Abstract
STUDY QUESTION
What is the current state-of-the-art methodology assessing decellularized extracellular matrix (dECM)-based artificial ovaries for treating ovarian failure?
SUMMARY ANSWER
Preclinical studies have demonstrated that decellularized scaffolds support the growth of ovarian somatic cells and follicles both in vitro and in vivo.
WHAT IS KNOWN ALREADY
Artificial ovaries are a promising approach for rescuing ovarian function. Decellularization has been applied in bioengineering female reproductive tract tissues. However, decellularization targeting the ovary lacks a comprehensive and in-depth understanding.
STUDY DESIGN, SIZE, DURATION
PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials were searched from inception until 20 October 2022 to systematically review all studies in which artificial ovaries were constructed using decellularized extracellular matrix scaffolds. The review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Two authors selected studies independently based on the eligibility criteria. Studies were included if decellularized scaffolds, regardless of their species origin, were seeded with ovarian cells or follicles. Review articles and meeting papers were removed from the search results, as were articles without decellularized scaffolds or recellularization or decellularization protocols, or control groups or ovarian cells.
MAIN RESULTS AND THE ROLE OF CHANCE
The search returned a total of 754 publications, and 12 papers were eligible for final analysis. The papers were published between 2015 and 2022 and were most frequently reported as coming from Iran. Detailed information on the decellularization procedure, evaluation method, and preclinical study design was extracted. In particular, we concentrated on the type and duration of detergent reagent, DNA and extracellular matrix detection methods, and the main findings on ovarian function. Decellularized tissues derived from humans and experimental animals were reported. Scaffolds loaded with ovarian cells have produced estrogen and progesterone, though with high variability, and have supported the growth of various follicles. Serious complications have not been reported.
LIMITATIONS, REASONS FOR CAUTION
A meta-analysis could not be performed. Therefore, only data pooling was conducted. Additionally, the quality of some studies was limited mainly due to incomplete description of methods, which impeded specific data extraction and quality analysis. Several studies that used dECM scaffolds were performed or authored by the same research group with a few modifications, which might have biased our evaluation.
WIDER IMPLICATIONS OF THE FINDINGS
Overall, the decellularization-based artificial ovary is a promising but experimental choice for substituting insufficient ovaries. A generic and comparable standard should be established for the decellularization protocols, quality implementation, and cytotoxicity controls. Currently, decellularized materials are far from being clinically applicable to artificial ovaries.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the National Natural Science Foundation of China (Nos. 82001498 and 81701438). The authors have no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
This systematic review is registered with the International Prospective Register of Systematic Reviews (PROSPERO, ID CRD42022338449).
Keywords: decellularization, artificial ovary, ovarian failure, extracellular matrix, ovarian tissue transplantation, methodology
WHAT DOES THIS MEAN FOR PATIENTS?
Ovarian failure will not only lead to fertility loss but can also affect a woman’s overall health and quality of life. One of the strategies under development to treat ovarian failure is the construction of artificial ovaries by encapsulating healthy ovarian cells or follicles into ovarian scaffolds. Decellularized scaffolds are created by the removal of the cells while preserving the natural tissue matrix; they have been widely studied in tissue regeneration research and have been applied in clinical practice. This systematic review was conducted to evaluate whether the decellularization-based artificial ovary can be used to restore ovarian function. Specifically, we provide detailed information on the ovarian decellularization procedure, evaluation method, and preclinical study design. The decellularized scaffolds loaded with ovarian cells can produce estrogen and progesterone, though with high variability, and support the growth of follicles, without reports of serious complications. Thus, the decellularization-based artificial ovary may be a promising choice for restoring ovarian function and improving female fertility in the future.
Introduction
Ovarian failure is characterized by the disruption of both endocrine and reproductive ovarian function and has gained increased interest in reproductive medicine, oncofertility, and organ aging (Mauri et al., 2020). Ovarian failure can be attributed to chemotherapy, radiotherapy, natural aging, or genetic predisposition (Sükür et al., 2014; Qin et al., 2015; Chemaitilly et al., 2017; Cui et al., 2018). An exhausted ovary will not only lead to fertility loss but can also increase the risks of cardiovascular disease, osteoporosis, and urogenital atrophy (Proserpio et al., 2020). As the average lifespan of women has exceeded 80 years, a naturally menopausal woman will spend almost 40% of her lifetime in the post-menopausal phase (Takahashi and Johnson, 2015). However, young women with primary ovarian insufficiency experience menopause even earlier. Ovarian failure can considerably affect a woman’s overall health, work productivity and quality of life. Accordingly, minimizing the adverse effects of ovarian failure is important and urgent.
Common treatments for ovarian failure include pharmacological medication, which mainly involves the supplementation of estrogen alone or estrogen–progestogen combinations. However, hormone replacement therapy should be implemented with particular caution, as the dosage, frequency, and time frame require individualization (Kling et al., 2019; Flores et al., 2021). Novel strategies such as ovarian tissue cryopreservation (OTC) and ovarian tissue transplantation (OTT) have arisen over the last two decades (Donnez et al., 2004), particularly for restoration of fertility after cancer treatments. To date, OTC and OTT have led to 189 deliveries and have been shown to restore ovarian function for many years (Donnez and Dolmans, 2017, 2018; Khattak et al., 2022). However, some groups and countries, such as the American Society of Clinical Oncology, consider OTC an experimental technique (Oktay et al., 2018). The method is also hampered by the risk of tumor reoccurrence due to hidden malignant cells within tissue grafts (Stern et al., 2014; Fajau-Prevot et al., 2017).
An artificial ovary is also a promising approach for rescuing ovarian function (Cho et al., 2019). It is constructed by encapsulating healthy ovarian cells or follicles in scaffolds to replace failed ovaries (Amorim and Shikanov, 2016). Various polymers have been utilized to create scaffolds to fabricate biomimetic functional ovaries. Synthetic polymers, such as gelatin-methacryloyl and polyethylene glycol, are easily manufactured and show enhanced mechanical properties but have limited cell adhesion sites (Mendez et al., 2018; Wu et al., 2022a). In contrast, alginate and fibrin are the two most commonly used natural materials as they have a large diversity of integrin-binding motifs and are more biocompatible. Decellularized ovaries are also based on these individual extracellular matrix (ECM) components and have the advantage of retaining the tissue inner spatial distribution of the tissue as well as its vascularization channels and mechanical properties. Decellularized ovaries are therefore attracting much attention in the field of organ regeneration (Kim et al., 2021b).
Decellularization refers to the removal of the cellular compartments while preserving the natural ECM with optimal porosity, stiffness and elasticity, thus yielding decellularized ECM (dECM) constructs (Fig. 1) (Saldin et al., 2017; Wu et al., 2022b). Decellularization has been widely studied in bone, heart, dermal tissues, and small intestinal submucosa, in both basic research and clinical practice (Bejleri and Davis, 2019; Xu et al., 2020; Datta et al., 2021). For example, commercial dECM products have been approved for pericardial reconstruction and have demonstrated good performance (van Rijswijk et al., 2020; Mohamed et al., 2021). Acellular dermal matrices are also available to promote breast reconstruction following mastectomy for breast cancer (Folli et al., 2018). Similar to the aforementioned dECM materials, decellularized ovarian scaffolds can also provide tissue-specific biomechanical cues to facilitate cell growth and can therefore be an ideal platform to support follicular development and restoration of ovarian function (Hoshiba, 2021). This encouraging method has been commonly discussed in female reproduction bioengineering topics (Gandolfi et al., 2020; Kim et al., 2021b; Francés-Herrero et al., 2022). However, a comprehensive and in-depth understanding of bioengineered ovaries is currently lacking. Since the first successful decellularization of human and bovine ovarian tissues in 2012, an increasing number of relevant papers has been published in recent years (Laronda et al., 2015). As many more different strategies have been proposed, there is an urgent need to compile previous work and to guide future studies.
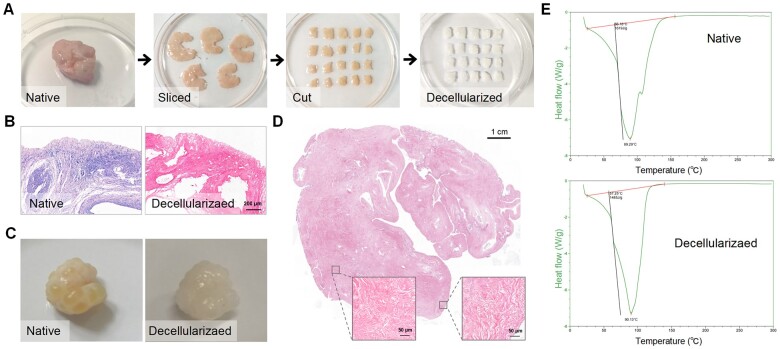
Figure 1.
The process and evaluation of extracellular matrix (ECM)-based scaffolds. (A) The color of the ovarian cortical strips turns from red to white after decellularization but displays comparable shapes. (B) H&E staining of the ovarian cortical strips. (C) The change in an intact porcine ovary after decellularization. (D) H&E staining shows the absence of basophilic materials in the decellularized tissues. (A–D) Reprinted with permission from Wu et al. (2022b). (E) Representative images of thermal analysis characterization; the different slopes indicate the change of ECM ultrastructure or motifs after decellularization. ECM, extracellular matrix; H&E, hematoxylin and eosin.
This systematic review summarizes the recent progress in constructing artificial ovaries based on dECM scaffolds, elucidates its application in restoring ovarian function, and provides a theoretical basis for future optimizations and improvements.
Materials and methods
Protocol and registration
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42022338449) and was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol (Moher et al., 2009). This systematic review aims to answer the question ‘What is the current state-of-the-art methodology to assess dECM-based artificial ovaries for treating ovarian failure?’ The search terms were based on a PICO (population, intervention, comparison and outcome) framework: animals and humans (P) with dECM-based artificial ovaries (I) as compared with controls (C) to support follicular growth or restore ovarian function (O) (Schardt et al., 2007).
Literature search
A systematic search was conducted in the PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials electronic medical databases from inception until 20 October 2022. We used the following search keywords to maximally cover the relevant literature: ‘decellularization’, ‘acellular’, ‘recellularization’, ‘ovary’, ‘ovarian tissue’, and ‘follicle’ (Supplementary Table S1). Articles were identified using MeSH headings and keywords combined with Boolean operators. There was no restriction on the date or publication status.
Eligibility criteria
Studies were included if the decellularized scaffolds were seeded with ovarian cells or follicles. For example, scaffolds derived from amniotic membranes and loaded with ovarian cells were included. Studies were excluded if: (i) the ovarian dECM scaffolds were not loaded with ovarian cells, (ii) the decellularization protocol was not described, (iii) the scaffold was not obtained by decellularization, (iv) no control group was established, (v) it was a repeated record, or (vi) it was a non-English language paper. Studies describing the changes before and after decellularization without control groups were excluded because the alterations might be confounded by placebo effects, or research bias or other changes in the experiment site (Grimes and Schulz, 2002). Different original articles by the same group using the same decellularization method were defined as repeated records and only the most recent version was retained.
Study selection and data collection
Two reviewers (T.W. and K.-C.H.) independently searched the electronic medical databases and selected studies based on the eligibility criteria (Fig. 2). Discrepancies between the selected studies by both authors were discussed in a consensus meeting with the senior authors (J.-J.Z. and S.-X.W.) providing a binding verdict.
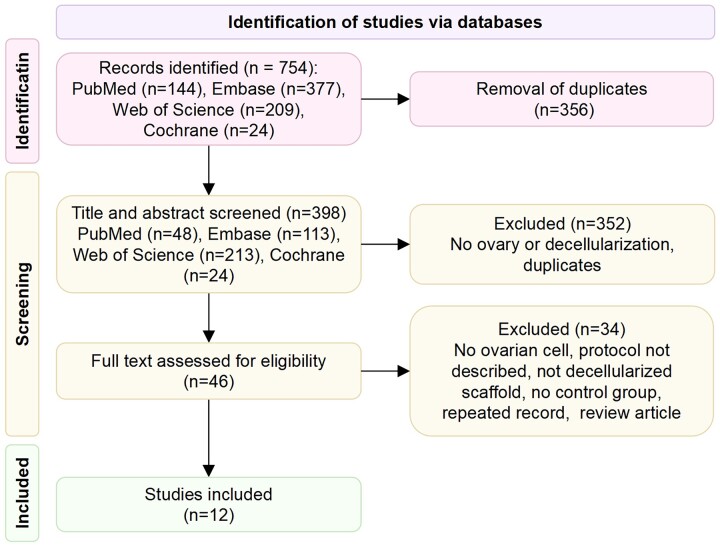
Figure 2.
Flow diagram of study selection on 20 October 2022.
Data extraction
Data were independently extracted by two reviewers (T.W. and J.-F.Y.). The outcomes of interest covered most ovary decellularization details and were classified into three groups: decellularization procedure, evaluation method, and preclinical study design. Specifically, the following information was recorded: author, publication year, country, animal species, preprocessing program, type and duration of detergent reagent and enzyme, biocompatibility, DNA and ECM detection methods, seeding cells, experimental grouping, and main findings.
The risk of bias was assessed by two independent blinded reviewers (T.W. and J.-F.Y.) using the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) tool (Hooijmans et al., 2014). The SYRCLE tool addresses selection bias, performance bias, attrition bias, detection bias and reporting bias. Each item of a study is assigned ‘yes’ (low risk of bias), ‘no’ (high risk of bias), or ‘unclear’ (insufficient details).
Results
Included articles
The initial electronic database search returned 754 papers, of which 398 remained after duplicates had been removed. There were 46 full-text studies remaining for assessment of the eligibility criteria. After full-text screening, 34 studies were excluded as they did not meet the inclusion criteria and 12 studies were included (Fig. 2, Supplementary Table S2). All 12 studies performed decellularization and recellularized dECM scaffolds with cells and compared them with the control groups. The studies were appraised for risk of bias (Supplementary Table S3). The allocation concealment was unclear in all studies, and the lack of information on housing of animals and observer blinding to the interventions resulted in a risk of performance bias. The attrition bias was either low risk (n = 5) or unclear (n = 7) in the included studies. The risks of selective reporting and other biases were low for most studies. The studies were published between 2015 and 2022 and were conducted in five countries, most frequently in Iran (n = 7), followed by China (n = 2), Belgium (n = 1), the USA (n = 1), and Italy (n = 1).
Characteristics of decellularization approaches
Of the 12 papers, 10 used one species (3 studies each used human and porcine tissue, respectively, 2 studies used mouse tissue, and 1 study each used bovine and sheep tissue, respectively). Two studies utilized tissues from humans and other species simultaneously (Table 1). The most common source of dECM scaffolds was ovarian tissues (n = 8), followed by amniotic membranes (n = 2), greater omentum (n = 1), and peritoneal membranes (n = 1) (Motamed et al., 2017; Sarabadani et al., 2021; Haghshenas et al., 2022). In seven studies, different regions of tissues (six ovaries and one amniotic membrane) were dissected and subsequently minced (Supplementary Table S4) (Laronda et al., 2015; Motamed et al., 2017; Hassanpour et al., 2018; Nikniaz et al., 2021; Chiti et al., 2022; Zheng et al., 2022; Wu et al., 2022b). The greater omentum and amniotic membrane were directly cut into small fragments (Fazelian-Dehkordi et al., 2022; Haghshenas et al., 2022). Four studies conducted freeze–thaw cycles. Fazelian-Dehkordi et al., (2022) performed the most complicated preprocessing, with six steps that spanned more than 2 days.
Table 1.
Characteristics of decellularization procedures.
| Study ID | Species | Tissue | Detergent reagent | Enzyme |
|---|---|---|---|---|
| Alaee et al. (2021) | Mouse | Ovary | 1% SLES (4 h) | Not used |
| Chiti et al. (2022) | Bovine | Ovary |
|
0.05% trypsin/0.02% EDTA (1 h) |
| Fazelian-Dehkordi et al. (2022) | Sheep | Great omentum |
|
Not used |
| Haghshenas et al. (2022) | Human | Amniotic membrane |
|
Not used |
| Hassanpour et al. (2018) | Human | Ovary |
|
500 U/ml DNase I (24 h) |
| Laronda et al. (2015) | Human and bovine | Ovary |
|
Not used |
| Motamed et al. (2017) | Human | Amniotic membrane | Not used | 0.25% trypsin/0.02% EDTA (2 h) |
| Nikniaz et al. (2021) | Human and porcine | Ovary |
|
Not used |
| Pennarossa et al. (2021) | Porcine | Ovary |
|
Not used |
| Sarabadani et al. (2021) | Mouse | Peritoneal membrane |
|
|
| Wu et al. (2022b) | Porcine | Ovary |
|
RNase/DNase 80 U/ml (6 h) |
| Zheng et al. (2022) | Porcine | Ovary |
|
50 U/ml DNase I/1 U/ml RNase (12.5 h) |
EDTA, ethylene diamine tetraacetic acid; SDC, sodium deoxycholate; SDS, sodium dodecyl sulfate; SLES, sodium lauryl ester sulfate; PBS, phosphate-buffered saline; PMSF, phenylmethylsulfonyl fluoride.
Decellularization is greatly affected by detergent type, exposure time, and incubation temperature. Balancing these factors to sufficiently remove cells and preserve the ECM is vital for decellularization. Four studies used solo chemicals for decellularization, where two studies each used sodium dodecyl sulfate (SDS) and sodium lauryl ester sulfate (SLES), respectively. Seven studies used detergent combinations (Nikniaz et al., 2021; Pennarossa et al., 2021; Sarabadani et al., 2021; Chiti et al., 2022; Fazelian-Dehkordi et al., 2022; Zheng et al., 2022; Wu et al., 2022b). From the perspective of chemical types, ionic detergents (SDS, sodium deoxycholate, SLES) were the most commonly used reagents (n = 10) (Laronda et al., 2015; Hassanpour et al., 2018; Alaee et al., 2021; Nikniaz et al., 2021; Pennarossa et al., 2021; Chiti et al., 2022; Haghshenas et al., 2022; Zheng et al., 2022; Wu et al., 2022b), and five studies used non-ionic detergents, mainly Triton X-100 (Nikniaz et al., 2021; Pennarossa et al., 2021; Chiti et al., 2022; Zheng et al., 2022; Wu et al., 2022b). Less frequently used detergents included ammonium hydroxide and 2-propanol. Trypsin/EDTA and DNase/RNase solution were equally frequently used enzymes following detergents (n = 6) (Motamed et al., 2017; Hassanpour et al., 2018; Sarabadani et al., 2021; Chiti et al., 2022; Zheng et al., 2022; Wu et al., 2022b). In three studies, the dECM scaffolds were further processed into hydrogels, which were easier to use and apply (Supplementary Table S4) (Chiti et al., 2022; Haghshenas et al., 2022; Zheng et al., 2022). Regarding the operation duration, most studies completed decellularization within several days, except for those that used intact ovaries, where the operation spanned >3 weeks (Laronda et al., 2015; Hassanpour et al., 2018).
Efficiency evaluation methods
The accepted standard for evaluating decellularization efficacy is: (i) maximal 50 ng/mg DNA per dry weight ECM, (ii) maximum 200-bp DNA fragment size, and (iii) negative histology for nuclear materials in tissues stained with 4′,6-diamidino-2-phenylindole (DAPI) or hematoxylin and eosin (H&E) (Crapo et al., 2011). The DNA content within scaffolds was qualitatively described and quantitatively detected in all 12 studies (Table 2). However, DNA content >50 ng/mg was observed in two studies, which indicated insufficient decellularization (Pennarossa et al., 2021; Haghshenas et al., 2022). Resident ECM proteins were observed using connective tissue staining, such as Masson trichrome, Alcian blue, and periodic acid-Schiff staining, in seven studies. Collagen type I and IV, laminin, and fibronectin were analyzed by immunological methods in three studies (Laronda et al., 2015; Hassanpour et al., 2018; Wu et al., 2022b). Glycosaminoglycans were quantitatively determined in four studies (Motamed et al., 2017; Chiti et al., 2022; Fazelian-Dehkordi et al., 2022; Haghshenas et al., 2022). Other staining techniques such as Gomori (n = 2), Heidenhain’s Azan (n = 1), Mallory (n = 1) and orcein (n = 1) were also reported. In all studies, the ultrastructure was evaluated by scanning electron microscope (Supplementary Table S4). Raman spectrum was calculated in one study to analyze the DNA and ECM (Alaee et al., 2021). Additionally, three studies tested the rheological properties of the dECM hydrogels (Chiti et al., 2022; Haghshenas et al., 2022; Zheng et al., 2022). Eight studies routinely examined cytotoxicity and biocompatibility to rule out unwanted adverse effects (Motamed et al., 2017; Hassanpour et al., 2018; Nikniaz et al., 2021; Pennarossa et al., 2021; Fazelian-Dehkordi et al., 2022; Haghshenas et al., 2022; Zheng et al., 2022; Wu et al., 2022b).
Table 2.
Evaluation of the decellularized extracellular matrix scaffolds.
| Study ID | DNA examination |
ECM examination |
||
|---|---|---|---|---|
| Description | Quantification (ng/mg) | Description | Quantification | |
| Alaee et al. (2021) | H&E, Hoechst, Raman microscope | 21.45 ± 3.36 | MT, AB, Raman microscope | Not reported |
| Chiti et al. (2022) | Not reported | + (dng) | Not reported | Collagen (not significant, 30.67 ± 0.2 μg/mg), GAG (dng) |
| Fazelian-Dehkordi et al. (2022) | H&E, Hoechst | <50 | Aldehyde fuchsine, PAS, oil red, AB, methylene blue. | VEGF (500 ng/l), GAG (dng) |
| Haghshenas et al. (2022) | H&E, DAPI | 114 ± 32.46 | MT, AB | Collagen (not significant), GAG (decrease, 166.2 ± 5.87 μg/mg) |
| Hassanpour et al. (2018) | H&E, Hoechst | 40 ± 7.33 | Heidenhain’s AZAN, MT, Gomori, AB, IHC (COL1, COL4, LAM, FN) | Not reported |
| Laronda et al. (2015) | H&E, DAPI | + (dng) | IF (COL1, COL4, LAM, FN) | Not reported |
| Motamed et al. (2017) | H&E | 39.38 ± 4.04 | MT | GAG (decrease, 43 ± 3.08 μg/mg) |
| Nikniaz et al. (2021) | H&E, DAPI | + (dng) | Orcein, MT | Not reported |
| Pennarossa et al. (2021) | H&E, DAPI | 50 ± 30 | MT, Mallory, AB, Gomori | Not reported |
| Sarabadani et al. (2021) | H&E, DAPI | 8.98 | MT, PAS | Not reported |
| Wu et al. (2022b) | H&E, DAPI, gel electrophoresis | 12.86 ± 1.707 | MT, AB, PAS, sirius red, IHC (COL1, COL2, COL3, COL4, FN, LAM, AMH, TGFB, BMP15, CTGF) | Collagen (not significant), length, width, straightness of collagen |
| Zheng et al. (2022) | H&E, DAPI | 48.48 ± 1.88 | MT, Toluidine blue | Not reported |
AB, Alcian blue; AMH: anti-Mullerian hormone; BMP, bone morphogenetic protein; COL, collagen; CTGF, connective tissue growth factor; DAPI, 4′,6-diamidino-2-phenylindole; dng, data not given; ECM, extracellular matrix; FN, fibronectin; H&E, hematoxylin and eosin; IF, immunofluorescence; IHC: immunohistochemistry; GAG, glycosaminoglycans; LAM, laminin; MT, Masson trichrome; PAS, periodic acid-Schiff; TGFB, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Functional restoration of dECM-based artificial ovaries
The ovarian scaffolds were successfully recellularized with follicles (n = 6), ovarian somatic cells (n = 5), cumulus–oocyte complex (n = 1), and epigenetically erased dermal fibroblasts (n = 1) (Supplementary Table S4). Mouse cells were most commonly used (n = 10). Eight groups cultured preantral follicles for 7–12 days on dECM scaffolds in vitro (Alaee et al., 2021; Chiti et al., 2022). The seeding cells or follicles were mainly assessed based on the follicle survival rate, growth diameter, antrum formation, oocyte maturation, hormone secretion, and mRNA/protein markers (Table 3) (Laronda et al., 2015; Motamed et al., 2017; Hassanpour et al., 2018; Alaee et al., 2021; Sarabadani et al., 2021; Haghshenas et al., 2022; Zheng et al., 2022). Three studies demonstrated increased estradiol (E2) or progesterone (P4) (Motamed et al., 2017; Alaee et al., 2021; Sarabadani et al., 2021). Four studies evaluated in vivo follicular development or ovarian function. Similar to the in vitro results, the dECM-based artificial ovaries contributed to higher E2 or P4 or inhibin A compared with those in ovariectomized mice (Laronda et al., 2015; Hassanpour et al., 2018; Zheng et al., 2022). The molecular marker growth differentiation factor (GDF) 9 and GDF15, specifically expressed in oocytes, were the most studied markers (n = 2 each) (Motamed et al., 2017; Alaee et al., 2021; Pennarossa et al., 2021). Altogether, all studies demonstrated the feasibility and benefits of dECM-based artificial ovaries.
Table 3.
Preclinical study design of decellularized extracellular matrix scaffolds.
| Study ID | Grouping | Main finding |
|---|---|---|
| Alaee et al. (2021) |
|
|
| Chiti et al. (2022) |
|
|
| Fazelian-Dehkordi et al. (2022) |
|
|
| Haghshenas et al. (2022) |
|
|
| Hassanpour et al. (2018) |
|
|
| Laronda et al. (2015) |
|
|
| Motamed et al. (2017) |
|
|
| Nikniaz et al. (2021) |
|
Higher ratio of follicular recovery in ② than ①. |
| Pennarossa et al. (2021) |
|
|
| Sarabadani et al. (2021) |
|
|
| Wu et al. (2022b) |
|
|
| Zheng et al. (2022) |
|
|
Bax, Bcl2 associated x; Bcl2, Bcl2 apoptosis regulator; Bmp, bone morphogenetic protein; Cyp, Cytochrome P450; DAPI, 4′,6-diamidino-2-phenylindole; DAZL, deleted in azoospermia like; dECM, decellularized extracellular matrix; E2, estradiol; EpiE, epigenetically erased dermal fibroblast; ER, estrogen receptor; FBS, fetal bovine serum; FSH, follicle stimulating hormone; Gdf, growth differentiation factor; GVBD, germinal vesicle breakdown; H&E, hematoxylin and eosin; INH, inhibin; nsd, not significant difference; MII, metaphase II; OSC, ovarian comatic cell; OVX, ovariectomy; P4, progesterone; PR, progestogen receptor; Star, steroidogenic acute regulatory; Thy1, Thy-1 cell surface antigen; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; Vim, vimentin; ZP, zona pellucida glycoprotein.
Discussion
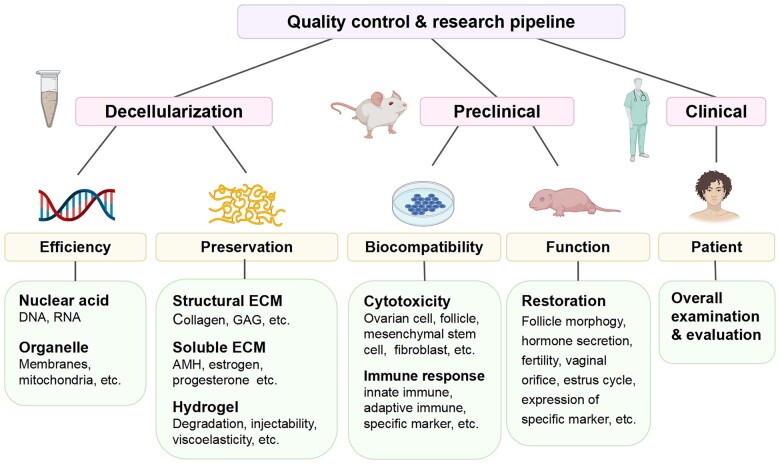
This systematic review suggests that dECM materials hold promise for constructing artificial ovaries and counteracting ovarian failure. A total of 12 studies were included and the decellularization methods and evaluation parameters varied greatly. An optimal reproducible and standardized procedure is a prerequisite for future clinical application (Fig. 3) (Naso and Gandaglia, 2022). Both preclinical and clinical trials should comply with the quality control and research pipelines. For these reasons, the evaluations of the following characteristics of ovarian-specific dECM scaffolds are proposed: (i) DNA removal efficiency; (ii) ECM preservation; (iii) cell debris residues; (iv) biocompatibility; and (v) restoration of ovarian function.
Figure 3.
Quality controls and clinical directions for decellularized ovaries. AMH, anti-Mullerian hormone; DNA, deoxyribonucleic acid; ECM, extracellular matrix; GAG, glycosaminoglycan; RNA, ribonucleic acid.
Effective decellularization is reflected by the adequate removal of cellular components and good preservation of ECM proteins (Luo et al., 2019). The tissue type, animal species, chemical reagents, and exposure time all affect the efficiency and should be balanced (Eivazkhani et al., 2019). For example, minimizing the detergent concentration contributes to more retention of ECM, but it might also cause insufficient cell removal, risking a relevant immune response after in vivo implantation (Luo et al., 2019; Chakraborty et al., 2020). Regarding the DNA evaluation methods, it was interesting that only one study performed electrophoresis, while all studies conducted H&E/DAPI staining and DNA extraction. We speculated that this might be due to the complex manipulation processes compared with histological staining and DNA quantification (Lee et al., 2012). However, neither DNA staining nor quantification can substitute for the evaluation of DNA size, as the two methods occasionally cannot detect the minimal virus DNA contents, which may nevertheless elicit an immune response (Chakraborty et al., 2020). Furthermore, peracetic acid/ethanol treatment eliminates small DNA debris (Hodde and Hiles, 2002). Altogether, there should be more focus on comprehensive assessment of DNA residues, especially DNA fragments.
ECM is composed of structural and soluble components. The former includes collagen, laminin, fibronectin and elastin (Naba et al., 2017; Yuzhalin et al., 2018). The Masson trichrome and Heidenhain’s Azan staining method mainly detects fibrillar collagens (Polat et al., 2007). Mallory staining can distinguish collagens from elastin (Chambrone et al., 2015), while the Alcian blue staining is specific for glycosaminoglycans (Mead, 2020). Immunological methods can reveal the ECM protein distribution via antigen-antibody binding. However, the fact that various methods serve similar purposes and produce repeated results remains an issue. Most studies neglect other equally important molecules such as soluble hormones and growth factors, and properties such as stiffness (Fazelian-Dehkordi et al., 2022; Wu et al., 2022b). To address these problems, it is suggested that dECM undergo enzyme-linked immunosorbent assay (ELISA), stress relaxation testing and atomic force microscopy. Ovaries are important endocrine glands that secrete estrogen, progesterone, and anti-Müllerian hormone (Leong, 2018). Nevertheless, the amounts of these components within dECM scaffolds remain obscure although they are expected to facilitate the growth of seeding cells. Recently, ECM mechanical cues were demonstrated to underlie the development of polycystic ovary syndrome and in vitro activation (Lunde et al., 2001; Wood et al., 2015; Kawamura et al., 2016). ECM accumulation along with aging leads to a fibrotic ovary and worsens follicle development (Shah et al., 2018), which underlines the fact that dECM rigidity should be tested and modified for artificial ovary construction (Chiti et al., 2018).
The dECM-derived artificial ovaries demonstrate great potential for restoring ovarian function. In most of the included studies, preantral follicles formed the antral cavity, underwent maturation, and produced estradiol after reseeding on the dECM scaffolds. Many groups achieved healthy follicle development both in vitro and in vivo. Nevertheless, further progress concerning any pregnancy or live birth of experimental animals with the dECM scaffolds has never been reported, which would be a milestone in the field of decellularized materials. Additionally, more in-depth studies concerning safety are needed.
Future perspectives
In addition to direct application as solid platforms, dECM-based materials can also be produced via 3D printing and microfluidic chips. Such bioengineering approaches have recently been applied in the vagina and for ovary regeneration (Hou et al., 2021; Zheng et al., 2022). After a series of lyophilization, pulverization, digestion, and solubilization, the dECM powder is formed into a hydrogel, serving as a printable bioink (Kim et al., 2019). Meanwhile, the rheological, flow and gelatin behaviors of the bioinks are characterized to aid parameter optimization during printing (Das et al., 2019). The bioink extrusion speed, nozzle routing, strut distance, and layer height should be coordinated. Finally, the dECM hydrogel is shaped via 3D printing and a biomimetic ovary mimicking the actual cell arrangement is precisely fabricated (Laronda et al., 2017). Mixing dECM hydrogel with collagens is expected to create a more rigid environment similar to that of ovary fibrosis and the cortex of polycystic ovary syndrome (Filatov et al., 2015; Ouni et al., 2020; Kim et al., 2021a). The combination of dECM and hyaluronic acid is suitable for the survival of the cumulus cell-oocyte complex, which is essential for ovulation and fertilization (Briggs et al., 2015; Serati et al., 2015). When integrating with microfluidic chips, the decellularization and recellularization manipulation can be reproduced directly in the devices or used as medium to fill the chips (Hong et al., 2017; Palikuqi et al., 2020; Bhatt et al., 2022). The latter provides a dynamic stimulus to the cumulus cell–oocyte complex or denuded oocytes, which improves the outcomes of oocyte maturation and IVF (Nagashima et al., 2018; Podwin et al., 2020; Healy et al., 2021; Sadeghzadeh Oskouei et al., 2021). Altogether, the integration of these advanced culture systems will facilitate the development of ovarian regeneration and drug-testing models.
Even though decellularization eliminates most immunogenic substances, adverse effects that include inflammatory reactions, fibrosis and calcification have been recorded (Padalino et al., 2016; Woo et al., 2016; Hofmann et al., 2017). Most studies did not focus adequately on evaluating the residual antigenicity. α galactosidase (αGal) is the major xenogeneic antigen that causes rejection-related responses, but no included studies examined the αGal level (Li et al., 2021). It is also suggested that dECM is elevated for the release of interleukins, chemokines and other cytokines; therefore, blockade intervention is recommended in xenotransplantation (Islam et al., 2021). Antigenicity is also associated with the decellularization protocols. Prolonged operation times and increased detergent concentrations are used to reinforce decellularization efficiency. However, these adjustments may further expose the hidden antigenic motifs in ECM components, such as laminin, aggrecan, versican, collagen types I and IV, and hyaluronan (Chakraborty et al., 2020). The 7S domain constitutes the amino-terminal end of type IV collagen; when exposed after decellularization, it will cause a neutrophil chemotactic response (Senior et al., 1989). Hyaluronan is an abundant ECM component enriched in follicular fluid and ovarian stroma. However, it also has diverse roles in chronic inflammation and immune cell activation and can even cause autoimmune diseases (Johnson et al., 2018; Nagy et al., 2019). The matrikines refer to a group of ECM fragments and are inactive in most cases. However, structural and conformational alterations in ECM proteins may result in matrikine release by proteolysis, which contributes to fibrosis, cancer, and aging (Abdul Roda et al., 2015; Jariwala et al., 2022). In some circumstances, these minor alterations occur in ECM ultrastructure and cannot be observed via histological staining or electrical microscopy. In such cases, thermal analysis characterization using differential scanning calorimetry can be used (Samouillan et al., 1999; Wu et al., 2022b). To summarize, appropriate selection criteria are a prerequisite to identify the antigenic motif or matrikine on xenogeneic decellularized tissue to avoid the possibility of interspecies reaction upon clinical application.
Conclusion
To our knowledge, this is the first systematic review to provide a broad overview on the current state-of-the-art of dECM-based-artificial ovaries. Artificial ovaries provide promising opportunities to restore ovarian function, yet animal studies and preclinical applications of dECM-derived artificial ovaries have only just begun. It is important to comprehensively assess the decellularized scaffolds and demonstrate their effectiveness. Standardizing decellularization protocols and the implementation of quality controls and cytotoxicity measurements will enable the construction of generic and comparable dECM-based artificial ovaries in the future.
Supplementary Material
Contributor Information
Tong Wu, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Ke-Cheng Huang, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Jin-Feng Yan, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan, China.
Jin-Jin Zhang, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Shi-Xuan Wang, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
All data used for the study have been included in the article and Supplementary Material.
Authors’ roles
T.W. and S.-X.W. conceptualized the study. T.W. and K.-C.H. performed the search and assessed studies for eligibility. T.W. and J.-F.Y. extracted the data and conducted the quality appraisal with secondary reviews from J.-J.Z. and S.-X.W. T.W. wrote the first draft of the manuscript, with inputs from K.-C.H., J.-F.Y., J.-J.Z. and S.-X.W. J.-J.Z. and S.-X.W. coordinated and supervised the planning and execution. All authors provided inputs into the subsequent editing of the article.
Funding
This study was funded by the National Natural Science Foundation of China (Nos. 82001498 and 81701438).
Conflict of interest
The authors declare no competing interests.
References
- Abdul Roda M, Fernstrand AM, Redegeld FA, Blalock JE, Gaggar A, Folkerts G.. The matrikine PGP as a potential biomarker in COPD. Am J Physiol Lung Cell Mol Physiol 2015;308:L1095–L1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaee S, Asadollahpour R, Hosseinzadeh Colagar A, Talaei-Khozani T.. The decellularized ovary as a potential scaffold for maturation of preantral ovarian follicles of prepubertal mice. Syst Biol Reprod Med 2021;67:413–427. [DOI] [PubMed] [Google Scholar]
- Amorim CA, Shikanov A.. The artificial ovary: current status and future perspectives. Future Oncol 2016;12:2323–2332. [DOI] [PubMed] [Google Scholar]
- Bejleri D, Davis ME.. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater 2019;8:e1801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A, Dhiman N, Giri PS, Kasinathan GN, Pati F, Rath SN.. Biocompatibility-on-a-chip: Characterization and evaluation of decellularized tendon extracellular matrix (tdECM) hydrogel for 3D stem cell culture in a microfluidic device. Int J Biol Macromol 2022;213:768–779. [DOI] [PubMed] [Google Scholar]
- Briggs DC, Birchenough HL, Ali T, Rugg MS, Waltho JP, Ievoli E, Jowitt TA, Enghild JJ, Richter RP, Salustri A. et al. Metal ion-dependent heavy chain transfer activity of TSG-6 mediates assembly of the cumulus-oocyte matrix. J Biol Chem 2015;290:28708–28723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty J, Roy S, Ghosh S.. Regulation of decellularized matrix mediated immune response. Biomater Sci 2020;8:1194–1215. [DOI] [PubMed] [Google Scholar]
- Chambrone L, Chambrone LA, Tatakis DN, Costa Hanemann JA, Shibli JA, Nevins M.. Wound healing of the laterally positioned flap: a histomorphometric assessment. Int J Periodontics Restorative Dent 2015;35:785–792. [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, Klosky JL, Barnes N, Clark KL, Farr JB. et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab 2017;102:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, Van Ruymbeke E, Champagne SD, Donnez J, Amorim CA.. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet 2018;35:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti MC, Vanacker J, Ouni E, Tatic N, Viswanath A, Des Rieux A, Dolmans MM, White LJ, Amorim CA.. Ovarian extracellular matrix-based hydrogel for human ovarian follicle survival in vivo: a pilot work. J Biomed Mater Res B Appl Biomater 2022;110:1012–1022. [DOI] [PubMed] [Google Scholar]
- Cho E, Kim YY, Noh K, Ku SY.. A new possibility in fertility preservation: the artificial ovary. J Tissue Eng Regen Med 2019;13:1294–1315. [DOI] [PubMed] [Google Scholar]
- Crapo PM, Gilbert TW, Badylak SF.. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Stern C, Hickey M, Goldblatt F, Anazodo A, Stevenson WS, Phillips KA.. Preventing ovarian failure associated with chemotherapy. Med J Aust 2018;209:412–416. [DOI] [PubMed] [Google Scholar]
- Das S, Kim SW, Choi YJ, Lee S, Lee SH, Kong JS, Park HJ, Cho DW, Jang J.. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater 2019;95:188–200. [DOI] [PubMed] [Google Scholar]
- Datta S, Rameshbabu AP, Bankoti K, Roy M, Gupta C, Jana S, Das AK, Sen R, Dhara S.. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater Sci Eng C Mater Biol Appl 2021;119:111604. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A.. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004;364:1405–1410. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM.. Fertility preservation in women. N Engl J Med 2017;377:1657–1665. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM.. Natural hormone replacement therapy with a functioning ovary after the menopause: dream or reality? Reprod Biomed Online 2018;37:359–366. [DOI] [PubMed] [Google Scholar]
- Eivazkhani F, Abtahi NS, Tavana S, Mirzaeian L, Abedi F, Ebrahimi B, Montazeri L, Valojerdi MR, Fathi R.. Evaluating two ovarian decellularization methods in three species. Mater Sci Eng C Mater Biol Appl 2019;102:670–682. [DOI] [PubMed] [Google Scholar]
- Fajau-Prevot C, Le Gac YT, Chevreau C, Cohade C, Gatimel N, Parinaud J, Leandri R.. Ovarian mucinous cystadenoma after ovarian graft. Obstet Gynecol 2017;129:1035–1036. [DOI] [PubMed] [Google Scholar]
- Fazelian-Dehkordi K, Talaei-Khozani T, A SFM.. Three-dimensional in vitro maturation of rabbit oocytes enriched with sheep decellularized greater omentum. Vet Med Sci 2022;8:2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov MA, Khramova YV, Semenova ML.. In vitro mouse ovarian follicle growth and maturation in alginate hydrogel: current state of the art. Acta Nat 2015;7:48–56. [PMC free article] [PubMed] [Google Scholar]
- Flores VA, Pal L, Manson JE.. Hormone therapy in menopause: concepts, controversies, and approach to treatment. Endocr Rev 2021;42:720–752. [DOI] [PubMed] [Google Scholar]
- Folli S, Curcio A, Melandri D, Bondioli E, Rocco N, Catanuto G, Falcini F, Purpura V, Mingozzi M, Buggi F. et al. A new human-derived acellular dermal matrix for breast reconstruction available for the European market: preliminary results. Aesthetic Plast Surg 2018;42:434–441. [DOI] [PubMed] [Google Scholar]
- Francés-Herrero E, Lopez R, Hellström M, De Miguel-Gómez L, Herraiz S, Brännström M, Pellicer A, Cervelló I.. Bioengineering trends in female reproduction: a systematic review. Hum Reprod Update 2022;28:798–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi F, Ghiringhelli M, Brevini TAL.. Bioengineering the ovary to preserve and reestablish female fertility. Anim Reprod 2020;16:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF.. Bias and causal associations in observational research. Lancet 2002;359:248–252. [DOI] [PubMed] [Google Scholar]
- Haghshenas M, Tavana S, Zand E, Montazeri L, Fathi R.. Mouse ovarian follicle growth in an amniotic membrane-based hydrogel. J Biomater Appl 2022;37:563–574. [DOI] [PubMed] [Google Scholar]
- Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z.. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther 2018;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy MW, Dolitsky SN, Villancio-Wolter M, Raghavan M, Tillman AR, Morgan NY, DeCherney AH, Park S, Wolff EF.. Creating an artificial 3-dimensional ovarian follicle culture system using a microfluidic system. Micromachines (Basel) 2021;12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodde J, Hiles M.. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol Bioeng 2002;79:211–216. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Schmiady MO, Burkhardt BE, Dave HH, Hübler M, Kretschmar O, Bode PK.. Congenital aortic valve repair using CorMatrix(®): a histologic evaluation. Xenotransplantation 2017;24:e12341. [DOI] [PubMed] [Google Scholar]
- Hong Y, Koh I, Park K, Kim P.. On-chip fabrication of a cell-derived extracellular matrix sheet. ACS Biomater Sci Eng 2017;3:3546–3552. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW.. SYRCLEs risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiba T. Decellularized extracellular matrix for cell biology. Curr Protoc 2021;1:e318. [DOI] [PubMed] [Google Scholar]
- Hou C, Zheng J, Li Z, Qi X, Tian Y, Zhang M, Zhang J, Huang X.. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int J Biol Macromol 2021;180:177–186. [DOI] [PubMed] [Google Scholar]
- Islam R, Islam MM, Nilsson PH, Mohlin C, Hagen KT, Paschalis EI, Woods RL, Bhowmick SC, Dohlman CH, Espevik T. et al. Combined blockade of complement C5 and TLR co-receptor CD14 synergistically inhibits pig-to-human corneal xenograft induced innate inflammatory responses. Acta Biomater 2021;127:169–179. [DOI] [PubMed] [Google Scholar]
- Jariwala N, Ozols M, Bell M, Bradley E, Gilmore A, Debelle L, Sherratt MJ.. Matrikines as mediators of tissue remodelling. Adv Drug Deliv Rev 2022;185:114240. [DOI] [PubMed] [Google Scholar]
- Johnson P, Arif AA, Lee-Sayer SSM, Dong Y.. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front Immunol 2018;9:2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Hsueh AJ.. Activation of dormant follicles: a new treatment for premature ovarian failure?, Curr Opin Obstet Gynecol 2016;28:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, Silber S, Gook D, Demeestere I, Bystrova O. et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Hum Reprod Update 2022;28:400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim M, Hwang DG, Shim IK, Kim SC, Jang J.. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue constructs. J Vis Exp 2019;e60434. [DOI] [PubMed] [Google Scholar]
- Kim J, Kong JS, Kim H, Han W, Won JY, Cho DW.. Maturation and protection effect of retinal tissue-derived bioink for 3D cell printing technology. Pharmaceutics 2021a;13:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Kim YY, Kim H, Ku SY.. Recent advancements in engineered biomaterials for the regeneration of female reproductive organs. Reprod Sci 2021b;28:1612–1625. [DOI] [PubMed] [Google Scholar]
- Kling JM, Dowling NM, Bimonte-Nelson HA, Gleason CE, Kantarci K, Manson JE, Taylor HS, Brinton EA, Lobo RA, Cedars MI. et al. Impact of menopausal hormone formulations on pituitary-ovarian regulatory feedback. Am J Physiol Regul Integr Comp Physiol 2019;317:R912–R920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK.. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015;50:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN.. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 2017;8:15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PY, Costumbrado J, Hsu CY, Kim YH.. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp 2012;e3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong I. Reproductive endocrinology: restoring ovarian function. Nat Rev Endocrinol 2018;14:66. [DOI] [PubMed] [Google Scholar]
- Li P, Walsh JR, Lopez K, Isidan A, Zhang W, Chen AM, Goggins WC, Higgins NG, Liu J, Brutkiewicz RR. et al. Genetic engineering of porcine endothelial cell lines for evaluation of human-to-pig xenoreactive immune responses. Sci Rep 2021;11:13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde O, Djøseland O, Grøttum P.. Polycystic ovarian syndrome: a follow-up study on fertility and menstrual pattern in 149 patients 15-25 years after ovarian wedge resection. Hum Reprod 2001;16:1479–1485. [DOI] [PubMed] [Google Scholar]
- Luo Y, Lou D, Ma L, Gao C.. Optimizing detergent concentration and processing time to balance the decellularization efficiency and properties of bioprosthetic heart valves. J Biomed Mater Res A 2019;107:2235–2243. [DOI] [PubMed] [Google Scholar]
- Mauri D, Gazouli I, Zarkavelis G, Papadaki A, Mavroeidis L, Gkoura S, Ntellas P, Amylidi AL, Tsali L, Kampletsas E.. Chemotherapy associated ovarian failure. Front Endocrinol (Lausanne) 2020;11:572388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead TJ. Alizarin red and alcian blue preparations to visualize the skeleton. Methods Mol Biol 2020;2043:207–212. [DOI] [PubMed] [Google Scholar]
- Mendez U, Zhou H, Shikanov A.. Synthetic PEG hydrogel for engineering the environment of ovarian follicles. Methods Mol Biol 2018;1758:115–128. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Patel AJ, Mazhar K, Jeeji R, Ridley PD, Balacumaraswami L.. Anterior leaflet replacement and reconstruction with Admedus Cardiocel™ decellularized pericardial patch in tricuspid valve endocarditis. J Surg Case Rep 2021;2021:rjab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed M, Sadr Z, Valojerdi MR, Moini A, Oryan S, Totonchi M, Ebrahimi B, Maroufizadeh S, Taghiabadi E, Fathi R.. Tissue engineered human amniotic membrane application in mouse ovarian follicular culture. Ann Biomed Eng 2017;45:1664–1675. [DOI] [PubMed] [Google Scholar]
- Naba A, Pearce OMT, Del Rosario A, Ma D, Ding H, Rajeeve V, Cutillas PR, Balkwill FR, Hynes RO.. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res 2017;16:3083–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima JB, El Assal R, Songsasen N, Demirci U.. Evaluation of an ovary-on-a-chip in large mammalian models: species specificity and influence of follicle isolation status. J Tissue Eng Regen Med 2018;12:e1926–e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Kuipers HF, Marshall PL, Wang E, Kaber G, Bollyky PL.. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol 2019;78–79:292–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso F, Gandaglia A.. Can heart valve decellularization be standardized? A review of the parameters used for the quality control of decellularization processes. Front Bioeng Biotechnol 2022;10:830899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikniaz H, Zandieh Z, Nouri M, Daei-Farshbaf N, Aflatoonian R, Gholipourmalekabadi M, Jameie SB.. Comparing various protocols of human and bovine ovarian tissue decellularization to prepare extracellular matrix-alginate scaffold for better follicle development in vitro. BMC Biotechnol 2021;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW.. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- Ouni E, Bouzin C, Dolmans MM, Marbaix E, Pyr Dit Ruys S, Vertommen D, Amorim CA.. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum Reprod 2020;35:1391–1410. [DOI] [PubMed] [Google Scholar]
- Padalino MA, Castaldi B, Fedrigo M, Gallo M, Zucchetta F, Vida VL, Milanesi O, Angelini A, Stellin G.. Porcine intestinal submucosa (CorMatrix) for semilunar valve repair in children: a word of caution after midterm results. Semin Thorac Cardiovasc Surg 2016;28:436–445. [DOI] [PubMed] [Google Scholar]
- Palikuqi B, Nguyen DT, Li G, Schreiner R, Pellegata AF, Liu Y, Redmond D, Geng F, Lin Y, Gómez-Salinero JM. et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020;585:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarossa G, De Iorio T, Gandolfi F, Brevini TAL.. Ovarian decellularized bioscaffolds provide an optimal microenvironment for cell growth and differentiation in vitro. Cells 2021;10:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podwin A, Lizanets D, Przystupski D, Kubicki W, Śniadek P, Kulbacka J, Wymysłowski A, Walczak R, Dziuban JA.. Lab-on-chip platform for culturing and dynamic evaluation of cells development. Micromachines (Basel) 2020;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat ZA, Ozçelik S, Vural A, Saygi G.. [Observations on Acanthamoeba trophozoites in axenic cultures and their staining characteristics with different stains]. Turkiye Parazitol Derg 2007;31:7–13. [PubMed] [Google Scholar]
- Proserpio P, Marra S, Campana C, Agostoni EC, Palagini L, Nobili L, Nappi RE.. Insomnia and menopause: a narrative review on mechanisms and treatments. Climacteric 2020;23:539–549. [DOI] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL, Chen ZJ.. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update 2015;21:787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghzadeh Oskouei B, Zargari S, Shahabi P, Ghaffari Novin M, Pashaiasl M.. Design and microfabrication of an on-chip oocyte maturation system for reduction of apoptosis. Cell J 2021;23:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF.. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater 2017;49:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samouillan V, Dandurand-Lods J, Lamure A, Maurel E, Lacabanne C, Gerosa G, Venturini A, Casarotto D, Gherardini L, Spina M.. Thermal analysis characterization of aortic tissues for cardiac valve bioprostheses. J Biomed Mater Res 1999;46:531–538. [DOI] [PubMed] [Google Scholar]
- Sarabadani M, Tavana S, Mirzaeian L, Fathi R.. Co-culture with peritoneum mesothelial stem cells supports the in vitro growth of mouse ovarian follicles. J Biomed Mater Res A 2021;109:2685–2694. [DOI] [PubMed] [Google Scholar]
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P.. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior RM, Hinek A, Griffin GL, Pipoly DJ, Crouch EC, Mecham RP.. Neutrophils show chemotaxis to type IV collagen and its 7S domain and contain a 67 kD type IV collagen binding protein with lectin properties. Am J Respir Cell Mol Biol 1989;1:479–487. [DOI] [PubMed] [Google Scholar]
- Serati M, Bogani G, Di Dedda MC, Braghiroli A, Uccella S, Cromi A, Ghezzi F.. A comparison between vaginal estrogen and vaginal hyaluronic for the treatment of dyspareunia in women using hormonal contraceptive. Eur J Obstet Gynecol Reprod Biol 2015;191:48–50. [DOI] [PubMed] [Google Scholar]
- Shah JS, Sabouni R, Cayton Vaught KC, Owen CM, Albertini DF, Segars JH.. Biomechanics and mechanical signaling in the ovary: a systematic review. J Assist Reprod Genet 2018;35:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, Jobling T.. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod 2014;29:1828. [DOI] [PubMed] [Google Scholar]
- Sükür YE, Kıvançlı IB, Ozmen B.. Ovarian aging and premature ovarian failure. J Turk Ger Gynecol Assoc 2014;15:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TA, Johnson KM.. Menopause. Med Clin North Am 2015;99:521–534. [DOI] [PubMed] [Google Scholar]
- van Rijswijk JW, Talacua H, Mulder K, van Hout GPJ, Bouten CVC, Gründeman PF, Kluin J.. Failure of decellularized porcine small intestinal submucosa as a heart valved conduit. J Thorac Cardiovasc Surg 2020;160:e201–e215. [DOI] [PubMed] [Google Scholar]
- Woo JS, Fishbein MC, Reemtsen B.. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc Pathol 2016;25:12–17. [DOI] [PubMed] [Google Scholar]
- Wood CD, Vijayvergia M, Miller FH, Carroll T, Fasanati C, Shea LD, Brinson LC, Woodruff TK.. Multi-modal magnetic resonance elastography for noninvasive assessment of ovarian tissue rigidity in vivo. Acta Biomater 2015;13:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Gao YY, Su J, Tang XN, Chen Q, Ma LW, Zhang JJ, Wu JM, Wang SX.. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022a;25:170–178. [DOI] [PubMed] [Google Scholar]
- Wu T, Gao YY, Tang XN, Zhang JJ, Wang SX.. Construction of artificial ovaries with decellularized porcine scaffold and its elicited immune response after xenotransplantation in mice. J Funct Biomater 2022b;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chen C, Xu Z, Chen F, Yu Y, Hong X, Xu S, Chen J, Ding Q, Chen H.. Ureteral reconstruction with decellularized small intestinal submucosa matrix for ureteral stricture: a preliminary report of two cases. Asian J Urol 2020;7:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzhalin AE, Urbonas T, Silva MA, Muschel RJ, Gordon-Weeks AN.. A core matrisome gene signature predicts cancer outcome. Br J Cancer 2018;118:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Liu Y, Hou C, Li Z, Yang S, Liang X, Zhou L, Guo J, Zhang J, Huang X.. Ovary-derived decellularized extracellular matrix-based bioink for fabricating 3D primary ovarian cells-laden structures for mouse ovarian failure correction. Int J Bioprint 2022;8:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for the study have been included in the article and Supplementary Material.