Promega) by direct PCR T-A cloning. By extensive colony hybridization and DNA dot blot hybridization analyses, we identified 10 distinct clones from the [lpsn]5 library and 7 clones from the [lpsd]5 library which were shown potentially to be differentially expressed. The insert DNAs from these 17 clones were sequenced, and the sequences obtained were analyzed for sequence similarity in GenBank. One clone (TB54) from the [lpsd]5 library displayed 98% sequence identity to murine caveolin-1 mRNA over the 267-bp cDNA insert. Since caveolin-1 is a principal component of caveolae, and there is now strong evidence that would implicate this gene product as an important regulatory component of signal transduction (21, 27), and since caveolin-1 has been reported to interact with many signaling proteins as well (6), we elected to focus initially on this clone, TB54. Future studies will address other clones that have been identified to be differentially expressed in lpsn and lpsd mice.
Northern blot hybridization analysis of caveolin-1 mRNA.
A PCR DNA product of 517 bp of the caveolin-1 coding region was used as a probe for Northern analyses. Total RNAs of SVC3H (lpsd) and 776-eB/FeJ (lpsn) cells were isolated with RNeasy kits (QIAGEN). Poly(A)+ RNAs were further isolated with Oligotex mRNA kits (QIAGEN). The isolated mRNAs of SVC3H and 776-eB/FeJ cells (0.6 μg each) were then fractionated by agarose-formaldehyde gel electrophoresis (26) and transferred to nylon membranes. AlkoPhos direct labeling and CDP-Star chemifluorescent detection systems (Amersham) were used for Northern blot hybridization analysis.
Immunoblot analysis of cellular caveolin-1 protein levels.
Total protein present in the cell lysates was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membranes (Millipore), immunoblotted with rabbit polyclonal anti-caveolin-1 antibody and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Transduction Laboratories), and detected by chemifluorescent reagents (Amersham).
RESULTS
Cloning and sequence analyses of caveolin-1 cDNA.
The TB54 plasmid DNA isolated from the [lpsd]5 library and established to manifest 98% sequence homology with mouse caveolin-1 in the 267-bp insert (data not shown) was purified with a plasmid DNA purification kit (QIAGEN), and the DNA insert was labeled with [α-32P]ATP using the multiprime DNA labeling system (Amersham). The labeled DNA was then used as a probe to screen the lpsd SVC3H cDNA library constructed in the λgt22A vector (SuperScript Lambda system for cDNA library; Gibco BRL). Four isolates were obtained. λgt11 reverse and forward primers were used to generate PCR products of these four isolates. The PCR DNA products of all four clones show similar sizes of about 2.5 kb. One clone was selected for further subcloning and sequencing. Again, λgt11 forward and reverse primers were used to generate PCR products of the insert DNA cloned in the λ vector with Platinum High Fidelity Tag polymerase (Gibco BRL). The PCR DNA product was ligated directly with the T-A cloning vector pGEM-T Easy, and the subclone plasmid DNA, pTB548, was purified and mapped by restriction analysis. Deletion subclones were generated, and the insert DNAs were sequenced. The DNA sequence data show that the insert cDNA of TB548 has 2,482 bp, which encodes a complete sequence of the mouse caveolin-1 gene near the 5′ end of the mRNA.
Northern blot hybridization analyses of caveolin-1 mRNA.
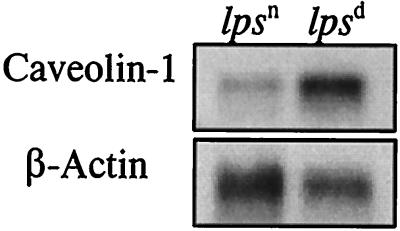
The caveolin-1 probe was shown to hybridize to a single mRNA band, about 2.5 kb, in both lpsn and lpsd fibroblast cell lines but with substantially different hybridization intensities. As demonstrated by the data in Fig. 1, much higher levels of expression of caveolin-1 mRNA were found in LPS-hyporesponsive SVC3H fibroblasts than in LPS-responsive 776-eB/FeJ fibroblasts, especially when the caveolin-1 mRNA was normalized relative to the control β-actin mRNA in the two cell preparations. These results further confirm the previous DNA dot blot analysis finding that the caveolin-1 gene is, in fact, differentially expressed to higher levels in LPS-hyporesponsive SVC3H cells than in LPS-responsive 776-eB/FeJ cells. To our knowledge, this is the first report that shows a potential relationship of LPS hyporesponsiveness and caveolin-1 hyperexpression in endotoxin research.
FIG. 1.
Northern blot analysis of caveolin-1 in lpsn and lpsd cells. Isolated poly(A)+ RNAs of 776-eB/FeJ (lpsn) and SVC3H (lpsd) fibroblasts (0.6 μg each) were probed with the caveolin-1 gene. The same blot was stripped and probed again with β-actin as a control.
Detection of caveolin-1 mRNA in macrophages.
Macrophages are well recognized to play a central role in host defense in response to infection. In this respect, LPS induces both high-level cytokine expression and NO production and also regulates surface receptor expression in macrophages. Since the question of expression of caveolin-1 in mouse macrophages is currently considered somewhat controversial (17–19, 32), it would, of course, be important to clarify whether mouse macrophages even express caveolin-1 mRNA. We first synthesized a reverse primer, p1 (5′-GGG AGA ACA GAC ATG TCT TG-3′ and a forward primer, p2 (5′-TAC GAT CTT CGG CAT CCC AAT-3′), that frame 1,957 bp of the caveolin-1 cDNA. Using this primer pair, we obtained almost exactly the predicted size of the DNA product by reverse transcription (RT)-PCR with total RNAs isolated from both the RAW264.7 and the J774.1 macrophage-like cell lines, as well as from primary TG-elicited peritoneal macrophages of C3HeB/FeJ (lpsn) and C3H/HeJ (lpsd) mice (data not shown). These RT-PCR products are very similar in size to those found in the fibroblast cell lines (data not shown). In addition to the 1,957-bp PCR product, however, we also obtained a DNA product of about 1,300 bp with primers p1 and p2 in all cells except in J774.1. We have cloned this 1,300-bp DNA fragment from SVC3H cells, and DNA sequence analysis shows that the 1,300-bp DNA fragment also codes for caveolin-1, with the sequence truncated at a position downstream of the primary caveolin-1 coding sequence. It is possible that the mRNA coding for the 1,300-bp RT-PCR DNA product may be the product of some posttranscriptional modification events.
Effect of LPS on caveolin-1 expression in RAW264.7 cells.
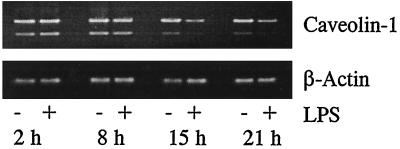
Since caveolin-1 message was readily detected in virtually all of the mouse macrophage populations tested, we investigated further whether LPS stimulation can affect caveolin-1 mRNA expression in these cells. RAW264.7 is one of the most widely used murine macrophage-like cell lines that has been investigated in studies of LPS stimulation. To determine whether caveolin-1 mRNA levels can be modified in the presence of LPS, RAW264.7 cells were cultured in 6-well plates (5 × 105 cells per well) overnight and then incubated with LPS (100 ng/ml) for periods of time from 2 to 24 h. Total RNA in each well after LPS stimulation was prepared with Trizol reagent (Gibco BRL). Caveolin-1 message was then analyzed by RT-PCR with primers p1 and p2. The results of these experiments show that, after incubation of RAW264.7 cells with LPS for 15 to 24 h, mRNA levels were markedly reduced compared to those obtained without LPS stimulation. The results of LPS down-regulation of caveolin message were confirmed by individual post-LPS-stimulation time course experiments (Fig. 2). It is also of interest that the decrease in levels of the truncated caveolin-1 message (the 1,300-bp PCR product) is also time dependent in RAW264.7 cells and is observed both in the presence and in the absence of LPS stimulation, with perhaps an even more rapid rate of decrease in the presence of LPS relative to the untreated control cells. The significance of this observation is unclear at present.
FIG. 2.
Effect of LPS on caveolin-1 expression in RAW264.7 cells. RAW264.7 cells were incubated with 100 ng of LPS/ml for various lengths of time. Total RNAs were isolated with Trizol reagent. Equal amounts of total RNAs were reverse transcribed using oligo(dT)16 as the primer. The caveolin-1-specific primers p1 and p2 were used in PCR amplification. Equal amounts of PCR products were subjected to agarose gel electrophoresis.
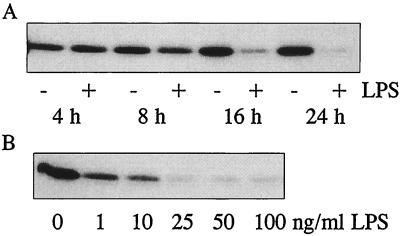
To further investigate the effect of LPS on caveolin-1 at the protein level, RAW264.7 cells were incubated with LPS to determine both the time course (4 to 24 h at 100 ng of LPS/ml) (Fig. 3A) and the concentration dependence (0 to 100 ng of LPS/ml for 24 h) (Fig. 3B) by immunoblotting with anti-caveolin-1 antibody. We found that the levels of caveolin-1 protein in RAW264.7 cells were also, as expected, substantially reduced in the total-cell lysate after stimulation of these cells with LPS, in both a time- and a dose-dependent manner. The results of the immunoblot studies strongly support the conclusion that cellular caveolin-1 levels can be highly regulated by LPS stimulation in RAW264.7 cells.
FIG. 3.
Effect of LPS on caveolin-1 protein expression. RAW264.7 cells were incubated with 100 ng of LPS/ml for 4 to 24 h (A) or with 1 to 100 ng of LPS/ml for 24 h (B). Equal volumes of total-cell lysates (20 μg of protein per sample) were subjected to SDS-PAGE, transferred to Immobilon-P membranes, and analyzed by immunoblotting using rabbit anti-caveolin-1 antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG. The immunoblots were developed by enhanced chemifluorescence. The protein concentrations in each cell lysate were comparable as analyzed by bicinchoninic acid reagents (Pierce).
Effect of LPS on caveolin-1 expression in TG-elicited peritoneal macrophages.
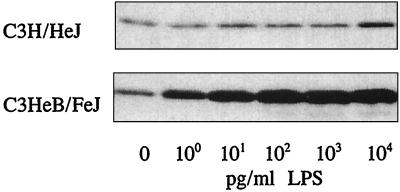
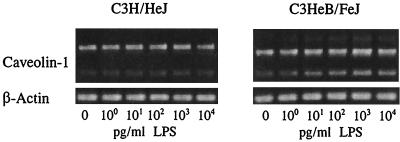
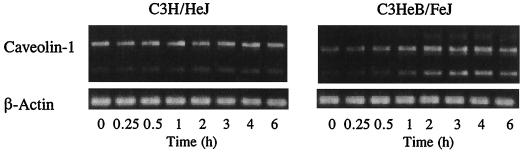
We have also investigated whether LPS affects caveolin-1 mRNA expression in primary cultures of TG-elicited mouse peritoneal macrophages. Cells were plated in 6-well plates at 2 × 106 cells/well and incubated at 37°C for 2 h. Nonadherent cells were removed by gentle washing, and the remaining adherent cells were incubated with LPS at 1.0 pg/ml to 10 ng/ml for 6 h for concentration-dependent studies (Fig. 4), and with LPS at 10 ng/ml for 15 min to 6 h for time-dependent studies (Fig. 5). The expression of caveolin-1 protein was analyzed by immunoblotting with anti-caveolin-1 antibody as described for Fig. 3. The results show that, in LPS-responsive primary macrophages of C3HeB/FeJ origin, caveolin-1 protein expression was highly up-regulated at test concentrations as low as 1.0 pg of LPS/ml. However, LPS concentrations at least 4 orders of magnitude higher (104 pg/ml) were required to stimulate the LPS-hyporesponsive primary macrophages of C3H/HeJ origin (Fig. 4) to manifest even a modestly significant increase in cellular caveolin-1 expression. The results of time-dependent studies also show that at 10 ng of LPS/ml, the up-regulation of caveolin-1 protein levels in C3HeB/FeJ peritoneal macrophages could be detected 2 h post-LPS treatment, while that in C3H/HeJ peritoneal macrophages was detected 4 to 6 h post-LPS treatment (Fig. 5). The results of these immunoblot studies show clearly that caveolin-1 protein levels in lpsn primary macrophages are regulated by LPS even at extremely low LPS concentrations (e.g., 1.0 pg/ml) but that regulation in C3H/HeJ macrophages is markedly less sensitive.
FIG. 4.
Dose-dependent effect of LPS on caveolin-1 protein expression in TG-elicited peritoneal macrophages prepared from C3H/HeJ and C3HeB/FeJ mice. Cells were incubated with LPS at 1.0 pg/ml to 10 ng/ml for 6 h. Cellular caveolin-1 protein levels were analyzed by immunoblotting with anti-caveolin-1 antibody as described for Fig. 3.
FIG. 5.
Time-dependent effect of LPS on caveolin-1 protein expression in TG-elicited peritoneal macrophages prepared from C3H/HeJ and C3HeB/FeJ mice. Cells were incubated with 10 ng of LPS/ml for 15 min to 6 h. Caveolin-1 protein expression was analyzed by immunoblotting as described for Fig. 3.
We initially determined by RT-PCR analyses that caveolin-1 mRNA levels in primary cultures of TG-elicited peritoneal macrophages of C3HeB/FeJ mice, but not in those of C3H/HeJ mice, were up-regulated by LPS stimulation at 25 to 100 ng/ml after 16 h of incubation (data not shown). However, the above results (Fig. 4 and 5) show that cellular caveolin-1 protein levels were affected by LPS at much lower concentrations and at earlier times. We therefore further studied the effect of LPS on caveolin-1 mRNA expression at the same LPS concentrations and time course as described for Fig. 4 and 5 by RT-PCR analyses. The results of these studies are shown in Fig. 6 and 7 for concentration- and time-dependent experiments, respectively. Although the results of RT-PCR analysis for the up-regulation of caveolin-1 expression by LPS were found not to be as marked as the protein immunoblot data shown in Fig. 4 and 5, they clearly confirm detectable up-regulation of both the 1,957- and 1,300-bp PCR products, reflecting the regulation of caveolin-1 mRNA expression in primary cultures of lpsn TG-elicited peritoneal macrophages by LPS. It is of potential interest, once again, that the truncated caveolin-1 message that produces the 1,300-bp PCR product appears to be more sensitive to LPS-dependent regulation than the message that produces the 1,957-bp PCR product. In contrast, we have observed little detectable change in levels of either caveolin-1 mRNA in primary cultures of C3H/HeJ macrophages in response to LPS.
FIG. 6.
Dose-dependent effect of LPS on caveolin-1 mRNA expression in elicited peritoneal macrophages. TG-elicited peritoneal macrophages were prepared and treated with LPS at 1 pg/ml to 10 ng/ml for 6 h. Caveolin-1 mRNA expression was analyzed by RT-PCR as described for Fig. 2.
FIG. 7.
Time-dependent effect of LPS on caveolin-1 mRNA expression in elicited peritoneal macrophages. TG-elicited peritoneal macrophages were prepared and treated with 10 ng of LPS/ml for 15 min to 6 h. Caveolin-1 mRNA expression was analyzed by RT-PCR as described for Fig. 2.
DISCUSSION
The findings summarized in Results show differential expression of caveolin-1 in lpsn and lpsd fibroblasts and indicate that expression of this gene and gene product can be markedly up-regulated by LPS in primary cultures of TG-elicited peritoneal macrophages of lpsn mice, but not of lpsd mice, at extremely low concentrations of LPS. Importantly, the effect of LPS on caveolin-1 in C3HeB/FeJ macrophages is totally different from that observed in RAW264.7 cells. These results indicate that the effects of LPS stimulation on caveolin-1 expression differ not only between lpsn and lpsd cells, but also among lpsn cells from different sources. These findings support the concept that caveolin-1 may well serve as one of the important cellular contributing factors that ultimately dictate the well-recognized diverse phenotypic responses of macrophages to LPS stimulation.
In recent studies, Wang et al. (32) and Kitchens et al. (18) reported that, in CD14-expressing THP-1 and normal human monocytes, glycosylphosphatidylinositol (GPI)-anchored CD14- dependent internalization of LPS appears to occur predominantly via non-clathrin-coated “caveola-like” plasma membrane invaginations. The authors indicated, however, that in THP-1 cells, membrane invaginations were more “tubular” in structure than were classical “flask-shaped” caveolae. These investigators nevertheless cautioned that these cells might not contain caveolae. However, Matveev et al. (19) have reported that caveolin-1 is associated with cholesteryl ester uptake in THP-1 macrophages. Kiss and Geuze (17), using antibody against caveolin-1, found that caveolae, the omega-shaped plasma membrane invaginations, were abundantly present in the plasma membranes of elicited rat peritoneal macrophages. They also found that, in elicited macrophages, caveolae could be observed to “pinch off” from the plasma membrane. Based on those findings, these authors suggested that caveolae might well function as alternative carriers in endocytotic processes of these cells. Very recently, caveolin-1 has been suggested to serve a primary role in organizing “preassembled signaling complexes” at the plasma membrane (21). Collectively, these results, when considered within the framework of our own studies reported here showing major effects of LPS on cellular caveolin-1 expression, strongly support the conclusion that caveolae, and more specifically caveolin-1, may well be involved as critical regulatory components in cellular responses to LPS, leading to signaling and/or internalization in mouse macrophages.
While there is no question but that the mutational defect in TLR4 in C3H/HeJ mice, affecting innate LPS recognition, is highly important in the regulation of LPS cellular triggering events, it remains inconclusive in terms of full manifestation of the lpsd phenotype (31). Our new findings that caveolin-1 is differentially expressed in LPS-responsive and -hyporesponsive cells tend to support the conclusion that the C3H/HeJ defect may also be affected by gene products other than TLR4, a conclusion that has a solid scientific basis in the recently published studies of Vogel et al. (31). It is of some interest that Toll-like receptor 2 (TLR2) was also identified as a potential membrane receptor/transducer for LPS signal transduction in tlr2-transfected human embryonic kidney cells (16, 35). While this may well be true in appropriately transfected cells, Heine et al. (10) reported that expression of functional TLR2 would not be essential for the LPS-sensitive CD14-transfected Chinese hamster ovary fibroblast response to LPS. More recently, Underhill et al. (30) demonstrated that in macrophages, TLR2 primarily mediates signals from yeast and gram-positive bacteria, but not from gram-negative bacteria and LPS.
As pointed out above, caveolin-1, a dynamic integral membrane protein, has been shown to interact with, and to suppress, the activities of various signaling proteins (6, 7, 21) and has been suggested as a possible candidate for a tumor suppressor gene (7). We have found that cellular caveolin-1 levels are significantly higher in LPS-hyporesponsive fibroblasts than in LPS-responsive fibroblasts. Although many studies have demonstrated that LPS regulates the expression of many genes, our data show for the first time that LPS regulates caveolin-1 expression levels in normal LPS-responsive mouse macrophages. Taken together, therefore, these studies suggest a potentially important role for this protein in regulating host responsiveness to LPS.
We hypothesize, based upon these findings, that molecular regulation of caveolin-1 gene expression may serve as a “gatekeeper” in defining the specific consequences of interactions of LPS with LPS receptors at the macrophage surface membrane (i.e., cellular internalization versus signaling) that will ultimately define the phenotypic response of this host immune-inflammatory cell to LPS and probably to gram-negative, LPS-containing microbes as well. The potentially differential roles of the two caveolin-1 messages observed in these studies, one producing a 1,957-bp PCR product and the other a truncated 1,300-bp PCR product, also may well merit additional investigation. In any case, these findings add to the complexity of the pathways that dictate both triggering and regulation of LPS-mediated events in host immune-inflammatory cell responses.
ACKNOWLEDGMENTS
This work was supported by NIH grant R37AI23447.
We authors thank Eleanor Zuvanich and Wei Cui, Jiangjun Gao, and Fuan Wang for helping with the preparation of macrophages, and we thank Alexander Shnyra and John Gray for constructive advice in the preparation of figures. Special thanks are extended to Chia Y. Lee for helpful discussions in the course of this work.
REFERENCES
- 1.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 2.Amura C R, Chen L-C, Hirohashi N, Lei M-G, Morrison D C. Two functionally independent pathways for lipopolysaccharide-dependent activation of mouse peritoneal macrophages. J Immunol. 1997;159:5079–5083. [PubMed] [Google Scholar]
- 3.Ausubel F M, Kingston R E, Seidman J G, Struhl K, Moore D D, Brent R, Smith J A, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1999. [Google Scholar]
- 4.Brooke M S. Conversion of immunological paralysis to immunity by endotoxin. Nature. 1965;206:635–636. doi: 10.1038/206635a0. [DOI] [PubMed] [Google Scholar]
- 5.Chow J C, Young D W, Golenbock D T, Crist W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 6.Couet J, Sargiacomo M, Lisanti M P. Interaction of a receptor tyrosine kinase, EGF-R, with caveolin. Caveolin binding negatively regulates tyrosine and serine/threoline kinase activities. J Biol Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 7.Engelman J A, Zhang X L, Galbiati F, Lisanti M P. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3) FEBS Lett. 1998;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- 8.Glenney J R, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman S A, Morrison D C. Lipopolysaccharide receptors on lymphocytes. I. Lack of immunologic recognition of a putative LPS receptor on LPS-responder lymphocytes by LPS-nonresponder mice. J Immunol. 1985;135:1906–1910. [PubMed] [Google Scholar]
- 10.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 11.Hoffmann J A, Kafatos F C, Janeway C A, Jr, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Jin F-Y, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 14.Jin F-Y, Nathan C F, Ding A. Paradoxical preservation of a lipopolysaccharide response in C3H/HeJ macrophages: induction of matrix metalloproteinase-9. J Immunol. 1999;162:3596–3600. [PubMed] [Google Scholar]
- 15.Kang A D, Wong P M C, Chen H, Castagan R, Chung S-W, Sultzer B M. Restoration of lipopolysaccharide-mediated B-cell response after expression of a cDNA encoding a GTP-binding protein. Infect Immun. 1996;64:4612–4617. doi: 10.1128/iai.64.11.4612-4617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss A L, Geuze H J. Caveolae can be alternative endocytotic structures in elicited macrophages. Eur J Cell Biol. 1997;73:19–27. [PubMed] [Google Scholar]
- 18.Kitchens R L, Wang P-Y, Munford R S. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J Immunol. 1998;161:5534–5545. [PubMed] [Google Scholar]
- 19.Matveev S, van der Westhuyzen D R, Smart E J. Co-expression of scavenger receptor-B1 and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J Lipid Res. 1999;40:1647–1654. [PubMed] [Google Scholar]
- 20.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda T, Akira S. Endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 21.Okamato T, Schlegel A, Scherer P E, Lisanti M P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 22.Poltorak A, He X, Smirnova I, Liu M-Y, van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutation in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi S T, Larivière L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutation in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstreich D L. Genetic control of endotoxin response: C3H/HeJ mouse. In: Berry L J, editor. Handbook of endotoxin. 3. Cellular biology of endotoxin. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. pp. 82–122. [Google Scholar]
- 25.Rothberg K G, Houser J E, Donzell W C, Ying Y-S, Glenney J R, Anderson R G W. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Shaul P W, Anderson R G W. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 28.Sultzer B M. Genetic control of leukocyte responses to endotoxin. Nature. 1968;219:1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- 29.Tang Z L, Okamoto T, Boontrakulpoontawee P, Katada T, Otsuka A J, Lisanti M P. Identification, sequence, and expression of an invertebrate caveolin gene family from the nematode Caenorhabditis elegans. J Biol Chem. 1997;272:2437–2445. doi: 10.1074/jbc.272.4.2437. [DOI] [PubMed] [Google Scholar]
- 30.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 31.Vogel S N, Johnson D, Perera P-Y, Medvedev A, Larivière L, Qureshi S T, Malo D. Functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 32.Wang P-Y, Kitchens R L, Munford R S. Bacterial lipopolysaccharide binds to low-density domains of the monocyte-macrophage plasma membrane. J Inflamm. 1996;47:126–137. [PubMed] [Google Scholar]
- 33.Wong P M C, Kang A, Chen H, Yuan Q, Fan P, Sultzer B M, Kan Y W, Chung S-W. Lpsd/Ran of endotoxin-resistant C3H/HeJ mice is defective in mediating lipopolysaccharide endotoxin responses. Proc Natl Acad Sci USA. 1999;96:11543–11548. doi: 10.1073/pnas.96.20.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson J, Kelly K, Largen M, Taylor B A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978;120:422–424. [PubMed] [Google Scholar]
- 35.Yang R B, Mark M R, Gray A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski H J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]