Abstract
Background
To investigate whether whole‐brain radiotherapy (WBRT) decreases lymphocyte counts and evaluate the impact of treatment‐related lymphopenia on survival in patients with brain metastasis.
Methods
Medical records from 60 small‐cell lung cancer patients treated with WBRT from January 2010 to December 2018 were included in the study. Total lymphocyte count (TLC) was obtained pre and post treatment (within 1 month). We performed linear and logistic regression analyses to identify predictors of lymphopenia. The association between lymphopenia and survival was analyzed using Cox regression analysis.
Results
Thirty‐nine patients (65%) developed treatment‐related lymphopenia. The median TLC decrease was −374 cells/μL (interquartile range −50 to −722, p < 0.001). Baseline lymphocyte count was a significant predictor of TLC difference and percentage change in TLC. Logistic regression analysis found male sex (odds ratio [OR] 0.11, 95% confidence interval [CI] 0.00–0.79, p = 0.033) and higher baseline lymphocyte count (OR 0.91, 95% CI 0.82–0.99, p = 0.005) were associated with a lower risk of developing ≥grade 2 treatment‐related lymphopenia. Cox regression analysis showed that age at brain metastasis (hazard ratio [HR] 1.03, 95% CI 1.01–1.05, p = 0.013), ≥grade 2 treatment‐related lymphopenia, and percentage change in TLC (per 10%, HR 0.94, 95% CI 0.89–0.99, p = 0.032) were prognostic factors of survival.
Conclusions
WBRT decreases TLC and the magnitude of treatment‐related lymphopenia is an independent predictor of survival in small‐cell lung cancer patients.
Keywords: brain metastasis, small‐cell lung carcinoma, treatment‐related lymphopenia, whole‐brain radiotherapy
We found palliative whole‐brain radiotherapy reduces blood total lymphocyte count and equal or greater than grade 2 radiation‐induced lymphopenia is associated with worse survival in multivariate analysis.
INTRODUCTION
Radiation therapy (RT), commonly used to treat cancer, affects the hematopoietic system. Circulating lymphocyte populations are highly sensitive to radiation, often undergoing apoptosis in response. 1 Irradiation to circulating lymphocytes may be one pathophysiology by which radiation‐induced lymphopenia develops. 2 A growing body of literature suggests total lymphocyte count (TLC) is associated with survival rates in many malignancies. Grossman et al. 3 demonstrated that a severe reduction of CD4 counts, with parallel changes in TLCs, was associated with a shorter median survival time in patients with high‐grade glioma treated with radiation and temozolomide. Similar results have been reported in locally advanced pancreatic cancer and stage I–III esophageal carcinoma. Wild et al. 4 found severe lymphopenia (TLC < 500 cells/μL) after the initiation of chemoradiation independently predicted progression‐free survival and overall survival in patients with locally advanced pancreatic adenocarcinoma. Deng et al. 5 demonstrated esophageal cancer patients with grade 4 lymphopenia during concurrent chemoradiation treatment had poorer survival outcomes than grade 0–3 lymphopenia patients.
Substantial literature on the prognostic value of lymphopenia when treating primary solid tumors exists but less work exists on evaluating its role in palliative treatment. A study of overall survival in breast cancer patients with brain metastases concluded that pretreatment lymphopenia (<700 cells/uL) was a prognostic factor of poor outcome. 6 Given the limited data on the role of treatment‐related lymphopenia in the setting of palliative RT, we investigated the impact of RT on lymphocyte count and survival outcome in a cohort of patients with brain metastases.
Brain metastases are present at diagnosis in approximately 20% of patients with small‐cell lung cancer (SCLC), and the brain is a frequent site of failure after initial chemotherapy or chemoradiotherapy for both extensive and limited‐stage disease. Whole‐brain radiotherapy (WBRT) is a standard treatment modality for such patients. In this study, we explored whether WBRT decreases lymphocyte count in SCLC patients. We further evaluated predictive factors for lymphocyte count change. Finally, we examined the association of posttreatment lymphocyte count with survival outcome. Our study helps to elucidate whether WBRT reduces lymphocyte count and the association between the degree of lymphopenia and survival time.
MATERIALS AND METHODS
Study population
This study was reviewed and approved by the Institutional Review Board of Taipei Veteran General Hospital (No. 2019‐03‐009CC). We retrospectively investigated SCLC patients treated with WBRT between January 2010 and December 2018. Eligible patients were required to have a recorded complete blood count (CBC) with differential count (DC) value within 1 month prior to initiating RT and within 1 month after completing RT. A total of 83 patients were identified. Patients who underwent prophylactic cranial irradiation (n = 3), who simultaneously received irradiation to other body sites (bone = 6, thorax = 4), or who did not receive a complete treatment course (n = 10) were excluded. As a result, 60 patients were included in the final study analysis. Figure 1 shows the study flowchart. The demographic information collected from patients included age at brain metastasis, sex, Karnofsky performance status (KPS), and smoking status.
FIGURE 1.
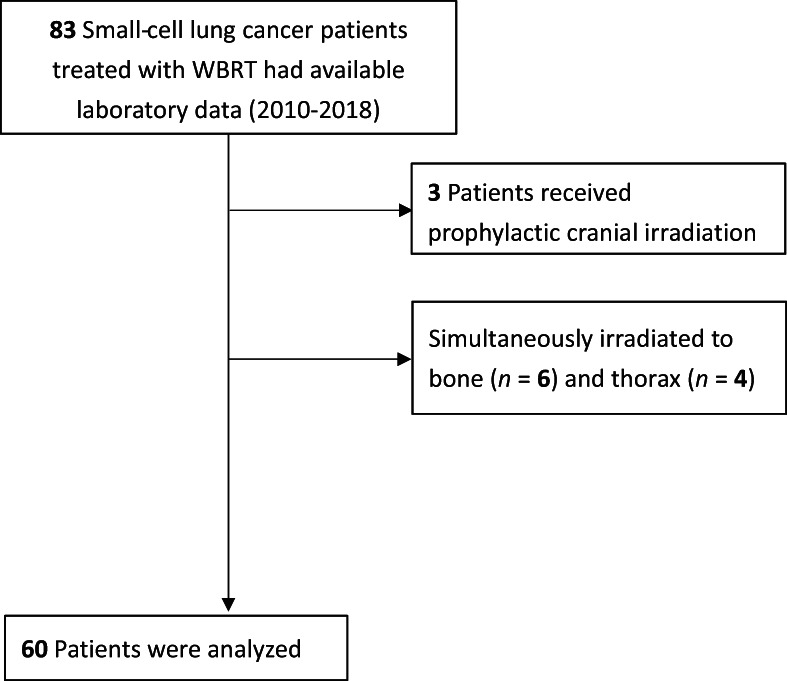
Study flowchart of including and excluding patients with small‐cell lung cancer receiving whole‐brain radiotherapy (WBRT).
Lymphocyte count assessment
The medical records of the 60 patients included were reviewed. Baseline TLC data were obtained based on the CBC/DC data within 1 month of RT initiation. If several CBC/DC values were available, the closest value to the start of RT was used. Posttreatment TLC data were taken from CBC/DC values obtained within 1 month after the end of RT; the value obtained closest to the end of RT was used for analysis. Lymphocyte count differences were calculated as posttreatment TLC subtracting baseline TLC, and percentage change was calculated by dividing the lymphocyte count difference by baseline TLC. Treatment‐related lymphopenia severity was classified using the Common Terminology Criteria for Adverse Events (version 5.0). TLC from the lower limit of the normal range to 800 cells/μL was defined as grade 1, 800–500 cells/μL as grade 2, 500–200 cells/μL as grade 3, and <200 cells/μL as grade 4.
Radiotherapy
External beam RT was delivered using either a three‐dimensional technique or intensity‐modulated radiotherapy. Patients were simulated with computed tomography for RT planning.
Statistical analysis
Patient baseline characteristics were summarized using descriptive statistics. The effect of RT on TLC and posttreatment TLC was determined using the Wilcoxon signed‐rank test. Overall survival (OS) was defined as the time from the start date of RT to the date of death. OS probability was estimated using the Kaplan–Meier method and graphed by stratifying for variables. Univariate and multivariate analyses to identify predictors for percentage change in lymphocyte count and posttreatment TLC severity were performed using linear and logistic regression, respectively. Cox regression analysis was used to assess the association between percentage change in lymphocyte count or posttreatment TLC severity and OS. An interaction test was performed to examine the interaction between the variables. Radiation Therapy Oncology Group recursive partitioning analysis (RPA) class was a widely accepted prognostic parameter. We included RPA in the analysis. 7 Two‐sided p values are reported, with a p value <0.05 considered statistically significant. Statistical analyses were performed with the software R (version 4.1.3, R Foundation for Statistical Computing).
RESULTS
Baseline characteristics of patients and laboratory data
Sixty eligible patients were identified; baseline demographic information is summarized in Table 1. The median age at brain metastasis was 67 years (interquartile range [IQR] 60–75 years). Fifty‐three patients were male (88%) and 66.7% of the patients had a KPS below 70. The median radiation dose to the whole brain was 30 Gy (IQR 30–35 Gy). Sixty‐five percent of the patients received concurrent chemotherapy.
TABLE 1.
Baseline and treatment characteristics of all patients.
| Characteristics | Number (%) or median [IQR] |
|---|---|
| Total patients number | 60 |
| Age at brain metastasis (years) | 67 [60, 75] |
| Gender | |
| Male | 53 (88.3) |
| Female | 7 (11.7) |
| KPS | |
| ≥70 | 20 (33.3) |
| <70 | 40 (66.7) |
| Smoking status | |
| Current smoker | 33 (55%) |
| Former smoker | 24 (40%) |
| Never smoker | 3 (5%) |
| Radiotherapy dose (Gy) | 30 [30, 35] |
| Radiotherapy regimen | |
| Conventional | 52 (86.7) |
| SIB | 8 (13.3) |
| Concurrent with chemotherapy | |
| Yes | 39 (65.0) |
| No | 21 (35.0) |
| Steroid use | 46 (76.7) |
| Laboratory values | |
| Baseline lymphocyte count (cells/μL) | 1101.55 [734.2, 1717.6] |
| Post treatment lymphocyte count (cells/μL) | 855.20 [539.6, 1104.4] |
| Lymphopenia grading (CTCAE) | |
| Grade 0 | 21 (35.0) |
| Grade 1 | 11 (18.3) |
| Grade 2 | 15 (25.0) |
| Grade 3 | 11 (18.3) |
| Grade 4 | 2 (3.3) |
Abbreviations: CTCAE, common terminology criteria for adverse events; KPS, karnofsky performance status; SIB, simultaneous integrated boost.
Laboratory data are provided in Table 1. The median value of the baseline TLC was 1102 cells/μL (IQR 734–1718 cells/μL). The median value of posttreatment TLC was 855 cells/μL (IQR 540–1104 cells/μL), and the median value of the TLC difference was −374 cells/μL (IQR 50–722, p < 0.001). Overall, TLCs were reduced by a median of 31.2%. Sixty‐five percent of patients developed treatment‐related lymphopenia: 18% of patients had grade 1, 25% had grade 2, 18% had grade 3, and 3% had grade 4.
Associations between patient characteristics and TLC difference
The univariate and multivariate associations between patient characteristics and TLC difference are presented in Table 2. Baseline lymphocyte count was the only significant predictor of TLC difference and percentage change in lymphocyte count in both univariate (p < 0.001) and multivariate analyses (p < 0.001). An increase in baseline TLC by 100 cells/μL further decreased 74.81 cells/μL and lymphocyte count by 4.16% after WBRT.
TABLE 2.
Lymphocyte count differences and percentage change in lymphocyte count with patient characteristics
| Lymphocyte count differences (cells/μL) | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Characteristic | Estimates | 95% CI | p | Estimates | 95% CI | p |
| Age at BM | −9.96 | −26.77 – 6.86 | 0.241 | −5.99 | −15.77 – 3.80 | 0.225 |
| Sex (male vs. female) | 196.80 | −355.35 – 748.94 | 0.478 | 284.09 | −32.36 – 600.54 | 0.077 |
| Baseline lymphocyte count (per 100 cells/μL) | −74.81 | −88.54 – −61.08 | <0.001 | −76.18 | −90.35 – −62.00 | <0.001 |
| RT dose (per Gy) | 26.19 | −16.16 – 68.53 | 0.221 | 2.66 | −22.31 – 27.63 | 0.832 |
| Concurrent CT | −181.20 | −551.40 – 189.00 | 0.331 | 80.88 | −136.56 – 298.32 | 0.459 |
| Steroid | −129.77 | −549.31 – 289.76 | 0.538 | −171.35 | −427.88 – 85.18 | 0.186 |
| KPS (per 10) | 25.23 | −132.21 – 182.67 | 0.750 | 2.94 | −94.06 – 99.94 | 0.952 |
| Percentage change in lymphocyte count (%) a | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | |||||
| Characteristic | Estimates | 95% CI | p | Estimates | 95% CI | p |
| Age at BM | −0.92 | −2.41 – 0.57 | 0.222 | −0.74 | −2.05 – 0.57 | 0.262 |
| Sex (male vs. female) | 36.01 | −12.20 – 84.23 | 0.140 | 40.58 | −1.81 – 82.98 | 0.060 |
| Baseline lymphocyte count (per 100 cells/μL) | −4.16 | −5.98 – −2.34 | <0.001 | −4.13 | −6.03 – −2.23 | <0.001 |
| RT dose (per Gy) | 2.05 | −1.72 – 5.81 | 0.281 | 0.59 | −2.75 – 3.94 | 0.723 |
| Concurrent CT | −15.01 | −47.84 – 17.83 | 0.364 | −1.10 | −30.23 – 28.03 | 0.940 |
| Steroid | −9.04 | −46.26 – 28.18 | 0.629 | −2.75 | −37.11 – 31.62 | 0.873 |
| KPS (per 10) | 7.34 | −6.49 – 21.17 | 0.292 | 6.24 | −6.76 – 19.23 | 0.340 |
Abbreviations: BM, brain metastasis; CI, confidence interval; CT, chemotherapy; KPS, Karnofsky performance status; RT, radiotherapy.
Percentage change in lymphocyte count = [(posttreatment lymphocyte count – baseline lymphocyte count)/baseline lymphocyte count] × 100%.
Associations between patient characteristics and treatment‐related lymphopenia
The median value of lymphocyte count after WBRT was 855 cells/μL. Based on this, treatment‐related lymphopenia greater than or equal to grade 2 (<800 cells/μL) was used as the endpoint for univariate and multivariate logistic regression analyses; the results are provided in Table 3. In the univariate analysis, male sex (odds ratio [OR] 0.14, 95% confidence interval [CI] 0.01–0.88, p = 0.035) and increased baseline lymphocyte count (per 100 cells/μL) (OR 0.90, 95% CI 0.82–0.97, p = 0.011) were significant protective factors against ≥grade 2 treatment‐related lymphopenia. In multivariate analysis, male (OR 0.11, 95% CI 0.00–0.79, p = 0.033) and higher baseline lymphocyte count (per 100 cells/μL) (OR 0.91, 95% CI 0.82–0.99, p = 0.005) remained associated with a lower risk of ≥grade 2 treatment‐related lymphopenia after adjusting for other factors.
TABLE 3.
Univariate and multivariate analyses of factors associated with greater than or equal to grade 2 treatment‐related lymphopenia
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p | OR | 95% CI | p |
| Age at BM | 1.01 | 0.96–1.06 | 0.777 | 1.01 | 0.96–1.07 | 0.776 |
| Baseline Lymphocyte count (per 100 cells/μL) | 0.90 | 0.82–0.97 | 0.011 | 0.91 | 0.82–0.99 | 0.005 |
| Sex (male vs. female) | 0.14 | 0.01–0.88 | 0.035 | 0.11 | 0.00–0.79 | 0.033 |
| RT dose (per Gy) | 1.10 | 0.97–1.27 | 0.141 | 1.12 | 0.95–1.27 | 0.252 |
| Concurrent CT | 0.48 | 0.16–1.39 | 0.179 | 0.36 | 0.11–1.59 | 0.285 |
| Steroid | 2.14 | 0.64–7.91 | 0.227 | 2.52 | 0.56–15.22 | 0.276 |
| KPS (per 10) | 1.00 | 0.95–1.04 | 0.909 | 1.13 | 0.66–2.09 | 0.583 |
Abbreviations: BM, brain metastasis; CI, confidence interval; CT, chemotherapy; KPS, Karnofsky performance status; OR, odds ratios; RT, radiotherapy.
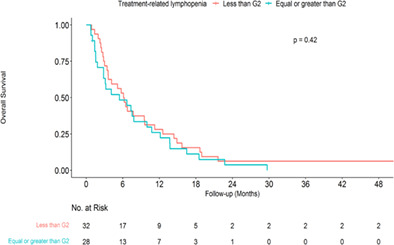
Associations between treatment‐related lymphopenia and overall survival
The median OS for all patients was 6.03 months (IQR 2.73–12.47 months). Kaplan–Meier survival curves stratified by RPA and ≥grade 2 treatment‐related lymphopenia are presented in Figures 2 and 3, respectively. RPA is a significant predictor of OS in our data (p = 0.003, log‐rank test; Figure 2). Table 4 presents univariate and multivariate associations between patient characteristics, treatment‐related lymphopenia, and OS. RPA (hazard ratio [HR] 1.70, 95% CI 1.18–2.44, p = 0.004), age at brain metastasis (HR 1.03, 95% CI 1.01–1.06, p = 0.008), and KPS (HR 0.76, 95% CI 0.60–0.96, p = 0.022) were significant prognostic factors of OS in univariate analyses. Multivariate analysis confirmed age at brain metastasis (HR 1.03, 95% CI 1.01–1.06, p = 0.007) and identified percentage change in lymphocyte count (per 10%, HR 0.94, 95% CI 0.89–0.99, p = 0.032) as factors significantly associated with OS. In addition, ≥grade 2 treatment‐related lymphopenia was a strong predictor of OS (HR 2.02, 95% CI 1.09–3.74, p = 0.025) in the multivariate analysis. The results of the interaction test showed that there was a significant interaction between ≥grade 2 treatment‐related lymphopenia and RPA (p = 0.006), age at brain metastasis (p < 0.001), and KPS (p = 0.018), as shown in Supporting Information Table S1.
FIGURE 2.
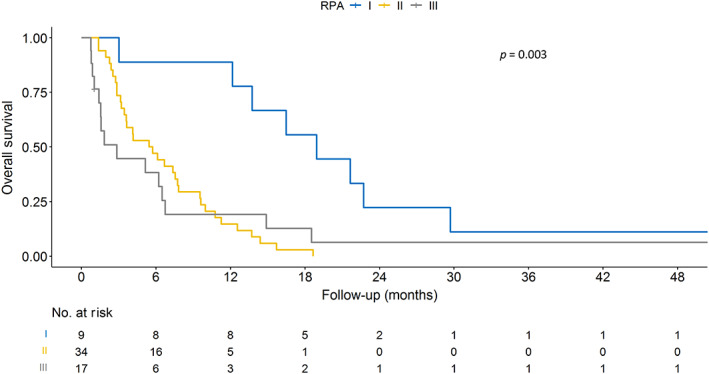
Kaplan–Meier plot of survival stratified by recursive partitioning analysis (RPA).
FIGURE 3.
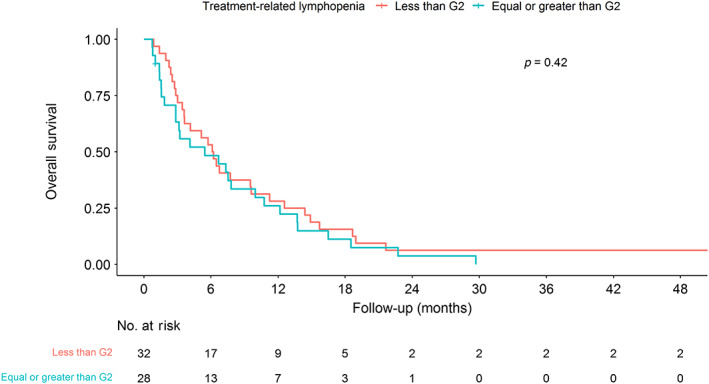
Kaplan–Meier plot of survival stratified by grade 2 (G2) treatment‐related lymphopenia.
TABLE 4.
Univariate and multivariate Cox regression of overall survival
| Univariate | Multivariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| RPA | 1.70 | 1.18–2.44 | 0.004 | 1.39 | 0.71–2.73 | 0.336 | 1.75 | 0.87–3.52 | 0.114 |
| Age at BM | 1.03 | 1.01–1.06 | 0.008 | 1.03 | 1.01–1.05 | 0.013 | 1.03 | 1.01–1.06 | 0.007 |
| Sex (male vs. female) | 1.16 | 0.49–2.73 | 0.730 | 2.16 | 0.67–6.93 | 0.195 | 2.37 | 0.70–7.95 | 0.163 |
| KPS (per 10) | 0.76 | 0.60–0.96 | 0.022 | 0.86 | 0.62–1.17 | 0.333 | 0.88 | 0.65–1.19 | 0.405 |
| Concurrent CT | 0.79 | 0.46–1.37 | 0.410 | 0.71 | 0.39–1.29 | 0.263 | 0.76 | 0.42–1.37 | 0.361 |
| Baseline lymphocyte count (per 100 cells/μL) | 1.00 | 0.96–1.03 | 0.817 | 0.97 | 0.93–1.01 | 0.183 | 1.01 | 0.97–1.05 | 0.648 |
| Percentage change in lymphocyte count (per 10%) a | 0.97 | 0.93–1.01 | 0.141 | 0.94 | 0.89–0.99 | 0.032 | |||
| ≥G2 treatment‐related lymphopenia | 1.24 | 0.74–2.09 | 0.421 | 2.02 | 1.09–3.74 | 0.025 | |||
Abbreviations: BM, brain metastasis; CI, confidence interval; CT, chemotherapy; G2, grade 2; HR, hazard ratio; KPS, Karnofsky performance status; RPA, recursive partitioning analysis.
Percentage change in lymphocyte count = [(posttreatment lymphocyte count – baseline lymphocyte count)/baseline lymphocyte count] × 100%.
DISCUSSION
This retrospective study demonstrates that WBRT as a palliative treatment decreases TLC by a median of 31.2% within 1 month after completion of RT in SCLC patients. Furthermore, the TLC reduction is not associated with concurrent steroid or chemotherapy administration. A relative decrease in TLC and ≥grade 2 lymphopenia are also associated with worse survival. This finding is consistent with previous reports that treatment‐related lymphopenia is correlated with poor survival outcomes. 3 , 8 , 9 , 10
The phenomenon of radiation‐induced lymphopenia across various disease sites and tumor types has been summarized in a systematic review that excluded studies of lymphopenia after palliative radiation for brain metastasis and bone metastasis. 11 Radiation‐induced lymphopenia can result from direct hits to lymphoid organs, to lymphopoiesis sites, such as bone marrow, and to circulating lymphocytes. The brain harbors minimal lymphatics, and the skull has little bone marrow. 12 The insult to lymphocytes in WBRT is most likely from radiation to the circulating lymphocyte within treatment fields. Yovino et al. 2 found that after 30 fractions of 2 Gy to the brain in radiotherapy for gliomas, 99% of circulating lymphocytes received ≥0.5 Gy, a dose that can lead to a reduction in the number of lymphocytes. 13 Our results support the proposed mechanism that a palliative brain radiotherapy regimen decreases TLC.
Compared with previous work on patients with high‐grade glioma, the treatment‐related lymphopenia observed in our study was less severe. Grossman and colleagues 3 found the median percentage reduction in CD4 counts at 2 months was 69%, compared with the 31.2% reduction in TLC in our study. This difference is likely related to the larger fraction number used in glioma compared with that used in WBRT. A reduction in fraction number reduces the total volume of blood exposed to radiation over the treatment course. Although lower fraction number and dose of WBRT than that of glioma, the field size of WBRT is larger. A study investigating palliative RT on circulating lymphocyte count in patients receiving immune checkpoint inhibitors found a reduction of absolute lymphocyte count of 48 cells/mL in brain‐directed RT. 14 Together with the finding, it suggests that WBRT, at least in part, would contribute the treatment‐related lymphopenia.
Predictors of treatment‐related lymphopenia have been identified in several studies. Rudra and colleagues 15 found that in glioblastoma patients receiving chemoradiation older age, lower KPS, female sex, lower baseline TLC, and higher brain volume receiving 10Gy to 50Gy were all associated with acute severe lymphopenia in univariate analyses, with only older age, female sex, and brain volume receiving 25Gy were significant in a subsequent multivariate analysis. Consistent with that study, our results also showed that female sex was a significant predictor for ≥grade 2 treatment‐related lymphopenia. In contrast, in a population of hepatocellular carcinoma patients sex was not a predictive factor for developing lymphopenia. 16 Whether or not there are sex differences in radiosensitivity remains controversial. In an in vitro study, higher radiosensitivity, measured by clonogenic survival assays, was observed in female fibroblast cell lines. 17 However, Schuster and colleagues 18 reported no significant differences in radiosensitivity between sex in a population of advanced rectal cancer patients. Additionally, the study found that leukocyte counts decreased more in women than in men. Further research is needed to explore the effect of sex on treatment‐related lymphopenia.
Aside from sex, we identified that the higher TLCs were at baseline, the more lymphocytes were depleted after treatment. Despite that fact, the baseline TLC was independently associated with ≥grade 2 treatment‐related lymphopenia in univariate and multivariate analyses (OR = 0.91, p = 0.005). Grossman et al. 3 found that the median percentage reduction in CD4 counts at 2 months after treatment was 72% in patients with baseline CD4 counts ≥500 cells/mm3 and 49% in patients with baseline CD4 counts ≤500 cells/mm3. Our results support the previous studies in that although a higher baseline TLC leads to a greater reduction in lymphocytes, it is a protective factor against severe treatment‐related lymphopenia. 3 , 15 , 16
Patients treated with WBRT are frequently given steroids or chemotherapy and either may affect lymphocyte count. Similar to the previous studies working on high‐grade glioma patients, 15 , 19 our data showed that steroid administration was not correlated with either ≥grade 2 treatment‐related lymphopenia or the TLC difference. Furthermore, concurrent chemotherapy administration was not associated with treatment‐related lymphopenia in our analysis. There are some possible reasons for this. First, previous research has shown that lymphocytes are more sensitive to radiation therapy than leukocytes, and therefore the impact of chemotherapy on lymphocytes may not have been observed because TLC is more responsive to radiation than neutrophils. 20 Second, the limited sample size of the study may have affected its statistical power. Finally, the various regimens of chemotherapy used in the study may have affected the correlation between chemotherapy and TLC. In addition, Campian and colleagues 8 observed mostly unchanged of TLC after receiving neoadjuvant chemotherapy in stage III non‐small‐cell lung cancer patients. This suggests that radiation is a vital factor for lymphocyte depletion, regardless of with out without concurrent chemotherapy.
The present study supports previous research showing that treatment‐related lymphopenia is independently associated with a worse outcome, whereas baseline lymphocyte status is not. 3 , 4 , 15 , 16 In the multivariate analyses, three independent predictors of OS were identified: age at brain metastasis (HR 1.03, p < 0.05), ≥grade 2 treatment‐related lymphopenia (HR 2.02, p = 0.025) and percentage change in lymphocyte count (HR 0.94, p = 0.03) (Table 4). Lymphopenia stood out as a significant prognostic factor for OS even when adjusting for RPA. Notably, we found a significant interaction between ≥grade 2 treatment‐related lymphopenia with RPA, age at brain metastasis, and KPS. These data suggested that patients with a high RPA score, older age, and low KPS, which often indicates relative frailty, may be particularly susceptible to the adverse effects of lymphopenia on outcomes. For extensive‐stage SCLC patients, overall prognosis is poor; our results suggest WBRT reduces lymphocytes and the magnitude of the reduction is correlated with outcome.
This study was limited by its retrospective nature. Obtaining CBC data at a preplanned time point for our population was challenging, resulting in inconsistent evaluation time. However, we set a limit duration for data collection to minimize possible bias. In addition, the uneven distribution of sex in the study population could have affected the sensitivity and specificity of predictions. Furthermore, our patients were drawn over a period of 9 years and patients likely received temporally heterogenous treatments given evolving treatment strategies. Nonetheless, this is the first study investigating treatment‐related lymphopenia in SCLC patients receiving WBRT and the potential association between this phenomenon and OS.
No randomized trial has compared the prognosis of SCLC patients with brain metastasis treated with supportive care or WBRT. Although WBRT has shown a 50% response rate in an EORTC phase II study 21 and a small survival benefit in elderly patients not receiving chemotherapy in a national cancer database (NCDB) analysis, 22 patients in the RPA class III group have a poor prognosis with a median survival time of 2.3 months, 23 therefore WBRT should be carefully weighed against supportive care. Due to the high incidence of intracranial dissemination and rapid progression of intracranial disease in SCLC, WBRT remains the standard of care in routine clinical practice. Our findings offer a novel predictor for survival outcomes in SCLC patients receiving WBRT, which may serve as guidance for treatment decision‐making.
In conclusion, we demonstrate that WBRT causes lymphopenia in SCLC patients with brain metastasis. In addition, our data suggest ≥grade 2 treatment‐related lymphopenia and percentage change in lymphocytes are independent predictors of OS. The identification of such prognostic factors among SCLC patients with brain metastasis can help physicians to optimize individualized treatment.
AUTHOR CONTRIBUTIONS
Study conception and design: Yu‐Wen Hu and Yu‐Jung Lin. Data analysis: Yu‐Jung Lin and Yu‐Wen Hu. Manuscript writing and editing: Yu‐Jung Lin and Yu‐Wen Hu. Critical feedback and revision of manuscript: All authors. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors report there are no competing interests to declare.
Supporting information
SUPPORTING INFORMATION TABLE S1. Cox proportional hazards model with interaction test between ≥grade 2 treatment‐related lymphopenia and recursive partitioning analysis, age at brain metastasis, and Karnofsky performance status
ACKNOWLEDGMENTS
None.
Lin Y‐J, Kang Y‐M, Wu Y‐H, Chen Y‐W, Hu Y‐W. Lymphocytopenia and survival after whole‐brain radiotherapy in patients with small‐cell lung cancer. Thorac Cancer. 2023;14(14):1268–1275. 10.1111/1759-7714.14868
REFERENCES
- 1. Schrek R. Qualitative and quantitative reactions of lymphocytes to X‐rays. Ann N Y Acad Sci. 1961;95(2):839–48. [DOI] [PubMed] [Google Scholar]
- 2. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment‐related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high‐grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation‐related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deng W, Xu C, Liu A, van Rossum PS, Deng W, Liao Z, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation‐induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019;133:9–15. [DOI] [PubMed] [Google Scholar]
- 6. Claude L, Perol D, Ray‐Coquard I, Petit T, Blay J‐Y, Carrie C, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol. 2005;76(3):334–9. [DOI] [PubMed] [Google Scholar]
- 7. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51. [DOI] [PubMed] [Google Scholar]
- 8. Campian JL, Ye X, Brock M, Grossman SA. Treatment‐related lymphopenia in patients with stage III non‐small‐cell lung cancer. Cancer Invest. 2013;31(3):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho O, Oh Y‐T, Chun M, Noh OK, Lee H‐W. Radiation‐related lymphopenia as a new prognostic factor in limited‐stage small cell lung cancer. Tumor Biol. 2016;37(1):971–8. [DOI] [PubMed] [Google Scholar]
- 10. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte‐sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation‐induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 12. Hayman JA, Callahan JW, Herschtal A, Everitt S, Binns DS, Hicks RJ, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT‐PET imaging. Int J Radiat Oncol Biol Phys. 2011;79(3):847–52. [DOI] [PubMed] [Google Scholar]
- 13. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 8th ed. Philadelphia, PA: Wolters Kluwer; 2018. [Google Scholar]
- 14. Pike LR, Bang A, Mahal BA, Taylor A, Krishnan M, Spektor A, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD‐1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103(1):142–51. [DOI] [PubMed] [Google Scholar]
- 15. Rudra S, Hui C, Rao YJ, Samson P, Lin AJ, Chang X, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2018;101(1):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byun HK, Kim N, Park S, Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. 2019;195(11):1007–17. [DOI] [PubMed] [Google Scholar]
- 17. Alsbeih G, Al‐Meer RS, Al‐Harbi N, Judia SB, Al‐Buhairi M, Venturina NQ, et al. Gender bias in individual radiosensitivity and the association with genetic polymorphic variations. Radiother Oncol. 2016;119(2):236–43. [DOI] [PubMed] [Google Scholar]
- 18. Schuster B, Hecht M, Schmidt M, Haderlein M, Jost T, Büttner‐Herold M, et al. Influence of gender on radiosensitivity during radiochemotherapy of advanced rectal cancer. Cancer. 2021;14(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen DF, Fergus S, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high‐grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000–7. [DOI] [PubMed] [Google Scholar]
- 20. Zachariah B, Jacob S, Gwede C, Cantor A, Patil J, Casey L, et al. Effect of fractionated regional external beam radiotherapy on peripheral blood cell count. Int J Radiat Oncol Biol Phys. 2001;50(2):465–72. [DOI] [PubMed] [Google Scholar]
- 21. Postmus PE, Haaxma‐Reiche H, Gregor A, Groen HJ, Lewinski T, Scolard T, et al. Brain‐only metastases of small cell lung cancer; efficacy of whole brain radiotherapy. An EORTC phase II study. Radiother Oncol. 1998;46(1):29–32. [DOI] [PubMed] [Google Scholar]
- 22. Renz P, Hasan S, Wegner RE. Survival outcomes after whole brain radiotherapy for brain metastases in older adults with newly diagnosed metastatic small cell carcinoma: a national cancer database (NCDB) analysis. J Geriatr Oncol. 2019;10(4):560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Videtic GM, Adelstein DJ, Mekhail TM, Rice TW, Stevens GH, Lee S‐Y, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small‐cell lung cancer–only brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(1):240–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION TABLE S1. Cox proportional hazards model with interaction test between ≥grade 2 treatment‐related lymphopenia and recursive partitioning analysis, age at brain metastasis, and Karnofsky performance status


