Abstract
Vaccination has been proposed for the prevention of disease due to enterohemorrhagic Escherichia coli (EHEC), but the immune response following human infection, including the choice of potential antigens, has not been well characterized. To study this, sera were obtained from five pediatric patients with acute diarrhea caused by E. coli O157:H7 0, 8, and 60 days after hospitalization. These sera were used to examine the immune response to four different EHEC virulence factors: Tir (translocated intimin receptor, which is inserted into the host cell membrane), intimin (bacterial outer membrane protein which binds to Tir), EspA (secreted protein which forms filamentous structures on EHEC surface), and EspB (inserted into the host membrane and cytoplasm). The response to O157:H7 lipopolysaccharide was also examined. Sera were assayed against purified recombinant proteins using immunoblot analysis and by enzyme-linked immunosorbent assay to determine the sera's titers to each of the antigens in all patients. We found that there was little reaction to EspA, EspB, and intimin in the acute-phase sera, although there was some reactivity to Tir. By day 8, titers of antibody to all four virulence factors were present in all patients, with a very strong response against Tir (up to a titer of 1:256,000), especially in hemolytic-uremic syndrome patients, and lesser strong responses to the other three antigens. The titer to the antigens 60 days after hospitalization was decreased but was still highest for Tir. These results suggest that there is a strong immune response to Tir, and to a lesser extent to the other three virulence factors, following EHEC disease, indicating that these bacterial molecules are potential vaccine candidates for preventing EHEC disease. They also suggest that bacterial virulence factors that are inserted into host cells during infection by type III secretion systems (Tir or EspB) are still recognized by the host immune response.
Enterohemorrhagic Escherichia coli (EHEC) (also called verotoxigenic E. coli) is an important cause of diarrhea. This organism also produces a toxin which plays a role in disease (verotoxin or Shiga-like toxin [SLT]). Most patients with EHEC infection recover uneventfully within a few days, but in about 8% of cases (23) the diarrhea is followed by hemolytic-uremic syndrome (HUS), a life-threatening complication with a substantial morbidity in survivors (19, 25). HUS appears to be caused by the interaction of SLT with endothelial cells. Worldwide, many serotypes of EHEC have been described, but in North America one type predominates, E. coli O157:H7 (11, 12). Epidemiological evidence shows that EHEC O157:H7 strains are present in the feces of healthy cattle, providing indirect evidence that EHEC O157:H7 can colonize the bovine intestine without causing disease.
Because of the potential seriousness of EHEC infection (4), vaccination has been proposed, either to prevent the disease in humans or to reduce colonization in cattle. However, the immunological response to EHEC infection has not been well characterized. It is known that patients infected with E. coli O157:H7 and other serotypes of EHEC develop an antibody response to O lipopolysaccharide (O LPS), and there is evidence that antibodies to O157 may prevent colonization (3, 6, 20). There are, however, many serotypes of EHEC that cause HUS, and a monovalent LPS vaccine would not cover them all. The extent to which there is an immunologic response to SLT is controversial (16).
EHEC produces several recently described virulence determinants, which enables it to colonize the large bowel and cause disease (7, 9). Several of its virulence factors are secreted by a type III secretion system which delivers virulence factors directly into host cells (13). These factors include EspA, which forms filamentous structures on the bacterial surface bridging to the host cells' surface (18). These structures may deliver other virulence factors directly into the host cell from EHEC. EspB is delivered primarily into the host cell membrane, where it becomes an integral membrane protein (26). EspB, along with EspD, probably forms a pore structure through which other bacterial effectors, such as Tir, gain access to the host cell. Additionally, a small fraction of EspB is delivered into the host cytosol. Tir is a bacterial molecule that uses the type III secretion system, EspA, EspB, and EspD for delivery into the host cell membrane. Once translocated into the host cell, Tir then functions as the receptor for intimin, which is an integral outer membrane protein of EPEC and EHEC (17). Tir-intimin binding attaches EHEC to the intestinal cell surface and triggers actin cytoskeletal rearrangements beneath adherent EHEC, resulting in pedestal formation.
There is no good animal model for EHEC pathogenesis, and thus much of our knowledge about EHEC pathogenesis and virulence comes from studies with related pathogens that cause pedestal formation and diarrhea using homologous proteins. Enteropathogenic E. coli (EPEC) adheres to the small bowel but causes morphological changes on the intestinal surface similar to those caused by EHEC (7), in addition to causing severe neonatal diarrhea in children. Using EPEC, it has been shown that intimin and EspB are key virulence factors in human volunteers (8). Additionally, using rabbits infected with rabbit EPEC O103, it has been shown that EspA and EspB are essential virulence factors needed for pedestal formation and infantile rabbit diarrhea (1), while another group has recently shown that Tir is a virulence factor in the rabbit infection model (21). Finally, using Citrobacter rodentium, it has recently been shown that EspB and Tir are virulence factors in this related infection model (unpublished data). Collectively, these studies indicate that pedestal formation, mediated by intimin, EspA, EspB, and Tir, is an essential process for disease and that these four molecules are essential virulence factors.
Given the key roles that EspA, EspB, Tir, and intimin play in EHEC adherence, pedestal formation, and presumably virulence and their different locations—either on the bacterial surface (EspA and intimin) or inserted into the host cell (EspB and Tir)—we examined whether EHEC-infected patients raise an immune response to these virulence factors.
MATERIALS AND METHODS
Sera.
Sera were obtained from five patients with acute diarrhea due to E. coli O157:H7, two of whom developed HUS (Table 1). Acute-phase sera were taken on the patients' initial presentation to the emergency department, and convalescent-phase sera were obtained 8 and 60 days after the acute-phase specimen. The timing of these specimens in relation to the onset of illness is shown in Table 1. Twenty-five sera from patients between 1 and 16 years of age, without a history of recent diarrhea, were used as controls to determine the breakpoint of the O157 antibody tests. Three random sera were selected from these for controls in the Tir, EspA, EspB, and intimin antibody assays.
TABLE 1.
Antibody response of patient sera to O157 LPS antigen
| Patient | Serum 1
|
Serum 2
|
Serum 3
|
HUS | Patient age (mo)e | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daya | ELISA ford:
|
HAb | Day | ELISA for:
|
HA | Day | ELISA for:
|
HA | ||||||
| IgG | IgM | IgG | IgM | IgG | IgM | |||||||||
| 95-8160 | 2 | − | − | Neg | 10 | − | − | 1,000 | 62 | − | − | Neg | No | 57 |
| 96-9101 | 4 | − | − | Neg | 12 | + | + | 16,000 | 64 | + | + | 1,000 | Noc | 120 |
| 97-8005 | 4 | − | − | Neg | 12 | + | + | 4,000 | 64 | + | − | Neg | No | 120 |
| 95-8050 | 2 | − | − | Neg | 10 | + | + | 4,000 | 62 | + | − | Neg | Yes | 47 |
| 95-8001 | 3 | + | + | 4,000 | 11 | + | + | 16,000 | 63 | + | + | 4,000 | Yes | 46 |
Days after onset of diarrhea that specimen was taken.
Hemagglutination inhibition expressed as reciprocal titer. Neg, no hemagglutination inhibition observed.
Fragmentation hemolysis without anemia.
−, negative; +, positive.
Age at admission to hospital.
Purification of EHEC secreted proteins.
Wild-type EHEC O157:H7 or a type III secretion mutant (sepB) which does not secrete Tir, EspA, or EspB (14) was grown in Luria-Bertani (LB) medium overnight. The culture was then diluted 1:10 into M-9 minimal medium supplemented with 44 mM NaHCO2, 8 mM MgSO4, 0.4% glucose, and 0.1% Casamino Acids, culture conditions which optimize type III secretion. Cultures were grown standing at 37°C in 5% CO2 to an optical density at 600 nm of 0.7 to 0.8. The growth supernatant was concentrated 100-fold by ultrafiltration (Amicon 30 device); the total protein concentration was verified by the bicinchoninic acid protein assay method.
Purification of recombinant EHEC proteins.
A clinical isolate of E. coli serotype O157:H7 was used as the source of DNA. espA, espB, tir, and the region of eae encoding the 280 carboxyl-terminal amino acids of intimin were amplified from chromosomal DNA using PCR to introduce unique restriction sites, followed by cloning into pCR2.1 TOPO (Invitrogen). The resulting plasmids were cleaved with BamHI/SalI (espA), BamHI/XhoI (expB), or XhoI (tir) and then ligated into pGEX-6P-1 (Pharmacia) to create glutathione S-transferase (GST) fusions. Plasmids were then electroporated into the expression strain E. coli BL21 (Pharmacia) and grown in LB medium containing ampicillin (10 μg/ml) overnight. This culture was diluted 1:10 into fresh LB-ampicillin medium and grown for an additional 1 h at 37°C, with fusion protein expression being induced by adding 100 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to a concentration of 0.3 mM and continuing incubation about 4 h. Bacteria were pelleted (15 min at 6,100 × g), and the pellet was resuspended in Tris-buffered saline (TBS) prior to sonication to lyse bacteria. The supernatant was centrifuged (10,000 × g for 15 min at 4°C) to remove intact cells and insoluble material. The supernatant was then mixed with a 50% slurry of glutathione-agarose beads (Sigma) for 30 min at 4°C. Recombinant proteins were eluted by adding 50 mM Tris-HCl (pH 8.0)–10 mM reduced glutathione. The purity of the proteins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining.
Immunoblot analysis.
Supernatants containing the EHEC secreted proteins (EspA, EspB, and Tir) or the supernatant from the sepB type III secretion mutant and purified EHEC recombinant GST fusion proteins were resolved by PAGE (10% polyacrylamide). Proteins were transferred to nitrocellulose, and immunoblots were blocked in 5% nonfat dried milk in TBS, pH 7.2, containing 0.1% Tween 20 (TBS-T) overnight at 4°C and then incubated with patient antisera (1:10,000 dilution) for 2 h. Membranes were washed three times with 0.5% nonfat dried milk in TBS-T and incubated with a 1:10,000 dilution of horseradish peroxidase-conjugated rabbit anti-human immunoglobulin G. Antigen-antibody complexes were visualized with the enhanced chemiluminescence detection kit (Amersham). Control immunoblots were performed with sera obtained from patients admitted for non-E. coli disease. All of these blots showed no detectable reactivity to the EHEC secreted proteins.
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were used to determine the titers of antibodies in patient sera. One hundred microliters of EHEC GST fusion proteins of EspA (0.16 μg/well), EspB (0.24 μg/well), Tir (0.1 μg/well), and intimin (0.187 μg/well) was used to coat individual wells in microtiter plates and incubated overnight at 4°C. Wells were washed three times and blocked with 0.5% nonfat dried milk in phosphate-buffered saline. Serial dilutions of sera were then added to each well and incubated for 2 h at 37°C, washed, and blocked as described above. Peroxidase-conjugated rabbit anti-human immunoglobulin G antibodies were diluted 1:5,000 in 0.5% milk in phosphate-buffered saline, and 100 μl was added to each well. After 1 h of incubation at 37°C, wells were washed three times, followed by three washes with distilled water. ELISAs were read at a wavelength of 492 nm. Control sera from patients admitted for non-E. coli disease had titers of <1/50, which is similar to background readings.
O157 antibody tests.
Hemagglutination inhibition (2) and enzyme immunoassay (3, 10) tests for antibody to O157 LPS were performed as previously described.
RESULTS
Immunoblot analysis of patients' sera to EHEC supernatant.
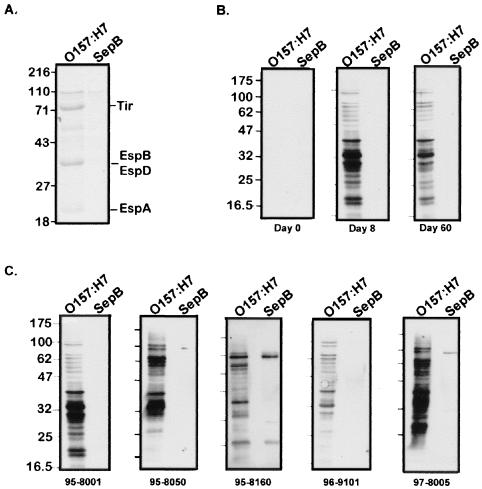
To determine if the patients' sera contained antibodies to EHEC secreted proteins, immunoblots were performed with EHEC supernatant probed with sera taken from the five patients at days 0, 8, and 60 following hospitalization (Fig. 1B). As a control, EHEC supernatant proteins collected from a type III secretion mutant (sepB) which does not secrete Tir, EspA, or EspB was used (Fig. 1A). There was little reaction to either supernatant with the serum obtained at day 0 (patient 95-8001). However, by day 8 a strong reaction to several supernatant proteins from EHEC, but not from the type III mutant, was seen. The response at day 60 was similar but dampened. Similar results were seen with all five patients' sera at day 0, day 8 (Fig. 1C), and day 60. In the sera of three patients (patients 95-8050, 95-8160, and 97-8005), there was also reactivity against an approximately 100-kDa supernatant protein whose secretion was independent of the type III system. This protein is likely EspP, which is the other predominant secreted protein of EHEC (Fig. 1A) that comigrates with this band and uses an autoproteolytic mechanism for secretion (5). This protein is not involved in pedestal formation. These results indicate that EHEC proteins secreted by its type III system are strongly immunogenic.
FIG. 1.
Immunoblot analysis of patient serum reactivity against EHEC secreted proteins. (A) Secreted proteins from wild-type EHEC O157:H7 and the type III secretion mutant, SepB. (B) Immunoreactivity of sera from patient 95-8001 against secreted proteins from O157:H7 or SepB at days 0, 8, and 60 posthospitalization. (C) Immunoblots showing reactivities of patients' sera at day 8 posthospitalization. The patient identification number is indicated beneath each blot. Culture supernatants were concentrated by trichloroacetic acid precipitation, resolved by SDS–12% PAGE, and either stained with Coomassie blue (A) or transferred to nitrocellulose and probed with patients' sera (B and C). Molecular mass markers (in kilodaltons) are shown to the left of each panel.
Immunoblot analysis of patients' sera to EHEC secreted proteins and intimin.
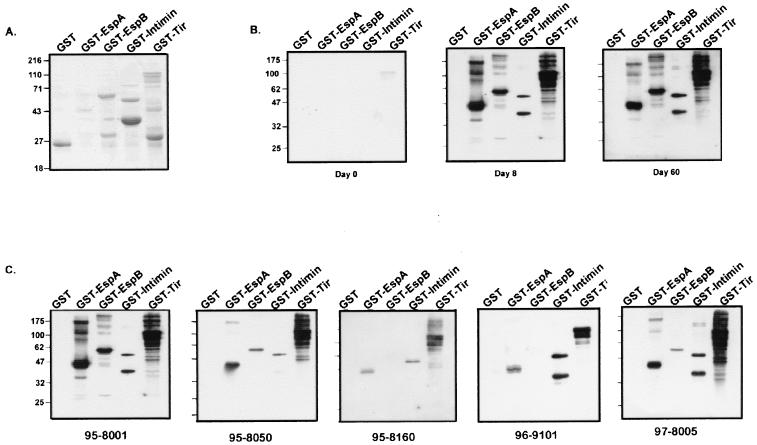
To determine which type III secreted proteins were being recognized, we determined the reactivities of the sera to various purified EHEC secreted proteins. Because the recombinant proteins were made in a non-O157 E. coli strain, any potential LPS cross-reactivity would be removed. EspA, EspB, Tir, and the 280 carboxyl-terminal amino acids of intimin were fused to GST, and the recombinant fusion proteins were expressed in nonpathogenic E. coli and purified with glutathione beads. Although intimin is an outer membrane protein not secreted by the type III system, because it binds to Tir and is a key virulence factor we also included it. EspD, the other type III EHEC secreted protein, was not included as we were unable to produce a recombinant form of it, despite repeated attempts, perhaps due to its hydrophobic nature. Each recombinant fusion protein was separated by SDS-PAGE, and immunoblot analysis with the patients' sera was performed (Fig. 2). The results for one patient, patient 95-8001, are shown in Fig. 2B. Little reaction was seen to any of the EHEC proteins at day 0, although there was faint reactivity to Tir. Day 8 serum strongly reacted with all four GST fusion proteins but not with GST alone, with the Tir response being the strongest. Day 60 serum showed a reaction similar to that seen with day 8 serum but weaker. Similar results were seen with sera from all five patients (reactivity with day 8 serum is shown in Fig. 2). In all cases, the reaction to Tir was the strongest, followed by reactions to intimin and EspA, and the anti-EspB response was the weakest. These results suggest that each of these four EHEC virulence factors is recognized by the immune response, with the anti-Tir response being the strongest.
FIG. 2.
Immunoblot analysis of patient serum reactivity against recombinant EHEC virulence proteins. (A) Purified GST fusions to EHEC virulence proteins separated by PAGE and stained with Coomassie blue. (B) Immunoreactivity of serum from patient 95-8001 against GST-EHEC fusion proteins at days 0, 8, and 60 posthospitalization. (C) Immunoreactivity of patients' sera at 8 days posthospitalization. GST and GST-EHEC fusion proteins were purified as described in Materials and Methods and resolved by SDS–12% PAGE, followed by either staining with Coomassie blue (A) or immunoblotting with patients' sera (B and C). Molecular mass markers (in kilodaltons) are shown to the left of each panel.
ELISA of responses of patients' sera to EHEC secreted proteins and intimin.
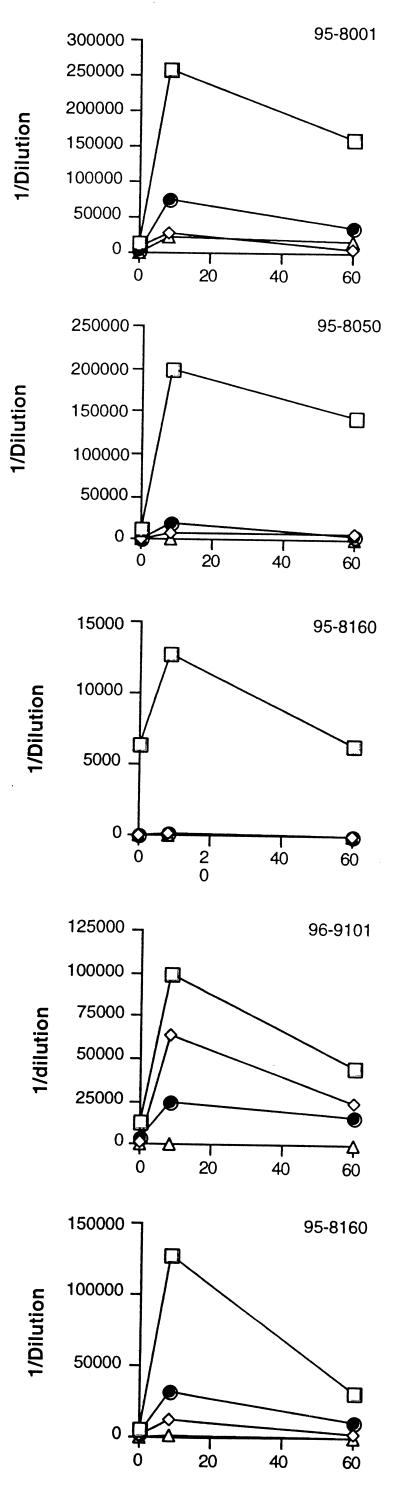
To obtain a more quantitative measure of the response to each EHEC protein, ELISAs were performed with each purified fusion protein. (ELISAs performed with EHEC supernatants were not included, as O157 LPS contamination in the supernatant could not be avoided.) In Fig. 3, ELISA results with all five patients' sera are shown. In all five day 8 samples, the titer of antibody against Tir was greatest, ranging from 1:6,400 to 1:256,000. Similar to the immunoblot results, reactivity to intimin and EspA was intermediate, with titers of antibody to EspB being the lowest in all five patients.
FIG. 3.
Individual patient antibody response to EHEC antigens (y axis) at days 0, 8, and 60 posthospitalization (x axis). ELISAs were performed to detect the recombinant GST fusion proteins, and titers of the following were determined: Tir (squares), intimin (diamonds), EspA (circles), and EspB (triangles). The figure shows data from one representative experiment, and experiments were repeated at least three times.
In Fig. 3, the antibody responses of all the patients at the three collection times to Tir, EspA, intimin, and EspB can be calculated. The response to Tir was strongest, followed by the responses to EspA (titers ranging from 1:100 to 1:7,500) and intimin (titers ranging from 0 to 1:25,600) and then the reaction to EspB (titers ranging from 0 to 1:20,000).
As expected, all five patients also responded to O157 antigen (Table 1). Patient 95-8160 was antigen positive by hemagglutination inhibition but antigen negative by enzyme immunoassay.
DISCUSSION
Given the incidence of EHEC-associated diseases, there is increased interest in developing strategies to prevent these diseases. One potential strategy would be to develop a human EHEC vaccine that would prevent primary EHEC infection, thereby blocking progression to severe sequelae in those with established infection. This strategy requires an increased understanding of EHEC molecules that could be used as vaccine candidates. This includes identification of such molecules, determining their antigenicity and immunogenicity, and testing their potential in vaccines.
To this end, we focused on key EHEC virulence factors (EspA, EspB, Tir, and intimin) that are essential for bacterial interactions with host cells and disease, since these represent EHEC-specific factors that are not present in normal flora yet are conserved among EHEC isolates. These four proteins represent interesting vaccine candidates since intimin is an integral outer membrane protein, EspA is part of a filamentous structure on the bacterial surface, and EspB and Tir are delivered to the host cell. Blocking any of these proteins should prevent pedestal formation, inhibit adherence, and ultimately block disease.
Several reactive bands were seen when EHEC supernatants were probed with patient sera. It is unlikely that these multiple bands represent O157 LPS, since equal amounts of the supernatant from the type III mutant did not produce these reactive bands. It is possible that they represent breakdown forms of the type III secreted proteins, since breakdown forms of Tir have been reported (17). In Salmonella spp., several other pathogenicity islands and islets have been identified which encode effectors secreted by a type III system encoded on a distant genetic locus. It is possible that these multiple bands represent other type III effectors encoded elsewhere on the EHEC genome. It is unlikely that they play a role in pedestal formation, since the EHEC locus for enterocyte effacement contains all the necessary genetic factors to form pedestals in nonpathogenic E. coli (22). However, they may have other effects necessary for full virulence.
The results seen in Fig. 3 demonstrate that all five of the patients with EHEC infection developed a significant antibody response to Tir and to EspA. All patients except patient 95-8160 also produced a detectable antibody response to intimin and to EspB. The five patients differ in the overall magnitude of response, but in all five patients the response to Tir is greater than that to the other three proteins (note differences in scales within Fig. 3), and in the case of patient 95-8160, Tir was the only secretory protein to which there was a detectable response. In each assay system the amount of protein incorporated into the solid phase was standardized. Although there are differences in molecular weight between the proteins, these are unlikely to explain the amount by which the response to Tir exceeds that to the other three proteins. Instead, this difference can be attributed to a greater immunologic response to Tir than the other three molecules. Two of the patients developed HUS (patients 95-8050 and 95-8001). The antibody responses of these patients do not appear to differ from those of the three who did not develop HUS. All five patients developed the expected response to O157 LPS (Table 1).
There appears to be some antibody in some of the early samples, especially antibody to Tir, although in all cases there was a rise in titer between the first and the second specimens, with the exception of titers of antibodies to intimin and EspB in patient 95-8160. The first serum was taken from patients 2 to 4 days after the onset of diarrhea (Table 1). The incubation period of EHEC is thought to be about 3 to 5 days. It can therefore be estimated that the first sera were collected between 5 and 9 days after infection, by which time there might be an early primary antibody response.
A very recent study of Brazilian mothers and children found anti-Tir in the colostrum and in the serum (24). In our study, anti-Tir was present in all the patients at 60 days, but the titer of this antibody was less than that for the day 8 sera. Long-term follow-up of patients with acute infection would be necessary to determine whether detectable anti-Tir persists or whether the presence of the antibody in a random population in Brazil reflects repeated exposure to Tir-producing pathogens in a different epidemiologic situation. In a second recent study, antibodies to Tir, EspA, and intimin were found, by Western blotting, in a series of EHEC-infected patients (15). However, in that study the recombinant proteins used were from EPEC, and these proteins differ significantly from EHEC proteins, which could affect reactivity. This study is the first to define quantitatively the antibody response by serial observation of individual O157:H7 patients to O157:H7 antigens. Both studies indicate that Tir is highly immunogenic. However, at least with tissue culture models, most of Tir is inserted directly into host cells. This also indicates that type III effectors that are delivered to host cells can still be recognized by the host immune system. However, we cannot rule out the possibility that Tir is secreted by the bacterium during infection or is released by bacterial lysis. Whichever the case may be, Tir makes a promising vaccine candidate, given its essential role in adherence and disease and the robust antibody response it elicits during disease.
In conclusion, we have shown that EHEC patients raise a significant antibody response to EHEC virulence factors, especially Tir. Although this indicates that these factors may be potential vaccine candidates for both humans and cattle, additional studies need to be done to demonstrate that antibodies to these virulence factors are capable of preventing either disease or carrier status.
ACKNOWLEDGMENTS
We thank Rebekah Devinney for her help with figures and critical reading and Lucie Hyde for technical assistance.
This work was supported by operating grants to B.B.F. from the Medical Research Council of Canada, the Canadian Bacterial Disease Network Center of Excellence, and the Beef Industry Development Fund and a Howard Hughes International Research Scholar award. B.B.F. is an MRC Scientist.
REFERENCES
- 1.Abe A, Heczko U, Hegele R, Finlay B. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J Exp Med. 1998;188:1907–1916. doi: 10.1084/jem.188.10.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitzan M, Karch H. Indirect hemagglutination assay for diagnosis of Escherichia coli O157 infection in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1174–1178. doi: 10.1128/jcm.30.5.1174-1178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitzan M, Moebius E, Ludwig K, Müller-Wiefel D E, Heeseman J, Karch H. High incidence of serum antibodies to Escherichia coli O157 lipopolysaccharide in children with hemolytic-uremic syndrome. J Pediatr. 1991;119:380–385. doi: 10.1016/s0022-3476(05)82049-9. [DOI] [PubMed] [Google Scholar]
- 4.Brotman M, Giannella R A, Alm P F, Bauman H, Bennett A R, Black R E, Bruhn C M, Cohen M B, Gorbach S L, Kaper J B, Roberts M R, Staneck J L, Taylor S, Troutt H F, Bell B P, Buchanan R L, Durham K, Feng P, Forman C T, Galler R G, Hall R B, Hancock D D, Hollingsworth J, Karmali M A, et al. Consensus conference statement: Escherichia coli O157:H7 infections—an emerging national health crisis, July 11–13, 1994. Gastroenterology. 1995;108:1923–1934. [PubMed] [Google Scholar]
- 5.Brunder W, Schmidt H, Karsh H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 6.Chart H, Scotland S M, Rowe B. Serum antibodies to Escherichia coli serotype O157:H7 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1989;27:285–290. doi: 10.1128/jcm.27.2.285-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel G, Phillips A D, Rosenshine I, Dougan G, Kaper J B, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 10.Greatorex J S, Thorne G M. Humoral immune responses to Shiga-like toxins and Escherichia coli O157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J Clin Microbiol. 1994;32:1172–1178. doi: 10.1128/jcm.32.5.1172-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin P, Tauxe R. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 12.Griffin P M. Epidemiology of Shiga toxin-producing Escherichia coli in humans in the United States. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: ASM Press; 1998. pp. 15–22. [Google Scholar]
- 13.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis K, Kaper J. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins C, Chart H, Smith H R, Hartland E L, Batchelor M, Delahay R M, Dougan G, Frankel G. Antibody response of patients infected with verocytotoxin-producing Escherichia coli to protein antigens encoded on the LEE locus. J Med Microbiol. 2000;49:97–101. doi: 10.1099/0022-1317-49-1-97. [DOI] [PubMed] [Google Scholar]
- 16.Karmali M A. The nature of immunity to the Escherichia coli Shiga toxin and options for toxoid immunisation. J Med Sci Biol. 1998;51(Suppl.):S26–S35. doi: 10.7883/yoken1952.51.supplement1_s26. [DOI] [PubMed] [Google Scholar]
- 17.Kenny B, Devinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 18.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loirat C, Sonsino E, Moreno M, Pillion G, Mercoer J C, Beaufils F, Mathieu H. Hemolytic-uremic syndrome: an analysis of the natural history and prognostic features. Acta Paediatr Scand. 1984;73:505–514. doi: 10.1111/j.1651-2227.1984.tb09962.x. [DOI] [PubMed] [Google Scholar]
- 20.Luzzi I, Tozzi A E, Rizzoni G, Niccolini A, Bendetti I, Minelli F, Caprioli A. Detection of serum antibodies to the lipopolysaccharide of Escherichia coli O103 in patients with hemolytic-uremic syndrome. J Infect Dis. 1995;171:514–515. doi: 10.1093/infdis/171.2.514. [DOI] [PubMed] [Google Scholar]
- 21.Marches O, Nougayrede J P, Boullier S, Mainil J, Charlier G, Raymond I, Pohl P, Boury M, De Rycke J, Milon A, Oswald E. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun. 2000;68:2171–2182. doi: 10.1128/iai.68.4.2171-2182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 23.Rowe P C, Orrbine E, Lior H, Wells G, Yetisir E, Clulow M, McLaine P N. Risk of hemolytic uremic syndrome after sporadic Escherichia coli O157:H7 infection: results of a Canadian collaborative study. J Pediatr. 1998;132:777–781. doi: 10.1016/s0022-3476(98)70303-8. [DOI] [PubMed] [Google Scholar]
- 24.Sanches M I, Keller R, Hartland E L, Figueiredo D M, Batchelor M, Martinez M B, Dougan G, Careiro-Sampaio M M, Frankel G, Trabulsi L R. Human colostrum and serum contain antibodies reactive to the intimin-binding region of the enteropathogenic Escherichia coli translocated intimin receptor. J Pediatr Gastroenterol Nutr. 2000;30:73–77. doi: 10.1097/00005176-200001000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Van Dyck M, Proesmans W C, Depraetere M. Hemolytic uremic syndrome in childhood: renal function ten years later. Clin Nephrol. 1988;29:109–112. [PubMed] [Google Scholar]
- 26.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]