Abstract
Infection with Chlamydia pneumoniae, a causative agent of acute and chronic respiratory diseases, has recently been implicated as a potential risk factor in atherosclerosis. Atherosclerotic lesions are characterized by monocyte infiltration, which may be regulated by the chemokine monocyte chemotactic protein 1 (MCP-1). We have previously shown that C. pneumoniae infection stimulates MCP-1 production in human endothelial cells, an event which may be specific to this species of Chlamydia, since Chlamydia trachomatis infection fails to induce this response. To examine the underlying mechanisms by which C. pneumoniae infection induces MCP-1 production in endothelial cells, the present study investigated the role of transcription factor NF-κB in MCP-1 mRNA expression. Human umbilical vein endothelial cells (HUVEC) were infected with the coronary isolate C. pneumoniae A-03 or with C. trachomatis L2, and MCP-1 mRNA expression was assessed after different periods of infection by reverse transcription-PCR. Expression of MCP-1 mRNA in C. pneumoniae-infected HUVEC was significantly elevated as early as 1 h postinfection and increased dramatically by 12 and 24 h compared to baseline controls. Nuclear translocation of NF-κB occurred by 30 min of infection, as determined by electrophoretic mobility shift assays and immunofluorescence staining. Treatment of C. pneumoniae-infected HUVEC with parthenolide, a specific inhibitor of NF-κB activation, suppressed MCP-1 mRNA expression. In contrast, infection with C. trachomatis L2 did not induce MCP-1 mRNA in infected HUVEC and failed to activate NF-κB. Results from this study demonstrate a requirement for NF-κB activation in stimulation of MCP-1 gene expression by C. pneumoniae in human endothelial cells. Furthermore, the data suggest that, within the genus Chlamydia, functionally distinct signaling pathways leading to NF-κB activation are utilized by C. pneumoniae in endothelial cells during infection.
Chlamydia pneumoniae is a pathogen causing respiratory infections such as sinusitis, bronchitis, and pneumonia (12, 13). Chronic respiratory infections with this organism may also develop following acute illness in spite of appropriate antibiotic therapy (15). A potential role for C. pneumoniae in atherosclerosis has been suggested by studies documenting an association between patients with coronary artery disease and increased antibody titers to this organism (30, 38, 39). Furthermore, the presence of C. pneumoniae in atherosclerotic lesions has been shown by different techniques, such as electron microscopy, immunocytochemistry, PCR, and culture (reviewed in references 3, 40, and 43). Additional evidence supporting a relationship between C. pneumoniae and atherosclerosis has come from in vitro experiments showing the ability of this organism to replicate in cells of the vascular wall (i.e., endothelial cells, aortic smooth muscle cells, and macrophages) (9, 10). A causal role for this organism in atherogenesis has recently been strengthened by in vivo studies documenting the ability of C. pneumoniae to induce atheromatous-like lesions in the aortas of infected rabbits (7, 25). Still, the mechanisms by which C. pneumoniae might contribute to the pathogenesis of atherosclerosis remain unclear.
A major inflammatory event that takes place during atherosclerosis is the accumulation of monocytes from the circulation into the arterial intima (37). Monocyte chemotactic protein 1 (MCP-1), a member of the C-C chemokine family, has been proposed to play an important role in the early events of atherogenesis. Recent studies suggest that mice deficient in MCP-1 are less susceptible to experimental atherosclerosis (11, 14). MCP-1 is a 14-kDa glycoprotein secreted by many cells, including endothelial cells and vascular smooth muscle cells, which can be transcriptionally activated by different stimuli, such as tumor necrosis factor alpha, interleukin-1, and lipopolysaccharide (LPS) (2). A variety of signaling mechanisms are involved in the intracellular activation of MCP-1 gene expression by these stimuli, including activation of phospholipase C, generation of diacylglycerol, and activation of protein kinase C and tyrosine kinases (42). Subsequent events include generation of reactive oxygen intermediates and activation of transcription factor NF-κB (34). There is evidence that NF-κB is a major regulator of the transcriptional activation of MCP-1 in cytokine-activated human endothelial cells (27). In addition, other studies have shown that NF-κB is activated during the early stages of atherosclerosis and may function as a point of convergence of the diverse risk factors associated with this disease (4).
Previous studies from this laboratory have shown that C. pneumoniae induces MCP-1 secretion from human endothelial cells and promotes the transendothelial migration of monocytes in vitro (31, 32). Interestingly, infection with Chlamydia trachomatis does not result in stimulation of MCP-1 (32). This divergence between species may reflect the presence of specific features in C. pneumoniae that activate different signaling pathways within human endothelial cells. To provide a better understanding of the underlying mechanisms involved in the C. pneumoniae-dependent activation of MCP-1 in infected endothelial cells, this study investigated the role of NF-κB in MCP-1 gene expression.
MATERIALS AND METHODS
Chlamydia isolates.
C. pneumoniae A-03 (ATCC VR-1452) was isolated from an atheroma of a patient with coronary artery disease (35) and propagated in HEp-2 cell cultures (ATCC CCL-23) as previously described (31). C. trachomatis L2/434 was kindly provided by James B. Mahoney, McMaster University Regional Virology and Chlamydiology Laboratory, Hamilton, Ontario, Canada.
Endothelial cell cultures.
Human umbilical vein endothelial cells (HUVEC) (ATCC 1730-CRL) were cultured in 75-cm2 flasks and maintained in Ham's F12K medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin-amphotericin B (Fungizone) mix, 30 μg of endothelial cell growth supplement per ml, and 100 μg of heparin (Sigma, St. Louis, Mo.) per ml. Prior to infection, HUVEC were transferred into gelatin-coated 24-well plates at 2 × 105 cells/well (MCP-1 experiments) or 6-well plates at 5 × 105 cells/well (NF-κB experiments) and incubated overnight at 37°C with 5% CO2.
Infection protocol.
HUVEC monolayers in 24- or 6-well plates were inoculated separately with C. pneumoniae A-03 or C. trachomatis L2 suspended in Ham's medium without antibiotics. Cells grown in 24-well plates received 2 × 105 inclusion-forming units per well, while cells grown in 6-well plates were inoculated with 5 × 105 inclusion-forming units per well, resulting in a multiplicity of infection (MOI) of 1:1 for each case. The inocula of both chlamydial species contained equivalent amounts of LPS as determined by the Limulus amebocyte lysate test for endotoxin (Sigma), and 2-keto-3-deoxyoctonate, measured by the method of Karkhanis et al. (20). Such controls were performed to rule out possible differences in the numbers of total chlamydial particles (i.e., infectious and noninfectious) between C. pneumoniae and C. trachomatis inocula. Following inoculation, infection of HUVEC was followed by centrifugation as described (31). Mock-infected controls were also included, which consisted of HUVEC treated with crude lysates of HEp-2 cells. For the MCP-1 experiments, uninfected, mock-infected, and infected cells were incubated for 1, 2, 4, 8, 12, and 24 h at 37°C in 5% CO2 before total RNA isolation. For the NF-κB experiments, cells were incubated for 30 min before nuclear protein extraction. The response of HUVEC to stimulation with 500 U of human recombinant tumor necrosis factor alpha (TNF-α; Promega, Madison, Wis.) per ml was used as a positive control for MCP-1 gene expression and NF-κB activation.
RT-PCR.
For reverse transcription-PCR (RT-PCR), total RNA from HUVEC cultured in 24-well plates was isolated using RNeasy minikits following the manufacturer's procedures (Qiagen, Santa Clarita, Calif.). The amount of RNA was measured with a spectrophotometer and determined to be 16 to 20 μg. Reverse transcription was performed at 42°C for 20 min in 10 μl of reaction mixture containing 0.2 μg of total RNA, 5 mM MgCl2, 1× reverse transcription buffer (10 mM Tris-HCl, 50 mM KCl, 0.1% Triton X-100), 1 mM each of the four deoxynucleoside triphosphates (dNTPs), 20 U of recombinant RNasin RNase inhibitor, 15 U of avian myeloblastosis virus reverse transcriptase, and 0.5 μg of oligo(dT)15 (Promega). Reactions were stopped by heating at 99°C and cooling at 4°C for 5 min. Subsequently, 2 μl of cDNA products was amplified in 50 μl of 50 mM KCl–10 mM Tris-HCl–2.5 mM MgCl2–0.2 mM each dNTP–1 U of AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, Calif.)–0.45 μM human MCP-1 primers. As internal controls for RT-PCR, 2 μl of cDNA products was also amplified in separate reactions containing β-actin primers. The concentrations of PCR reagents and primers for β-actin were the same as for MCP-1. Primers were purchased from R&D Systems, Minneapolis, Minn. The sequences of the forward and reverse primers for MCP-1 were 5′-CAGCCAGATGCAATCAATGC-3′ and 5′-GTGGTCCATGGAATCCTGAA-3′, respectively. The sequences of the forward and reverse primers for β-actin were 5′-CTACAATGAGCTGCGTGTGG-3′ and 5′-AAGGAAGGCTGGAAGAGTGC-3′, respectively. The parameters for PCR were as follows: 94°C for 10 min and 25 cycles of 30 s of denaturation at 94°C, 22 s of annealing at 70°C, and 30 s of extension at 72°C. RT-PCR product sizes for MCP-1 and β-actin were 198 and 528 bp, respectively. To control for the presence of PCR inhibitors, cDNA samples were also coamplified with MCP-1 or β-actin PCR-positive control templates, which generated products of 320 and 528 bp, respectively (R&D Systems). The concentration of template added in each PCR reaction was 5.0 pg/μl.
Densitometric analysis of MCP-1 and β-actin RT-PCR products.
Densitometry of MCP-1 and β-actin RT-PCR products was performed with the AlphaImager 2000 software. Densitometric values of β-actin bands were used to standardize the results. Increases in MCP-1 mRNA levels in experimental groups were expressed as the ratio of MCP-1 to β-actin RT-PCR products. The numerical data were subjected to analysis of variance followed by the Tukey-Kramer multiple-comparison test. A P value of <0.05 was used to determine statistical significance for all analyses.
Nuclear protein extraction.
Nuclear protein extracts were obtained by a modification of the procedures described by Dignam et al. (6). Briefly, HUVEC cultured in six-well plates (total of 3 × 106 cells for each experimental condition) were washed with ice-cold phosphate-buffered saline (PBS), removed by gentle scraping, and centrifuged at 1,200 rpm for 10 min at 4°C. Cell pellets were resuspended in 400 μl of ice-cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride) and allowed to swell on ice for 15 min. Cells were then lysed by addition of 25 μl of 10% Nonidet P-40 with vigorous vortexing for 10 s. Nuclear pellets were collected by microcentrifugation at 14,000 rpm for 30 s at 4°C. Supernatants were removed, and nuclear pellets were resuspended in 50 μl of ice-cold buffer C (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride) for 20 min on ice with periodic mixing. Nuclear extracts were centrifuged at 14,500 rpm for 5 min to pellet insoluble material. Supernatants containing nuclear proteins were collected and frozen at −80°C. Protein concentrations were 1 to 2 pg/μl as determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.).
EMSA.
For the electrophoretic mobility shift assay (EMSA), a double-stranded oligonucleotide containing the NF-κB consensus sequence (underlined) (5′-GTGAGGGGACTTTCCCAGGC-3′; Promega Corp.) was end labeled with [γ-32P]ATP (3,000 Ci/mmol at 10 mCi/ml; Amersham Corp., Arlington Heights, Ill.) as described previously (26). Binding reactions were performed at room temperature for 30 min in 15 μl of a mixture containing 5 μg of nuclear protein and 35 fmol (∼50,000 cpm) of oligonucleotide in binding buffer [4% glycerol, 1 mM MgCl2, 0.5 mM EDTA (pH 8.0), 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris (pH 7.6), and 50 μg of poly(dI-dC) (Pharmacia, Piscataway, N.J.) per ml] (26). Antibodies to NF-κB p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), p68 (RelB), and p75 (c-Rel) (Santa Cruz Biotechnology, Santa Cruz, Calif.) were used for supershift assays. Reaction products were separated in 4% nondenaturing polyacrylamide gels and analyzed by autoradiography.
Immunofluorescent staining for NF-κB.
HUVEC grown on gelatin-coated glass coverslips were infected by centrifugation with C. pneumoniae A-03 or C. trachomatis L2 at an MOI of 1:1. After 30 min of infection, cells were fixed for 5 min with methanol at −20°C. To suppress nonspecific binding of immunoglobulin G (IgG), cells were initially incubated for 20 min with 1% bovine serum albumin (BSA) at room temperature. Incubation with rabbit polyclonal IgG antibody against the p65 component of NF-κB (2 μg/ml in PBS with 1% BSA) was carried out for 1 h at room temperature. Monolayers were then washed three times with PBS for 5 min each. This was followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (5 μg/ml in PBS with 1% BSA) for 45 min. Antibodies were purchased from Santa Cruz Biotechnology. Coverslips with stained cells were mounted in 80% glycerol in PBS and examined by confocal microscopy under an ×1,000 objective.
Treatment of C. pneumoniae-infected HUVEC with the NF-κB inhibitor parthenolide.
The effects of NF-κB inhibition on MCP-1 mRNA production were examined by treatment of infected HUVEC with parthenolide, a compound that has been shown to suppress NF-κB activation (16). Prior to infection, HUVEC grown on 24-well plates were preincubated for 1 h with 50 μM of parthenolide (Biomol, Plymouth Meeting, Pa.). Cells were then infected with C. pneumoniae A-03 in medium containing 50 μM parthenolide for 1 h, and expression of MCP-1 mRNA was examined by RT-PCR as described above. In separate experiments, HUVEC grown on six-well plates were preincubated with equivalent concentrations of parthenolide for 1 h, infected with C. pneumoniae A-03, and incubated for an additional 30 min in the presence of the inhibitor prior to examination of NF-κB by EMSA.
RESULTS
Expression of MCP-1 mRNA in HUVEC infected with C. pneumoniae or C. trachomatis.
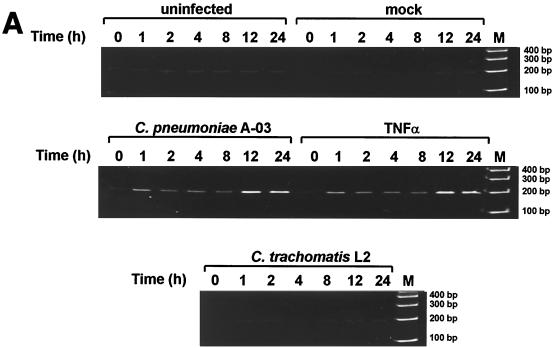
The kinetics of MCP-1 mRNA expression were examined by RT-PCR in HUVEC infected with C. pneumoniae or C. trachomatis. As shown in Fig. 1A, expression of MCP-1 mRNA in C. pneumoniae-infected cells was increased as early as 1 h postinfection compared to uninfected and mock-infected controls (198-bp RT-PCR product). MCP-1 gene expression remained elevated between 2 and 8 h of infection and peaked at 12 h postinfection. In contrast, infection of HUVEC with C. trachomatis L2 did not induce MCP-1 mRNA expression at any time point. Induction of MCP-1 gene expression by C. pneumoniae A-03 was comparable to that by 500 U of TNF-α per ml. Expression of β-actin mRNA, as an internal control for RT-PCR analyses, did not differ significantly among experimental groups throughout the 24-h period of incubation (data not shown).
FIG. 1.
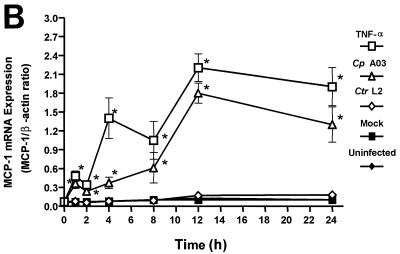
Expression of MCP-1 mRNA in HUVEC infected with C. pneumoniae or C. trachomatis. (A) RNA was isolated at 0, 1, 2, 4, 8, 12, and 24 h of incubation, and levels of MCP-1 mRNA were determined by RT-PCR as described in Materials and Methods. (Top panel) HUVEC were incubated with medium alone (uninfected) or crude lysates of HEp-2 cells (mock infected). (Middle panel) HUVEC were infected with C. pneumoniae A-03 at an MOI of 1:1 or treated with 500 U of TNF-α per ml as a positive control. (Bottom panel) Infection with C. trachomatis L2. The inoculum was equivalent to that of C. pneumoniae A-03. M, molecular size markers. (B) Levels of MCP-1 mRNA following infection of HUVEC with C. pneumoniae (Cp) or C. trachomatis (Ctr) were measured by plotting the densitometric MCP-1/β-actin RT-PCR product ratios. Data points represent the means ± standard errors of the mean of five separate experiments. ∗, P < 0.01.
Densitometric analysis of the bands representing MCP-1 RT-PCR products was performed as described in Materials and Methods to quantify increases in MCP-1 mRNA levels as a result of C. pneumoniae infection. Densitometry of β-actin RT-PCR products was performed to standardize the results, and levels of MCP-1 mRNA were expressed as MCP-1/β-actin ratios. The numerical values from this analysis were collected from five separate experiments and subjected to statistical analysis and are displayed in graphic form in Fig. 1B. Significant increases in MCP-1 mRNA expression were observed throughout the 24-h period of infection with C. pneumoniae A-03. Compared to mock-infected controls, these increases ranged from 5-fold at 1 h to 18-fold at 12 h postinfection. (P < 0.01). In contrast, levels of MCP-1 mRNA in response to C. trachomatis L2 did not increase above those in mock-infected controls.
Activation of NF-κB in HUVEC infected with C. pneumoniae or C. trachomatis.
To determine if differences in MCP-1 mRNA synthesis between C. pneumoniae and C. trachomatis were related to activation of the transcription factor NF-κB, infected HUVEC were examined by EMSA and immunofluorescence staining following 30 min of incubation. As shown in Fig. 2, infection of HUVEC with C. pneumoniae A-03 resulted in an increase in the nuclear translocation of NF-κB compared to mock-infected cells. In contrast, C. trachomatis L2 did not induce NF-κB activation above that in mock-infected cells. TNF-α-induced NF-κB activation served as a positive control.
FIG. 2.
Translocation of NF-κB determined by EMSA in HUVEC infected with C. pneumoniae or C. trachomatis. Mock-infected HUVEC were incubated with crude lysates of HEp-2 cells. Infection with C. pneumoniae A-03 or C. trachomatis L2 was performed at an MOI of 1:1. Treatment of HUVEC with 500 U of TNF-α per ml served as a positive control. Nuclear extracts were prepared after 30 min of incubation. EMSAs were performed with a 32P-labeled oligonucleotide containing the NF-κB consensus sequence (see Materials and Methods). C. pneumoniae A-03 and TNF-α caused an increase in NF-κB DNA-binding activities of nuclear protein complexes compared to the levels in mock-infected cells and C. trachomatis L2-infected cells.
The components of the NF-κB complex in C. pneumoniae A-03-infected HUVEC were identified by supershift assays with antibodies to the p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), p65 (RelB), and p75 (c-Rel) members of the NF-κB family (Fig. 3). Supershifts of NF-κB complexes occurred only with the addition of antibodies to p50 or p65 (open and solid arrows, respectively). These results demonstrate that NF-κB complexes from HUVEC infected with C. pneumoniae A-03 are composed of p50-p65 heterodimers.
FIG. 3.
Supershift analysis of NF-κB-binding complexes. DNA-binding reactions with nuclear extracts from HUVEC infected with C. pneumoniae A-03 were incubated with 32P-labeled NF-κB oligonucleotide in the presence of antibodies (Ab) to NF-κB proteins p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), p68 (RelB), and p75 (c-Rel). The solid arrowhead indicates the NF-κB band. Supershifts of p50 and p65 are indicated by the open and solid arrows, respectively.
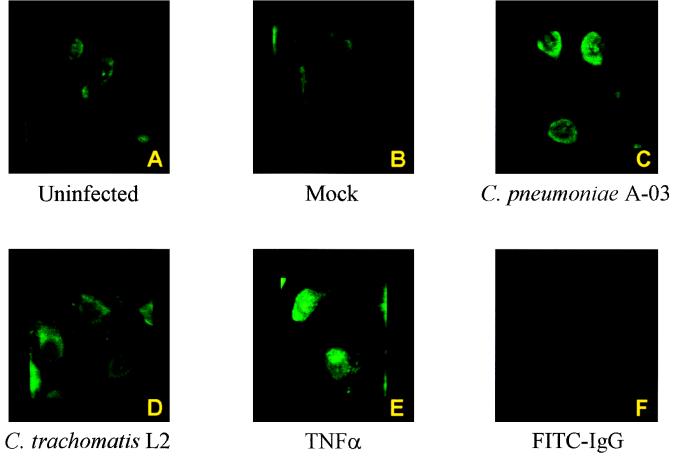
The p65 component of NF-κB has been shown to be the principal regulator of transcriptional activation (41). Since p65 was one of the primary components identified in the NF-κB complex activated by C. pneumoniae, the subcellular localization of p65 in infected HUVEC was analyzed using immunofluorescence staining and confocal microscopy. As shown in Fig. 4, uninfected and mock-infected cells (Fig. 4A and B, respectively) displayed only cytoplasmic staining for NF-κB p65. In contrast, nuclear staining for NF-κB was observed in HUVEC infected with C. pneumoniae A-03 (Fig. 4C). Similar to uninfected and mock-infected cells, no nuclear NF-κB was detected in cells infected with C. trachomatis L2. HUVEC treated with 500 U of TNF-α per ml as a positive control displayed strong nuclear staining for NF-κB p65 (Fig. 4E). Figure 4F represents a control in which fixed monolayers of C. pneumoniae A-03-infected HUVEC were incubated only with the FITC-conjugated anti-rabbit IgG. As expected, no staining was observed in these cells.
FIG. 4.
Immunofluorescent staining of NF-κB p65 in HUVEC infected with C. pneumoniae or C. trachomatis. HUVEC grown on gelatin-coated glass coverslips were infected with C. pneumoniae A-03 or C. trachomatis L2 at an MOI of 1:1. After 30 min of infection, cells were processed for immunofluorescent staining as described in Materials and Methods with rabbit polyclonal antibody against the p65 component of NF-κB. Stained cells were immediately examined by confocal microscopy (×1,000). Absence of nuclear NF-κB p65 is observed in uninfected, mock-infected, and C. trachomatis L2-infected cells (panels A, B, and D, respectively). Nuclear localization of NF-κB is observed in HUVEC infected with C. pneumoniae A-03 (panel C) and to a greater extent in cells treated with 500 U of TNF-α per ml for 30 min as a positive control (panel E). Panel F represents a control to rule out possible nonspecific binding of the FITC-conjugated anti-rabbit IgG in C. pneumoniae A-03-infected HUVEC.
Inhibition of NF-κB prevents C. pneumoniae-induced MCP-1 gene expression in HUVEC.
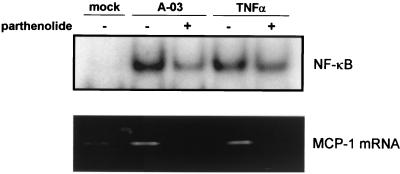
The relationship of NF-κB activation to induction of MCP-1 mRNA expression in C. pneumoniae-infected HUVEC was investigated further with parthenolide, a specific inhibitor of NF-κB that prevents the inducible degradation of IκBα and IκBβ proteins (16). As shown in Fig. 5, C. pneumoniae-induced activation of NF-κB at 30 min postinfection was markedly decreased by incubation of cells with 50 μM parthenolide (top panel). Treatment with parthenolide resulted in a similar reduction in TNF-α-induced NF-κB activation. The effects of parthenolide on MCP-1 gene expression in HUVEC infected with C. pneumoniae are also depicted in Fig. 5 (bottom panel). Expression of MCP-1 mRNA induced by C. pneumoniae A-03 at 1 h postinfection was decreased to mock-infected levels with 50 μM parthenolide. These data suggest that NF-κB activation is required for induction of MCP-1 gene expression by C. pneumoniae in HUVEC.
FIG. 5.
Effects of parthenolide on NF-κB activation and MCP-1 gene expression in HUVEC infected with C. pneumoniae. (Top panel) Prior to infection, HUVEC grown on six-well plates were preincubated with 50 μM parthenolide for 1 h. Cells were then infected with C. pneumoniae A-03 at an MOI of 1:1 or treated with 500 U of TNF-α per ml in medium containing 0 or 50 μM parthenolide for 30 min before nuclear protein extraction. NF-κB activation was examined by EMSA. (Bottom panel) HUVEC grown on 24-well plates were preincubated with 50 μM parthenolide for 1 h. Cells were then infected with C. pneumoniae A-03 at an MOI of 1:1 or treated with 500 U of TNF-α per ml in medium containing 0 or 50 μM parthenolide for 1 h before total RNA isolation. Expression of MCP-1 mRNA was examined by RT-PCR.
DISCUSSION
The present study demonstrates that infection of human endothelial cells with C. pneumoniae results in rapid induction of MCP-1 mRNA expression, an event that appears to be dependent on early activation of NF-κB. Stimulation of MCP-1 gene expression occurred in a time-dependent fashion, with increased levels of MCP-1 mRNA observed as early as 1 h postinfection. C. pneumoniae stimulated translocation of NF-κB in infected HUVEC at 30 min of incubation, as determined by EMSA as well as immunofluorescent staining of NF-κB p65. Supershift analyses revealed that the components of the NF-κB complex in C. pneumoniae-infected HUVEC were p50-p65 heterodimers. These results confirm those from a recent study in which p50-p65 heterodimers were identified as the active NF-κB components present in smooth muscle cells and endothelial cells infected with C. pneumoniae (5). In our study, a functional role for NF-κB in induction of MCP-1 gene expression by C. pneumoniae was demonstrated by experiments using parthenolide, a compound that has been shown to prevent the inducible degradation of IκBα and IκBβ proteins (16). Treatment with parthenolide suppressed C. pneumoniae-induced NF-κB activation and completely abrogated MCP-1 mRNA expression in infected HUVEC.
As opposed to C. pneumoniae A-03, C. trachomatis L2 did not stimulate MCP-1 mRNA synthesis in infected HUVEC, which was consistent with an inability of this species to activate NF-κB. These results confirm our earlier findings of a failure of C. trachomatis L2 to stimulate MCP-1 protein production despite significant replication of this organism in HUVEC (32). At the MOIs used in the present study, we have found that average C. trachomatis L2 growth titers in infected HUVEC are 100-fold higher than those of C. pneumoniae, indicating that the differences in endothelial cell activation between the two species are not related to poor replication by C. trachomatis L2. In addition, possible discrepancies in the total numbers of chlamydial particles (i.e., infectious and noninfectious) between C. pneumoniae and C. trachomatis inocula were ruled out by measurements of similar amounts of LPS, as determined by levels of endotoxin and 2-keto-3-deoxyoctonate.
The basis for the differences observed between chlamydial species in the NF-κB-mediated activation of MCP-1 in HUVEC reported herein remains unclear. A possible scenario is that C. pneumoniae may possess specific features required for attachment and uptake in endothelial cells that trigger distinct signal transduction components linked to the activation of NF-κB. A recent study showed that C. pneumoniae but not C. trachomatis significantly exacerbated atherosclerosis in low-density lipoprotein receptor-deficient mice fed a high-cholesterol diet, suggesting that C. pneumoniae may possess a unique atherogenic property (18). Since NF-κB is considered a major regulator of endothelial dysfunction during atherogenesis (4), it is possible that the C. pneumoniae-dependent activation of NF-κB plays an important role in the development of atherosclerosis. It should be mentioned, however, that the differences between these two Chlamydia species may also be cell specific, since previous work has shown that C. trachomatis L2 activates NF-κB in epithelial cells (36).
Recent experiments performed in vitro with C. pneumoniae have been compatible with the pathological characteristics of atherosclerosis in humans (1, 8–10, 17, 19, 29). In addition, cell culture studies have provided a better understanding of the mechanisms involved in the generation of atherosclerotic lesions described in animal models. Initial reports documenting the susceptibility of endothelial cells, aortic smooth muscle cells, and macrophages to productive infection with C. pneumoniae suggested the ability of this organism to localize and survive within the arterial wall (9, 10). Transfer of C. pneumoniae infection from mononuclear phagocytes to endothelial cells by cell-to-cell spread suggests that dissemination from the respiratory tract to the arteries could occur via circulating monocytes (8). Infection of monocyte-derived macrophages with this organism in the presence of low-density lipoprotein leads to foam cell formation and accumulation of cholesteryl esters (19). In addition, macrophages exposed to C. pneumoniae increase the production of proinflammatory cytokines such as TNF-α and interleukin-1 (17), which may contribute to endothelial cell activation and tissue damage. Recently, data have also shown that C. pneumoniae infection induces a procoagulant phenotype in smooth muscle cells and endothelial cells by increasing the expression of tissue factor and plasminogen activator inhibitor 1 (5). Lastly, the development of a persistent state in cell culture with noninfectious but viable C. pneumoniae (1, 29) correlates with the ability of this organism to cause a chronic infection within arterial tissues, an event which may be critical for the maintenance of a long-term inflammatory response.
Elucidation of the bacterial and cellular components involved in the activation of MCP-1 by C. pneumoniae in endothelial cells requires further investigations. Previous data demonstrated a role for a heat-labile component of C. pneumoniae, since UV-inactivated but not heat-inactivated organisms retained the ability to stimulate MCP-1 secretion from HUVEC (31, 32). These results also suggested that chlamydial LPS may not be required for this response. Possible candidates involved in attachment of C. pneumoniae to endothelial cells may include surface-exposed outer membrane proteins such as Omp4 and Omp5 (21). A recent report has shown that tyrosine phosphorylation of endothelial cell proteins occurs within 5 min of C. pneumoniae infection, suggesting that bacterial attachment may be sufficient to trigger an endothelial inflammatory response (24). In addition, C. pneumoniae infection caused rapid phosphorylation of mitogen-activated protein kinase, which could be an important upstream mediator of the NF-κB signaling cascade (24, 28). Maintenance of a chronic inflammatory response may involve ongoing activation of endothelial cells by C. pneumoniae antigens. Of interest in the immunopathogenesis of chlamydial infections is heat shock protein 60 (Hsp-60), a delayed-type hypersensitivity antigen implicated in the chronic inflammatory response of trachoma (33). Recent studies have shown that chlamydial Hsp-60 localizes in human atheromas and triggers activation of NF-κB in human endothelial cells, smooth muscle cells, and macrophages in vitro, supporting a contribution of this antigen to the pathogenesis of atherosclerosis (22, 23).
In summary, despite the numerous reports implicating C. pneumoniae in atherosclerosis, a causal role remains to be established. Our data suggest that activation of NF-κB and the resulting expression of MCP-1 by C. pneumoniae in human endothelial cells may participate in monocyte-macrophage recruitment during the early stages of atherogenesis. These events may also contribute to the exacerbation of a previously established atherosclerotic process. The differences observed between Chlamydia species, documented in this report, suggest that C. pneumoniae may possess specific features that function as triggering mechanisms in the activation of inflammatory mediators involved in atherosclerosis. Analysis of the signaling pathways involved in NF-κB activation of MCP-1 by C. pneumoniae may help identify potentially unique intracellular mechanisms utilized by this organism in human endothelial cells. The identification of NF-κB as a critical transcription factor in C. pneumoniae-induced MCP-1 gene expression may provide insights into specific therapeutic strategies for the treatment of diseases associated with this organism, including atherosclerosis.
ACKNOWLEDGMENTS
We thank Jon B. Klein, Director, Core Confocal Microscopy Laboratory, University of Louisville, and Pat Y. Coxon, Division of Nephrology, University of Louisville, for her assistance in acquiring the confocal microscope images.
REFERENCES
- 1.Airenne S, Surcel H-M, Alakärppä H, Laitinen K, Paavonen J, Saikku P, Laurila A. Chlamydia pneumoniae infection in human monocytes. Infect Immun. 1999;67:1445–1449. doi: 10.1128/iai.67.3.1445-1449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemotactic and inflammatory cytokines—CXC and CC proteins. Adv Exp Med Biol. 1993;353:1–10. doi: 10.1007/978-1-4615-2952-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Campbell L A, Kuo C-C, Grayston J T. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–579. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins T. Endothelial nuclear factor-κB and the initiation of the atherosclerotic lesion. Lab Investig. 1993;68:499–508. [PubMed] [Google Scholar]
- 5.Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba D C. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-κB and induces tissue factor and PAI-1 expression. Circulation. 1999;100:1369–1373. doi: 10.1161/01.cir.100.13.1369. [DOI] [PubMed] [Google Scholar]
- 6.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1488. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. Infect Immun. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaydos C A, Pham D G, Quinn T C. Chlamydia pneumoniae infections in macrophages and coronary artery endothelial cells. In: Stary A, editor. Proceedings of the Third Meeting of the European Society for Chlamydia Research. Bologna, Italy: Società Editrice Esculapio; 1996. p. 223. [Google Scholar]
- 9.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godzik K L, Obrien E R, Wang S K, Kuo C-C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot C H. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Investig. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayston J T. Chlamydia pneumoniae strain TWAR pneumonia. Annu Rev Med. 1992;43:317–323. doi: 10.1146/annurev.me.43.020192.001533. [DOI] [PubMed] [Google Scholar]
- 13.Grayston J T, Aldous M B, Easton A, Wang S-P, Kuo C-C, Campbell L A, Altman J. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J Infect Dis. 1993;168:1231–1235. doi: 10.1093/infdis/168.5.1231. [DOI] [PubMed] [Google Scholar]
- 14.Gu L, Okada Y, Clinton S K, Gerard C, Sukhova G K. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschlag M R, Chirgwin K, Roblin P M, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–182. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 16.Hehner S P, Heinrich M, Bork P M, Vogt M, Ratter F. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκB-β. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann M, Susa M, Simnacher V, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H, Pierce G N, Zhong G. The atherogenic effects of Chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Investig. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalayoglu M V, Byrne G I. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–729. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 20.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen K, Madsen A S, Mygind P, Christiansen G, Birkelund S. Identification of two novel genes encoding 97- to 99-kilodalton outer membrane proteins of Chlamydia pneumoniae. Infect Immun. 1999;67:375–383. doi: 10.1128/iai.67.1.375-383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-α and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 23.Kol A, Bourcier T, Lichtman A H, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Investig. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krüll M, Klucken A C, Wuppermann F N, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hegemann J H, Jantos C A, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162:4834–4841. [PubMed] [Google Scholar]
- 25.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentsch A B, Shanley T P, Sarma V, Ward P A. In vivo suppression of NF-κB and preservation of IκBα by interleukin-10 and interleukin-13. J Clin Investig. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin T, Cardarelli P M, Parry G C N, Felts K A, Cobb R R. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-κB and AP-1. Eur J Immunol. 1997;27:1091–1097. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 28.May M J, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S J, Miller R D, Ramirez J A, Summersgill J T. Inhibition of Chlamydia pneumoniae replication in HEp-2 cells by interferon-γ: role of tryptophan catabolism. J Infect Dis. 1998;177:1326–1331. doi: 10.1086/515287. [DOI] [PubMed] [Google Scholar]
- 30.Melnick S L, Shahar E, Folsom A R, Grayston J T, Sorlie P D, Wang S-P, Szklo M. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 31.Molestina R E, Dean D, Miller R D, Ramirez J A, Summersgill J T. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect Immun. 1998;66:1370–1376. doi: 10.1128/iai.66.4.1370-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molestina R E, Miller R D, Ramirez J A, Summersgill J T. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry G C N, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain κB-like sites that specifically bind c-Rel-p65 heterodimers. J Biol Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- 35.Ramirez J A the Chlamydia pneumoniae/Atherosclerosis Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen S J, Sen C K, Stephens R S. Temporal expression of interleukin-8 in Chlamydia-infected epithelial cells. In: Stephens R S, Byrne G I, Christiansen G, Clarke I N, Grayston J T, Rank R G, Ridgway G L, Saikku P, Schachter J, Stamm W E, editors. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. Berkeley, Calif: University of California Printing Services; 1998. pp. 411–414. [Google Scholar]
- 37.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 38.Saikku P, Mattila K, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 39.Saikku P, Leinonen M, Tenkanen L, Ekman M R, Linnanmäki E, Manninen V, Mänttäri M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 40.Saikku P. Chlamydia pneumoniae and atherosclerosis—an update. Scand J Infect Dis Suppl. 1997;104:53–56. [PubMed] [Google Scholar]
- 41.Schmitz M L, Baeuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shyy Y-J, Li Y-S, Kolattukudy P E. Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem Biophys Res Commun. 1993;192:693–699. doi: 10.1006/bbrc.1993.1470. [DOI] [PubMed] [Google Scholar]
- 43.Taylor-Robinson D, Thomas B J. Chlamydia pneumoniae in arteries: the facts, their interpretation, and future studies. J Clin Pathol. 1998;51:793–797. doi: 10.1136/jcp.51.11.793. [DOI] [PMC free article] [PubMed] [Google Scholar]