Abstract
Innate immunity is important for the integrity of the host against potentially invasive pathogenic microorganisms in the environment. Antibiotic peptides with broad antimicrobial activity are part of the innate immune system. We investigated the presence of the cathelicidin, human cationic antimicrobial protein (hCAP-18), in the male reproductive system. We found strong expression of the hCAP-18 gene by in situ hybridization and hCAP-18 protein, as detected by immunohistochemistry, in the epithelium of the epididymis, but not in the testis. The highest expression in the epididymis was in the caudal part. Western blotting showed a doublet band, the upper part corresponding to the size of hCAP-18 in plasma and neutrophils. Using a specific enzyme-linked immunosorbent assay (ELISA), levels of 86.5 ± 37.8 μg/ml (mean ± standard deviation; range, 41.8 to 142.8 μg/ml; n = 10) were detected in seminal plasma from healthy donors, which is 70-fold higher than the level in blood plasma. Flow cytometry and immunocytochemistry revealed the presence of hCAP-18 on spermatozoa. ELISA measurement showed levels of 196 ng/106 spermatozoa, corresponding to 6.6 × 106 molecules of hCAP-18 per spermatozoon. Our results suggest a key role for hCAP-18 in the antibacterial integrity of the male reproductive system. The attachment of hCAP-18 to spermatozoa may implicate a role for hCAP-18 in conception.
The integrity of the human reproductive system against potentially invasive pathogenic microorganisms is crucial. Readily available, preformed antimicrobial proteins of the nonadaptive immune system serve as the body's first line of defense (5), while the adaptive immune system becomes involved if pathogens start to invade. In recent years, several components of the human nonadaptive immune system have been isolated and characterized, among them the only member of the cathelicidins known to exist in humans, the human cationic antimicrobial protein (hCAP-18) (4, 10). This protein is synthesized in neutrophil progenitors in the bone marrow and stored in the specific granules of mature neutrophils (15). It is synthesized as an 18-kDa proprotein from which a 5-kDa C-terminal fragment, LL-37, bearing all of the hitherto known biological activity, is cleaved (8). LL-37 has lipopolysaccharide-binding properties and manifests antibacterial effect against a wide range of bacterial species (11, 20). Recently, expression of hCAP-18 has also been demonstrated in the epithelium of several organs, including the vagina, cervix, mouth, and lung (2, 6). The cDNA encoding hCAP-18 has been detected in a library prepared from the human testis, suggesting that the gene is expressed here (1).
In the present study, we investigated the presence of hCAP-18 in the male reproductive system. We found expression in the epithelium of the epididymis by in situ hybridization and by immunohistochemistry. High levels of hCAP-18 were found in seminal plasma and in association with spermatozoa, but we were unable to detect expression of the hCAP-18 gene or the presence of hCAP-18 protein in the testis. Our findings suggest a key role for hCAP-18 in the innate immunity of the male reproductive system.
MATERIALS AND METHODS
Tissue samples.
Formalin-fixed and paraffin-embedded archival human tissues, including samples from the testis (n = 7) and epididymis (n = 7), were used, originating from men undergoing surgical castration as treatment for prostate cancer in advanced stages. Seminal vesicles (n = 5) and prostate (n = 5) were obtained from men undergoing curative treatment for localized prostate cancer or benign hyperplasia of the prostate by retropubic prostatectomy. Biopsies from the urethra (n = 2) was obtained from patients undergoing cystoscopy as surveillance after cancer of the urinary bladder. Tissue samples were put in 4% buffered formaldehyde within 15 min after removal at surgery. After an overnight immersion fixation, tissue specimens were paraffin embedded and 4-μm-thick sections were mounted on Superfrost Plus glass slides (Mentzelglazer) prior to immunohistochemistry.
Seminal plasma and spermatozoa.
Freshly ejaculated semen was collected from 10 healthy volunteers at an outpatient fertility clinic and allowed to liquefy for 1 h at room temperature. Some ejaculates were collected in phosphate-buffered saline (PBS) containing 4 M urea and 10 mM EDTA to prevent proteolytic degradation. After centrifugation for 5 min at 800 × g, the seminal plasma was collected and stored at −70°C until used in assays. The pelleted spermatozoa were washed five times in PBS (pH 7.2) and, after resuspension in PBS, counted in a Bürker-Türk chamber. Smears of spermatozoa, intended for immunocytochemistry, were made on Superfrost Plus glass slides and kept at −70°C until use. Pelleted spermatozoa were also lysed with 1% Triton X-100 and 10 mM benzamidine in PBS, pH 7.2, for 20 min on ice. The supernatants were collected and stored at −70°C until analyzed by enzyme-linked immunosorbent assay (ELISA) or Western blotting.
Plasma.
Blood was obtained from healthy volunteers and used to prepare human plasma anticoagulated with EDTA.
Isolation of neutrophils from peripheral blood.
Human neutrophils were isolated from healthy volunteers as previously described (3). Briefly, after sedimentation with 2% Dextran T-500 (Pharmacia, Uppsala, Sweden) in isotonic NaCl, the leukocyte-rich supernatant was pelleted and resuspended in saline for subsequent centrifugation on Lymphoprep (Nycomed Pharma A/S, Oslo, Norway) at 400 × g for 30 min for removal of lymphocytes and monocytes. Remaining erythrocytes were lysed in ice-cold deionized water for 30 s. Tonicity was restored by addition of 1 volume of 1.8% NaCl. The cells were washed once and resuspended in the desired buffer. All steps were carried out at 4°C with the exception of the Dextran sedimentation (room temperature).
Stimulation of neutrophils.
Isolated neutrophils from peripheral blood were suspended in Krebs Ringer fosfate (10 mM NaH2PO4/Na2HPO4, 130 mM NaCl, 5 mM KCl, 0.95 mM CaCl2, 5 mM glucose) at a concentration of 1 × 107 cells/ml. Cells were preincubated at 37°C for 5 min and then stimulated with 1 μM ionomycin (Calbiochem, La Jolla, Calif.) for 20 min at 37°C. The stimulation was stopped by addition of 1 volume of ice-cold buffer. The cells were pelleted by centrifugation, and the supernatant containing the exocytosed material was harvested.
Preparation of RNA probes.
A 435-bp hCAP-18 full-length cDNA (4) was subcloned in pBluescript KS 11 and was used after linearization with BamHI and EcoRI as a template for in vitro transcription to generate 35S-labeled antisense and sense probes. After transcription, the RNA probes were ultrafiltered (Micron 100; Amicon Inc., Beverly, Mass.) before hybridization.
In situ hybridizations.
In situ hybridization was performed essentially as described previously (18). Briefly, 5-μm-thick sections were hybridized overnight with 2.5 × 106 cpm of 35S-labeled RNA probes at 55°C. After hybridization, the slides were washed under stringent conditions that included incubation with 50 μg of RNase A (Sigma) per ml for 30 min at 37°C and were then processed for autoradiography. Autoradiographic exposure was for 1 to 2 weeks. The specificity of the hCAP-18 probe was confirmed by Northern blot analysis (7).
Immunohistochemistry.
Deparaffinized tissue sections and smears of spermatozoa were incubated with a Protein A-purified polyclonal immunoglobulin G (IgG) fraction, raised in rabbits and against recombinant hCAP-18, and used at a final concentration of 0.5 μg/ml (14). The incubation was carried out for 60 min at room temperature, and all of the processing was performed using a staining machine (Dako TechMate 500/1000 Instruments; DAKO A/S, Glostrup, Denmark) and the manufacturer's detection kit (DAKO ChemMate Detection Kit Peroxidase/DAB, Rabbit/Mouse). Briefly, sections were treated with 0.3% H2O2 in methanol for 30 min at room temperature to quench endogenous peroxidase activity. A biotinylated mouse anti-rabbit IgG fraction was used as secondary antibody, followed by streptavidin-peroxidase conjugate and diaminobenzidine as chromogenic substrate. The sections were weakly counterstained with Mayer's hematoxylin solution.
For control purposes, adjacent tissue sections were processed with nonimmune rabbit IgG (DAKO) as replacement for, and at the same concentration as, the hCAP-18 antibodies.
SDS-PAGE, immunoblotting, and peptide synthesis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9) and immunoblotting (19) were performed with Bio-Rad systems according to the instructions of the manufacturer. After the transfer of proteins from the 14% polyacrylamide gel, the polyvinylidene fluoride membranes (Millipore, Bedford, Mass.) were blocked for 1 h with 5% skim milk in PBS. For visualization of hCAP-18, the membranes were incubated overnight with rabbit anti-hCAP-18 antibodies purified with protein A. The following day, the membranes were incubated for 2 h with porcine antibodies against rabbit Ig (DAKO) and visualized by diaminobenzidine and metal concentrate and Stable Substrate Buffer (Pierce, Rockford, Ill.). To determine the limit for detection of LL-37 in seminal plasma by Western blotting, a peptide was synthesized to immunize rabbits. LL-37 was synthesized by standard Fmoc chemistry on a Milligen/Biosearch 9050 peptide synthesizer (Milligen BioSearch, Cambridge, United Kingdom) using chemicals from Millipore (Millipore Intertech, Bedford, Mass.) and purified by reversed-phase high-pressure liquid chromatography systems according to the instructions of the manufacturer. The detection limit for LL-37 was 0.5 μg/ml.
ELISA.
A sandwich ELISA previously described (16) was used to quantitate the hCAP-18 in seminal plasma and in cell lysates.
Flow cytometry.
The presence of hCAP-18 on the surface of spermatozoa was analyzed using a FACScan flow cytometer (Becton Dickinson, San José, Calif.). A secondary fluorescein isothiocyanate-conjugated goat anti-rabbit antibody was used to detect the primary rabbit hCAP-18 antibody. In controls, the specific primary antibody was replaced with an IgG fraction from nonimmunized rabbits (DAKO) at the same concentration.
RESULTS
hCAP-18 expression in the epithelial cells of the epididymis.
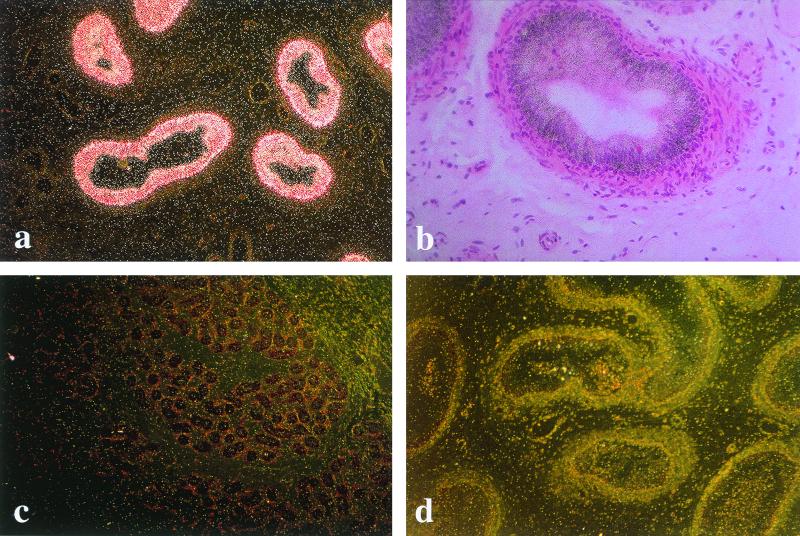
In situ hybridization showed strong expression of the hCAP-18 gene in the epithelial cells of the epididymis (Fig. 1a and b). Judging by the staining intensity, the expression levels were consistently higher in the caudal parts than in the corpus, and we detected only weak expression, if any, in the caput region (not shown). No expression of hCAP-18 could be detected in the testis (Fig. 1c), seminal vesicles, prostate, or urethra (not shown). Control experiments to verify specificity using the complementary sense probe yielded no signal in any of the tissues investigated (Fig. 1d).
FIG. 1.
In situ hybridization for the detection of hCAP-18 gene expression. (A) Dark-field microscopy showing expression of hCAP-18 in the epithelium of the epididymis. Bright deposit of silver grains indicates hCAP-18 gene expression in the epithelial cells. Magnification, ×24. (B) Bright-field microscopy showing expression of hCAP-18, as detected by black silver grains scattered over the epithelial cells of the epididymis. Magnification, ×39. (C) In the control, an antisense probe yields no labeling of the epithelium in a parallel section of the epididymis. Magnification, ×10. (D) Dark-field microscopy of testis, where no hCAP-18 expression is detected by in situ hybridization. Magnification, ×24.
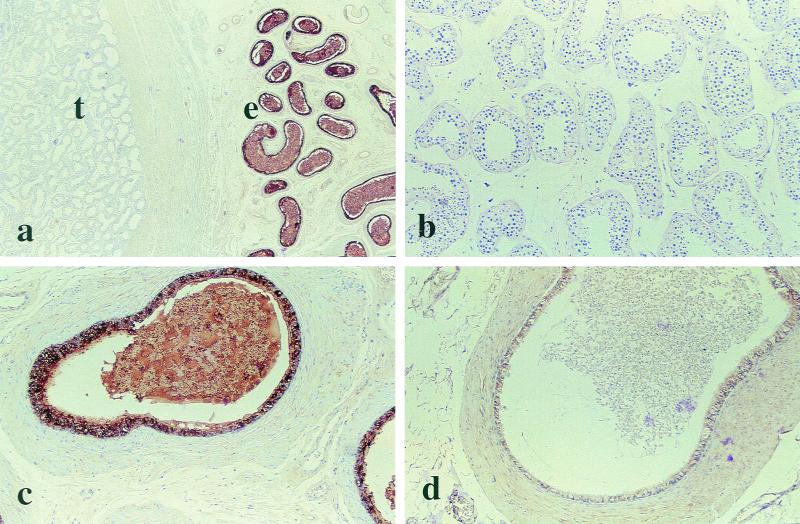
Immunohistochemistry showed intense labeling in the cytoplasm of the epithelial cells of the corpus and caudal regions of the epididymis, as well as in the vas deferens, indicating the presence of large quantities of hCAP-18 (Fig. 2). The majority of the epithelial cells in the caudal parts of the epithelium were very intensely labeled (Fig. 2a and c). The signals were much weaker in the epithelium of the corpus of the epididymis, and specific hCAP-18 staining was absent or very faint in the caput region (not shown). Seminal plasma present in the ducts of the corpus and caudal portions of epididymis showed strong immunoreactivity (Fig. 2c). No hCAP-18 could be detected in the testis (Fig. 2b), prostate, or urethra (not shown). However, there was faint cytoplasmic staining in the epithelium of the seminal vesicles (not shown). Replacement of the primary antibody with a nonimmunized IgG fraction at the same concentration resulted in loss of labeling (Fig. 2d).
FIG. 2.
Detection of hCAP-18 protein by immunohistochemistry. Binding of the specific antibody is detected by peroxidase, which yields a dark brown color reaction against a pale background. (A) hCAP-18 is detected in the epithelium of the epididymis (e) but not in the testis (t). Magnification, ×7. (B) Higher magnification of the testis, where no hCAP-18 can be detected by immunohistochemistry. Magnification, ×18. (C) Higher magnification of the epididymis, with strong labeling of the epithelial cells. Spermatozoa and seminal plasma present in the ducts also show the presence of hCAP-18. Magnification, ×29. (D) Control, where the specific antibody has been replaced by nonimmune serum, resulting in loss of labeling. Magnification, ×29.
hCAP-18 detection by Western blotting in seminal plasma.
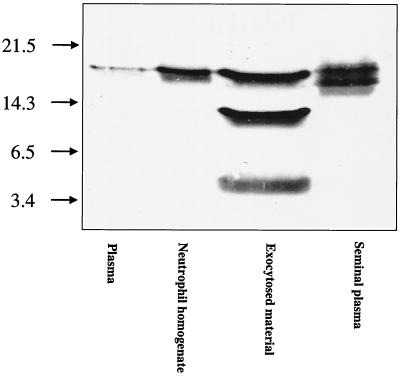
Plasma, neutrophil homogenates, exocytosed material from neutrophils, and seminal plasma were subjected to SDS-PAGE and Western blotting (Fig. 3). In plasma and neutrophil homogenates, a single band of 18 kDa was seen, corresponding to the hCAP-18 holoprotein. In exocytosed material from neutrophils, a 14-kDa and a 5-kDa band appeared in addition to the 18-kDa band, suggesting proteolytic cleavage of the holoprotein to form the 14-kDa cathelin fragment and the 5-kDa LL-37 fragment. In seminal plasma obtained without addition of protease inhibitors, a double band, consisting of one band at 18 kDa and one band corresponding to a protein with slightly lower molecular mass, was seen. No 14-kDa cathelin or 5-kDa LL-37 fragments could be detected by Western blotting in seminal plasma. Ejaculates obtained using strong inhibition of proteases resulted in the same doublet band, speaking against proteolytic modification of the hCAP-18 holoprotein after ejaculation (data not shown).
FIG. 3.
Western blot showing different patterns of hCAP-18 immunoreactivity in plasma, neutrophils, exocytosed material from neutrophils, and seminal plasma, respectively. In plasma and neutrophils, the hCAP-18 holoprotein is seen as a single band of 18 kDa. In exocytosed material from neutrophils, two products resulting from proteolytic cleavage of the holoprotein are seen: a 14-kDa band corresponding to the cathelin part and a 5-kDa band corresponding to LL-37. In seminal plasma, a doublet band is seen, an upper part of approximately 18 kDa and another that is slightly smaller.
hCAP-18 levels in seminal plasma.
A specific ELISA was used to determine the levels of hCAP-18 present in seminal plasma. The concentration was 86.5 ± 37.8 μg/ml (mean ± standard deviation; range, 41.8 to 142.8 μg/ml; n = 10).
Detection of hCAP-18 on the surface of spermatozoa.
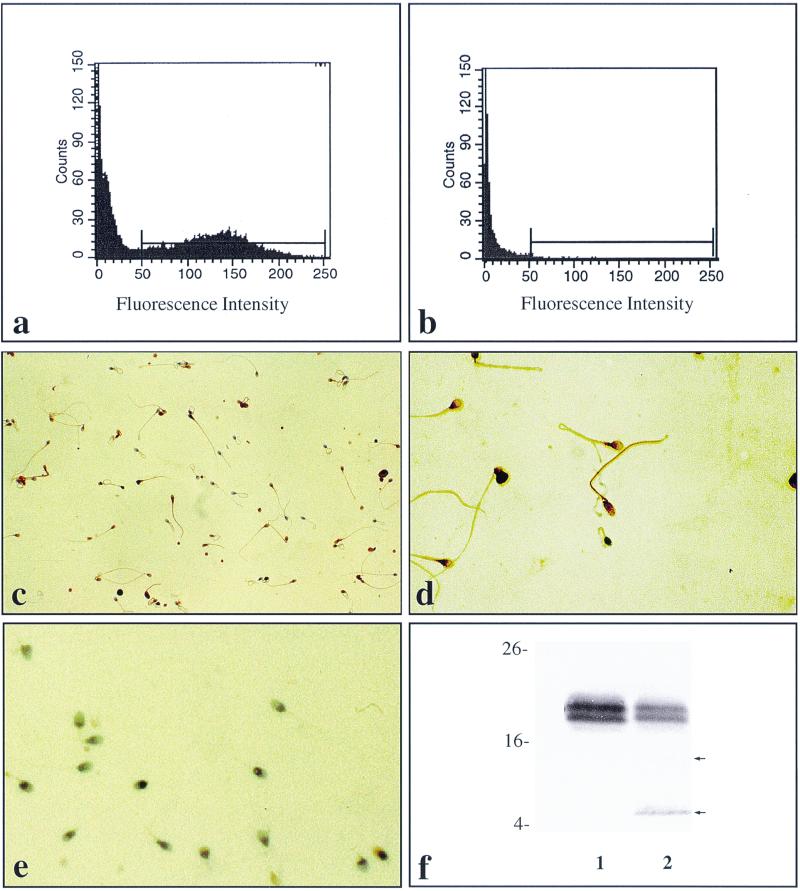
Flow cytometry suggested the presence of hCAP-18 on the surface of the cells. Replacement of the specific antibody with nonimmune rabbit IgG at the same concentration resulted in loss of labeling (Fig. 4a and b). Immunocytochemistry also revealed association of immunoreactive hCAP-18 with the spermatozoa, with a predominant location being the neck and tail region. Control experiments performed to verify the specificity of the staining reactions by replacement of the specific antibody with nonimmune Ig resulted in loss of labeling (Fig. 4c to e). When lysates of spermatozoa were subjected to SDS-PAGE and Western blotting, a doublet band of approximately 18 kDa was seen, similar to the one seen in seminal plasma (Fig. 4f). Interestingly, a weak band of 14 kDa, corresponding in size to the cathelin fragment, and a stronger band of 5 kDa, corresponding in size to LL-37, was consistently observed, suggesting cleavage of the holoprotein by a protease(s) present in spermatozoa during the lysing process.
FIG. 4.
Detection of hCAP-18 on the surface of spermatozoa. (A) Flow cytometry showing the presence of hCAP-18 on the surface of spermatozoa. (B) Flow cytometry of spermatozoa, where the specific primary antibody has been replaced by nonimmune IgG at the same concentration, resulting in loss of labeling. (C) Immunocytochemistry showing the presence of hCAP-18 on the surface of spermatozoa. Binding of specific antibody is visualized by a peroxidase reaction, resulting in brown deposits. Magnification, ×25. (D) Higher magnification (×40 objective) of spermatozoa immunolabeled for hCAP-18, showing immunoreactivity with a predominance in the neck and tail region of the spermatozoa. (E) Control, where the primary antibody has been replaced by nonimmune IgG, resulting in loss of labeling. A ×25 objective was used. The cells were counterstained with Mayer's hematoxylin. (F) Western blot of lysates of washed spermatozoa. An 18-kDa immunoreactive double band corresponding to hCAP-18 in seminal plasma (1) as well as in homogenates obtained from washed spermatozoa (2) is seen. In addition, the lane holding lysed spermatozoa shows a faint 14-kDa band and a stronger 5-kDa band corresponding in size to the cathelin fragment and LL-37, respectively (arrows).
The amounts of hCAP-18 detected in washed pellets of spermatozoa by ELISA measurement was 196 ng (mean; range, 44 to 463 ng; n = 5)/106 spermatozoa, corresponding to 6.6 × 106 hCAP-18 molecules per spermatozoon.
DISCUSSION
In the present study, we show that hCAP-18 is expressed in the epithelium of the caput and caudal parts of the epididymis and that the protein is present at high concentrations in seminal plasma. We also show a strong association of the protein with the surface of spermatozoa.
Our findings contrast with the previously reported expression of hCAP-18 in human testis (1). However, in that study, hCAP-18 was detected at the cDNA level, and a possible explanation for the result could be contamination of the testis cDNA with cDNA from the epididymis.
Widespread expression of hCAP-18 has been found in the epithelium lining of several different organs, for example, the lung (2), the upper digestive tract, and, interestingly, the female reproductive system (6). Expression of the hCAP-18 gene seems to be not only constitutive but also induced during inflammation, for example, in keratinocytes (7). Constitutive expression of hCAP-18 in the epididymis may serve as a barrier against ascending bacteria. In seminal plasma, a double band was seen, rather than the single band seen in neutrophils. Inhibition of proteases at ejaculation did not interfere with the appearance of the double band (data not shown). This may suggest that hCAP-18 is subject to posttranslational modification in the epithelial cells of the epididymis or in semen prior to ejaculation.
In blood, hCAP-18 is present at a plasma concentration of 1.2 μg/ml (16), which is 20-fold higher than for other specific granule proteins of neutrophils. Despite this, the level in seminal plasma was 70-fold higher than that in blood plasma, which may indicate a key role for hCAP-18 in the context of reproduction.
It has previously been reported that human seminal plasma has some, but not very strong, antibacterial activity (12). In general, for this family of antimicrobial proteins, the cathelicidins, the N-terminal cathelin segment must be removed to unleash the microbicidal activity of the C-terminal part (13, 21). The high levels of hCAP-18 found in our investigation indicate a potential for very high antibacterial activity if there is access to an active protease which can liberate the antibacterial LL-37 fragment from the holoprotein. Using synthetic LL-37 to determine the detection limit for the antibacterial fragment by ELISA, it was shown that less than 1% of hCAP-18 is cleaved in seminal plasma, generating LL-37 (data not shown). However, it cannot be ruled out that the holoprotein itself possesses antibacterial activity at the high concentrations present in seminal plasma.
Almost all the hCAP-18 present in the blood plasma is bound to lipoproteins, through hydrophobic interactions with its lipopolysaccharide-binding C-terminal part (21). The reason for this may be to have a reservoir of microbicidal peptides or proteins, and it may serve as a form of protection against the cytotoxic effects of these peptides. Similar mechanisms may be present in seminal plasma, especially since the hCAP-18 concentrations are so much higher and protection against cytotoxic effects is all the more important.
There may be other antimicrobial proteins in seminal plasma. The beta defensin hBD-1 occurs in the human testis (13), and the antibacterial peptide seminal plasmin has been isolated in seminal plasma from cattle (17).
A significant amount of hCAP-18 is associated with the spermatozoa. Neutrophils, which are recognized as a cellular source of hCAP-18, contain 0.627 μg of hCAP-18/106 cells (16), compared to 0.196 μg of hCAP-18/106 spermatozoa. It is not unlikely that the spermatozoa carry hCAP-18 with them on their way to the ovum and that the hCAP-18 provides protection against microorganisms during fertilization. When the acrosome of the spermatozoon reacts with the zona pellucida of the ovum, at least one serine protease, acrosin, is released, which may possibly cleave off LL-37 from the cathelin.
ACKNOWLEDGMENTS
This study was supported by grants from the Greta & Johan Kock Foundations, the Th C Berg Foundation, the Magnus Bergvall Foundation, the Alfred Österlund Foundation, the Tore Nilsson Foundation, the Malmö General Hospital Cancer Foundation, the Alfred Benzon Foundation, the Danish Medical Research Council, the Amalie Jørgensen Foundation, the Swedish Medical Research Council, Fundacion Federico S.A., and the Welander-Finsen Foundation.
We thank Elise Nilsson, Birgitta Frohm, Pia Andersson, and Hanne Kidmose for skillful technical assistance.
REFERENCES
- 1.Agerberth B A, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig. 1968;97:77–89. [PubMed] [Google Scholar]
- 4.Cowland J B, Johnsen A H, Borregaard N. hCAP-18, a cathelin/pro-bactenectin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 5.Elsbach P, Weiss J, Levy O. Oxygen-independent antimicrobial systems of phagocytes. In: Gallin J I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd ed. Philadelphia, Pa: Lippincott, Williams, & Wilkins Co.; 1999. pp. 801–817. [Google Scholar]
- 6.Frohm-Nilsson M, Sandstedt B, Sørensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frohm-Nilsson M, Agerberth B, Ahangari G, Ståhle-Bäckdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson G H, Agerberth B A, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Larrick J W, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J M, Warren S H, Wright S C. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–240. [PubMed] [Google Scholar]
- 11.Larrick J W, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright S C. Antimicrobial activity of rabbit CAP 18-derived peptides. Antimicrob Agents Chemother. 1993;37:2534–2539. doi: 10.1128/aac.37.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mårdh P A, Colleen S. Antimicrobial activity of human seminal fluid. Scand J Urol Nephrol. 1975;9:17–23. doi: 10.3109/00365597509139907. [DOI] [PubMed] [Google Scholar]
- 13.Scocchi M, Skerlavaj B, Romeo D, Gennaro R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–595. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 14.Sitaram N, Krishnakumari V, Nagaraj R. The antibacterial peptide seminal plasmin alters permeability of the inner membrane of E. coli. FEBS Lett. 1992;303:265–268. doi: 10.1016/0014-5793(92)80535-o. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen O, Arnljots K, Cowland J, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18 is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 16.Sørensen O, Cowland J B, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen O, Bratt T, Johnsen A H, Thorup-Madsen M, Borregaard N. The human antibacterial cathelicidin, hCAP 18, is bound to lipoproteins in plasma. J Biol Chem. 1999;274:22445–22451. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 18.Ståhle-Bäckdahl M, Sudbeck B D, Eisen A Z, Welgus H G, Parks W C. Expression of 92 kDa type IV collagenase in eosinophils associated with basal cell carcinoma. J Investig Dermatol. 1992;99:497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R. Activities of L-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanetti M, Litteri L, Griffiths G, Gennaro R, Romeo D. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J Immunol. 1991;146:4295–4300. [PubMed] [Google Scholar]
- 22.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]