Abstract
Helicobacter hepaticus has been reported to induce colitis, hepatitis, and hepatocellular carcinoma in several different murine models. The aim of this study was to determine if H. hepaticus will cause colitis in monoassociated mice lacking the interleukin-10 gene (IL-10−/− mice) and potentiate colitis in specific-pathogen-free (SPF) IL-10−/− mice. Germfree IL-10−/− mice on either a mixed (C57BL/6 × 129/Ola) or inbred (129/SvEv) genetic background were monoassociated with H. hepaticus ATCC 51448 by oral feeding and rectal enemas. In a second experiment, germfree IL-10−/− mice were colonized with stool from SPF mice that harbored or did not harbor endogenous H. hepaticus. After 7 to 9 weeks of colonization, weight loss and mortality were assessed, the colon was isolated for histology and IL-12 secretion, and mesenteric lymph node cells were assessed for T-cell activation markers. It was found that IL-10−/− mice monoassociated with H. hepaticus for up to 16 weeks showed almost no histologic colitis or increased IL-12 production. SPF IL-10-knockout mice had no significant difference in weight loss, mortality rate, histologic scores, colonic IL-12 secretion, or T-cell activation with or without H. hepaticus. We conclude that H. hepaticus does not induce or potentiate disease in our IL-10−/− mice and therefore is not required to induce colitis in genetically susceptible hosts.
The pathogenesis of chronic inflammatory bowel diseases is still poorly understood. In the last decade experimental models of chronic intestinal inflammation have clarified the concept that a combination of genetic, environmental, and immunologic factors are necessary to induce and perpetuate these chronic, spontaneously relapsing diseases (10, 25).
Results in a number of rodent models have conclusively documented that luminal bacteria are required for chronic intestinal inflammation (27). For example, mice that lack the interleukin-10 gene (IL-10−/− mice) develop a spontaneous, lethal enterocolitis when housed in a conventional environment (18). However, when these animals are kept under specific-pathogen-free (SPF) conditions, they develop nonlethal inflammation restricted to the colon (18, 30), and in a germfree (sterile) state, IL-10−/− mice fail to develop colitis or mucosal immune activation (30). These findings elegantly demonstrate how commensal intestinal bacteria can induce and perpetuate colitis in a genetically susceptible host. This concept has been confirmed in several other genetically engineered colitis models, such as HLA-B27 transgenic rats (22, 33) IL-2−/− mice (24, 29), mice lacking the T-cell receptor α gene (8), and Tgɛ26 transgenic mice (34), as well as by the absence of chronic small intestinal ulceration in Lewis rats exposed to indomethacin (26).
Although these findings suggest that normal intestinal flora provide the constant antigenic drive for chronic intestinal inflammation in susceptible hosts, the possibility of a persistent pathogenic microbial infection, or an unconventional pathogen which is difficult to detect, has not been excluded in either human inflammatory bowel disease or rodent models (3). Among those pathogens considered potential candidates to induce chronic intestinal inflammation are Helicobacter species (2). Helicobacter hepaticus is a widespread contaminant of murine colonies (31), including genetically engineered mice with colitis, such as our IL-10−/− mice (30). H. hepaticus causes chronic active hepatitis, hepatocellular adenoma, and carcinoma, especially in A/JCr mice (13, 37). A/JCr mice experimentally infected with H. hepaticus sporadically develop cecal inflammation (typhlitis) (15, 38), as do CD45 RBhi reconstituted SCID mice (3). Also, mice which lack functional T lymphocytes, such as athymic nude mice (36), SCID/NCr mice (20), and RAG-2−/− mice (35), develop colitis after colonization with H. hepaticus. Other Helicobacter species are also associated with colitis in SCID mice, such as Helicobacter bilis, resulting in a proliferative typhlitis and colitis (17, 32). Of considerable interest is that a novel urease-negative intestinal Helicobacter species was cultivated from cotton-top tamarins with chronic colitis (28), whereas recently another urease-negative Helicobacter species was isolated and associated with colitis in IL-10−/− mice (14).
The aim of the present studies was to investigate whether H. hepaticus plays an essential or potentiating pathogenetic role in the development of colitis in susceptible mice with functioning T cells. Colitis was quantified by clinical, histologic, and immunologic parameters in germfree IL-10−/− mice, IL-10−/− mice monoassociated with H. hepaticus ATCC 51448, or IL-10−/− mice colonized with SPF luminal bacteria with or without endogenous H. hepaticus recovered from IL-10−/− mice (30). All bacterial colonizations were performed by the physiologic route of several oral feedings plus rectal swabs.
MATERIALS AND METHODS
Animals.
Two sublines of germfree IL-10−/− mice were used in these studies. One subline is on a mixed (C57BL/6 × 129/Ola) background. The other is on the 129/SvEv inbred background. Both sublines were provided by D. Rennick, DNAX, Palo Alto, Calif., and were rederived into germfree conditions by E. Balish. The genotype was confirmed by analysis of tail tip digests using the PCR as described previously (30).
Study design.
We report here the results of two separate studies designed to evaluate the role of H. hepaticus in development of colitis in IL-10−/− mice. In the first study, 2-month-old germfree C57BL/6 × 129/Ola IL-10−/− mice were transferred to SPF housing conditions. We have previously determined that colonization of mice at this age with SPF bacteria induced optimal colitis (30). Transferred mice were maintained for 4 to 5 days in cages that contained sterile bedding and were fed autoclaved food and water. Afterwards, bedding from Helicobacter-free SPF mice was added to each cage in order to colonize all mice with the same SPF flora. Two days later, each mouse received fecal contents from either endogenous H. hepaticus-positive or H. hepaticus-negative mice. The fecal pellets were solubilized in sterile phosphate-buffered saline (PBS) and administered to each recipient mouse by oral feeding and rectal swabs. This procedure was repeated three times during a 1-week period. The donors of H. hepaticus-positive stool were C57BL/6 × 129 Ola IL-10−/− mice housed in SPF conditions that were found to harbor endogenous H. hepaticus as detected by PCR. This H. hepaticus strain was the strain originally found in our IL-10−/− mice (30). Mice were sacrificed 1, 3, or 8 weeks after PCR detection of H. hepaticus DNA in fecal pellets. Tissue were collected for histology, intestinal fragment culture, and flow cytometry of mesenteric lymph node cells.
For the second part of the experiment, germfree IL-10−/− mice on the mixed (C57BL/6 × 129 Ola) background and germfree IL-10−/− inbred 129/SvEv mice 2 months of age were colonized with a well-characterized murine H. hepaticus strain (ATCC 51448) (15), which was kindly provided by D. Schauer, Massachusetts Institute of Technology, Cambridge. The animals were colonized by oral gavage twice using 1 ml of an H. hepaticus suspension prepared to a McFarland turbidity standard of 1.0 in PBS. The presence of H. hepaticus in the stool was documented at the start of as well as during the study and at necropsy by culture as well as by PCR, as described below. The mice were sacrificed 9 weeks after successful colonization as documented by PCR and culture of fecal pellets. Control littermates remained germfree. Also included in the study were IL-10−/− 129/SvEv mice which were colonized with H. hepaticus at birth as the offspring of IL-10−/− mice that were previously monoassociated with H. hepaticus (ATCC 51448) and which were kept in a separate isolator. These animals were killed at 16 weeks of age. Studies were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Cecal bacterial culture.
The cecal contents of each mouse were homogenized in 1 ml of sterile PBS, after which the slurry was passed through a 0.45-μm syringe tip filter. The filtrate was then cultured for H. hepaticus at 37°C on blood agar supplemented with trimethoprim, vancomycin, and polymyxin (Remel Labs, Lenexa, Kans.) under microaerophilic conditions as described previously (13).
PCR of stool and cecal content.
Helicobacter DNA was identified in stool or in cecal contents at necropsy by PCR amplification using primers B38 and B39 as described by Shames et al. (31). The presence or absence of other Helicobacter species was determined by PCR using consensus primers C97 and C98, recognizing a broad panel of Helicobacter species as described by Fox et al. (12). To confirm the presence of bacterial DNA in all specimens, a PCR using primers PC5 and P3, detecting conserved bacterial 16S ribosomal DNA, was performed as described previously (39). All primers sequences used are shown in Table 1.
TABLE 1.
Sequences of oligonucleotide primers used in PCR amplifications
| Primer | Sequence |
|---|---|
| B38 | 5′ GCATTTGAAACTGTTACTCTG 3′ |
| B39 | 5′ CTGTTTTCAAGCTCCCCGAAG 3′ |
| C97 | 5′ GCTATGACGGGTATCC 3′ |
| C98 | 5′ GATTTTACCCCTACACCA 3′ |
| PC5 | 5′ TACCTTGTTACGACTT 3′ |
| P3 | 5′ AGGATTAGATACCCTTTAG 3′ |
Histopathology.
Sections of stomach, duodenum, ileum, various parts of the colon (representing cecum and proximal and distal colon), and liver were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin for histologic scoring. Scoring was conducted in a blinded fashion on a validated scale of 0 to 4, with 0 representing no inflammation and 4 representing severe inflammation characterized by widespread infiltration with inflammatory cells, crypt hyperplasia, distorsion of architecture, and the presence of ulcers and crypt abscesses as previously described and validated (22, 30). The total colonic histology score was determined by adding the scores for each section of the large intestine divided by the total number of sections examined.
Colon cultures.
Cultures of colon fragments were prepared according to published methods (4) as described previously (30). Between 50 and 100 mg of tissue per well of a 24-well tissue culture plate (Costar) was cultured in duplicate or triplicate, as tissue permitted, and cultured in 1 ml of complete RPMI medium as described previously (30). The cultures were incubated at 37°C for 18 h. Supernatants were collected and stored at −20°C until assayed.
IL-12 assay.
Immunoreactive IL-12 p40 in colon culture supernatants was measured by ELISA, using the commercially available antibodies C15.6 and biotinylated C17.8 (Pharmingen, San Diego, Calif.) for capture and detection, respectively of the IL-12 p40 subunit, as previously described (30).
Lymphoid cell preparations and flow cytometry.
Mesenteric lymph nodes (MLN) were isolated and single-cell preparations were prepared by gentle teasing, as previously described (5). Cells were washed and resuspended in complete medium RPMI 1640 (Tissue Culture Facility, University of North Carolina Lineberger Cancer Center, Chapel Hill) supplemented with 5% heat-inactivated fetal calf serum (Irvine Scientific, Santa Ana, Calif.), 2 mM l-glutamine, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, and 50 μg of gentamicin (Sigma, St. Louis, Mo.) per ml. The proportion of CD4+ MLN cells that display an activated and/or memory phenotype was analyzed by two-color flow cytometry using rat monoclonal anti-mouse CD44, anti-mouse CD45RB, and anti-mouse CD62L (L-selectin), as described previously (5), as well as anti-CD69 (H1.2F3; Pharmingen).
Statistics.
Statistical analysis was performed using a commercial software package (InStat; GraphPad Software, San Diego, Calif.). Normally distributed data are expressed as the mean ± standard deviation, and differences among groups were identified by the Student t test; significance was set at a P value of <0.05. For nonnormally distributed categorical data, comparison of median values was achieved using a Kruskal-Wallis one-way analysis of variance on ranks, and group differences were identified by Dunn's multiple comparison procedure.
RESULTS
Germfree mice transferred to SPF conditions colonized with or without H. hepaticus.
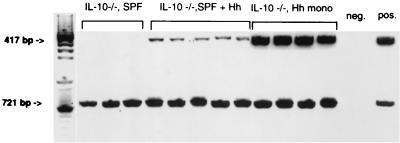
Germfree IL-10−/− mice were transferred into SPF housing conditions and then colonized with intestinal microorganisms from mice that harbor endogenous H. hepaticus or from mice found to be free of this organism as determined by PCR. Within 1 week of administering the third dose of intestinal microorganisms by oral feeding and rectal swabs, H. hepaticus DNA was detectable in stool samples of the transferred mice. H. hepaticus DNA remained in fecal pellets of these mice throughout the study period and was also documented in cecal contents when the mice were sacrificed 8 to 9 weeks after first confirmation of H. hepaticus DNA in fecal pellets (Fig. 1). In addition, fecal pellets and cecal contents of SPF mice colonized with bacteria free of H. hepaticus remained H. hepaticus negative by PCR. We excluded other known Helicobacter species by performing PCR using primers that recognize consensus sequences of a panel of Helicobacter species (12).
FIG. 1.
PCR analysis of H. hepaticus DNA (417 bp) and conserved bacterial 16S ribosomal DNA (721 bp) from cecal contents collected at necropsy from IL-10−/− mice transferred from germfree to SPF conditions without H. hepaticus for 8 weeks before sacrifice (IL-10−/−, SPF) (n = 3), from IL-10−/− mice transferred from germfree to SPF conditions with H. hepaticus for 8 weeks before sacrifice (IL-10−/−, SPF + Hh) (n = 5), or from germfree IL-10−/− mice monoassociated with H. hepaticus ATCC 51488 for 9 weeks before sacrifice (IL-10−/−, Hh mono) (n = 4). DNA extracted from a culture of H. hepaticus ATCC 51488 served as the positive control (pos.), and the PCR mix without DNA served as the negative control (neg.).
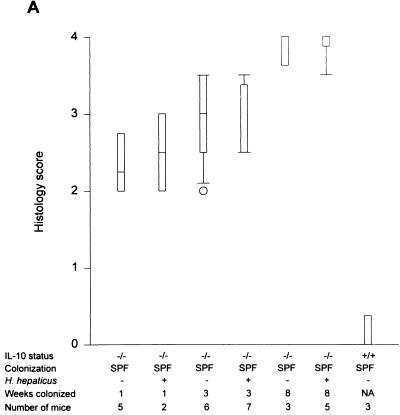
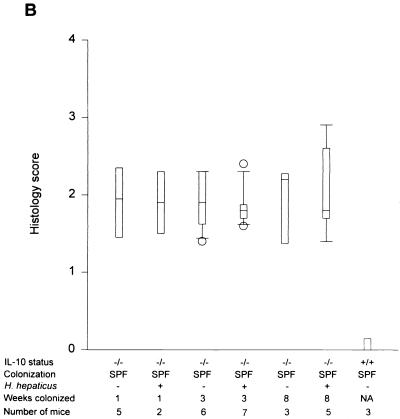
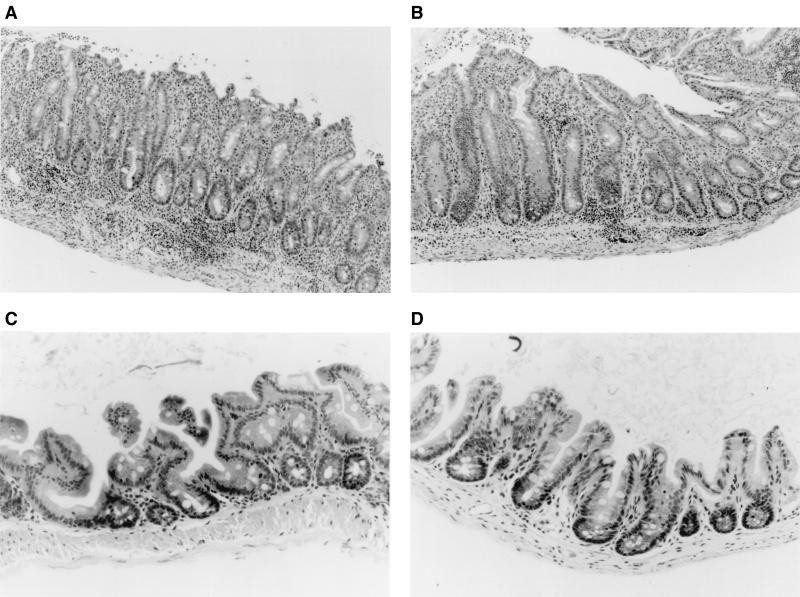
Within 1 week after colonization with SPF flora without Helicobacter, C57BL/6 × 129/Ola IL-10−/− mice developed a significant but moderate mucosal inflammation, especially prominent in the cecum (median of 2.25 in SPF versus 0 in germfree IL-10−/− mice; P < 0.001) (Fig. 2A), which progressively increased to submucosal involvement by 3 weeks (median score of 3.0) and severe pancolitis with transmural inflammation of the cecum by 8 weeks of bacterial colonization (median of 4.0) (Fig. 2A and 3). H. hepaticus-infected SPF IL-10−/− mice had almost identical histologic scores (cecal medians: 1 week, 2.25; 3 weeks, 2.5; 8 weeks, 4.0); there were no significantly different inflammatory scores in the H. hepaticus-negative group at any time point (Fig. 2A). There was a similar rapid increase in total colonic inflammation after 1 week of colonization with H. hepaticus-negative SPF flora. Total colonic histology scores from these mice also did not differ from those for SPF IL-10−/− mice colonized with H. hepaticus at any time point (Fig. 2B). By 8 weeks after SPF exposure, all IL-10−/− mice showed similar degrees of weight loss (2.4 ± 1.5 g in H. hepaticus-negative mice versus 2.7 ± 2.2 g in H. hepaticus-positive mice). Of note, two of five mice in the 8-week H. hepaticus-negative group died, versus no mortality in the H. hepaticus-positive group. Liver abnormalities were not found by histologic assessment in either group. These results indicate that endogenous H. hepaticus colonization did not potentiate or accelerate the progressive colitis that develops in germfree IL-10−/− mice after colonization with SPF bacteria.
FIG. 2.
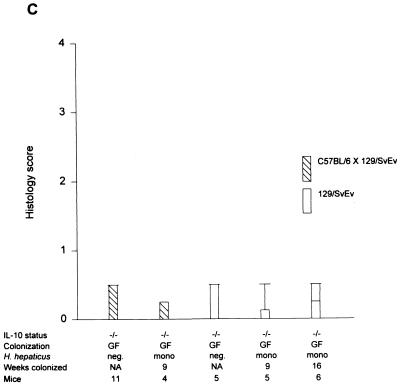
Cecal (A) and total colonic (B) inflammatory scores of IL-10−/− mice on a mixed (C57BL/6 × 129/Ola) background after 1, 3, and 8 weeks of SPF colonization in the presence or absence of H. hepaticus. Inflammatory scores for SPF IL-10+/+ mice are also shown. Box plots represent median values, 25th and 75th percentiles (boxes), 10th and 90th percentiles (error bars), and outlying values (circles). (C) Cecal histology scores of IL-10−/− mice on either a mixed (C57BL/6 × 129/Ola) background or a 129 SvEv background monoassociated with H. hepaticus for 9 and 16 weeks. Inflammatory scores of germfree IL-10−/− mice are also shown. NA, not applicable.
FIG. 3.
Representative photomicrographs of tissue sections from the cecum of an IL-10−/− mouse colonized for 8 weeks with H. hepaticus-negative SPF flora (A), an IL-10−/− mouse colonized for 8 weeks with H. hepaticus-positive SPF flora (B), a gnotobiotic IL-10−/− mouse monoassociated with H. hepaticus for 16 weeks since birth (C), and an IL-10+/+ SPF mouse (D). Note the extensive transmural inflammation in sections of the cecum of SPF IL-10−/− mice with or without H. hepaticus. Only very mild cecal inflammation with a few submucosal lymphocytes was observed in some IL-10−/− mice monoassociated with H. hepaticus.
Monoassociation with H. hepaticus.
Germfree IL-10−/− mice were successfully monoassociated with H. hepaticus (ATCC 51448) by oral feeding and rectal swabbing, as confirmed by PCR analysis of the stool during the study and of cecal contents at necropsy (Fig. 1). The persistence of H. hepaticus colonization was also confirmed by anaerobic culture in all animals at the end of the study period. The absence of contamination with other organisms was confirmed by Gram staining and aerobic and anaerobic culture. C57BL/6 × 129/Ola IL-10−/− mice monoassociated with H. hepaticus for 9 weeks developed no evidence of colitis (Fig. 2C and 3). Other organs, including the liver and stomach, also did not show any histologic abnormalities (data not shown). These results were confirmed with gnotobiotic IL-10−/− mice on an inbred 129SvEv genetic background, which did not develop colitis at 9 weeks after H. hepaticus monoassociation (cecal median score of 0) and which showed only minimal inflammation of the cecum at 16 weeks of age after monoassociation with H. hepaticus since birth (cecal median score of 0.25) (Fig. 2C and 3) (this result was not significantly different from that for germfree controls).
Colonic IL-12 secretion.
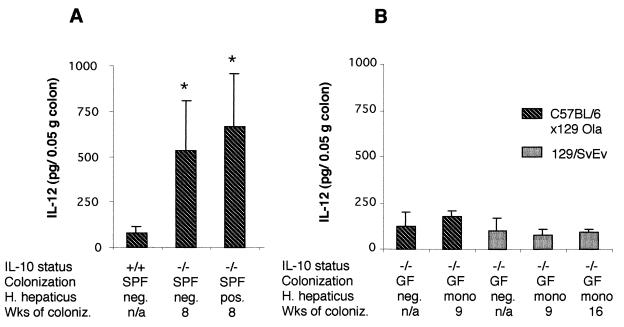
IL-12 has been shown to play a pivotal role in the intestinal inflammation that develops in several different murine models of colitis, including the colitis that occurs spontaneously in IL-10−/− mice (7, 9, 21). In a previous study designed to determine if luminal bacteria are required for development of colitis in IL-10−/− mice, we identified IL-12 in supernatants of colon fragment cultures from SPF but not germfree IL-10−/− mice (30). In the present studies, we asked whether or not H. hepaticus alone or H. hepaticus as one of the luminal bacterial species influenced the production of colonic IL-12 in IL-10−/− mice. The results in Fig. 4A demonstrate detection of significantly higher levels of colonic IL-12 in SPF IL-10−/− mice than in SPF IL-10+/+ mice, in both the presence and absence of H. hepaticus colonization. H. hepaticus colonization of SPF IL-10−/− mice did not increase colonic IL-12 levels. Colonic IL-12 in SPF IL-10−/− mice did not show significant differences at the various time points, and at no time were differences seen in mice with or without H. hepaticus (data not shown). Colonic fragment culture supernatants from IL-10−/− mice monoassociated with H. hepaticus in both strains of IL-10−/− mice contained only low levels of IL-12, which were not significantly different from the amounts of IL-12 detected in colonic supernatants from germfree IL-10−/− mice (Fig. 4B). IL-12 levels in colon culture supernatants from IL-10−/− mice monoassociated with H. hepaticus for 16 weeks since birth also did not significantly differ from those of germfree littermates (data not shown).
FIG. 4.
IL-12 concentrations measured in supernatants of colon fragment cultures of IL-10−/− mice 8 weeks after SPF colonization with or without H. hepaticus (A) and in IL-10−/− mice 9 and 16 weeks after monoassociation with H. hepaticus or in germfree mice (B). Values for SPF IL-10+/+ mice are also shown (A). Values (means ± standard deviations) represent picograms of p40 IL-12 per 50 mg of tissue as detected by enzyme-linked immunosorbent assay. ∗, P < 0.05 compared to colonic IL-12 levels from SPF IL-10+/+ mice.
T-cell activation of CD4+ MLN cells.
As shown in Table 2, we detected statistically significant albeit limited differences in the expression of some but not all T-cell activation markers among gnotobiotic IL-10−/− mice on the 129/SvEv background. We observed higher proportions of CD69+ CD4+ T cells and of CD4+ T cells that express high levels of CD44 and low levels of CD45RB in MLN of some H. hepaticus-monoassociated IL-10−/− mice than in IL-10−/− germfree mice. We did not observe significant differences when we compared the expression of these cell surface molecules on CD4+ MLN T cells from H. hepaticus-monoassociated IL-10−/− mice on a C57BL/6 × 129/Ola background with those in germfree IL-10−/− on the same background (data not shown). It is interesting, however, that MLN from germfree 129/SvEv IL-10−/− mice contain a significantly higher proportion of CD4+ T cells (42.7% ± 1.2%) than do germfree IL-10−/− mice on a C57BL/6 × 129/Ola background (30.9% ± 4.9%) (P < 0.005). In SPF IL-10−/− mice there was not a significant increase in T-cell activation with H. hepaticus colonization. However, there were fewer CD44hi T cells in SPF IL-10−/− mice colonized with H. hepaticus.
TABLE 2.
Expression of cell surface activation markers on CD4+ MLN T cells from IL-10−/− mice in the absence or presence of H. hepaticus
| Mice | Status and time of sacrifice | % (mean ± SD)a of CD4+ T cells expressing the following phenotype:
|
|||
|---|---|---|---|---|---|
| CD69pos | CD44hi | CD45RBlo | CD62Lneg | ||
| 129/SvEv IL-10−/− | Germfree | 7.7 ± 1.5 | 1.6 ± 0.5 | 27.7 ± 13.6 | 29.6 ± 3.8 |
| H. hepaticus monoassociated, 9 wk | 8.1 ± 1.4 | 3.5** ± 2.1 | 42.7** ± 7.2 | 29.3 ± 5.2 | |
| H. hepaticus monoassociated, 16 wk | 9.7** ± 1.0 | 2.3** ± 0.5 | 31.9 ± 5.5 | 27.6 ± 2.8 | |
| C57BL/6 × 129/Ola IL-10−/− | SPFb, H. hepaticus negative, 8 wk | 11.1 ± 1.1 | 12.8 ± 2.0 | 44.1 ± 4.2 | 46.8 ± 4.6 |
| SPF, H. hepaticus positive, 8 wk | 11.4 ± 3.5 | 7.8* ± 3.9 | 50.9 ± 8.3 | 50.0 ± 6.1 | |
**, P < 0.01 versus germfree; *, P < 0.05 versus SPF, H. hepaticus negative.
Mice were colonized with SPF flora.
DISCUSSION
Spontaneous H. hepaticus infection has been detected in IL-10−/− mice with colitis, including our conventional colony of C57BL/6 × 129/Ola IL-10−/− mice (30). To establish if H. hepaticus that is endogenous to our colony could induce and/or potentiate colitis in IL-10−/− mice, we compared the time course of development of colitis in germfree IL-10−/− mice colonized with the endogenous H. hepaticus 2 days after transfer to an SPF environment with that in littermates similarly conventionalized but remaining free of H. hepaticus. Our results show that adolescent and young adult IL-10−/− mice develop a rapid onset of colitis within 1 week after colonization with SPF flora, with no influence on either the aggressiveness of inflammation or the rate of progression when endogenous H. hepaticus was persistently present. The lack of a potentiating or accelerating effect of native H. hepaticus on colonic inflammation is most definitely evident at 1 week, when cecal inflammation is relatively mild, so that a potentiating effect could be more easily recognized. Since progressive, severe typhlitis and colitis developed in the absence of H. hepaticus, as determined by PCR, we conclude that the endogenous H. hepaticus in our IL-10−/− mice is not required to induce colitis, confirming earlier observations (30). Novel features of the present study which advance our previous observations that SPF bacteria devoid of H. hepaticus can induce aggressive colitis in C57BL/6 × 129 Ola IL-10−/− mice (30) are a direct comparison of IL-10−/− mice colonized with SPF bacteria with and without H. hepaticus, monoassociation with a defined H. hepaticus strain, and the failure to detect other Helicobacter species in the SPF flora by PCR using Helicobacter consensus primers (12). We can therefore also conclude that colitis in our colony of an SPF IL-10−/− mouse strain is not caused by (known) Helicobacter species but is more likely the result of an interplay between commensal, nonpathogenic intestinal bacteria in a susceptible host which has a defect in immune homeostasis. This hypothesis is consistent with observations in a number of models, including HLA-B27 transgenic rats (22), IL-2−/− mice (5, 29), and mice lacking the T-cell receptor α gene (8). We have documented by PCR the absence of Helicobacter species in our SPF HLA-B27 transgenic rats (R. K. Sellon, unpublished data) and in SPF IL-2−/− and Tgɛ26 transgenic mice, all of which develop active Th1-mediated colitis (22, 29, 34).
Our monoassociation results indicate that at least the strain of H. hepaticus that we investigated is not able to induce colitis in our IL-10−/− mice in the absence of other bacteria, since selective colonization with H. hepaticus for at least 9 to 16 weeks did not induce aggressive experimental colitis in gnotobiotic IL-10−/− mice either on a mixed C57BL/6 × 129/Ola background or on an inbred 129/SvEv background. Monoassociated IL-10−/− mice had no increased colonic histologic scores or IL-12 production relative to SPF IL-10+/+ or germfree IL-10−/− controls. Persistent H. hepaticus colonization was documented by PCR and culture. It should be noted that in IL-10−/− mice, no single bacterial species has been shown to induce colitis, although HLA-B27 transgenic rats monoassociated with Bacteroides vulgatus develop colonic inflammation, establishing a precedent for a single organism to induce immune-mediated colitis in genetically engineered rodents (23).
Our results concerning the role of H. hepaticus in IL-10−/− mice are different from previous reports. Fox et al. (16) colonized germfree Swiss Webster mice with the same strain of H. hepaticus used in this study and reported persistent hepatitis and enterocolitis. These differences could be explained by genetic host factors. Another reason for the divergent results is the longer duration of H. hepaticus colonization in Swiss Webster mice, in which minimal to mild colitis developed only after 28 to 33 weeks of colonization. In the present study, monoassociated IL-10−/− mice were examined after 9 weeks of colonization with H. hepaticus. Although the kinetics of colitis development can be different in monoassociated mice, we wanted to explore the effect of H. hepaticus within the time frame that colitis develops in IL-10−/− mice moved from germfree to SPF conditions. However, only minimal inflammation of the cecum was observed in 16-week-old IL-10−/− mice that were monoassociated with H. hepaticus at birth.
Even if H. hepaticus is not required to induce colitis in IL-10−/− mice, this organism could potentiate inflammation induced by nonpathogenic commensal bacteria. We therefore compared histologic and immunologic parameters between IL-10−/− mice at several times after transfer to a SPF environment in the presence or absence of endogenous H. hepaticus. Longer exposure of IL-10−/− mice to SPF flora worsened the severity of colitis, especially in the cecum. However, our results consistently reveal that the persistent presence of H. hepaticus did not potentiate typhlitis and colitis at the onset or advanced stage of disease in these mice as determined by blinded histologic scores for different regions of the cecum and colon and levels of colonic IL-12.
Our results are in contrast to a recent study by Kullberg et al. (19), who reported that H. hepaticus triggered colitis in SPF IL-10−/− mice through IL-12- and gamma interferon-dependent mechanisms. Several differences in the experimental designs of the two studies could explain these discrepant results. One potential explanation for the different outcomes is that we colonized our IL-10−/− mice with an endogenous H. hepaticus strain, whereas Kullberg et al. (19) used the original Frederick isolate (ATCC 51488) derived from A/JCr mice (13, 37). However, we clearly demonstrated that monoassociation of our IL-10−/− mice with the same H. hepaticus strain (ATCC 51488) used by Fox et al. (16) and Kullberg et al. (19) did not result in significant colitis. An additional difference in the two studies is the method of H. hepaticus colonization. In the study of Kullberg et al., IL-10−/− mice were inoculated with H. hepaticus intraperitoneally and intragastrically (19), whereas in our study, oral feeding and enemas containing SPF enteric flora with H. hepaticus were used. We chose the oral-fecal colonization method because this mode of transmission more accurately reflects physiologic transfer of this organism than does a parenteral route (16, 19). An important methodologic difference which could explain the discrepancy between our results and those of Kullberg et al. (19) is that we colonized germfree IL-10−/− mice with SPF bacteria with or without H. hepaticus at 2 months of age, whereas the IL-10−/− mice in the previous study were born in an SPF environment and colonized with H. hepaticus at 6 to 9 weeks of age. We have demonstrated that older germfree mice colonized with SPF bacteria develop more aggressive cecal inflammation and colitis than mice born into an SPF environment (30). Another reason for the divergent results with the study of Kullberg et al. could be differences in the composition of the intestinal flora, other than H. hepaticus, in the animal facilities of the two institutions. However, to date specific inducing organisms in IL-10−/− mice still need to be clarified.
It is also possible that failure to induce and potentiate colitis in our IL-10−/− mice, in contrast to the results of Kullberg et al. (19), is due to genetic differences between the strains of IL-10−/− mice used in the two studies. We investigated IL-10−/− mice on a mixed C57BL/6 × 129 Ola background as well as inbred 129/SvEv IL-10−/− mice, whereas Kullberg et al. used inbred C57BL/10SgSnAi IL-10−/− mice (19). Berg et al. (1) have demonstrated important differences in the susceptibilities of inbred IL-10−/− mice on different genetic backgrounds to colitis and colon cancer. In the studies 129/SvEv IL-10−/− mice developed aggressive inflammation, whereas C57/BL6 IL-10−/− mice had mild, delayed-onset disease. IL-10−/− mice on a mixed background developed intermediate disease (1). Differences in genetic background as well as other host factors mentioned above could explain why the SPF C57BL/10 IL-10−/− mice without H. hepaticus used by Kullberg et al. had very little colitis. In support of our observations, a preliminary report by Czinn et al. showed no potentiation of colitis in SPF IL-10−/− mice on a C57BL/10 background by colonization with H. hepaticus strain ATCC 51488 (6).
Except for the development of colitis in older Swiss Webster (16) and A/JCr mice (15, 37), most of the initial studies reporting H. hepaticus-induced colitis and hepatitis focused on immunodeficient mice, such as nude (11, 36) and SCID (20) mice, which lack functional T cells, demonstrating that H. hepaticus can induce disease in the absence of T cells. However, the worsening of colitis in SCID mice reconstituted with CD45 RBhi CD4+ T cells (3) and the findings of Kullberg et al. in C57BL/10 IL-10−/− mice (19) indicate not only that immunocompetent mice can develop colitis in the presence of this organism but also that disease can occur in animals that have functional T lymphocytes. In immunocompetent mice, H. hepaticus-induced colitis or chronic active hepatitis is accompanied by a Th1 cytokine response (19, 38). Not only did H. hepaticus infection not induce or potentiate colitis in our IL-10−/− mice, but it also did not significantly affect the levels of mucosal Th1-associated cytokines. Colonic culture IL-12 concentrations were no different in SPF mice with or without H. hepaticus infection and were not increased by monoassociation of germfree IL-10−/− mice with H. hepaticus. Increased colonic culture IL-12 concentrations in SPF IL-10−/− mice indicate a vigorous Th1 cytokine response following SPF colonization with normal resident bacteria in the absence of H. hepaticus. However, this Th1 response in SPF IL-10−/− mice was not influenced by the presence of H. hepaticus at any time during the development of colitis.
In conclusion, oral-fecal colonization with H. hepaticus (ATCC 51448) did not induce significant colitis in monoassociated IL-10−/− mice, nor was colitis potentiated in SPF IL-10−/− mice cocolonized with endogenous H. hepaticus, when mice were monitored to the aggressive stage of disease. This study indicates that H. hepaticus is not required for induction of colitis in IL-10−/− mice and suggests that the ability of H. hepaticus to induce colitis depends on the interaction between this organism and genetic or immunologic host factors.
ACKNOWLEDGMENTS
We thank Lisa Holt for her help and technical guidance during the PCR analysis. We are also grateful for the technical support of Julie Kwan with MLN isolation and flow cytometry analysis.
This work was supported by NIH grants KO8 553773, DK40249, DK533047, DK 34987, and AI 01122 and by the Crohn's and Colitis Foundation of America. Support for derivation of the germfree IL-10−/− mice on a 129/SvEv background was provided by NIH grant DK 50980 (Peter Ernst, principal investigator).
REFERENCES
- 1.Berg D J, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10 deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser M J. Microbial causation of the chronic idiopathic inflammatory bowel diseases. Inflamm Bowel Dis. 1997;3:225–229. [PubMed] [Google Scholar]
- 3.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke S J, Stokes C R. The intestinal and serum humoral immune response to systemically and orally administered antigens in liposomes. I. The response to liposome-entrapped soluble proteins. Vet Immunol Immunopathol. 1992;32:125–138. doi: 10.1016/0165-2427(92)90074-z. [DOI] [PubMed] [Google Scholar]
- 5.Contractor N V, Bassiri H, Reya T, Park A Y, Baumgart D C, Wasik M A, Emerson S G, Carding S R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385–394. [PubMed] [Google Scholar]
- 6.Czinn S J, Zagorski B M, Spencer D M, Garhart C, Levine A D. Superinfection with Helicobacter hepaticus does not alter the natural course of colitis in IL-10 deficient mice. Gastroenterology. 1998;114:A958. [Google Scholar]
- 7.Davidson N J, Hudak S A, Lesley R E, Menon S, Leach M W, Rennick D M. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 8.Dianda L, Hanby A M, Wright N A, Sebesteny A, Hayday A C, Owen M J. T cell receptor-alpha beta deficient deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrhardt R O, Ludviksson B R, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol. 1997;158:566–573. [PubMed] [Google Scholar]
- 10.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 11.Foltz C J, Fox J G, Cahill R, Murphy J C, Yan L, Shames B, Schauer D B. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 12.Fox J G, Dewhirst F E, Shen Z, Feng Y, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 13.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox J G, Gorelick P L, Kullberg M C, Zhongming G E, Dewhirst F E, Ward J M. A novel urease-negative Helicobacter species associated with colitis and typhlitis in interleukin-10 deficient mice. Infect Immun. 1999;67:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin C, Riley L, Livingston R, Beckwith C, Besch-Williford C, Hook R. Enterohepatic lesions in SCID mice infected with Helicobacter bilis. Lab Anim Sci. 1998;48:334–339. [PubMed] [Google Scholar]
- 18.Kuhn R, Lohler J, Rennick D, Rajewski K, Muller W. Interleukin-10 deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 19.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Fox J G, Whary M T, Shames B, Zhao Z. SCID/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477–5484. doi: 10.1128/iai.66.11.5477-5484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurath M F, Fuss I, Kelsall B L, Stuber E, Strober W. Antibodies to interleukin-12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rath H C, Herfarth H H, Ikeda J S, Grenther W B, Hamm T E, Balish E, Taurog J D, Hammer R E, Wilson K H, Sartor R B. Normal bacteria stimulate colonic, gastric, and systemic inflammation in HLA-B27/β2m transgenic rats. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath H C, Wilson K, Sartor R B. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect Immun. 1999;67:2969–2974. doi: 10.1128/iai.67.6.2969-2974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 25.Sartor R. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 26.Sartor R, Bender D, Grenther T, Holt L. Absolute requirement for ubiquitous luminal bacteria in the pathogenesis of chronic intestinal inflammation. Gastroenterology. 1994;106:A767. [Google Scholar]
- 27.Sartor R B. Enteric microflora in IBD: pathogens or commensals? Inflamm Bowel Dis. 1997;3:230–235. [PubMed] [Google Scholar]
- 28.Saunders K E, Shen Z, Dewhirst F E, Paster B J, Dangler C A, Fox J G. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J Clin Microbiol. 1999;37:146–151. doi: 10.1128/jcm.37.1.146-151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz M, Tonkonogy S L, Sellon R K, Veltkamp C, Godfrey V L, Kwon J, Grenther W B, Balish E, Horak I, Sartor R B. Interleukin-2 deficient mice raised under germfree conditions develop mild, focal intestinal inflammation. Am J Physiol. 1999;276:G1461–G1472. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 30.Sellon R K, Tonkonogy S L, Schultz M, Dieleman L A, Grenther W, Balish E, Rennick D M, Sartor R B. Resident enteric bacteria are necessary for the development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shames B, Fox J G, Dewhirst F, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shomer N H, Dangler C A, Schrenzler M D, Fox J G. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect Immun. 1997;65:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents the development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veltkamp C, Tonkonogy S, de Jong Y, Dieleman L, Balish E, Terhorst C, Sartor R. Continuous luminal bacterial stimulation is essential for colitis in TGɛ26 mice after bone marrow transplantation or T cell transfer. Gastroenterology. 1999;116:A838. [Google Scholar]
- 35.von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161:5673–5680. [PubMed] [Google Scholar]
- 36.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 37.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 38.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson K H, Blitchington R, Greene R C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 1990;28:1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]