Abstract
We introduce a versatile strategy for the bioreversible modification of proteins. Our strategy is based on a tricomponent molecule, synthesized in three steps, that incorporates a diazo moiety for chemoselective esterification of carboxyl groups, a pyridyl disulfide group for late-stage functionalization with thiolated ligands, and a self-immolative carbonate group for esterase-mediated cleavage. Using cytochrome c (Cyt c) and the green fluorescent protein (GFP) as models, we generated protein conjugates modified with diverse domains for cellular delivery that include a small molecule, targeting and cell-penetrating peptides (CPPs), and a large polysaccharide. As a proof of concept, we used our strategy to effect the delivery of proteins into the cytosol of live mammalian cells in the presence of serum. The cellular delivery of functional Cyt c, which induces apoptosis, highlighted the advantage of bioreversible conjugation on a carboxyl group versus irreversible conjugation on an amino group. The ease and utility of this traceless modification provide new opportunities for chemical biologists.
TOC Graphic
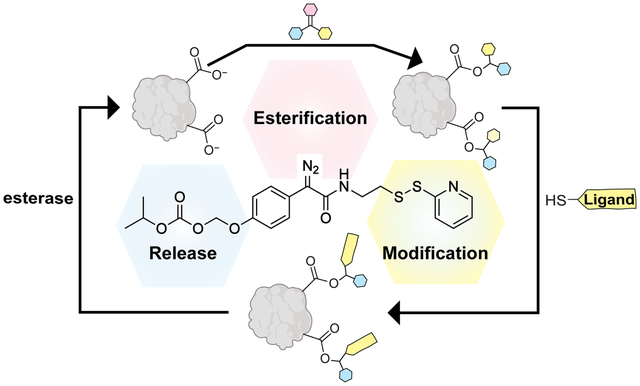
Chemical protein modification has emerged as an invaluable tool for basic and translational research in chemical biology.1–7 Ideal modifications are “traceless,” that is, they bestow a desirable attribute but ultimately leave behind no atoms that could have deleterious consequences for structure or function. The extant repertoire of bioreversible strategies is small. Cys and Lys residues have been the primary targets. A common approach is to attach a ligand to Cys via a mixed disulfide that is then reduced in the cytosol.8–13 Cys has, however, a low abundance in human proteins (2.4%)14 and is often sequestered in the hydrophobic core or disulfide bonds, thus being unavailable for modification.15 In comparison with Cys, Lys is more abundant (5.0%)14 and solvent-accessible,15,16 and some emerging strategies can modify Lys reversibly.17–19 Nonetheless, the available options for traceless protein modification are scant and lack modularity.
The high abundance and solvent accessibility15 of Glu (6.4%)14 and Asp (4.5%)14 residues in human proteins make carboxyl groups (total: 10.9%)14 an attractive target for conjugation. Herein, we introduce a chemoselective, modular, and bioreversible strategy for the modification of protein carboxyl groups. Our strategy relies on a compound that includes domains for (1) protein conjugation, (2) late-stage modification, and (3) traceless removal (Figure 1A). Specifically, we report that diazo compound 1 reacts with protein carboxyl groups in aqueous solution to install activated mixed disulfides, which allow facile diversification via thiol–disulfide interchange. The resultant modifications are tracelessly cleaved by endogenous esterases (Figure 1B).
Figure 1.
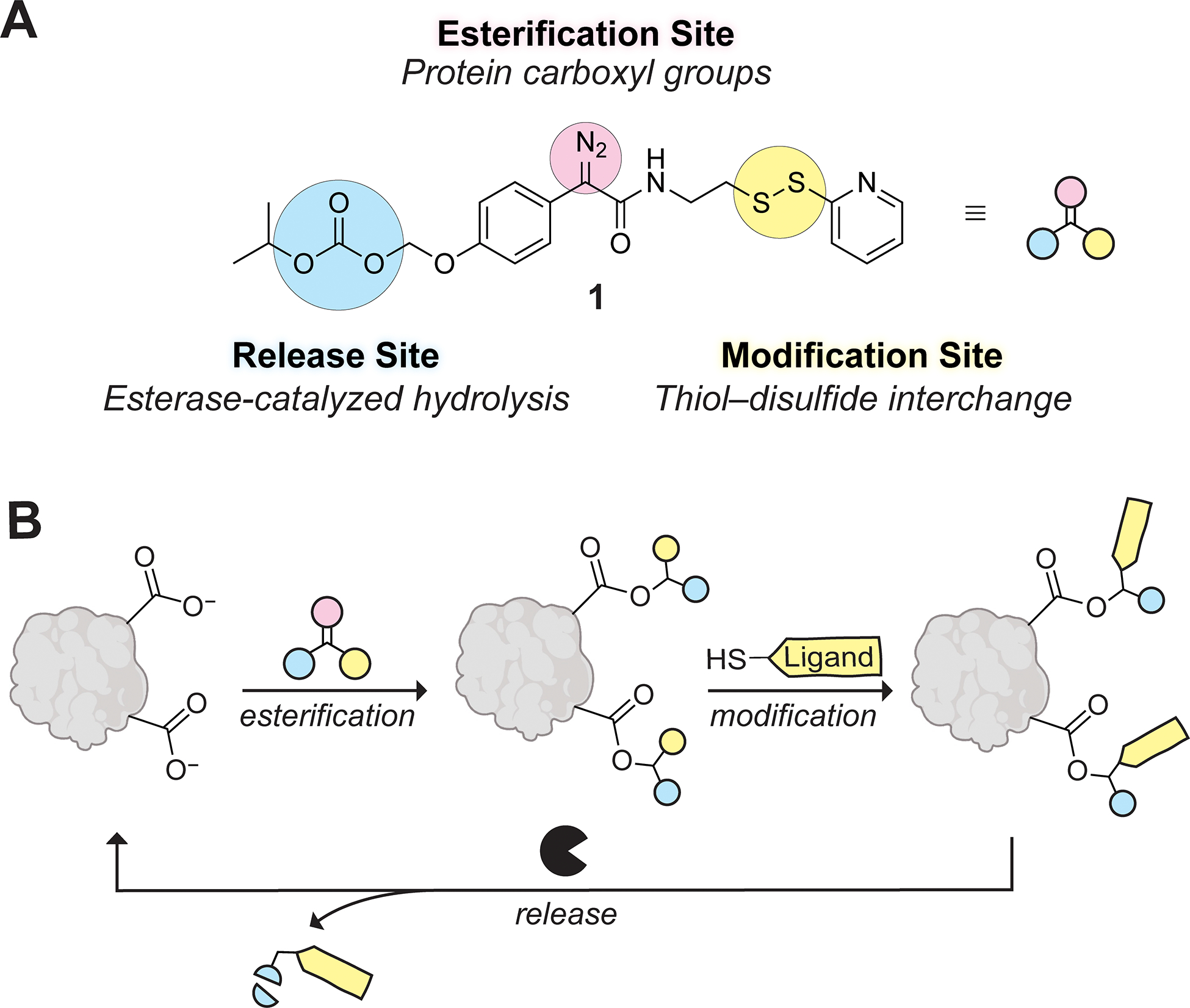
(A) Design of diazo compound 1 with its three strategic domains highlighted in different colors. (B) Scheme for the bioreversible late-stage modification of protein carboxyl groups.
We chose to apply our strategy to the traceless delivery of proteins into mammalian cells—a research area that lacks reagents.20 Proteins have been delivered into cells via site-directed mutagenesis,21,22 conjugation of transduction domains (e.g., CPPs),23–25 and encapsulation in cationic lipid carriers.26 These strategies have shown promise but, with the exception of disulfide-linked CPPs, typically require the irreversible modification of native proteins, which can compromise protein function27 and be problematic for applications that involve live cells.28 For example, upon Lys amidation, PEGylated IFNα2 loses 93% of its activity.29 Addressing these limitations, ongoing efforts are seeking reversible modification strategies.27–29
Recently, we reported on α-aryl-α-diazoacetamides—a new class of bioreversible modification reagents that O-alkylate protein carboxyl groups chemoselectively under physiological conditions.30–32 Such esterification makes the protein surface more cationic and hydrophobic, enabling the delivery of proteins into the cytosol.31,32 Still, the successful delivery of GFP, which is an anionic protein with Z = −9 (where Z refers to Arg + Lys − Asp − Glu), requires the esterification of a dozen or so carboxyl groups.31 Such extensive modification can compromise conformational stability and aqueous solubility of a protein.33
To enable protein delivery with fewer pendant esters, we designed a diazo compound that enables the addition of highly potent delivery domains. Specifically, we supplied diazo compound 1 with a pyridyl disulfide, which allows for rapid thiol–disulfide interchange under mild conditions.34,35 For traceless ester hydrolysis, we installed an alkyloxycarbonyloxymethyl (AOCOM) group, which is found in prodrugs36,37 such as tenofovir disoproxil.38
We synthesized diazo compound 1 by palladium-catalyzed C−H arylation followed by aminolysis (Scheme 1).39 First, AOCOM-functionalized aryl iodide (S1) was synthesized in one step from commercially available starting materials. The coupling of S1 to N-succinimidyl 2-diazoacetate (S2) proceeded in 81% yield despite the reported incompatibility of p-substituted electron-rich aryl groups in C–H arylation.39,40 O→N acyl transfer of AOCOM-functionalized diazoester S3 with (S)-2-pyridylthiocysteamine in wet tetrahydrofuran provided diazo compound 1 in 47% yield. As a control, we synthesized diazo compound 2, which lacks the AOCOM moiety, using 4-iodotoluene (S1′).
Scheme 1.
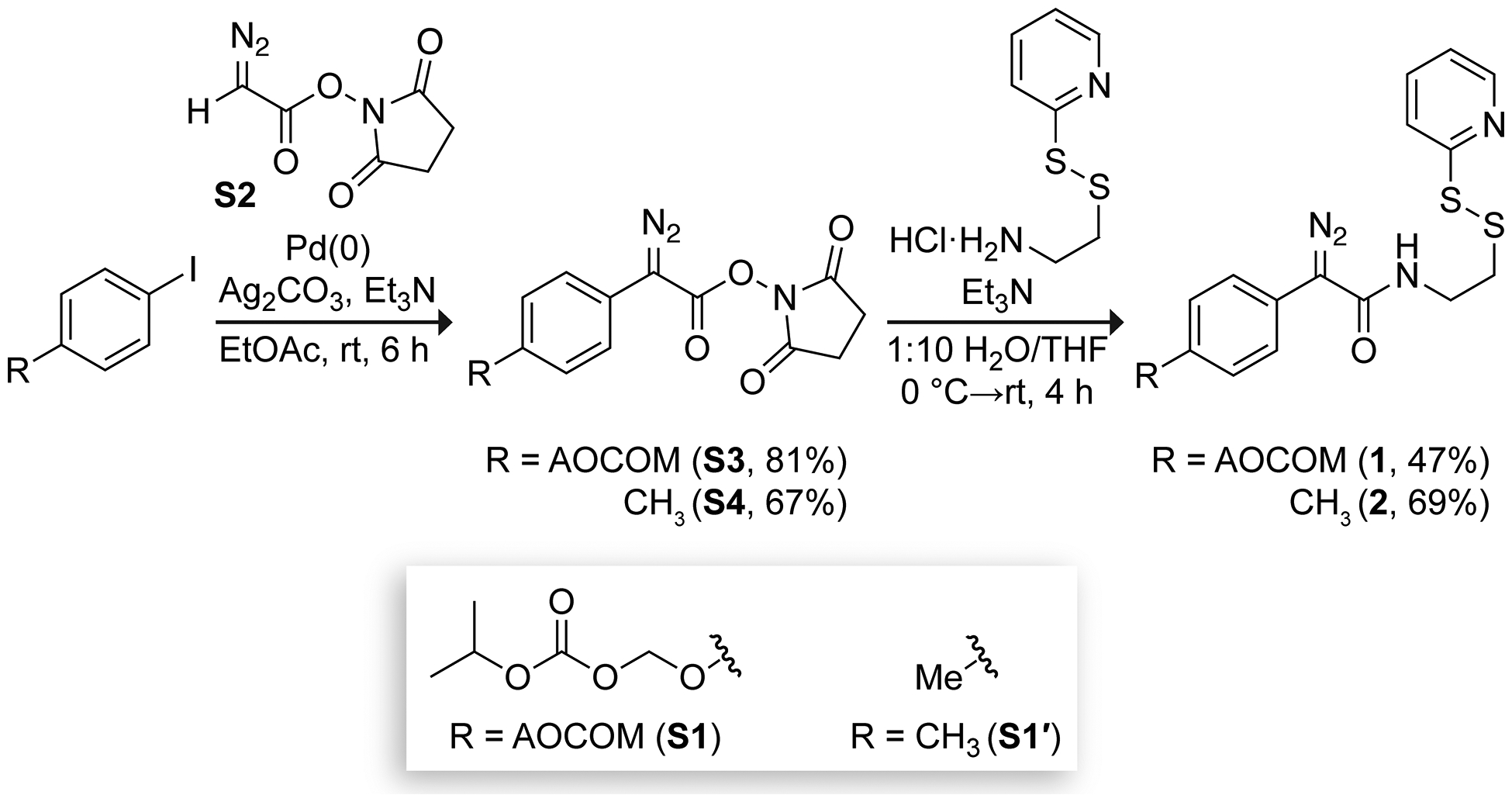
Synthetic Route to Diazo Compounds 1 and 2.
To validate our strategy, we chose two representative proteins with contrasting properties: Cyt c, which is a small, cationic (Z = +6) protein with no reactive Cys residues, and GFP, which is a larger, anionic protein with two reactive Cys residues (Figure 2A). We chose thiolated ligands relevant to protein delivery applications: 1-(2-mercaptoethyl)guanidine (HS-guan),41,42 CPPs cyclic deca-arginine (HS-cR10)10,12,13,43 and linear TAT (HS-TAT),23 integrin-targeting ligand cyclic RGD (HS-cRGD),44 as well as a polysaccharide (HS-dextran (110 K)),45 which represent small molecules, peptides, and macromolecules, respectively. We confirmed the degree of labeling with Q-TOF MS, MALDI-TOF MS, or SDS-PAGE, depending on the nature of the ligand.
Figure 2.
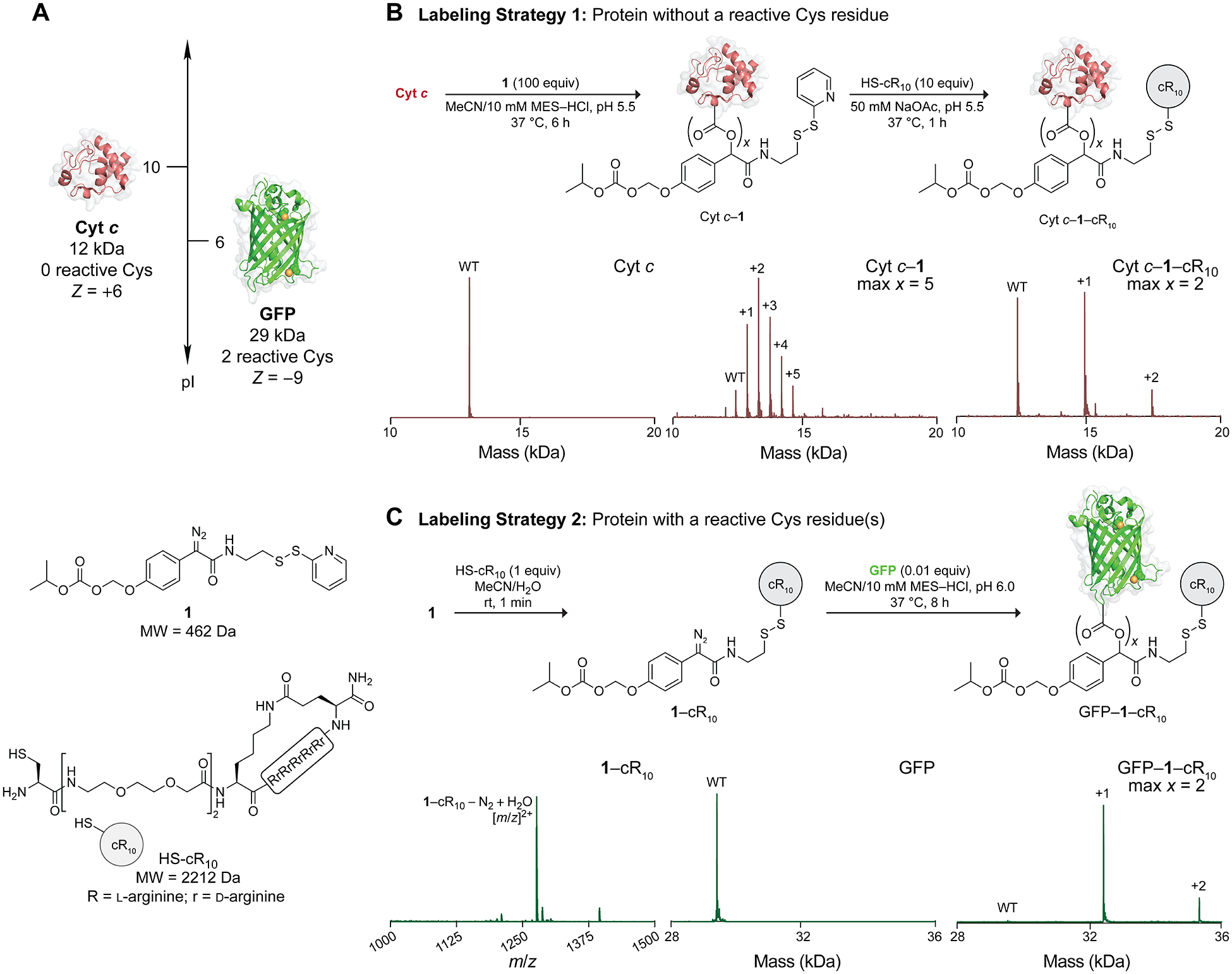
(A) Model proteins and structures of 1 and HS-cR10. (B, C) Labeling strategies for proteins without and with reactive Cys residues. Corresponding Q-TOF MS data are shown under the labeling scheme. The numbers +1, etc. refer to the number of labels; “max x” refers to the maximum number of labels; “WT” refers to the native protein. Expected and observed masses are listed in the Supporting Information (Figures S7, S8, S16, and S17)
We began by optimizing the labeling conditions for a protein without reactive Cys residues, represented by Cyt c.46 Using labeling strategy 1 (Figure 2B), Cyt c was esterified with 100 equiv of 1 (~7 equiv per carboxyl group) at pH 5.5 for 6 h twice, which resulted in up to five labels of 1 on Cyt c (Cyt c–1) (Figure S7). Cyt c–1 (1 equiv) was then incubated with a minimal amount of thiolated ligands (5–20 equiv) at pH 5.5 until all Cyt c–1 was consumed. Using these conditions, Cyt c was modified with HS-cR10 (2 labels, Z = +30, Cyt c–1–cR10) (Figure S8) or HS-cRGD (6 labels, Z = +12) (Figure S9). We also conjugated Cyt c with HS-dextran (110 K) (Figure S10) to demonstrate that our labeling strategy is compatible with a macromolecule.
Next, we modified GFP, a model protein with two reactive Cys residues, using labeling strategy 2 (Figure 2C). Because Cys residues can react with the pyridyl disulfide group (Figure S13), 1 (1 equiv) was premixed with thiolated ligands (1–2 equiv) for 1 min prior to the protein esterification step. The resultant mixture (100 equiv per protein, ~4 equiv per carboxyl group) was then used to esterify GFP at pH 6.0 for 8 h. Using labeling strategy 2, GFP was successfully conjugated with HS-cR10 (2 labels, Z = +15, GFP–1–cR10) (Figures S17 and S18), HS-guan (4 labels, Z = −1) (Figure S19), or HS-TAT (2 labels, Z = +11, GFP–1–TAT) (Figure S20). Notably, the ability to generate 1–TAT in situ highlights the high chemoselectivity of diazo compound 1, which does not react with the sulfhydryl, amino, or low-pKa C-terminal carboxyl group of HS-TAT (Figure S20). Thus, premixing 1 with desired ligands can generate functional diazo compounds. The optimized labeling strategies were also used to generate Cyt c and GFP conjugates with 2 (Figures S7–S10 and S17–S20, respectively).
Then, we turned our attention to the bioreversibility of our strategy. Because of the self-immolative nature of the AOCOM moiety, we envisioned that esterase cleavage of the carbonate group would trigger cleavage of the pendant ester bond through the formation of a quinone methide (QM-1) along with CH2O and CO2, thereby converting protein conjugates back to their native form (Figure 3A). To investigate if esterase-catalyzed hydrolysis of the carbonate bond can initiate the release of the parent carboxyl group, we synthesized a model small molecule (MGA–1) by esterifying O-(2-methoxyethyl)glycolic acid (MGA) with 1. MGA–1 was coincubated with pig liver esterase (PLE) and biological nucleophiles (e.g., reduced glutathione (GSH)) overnight to generate QM-1 (Figures S21–S25). Using LC-MS, we observed masses that correspond to those of QM-1 trapped with water (QM-1–OH), GSH (QM-1–GSH), or 2-mercaptopyridine (QM-1–pyS). These data show that an esterase can release the model acid in a traceless manner.
Figure 3.
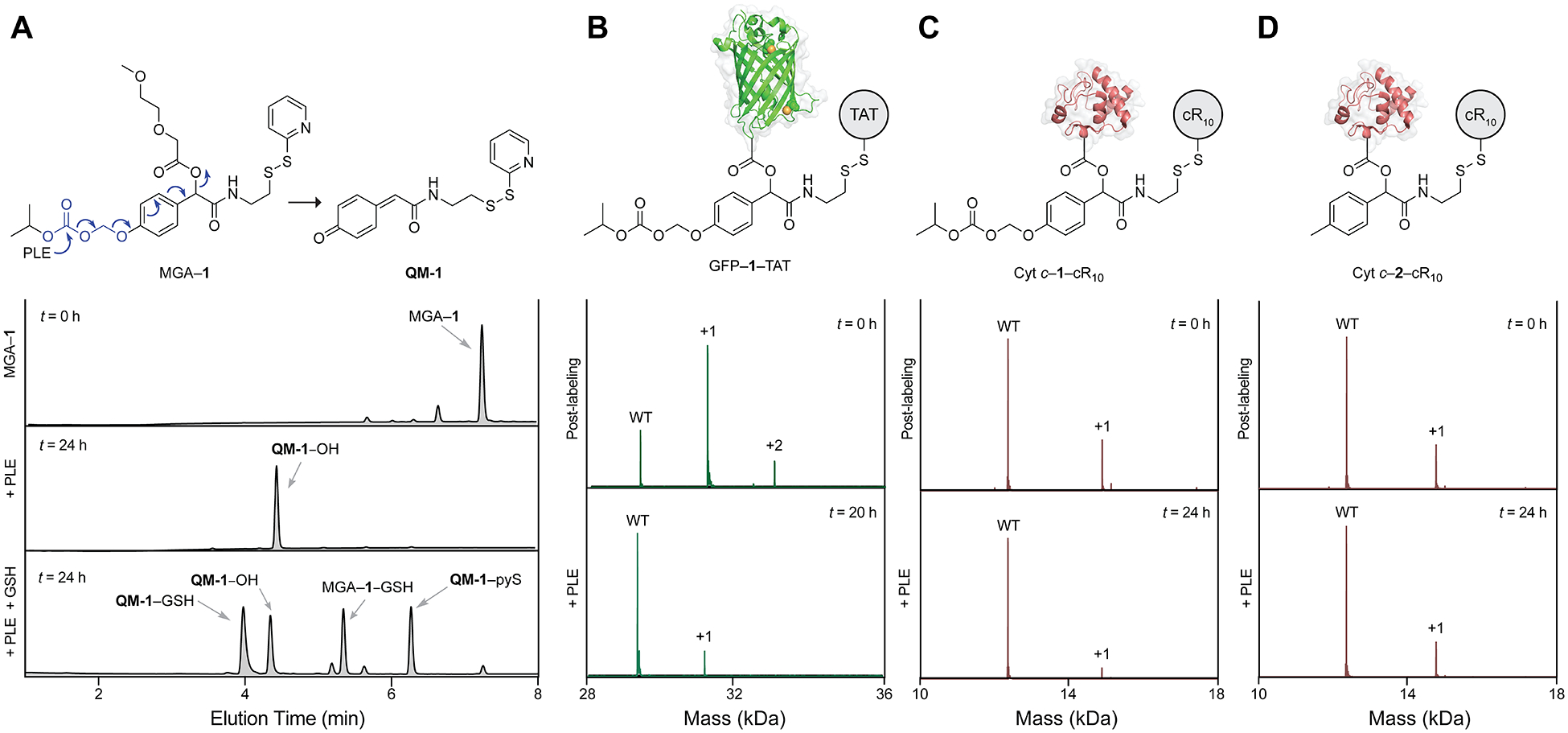
Esterase cleavage. (A) LC-MS analysis of PLE-mediated cleavage of MGA–1. (B–D) Q-TOF MS data of GFP–1–TAT, Cyt c–1–cR10, and Cyt c–2–cR10 before and after incubation with PLE. “WT” refers to the native protein. For experimental details, see Figures S21–S32.
Similarly, we treated Cyt c–1–cR10 and GFP–1–TAT with PLE in the absence of reducing agents. PLE efficiently removed the modifications from both proteins (Figures 3B,C). Under the same conditions, PLE-mediated cleavage of Cyt c–2–cR10 resulted in a slower loss of labels (Figure 3D), likely due to limited access of the esterase to the pendent ester compared to the carbonate group. Overall, GFP–1–TAT, Cyt c–1–cR10, and other conjugates were regenerated to their native forms upon treatment with either PLE, cell lysate, or hydrolysis alone (Figures S26–S32 and S35–S40). Note that, in the presence of cell lysate, which contains endogenous reductants, the disulfide bonds in Cyt c–1–cR10 were reduced (Figures S30–S32) to thiols prior to the loss of labels. These experiments validate the use of the AOCOM moiety for traceless release.
To apply our bioreversible strategy to protein delivery into live cells, we used one of the most efficient of known CPPs,47 cR10 (Figure 4A).10,12,13,43 GFP–1–cR10 and Cyt c–F–1–cR10 (where Cyt c–F refers to fluorescein-labeled Cyt c), as well as the respective conjugates modified with 2 were added to live HeLa or M21 melanoma cells48,49 in medium containing fetal bovine serum (FBS). Based on live-cell epifluorescence imaging (Figure 4B), all the cR10-modified conjugates were internalized into the cytosol within 2.5 h, whereas unesterified proteins did not cross the cell membrane (Figures S43 and S44 for GFP and S47–S49 for Cyt c).
Figure 4.
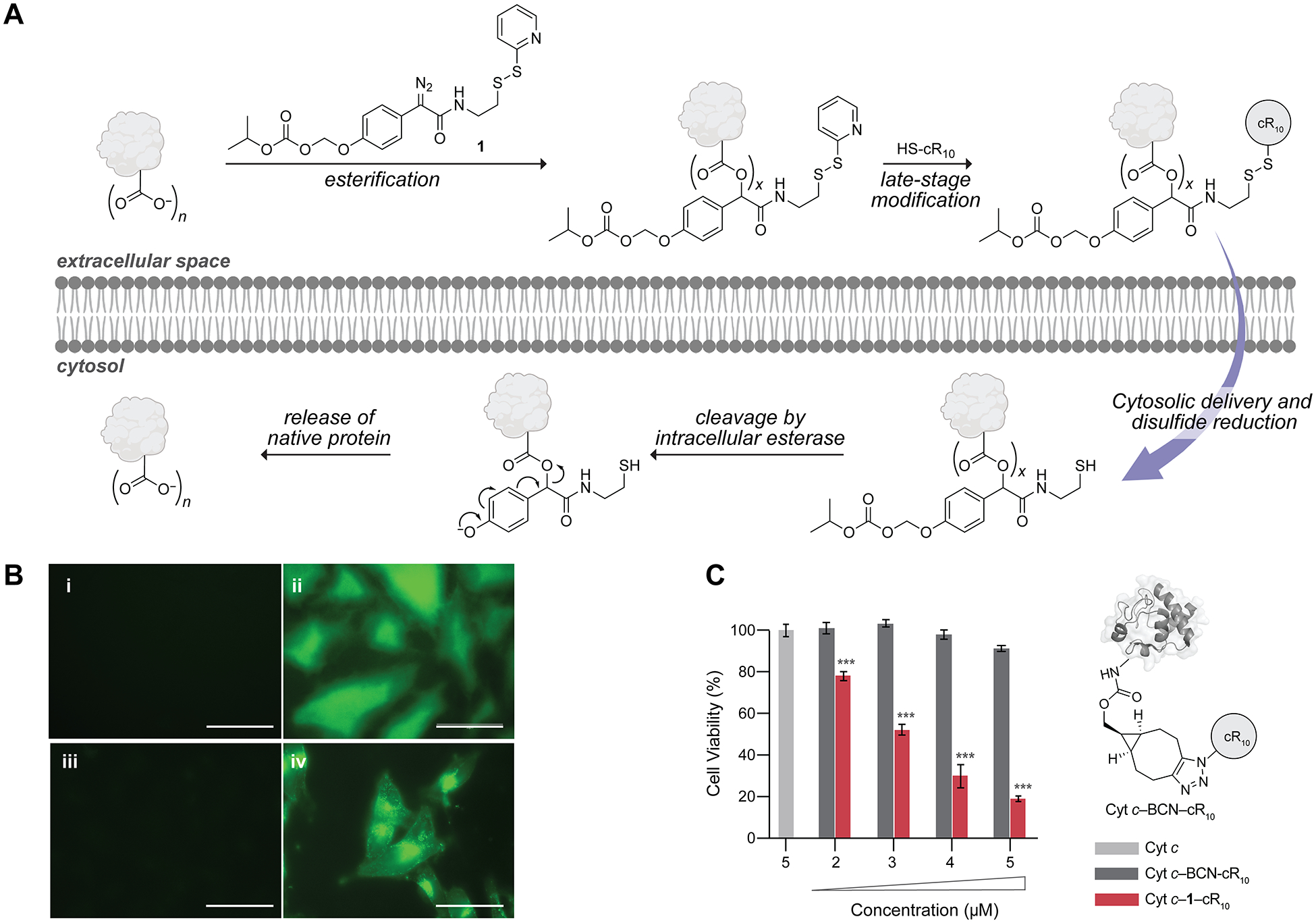
Cellular delivery of proteins. (A) Putative mechanism of cytosolic protein delivery via bioreversible modification with cR10. n refers to the total number of carboxyl groups; x refers to the number of ester labels. (B) Epifluorescence images of 5-μM (i) GFP, (ii) GFP–1–cR10, (iii) Cyt c–F, and (iv) Cyt c–F–1–cR10 incubated with live HeLa cells for 1.5 h (i, ii) or M21 cells for 2.5 h (iii, iv) in the presence of FBS-supplemented DMEM. λex = 488 nm and λem = 500 nm. Scale bars: 50 μm. (C) Viability of M21 cells upon a 41-h treatment with Cyt c (light gray), Cyt c–BCN–cR10 (dark gray, schematic view is shown), or Cyt c–1–cR10 (red). Values are the mean ± SD; ***p ≤ 0.001. For experimental details, see Figures S43, S44, and S47–S49.
Our strategy leads to the regeneration of a native protein in the presence of cellular esterases. This attribute was apparent in cytotoxicity experiments with Cyt c, a protein that induces apoptosis by activating a caspase-dependent proteolytic cascade in the cytosol (Figures S52 and S53).46,50–54 We compared the activity of Cyt c–1–cR10 in live cells with that of Cyt c conjugated with cR10 via irreversible Lys amidation (Cyt c–BCN–cR10) (Figure S54). Specifically, we assessed the toxicity of each conjugate to M21 cells using an MTS assay for metabolic activity. Surprisingly, the bioreversible Cyt c–1–cR10 conjugate, but not the irreversible Cyt c–BCN–cR10 conjugate, induced dose-dependent cytotoxicity (Figures 4C, S55, and S56), despite the high cytosolic uptake of both conjugates (Figures 4B, S50, and S51). These findings demonstrate that efficient protein delivery is necessary but not sufficient for biological activity.51 MS analysis of the proteolyzed irreversible conjugate revealed that Lys86 is modified (Figure S57). This residue is at the interface in the Cyt c Apaf-1 complex, which is required to initiate Cyt c-dependent apoptosis.46,52 The irreversible introduction of cR10 at Lys86 likely disrupts the formation of this complex. These data illustrate an advantage of delivering a protein with our bioreversible strategy.
In conclusion, we have developed a versatile, general late-stage strategy for protein conjugation. rDNA technology is not required, modifications are done under mild conditions, and the number of labels can be tuned by users. Traceless release is achieved by the esterase-mediated formation of a quinone methide. The scaffold can be modified to enable release by other triggers, including small molecules.55,56
In essence, our strategy enables the bioreversible installation of disulfide bonds and sulfhydryl groups on virtually any protein of interest. Its utility is apparent in the traceless cytosolic delivery of proteins. Other applications, such as the reversible decoration of proteins of interest with targeting ligands and pharmacokinetic enhancers, are evident.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Keith M. Cheah (Massachusetts Institute of Technology) for GFP production. We thank the Koch Institute’s Robert A. Swanson (1969) Biotechnology Center, specifically the High Throughput Sciences core facility, for providing HeLa and CHO-K1 cells. The M21 cell line was a kind gift from Dr. Oscar Ortiz (Merck KGaA, Darmstadt, Germany). We thank Prof. Norbert Sewald (Universität Bielefeld) for advice with the M21 cell line. Figures 1 and 4 and the TOC graphic were created with software from BioRender.com.
Funding
J.V.J. was supported by a postdoctoral fellowship from the Ludwig Center at the Koch Institute for Integrative Cancer Research at MIT and a Life Sciences Research Foundation fellowship sponsored by the Shurl and Kay Curci Foundation.
Y.D.P. was supported by NSF Graduate Research Fellowship 4000143422. L.W.E. was supported by NIH postdoctoral fellowship F32 GM126844. This work was supported by NIH grants R01 GM044783, R01 CA073808, and P30 CA014051.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/xxxx.xxxxxxx.
Experimental procedures, compound characterization data, and Figures S1–S57 (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Baslé E; Joubert N; Pucheault M Protein chemical modification on endogenous amino acids. Chem. Biol 2010, 17, 213–227. [DOI] [PubMed] [Google Scholar]
- (2).Stephanopoulos N; Francis MB Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol 2011, 7, 876–884. [DOI] [PubMed] [Google Scholar]
- (3).Hermanson GT, Bioconjugate Techniques, 3rd ed. Academic Press: Waltham, MA, 2013. [Google Scholar]
- (4).Lundblad RL, Chemical Reagents for Protein Modification. CRC Press: Boca Raton, FL, 2014. [Google Scholar]
- (5).Shannon DA; Weerapana E Covalent protein modification: The current landscape of residue-specific electrophiles. Curr. Opin. Chem. Biol 2015, 24, 18–26. [DOI] [PubMed] [Google Scholar]
- (6).Wright TH; Vallée MRJ; Davis BG From chemical mutagenesis to post-expression mutagenesis: A 50 year odyssey. Angew. Chem., Int. Ed 2016, 55, 5896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).deGruyter JN; Malins LR; Baran PS Residue-specific peptide modification: A chemist’s guide. Biochemistry 2017, 56, 3863–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Robbins PB; Oliver SF; Sheu SM; Goodnough JB; Wender P; Khavari PA Peptide delivery to tissues via reversibly linked protein transduction sequences. Biotechniques 2002, 33, 190–192. [DOI] [PubMed] [Google Scholar]
- (9).David Y; Vila-Perelló M; Verma S; Muir TW Chemical tagging and customizing of cellular chromatin states using ultrafast trans-splicing inteins. Nat. Chem 2015, 7, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Herce HD; Schumacher D; Schneider AFL; Ludwig AK; Mann FA; Fillies M; Kasper M-A; Reinke S; Krause E; Leonhardt H; Cardoso MC; Hackenberger CPR Cell-permeable nanobodies for targeted immunolabelling and antigen manipulation in living cells. Nat. Chem 2017, 9, 762–771. [DOI] [PubMed] [Google Scholar]
- (11).Slastnikova TA; Ulasov AV; Rosenkranz AA; Sobolev AS Targeted intracellular delivery of antibodies: The state of the art. Front. Pharmacol 2018, 9, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gui W; Ott CA; Yang K; Chung JS; Shen S; Zhuang Z Cell-permeable activity-based ubiquitin probes enable intracellular profiling of human deubiquitinases. J. Am. Chem. Soc 2018, 140, 12424–12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schneider AFL; Wallabregue ALD; Franz L; Hackenberger CPR Targeted subcellular protein delivery using cleavable cyclic cell-penetrating peptides. Bioconjugate Chem. 2019, 30, 400–404. [DOI] [PubMed] [Google Scholar]
- (14).Echols N; Harrison P; Balasubramanian S; Luscombe NM; Bertone P; Zhang Z; Gerstein M Comprehensive analysis of amino acid and nucleotide composition in eukaryotic genomes, comparing genes and pseudogenes. Nucleic Acids Res. 2002, 30, 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Marino SM; Li Y; Fomenko DE; Agisheva N; Cerny RL; Gladyshev VN Characterization of surface-exposed reactive cysteine residues in Saccharomyces cerevisiae. Biochemistry 2010, 49, 7709–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Abbasov ME; Kavanagh ME; Ichu T-A; Lazear MR; Tao Y; Crowley VM; Am Ende CW; Hacker SM; Ho J; Dix MM; Suciu R; Hayward MM; Kiessling LL; Cravatt BF A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem 2021, 13, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Maier K; Wagner E Acid-labile traceless click linker for protein transduction. J. Am. Chem. Soc 2012, 134, 10169–10173. [DOI] [PubMed] [Google Scholar]
- (18).Liu X; Zhang P; Rödl W; Maier K; Lächelt U; Wagner E Toward artificial immunotoxins: Traceless reversible conjugation of RNase A with receptor targeting and endosomal escape domains. Mol. Pharmacol 2017, 14, 1439–1449. [DOI] [PubMed] [Google Scholar]
- (19).He M; Li J; Han H; Borges CA; Neiman G; Røise JJ; Hadaczek P; Mendonsa R; Holm VR; Wilson RC; Bankiewicz K; Zhang Y; Sadlowski CM; Healy K; Riley LW; Murthy N A traceless linker for aliphatic amines that rapidly and quantitatively fragments after reduction. Chem. Sci 2020, 11, 8973–8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Horn JM; Obermeyer AC Genetic and covalent protein modification strategies to facilitate intracellular delivery. Biomacromolecules 2021, 22, 4883–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fuchs SM; Raines RT Arginine grafting to endow cell permeability. ACS Chem. Biol 2007. 2, 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Smith BA; Daniels DS; Coplin AE; Jordan GE; McGregor LM; Schepartz A Minimally cationic cell-permeable miniature proteins via α-helical arginine display. J. Am. Chem. Soc 2008, 130, 2948–2949. [DOI] [PubMed] [Google Scholar]
- (23).Schwarze SR; Ho A; Vocero-Akbani A; Dowdy SF In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [DOI] [PubMed] [Google Scholar]
- (24).Nischan N; Herce HD; Natale F; Bohlke N; Budisa N; Cardoso MC; Hackenberger CPR Covalent attachment of cyclic TAT peptides to GFP results in protein delivery into live cells with immediate bioavailability. Angew. Chem., Int. Ed 2015, 54, 1950–1953. [DOI] [PubMed] [Google Scholar]
- (25).LaRochelle JR; Cobb GB; Steinauer A; Rhoades E; Schepartz A Fluorescence correlation spectroscopy reveals highly efficient cytosolic delivery of certain penta-Arg proteins and stapled peptides. J. Am. Chem. Soc 2015, 137, 2536–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zuris JA; Thompson DB; Shu Y; Guilinger JP; Bessen JL; Hu JH; Maeder ML; Joung JK; Chen Z-Y; Liu DR Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol 2015, 33, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lieser RM; Yur D; Sullivan MO; Chen W Site-specific bioconjugation approaches for enhanced delivery of protein therapeutics and protein drug carriers. Bioconjugate Chem. 2020, 31, 2272–2282. [DOI] [PubMed] [Google Scholar]
- (28).Baumann AL; Hackenberger CPR Tag and release: Strategies for the intracellular cleavage of protein conjugates. Curr. Opin. Chem. Biol 2019, 52, 39–46. [DOI] [PubMed] [Google Scholar]
- (29).Veronese FM; Mero A The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [DOI] [PubMed] [Google Scholar]
- (30).Mix KA; Raines RT Optimized diazo scaffold for protein esterification. Org. Lett 2015, 17, 2358–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mix KA; Lomax JE; Raines RT Cytosolic delivery of proteins by bioreversible esterification. J. Am. Chem. Soc 2017, 139, 14396–14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ressler VT; Mix KA; Raines RT Esterification delivers a functional enzyme into a human cell. ACS Chem. Biol 2019, 14, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cheah KM; Jun JV; Wittrup KD; Raines RT Host−guest complexation by β-cyclodextrin enhances the solubility of an esterified protein. Mol. Pharm 2022, 19, 3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).van der Vlies AJ; O’Neil CP; Hasegawa U; Hammond N; Hubbell JA Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjugate Chem. 2010, 21, 653–662. [DOI] [PubMed] [Google Scholar]
- (35).Geven M; Luo H; Koo D; Panambur G; Donno R; Gennari A; Marotta R; Grimaldi B; Tirelli N Disulfide-mediated bioconjugation: disulfide formation and restructuring on the surface of nanomanufactured (microfluidics) nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 26607–26618. [DOI] [PubMed] [Google Scholar]
- (36).Thomas JD; Sloan KB Overcoming steric effects in the coupling reaction of alkyloxycarbonyloxymethyl (AOCOM) halides with phenols: An efficient synthesis of AOCOM phenolic prodrugs. Tetrahedron Lett. 2007, 48, 109–112. [Google Scholar]
- (37).Rautio J; Kumpulainen H; Heimbach T; Oliyai R; Oh D; Järvinen T; Savolainen J Prodrugs: Design and clinical applications. Nat. Rev. Drug Discovery 2008, 7, 255–270. [DOI] [PubMed] [Google Scholar]
- (38).Shaw J-P; Sueoka CM; Oliyai R; Lee WA; Arimilli MN; Kim CU; Cundy KC Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res 1997, 14, 1824–1829. [DOI] [PubMed] [Google Scholar]
- (39).Jun JV; Raines RT Two-step synthesis of α-aryl-α-diazoamides as modular bioreversible labels. Org. Lett 2021, 23, 3110–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Yu Z; Mendoza A Enantioselective assembly of congested cyclopropanes using redox-active aryldiazoacetates. ACS Catal. 2019, 9, 7870–7875. [Google Scholar]
- (41).Pantos A; Tsogas I; Paleos CM Guanidinium group: A versatile moiety inducing transport and multicompartmentalization in complementary membranes. Biochim. Biophys. Acta—Biomembr 2008, 1778, 811–823. [DOI] [PubMed] [Google Scholar]
- (42).Wexselblatt E; Esko JD; Tor Y On guanidinium and cellular uptake. J. Org. Chem 2014, 79, 6766–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Schneider AFL; Kithil M; Cardoso MC; Lehmann M; Hackenberger CPR Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives. Nat. Chem 2021, 13, 530–539. [DOI] [PubMed] [Google Scholar]
- (44).Kapp TG; Rechenmacher F; Neubauer S; Maltsev OV; Cavalcanti-Adam EA; Zarka R; Reuning Y; Notni J; Wester H-J; Mas-Moruno C; Spatz J; Geiger B; Kessler H A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep 2017, 7, 39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chyan W; Kilgore HR; Raines RT Cytosolic uptake of large monofunctionalized dextrans. Bioconjugate Chem. 2018, 29, 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Alvarez-Paggi D; Hannibal L; Castro MA; Oviedo-Rouco S; Demicheli V; Tórtora V; Tomasina F; Radi R; Murgida DH Multifunctional cytochrome c: Learning new tricks from an old dog. Chem. Rev 2017, 117, 13382–13460. [DOI] [PubMed] [Google Scholar]
- (47).Kalafatovic D; Giralt E Cell-penetrating peptides: Design strategies beyond primary structure and amphipathicity. Molecules 2017, 22, 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Cheresh DA; Spiro RC Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem 1987, 262, 17703–17711. [PubMed] [Google Scholar]
- (49).Felding-Habermann B; Mueller BM; Romerdahl CA; Cheresh DA Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J. Clin. Investig 1992, 89, 2018–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Morales-Cruz M; Cruz-Montañez A; Figueroa CM; González-Robles T; Davila J; Inyushin M; Loza-Rosas SA; Molina AM; Muñoz-Perez L; Kucheryavykh LY; Tinoco AD; Griebenow K Combining stimulus-triggered release and active targeting strategies improves cytotoxicity of cytochrome c nanoparticles in tumor cells. Mol. Pharmaceutics 2016, 13, 2844–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Mout R; Ray M; Yesilbag Tonga G; Lee Y-W; Tay T; Sasaki K; Rotello VM Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano 2017, 11, 2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).González-Arzola K; Velázquez-Cruz A; Guerra-Castellano A; Casado-Combreras MÁ; Pérez-Mejías G; Díaz-Quintana A; Díaz-Moreno I; De la Rosa MÁ New moonlighting functions of mitochondrial cytochrome c in the cytoplasm and nucleus. FEBS Lett. 2019, 593, 3101–3119. [DOI] [PubMed] [Google Scholar]
- (53).Delinois LJ; Peón H; Villalobos-Santos JC; Ramírez-Paz J; Miller J; Griebenow KH; Tinoco AD A cytochrome c–chlorotoxin hybrid protein as a possible antiglioma drug. ChemMedChem 2020, 15, 2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Tai W; Zhao P; Gao X Cytosolic delivery of proteins by cholesterol tagging. Sci. Adv 2020, 6, eabb0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Rokita SE, Ed. Quinone Methides. John Wiley & Sons: Hoboken, NJ, 2009. [Google Scholar]
- (56).For example, a phosphine or trans-cyclooctene can trigger release via imino-quinone-methide formation from an aryl azide within this scaffold; (Petri YD; Gutierrez CS; Raines RT Chemoselective caging of carboxyl groups for on-demand protein activation with small molecules. Angew. Chem., Int. Ed, submitted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


