ABSTRACT
Pneumothorax is a frequently encountered entity in pulmonary practice and can be primary or secondary. Traumatic and iatrogenic causes also account for a minority of cases presenting to the chest physician. The most common therapeutic intervention done is a tube thoracostomy in all but the mildest of cases. Pneumothorax ex vacuo is a distinctly uncommon entity that differs considerably from the rest of the pneumothorax cases in its pathogenesis, clinical manifestations, radiological findings, and management. Pneumothorax in this entity results from the sucking in of air into the pleural space caused by an exaggerated negative intrapleural pressure, which is most frequently secondary to acute lobar collapse. Symptoms attributable to pneumothorax per se are distinctly mild and the vital aspect of treatment is to relieve the bronchial obstruction. Tube thoracostomy fails to relieve the pneumothorax in such cases and should be avoided. We share three cases of pneumothorax ex vacuo encountered in our institution and alert clinicians of the presentation, radiology, and management of this uncommon condition.
KEY WORDS: lung collapse, pneumothorax ex vacuo, tube thoracostomy
INTRODUCTION
Pneumothorax is frequently encountered in pulmonary practice and is generally an urgent situation that requires prompt interventions upon diagnosis. Pneumothorax is divided into primary and secondary. A primary pneumothorax occurs in the absence of significant identifiable lung disease. On the other hand, secondary pneumothorax occurs in the presence of identifiable existing lung pathology. The presence of a pneumothorax usually implies that a communication with the pleural space and airways (bronchial, bronchiolar, or alveolar leak) or atmosphere (trauma or iatrogenic procedures) has occurred. In the absence of spontaneous closure of the air leak, the amount of air in the pleural space can increase markedly and a one-way valve is formed, leading to a tension pneumothorax. Unless reversed by effective treatment, this situation can progress and potentially cause cardiopulmonary compromise and death. Symptoms of pneumothorax typically include chest pain and shortness of breath. A definite diagnosis of a pneumothorax requires a chest X-ray, ultrasound examination of the chest, or computed tomography (CT) scan. Small spontaneous pneumothoraces typically resolve without treatment and require only monitoring.
Pneumothorax ex vacuo is a distinctly uncommon entity and has been traditionally observed to occur as a consequence of acute pulmonary lobar collapse.[1,2] The bronchial obstruction that precipitates and leads to pneumothorax is typically acute and is usually secondary to mucoid impaction, aspirated foreign bodies, or malpositioned endotracheal tubes. Rarely, tumour fragmentation and proximal migration of a detached fragment can result in a similar situation. The term pneumothorax ex vacuo has been expanded to include scenarios when a lung is unable to re-expand following drainage of pleural fluid and results in loculated pneumothorax[3,4]: a phenomenon characteristically seen in malignant pleural effusions with fibrinous pleural peel. The malignant cell infiltration creates a fibrinous peel limiting re-expansion of the lung and resulting in negative pleural pressure and suction of tissue air into the pleural space, similar to acute lobar collapse. The seal between the visceral and parietal pleura remains intact at the sites of the aerated lobes, causing the pneumothorax ex vacuo to surround only the collapsed lobe. Similar situations arise in tuberculous pleural effusions[5] and recurrent hepatic hydrothorax.[6] We share three cases of pneumothorax ex vacuo encountered in our institution in the past few years.
CASE REPORT
The three cases occurred in three different clinical scenarios and had different aetiologies.
Case 1
The first case occurred in a 22-year-old man who presented with cough, fever, and loss of weight for two months. He had acute worsening of symptoms with left-sided chest pain and dyspnoea for a day. Chest radiograph showed left hilar prominence with weil-like opacification of the left upper and mid zones consistent with left upper lobe collapse [Figure 1]. A shallow pneumothorax limited to the left upper zone was noted. Computed tomography (CT) of the thorax confirmed left upper lobe bronchial obstruction, left upper lobe collapse, and small pneumothorax limited to the left upper thorax adjacent to the collapsed left upper lobe [Figure 2]. Bronchoscopy revealed thick exudates and granulations occluding the left upper lobe bronchial orifice which were partially cleared with endobronchial interventions [Figure 3]. Bronchoalveolar lavage (BAL) for acid-fast bacilli was positive. Luminal patency was achieved and chest radiograph after four hours of procedure showed total clearance of the pneumothorax. He responded well to anti-tuberculosis chemotherapy with no further lobar collapse or pneumothorax. A check bronchoscopy after three months revealed good restoration of luminal patency and clearance of granulations.
Figure 1.
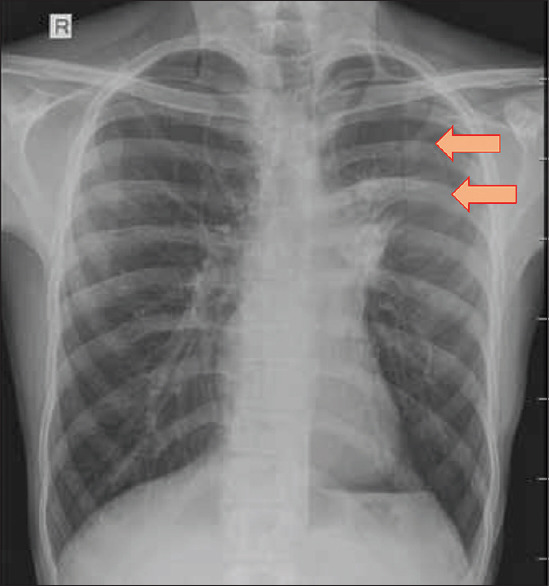
Chest radiograph of the second patient showing left hilar prominence, left upper lobe collapse and shallow pneumothorax limited to left upper zone
Figure 2.
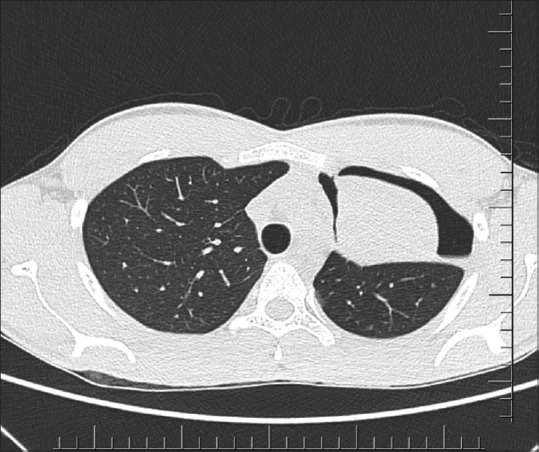
Axial CT thorax image of the second patient showing left upper lobe collapse and shallow pneumothorax surrounding the collapsed lung
Figure 3.
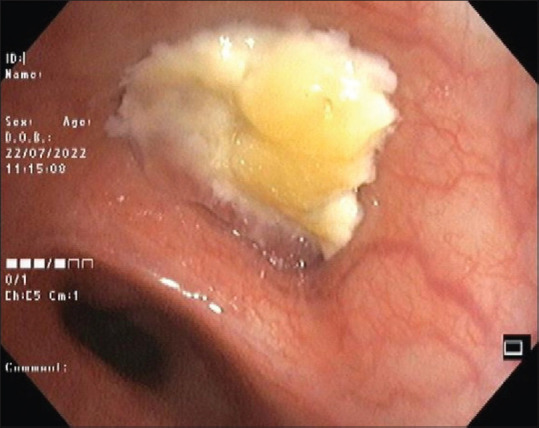
Bronchoscopic image of the second patient showing mucoid impaction in the left upper lobe bronchus causing total occlusion of the lumen
Case 2
The second case was a 19-year-old hotel management student who was asthmatic. He presented with throat itching, productive cough, chest discomfort, and breathlessness for two weeks. He was not tachypnoeic or hypoxic at presentation. Absent breath sounds in the left upper chest area were noticed. Chest radiograph and CT thorax showed left upper lobe collapse with small left apical pneumothorax and left main bronchial obstruction. Bronchoscopy revealed mucoid impaction which was cleared, restoring bronchial patency. Lung expansion and resorption of pneumothorax was noted as early as one hour after the procedure. Serum IgE level was >5000 units and Aspergillus skin prick test was positive. A diagnosis of allergic bronchopulmonary aspergillosis, mucoid impaction, and left pneumothorax ex vacuo was made. He was started on a combination of medium-dose inhaled steroid and a long-acting beta 2 agonist inhaler with montelukast. Systemic steroids were initiated at a dose of 30 mg of prednisolone and was tapered off, guided by serum IgE levels. He had good asthma control, normal lung function, and no further exacerbations that would have required emergency visits in the next year. Systemic steroids were discontinued as early as the fourth month and systemic antifungals were not required. He continues to be on follow-up and no further mucoid impaction and pneumothorax has been noted.
Case 3
The last case was a ventilated patient who had a cerebrovascular accident, recurrent aspiration pneumonias, and poor conscious level. The patient was a 78-year-old man with systemic hypertension and type 2 diabetes mellitus. He was hospitalised for a cerebrovascular accident with left hemiplegia 12 days prior to presentation and had aspirations progressing to pneumonia and respiratory failure, needing mechanical ventilation. After eight days of mechanical ventilation, sudden worsening of oxygenation and elevation of peak airway pressures were noted while on ventilatory support which prompted an emergency chest radiograph. Right upper lobe collapse with shallow right apical pneumothorax was seen. Bedside bronchoscopy was done through the endotracheal tube, and right upper lobe secretions were cleared. Collapse and pneumothorax resolved instantaneously. He was slowly weaned off from mechanical ventilatory support after improvement of his pharyngeal function and had a prolonged hospital stay with neuro and pulmonary rehabilitation assistance. Further significant aspiration episodes did not occur during his hospital stay.
Table 1 summarises the clinical characteristics of the three cases.
Table 1.
Summary of the three cases who presented with pneumothorax ex vacuo
| Serial number | Age and gender | Aetiology of bronchial obstruction | Collapsed lobe | Treatment given and result |
|---|---|---|---|---|
| Case 1 | 22/Male | Endobronchial tuberculosis | Left upper lobe | Bronchoscopic clearance; successful restoration of luminal patency and prompt resorption of pneumothorax |
| Case 2 | 19/Male | Allergic bronchopulmonary aspergillosis with mucoid impaction | Left upper lobe | Bronchoscopic clearance; successful restoration of luminal patency and prompt resorption of pneumothorax |
| Case 3 | 78/Male | Cerebrovascular accident (CVA), aspiration pneumonia with aspirated secretions | Right upper lobe | Bronchoscopic clearance; successful restoration of luminal patency and prompt resorption of pneumothorax |
DISCUSSION
In the present manuscript, we shared three cases of pneumothorax ex vacuo, a rare and distinct entity having specific diagnostic and therapeutic implications. The aetiology was different in the three cases: tuberculosis, allergic bronchopulmonary aspergillosis with mucoid impaction, and ventilator-associated pneumonia with acute lobar collapse in cases 1, 2, and 3, respectively. The second case was previously published as the first reported pneumothorax ex vacuo in a patient with allergic bronchopulmonary aspergillosis.[7]
The pathophysiology of pneumothorax ex vacuo has been narrated in previous reports.[1,2] Acute collapse of the obstructed lobe results in powerful elastic recoil of the lobe and a significant increase in negative intrapleural pressure, reaching magnitudes of up to −50 mmHg surrounding the collapsed lobe. This may be particularly pronounced in mechanically-ventilated subjects with high FiO2 and relatively dry gases. The resultant in-drawing of gas into the pleural space by a suction effect creates the pneumothorax. The constitution of pneumothorax is predominantly nitrogen-derived from the ambient tissues and blood. Thus, the designation pneumothorax ex vacuo indicates that the gas in the pleural space has arisen from this relative vacuum phenomenon rather than from an alveolo-pleural connection. The accumulation of gas and hence the pneumothorax is limited to the area around the collapsed lobe while the seal between the visceral and parietal pleura of the adjacent lobe or lobes remains intact.
Clinically, the presentation is dominated by features of acute lobar collapse rather than the pneumothorax. The presentation in aspirated foreign bodies is usually dramatic, although the history may not be forthcoming in paediatric subjects. In mechanically-ventilated subjects, worsening hypoxia and pulmonary compliance ensues and chest roentgenography or bedside chest ultrasonography is required for detection. Radiologically, features of lobar collapse (volume loss) predominate, as manifested by loss of aeration and shift of fissures and mediastinum. It is likely that pneumothorax ex vacuo could occur from acute collapse of any lobe; however, all three cases reported by Berdan et al.[1] and two of three cases reported by Woodring et al.[2] involved the upper lobe of the right lung. The effect of gravity and consequent higher negative intrapleural pressure in the upper zones of thorax make the entity more likely in upper lobe collapse. The pneumothorax is shallow and occurs adjacent to the collapsed lobe. As opposed to usual pleural pathologies, the pneumothorax is often limited by lobar outlines. In recent years, bronchoscopic lung volume reduction procedures are becoming an important cause of pneumothorax ex vacuo.[8]
The critical need of recognising this entity is for treatment planning. The pleural air collection in pneumothorax ex vacuo spontaneously resolves when the bronchial obstruction is relieved and the lobe re-expands. Treatment aimed to relieve the bronchial obstruction holds the priority, either by bronchoscopic measures or conservative approaches, rather than inserting a chest tube into the pleural space. Tube thoracostomy fails in these conditions as the primary pathology and mechanism of pneumothorax remain uncorrected.[9,10] Chest tubes are not beneficial to asymptomatic patients with malignant lung parenchymal disease and pneumothorax ex vacuo. Malignant infiltration of the lung tissue leads to poor lung compliance and unresponsiveness to chest tubes.[3,4] The therapy of choice in such patients, if their health status permits, is a parietal pleurectomy; this will remove the fibrinous peel and allow lung expansion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Berdan WE, Dee GJ, Abramson ST, Altman RP, Wung JT. Localized pneumothorax adjacent to a collapsed lobe: A sign of bronchial obstruction. Radiology. 1984;150:691–94. doi: 10.1148/radiology.150.3.6695068. [DOI] [PubMed] [Google Scholar]
- 2.Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest. 1996;110:1102–5. doi: 10.1378/chest.110.4.1102. [DOI] [PubMed] [Google Scholar]
- 3.Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170:943–6. doi: 10.2214/ajr.170.4.9530040. [DOI] [PubMed] [Google Scholar]
- 4.Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with ex vacuo pneumothorax after thoracentesis. Acad Radiol. 2005;12:980–6. doi: 10.1016/j.acra.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Rojas ORG, Weatherhead M, Hicks M, Dado C, Adam A. Pneumothorax Ex vacuo as initial presentation of endobronchial tuberculosis by Mycobacterium Bovis. Crit Care Med. 2022;50:S–358. [Google Scholar]
- 6.Kim YS, Susanto I, Lazar CA, Zarrinpar A, Eshaghian P, Smith MI, et al. Pneumothorax ex-vacuo or “trapped lung”in the setting of hepatic hydrothorax. BMC Pulm Med. 2012;12:78. doi: 10.1186/1471-2466-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajesh V, Divya R, Augustine J, Cleetus M. Flash pneumothorax in a young gentleman. Curr Med Res Pract. 2018;8:147–9. [Google Scholar]
- 8.Brahmandam S, Gauhar UA. Pneumothorax ex vacuo following bronchoscopic lung volume reduction. Am J Respir Crit Care Med. 2022;205:A4150. [Google Scholar]
- 9.Galvis AG, Bowen A, Oh KS. Nonexpandable lung after drainage of pneumothorax. AJR. 1981;136:1224–6. doi: 10.2214/ajr.136.6.1224. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka F, Ezaki H, lsobe J, Ueno Y, Inoue R, Ito M, et al. Four cases of spontaneous pneumothorax with no reexpansion of collapsed lung despite chest tube drainage. Kyobu Geka. 1992;45:515–8. [PubMed] [Google Scholar]


