Abstract
Background:
Staging laparoscopy (SL) is used to avoid resection failure and thus increase the curative resection rate. SL utilization in extra‐hepatic biliary tumors (EHBT) is variable.
Methods:
Data from 1090 patients with potentially resectable EHBT including gallbladder (GBC), distal (DC), and hilar (HC) subtypes were retrospectively collected from 10 academic centers (2000‐2015).
Results:
The SL utilization rate increased over time and was significantly higher in GBC than DC and HC. SL yield was 16.8% and did not differ between groups or over time. In patients undergoing attempted resection with prior SL, the curative resection rate did not differ between subtypes. In patients undergoing attempted resection without prior SL, the curative resection rate was less in GBC compared with DC or HC. After matching cohorts by inverse probability weighting, prior SL was associated with curative resection in GBC only (odds ratio [OR], 2.41, 95% CI, 1.36‐4.27). On multivariable regression analysis, elevated carbohydrate antigen 19‐9 (CA 19‐9), low serum albumin, and GBC were strong predictors of distant disease on SL. After categorizing patients undergoing SL into low, intermediate, and high‐risk groups based on these parameters, SL yield improved progressively from 10.0% to 19.6% to 52.6%.
Conclusions:
We recommend routine SL for patients with GBC, particularly with elevated CA19‐9 level and/or decreased serum albumin.
Keywords: biliary cancer, cholangiocarcinoma, staging laparoscopy
1 |. INTRODUCTION
Extra‐hepatic biliary tumors (EHBT) represent a subtype of cholangiocarcinoma that arises from the gallbladder or the extra‐hepatic biliary tree. The estimated incidence of EHBT was 3.5 cases per 100,000 persons in 2016 in the United States.1,2 Prognosis for patients with disease beyond stage 0 or I is poor, and surgical resection is the only curative modality. The majority of patients, however, present in a delayed fashion with unresectable disease.3 Recent advances in imaging, such as positron emission tomography (PET), have led to the improved preoperative determination of resectability.4,5 However, up to approximately 46% of patients are subjected to nontherapeutic exploratory laparotomy (EL) after the discovery of metastases.6,7 Staging laparoscopy (SL) can detect radiographically occult disseminated disease, and therefore, SL may assist in reducing failed ELs and thus increasing the rate of curative resection. SL also confers clinical benefit by decreasing length of hospitalization, postoperative morbidity, and time to systemic therapy.8–10 Some investigators argue, however, that SL increases operative times, resource utilization, and overall costs.11
A few extensive studies have evaluated the utility of SL among the three subtypes of EHBT in the era of modern imaging and thus the use of SL in these cancers is controversial. We, therefore, sought to define the utility of SL in the gallbladder (GBC), distal (DC), and hilar (HC) EHBT to determine which subtypes may merit more routine vs more selective SL use in a modern cohort of patients taken to the operating room with curative therapeutic intent.
2 |. MATERIALS AND METHODS
2.1 |. Study population
The United States Extra‐Hepatic Biliary Malignancy Consortium includes data from 10 academic institutions (Emory University, Johns Hopkins Hospital, University of Louisville, New York University, Ohio State University, Stanford University, Vanderbilt University, Wake Forest University, Washington University in St. Louis, and the University of Wisconsin). Information on demographic, preoperative, intraoperative, and pathologic aspects was collected from patients diagnosed with potentially resectable EHBT between 2000 and 2015. Pathology staging was assigned per American Joint Committee on Cancer, Seventh Edition.12 Data regarding neoadjuvant and adjuvant therapies, preoperative imaging modalities, and postoperative outcomes were also collected. Institutional review board approval was obtained from each institution.
2.2 |. Primary and secondary study outcomes
A primary objective was to define the utilization rate and yield of SL in three subtypes of extra‐hepatic biliary malignancy. The yield was defined as the ratio of patients with positive SL divided by the total number of patients undergoing SL. An additional primary objective included assessment of the curative resection rate among patients who underwent SL vs upfront EL. Secondary objectives included determining preoperative variables predictive of distant disease on SL.
2.3 |. Statistical analysis
All analyses were performed using IBM SPSS version 23.0.0.2 (IBM Corp, Armonk, NY) and GraphPad Prism version 7.03 (GraphPad Software, La Jolla, CA). Patient characteristics were summarized using descriptive statistics. Among the three types of EHBT, we compared the utilization rate and yield of SL, as well as the curative resection rate in patients with and without prior SL, using a two‐way analysis of variance with Tukey’s post hoc test for multiple comparisons. To analyze the association of SL with curative resection rate, we matched patients who did or did not undergo SL before attempted resection using inverse probability weighting (IPW). Weights were calculated by generating a logistic regression model to predict the probability of each patient who underwent attempted resection either receiving or not receiving SL on the basis of 15 defined preoperative variables to standardize the two groups. We then applied binary logistic regression modeling with SL as the independent variable and curative resection as the outcome.
To analyze the potential preoperative risk factors associated with distant disease on SL, patients with positive and negative SL were also matched by IPW on age, gender, comorbid condition, and neoadjuvant chemotherapy, and multivariable regression analysis was performed for tumor type, serum carbohydrate antigen 19‐9 (CA19‐9), total bilirubin, and albumin levels. Variable selection for this analysis was done using prior literature and biological and clinical plausibility. EHBT SL score was calculated by adding points for each of the predetermined variables. Missing values were assigned zero points. Scores were used to categorize patients into low, intermediate, and high risk of distant disease on SL. Within the score category, SL yield was defined as the ratio of patients with positive SL divided by the total number of patients undergoing SL. Statistical significance was set at P < 0.05.
3 |. RESULTS
1090 patients with potentially resectable EHBT were identified (Figure 1). Of this cohort, 250 (23%) underwent SL as part of their initial surgical plan. Among the 250 patients who underwent SL, 42 (17%) were unresectable. Among the 208 (83%) patients who proceeded to exploratory laparotomy (EL) after negative SL, 169 (81%) patients were resected and 39 (19%) failed. In the cohort of 840 patients who went straight for EL, 688 (82%) went on to curative resection while 152 (18%) failed.
FIGURE 1.
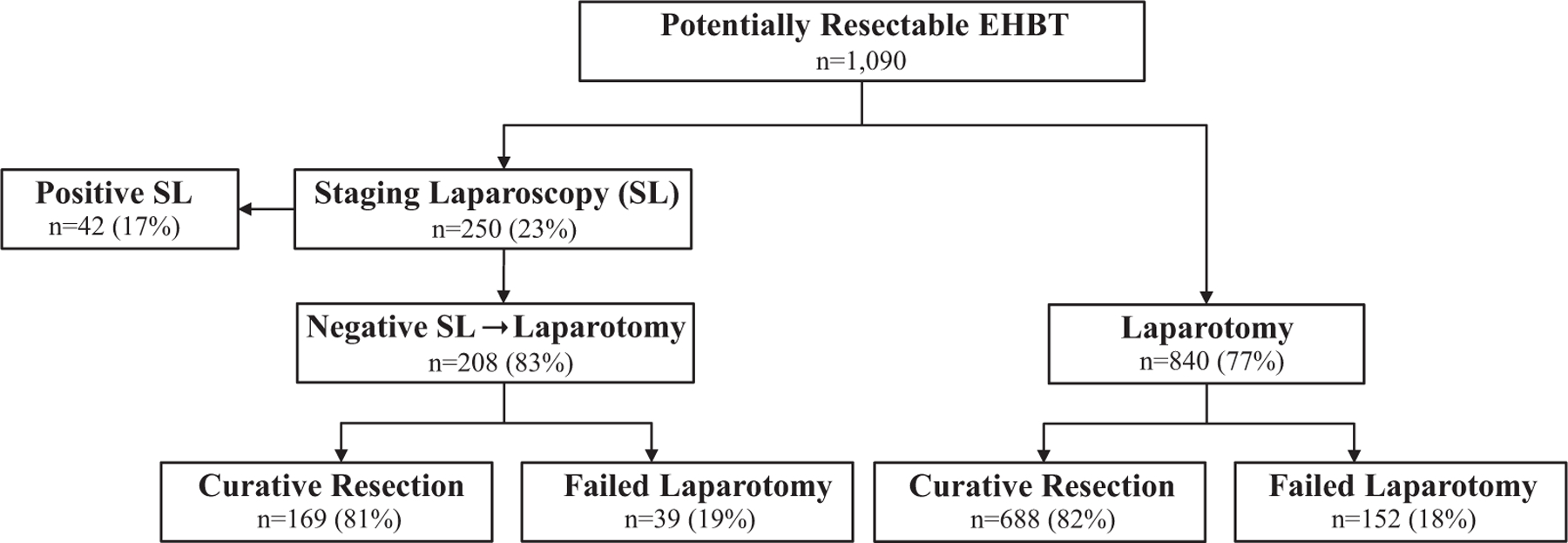
Surgical outcomes of patients with potentially resectable extra‐hepatic biliary tumor during the study period (2000‐2015). EHBT, extra‐hepatic biliary tumors; SL, staging laparoscopy
We divided the study into three time periods (2000‐2005, 2006‐2010, and 2011‐2015) to analyze the utilization rate and yield of SL over time. The number of patients in each group was similar (N = 353, 412, and 323, respectively). The utilization rate of SL increased over time and was significantly higher in GBC compared with DC (mean difference, +22.3%; P < 0.001) and HC subtypes (mean difference, +16.2%; P = 0.002; Figure 2A). The yield (defined as the ratio of patients with positive SL to the number of total patients who underwent SL) was 16.8% for EHBT overall and did not differ between GBC, DC, and HC subtypes (17.6%, 12.8%, and 17.2%, respectively; P = 0.82), and did not significantly change over time (Figure 2B). In patients who underwent SL before attempted resection, the curative resection rate tended to increase over time for GBC and HC but not DC and did not significantly differ between subtypes (Figure 2C). In patients who went straight to attempted resection without prior SL, the curative resection rate rose modestly over time for GBC and HC but not DC and tended to be less in GBC compared with DC (mean difference, −19.3%; P = 0.008) or HC (mean difference, −7.4%; P = 0.10; Figure 2D).
FIGURE 2.
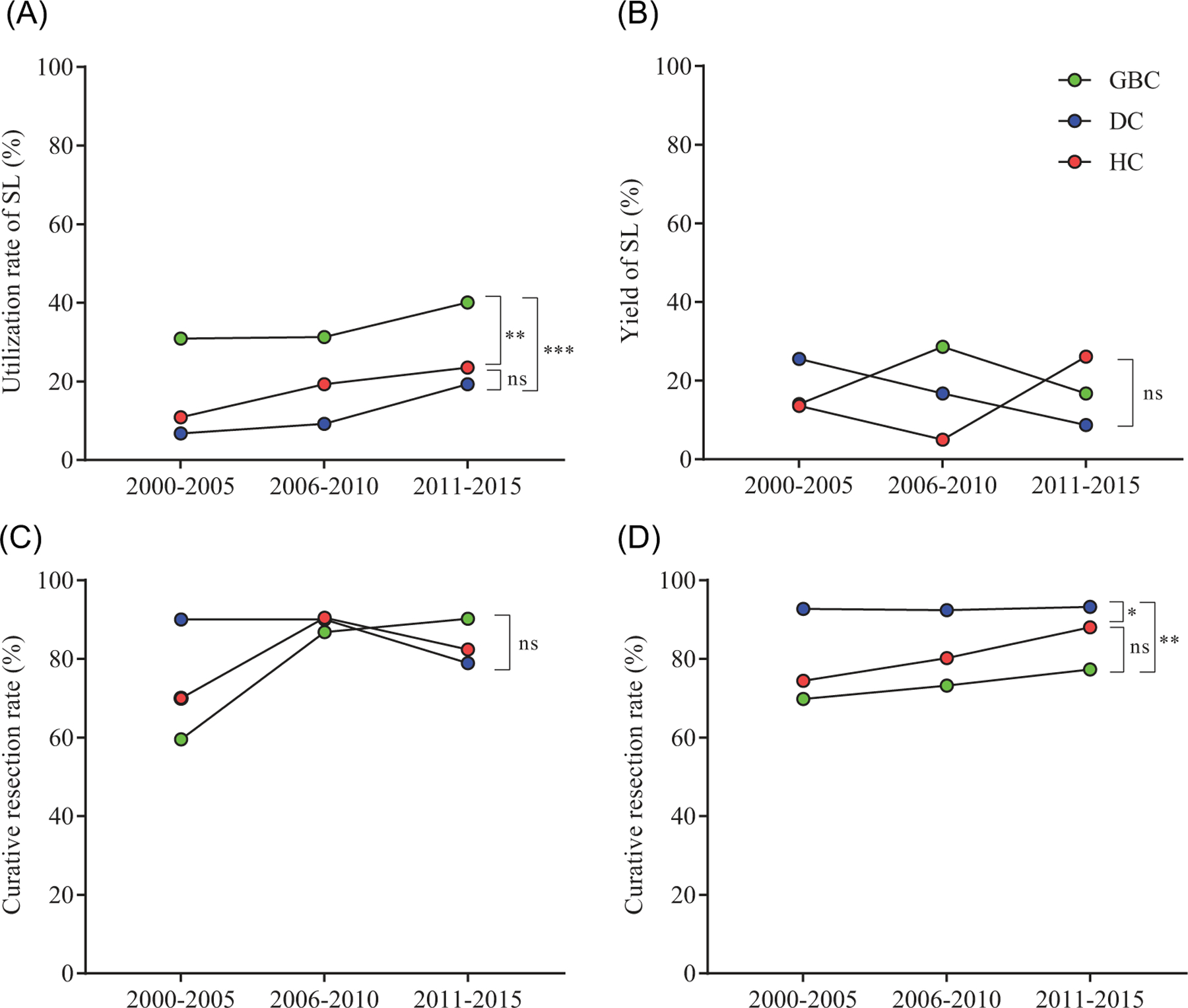
Trends in staging laparoscopy utilization (A) and yield (B) as well as curative resection rates with (C) and without (D) prior SL during the study period (2000‐2015). DC, distal cancer; GBC, gallbladder cancer; HC, hilar cancer; SL, staging laparoscopy; ns, not significant (P > 0.05); *P < 0.05; **P < 0.01; ***P < 0.001 using 2‐way ANOVA with Tukey’s multiple comparisons test
We then sought to determine if SL was associated with an increased probability of curative resection for EHBT, as the goal of SL in abdominal malignancy is to exclude patients with unresectable metastatic disease from undergoing failed resection. To mitigate treatment selection bias, we first matched the two cohorts of patients who underwent resection attempt with or without prior SL by IPW using 15 preoperative covariates. Adjusted clinical characteristics are shown in Table 1. After matching, we found that SL was not associated with curative resection in EHBT overall (odds ratio [OR], 0.97; 95% CI, 0.69‐1.37; Table 2). In patients with GBC, however, SL was associated with increased odds of curative resection ([OR] 2.41, 95% CI, 1.36‐4.27), while in DC, SL was associated with decreased odds of curative resection (OR, 0.35; 95% CI, 0.17‐0.72). In HC, SL did not appear to associate with the probability of undergoing a curative resection (OR, 0.96; 95% CI, 0.56‐1.65).
TABLE 1.
Adjusted preoperative baseline characteristics of patients with EHBT undergoing attempted resection with or without prior staging laparoscopy
| Characteristics | Adjusted using inverse probability weighting |
||
|---|---|---|---|
| SL before EL (n = 208) | Upfront EL (n = 840) | P | |
| Age, mean ± SD | 65.5 ± 10 | 65.6 ± 12 | 0.92 |
| Male sex, % | 55.5 | 55.5 | 0.99 |
| Comorbid condition,a % | 74.1 | 71.1 | 0.30 |
| Body mass index, mean ± SD | 27.4 ± 6.7 | 27.2 ± 6.3 | 0.75 |
| Neoadjuvant chemotherapy, % | 2.1 | 2.3 | 0.83 |
| Preoperative imaging, % | |||
| Ultrasound | 55.7 | 52.7 | 0.34 |
| CT | 92.8 | 93.3 | 0.80 |
| MRI/MRCP | 50.2 | 46.8 | 0.30 |
| PET/CT | 10.5 | 11.9 | 0.49 |
| ERCP/cholangiogram | 67.5 | 65.2 | 0.45 |
| CA 19–9 level, median (IQR) | 48.7 (185) | 68.0 (208.6) | 0.22 |
| Bilirubin, total, median (IQR) | 1.2 (3.2) | 1.3 (2.1) | 0.32 |
| Albumin, mean ± SD | 3.49 ± 0.80 | 3.55 ± 0.70 | 0.27 |
| Preoperative imaging diagnosis, % | 0.91 | ||
| Benign | 6.9 | 6.9 | |
| Malignant | 71.0 | 72.2 | |
| Indeterminate | 21.4 | 20.9 | |
| Emergency surgery, % | 1.3 | 1.3 | 1.00 |
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CT, computed tomography; EHBT, extra‐hepatic biliary tumor; EL, exploratory laparotomy; ERCP, endoscopic retrograde cholangiopancreatography; IQR, interquartile range; MRI/MRCP, magnetic resonance imaging/cholangiopancreatography; PET, positron emission tomography; SL, staging laparoscopy.
Includes hypertension, diabetes mellitus, congestive heart failure, prior cardiac event, and smoking history.
TABLE 2.
Adjusted odds of undergoing a curative resection with prior staging laparoscopy
| 95% CI |
||||
|---|---|---|---|---|
| Odds ratio (adjusted) | P | Upper limit | Lower limit | |
| EHBT (overall) | 0.97 | 0.87 | 0.69 | 1.37 |
| GBC | 2.41 | 0.003 | 1.36 | 4.27 |
| Distal | 0.35 | 0.005 | 0.17 | 0.72 |
| Hilar | 0.96 | 0.88 | 0.56 | 1.65 |
Abbreviations: EHBT, extra‐hepatic biliary tumor; GBC, gallbladder cancer.
Finally, we sought to determine if certain preoperative clinical and laboratory variables were predictive of SL‐detected distant disease. We matched patients who underwent a positive or negative SL by age, gender, body mass index, comorbid condition, and neoadjuvant chemotherapy, and then performed multivariable logistic regression analysis with preoperative characteristics. The diagnosis of GBC, increasing CA19‐9 level (0‐15, 15‐30, and >30 U/mL) and decreasing serum albumin level (>3.2, 2.8‐3.2, and <2.8 mg/dL), were strong independent predictors of a positive SL while preoperative total bilirubin was not (Table 3). This model had the moderate discriminative ability for the distant disease on SL (Figure 3A), with a C‐statistic of 0.75 (95% CI, 0.61‐0.89; P < 0.001). We then applied the ORs to develop a simple EHBT SL score ranging from 0 to 6 to categorize patients’ risk of distant disease on SL. An individual patient’s score was assigned by adding the points to each variable (Table 3). For patients within each score category (low risk, 0‐2; intermediate risk, 3‐4; and high risk, 5‐6), we then calculated the yield of SL (10.0%, 19.6%, and 52.6%, respectively) showing that stratifying patients based on the EHBT SL score corresponds to stepwise improvement in SL yield (Figure 3B).
TABLE 3.
Multivariable regression analysis of preoperative variables associated with unresectable disease on staging laparoscopy
| Odds ratio (adjusted) | P | 95% CI |
EHBTSL score | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| EHBT subtype | |||||
| Distal | Ref | Ref | – | – | 0 |
| Hilar | 1.46 | 0.40 | 0.61 | 3.53 | 0 |
| GBC | 3.12 | 0.005 | 1.42 | 6.88 | 2 |
| Total bilirubin, mg/dL | 1.03 | 0.41 | 0.96 | 1.09 | – |
| CA19–9 level, U/mL | |||||
| 0–15 | Ref | Ref | 0 | ||
| 15–30 | 2.03 | 0.12 | 0.98 | 5.64 | 1 |
| >30 | 3.66 | 0.001 | 1.73 | 7.76 | 2 |
| Albumin level, mg/dL | |||||
| >3.2 | Ref | Ref | 0 | ||
| 2.8–3.2 | 1.89 | 0.09 | 0.91 | 3.92 | 1 |
| <2.8 | 3.12 | 0.001 | 1.59 | 6.91 | 2 |
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; EHBT, extra‐hepatic biliary tumor; GBC, gallbladder cancer; SL, staging laparoscopy.
FIGURE 3.
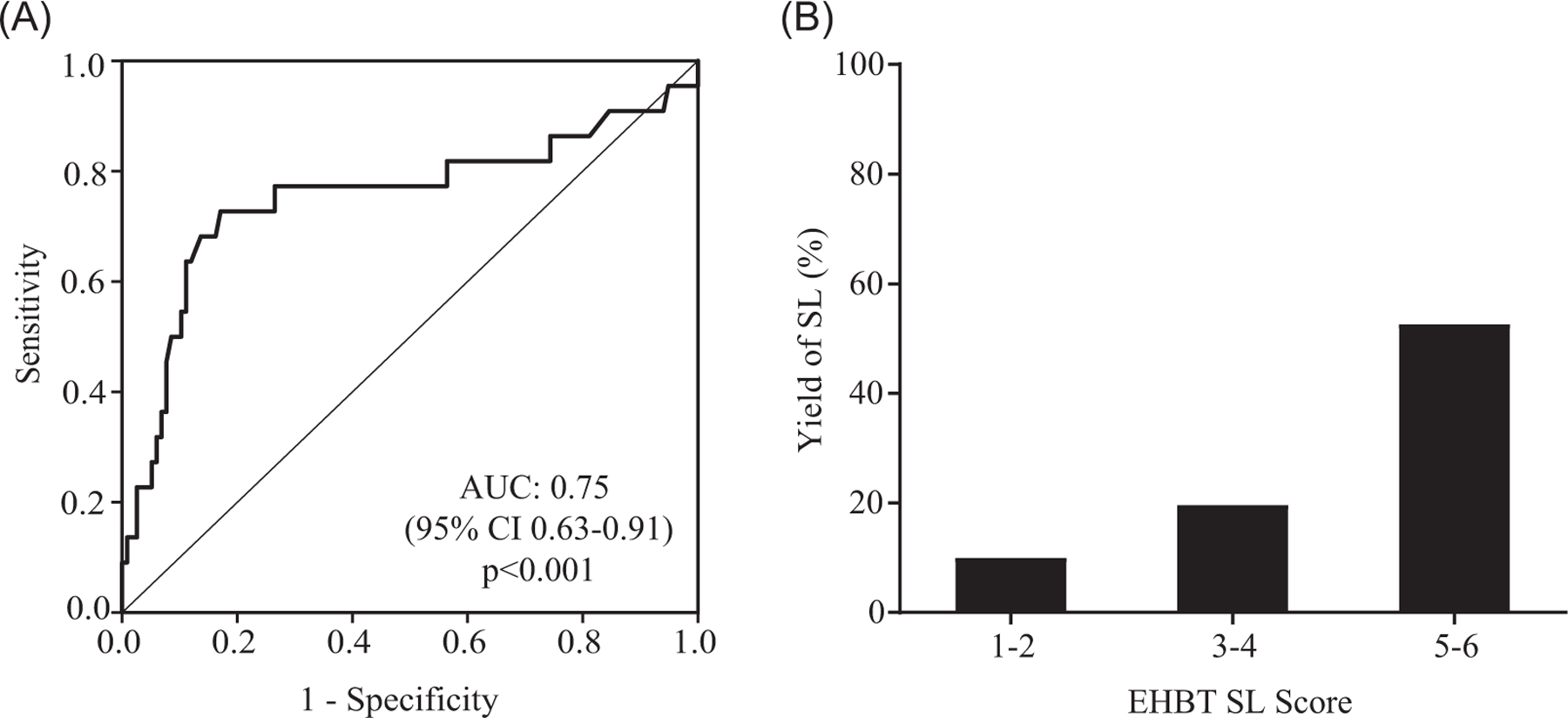
A, Receiver operating characteristic (ROC) of the predictive model for positive staging laparoscopy. B, The yield of staging laparoscopy (SL) stratified by extra‐hepatic biliary tumor (EHBT) SL score. AUC, area under the ROC curve
4 |. DISCUSSION
Despite the rational for its use, the clinical utility of SL in biliary cancer is variable. In a seminal study in 2002, Weber et al10 reported a yield of SL of 48% in patients with GBC and 36% in patients with locally advanced (T2/T3) but resectable hilar cholangiocarcinoma, suggesting that SL should be routinely pursued in both the groups. D’Angelica et al8 demonstrated a yield of SL of nearly 50% for GBC and 20%‐25% for peripheral and hilar cholangiocarcinoma. Several subsequent studies underscored that the utility of SL in hepatobiliary malignancy was dependent on the anatomical location of the primary tumor. Vollmer et al7 found that patients undergoing SL with GBC demonstrated the highest yield (55%), while those with ampullary malignancy demonstrated the lowest (0%). Goere et al6 reported a yield of SL in the gallbladder, intrahepatic HC, and extra‐hepatic cancer as 62%, 36%, and 25%, respectively, while White et al13 reported a yield of 9.8% for distal biliary cancer.
Contemporary studies have suggested that the yield of SL in biliary cancer has decreased over time. In 2011, Ruys et al14 reported a yield of SL of 14% in hilar cholangiocarcinoma, a significant decline from 41% reported in a previous study from their group in 2002.15 This difference was attributed to the improvement in high‐resolution imaging technology including MRI and PET/CT. In a modern, prospective study of the role of SL in 409 patients with GBC, Agarwal et al16 reported an overall yield of 23.2%, which was higher for patients with locally advanced vs early stage disease (25.2% vs 10.7%, respectively). The authors further suggested that improvements in SL could be made with the inclusion of laparoscopic ultrasound for deep parenchymal liver lesions and/or endoscopic or laparoscopic sampling of interaortocaval lymph nodes.16 In a recent meta-analysis (2017) comprising eight studies and 1062 patients undergoing SL for biliary cancer, the yield in GBC and HC was found to be 27.6% and 32.4%, respectively.17
In this study, we found that the utilization of SL for EHBT increased across 10 tertiary centers over the 15‐year time period and was significantly higher in GBC than DC or HC. Yet, the yield of SL overall was modest in comparison to prior studies (16.8%) and did not significantly change over time or between biliary cancer subtypes. The curative resection rates for patients undergoing SL before attempted resection appeared to trend upward with time for GBC and HC but not DC and it also did not significantly differ between subtypes. In addition, in patients that did not undergo SL, the curative resection rate appeared to increase modestly with time for GBC and HC and not DC but was significantly lower in patients with GBC. These trends in composite suggest that the utility of SL in EHBT is quite limited in the modern era, which is likely due in part to continued improvements in radiographic and clinical determination of resectability.
Owing to the modest yield of SL in EHBT overall, we sought to determine the cases in which it may provide the most utility. After matching patients on 15 preoperative demographic, clinical, biochemical, and imaging factors, we found that SL was associated with curative resection in patients with GBC but not HC. Unexpectedly, SL was associated with decreased odds of curative resection in DC, which is likely due to residual confounding that is not identifiable in our data set that remained even after standardization by IPW.
In this study, we also set out to define preoperative characteristics that may predict an increased risk of metastatic disease detectable by SL to potentially improve patient selection for the procedure. After matching patients who underwent a positive and negative SL by age, gender, body mass index, comorbid condition, and neoadjuvant chemotherapy, our analysis revealed that patients with GBC were at increased risk of detectable metastases on SL compared with DC and HC. In addition, increasing CA 19‐9 level and decreasing serum albumin were significant risk factors as well, in a gradient‐dependent manner, whereas preoperative total bilirubin level did not confer risk. These variables were chosen based on prior literature, as well as biological and clinical plausibility. CA 19‐9 level has been found to be an independent predictor of poor survival in patients with biliary tree malignancies18 and GBC specifically,19 and baseline CA 19‐9 level may predict the burden of disease in GBC, particularly above 20 units per milliliter.20 CA 19‐9 level also has been validated as a predictive marker for positive SL in potentially resectable pancreatic cancer,21 but to our knowledge, this study is the first demonstration of the predictive significance of CA 19‐9 in SL for potentially resectable EHBT. In addition, elevated serum albumin has been shown to be a favorable prognostic factor for survival in GBC,22,23 but to our knowledge, serum albumin has not been analyzed as a predictor in the context of SL for biliary malignancy. Serum albumin can be reduced in cases of bile tract cancer for several interrelated reasons, including extensive tumor burden, malnutrition, decreased functional status, response to chemotherapy, systemic inflammation, impaired liver function, and malignant ascites from peritoneal carcinomatosis. Irrespective of the underlying pathophysiology, decreasing serum albumin was a strong risk factor for metastatic disease on SL. We used these findings to develop a simple EHBT SL scoring system and found that an increasing score did correlate with increased risk of positive SL, which translated into improved SL yield (from 10.0% in the low‐risk group to 52.6% in the high‐risk group). If applied and validated in an external patient cohort, we believe that this scoring system could be used to improve the preoperative selection of patients with EHBT for staging laparoscopy.
This study has several limitations. First, it is limited by its retrospective design. We thus were unable to account for all of the specific preoperative radiographic and clinical criteria used by the clinicians at each institution in selecting patients to undergo SL. We attempted to control for selection bias by matching patients by inverse probability of treatment weighing with the maximum number of robust preoperative variables that were available in the data set. In addition, the specific details of a surgical technique for SL were not collected, and therefore, we were unable to ascertain whether surgeons performed a one‐stage or two‐stage procedure, or adjunctive SL approaches, such as nodal sampling, peritoneal lavage, exploration of the lesser sac, or endoscopic or laparoscopic ultrasound. Notably, however, this study included data from 10 high‐volume academic centers across the United States, which eliminates single institution bias and is more reflective of general practice patterns. The database was standardized to improve data quality and control and all the data were carefully scrutinized before inclusion for analysis.
5 |. CONCLUSIONS
Our multi‐institutional experience suggests the utility of SL in EHBT is limited in the modern era as the overall yield of SL was lower than prior reports and SL did not improve the curative resection rate in EHBT overall. However, SL did confer increased odds of undergoing a curative resection specifically in patients with GBC. In addition, several preoperative variables were identified as strong independent risk factors for distant disease detected on SL. On the basis of these results, we recommend that routine SL be considered for patients with GBC, particularly with elevated CA19‐9 and/or decreased serum albumin levels.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training program grant T32CA009621 from the National Cancer Institute (NCI) as part of the National Institutes of Health (NIH) for JTD, LXJ, and BK. This study was presented in part at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium; January 22, 2016; San Francisco, CA; and at the Society of Surgical OncologyAnnual Meeting; March 3, 2016; Boston, MA.
Funding information
National Cancer Institute as part of the National Institutes of Health (NIH), Grant/Award Number: T32 CA009621
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2.De Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med 1999;341(18):1368‐1378. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9(1):43‐57. [DOI] [PubMed] [Google Scholar]
- 4.Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol 2008;191(5):1448‐1457. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Kim MH, Lee TY, et al. Clinical role of 18F‐FDG PET‐CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol 2008;103(5):1145‐1151. [DOI] [PubMed] [Google Scholar]
- 6.Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 2006;20(5):721‐725. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer CM, Drebin JA, Middleton WD, et al. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg 2002;235(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol 2003;10(2):183‐189. [DOI] [PubMed] [Google Scholar]
- 9.Velanovich V, Wollner I, Ajlouni M. Staging laparoscopy promotes increased utilization of postoperative therapy for unresectable intra‐ abdominal malignancies. J Gastrointest Surg 2000;4(5):542‐546. [DOI] [PubMed] [Google Scholar]
- 10.Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002;235(3):392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper E, Kalb B, Martin DR, Kooby D, Adsay NV, Sarmiento JM. Staging laparoscopy for proximal pancreatic cancer in a magnetic resonance imaging‐driven practice: what’s it worth? HPB 2011;13(10):732‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, American Joint Committee on Cancer. AJCC Cancer Staging Handbook: from the AJCC cancer staging manual 7th ed., xix. New York: Springer; 2010:718. [Google Scholar]
- 13.White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg 2008;206(3):445‐450. [DOI] [PubMed] [Google Scholar]
- 14.Ruys AT, Busch OR, Gouma DJ, van Gulik TM. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol 2011;18(9):2647‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilleman EH, de Castro SM, Busch OR, et al. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg 2002;6(3):426‐430. discussion 430‐1. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer‐‐an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg 2013;258(2):318‐323. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y, Liu L, Yeolkar NV, Shen F, Li J, He Z. Diagnostic role of staging laparoscopy in a subset of biliary cancers: a meta‐analysis. ANZ J Surg 2017;87(1‐2):22‐27. [DOI] [PubMed] [Google Scholar]
- 18.Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol 2010;17(4):991‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Z, Si A, Yang J, et al. Elevation of CA19‐9 and CEA is associated with a poor prognosis in patients with resectable gallbladder carcinoma. HPB 2017;19(11):951‐956. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Lawrence A, Saxena R. Does CA 19‐9 have prognostic relevance in gallbladder carcinoma (GBC). J Gastrointest Cancer 2018;49(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 21.Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19‐9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol 2008;15(12):3512‐3520. [DOI] [PubMed] [Google Scholar]
- 22.Shiba H, Misawa T, Fujiwara Y, et al. Glasgow prognostic score predicts outcome after surgical resection of gallbladder cancer. World J Surg 2015;39(3):753‐758. [DOI] [PubMed] [Google Scholar]
- 23.Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrinogen‐to‐albumin ratio in gallbladder cancer patients. World J Gastroenterol 2018;24(29):3281‐3292. [DOI] [PMC free article] [PubMed] [Google Scholar]


