Abstract
Background
AZD7442 is a combination of extended half-life, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)−specific neutralizing monoclonal antibodies (tixagevimab and cilgavimab).
Methods
This phase 1, first-in-human, randomized, double-blind, placebo-controlled, dose-escalation study evaluated AZD7442 administered intramuscularly (300 mg) or intravenously (300, 1000, or 3000 mg) in healthy adults (aged 18–55 years). The primary end point was safety and tolerability. Secondary end points included pharmacokinetics and antidrug antibodies.
Results
Between 18 August and 16 October 2020, a total of 60 participants were enrolled; 50 received AZD7442, and 10 received placebo. Adverse events (all of mild or moderate intensity) occurred in 26 participants (52.0%) in the AZD7442 groups and 8 (80.0%) in the placebo group. No infusion or injection site or hypersensitivity reactions occurred. Tixagevimab and cilgavimab had mean half-lives of approximately 90 days (range, 87.0–95.3 days for tixagevimab and 79.8–91.1 days for cilgavimab) and similar pharmacokinetic profiles over the 361-day study period. SARS-CoV-2–specific neutralizing antibody titers provided by AZD7442 were maintained above those in plasma from convalescent patients with coronavirus disease 2019 (COVID-19).
Conclusions
AZD7442 was well tolerated in healthy adults, showing a favorable safety profile across all doses. Depending on the SARS-CoV-2 variant, pharmacokinetic analyses suggest the AZD7442 could offer protection for ≥6 months against symptomatic COVID-19 after a single 300-mg intramuscular administration.
Clinical trials registration
Keywords: SARS-CoV-2, COVID-19, monoclonal antibody, pharmacokinetics, phase 1, safety, tolerability
A single 300-mg intramuscular AZD7442 dose (150-mg each of tixagevimab/cilgavimab) exhibited an approximate 90-day half-life, was well tolerated in healthy adults (phase 1 study), and could offer protection for ≥6 months against some severe acute respiratory syndrome coronavirus 2 variants.
BACKGROUND
The burden of coronavirus disease 2019 (COVID-19) has been significantly reduced by vaccination [1–4]; however, some individuals are unable to mount a full immune response after vaccination and subsequently are still at risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19 [5–12]. For individuals unable to generate an adequate immune response to vaccination, those who have a breakthrough infection and are at high risk of severe disease, or those who cannot be vaccinated, SARS-CoV-2–specific neutralizing monoclonal antibodies present an option for preventing and treating COVID-19 [13].
AZD7442 (tixagevimab-cilgavimab), a combination of 2 human extended half-life SARS-CoV-2–specific neutralizing monoclonal antibodies, was derived from potent antibodies isolated from the B cells of SARS-CoV-2–infected individuals [14–17]. Tixagevimab (previously AZD8895) and cilgavimab (previously AZD1061) bind to 2 distinct epitopes of the SARS-CoV-2 spike protein receptor-binding domain, minimizing the potential of viral mutational escape [14–17]. Tixagevimab and cilgavimab have been engineered with a triple amino acid substitution (YTE) in their fragment crystallizable regions to prolong the serum half-life, providing extended protection against COVID-19 [18–20]. Another triple amino acid substitution present in both tixagevimab and cilgavimab, the triple modification (TM), decreases fragment crystallizable receptor and complement C1q binding to reduce the risk of antibody-dependent enhanced disease [16, 21].
Based on clinical studies, AZD7442 has been granted authorization for use as preexposure prophylaxis in several regions, including the United States, the European Union, the United Kingdom, Australia, and Canada, and for treatment in the European Union, Japan, and Canada [22–26].
Here, we report the results from the first-in-human, phase 1 study of the safety, tolerability, and pharmacokinetics of AZD7442, as well as assessment of AZD7442-derived anti–SARS-CoV-2 neutralizing antibody levels and antidrug antibodies (ADAs) to AZD7442, >12 months in healthy adults (NCT04507256).
METHODS
Trial Design
This phase 1, first-in-human, randomized, double-blind, placebo-controlled, dose escalation study evaluated the safety, tolerability, immunogenicity, and pharmacokinetics of AZD7442 in healthy adults aged 18–55 years inclusive (NCT04507256). The study started on 18 August 2020 and ended on 19 October 2021. All participants were enrolled at a single study center in the United Kingdom. All participants provided written informed consent.
The study was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Council for Harmonization Good Clinical Practice and AstraZeneca policy on Bioethics and Human Biological Samples. The National Research Ethics Service (London—Riverside Research Ethics Committee) and the Medicines and Healthcare Products Regulatory Agency reviewed and approved this clinical study.
The trial consisted of a screening period of up to 27 days (from day –28 to day –2) followed by a 2-day period before and after dosing on day 1 (day –1 to day 1). Participants were discharged on day 2 after completion of all safety evaluations. After discharge, participants underwent a 360-day follow-up period, during which they were monitored for adverse events (AEs), and serum samples were periodically collected to measure levels of tixagevimab-cilgavimab, anti–SARS-CoV-2 neutralizing antibodies, and ADAs to tixagevimab-cilgavimab. The data presented are from the final analysis through day 361.
Healthy adult participants were randomized 5:1 to receive a single dose of either AZD7442 (tixagevimab-cilgavimab; 100 mg/mL) or placebo in 5 fixed-dose cohorts. Cohort 1a received sequential 1.5-mL intramuscular injections of 150-mg tixagevimab followed by 150-mg cilgavimab (for a total AZD7442 dose of 300 mg) or 2 placebo administrations, with a 1–2-minute observational interval between injections. Cohorts 1b (150 mg of each antibody; 300 mg total), 2 (500 mg of each antibody; 1000 mg total), and 3 (1500 mg of each antibody; 3000 mg total) received 2 sequential intravenous administrations of either tixagevimab followed by cilgavimab, or 2 placebo administrations, with a 30-minute observational interval between infusions. Cohort 4 received a single intravenous infusion containing both tixagevimab (1500 mg) and cilgavimab (1500 mg), coadministered (total 3000 mg), or placebo (Supplementary Figure 1).
Cohort 1a received AZD7442 or placebo administered intramuscularly directly to the ventrogluteal region. AZD7442 was administered intravenously at a maximal rate of 20 mg/min in cohorts 1b, 2, and 3), and as a single intravenous infusion at 50 mg/min in cohort 4.
The AZD7442 starting dose of 300-mg administered intramuscularly used in this study was selected based on the antibody levels in serum predicted to neutralize SARS-CoV-2 for a duration of ≥5 months after the dose, according to AZD7442 80% inhibitory concentration against the USA-WA/1/2020 strain [16]. Sufficient safety margins were predicted for the highest dose used (3000 mg) based on the no AE dose observed level in a nonhuman primate toxicology study [16].
Treatment Allocation
Participants were randomized on day 1 and sequentially assigned randomization codes as they became eligible. Randomization lists and code break envelopes for each participant were produced by Calyx (London, UK).
Sentinel dosing was applied, with 2 participants per cohort randomized 1:1 to AZD7442 or placebo for the sentinel group. The remaining 10 participants were randomized 9:1 to AZD7442 or placebo 24 hours after the sentinel group, following principal study investigator review and confirmation of no safety concerns in the sentinel group, resulting in an overall 5:1 randomization to AZD7442 or placebo. Cohorts 2, 3, and 4 were dosed after the safety review of the previous cohort was complete, with a minimum of 8 participants' blinded safety data from the previous cohort used to determine the safety of dosing the next cohort. Blinded safety data from the 3000-mg intravenous sequentially dosed cohort 3 were evaluated by the Dose Escalation Committee before permitting coadministration of the antibodies intravenously in cohort 4 (Supplementary Figure 1).
Blinding
Investigators and participants were blinded to AZD7442 or placebo allocation but not to dose. The committee responsible for dose escalation decisions was blinded. AstraZeneca staff involved in the study were unblinded, and an unscheduled unblinding visit was permitted for participant COVID-19 vaccination.
Participants
Participants were aged 18–55 years inclusive at the time of screening, with negative SARS-CoV-2 quantitative reverse-transcription polymerase chain reaction and/or serology results before randomization, and they were considered healthy by medical history, physical examination, and baseline safety laboratory studies, according to the judgement of the investigator. Participants were excluded from the study if they had a known hypersensitivity or allergy to any dose component or previous hypersensitivity, infusion-related reaction, or severe adverse reaction to a monoclonal antibody. Participants were also excluded if they presented with acute illness (including fever >99.5°F [>37.5°C]), on the day of or before dosing. Any participant with a history of SARS-CoV-2 infection or symptoms of COVID-19 4 weeks before screening, or who had received a COVID-19 vaccination, was excluded. Full inclusion and exclusion criteria are included in the Supplementary Appendix.
End Points
The primary study end point was the safety and tolerability of AZD7442 administered intramuscularly or intravenously, assessed based on AEs, serious AEs (SAEs), safety laboratory parameters, 12-lead safety electrocardiographic findings, vital signs, and physical examination findings. Injection-site reactions were monitored for the intramuscular administration cohort and were recorded as AEs. The secondary safety end point was the incidence of ADA responses to AZD7442 over time. Treatment-emergent ADA were defined as either ADA negative at baseline and ADA positive at ≥ 1 postbaseline assessment with ADA titer ≥ 2-fold higher than the lowest detectable titer of the respective antibody, or as a baseline positive ADA titer that increased ≥ 4-fold during the study period.
The secondary pharmacokinetic end point was the single-dose pharmacokinetics of AZD7442 in serum, characterized with pharmacokinetic parameters and serum concentration-time profiles. Exploratory end points included the neutralizing antibody titers to SARS-CoV-2 in serum over time and the pharmacokinetics of tixagevimab and cilgavimab in nasal lining fluid (NLF), measured as nasal concentration-time profiles.
Assessments
AEs and SAEs were assessed by observation and questioning throughout the study period. AEs were spontaneously reported by the participant or reported in response to the open question from the study personnel, “Have you had any health problems since you were last asked?” Serum samples for pharmacokinetic and ADA assessments were collected before the dose (all cohorts), during and after infusion (intravenous cohorts only), at 8 hours after the dose and on days 2, 4, 6, 8, 15, 31, 61, 91, 151, 211, 271, and 361 (all cohorts). NLF samples were collected before the dose and on days 8, 31, 91, and 151. A full description of the methods used for pharmacokinetic, ADA, and SARS-CoV-2–specific neutralizing antibody titer assays is included in the Supplementary Appendix.
Statistical Analyses
Each of the 5 cohorts had 12 participants (10 AZD7442 and 2 placebo), for a total of 60 participants. No sample size calculations were performed owing to the descriptive nature of the analyses. No testing of a formal hypothesis was performed. Baseline, safety, and pharmacokinetic data, along with ADA and SARS-CoV-2–specific neutralizing antibody titers, were summarized by AZD7442 and pooled placebo groups, and by dose and administration route for AZD7442.
Missing data were not imputed. AEs were summarized descriptively for each dosing group, and AEs with unknown intensity, relationship to dosing, or seriousness were classed as “severe,” “related,” and “serious,” respectively.
RESULTS
Participants
Between 18 August and 16 October 2020, a total of 60 participants were enrolled in the study, with 10 participants randomized to receive AZD7442 in each cohort. All participants were randomized and dosed; 1 participant each in cohort 2 and the pooled placebo group withdrew consent, and 58 participants continued to the end of the study (Supplementary Figure 2). One participant met 1 of the exclusion criteria (history of squamous cell carcinoma) after being randomized and dosed as part of cohort 1b. Per study protocol, this participant continued with safety and pharmacokinetic and ADA assessments to ensure their safety. No study stopping criteria (Supplementary Appendix), were met and all randomized participants were included in the safety analysis set, with 49 participants who received AZD7442 included in the pharmacokinetic data analysis.
Similar baseline demographics were observed across the AZD7442 and placebo groups (Supplementary Table 1). The mean participant age was 39.4 and 38.1 years in the AZD7442 and placebo groups, respectively; 32 (64.0%) and 5 (50.0%), respectively, were male; and most were white (34 [68.0%] and 6 [60.0%]) and not of Hispanic or Latino ethnicity (49 [98.0%] and 10 [100%]). The mean body mass index (calculated as weight in kilograms divided by height in meters squared) ranged from 23.7 to 25.1 kg/m2 across all cohorts.
Safety of AZD7442
AEs were reported by 26 of 50 participants (52.0%) in the AZD7442 groups compared with 8 of 10 (80.0%) in the placebo group, and fewer participants who received 300-mg intramuscular AZD7442 reported AEs (2 of 10 [20.0%]), compared with those who received 300–3000-mg intravenous AZD7442 (5–7 from a total of 10 participants per cohort [50.0%–70.0%]). A total of 15 of 50 (30.0%) and 11 of 50 (22.0%) participants receiving AZD7442 reported AEs of mild and moderate intensity, respectively, compared with 6 of 10 (60.0%) and 2 of 10 (20.0%) receiving placebo. No severe AEs, SAEs, or AEs with an outcome of death were reported in any of the groups. In the 3000-mg intravenous AZD7442 group, 1 AE leading to dose interruption was reported. No dose-limiting toxicity occurred (Table 1).
Table 1.
Summary of Adverse Events From Dosing to Day 361 (Safety Analysis Set)a
| AE Category | Pooled Placebo (n = 10) | 300-mg AZD7442 Intramuscular (n = 10)b | 300-mg AZD7442 Intravenous Seq (n = 10)b | 1000-mg AZD7442 Intravenous Seq (n = 10)b | 3000-mg AZD7442 Intravenous Seq (n = 10)b | 3000-mg AZD7442 Intravenous Coadmin (n = 10)b | Total AZD7442 (n = 50) |
|---|---|---|---|---|---|---|---|
| Any AE | |||||||
| ȃParticipants, No. (%) | 8 (80.0) | 2 (20.0) | 5 (50.0) | 6 (60.0) | 7 (70.0) | 6 (60.0) | 26 (52.0) |
| ȃEvents, No.c | 14 | 3 | 15 | 14 | 16 | 10 | 58 |
| AE intensity, No. (%)d | |||||||
| ȃMild | 6 (60.0) | 2 (20.0) | 4 (40.0) | 3 (30.0) | 2 (20.0) | 4 (40.0) | 15 (30.0) |
| ȃModerate | 2 (20.0) | 0 (0) | 1 (10.0) | 3 (30.0) | 5 (50.0) | 2 (20.0) | 11 (22.0) |
| ȃSevere | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ȃAny AE leading to dose interruption, No. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0)e | 0 (0) | 1 (2.0) |
| ȃAny AE with the outcome of death, No. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: AE, adverse event; Coadmin, coadministered; SAE, serious AE; Seq, sequentially administered.
The summary includes AEs starting on or after dosing to day 361. Percentages are based on the total number of participants in the treatment group. No SAEs or deaths were observed in any of the groups, and no dose-limiting toxicity occurred.
300-mg AZD7442 included 150-mg tixagevimab and 150-mg cilgavimab; 1000-mg AZD7442, 500-mg tixagevimab and 500-mg cilgavimab; and 3000-mg AZD7442, 1500-mg tixagevimab and 1500-mg cilgavimab. Sequential administration involved 2 sequential intravenous administrations of tixagevimab followed by cilgavimab; coadministration, a single intravenous infusion containing both tixagevimab and cilgavimab.
Multiple events in the same category are counted multiple times in that category. Multiple events belonging to >1 category are counted multiple times in each of those categories.
Participants with multiple events in the same category were counted only once in that category. Participants with events in >1 category were counted once in each of those categories.
Approximately 40 minutes after the start of the second intravenous infusion (cilgavimab), a participant reported arthralgia and headache. The AE resolved after the infusion was stopped. The participant had no other symptoms, and electrocardiographic and vital signs were normal. After the participant was assessed, the infusion was restarted with no further recurrence of AEs.
The most frequently reported AE was headache, reported by 7 (14.0%) and 2 (20.0%) of the participants in the AZD7442 and placebo groups, respectively. Headaches of mild intensity were reported by 4 participants (8.0%) in the AZD7442 groups and 2 (20.0%) in the placebo group, with 3 participants (6.0%) in the AZD7442 groups reporting headaches of moderate intensity. Diarrhea and fatigue were reported by 3 participants (6.0%) in the AZD7442 groups; all were of mild intensity. No participants in the 300-mg intramuscular AZD7442 group reported headache, fatigue, or diarrhea of any severity. One participant in the 1000-mg intravenous AZD7442 group reported a skin irritation at the injection site, which was of mild intensity (Supplementary Table 2).
Mild COVID-19, defined by self-limiting symptoms not requiring any medications and not interfering with daily activities, was reported in 1 participant each in the AZD7442 300-mg intramuscular and the 3000-mg intravenous coadministered groups; both occurred approximately 1 year after dosing and resolved without complication (the specific variant was not confirmed, but Delta was prevalent at the time). There were no observed differences between the AZD7442 groups in mean hematology or clinical chemistry parameters over time, in shifts from normal to high/low for individual parameters, or in vital signs or electrocardiographic parameters.
Pharmacokinetics of Tixagevimab and Cilgavimab
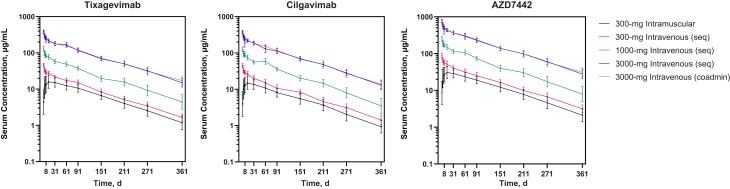
Tixagevimab and cilgavimab were quantifiable in serum over the 361-day study period, with similar profiles observed for each antibody (Figure 1). The mean half-lives calculated for both antibodies from all cohorts were approximately 90 days (range, 87.0–95.3 days for tixagevimab and 79.8–91.1 days for cilgavimab) (Figure 1 and Table 2). Half-lives of tixagevimab and cilgavimab were similar for both routes of administration and all doses.
Figure 1.
Geometric mean (± geometric standard deviation) serum concentrations of tixagevimab, cilgavimab, and AZD7442 (tixagevimab + cilgavimab) after single-dose intramuscular or intravenous administration to healthy adults (pharmacokinetic analysis set). Abbreviation: coadmin, coadministered; seq, sequential.
Table 2.
Pharmacokinetic Parameters for Tixagevimab and Cilgavimab After Single-Dose Intramuscular or Intravenous Administration of AZD7442 (Pharmacokinetic Analysis Set)
| 300-mg AZD7442 Intramuscular (n = 10)a | 300-mg AZD7442 Intravenous Seq (n = 10)a | 1000-mg AZD7442 Intravenous Seq (n = 10)a | 3000-mg AZD7442 Intravenous Seq (n = 10)a | 3000-mg AZD7442 Intravenous Coadmin (n = 10)a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Tixagevimab | Cilgavimab | Tixagevimab | Cilgavimab | Tixagevimab | Cilgavimab | Tixagevimab | Cilgavimab | Tixagevimab | Cilgavimab |
| AUCinf, geometric mean (geometric %CV), d·µg/mL | 2526 (29.75) | 2130 (31.25) | 3677 (13.75)b | 3276 (14.17)b | 9893 (12.58)c | 9712 (11.69)c | 31 850 (10.85) | 29 860 (11.71) | 31 850 (11.89) | 30 030 (11.82) |
| Cmax, geometric mean (geometric %CV), µg/mL | 16.52 (35.56) | 15.27 (38.53) | 53.71 (10.24) | 51.69 (12.31) | 162.2 (11.31) | 154.3 (14.66) | 505.8 (10.54) | 465.5 (11.09) | 447.8 (8.98) | 419.3 (11.62) |
| Tmax, median (min–max), d | 13.96 (3.05–29.99) | 13.98 (3.05–60.23) | 0.04 (0.02–0.33) | 0.02 (0.02–0.96) | 0.04 (0.02–0.05) | 0.02 (0.02–0.34) | 0.10 (0.06–0.13) | 0.06 (0.06–0.33) | 0.05 (0.05–0.05) | 0.05 (0.05–0.33) |
| t½, arithmetic mean (SD), d | 87.76 (14.56) | 79.78 (9.65) | 86.97 (5.20)b | 91.08 (9.15)b | 92.38 (17.23)c | 83.05 (16.22)c | 91.27 (7.83) | 88.52 (9.09) | 95.33 (11.06) | 87.17 (10.78) |
| Vz/F, arithmetic mean (SD), L | 7.77 (2.15) | 8.47 (2.83) | 5.15 (0.58)b | 6.04 (0.59)b | 6.81 (1.07)c | 6.24 (0.97)c | 6.23 (0.59) | 6.45 (0.69) | 6.51 (0.72) | 6.32 (0.78) |
| Vss, arithmetic mean (SD), L | NA | NA | 5.07 (0.48)b | 5.69 (0.47)b | 6.52 (0.97)c | 6.02 (0.88)c | 6.12 (0.60) | 6.31 (0.67) | 6.37 (0.69) | 6.29 (0.75) |
| F361, %d | 68.69 | 65.02 | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: %CV, percentage coefficient of variation; AUCinf, area under the concentration-time curve from time 0 to infinity; Cmax, maximum serum concentration; Coadmin, coadministered; F361d, bioavailability at day 361; NA, not applicable; SD, standard deviation; Seq, sequentially administered; t½, half-life associated with terminal slope of a semilogarithmic concentration-time curve; Tmax, time to maximum serum concentration; Vss, volume of distribution at steady state from an intravenous dose; Vz, volume of distribution following IV administration (based on terminal phase); Vz/F, volume of distribution (apparent) after extravascular administration (based on terminal phase).
300-mg AZD7442 included 150-mg tixagevimab and 150-mg cilgavimab; 1000-mg AZD7442, 500-mg tixagevimab and 500-mg cilgavimab; and 3000-mg AZD7442, 1500-mg tixagevimab and 1500-mg cilgavimab. Sequential administration involved 2 sequential intravenous administrations of tixagevimab followed by cilgavimab; coadministration a single intravenous infusion containing both tixagevimab and cilgavimab.
Note: n = 9; 1 participant met an exclusion criterion and was excluded from the analysis.
Note: n = 9; 1 participant had no samples beyond 1440 hours after the dose, owing to early termination.
Calculated as the single ratio of geometric mean AUCinf after intramuscular administration to geometric mean AUCinf after intravenous administration; thus, there was no %CV.
The maximum serum concentration (Cmax) and total systemic exposure (area under the concentration-time curve from time 0 to infinity) of tixagevimab and cilgavimab increased proportionally with the dose and were comparable when antibodies were sequentially administered or coadministered (Table 2). After 300-mg intramuscular administration, the maximum serum concentrations of 16.5 and 15.3 µg/mL for tixagevimab and cilgavimab, respectively, were reached at a median of 14 days for both antibodies (Table 2). Tixagevimab-cilgavimab serum levels were more variable in participants who received intramuscular AZD7442 when compared with intravenous, with interparticipant variability for total exposure and the maximum serum concentration of tixagevimab and cilgavimab ranging from 29.8% to 38.5% for intramuscular and 9.0% to 14.7% for intravenous doses (Table 2).
NLF Pharmacokinetics of AZD7442
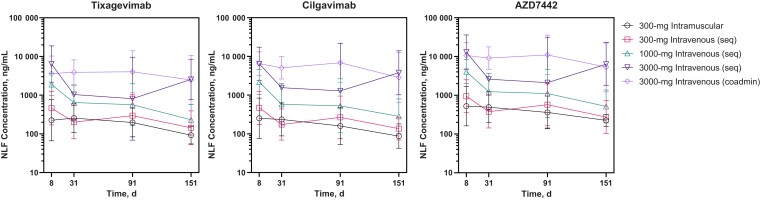
Median concentrations of tixagevimab and cilgavimab in the NLF of participants are displayed in Figure 2. Both tixagevimab and cilgavimab distributed into the upper respiratory tract after a single dose of AZD7442, and AZD7442 NLF concentrations increased in a dose-dependent manner. The geometric mean (standard deviation) NLF concentrations of tixagevimab and cilgavimab were 92.5 (49.2) and 87.5 (58.8) ng/mL, respectively, at day 151 after 300-mg intramuscular AZD7442 (Supplementary Table 3). AZD7442 NLF concentrations were comparable between sequential and coadministration intravenous cohorts. The relative levels of AZD7442 found in the NLF compared with serum levels (NLF:serum partition ratio) are displayed in Supplementary Figure 3 and ranged from 0.1% (day 91) to 22.3% (day 151; both with 3000-mg intravenous AZD7442 sequential dosing). The AZD7442 NLF-serum partition ratios observed were dose-independent, and values remained in the same range 8 and 151 days after the dose. Similar median partition ratios of approximately 1.8% were observed for AZD7442 at day 151 after 300-mg intramuscular or intravenous administration (Supplementary Table 4), showing the sustained distribution of AZD7442 to the upper respiratory tract >5 months.
Figure 2.
Geometric mean (± geometric standard deviation) concentrations of tixagevimab, cilgavimab, and AZD7442 (tixagevimab + cilgavimab) in NLF after a single AZD7442 dose (pharmacokinetic analysis set). Abbreviations: coadmin, coadministered; NLF, nasal lining fluid; seq, sequentially administered.
Antidrug Antibodies to AZD7442
Up to day 211, no ADAs were detected across any of the AZD7442 groups. At day 361, a single participant in the 300-mg intramuscular AZD7442 group tested positive for anti-tixagevimab ADAs, and 4 (40%) and 3 (30%) participants tested positive for anti-cilgavimab ADAs in the 300-mg intramuscular and intravenous cohorts, respectively (Supplementary Table 5). Six of the 7 (85.7%) had low ADA titers at the limit of detection (1:80 for tixagevimab and 1:40 for cilgavimab). Only 1 of the ADA-positive individuals had treatment-emergent ADAs, with a titer of 1:80 to cilgavimab.
SARS-CoV-2–Specific Neutralizing Antibody Titers
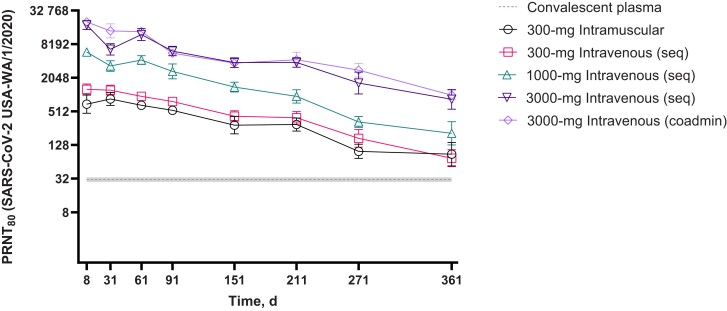
SARS-CoV-2–specific neutralizing antibody titers against the original USA-WA/1/2020 strain increased in a dose-dependent manner that paralleled AZD7442 concentrations and were maintained above the level of convalescent plasma over the 361-day trial period across all AZD7442 groups (Figure 3). Administering tixagevimab and cilgavimab together or sequentially did not affect the neutralizing antibody titers observed over time. Participants who received 300-mg intramuscular or intravenous AZD7442 exhibited 2.5–3-fold higher geometric mean neutralizing antibody titers (GMTs) at day 361 compared with the GMTs measured in convalescent plasma obtained from patients with COVID-19 who were admitted to the hospital >20 days after symptom onset (GMT, 30.8; n = 28) [16].
Figure 3.
Geometric mean (95% CI) neutralizing antibody titers against SARS-CoV-2 after single-dose intramuscular or intravenous administration of AZD7442 (tixagevimab + cilgavimab) to healthy adults compared with convalescent plasma (safety analysis set). Serum samples were from participants in the safety analysis set (all participants). Dashed line represents the GMT ± 95% CIs measured in convalescent plasma from patients with COVID-19 (GMT, 30.8; n = 28) [16]; error bars represent 95% CIs. Abbreviations: CI, confidence interval; coadmin, coadministered; COVID-19, coronavirus disease 2019; GMT, geometric mean titer; PRNT80, 80% plaque reduction neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; seq, sequentially administered.
DISCUSSION
This first-in-human phase 1 study showed that the combination of tixagevimab and cilgavimab (AZD7442) was well tolerated in healthy adults, and had a favorable safety profile at doses ranging from 300-mg intramuscular to 3000-mg intravenous. No dose-limiting toxicity occurred, and none of the study protocol stopping criteria were met. There were no reported incidences of infusion-related reactions (intravenous administration), injection-site reactions (intramuscular administration), or hypersensitivity reactions; with only 1 participant reporting an intravenous administration site irritation.
The ADA profile was unremarkable for AZD7442, with low-titer treatment-emergent ADA detected in only 1 participant, and ADA detected only at 12 months after the dose, with no detectable effect on pharmacokinetics. AZD7442 showed a predictable pharmacokinetic profile, with no interference when the antibodies were coadministered. Our pharmacokinetics model predicts that a minimum protective level is reached within approximately 6 hours based on activity against the original USA-WA/1/2020 strain. Both antibodies exhibited an extended half-life of approximately 90 days, which allowed the SARS-CoV-2–specific neutralizing antibody levels to remain above the GMT in convalescent plasma 12 months after dosing with AZD7442.
The extended half-lives of tixagevimab and cilgavimab support the protective effect of 300-mg intramuscular AZD7442 against COVID-19 for ≥6 months seen in the phase 3 PROVENT study [22], and facilitates the use of AZD7442 in COVID-19 prevention. Compared with AZD7442, other monoclonal antibodies currently or previously authorized for treatment of COVID-19 have shorter half-lives (11.5–49 days) [27–30] and require more frequent dosing [31]. As AZD7442 can be administered intramuscularly, it can be given quickly in an outpatient setting, compared with monoclonal antibodies which require intravenous infusion [27–30].
Initial safety and pharmacokinetic data from this study helped support selection of the 300-mg intramuscular AZD7442 dose for investigation in the phase 3 prophylaxis studies that confirmed the safety and efficacy profile of AZD7442 [22, 32]. The phase 3 PROVENT prophylaxis study, which randomized 3460 participants to dosing with AZD7442, demonstrated a 76.7% (95% confidence interval [CI], 46.0%–90.0%) relative risk reduction in developing symptomatic COVID-19 with 300-mg intramuscular AZD7442 versus placebo at primary analysis (median follow-up, approximately 3 months), and an 82.8% (95% CI 65.8%–91.4%) relative risk reduction at 6-month median follow-up. There were no evident safety concerns [32]. In the phase 3 TACKLE treatment study of 600-mg intramuscular AZD7442, which randomized 456 participants to treatment with AZD7442, AZD7442 was associated with a 50.5% (95% CI 14.6%–71.3%), 66.9% (95% CI 31.1%–84.1%), and an 88.0% (95% CI 9.4%–98.4%) relative risk reduction in the development of severe COVID-19 or death versus placebo when administered within 7, 5, and 3 days of symptom onset, respectively. The safety profile of AZD7442 was favorable [33].
AZD7442 has been shown to neutralize SARS-CoV-2 variants, including most Omicron subvariants, with a half-maximal inhibitory concentration (IC50) range of 4.0–806.0 ng/mL [16, 34–38]. The AZD7442 serum and NLF concentrations observed at days 361 and 151, respectively, exceeded the 10 ng/mL in vitro IC50 against the original SARS-CoV-2 strain, resulting in an expected >99.5% viral inhibition in serum and >90% in the upper respiratory tract [16]. The 4.0–806.0 ng/mL IC50 range observed for in vitro neutralization of some SARS-CoV-2 variants by AZD7442 [16, 34–38] suggests that AZD7442 lower respiratory tract lining fluid concentrations could neutralize those SARS-CoV-2 variants, including some Omicron subvariants, in the lower respiratory tract >5 months after administration. Emerging real-world evidence in immunocompromised populations indicates that a higher AZD7442 dose of 600 mg may be required for 6-month protection as preexposure prophylaxis [39, 40]. In solid organ transplant recipients, AZD7442 at 600 mg was well tolerated and associated with a lower incidence of breakthrough infections compared with matched controls [39]. In a predominantly (92%) immunocompromised population, 2 doses of 300-mg AZD7442 were associated with a significant decrease in the composite outcome of COVID-19 hospitalization and all-cause mortality compared with matched, vaccinated controls who were immunocompromised or at risk of severe disease if infected with SARS-CoV-2 [40].
Strengths of the study include the fact that it was a double-blind, randomized trial with long-term safety follow-up and a comprehensive immunologic and pharmacokinetic profile assessment of AZD7442. A wide range of doses (300–3000 mg) and different routes of administration (intramuscular and intravenous) were used in the study, which recruited a diverse study population.
Study limitations include the small sample size and the descriptive nature of the analyses. Phase 3 trials are assessing the efficacy and long-term safety of AZD7442 out to 15 months [32, 33, 41]. Participants were unblinded for COVID-19 vaccination, which could introduce bias in the safety results and affect long-term compliance with study procedures. Participants were unvaccinated at the start of the study, and we are therefore unable to draw conclusions on AZD7442 safety, tolerability, and pharmacokinetics in a vaccinated population. Assessment of time to reach minimum protective level was based on the initial serum pharmacokinetic samples that were collected approximately 8 and 24 hours after the dose, and there may be variation in the time required to reach a minimum protective level, depending on weight or body mass index.
Data from this first-in-human trial in healthy adults confirm the safety, high levels of SARS-CoV-2–specific neutralizing antibody titers provided by AZD7442, transudation to the nasal mucosae, and the extended half-life of, AZD7442 administered intramuscularly or intravenously. These data supported further investigation of AZD7442 in the prevention and treatment of COVID-19 in phase 3 clinical trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Pablo Forte-Soto, Parexel Early Phase Clinical Unit, London, United Kingdom.
Muna Albayaty, Parexel Early Phase Clinical Unit, London, United Kingdom; Patient Safety, Chief Medical Office, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom.
Dennis Brooks, Patient Safety, Chief Medical Office, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Rosalinda H Arends, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
John Tillinghast, Data Science and Artificial Intelligence, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Anastasia A Aksyuk, Translational Medicine, Vaccines & Immune Therapies, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Jerome Bouquet, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, South San Francisco, California, USA.
Cecil Chen, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, South San Francisco, California, USA.
Asfiha Gebre, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Robert J Kubiak, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Venkatesh Pilla Reddy, Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom.
Seth Seegobin, Biometrics, Vaccines & Immune Therapies, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom.
Katie Streicher, Translational Medicine, Vaccines & Immune Therapies, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Alison Templeton, Biometrics, Vaccines & Immune Therapies, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom.
Mark T Esser, Vaccines & Immune Therapies, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, USA.
Notes
Acknowledgments. The authors thank members of the AZD7442 bioanalytical group (AstraZeneca): Yue Huang, Anton Rosenbaum, Vivian Pettis, and Chong Kim for establishing the pharmacokinetics and antidrug antibody methods used in this study. They also thank Viroclinics Biosciences (Rotterdam, The Netherlands) for performing the severe acute respiratory syndrome coronavirus 2 live virus neutralization assays, and Pedro Garbes, Elaine Harrop, Sumit Markanday, and Swathi Ramesh for their contributions to the study. Medical writing support was provided by Matty Stone, MRes, and editorial support by Sharmin Saleque, MSc, and Joe Alling, BSc, all of Core Medica, London, United Kingdom, supported by AstraZeneca according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460).
Author contributions. J. T. supervised the statistical analysis conducted by Parexel International and contributed to the analysis of the neutralizing antibodies. All authors contributed to data interpretation and to the writing, editing and critical revision of the manuscript, and all reviewed and approved the manuscript for submission.
Disclaimer. The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, the ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Data sharing. Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca's data sharing policy, described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclaimer. The AstraZeneca group of companies allows researchers to submit a request to access anonymized participant-level clinical data, aggregate clinical or genomics data (when available), and anonymized clinical data.
Financial support. This work was supported by AstraZeneca. Funding to pay the Open Access publication charges for this article was provided by AstraZeneca.
References
- 1. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021; 373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. BMJ 2021; 374:n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narasimhan M, Mahimainathan L, Clark AE, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines 2021; 9:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022; 22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med 2021; 27:1744–51. [DOI] [PubMed] [Google Scholar]
- 8. Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021; 80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021; 70:1884–93. [DOI] [PubMed] [Google Scholar]
- 11. Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis 2021; 8:ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Institutes of Health . Anti-SARS-CoV-2 monoclonal antibodies.https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/. Accessed 1 February 2022.
- 14. Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020; 584:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zost SJ, Gilchuk P, Chen RE, et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat Med 2020; 26:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loo YM, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med 2022; 14:eabl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong J, Zost SJ, Greaney AJ, et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat Microbiol 2021; 6:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robbie GJ, Criste R, Dall'acqua WF, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57:6147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother 2017; 61:e01714–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Domachowske JB, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 2018; 37:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for Evusheld™ (tixagevimab co-packaged with cilgavimab).https://www.fda.gov/media/154701/download. Accessed 7 September 2022.
- 23. Australian Government Department of Health . Australian product information EVUSHELD™ tixagevimab and cilgavimab.https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2022-PI-01156-1&d=20220314172310101&d=20220314172310101. Accessed 14 March 2022.
- 24. Medicines and Healthcare Products Regulatory Agency . Summary of product characteristics for Evusheld.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1061470/gb-spc-Evusheld.pdf. Accessed 14 April 2022.
- 25. European Medicines Agency . Evusheld: EPAR—product information.https://www.ema.europa.eu/en/documents/product-information/evusheld-epar-product-information_en.pdf. Accessed 1 December 2022.
- 26. AstraZeneca . Evusheld™ receives Health Canada approval for treatment of Covid-19.https://www.astrazeneca.ca/en/media/press-releases/2022/evusheld–receives-health-canada-approval-for-treatment-of-covid.html. Accessed 30 November 2022.
- 27. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization (EUA) of sotrovimab.https://www.fda.gov/media/149534/download. Accessed 10 March 2022.
- 28. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration . Fact sheet for health care providers: emergency use authorization (EUA) of bamlanivimab and etesevimab.https://www.fda.gov/media/145802/download. Accessed 14 March 2022.
- 30. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for bebtelovimab.https://www.fda.gov/media/156152/download. Accessed 17 June 2022.
- 31. Medicines and Healthcare Products Regulatory Agency . Ronapreve 120 mg/mL solution for injection or infusion: summary of product characteristics.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1012415/revised-gb-spc-ronapreve-clean-120mg-ml12aug2021docx.pdf. Accessed 2 February 2022.
- 32. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med 2022; 386:2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10:985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 2022; 28:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022; 185:467–84.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature 2022; 604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022; 185:2422–33 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou T, Wang L, Misasi J, et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Science 2022; 376:eabn8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant 2022; 22:3130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young-Xu Y, Epstein L, Marconi VC, et al. Tixagevimab/cilgavimab for prevention of COVID-19 during the omicron surge: retrospective analysis of national VA electronic data. medRxiv [Preprint: not peer reviewed]. 29 May 2022. Available from:https://www.medrxiv.org/content/10.1101/2022.05.28.22275716v1. [Google Scholar]
- 41. ClinicalTrials.gov . Phase III double-blind, placebo-controlled study of AZD7442 for post-exposure prophylaxis of COVID-19 in adults (STORM CHASER).https://clinicaltrials.gov/ct2/show/NCT04625972. Accessed 1 February 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.