Abstract
Background
Respiratory syncytial virus (RSV) is associated with acute respiratory infection. We sought to identify RSV variants associated with prolonged infection.
Methods
Among healthy term infants we identified those with prolonged RSV infection and conducted (1) a human genome-wide association study (GWAS) to test the dependence of infection risk on host genotype, (2) a viral GWAS for association with prolonged RSV infection using RSV whole-genome sequencing, (3) an analysis of all viral public sequences, (4) an assessment of immunological responses, and (5) a summary of all major functional data. Analyses were adjusted for viral/human population structure and host factors associated with infection risk.
Results
We identified p.E123K/D and p.P218T/S/L in G protein that were associated with prolonged infection (Padj = .01). We found no evidence of host genetic risk for infection. The RSV variant positions approximate sequences that could bind a putative viral receptor, heparan sulfate.
Conclusions
Using analysis of both viral and host genetics we identified a novel RSV variant associated with prolonged infection in otherwise healthy infants and no evidence supporting host genetic susceptibility to infection. As the capacity of RSV for chronicity and its viral reservoir are not defined, these findings are important for understanding the impact of RSV on chronic disease and endemicity.
Keywords: RSV, GWAS, infection, population, prolonged, respiratory, viral
A comprehensive computational statistical analysis of both host and viral genetics provided compelling evidence for RSV viral persistence in healthy human infants, a finding of significant importance to understanding the impact of RSV on chronic disease and viral endemicity.
Human Orthopneumovirus, formerly known (and still referred to) as respiratory syncytial virus (RSV), results in significant global morbidity and mortality [1]. By the age of 2 to 3 years, nearly all children have been infected with RSV at least once [2]. RSV is a seasonal mucosal pathogen that primarily infects upper and lower respiratory tract epithelium, although it has been recovered from nonairway sources [3–8]. While RSV is mainly associated with acute respiratory infection, many RNA viruses can establish prolonged or persistent infection in some infected individuals [9]. Prolonged shedding of RSV, especially in young infants and following first infection, has been demonstrated, with longer average duration of viral shedding when polymerase chain reaction (PCR) is used to detect RSV [10]. While younger age and first infection are associated with protracted infection [2, 11], it is not known whether specific viral factors contribute to prolonged RSV infection in infants. This is important as prolonged infection may contribute to enhanced transmission and developmental changes to the early life airway epithelium. Furthermore, the reservoir of RSV infection is not understood, and it is possible that some RSV strains sustain a low level of ongoing viral circulation in the community until seasonal or other influences favor epidemic spread [12].
The objectives of this study were therefore to determine if there exist host or pathogen genetic risk alleles for RSV infection and to identify viral genetic variation associated with prolonged infection. These motivating questions are of fundamental interest in understanding viral and host genetic contributions that may underlie the development of chronic respiratory morbidity due to RSV, including asthma.
METHODS
The protocol and informed consent documents were approved by the Institutional Review Board at Vanderbilt University Medical Center (No. 111299). One parent of each participant in the cohort study provided written informed consent for participation in this study. The informed consent document explained study procedures and use of data and biospecimens for future studies, including genetic studies.
Among healthy term infants in a cohort specifically designed to capture first RSV infection we identified those with prolonged RSV infection and conducted (1) a human GWAS to test the dependence of first-year RSV infection risk on the genotype, (2) a viral genome-wide association study (GWAS) using RSV whole-genome sequencing to determine the relationship between viral genotypes and prolonged infant RSV infection, (3) an analysis of all viral public sequence data, (4) an assessment of the local immunological RSV responses, and (5) a summary of all the major functional data for the identified viral variant. Full details of the methods are included in the Supplementary Material.
RESULTS
Cohort Characteristics
The Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) cohort consisted of 1949 enrolled infants among whom there were 2093 in-person respiratory illness visits completed during the winter virus season, November-March, of each year (Supplementary Figure 1); the median (interquartile range) number of in-person respiratory illness visits per infant during this surveillance window was 1. There were 344 RSV PCR-positive samples from 325 individuals, which were sequenced. Prolonged infection was a priori defined as meeting criteria for acute respiratory infection with 2 or more RSV PCR-positive nasal samples ≥ 15 days between testing, with improvement in symptoms between testing. Thus, these infections do not represent severe infections, but prolonged infections. These infections were on average less severe compared with term healthy infant infection among the entire cohort as measured by an ordinal respiratory severity score (Supplemental Methods, Section 8.2) [12]. There were 19 infants who met the definition of prolonged infection with available viral sequencing used to confirm clonality of original and subsequent virus detections. The mean RSV cycle threshold (Ct) value of first infections was 25.9 (SD 7.1), and second detection was 31.6 (SD 5.4). All samples were analyzed together and raw values are reported without normalizing Ct to housekeeper genes. The mean number of days between detections was 29 (SD 21) days (Supplementary Figure 2). Table 1 lists the cohort characteristics of infants with prolonged RSV infection compared with other RSV infection and the entire cohort.
Table 1.
Characteristics of Infants With Prolonged RSV Infection Compared With Other RSV Infection and the Entire Cohort
| Characteristic | Prolonged RSV Infection (n = 19) |
RSV Infection (n = 342) |
Total (n = 1949) |
|---|---|---|---|
| Age at first RSV illness, mo, median (IQR) | 6 (4–6) | 4 (2–5) | NA |
| Illness respiratory severity score, median (IQR) | 2.0 (1.2–3.0) | 3.0 (2.0–4.0) | NA |
| RSV season | |||
| ȃ2012–2013 | 68 | 54 | 44 |
| ȃ2013–2014 | 32 | 46 | 56 |
| Self-reported race | |||
| ȃNon-Hispanic black | 37 | 13 | 18 |
| ȃNon-Hispanic white | 63 | 69 | 65 |
| ȃHispanic | 0 | 10 | 9 |
| ȃMultirace/ethnicity/other | 0 | 8 | 8 |
| Female sex | 53 | 44 | 48 |
| Second-hand smoke exposure | 21 | 23 | 47 |
| Health insurance | |||
| ȃMedicaid | 68 | 48 | 54 |
| ȃPrivate | 32 | 51 | 45 |
| ȃNone/unknown | 0 | 1 | 1 |
| Daycare and/or siblingsa | 84 | 78 | 66 |
Data are percentage except where indicated. Prolonged infection is defined as RSV PCR-positive samples with ≥15 days between testing and meeting criteria for acute respiratory infection.
Abbreviations: IQR, interquartile range; NA, not applicable; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
Presence of sibling or another child ≤ 6 years of age at home.
Host Genetic Analyses
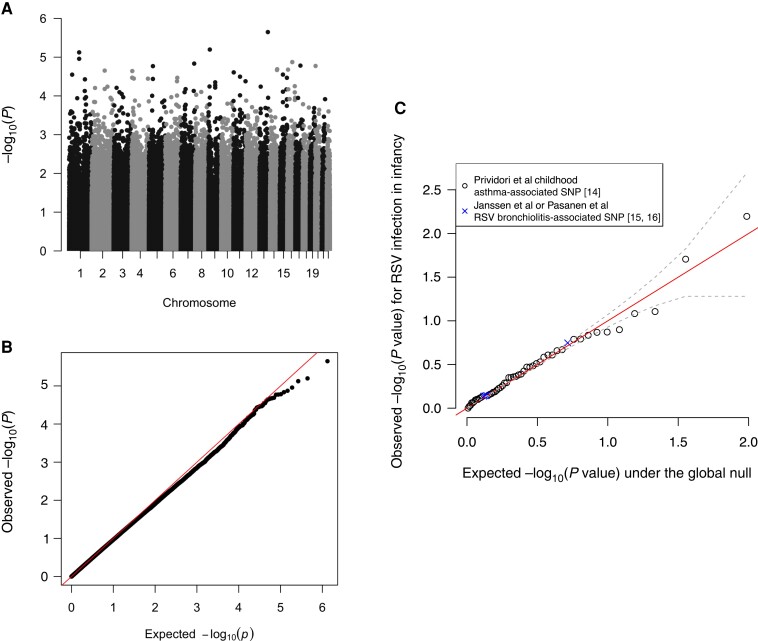
We explored whether RSV infection in infancy is a natural assignment (quasirandom) event and, unlike severity of early life RSV infection [13], occurs independently of host genetics. For the candidate single-nucleotide polymorphism (SNP) analysis, we considered childhood asthma- and RSV lower respiratory tract infection (LRTI)-associated SNPs identified in Pividori et al [14], Janssen et al [15], and Pasanen et al [16]. Associations between genotype at the resulting 54 SNPs (50 childhood asthma- and 4 RSV LRTI-associated SNPs) and RSV infection in infancy in our data are given in Figure 1. The data are consistent with little to no effect of genotype at these SNPs on RSV infection in infancy.
Figure 1.
Genetic analyses of RSV infection in infancy. A, The Manhattan plot shows no genome-wide significant associations (P value threshold of 5e−8). B, The Q-Q plot demonstrates that the observed P values are congruent with those expected under the null hypothesis that RSV infection in infancy is independent of host genotype. C, The association between the 54 selected childhood asthma- or RSV lower respiratory tract infection-associated SNPs and RSV infection in infancy in our data. The solid diagonol identity line (shown in red), and the dashed grey lines are ±1 standard deviation around the expected −log10 (P value). Abbreviations: RSV, respiratory syncytial virus; SNP, single nucleotide polymorphism.
We further investigated the possibility that the analysis was underpowered to identify associations with these SNPs by pooling information across SNPs to estimate the average genetic effect size [17]. We estimated the narrow-sense heritability of RSV infection during infancy on the latent liability scale (h2l), which if > 0 would indicate an accumulation of small genetic effects. We estimated h2l to be exactly 0, suggesting that, if present, infant RSV infection-related genetic signals are both small and sparse (Supplementary Material Section 8.6).
Population Structure
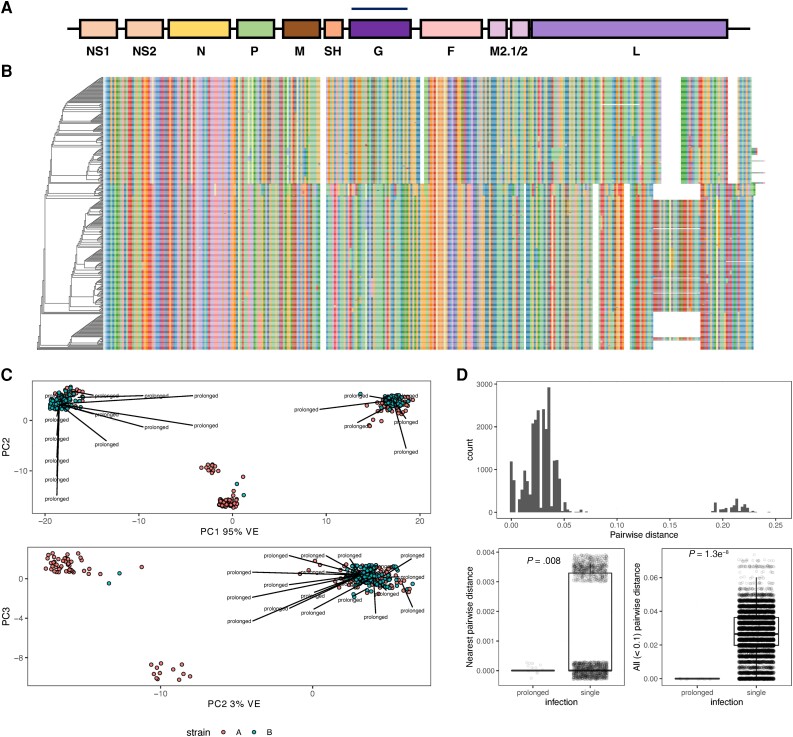
A summary of protein coding genes in RSV is illustrated in Figure 2A. Our analysis focused on F and G protein. The phylogenetic tree based on multiple sequence alignment of G protein amino acid sequences is shown in Figure 2B. One obvious feature causing a separation in genetic diversity is G protein partial gene duplication, which has emerged in recent years within RSV A strains [18]. RSV B strains with a homologous duplication have existed for 2 decades, although the selection process leading to emergence and clinical implications have not been entirely defined.
Figure 2.
Viral population structure. A, Linear map of the RSV genome. B, Phylogenetic tree based on multiple sequence alignment of G protein amino acid sequences. Color indicates amino acids. C, Principal component analysis. PCs 1–3 with labels indicating prolonged infections from different phylogenetic clades. D, A summary of every pairwise genetic distance between every viral sequence is shown (above). Genetic invariance in prolonged infections separated by at least 15 days was compared to other genetic variation within the most closely related sequences (below left) and within all possible closely related pairs (below right). Jitter applied for visualization. Abbreviations: G, glycoprotein; M, matrix protein; PC, principal component; RSV, respiratory syncytial virus; SH, small hydrophobic protein; VE, variance explained.
Principal component (PC) analysis was used for reducing the dimensionality of sequence data, where PC1 accounted for 95.19% of cumulative variance, and variance attributed to other PCs was roughly uniformly distributed (Figure 2C). We observed prolonged infections by viruses from different phylogenetic clades, rather than one specific clade (Figure 2C), indicating that these results are not confounded by latent clade membership.
Genetic Invariance of Prolonged Infection
Figure 2 D (upper graph) summarizes every pairwise genetic distance between every viral sequence, where small distances indicate pairs with closely related sequences. Figure 2D (lower left and right graphs), which summarize the difference in sequence similarity distributions between viruses from the same host and different hosts show that RSV sequences corresponding to initial and subsequent viral detections are nearly identical. These results support the conclusion that such cases are prolonged (ie, failure to clear) infections rather than new infections.
Variants in G Glycoprotein Significantly Associated With Prolonged Infection
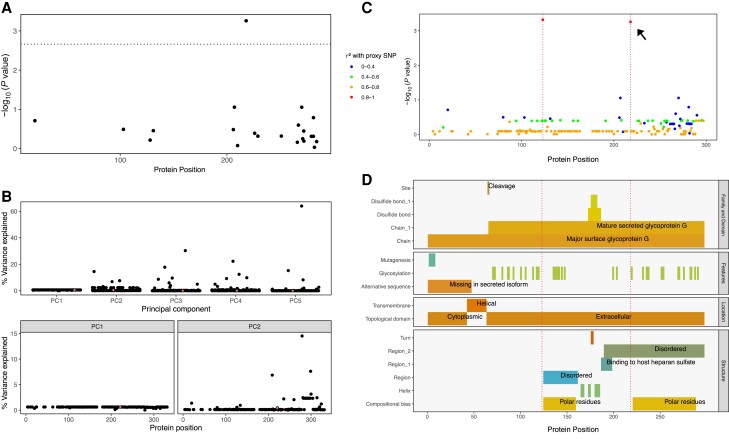
Variants at the amino acid level were assessed for their association with prolonged infection. The model consisted of the binary response (prolonged infection yes/no) and predictors: (1) viral genotype (reference/alternative amino acid); (2) viral PCs 1–5; (3) host sex and host features that have been previously demonstrated as significantly associated with infection; (4) self-reported race/ethnicity; (5) child-care attendance or living with another child ≤ 6 years of age at home [19]. A significant genetic association was identified between prolonged infection and the lead variant after Bonferroni correction for multiple testing (threshold for number of independent variants < 0.05/23 = 0.002), as shown in Figure 3A (P value = .0006).
Figure 3.
Viral genetic association with prolonged infection. A, Amino acid association with prolonged infection after multiple testing correction (significant threshold shown by dotted line). B, Variance explained within cohort. The effect of each variant on cohort structure is shown for PCs 1–2. The small percentage variance explained for a significantly associated lead variant supports a true positive. C, Variants in strong correlation were clumped for association testing using proxies for r2 ≥ 0.8. One significant association was identified (shown in A); the r2 values for all other variants show a single, highly correlated variant with the lead proxy (red), identifying p.E123K/D and p.P218T/S/L. D, Evidence for biological interpretation for every amino acid position is summarized. Dotted red lines indicate the positions at p.123 and p.218. Abbreviations: PC, principal component; SNP, single-nucleotide polymorphism.
To determine whether this association was simply due to population stratification between strains A and B, a subset analysis was performed using independently assessed clinical laboratory strain labels for A and B. The same direction of effect indicated that the association was not a false positive, although in this significantly smaller subanalysis the result was not significant.
To assess the possibility of a false-positive association due to population structure within our cohort, we assessed the magnitude of variance explained (VE) at every amino acid position. Figure 3B (upper graph) shows the VE by each amino acid in PCs 1–5. The cumulative proportion of variance for PCs 1–5 was 99.5% (PC1 = 95%, PC2 = 3%). The values are illustrated according to protein position in Figure 3B (lower left and right graphs). The lead association variant had 0.603% VE for PC1 and 0.458% VE for PC2, a negligible effect that precludes spurious association by allele frequency between populations.
After identifying a significant viral genetic association with prolonged infection, we quantified the correlation of variants with the lead variant. Clumping was performed by ranking based on minor allele frequency and with a cutoff threshold of r2 ≥ 0.8 (Supplementary Figure 3). The association model was repeated for all variants, defining protein p.E123K/D and p.P218T/S/L as candidate causal variants associated with prolonged infection, as shown in Figure 3C. No other variants were correlated with this outcome.
To determine whether p.E123K/D and p.P218T/S/L variant genotypes are novel and potentially influence viral fitness, we searched public worldwide viral data (1956 onward), yielding a total of 1084 G protein sequences. The variants were present at a low and stable frequency, without obvious temporal enrichment (Supplementary Figure 4). Thus, while historical data reveal no positive selective advantage attached to p.E123K/D and p.P218T/S/L, longstanding circulation and linkage in prolonged RSV infection suggest that these polymorphisms are present in the viral inoculum and do not arise through recurrent mutational events.
Due to multiple testing correction according to our analysis plan, an association also originally identified in F protein was rejected and therefore omitted from further discussion. For possible future relevance, the variant position was p.N116S (relative to strain A GenBank: AMN91253.1) (P = .0026; within F protein Padj = .021, combined F and G Padj = .082).
Functional Interpretation
Cell-attachment proteins of paramyxoviruses (G protein in RSV) span the viral envelope and form spike-like projections from the virion surface. RSV G protein is a type II integral membrane protein consisting of 298 amino acid residues comprising N-terminal cytoplasmic (p.1–43), transmembrane helical (p.43–63), and extracellular (p.64–298) domains (Figure 3D). RSV G protein ectodomain also exists in a soluble secreted form, p.66–298, which functions in immune evasion [20–22]. G protein interacts with the small hydrophobic protein [23] and, via the N-terminus, with matrix protein [24]. It has also been reported to form homo-oligomers [25]. The variant amino acid positions associated with prolonged infection reside in a portion of the G protein ectodomain of unassigned specific function and linearly noncontiguous with sequences that bind cell-surface heparan sulfate, which likely promotes RSV cell attachment (p.187–198) [20–22]. However, recent studies indicate that heparan sulfate is not present on the cell surface in the natural environment [1, 26, 27]. In addition, these positions do not contribute to known neutralization epitopes on G protein. Information available in PDB was insufficient to infer effects of p.E123K/D and p.P218T/S/L on local or regional protein structure. The potential effect on glycosylation is indeterminate. Lee et al [28] and Collarini et al [29] report on broadly neutralizing antibodies that bind to p.164–176 (conserved sequences shared by both RSV A and B subtypes) and p.190–204, as well as CD4 epitopes within the latter region. Figure 3D illustrates the position of the variants of interest relative to summarized known functional features.
DISCUSSION
In this study of term healthy infants, we found no evidence of host genetic susceptibility to RSV infection during infancy. This allowed our analysis to focus on elucidation of viral drivers of prolonged infection. A significant viral genetic association in the RSV G protein, p.E123K/D and p.P218T/S/L, with prolonged infant RSV infection was identified. These variants were not associated with severe disease, and public data reveal their consistent presence at low frequencies over the past 30 years, without evidence of enrichment by positive selective pressure over time. The 2 variants we identified in G are correlated with nonrandom association analogous to linkage disequilibrium in the human diploid genome and therefore are not likely to be random mutations, but instead coinherited in the infecting inoculum. This suggests an evolutionary benefit and raises the question of why such variants have maintained a stable but low frequency in the human population for at least 4 decades. These strains are a potential reservoir, emerging seasonally in response to immune, environmental, or other forces. Alternatively, the polymorphisms might recurrently arise de novo during infection of some individuals but are poorly transmissible because of suboptimal fitness. The possibility of viral mutational immune escape has been reported for infants who struggle to control primary RSV infections, allowing for prolonged viral replication and not previously described viral rebound [30].
The RSV variants associated with prolonged infection in our cohort, G p.E123K/D and p.P218T/S/L, lie in the surface region, and there are no known mechanistic features that directly overlap, although it is possible that variant positions approximate sequences that bind a putative viral receptor, heparan sulfate [21], in the G protein 3-dimensional structure. While immortalized cell lines abundantly express surface glycosaminoglycans, including heparan sulfate, it has been reported that RSV infects the apical aspect of ciliated respiratory epithelial cells, which lack detectable surface heparan sulfate [26, 27, 31]. G protein amino acid positions 123 and 218 are not part of known antibody neutralization epitopes or CD8+ cytotoxic T-cell epitopes (Figure 3D). However, p.164–176 and p.190–204 are bound by broadly neutralizing antibodies and CD4 epitopes are known within the region [28, 29]. Treatment and prophylaxis may be gained from the use of antibodies that target F and G proteins. In addition to heparan sulfate, interactions between G protein and CX3CR1, the receptor for the CX3C chemokine fractalkine, have been reported to modulate the immune response and facilitate infection [20–22, 27, 32–33] Furthermore, the mature secreted isoform of G protein (p.66–298) is thought to facilitate viral antibody evasion by acting as an antigen decoy and modifying the activity of leukocytes bearing Fc-γ receptors [34]. Our findings raise the interesting prospect that G protein variants associated with prolonged infection alter a key interaction at the immune interface between pathogen and host.
Although this study was not designed to define mechanisms underlying the association of G protein variants with prolonged infection, these sequence changes might dampen antiviral immune responses and thereby delay viral clearance [35]. It is possible that strains harboring G protein p.E123K/D and p.P218T/S/L variants are cleared more slowly and foster an immune environment of low-level chronic stimulation or exhaustion. We previously demonstrated that infants infected with RSV in their first year of life have dampened subsequent antiviral immune responses in early childhood [36] as well as changes in airway epithelial cell metabolism [37]. Altered immune responses are expected in extended infections by G protein variant strains [35], and we observed differences in the acute antiviral response between subjects with resolved and prolonged infection, specifically increased levels of types 1 and 2 interferon in nasal secretions; however, we could not make causal inference about variant sequences because of confounding by colinearity of these polymorphisms with RSV antigenic group.
While this study has a number of significant strengths, including one of few population-based surveillance studies of first RSV infections during infancy among term healthy infants, our findings are also subject to some limitations. First, this study was not designed with the primary intention to examine infection duration, and additional sampling following initial RSV infection was triggered by a repeat acute respiratory illness. Asymptomatic prolonged infections would therefore not have been captured. Second, our study cohort was small, necessitating focus on viral surface glycoproteins, F and G, due to their variability and importance in host immunity. A larger cohort with serial sampling would be required to diminish the impact of colinearity of viral genotypes with antigenic groups and to perform informative viral whole-genome analysis. Genome-wide information might elucidate other determinants of prolonged infection or pathogen fitness that mediate and/or modulate effects of phenotype-driving variations. Third, again due to small sample size, we could only investigate host genetic risk for infection, not prolonged infection. While we have not specifically assessed subjects for rare monogenic variants that may underlie immunodeficiency, our enrolment criteria included only infants who were term and otherwise healthy. While we performed an interaction analysis for the outcome of host asthma, host genetics, and pathogen genetics and found no significant interaction, our sample size is unlikely sufficient to exclude such an interaction. Lastly, while we do not expect a role for immune memory in these first-in-life RSV infections, we cannot exclude modulatory effects of maternal antibody, which we did not measure. Despite these limitations, the results are novel and represent an in-depth comprehensive computational statistical analysis of both host and viral genetics, providing compelling evidence for RSV viral strain persistence in healthy human infants, a finding of significant importance to understanding the impact of RSV on chronic disease and viral endemicity.
In summary, we identified a novel RSV viral variant associated with prolonged infection in healthy infants, but no evidence of host genetic susceptibility to infant RSV infection. Understanding host and viral mechanisms that contribute to prolonged infection will be important in crafting strategies to control the short- and long-term impact of RSV infection. The identification of RSV variants associated with prolonged infection might also improve vaccine design, particularly if these variants stimulate robust immunity or, in contrast, escape the immune response or induce immunopathologic conditions. The growing availability of large genomic and functional data sources provides opportunities for advancing our understanding of the pathogenesis of infant RSV infection, defining the contribution of viral genetic variants to acute and chronic disease, and informing the development of effective vaccines. As neither the capacity of RSV for prolonged infection in immunocompetent hosts nor a viral reservoir has been delineated, these results are of fundamental interest in understanding viral and host genetic contributions that may promote prolonged infection and influence development of chronic respiratory morbidity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support . This work was supported by the US National Institutes of Health(grant numbers U19 AI 095227, UG3/UH3 OD023282, UL1 TR002243), and Swiss National Science Foundation (SNSF IZSEZ0_191968) to T. V. H.; NIH U19 subaward to L. J. A.; SNSF 310030L_197721 to J. F.; and X01 HLG244 RS&G to E. L.).
Supplementary Material
Contributor Information
Dylan Lawless, Global Health Institute, School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
Christopher G McKennan, Department of Statistics, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Suman R Das, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Thomas Junier, Swiss Institute of Bioinformatics, Vital-IT Group, Lausanne, Switzerland.
Zhi Ming Xu, Global Health Institute, School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
Larry J Anderson, Department of Pediatrics, Emory University School of Medicine and Children's Healthcare of Atlanta, Atlanta, Georgia, USA.
Tebeb Gebretsadik, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Meghan H Shilts, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Emma Larkin, Division of Allergy, Immunology, and Pulmonary and Critical Care Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Christian Rosas-Salazar, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
James D Chappell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jacques Fellay, Global Health Institute, School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland; Biomedical Data Science Center, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Tina V Hartert, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. Hall CB, Weinberg GA, Iwane MK. et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 3. Bokun V, Moore JJ, Moore R. et al. Respiratory syncytial virus exhibits differential tropism for distinct human placental cell types with Hofbauer cells acting as a permissive reservoir for infection. PLoS One 2019; 14:e0225767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cubie HA, Duncan LA, Marshall LA, Smith NM. Detection of respiratory syncytial virus nucleic acid in archival postmortem tissue from infants. Pediatr Pathol Lab Med 1997; 17:927–38. [PubMed] [Google Scholar]
- 5. Nadal D, Wunderli W, Meurmann O, Briner J, Hirsig J. Isolation of respiratory syncytial virus from liver tissue and extrahepatic biliary atresia material. Scand J Infect Dis 1990; 22:91–3. [DOI] [PubMed] [Google Scholar]
- 6. O’Donnell DR, McGarvey MJ, Tully JM, Balfour-Lynn IM, Openshaw PJ. Respiratory syncytial virus RNA in cells from the peripheral blood during acute infection. J Pediatr 1998; 133:272–4. [DOI] [PubMed] [Google Scholar]
- 7. Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rohwedder A, Keminer O, Forster J, Schneider K, Schneider E, Werchau H. Detection of respiratory syncytial virus RNA in blood of neonates by polymerase chain reaction. J Med Virol 1998; 54:320–7. [DOI] [PubMed] [Google Scholar]
- 9. Randall RE, Griffin DE. Within host RNA virus persistence: mechanisms and consequences. Curr Opin Virol 2017; 23:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munywoki PK, Koech DC, Agoti CN. et al. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect 2015; 143:804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagga B, Harrison L, Roddam P, DeVincenzo J. Unrecognized prolonged viral replication in the pathogenesis of human RSV infection. J Clin Virol 2018; 106:1–6. [DOI] [PubMed] [Google Scholar]
- 12. Okiro EA, White LJ, Ngama M, Cane PA, Medley GF, Nokes DJ. Duration of shedding of respiratory syncytial virus in a community study of Kenyan children. BMC Infect Dis 2010; 10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larkin EK, Hartert TV. Genes associated with RSV lower respiratory tract infection and asthma: the application of genetic epidemiological methods to understand causality. Future Virol 2015; 10:883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med 2019; 7:509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssen R, Bont L, Siezen CLE. et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis 2007; 196:826–34. [DOI] [PubMed] [Google Scholar]
- 16. Pasanen A, Karjalainen MK, Bont L. et al. Genome-wide association study of polymorphisms predisposing to bronchiolitis. Sci Rep 2017; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golan D, Lander ES, Rosset S. Measuring missing heritability: inferring the contribution of common variants. Proc Natl Acad Sci 2014; 111:E5272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eshaghi A, Duvvuri VR, Lai R. et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS One 2012; 7:e32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas RG Jr. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294:414–9. [DOI] [PubMed] [Google Scholar]
- 20. Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol 1987; 68:2521–4. [DOI] [PubMed] [Google Scholar]
- 21. Feldman SA, Hendry RM, Beeler JA. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol 1999; 73:6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feldman SA, Audet S, Beeler JA. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol 2000; 74:6442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rixon HWM, Brown G, Murray J, Sugrue R. The respiratory syncytial virus small hydrophobic protein is phosphorylated via a mitogen-activated protein kinase p38-dependent tyrosine kinase activity during virus infection. J Gen Virol 2005; 86:375–84. [DOI] [PubMed] [Google Scholar]
- 24. Ghildyal R, Li D, Peroulis I. et al. Interaction between the respiratory syncytial virus G glycoprotein cytoplasmic domain and the matrix protein. J Gen Virol 2005; 86:1879–84. [DOI] [PubMed] [Google Scholar]
- 25. Collins PL, Mottet G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J Gen Virol 1992; 73:849–63. [DOI] [PubMed] [Google Scholar]
- 26. Monzon ME, Casalino-Matsuda SM, Forteza RM. Identification of glycosaminoglycans in human airway secretions. Am J Respir Cell Mol Biol 2006; 34:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson SM, McNally BA, Ioannidis I. et al. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog 2015; 11:e1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H-J, Lee J-Y, Park M-H, Kim J-Y, Chang J. Monoclonal antibody against G glycoprotein increases respiratory syncytial virus clearance in vivo and prevents vaccine-enhanced diseases. PLoS One 2017; 12:e0169139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collarini EJ, Lee FE-H, Foord O. et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 2009; 183:6338–45. [DOI] [PubMed] [Google Scholar]
- 30. Brint ME, Hughes JM, Shah A. et al. Prolonged viral replication and longitudinal viral dynamic differences among respiratory syncytial virus infected infants. Pediatr Res 2017; 82:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Bukreyev A, Thompson CI. et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 2005; 79:1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. Cx3c chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2001; 2:732–8. [DOI] [PubMed] [Google Scholar]
- 33. Jeong K-I, Piepenhagen PA, Kishko M. et al. Cx3cr1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PloS One 2015; 10:e0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bukreyev A, Yang L, Fricke J. et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol 2008; 82:12191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt ME, Varga SM. Modulation of the host immune response by respiratory syncytial virus proteins. J Microbiol 2017; 55:161–71. [DOI] [PubMed] [Google Scholar]
- 36. Chirkova T, Rosas-Salazar C, Gebretsadik T. et al. Effect of infant RSV infection on memory T cell responses at age 2–3 years. Front Immunol 2022; 13:826666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Connelly AR, Jeong BM, Coden ME. et al. Metabolic reprogramming of nasal airway epithelial cells following infant respiratory syncytial virus infection. Viruses 2021; 13:2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.