Abstract
Background:
The distribution of adverse pregnancy, birth and subsequent child developmental and health outcomes in the U.S. is characterized by pronounced racial (particularly Black-White) disparities. In this context, chronic stress exposure represents a variable of considerable importance, and the immune/inflammatory system represents a leading candidate biological pathway of interest. Previous pregnancy studies examining racial disparities in immune processes have largely utilized circulating cytokine levels, and have yielded null or mixed results. Circulating cytokines primarily represent basal secretion and do not necessarily represent functional features of immune responsivity and regulation. Thus, in order to conduct a more in-depth characterization of racial differences in functional immune properties during pregnancy, we utilized an ex vivo stimulation assay, a dynamic measure of immune function at the cellular level, to investigate Black-white racial differences in in mid- and late-gestation in i) pro-inflammatory (IL-6) responsivity of leukocytes to antigen [lipopolysaccharide (LPS)] challenge, and ii) regulation (dampening) of this pro-inflammatory response by glucocorticoids.
Method:
177 women (N=42 Black (24%), n=135 white (76%)) with a singleton, intrauterine pregnancy provided 20 ml venous blood in mid- (16.6± 2.4 wks) and late (33.3±1.1 wks) pregnancy. Maternal pro-inflammatory responsivity of leukocytes was quantified by assessing the release of the pro-inflammatory cytokine IL-6 in response to LPS stimulation, and regulation of the pro-inflammatory response was quantified by assessing the suppression of the stimulated IL-6 response after co-incubation with progressively increasing levels of dexamethasone [10−7, 10−6, 10−5M] (i.e., glucocorticoid receptor resistance (GRR)). A priori model covariates included maternal age, parity, SES (socioeconomic status), and pre-pregnancy BMI.
Results:
Maternal pro-inflammatory responsivity (LPS-stimulated IL-6) and GRR increased significantly across mid- and late gestation (adjusted β = 0.157, p= 0.007; β = 0.627, p<0.001, respectively). Across both time points in pregnancy Black women exhibited significantly higher LPS-stimulated IL-6 release and reduced glucocorticoid regulation of the IL-6 response (i.e., higher GRR) relative to white women, before and after adjusting for covariates (β = 0.381, p= 0.0030; β= 0.391, p=0.0075, respectively). There was no racial difference in the concentrations of circulating IL-6 (p=0.9199).
Conclusion:
Our findings support the hypothesis postulating significant racial (Black-white) differences in key functional properties of the maternal immune system in pregnancy, which were not apparent using circulating cytokine measures. These data elucidate a potentially important physiological mechanism underlying the transduction of environmental conditions into racial disparities in reproductive and subsequent child health outcomes, and the use of these ex vivo measures should be considered in future studies.
Keywords: inflammation, glucocorticoid receptor resistance, interleukin-6, racial disparities, Black, African American
1. Introduction
The distribution of adverse pregnancy, birth and subsequent child developmental and health outcomes in the U.S. is characterized by pronounced racial (particularly Black-White) disparities.1 This disparity persists even after accounting for sociodemographic and environmental factors,2 and has led to the “weathering hypothesis,” wherein chronic stress or repeated challenge/disadvantage accumulates over the lifespan, ultimately leading to increased vulnerability.3 In this context, the immune system (inflammation, and particularly the pro-inflammatory cytokine interleukin (IL)-6)) represents a leading candidate biological pathway of interest.4,5 Chronic stress can produce impairment in the glucocorticoid-cytokine feedback system, which diminishes inhibitory mechanisms designed to dampen immune cell proinflammatory cytokine production, thus maintaining or increasing inflammatory state.6 Previous studies examining racial disparities in immune processes have yielded mixed results.7,8 One feature of many of these studies is their reliance on circulating concentrations of pro-inflammatory cytokines as an indicator of immune state/function. A limitation of such use of circulating cytokine levels is that these levels do not adequately reflect key functional properties, such as the propensity of immune cells to mount an inflammatory response to antigen exposure, or the capacity to regulate this proinflammatory response.6
Thus, in order to conduct a more in-depth characterization of racial differences in functional immune properties during pregnancy, we conducted a study in a population of non-Hispanic Black and non-Hispanic white pregnant women using an ex vivo stimulation protocol to characterize racial differences across pregnancy in i) pro-inflammatory IL-6 responsivity of leukocytes to antigen [lipopolysaccharide (LPS)] challenge and ii) regulation (dampening) of this pro-inflammatory response by glucocorticoids. We hypothesized that Black pregnant women would exhibit a greater pro-inflammatory response to antigen challenge as well as a reduced capacity of cortisol to regulate/dampen this response (higher GRR) than white pregnant women, and that this difference would persist across pregnancy.
2. Materials and Methods
2.1. Participants
Two hundred women were enrolled from prenatal care clinics at the Magee Women’s Hospital of the University of Pittsburgh in Pittsburgh, PA., as part of a larger multi-site prospective cohort study (Measurement of Maternal Stress study (MOMS)). Women were eligible for the MOMS study if they were English speaking, >18 years age, with a singleton, intrauterine pregnancy, and less than 20 weeks pregnant. Exclusion criteria included major fetal congenital or chromosomal anomalies, or corticosteroid or progesterone treatment. Women at the Magee study site underwent additional study procedures, including the leukocyte ex vivo stimulation protocol. Once enrolled, participants attended two visits during pregnancy, at 12-20.6 (16.6± 2.4) and 32-35.6 (33.3±1.1) weeks gestation. The Institutional Review Board of the University of Pittsburgh approved the protocol. Of the 200 recruited women, one case of intra-uterine fetal demise was excluded from analyses, and of the remaining 199 women, complete ex vivo stimulation data was available in 185 women for at least one pregnancy assessment (n=157 at mid gestation, and n=145 at late gestation). Of these women, 177 self-reported being of non-Hispanic Black or white race/ethnicity, and they were included in this report.
2.2. Sociodemographic and Clinical Data
Standardized structured interviews were conducted for ascertainment of maternal sociodemographic characteristics (maternal age, education, race/ethnicity, parity). Women self-reported their race/ethnicity from the following categories: a) White, b) Black, c) Hispanic, d) other. Black-white disparities was the interest of these analyses; therefore, only women who reported as non-Hispanic Black or non-Hispanic white were included in the analyses (95% of the recruited population). Maternal socioeconomic status (SES) was defined as a combination of maternal educational level (assessed in categories from less than high school to advanced degree (master/doctorate) and coded into values from 1 to 3) and household income (originally assessed in categories from ≤ $15,000 to ≥ $100,000 and then coded into values from 1 to 4). The individual education level and household income were standardized (mean=0 and SD=1) and then summed for the combined SES index. Pre-pregnancy body-mass index (BMI; weight kg/height m2) was computed based on pre-pregnancy weight and height.
2.3. Fasting Blood Draw and Circulating Cytokine
A fasting maternal blood draw was collected at each study visit via antecubital venipuncture into 10-mL EDTA-coated Vacutainer tubes (BD Biosciences), which was spun within 30 minutes of collection at 2,000 x g at 4°C for 15 minutes. Plasma was harvested and stored at −80°C until the assay for circulating IL-6 was performed (via MSD V-Plex assay). Assays were performed in duplicate,9 and yielded reliable values (IL-6 intra-assay CV=5.54, inter-assay <10, lower limit of detection (LLD)=0.06 pg/mL).
2.4. Leukocyte Ex Vivo Stimulation.
Immune responsiveness and regulation were assessed using an ex vivo stimulation protocol, based on previously-published procedures.10 In brief, venous blood was collected in heparinized sterile tubes and diluted 10:1 with sterile phosphate buffered saline. 400μL aliquots of this mixture were dispensed into wells containing a) LPS (from Escherichia coli, 055:B5, Sigma-Aldrich; 60ng/mL) alone, and b) LPS (60ng/mL) co-incubated with serial dilutions of dexamethasone (Sigma-Aldrich; final concentration 10−7, 10−6, and 10−5M), on a 24-well plate. After 6-h of incubation at 37 °C and 5% CO2, the plates were centrifuged at 2000 x g at 4°C for 10 min, and the plasma supernatant was collected and stored at −80°C until analysis. IL-6 assays were conducted in duplicate using a commercially-available Multiplex Bead-Based Kit, the V-PLEX Proinflammatory Panel 1 (4-Plex, Human Panel; Millipore, Billerica, MA, USA), and plates were read on a Luminex FLEXMAP 3D System and analyzed using xPONENT® software (Luminex). Values below the detection limit were set to the plate-specific lowest standard concentration (2.4% of the sample). The assay yielded reliable values, with an intra-assay CV=6.02, LLD=0.06 pg/mL. Leukocyte release of IL-6 after co-incubation with LPS alone provided a marker of pro-inflammatory responsiveness to a standardized antigen challenge (referred to as stimulated IL-6). The ability of cortisol (represented by standard dexamethasone) to suppress or dampen stimulated IL-6 release across the four cortisol gradient exposures (LPS+0, LPS+10−7, LPS+10−6, and LPS+10−5M dexamethasone) was quantified using standard linear trapezoidal area under the curve (AUC) calculation, and provided a marker of the extent to which cortisol regulates the inflammatory response (i.e., glucocorticoid receptor resistance (GRR)), with higher IL-6 AUC indicating greater GRR.
2.5. Statistical Methods
Descriptive statistics were used to describe baseline maternal sociodemographic characteristics, and differences by race were tested using linear regression for continuous variables and Pearson’s chi-square for categorical variables. Circulating plasma, and ex vivo stimulated IL-6 concentrations were natural log-transformed, and all modeled betas, confidence intervals and p-values and model interpretations represent the Ln transformed values; however, the descriptive table and figure are presented in the untransformed form for ease of interpretation.
For the longitudinal analysis, a population-averaged model using generalized estimating equations (GEE) was utilized to model the population average IL-6 levels by race over gestation, with and without an interaction between the gestational visit status and race. The quasi-likelihood information criterion (QIC) was calculated to examine the GEE model fit and to select the exchangeable working matrix. The Huber–White standard error estimates were obtained to produce unbiased variance estimates of the regression coefficients.
The following a priori selected covariates were included in all adjusted models: maternal age, parity, SES, pre-pregnancy BMI. All statistical analyses were performed with SAS® Software Version 9.4. Results were considered statistically significant at the level of p < 0.05.
3. Results
3.1. Descriptive statistics
The maternal sociodemographic characteristics of the 177 participants are reported in Table 1, with values reported for all women and separated by race (24% identified as Black). None of the sociodemographic factors were significantly different in the subgroup of 23 women who were not included in these analyses (data not shown). There were, however, significant differences in sociodemographic variables by race, as reported in Table 1. The mean ± standard deviation mid- and late-gestation IL-6 concentrations (circulating IL-6, LPS-stimulated IL-6, and GRR) also are presented in Table 1.
Table 1:
Values presented as unadjusted mean ± standard deviation or N (%) for continuous and categorical variables, respectively. Black/white differences in continuous variables were tested by linear regression and differences in categorical variables were tested by chi square tests.
| Variable | All | Black | White | P | ||
|---|---|---|---|---|---|---|
| (n=177) | 42(24%) | 135(76%) | Black vs. white | |||
|
| ||||||
| Age (years) | 30.0±5.2 | 25.9±4.7 | 31.3±4.6 | <0.001 | ||
| SES index | 0.03±1.79 | −1.86±1.36 | 0.55±1.54 | <0.001 | ||
| Pre-pregnancy BMI (kg/m2) | 28.3±7.6 | 30.8±9.2 | 27.5±6.9 | 0.014 | ||
| Parity: | 0.002 | |||||
| 0 | 74(42.8%) | 9(21.4%) | 65(48.1%) | |||
| ≥1 | 103(58.2%) | 33(78.6%) | 70(51.9%) | |||
| mid gestation | late gestation | mid gestation | late gestation | mid gestation | late gestation | |
|
| ||||||
| Circulating plasma IL-6 (pg/mL) | 0.61±0.48 | 0.67±0.36 | 0.68±0.37 | 0.75±0.39 | 0.60±0.51 | 0.65±0.39 |
| Inflammatory responsivity: LPS-Stimulated IL-6 (pg/mL) | 3,535±2,391 | 3,978±2,581 | 4,701±2521 | 6,100±3,127 | 3,188±2,248 | 3,448±2,132 |
| Inflammatory regulation: GRR (IL-6 AUC) (pg/mL) | 4,649±4,045 | 7,512±4,939 | 6,455±4,815 | 10,066±6,152 | 4,083±3,614 | 6,867±4,386 |
SES= Socioeconomic Status, BMI= body mass index, GRR= Glucocorticoid Receptor Resistance, IL-6= Interleukin-6, AUC= Area Under the Curve, LPS= Lipopolysaccharide.
3.2. Change across gestation
Consistent across both races, circulating plasma IL-6 significantly increased from mid to late gestation, demonstrating the previously-described shift towards pro-inflammatory signaling from mid to late gestation (β = 0.166, p< 0.0001; adjusted β = 0.170, p<0.0001). Furthermore, both of the ex vivo stimulation measures significantly increased across pregnancy, with and without the consideration of a priori-specified covariates. Specifically, LPS-stimulated IL-6 and GRR significantly increased across gestation, indicating a gestational increase in pro-inflammatory responsivity and impaired regulation of this pro-inflammatory response from mid to late gestation (β = 0.153, p= 0.0067; adjusted β = 0.157, p= 0.0070; β = 0.609, p< 0.0001; adjusted β = 0.627, p<0.0001, respectively).
3.3. Racial differences
There was no racial difference in the unstimulated basal circulating plasma IL-6 levels in Black compared to white women (β = 0.159, p=0.056; adjusted β = −0.011, p=0.9199). However, as shown in Figure 1, the use of the more dynamic measures ex vivo stimulation measures of the immune system revealed significant racial differences. Specifically, across gestation, Black women exhibited significantly higher average LPS-Stimulated IL-6 release (β= 0.540, p< 0.001 ; adjusted β = 0.381, p= 0.0030) and a reduced average regulation of the IL-6 response (higher GRR) relative to white women, before and after adjusting for a priori covariates, maternal age, parity, SES, and pre-pregnancy BMI (β= 0.484, p< 0.0001 ; adjusted β= 0.391, p=0.0075). Additionally, this racial difference in immune responsivity and regulation was consistent across the mid and late-gestation measurement time points (pintereaction>0.05). To ensure that these observed racial differences in immune responsivity and regulation were independent of racial differences in maternal age and SES, we (a) repeated the analyses using only study visit, maternal age and SES as covariates in the model, (b) examined the association of maternal age and SES with the study outcomes separately within each of the two racial groups, and (c) re-ran the analyses in a subgroup of study participants matched across race for age and SES (data not shown). Each of these analyses confirm that finding that the effects of maternal race on the study outcomes are independent from those of maternal age and SES.
Figure 1:
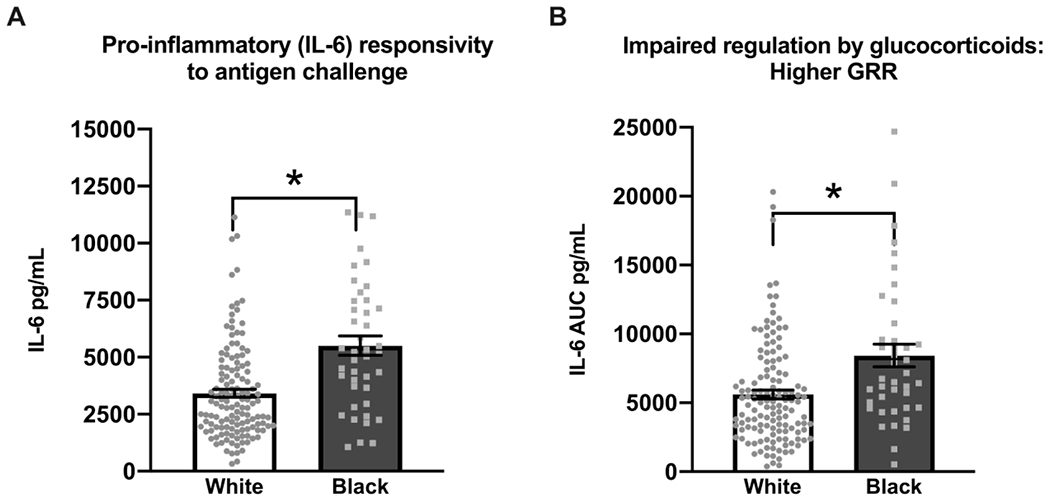
Black pregnant women exhibited significantly higher average gestational a) lipopolysaccharide (LPS) stimulated IL-6 response (unadjusted mean±standard error (SE)= 5441.1±399.3 vs. 3353.4± 168.1, β = 0.5395, p<0.0001; adjusted mean±SE= 5105.6±504.2 vs. 3435.7± 169.1, β = 0.3806, p= 0.0030) and b) reduced regulation of the IL-6 response (higher IL-6 AUC indicates impaired regulation by glucocorticoids and higher GRR) (unadjusted mean±SE=8258.0± 667.4 vs. 5486.1± 281.4, β = 0.4840, p<0.0001 ; adjusted mean±SE= 8230.3±829.2 vs. 5509.6± 303.0, β = 0.3912, p=0.0075) relative to white women. This racial difference was consistent across repeated mid- and late-gestation measurement. The data was modeled using Ln transformed IL-6 values, however raw untransformed IL-6 concentrations and mean±SE are presented in the figures for ease of interpretation.
4.1. Discussion
Our findings support the hypothesis postulating significant racial (Black-White) differences in key functional properties of the maternal immune system in pregnancy. Specifically, Black women exhibited a 48.6% greater pro-inflammatory response to antigen challenge (i.e. stimulated IL-6) and a 49.3% less capacity to regulate this response by glucocorticoids (i.e., GRR), compared to white women. These racial differences were not apparent using circulating cytokine measures, but became evident upon using ex vivo stimulation measures, thus emphasizing the need to characterize maternal inflammatory propensity using measures of functional properties, such as those in this study.
To the best of our knowledge, this is the first study in the context of pregnancy to demonstrate at the cellular level that Black women have increased immune responsivity and impaired immune regulation relative to white women. Furthermore, this racial difference was consistently evident across mid and late gestation. Previous health disparity studies in pregnancy have presented preliminary evidence for immune regulation and glucocorticoid resistance in pregnant Black women utilizing indirect measures of GRR, and they support our findings.8,11 Specifically, these studies were conducted using patterns of circulating leukocyte counts, cortisol and cytokine levels, and although these proxy-GRR measures were measured from circulation and not quantified at the cellular/receptor level, they reported higher GRR in black/minority pregnant women.8,11 Importantly, Gillespie et. al.11 linked a measure of chronic stress to impaired immune regulation and reported an association between racial discrimination and glucocorticoid resistance in Black pregnant women. Additionally, other studies have supported a similar pro-inflammatory profile in Black women during their pregnancy using downstream markers of inflammation such as circulating concentrations of CRP12 or through increased endogenous release of cytokines in response to a standardized social stress task.13
This study is strengthened by repeated measurement of multiple markers of inflammation across pregnancy. Beyond providing evidence for stable racial differences for immune dysregulation from mid- to late-gestation, our data demonstrates a within-subject gestational increase in immune responsivity (stimulated IL-6) and impaired immune regulation (increasing GRR) across all women. A gestational increase in GRR has previously been shown in a small cohort of 29 women,14 however, our data confirms these findings in a larger and more racially diverse cohort of 177 women. The mid- to late-gestation rise in the circulating pro-inflammatory cytokine, IL-6, has been previously reported in the larger MOMs cohort,15 and in this study, the concentrations of circulating cytokines were consistent across race at both study time-points.
5.1. Conclusion
Our findings support the hypothesis postulating significant racial (Black-white) differences in key functional properties of the maternal immune system in pregnancy. Notably, these differences were not apparent when using circulating cytokine measures. These data elucidate a potentially important physiological mechanism underlying the transduction of environmental conditions into racial disparities in reproductive and subsequent child health outcomes, and the use of these dynamic cellular level measures should be considered in the future studies.
Funding:
Funding for this study was provided by the NIH awards HHSN275201200007I--HHSN27500005. The preparation of this paper was additionally supported by the NIH grants R01 MD-010738, R01 AG-050455, and K99 HD-097302.
References:
- 1.Howell EA, Brown H, Brumley J, et al. Reduction of Peripartum Racial and Ethnic Disparities: A Conceptual Framework and Maternal Safety Consensus Bundle. J Obstet Gynecol Neonatal Nurs, 2018. 47(3): p. 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colen CG, Ramey DM, Cooksey EC, et al. Racial disparities in health among nonpoor African Americans and Hispanics: The role of acute and chronic discrimination. Soc Sci Med, 2018. 199: p. 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geronimus AT, The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis, 1992. 2(3): p. 207–21. [PubMed] [Google Scholar]
- 4.Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol, 2001. 15 Suppl 2: p. 17–29. [DOI] [PubMed] [Google Scholar]
- 5.Giurgescu C, Engeland CG, Templin TN, et al. Racial discrimination predicts greater systemic inflammation in pregnant African American women. Appl Nurs Res, 2016. 32: p. 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A, 2012. 109(16): p. 5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christian LM, At the forefront of psychoneuroimmunology in pregnancy: Implications for racial disparities in birth outcomes: PART 2: Biological mechanisms. Neurosci Biobehav Rev, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corwin EJ, Guo Y, Pajer K, et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology, 2013. 38(9): p. 1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Zhu J, and Van Eyk JE, Comparison of multiplex immunoassay platforms. Clin Chem, 2010. 56(2): p. 314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumsta R, Entringer S, Koper JW, et al. Glucocorticoid receptor gene polymorphisms and glucocorticoid sensitivity of subdermal blood vessels and leukocytes. Biol Psychol, 2008. 79(2): p. 179–84. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie SL and Anderson CM, Racial discrimination and leukocyte glucocorticoid sensitivity: Implications for birth timing. Soc Sci Med, 2018. 216: p. 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borders AE, Wolfe K, Qadir S, et al. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol, 2015. 35(8): p. 580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christian LM, Effects of stress and depression on inflammatory immune parameters in pregnancy. Am J Obstet Gynecol, 2014. 211(3): p. 275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz ER, Stowe ZN, Newport DJ, et al. Regulation of mRNA expression encoding chaperone and co-chaperone proteins of the glucocorticoid receptor in peripheral blood: association with depressive symptoms during pregnancy. Psychol Med, 2012. 42(5): p. 943–56. [DOI] [PubMed] [Google Scholar]
- 15.Ross KM, Miller G, Culhane J, et al. Patterns of peripheral cytokine expression during pregnancy in two cohorts and associations with inflammatory markers in cord blood. Am J Reprod Immunol, 2016. 76(5): p. 406–414. [DOI] [PubMed] [Google Scholar]


