Abstract
Sleep health tends to worsen during adolescence, partially due to pubertal-related changes that, in combination with social and psychological factors, can lead to long-lasting impairments in sleep health and affective functioning. Discrepant findings between subjective and objective measures of sleep in relation to affect have been reported in studies of adults; however, few investigations have assessed both subjective and objective sleep quality in a single sample, and fewer have examined this in the context of pubertal development. We aimed to 1) characterize pubertal associations with subjective sleep satisfaction, objective sleep efficiency, and objective and subjective sleep duration in adolescents; 2) examine the longitudinal association between daily affect and sleep metrics; and 3) test whether pubertal stage moderated this association. 89 participants (64% female, ages 13–20) completed an ecological momentary assessment (EMA) and actigraphy protocol. Independent of age, advanced pubertal stage was associated with lower subjective sleep satisfaction but not with objective sleep indices. Subjective sleep satisfaction was associated with within-person trajectories of negative affect, but not with positive affect. Pubertal stage and sleep satisfaction did not interact to predict within-day negative or positive affect. These findings are consistent with previous reports showing that objective and subjective sleep health are associated differently with puberty, and that subjective sleep health is associated with daily affect. Pubertal stage may be a more important indicator of subjective sleep quality in adolescence than is chronological age most likely due to hormonal changes and psychological adjustment to the physical changes associated with the pubertal transition.
Keywords: sleep quality, pubertal stage, ecological momentary assessment, actigraphy, multilevel modeling
Introduction
Insufficient sleep is prevalent in adolescence, affecting ~70% of high-school students (National Sleep Foundation, 2020). Studies recommend 8–10 hours of sleep (Paruthi et al., 2016) for adolescents 13–18 years of age; however, 12–18-year-old adolescents tend to sleep for only about 7 hours each night (Galland et al., 2018), which is associated with increased risk for psychological distress (Glozier et al., 2010). Although this low level of sleep duration is due in part to environmental factors (e.g., school start time), it is also influenced by biological changes that begin at the onset of puberty.
Researchers have documented consistent associations between pubertal development and changes in circadian rhythm, demonstrating that, compared to adolescents in earlier stages of puberty, adolescents in later stages of puberty have a delayed circadian phase (Boyar et al., 1972; Carskadon et al., 1993; Hagenauer et al., 2009; Jenni et al., 2005). This biological shift in adolescents’ circadian clock causes them to go to bed later which, when compounded by early school start times, results in a decrease in sleep duration (Crowley et al., 2018) and a greater difference between bedtimes during the week and bedtimes on the weekend (i.e., social jetlag) (Carvalho-Mendes et al., 2020). The endogenous shift towards eveningness in the circadian rhythm tends to correlate with self-reported circadian preference (Duarte & Menna-Barreto, 2022; Roenneberg et al., 2004). Circadian preference has been posited to influence various dimensions of sleep health, including daily sleep duration and sleep quality (Chan et al., 2020). Although the association between puberty and sleep duration has been examined, the association between puberty and sleep quality in adolescence has been understudied. Nonetheless, when examining dimensions of sleep health, a pattern of sleep-wakefulness that supports physical and mental well-being (Buysse, 2014), it is important to consider circadian preference, particularly in the context of associations with pubertal development.
Sleep quality is generally defined by an individual’s ratings of the previous night’s sleep experience (Krystal & Edinger, 2008), although exact definitions vary. Objective measures (e.g., polysomnography, actigraphy) tend to conflict with subjective reports of sleep (Choi et al., 2017). Despite the importance of assessing both objective and subjective measures of sleep health (Dewald et al., 2010), only a few studies have used both methods in the same participants in relation to pubertal development, and these investigations have yielded inconsistent results. For example, Holm et al. (2009) found that individuals in later stages of puberty objectively slept less than did those in earlier/pre-pubertal stages; however, in terms of subjective sleep quality, there were no differences based on pubertal stage. In a different sample, individuals in later stages of puberty reported poorer subjective sleep quality, but there was no association with objective sleep duration (Becker et al., 2019).
There are notable links between sleep health and affective problems (e.g., internalizing symptoms) during the transition through puberty that likely reflect a bidirectional relation between these two constructs. Although high levels of negative affect (NA) and low levels of positive affect (PA) are not necessarily problematic on a given day, a pattern of consistent high NA and low PA has been found to be associated with a diagnosis of depression, particularly in girls who are in mid to late puberty (Forbes et al., 2004). A meta-analysis examining naturalistically assessed sleep studies over time (i.e., through daily diary and actigraphy), mainly with adult samples, indicated that better sleep health predicts better next-day PA and lower levels of next-day NA (Konjarski et al., 2018). It is not clear, however, whether advanced pubertal stage moderates this association.
Present Study
In this study, our first aim was to use multimodal measures of naturalistic sleep quality and duration to characterize the associations between pubertal stage and sleep health. To measure subjective sleep health, we obtained daily self-reported measures of sleep duration and sleep satisfaction (i.e., quality) using ecological momentary assessment (EMA). To measure objective sleep health, we examined sleep duration and sleep efficiency (i.e., the proportion of total sleep time to time-in-bed) metrics from daily actigraphy. Whereas subjective sleep health dimensions of sleep duration and sleep quality were measured using ecological momentary assessment (EMA), objective sleep health dimensions of sleep duration and sleep quality (i.e., sleep efficiency) were measured with actigraphy. Sleep efficiency is commonly used as an objective index of sleep quality (Evans et al., 2021). Further, sleep efficiency has been shown to be related to subjective sleep quality more strongly than are other actigraphy-based metrics (Kaplan et al., 2017). Thus, for our first aim, we hypothesized that adolescents who are more advanced in puberty will have poorer subjective sleep satisfaction, lower objective sleep efficiency, and shorter subjective and objective sleep duration than will adolescents who are less advanced in puberty.
Importantly, a portion of our data were collected during the COVID-19 pandemic. The pandemic has had an unprecedented impact on daily routines in the U.S. (Becker et al., 2021). Thus, we adjusted for the effects of the pandemic on sleep quality and duration. We also adjusted for early adversity and perceived life stress, given that adversity during childhood (Kajeepeta et al., 2015) and higher levels of perceived stress have each been found to be associated with poorer sleep health (ten Brink et al., 2021). We also examined socioeconomic status (SES), given significant associations between sleep problems and household income (Stamatakis et al., 2008), as well as body mass index (BMI) given associations with puberty and sleep duration (Rutters et al., 2010).
Our second aim was to investigate the longitudinal association between sleep health and affect, and our third aim was to examine the moderating effects of advanced pubertal stage on the daily association between sleep health and affect. Across adolescents and adults, subjective measures of sleep tend to be related to affect more strongly than do objective measures of sleep (Konjarski et al., 2018). Thus, for our second aim, we hypothesized that poorer subjective sleep satisfaction or shorter duration (based on findings from our first aim) would be associated with higher NA and lower PA; and for our third aim, pubertal stage would moderate this association, such that this effect would be strongest for adolescents who are most advanced in puberty.
Methods
Participants and Procedure
A total of 174 adolescents participating in a larger longitudinal study (King et al., 2019) assessing the effects of early life stress on neurobiological development over puberty agreed to participate in an “at-home components” portion of the study. See Supplement (page S2) for recruitment procedures. All participants were recruited through print and online advertisements. For two weeks, participants were given an Actiwatch Spectrum Plus™ device (Phillips Respironics) to monitor sleep and downloaded a smartphone app, MetricWire, that prompted them to respond to two questions about their sleep every morning. Sleep diaries were administered to participants to supplement the actigraphy protocol (see Measures: Sleep). 112 participants had usable actigraphy and EMA sleep data; 91 of these participants completed actigraphy and EMA concurrently (overlapping at least 7 days). Two participants were missing pubertal data, bringing our sample to 89 participants. Given changes in circadian routines during the transition from grade school to post-high-school, our main analyses examined participants 13–18 years old (N=87), which excluded n=2 participants who were post-high-school. Supplementary analyses included these participants post-high-school. See Table 1 for demographic characteristics of the 89 participants who completed the EMA and actigraphy protocol concurrently. In accordance with the Declaration of Helsinki, participants and their parents provided informed written assent and consent, respectively. This study was approved by the Stanford University Institutional Review Board (IRB-27671) and all participants were compensated for their participation. See Supplement (page S2) for Participant Compensation and Adherence.
Table 1.
Participant Characteristics
| Variable (N=89) | M (SD) | Range |
|---|---|---|
| Sex: Female (%) | 64 | |
| Assessed during COVID-19: Yes (%) | 36 | |
| Age (years) | 15.51 (1.37) | 13–20 |
| Tanner Score | 4.33 (0.66) | 2–5 |
| Proportion of those in early puberty (stages 2–2.5) (%) | 5 | |
| Proportion of those in mid puberty (stages 3–3.5) (%) | 11 | |
| Proportion of those in late puberty (stages 4–4.5) (%) | 56 | |
| Proportion of those in post puberty (stage 5) (%) | 28 | |
| BMI | 22.02 (5.57) | 14.09–46.59 |
| Household Income (%) | ||
| less than $5,000 | 0 | |
| $5,001–$10,000 | 1 | |
| $10,001–$15,000 | 0 | |
| $15,001–$25,000 | 0 | |
| $25,001–$35,000 | 2 | |
| $35,001–$50,000 | 5 | |
| $50,001–$75,000 | 10 | |
| $75,001– $100,000 | 11 | |
| $100,001–$150,000 | 21 | |
| $150,000 or greater | 45 | |
| Not reported | 5 | |
| Race/Ethnicity (%) | ||
| White | 43 | |
| Black or African American | 8 | |
| Hispanic/Latino/Latinx | 8 | |
| Asian | 11 | |
| Biracial | 25 | |
| Other than the listed options | 5 | |
| Early Life Stress Severity | 6.32 (4.65) | 0–19 |
| Adolescent Life Stress Severity | 140.55 (42.61) | 56–246 |
| Assessed during COVID-19: Yes (%) | 36 | |
| Assessed during grade school: Yes (%) | 98 | |
| Interval between EMA and actigraphy start date (days) | 0.66 (1.46) | 0–7 |
Note. BMI = Body Mass Index; Choices for biological sex were Male or Female; EMA = Ecological Momentary Assessment
Measures
Pubertal stage.
To assess pubertal stage, participants rated their developmental stage using the Tanner Staging questionnaire (Morris & Udry, 1980) which measures developmental status based on participants’ endorsement of schematic drawings of secondary sex characteristics (pubic hair and breast development for females, pubic hair and testicular development for males) on a scale from 1 (no pubertal development) to 5 (adult level of pubertal development). We averaged the two ratings of the secondary sex characteristics to yield a composite measure of the participants’ pubertal stage. Self-reported Tanner staging has been found to be correlated with physicians’ physical examinations of pubertal development (Shirtcliff et al., 2009). In addition, adolescents’ self-reported Tanner stage and their circadian preference have been found to be associated with physician-measured Tanner stage and with the offset of salivary melatonin secretion (Carskadon et al., 1997).
Actigraphy assessment:
To objectively monitor sleep quality, participants were asked to wear an actigraphy device (Actiwatch Spectrum Plus) for two weeks to obtain reliable data (Buysse et al., 2006) while limiting the burden of assessments that participants were completing in the broader study. They were instructed to press an event marker for three seconds to mark the time they started trying to fall asleep and again as soon as they got out of bed to start their day. Participants were also instructed to complete a daily sleep diary over the same time period as they wore the device; the diary data were used only to guide the scoring of actigraphy data, as described in the Supplement (page S3). We configured the Actiwatches to have an epoch length of one minute to measure motor activity and light data. See Supplement for details about the hardware and software features (pages S2–3).
Actigraphy Scoring:
Actigraphy data were scored by two raters (JSK and SMC) to define rest, sleep, and wake periods with the Philips Respironics Actiware software (version 6.0.9) using criteria described in the Supplement (page S3). Summer months and school breaks were not excluded. Based on the literature examining the reliability of daily diary and actigraphy data, (Short et al., 2017) five weekday nights are typically recommended; however, we adopted a more liberal threshold to retain a sufficient sample size (see Fig. 1 for distribution of usable days), and required participants to have at least three days of usable data.
Fig. 1.
Distribution of Usable Days from Actigraphy and EMA
Objective Sleep Duration:
We operationalized sleep duration as the total number of 1-minute epochs scored as sleep.
Objective Sleep Efficiency:
Sleep efficiency is the percentage of time asleep (i.e., sleep duration) relative to the time allocated for sleep (i.e., scoring interval).
Subjective Sleep Duration:
Each morning participants were also asked via the EMA notification, “How many hours did you sleep last night?” Participants were given choices of “5 hours or less,” “6 hours,” “7 hours,” “8 hours,” “9 hours or more” for ease of participant responsiveness, which we coded as 5, 6, 7, 8, and 9 respectively.
Subjective Sleep Satisfaction:
We used EMA methods via a smartphone application, MetricWire, which sent a daily notification every morning at a fixed time for 2 weeks. At each morning prompt, participants were asked: “How restful or satisfying was your sleep last night?” to which they responded on a continuous scale from 1 (not at all) to 100 (very).
Daily Affect:
Consistent with the EMA protocol for sleep, participants received a prompt in the morning (i.e., the same time as the sleep prompt), the afternoon, and at night asking them “since the last prompt, please indicate the extent to which you felt [AFFECT ITEM]” on a scale from “not at all” to “very.” See Supplementary Material (page S9) for EMA protocol with list of affective items. The order in which these items were presented was randomized.
Potential Covariates
Circadian Preference:
The Children’s Morningness/Eveningness Preference (CMEP) scale is a 10-item measure of circadian preference (Carskadon et al., 1993). Scores range from 10 (extreme evening preference) to 42 (extreme morning preference). Questions such as “Is it easy for you to get up in the morning?,” “When’s the ideal time for you to take a test?” are designed to assess phase tendencies in children (Carskadon et al., 1993). 84 out of 89 participants completed the CMEP. Internal consistency of the CMEP in this sample was good (Cronbach’s α=0.82).
Body Mass Index (BMI):
We used BMI as a measure of healthy weight. Height and weight were obtained via a scale in the lab, and then used to calculate BMI. Given that males and females generally differ on BMI, we standardized BMI within each sex.
Early Life Stress (ELS):
To assess history of ELS, adolescents were interviewed at baseline of the broader longitudinal study about exposure to stressful experiences using a modified version of the Traumatic Events Screening Inventory for Children (Ribbe, 1996). As described in King et al. (2017), three coders blind to the adolescents’ subjective ratings rated the objective severity on a 5-point scale of each type of stressor. A cumulative objective stress severity score was calculated by summing the maximum severity scores for each type of stressor the adolescent endorsed; this method ensured that frequent but less severe events would not be overly weighted (King et al., 2017).
Perceived Current Stress:
To measure perceived severity of current life stressors, participants completed the Adolescent Stress Questionnaire (ASQ) (Byrne et al., 2007), which asks participants to rate subjective stress in different domains (e.g., home life, school performance, peer pressure, emerging adult responsibility) on a 1 to 5 scale (“not at all stressful to very stressful”). Total scores range from 58 – 290, with higher scores indicating greater experiences of stress. 87 of the 89 participants in our sample completed the ASQ, with excellent internal consistency (Cronbach’s α=0.97).
Household Income:
Parents reported on their household income for the past 12 months using the following categories: (1) less than $5,000, (2) $5,001–$10,000, (3) $10,001–$15,000, (4) $15,001–$25,000, (5) $25,001–$35,000, (6) $35,001–$50,000, (7) $50,001–$75,000, (8) $75,001– $100,000, (9) $100,001–$150,000, (10) $150,000 or greater. Participants from this sample were recruited from the San Francisco Bay area with income levels higher than many other national regions (King et al., 2020). Thus, we cannot generalize our findings to different income strata. Additionally, the racial and ethnic distribution of our sample, which is largely White and non-Hispanic may preclude generalization of our findings.
COVID-19 pandemic:
In our study, 36% of our sample were assessed during the COVID-19 pandemic. Although imprecise as a measure of the effects of COVID-19, we created a binary variable indicating whether participants were assessed prior to (i.e., before March 17th, 2020) (1) or during (0) the pandemic (i.e., after March 17th, 2020).
Analysis Aim 1
We averaged our repeated assessments of subjective sleep satisfaction, subjective sleep duration, objective sleep duration, and objective sleep efficiency over the two weeks to obtain more reliable estimates of these constructs. For each analysis, we first identified and removed outliers with respect to sleep duration, efficiency, and sleep satisfaction using a standard formula of 1.5 times the interquartile range beyond the upper or lower quartile. We conducted all analyses in R and R Studio version 4.1.2.
We standardized Tanner stage, age, and BMI within each sex, given expected differences in pubertal stage, age, and BMI between males and females. We considered BMI, ELS, perceived stress, household income, response rate to EMA prompts, biological sex (dummy-coded) and timing relative to COVID-19 (dummy-coded) as theoretically important variables that could be included in our models. Given the limited statistical power for identifying associations between various racial/ethnic groups and our response variables, we did not include this variable in our models; however, we present the breakdown of race/ethnicity in our descriptive tables. Continuous variables were standardized across the full sample.
To test the use of covariates in regression models, we followed standard approach to model fitting (Chambers, 1992; Streiner, 2016). First, we first tested the bivariate association between metrics of sleep health (i.e., subjective sleep satisfaction, subjective sleep duration, objective sleep duration, and objective sleep efficiency) and Tanner stage. Second, we tested whether each covariate significantly improved model fit using likelihood test ratios. We added variables as covariates if they were significantly (p<0.05) associated with the sleep outcome or improved model fit upon inclusion in the model with Tanner stage. We then repeated analyses replacing Tanner stage with chronological age to understand whether pubertal stage or chronological age contributes to our sleep variables of interest.
For each effect, we computed bootstrapped confidence intervals with 1000 samples and BayesFactor package in R (Morey & Rouder, 2018) to compare the alternative hypothesis with the null hypothesis. A BF between 1 and 3 indicates anecdotal evidence in favor of the alternative hypothesis; a BF between 3 and 10 indicates “moderate” or “substantial” evidence in favor of the alternative hypothesis; a BF between 10 and 30 indicates “strong” evidence in favor of the alternative hypothesis; and a BF > 30 indicates “very strong” evidence in favor of the alternative hypothesis (Kruschke & Liddell, 2018). For linear regression models, we used gvlma (Pena & Slate, 2019) to ensure that model assumptions were met.
Analyses Aims 2 and 3
83 of the 87 participants completed the EMA affect survey simultaneously with the EMA sleep survey. We averaged ratings of the negatively valenced items (sad, annoyed or angry, grouchy or cranky, worried, anxious, and bored, which were intercorrelated) to create an aggregate score for NA for each participant. Similarly, we averaged the positively valenced items (happy, cheerful, excited, and energetic, which were intercorrelated) to create an aggregate score for PA for each participant. Positively and negatively valenced items, and NA and PA aggregate scores, were negatively correlated with each other (Fig. 2).
Fig. 2.
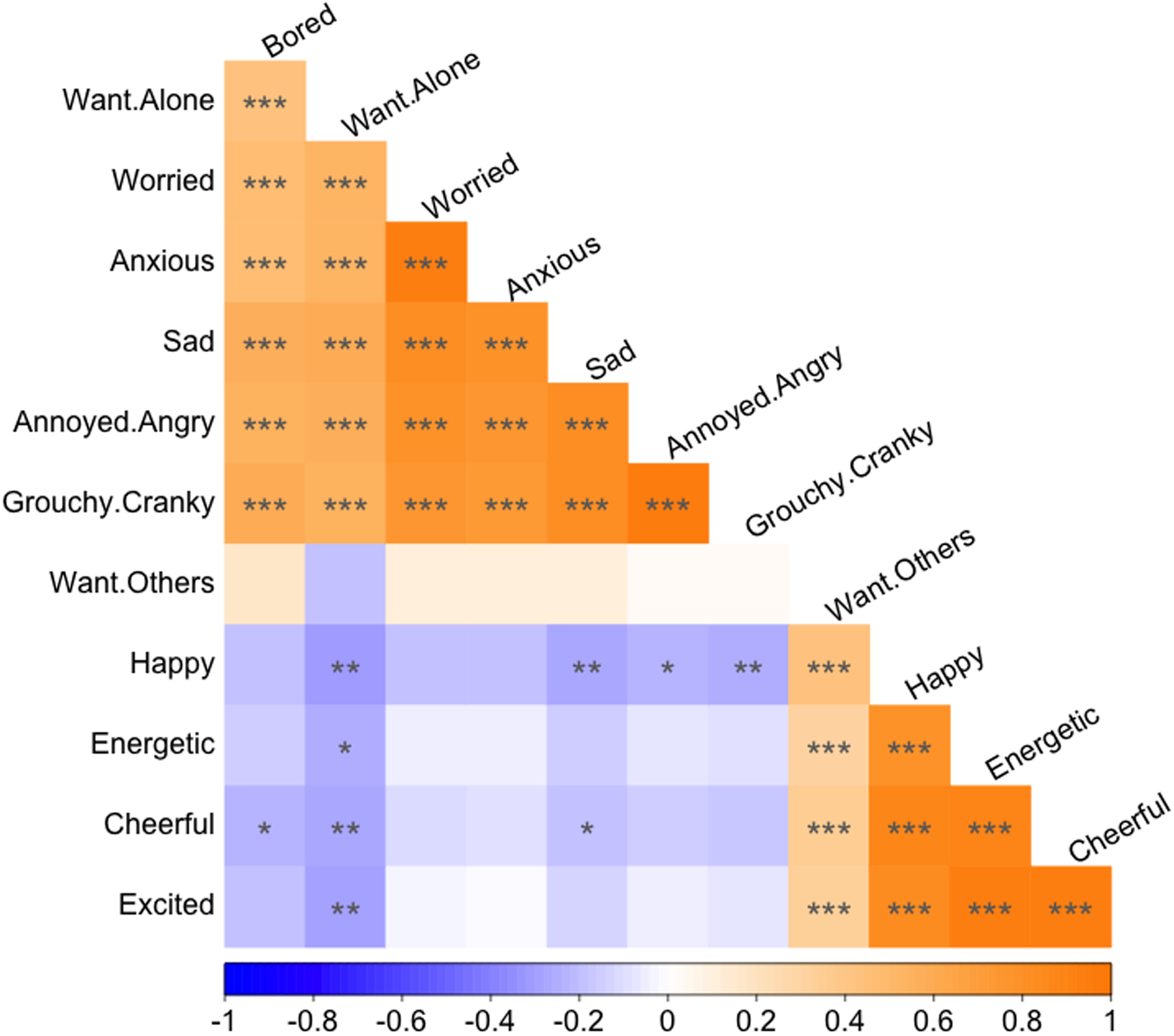
EMA Correlation Plot
To establish the temporal association between sleep and affect, we tested a three-level multilevel model (MLM) using the lmerTest package (Kuznetsova et al., 2017). To interpret the effects of time-varying variables, we disaggregated between- and within-person sources of variation of these variables (Howard, 2015); the between-person effect was operationalized as the sample-centered mean (e.g., trait-level sleep satisfaction) and the within-person effect was operationalized as the deviation from the person-centered mean (e.g., state-level sleep satisfaction). The first level was time-of-day (TOD) affect data coded as 0=morning, 1=afternoon, 2=evening. The second level was person-mean-centered daily sleep data, coded from 0=day one to 13=day 14 since baseline. The third level was sample-mean-centered individual difference data which included pubertal stage and the participant’s between-person sleep and affect data.
We included the interactions between pubertal stage and the within- and between-person sleep variables. We conducted models for NA and PA separately. However, to interpret the unique effect of sleep and puberty on affect, when NA was our dependent variable, we adjusted for within- and between-person effects of PA, and vice versa. For all models, we compared random intercept and random slopes based on model fit. If the model was too complex to converge, we interpreted the random intercept model.
Equation for the full model is notated below (with NA as our example dependent variable), where i=TOD; j=day; k=person. Equations for each level are noted in the Supplementary Material (Table S1).
Equation for full model:
Results
Associations among sleep health metrics and covariates
89 participants completed actigraphy and EMA concurrently with an average response rate of 66% to EMA sleep prompts, comparable to compliance rates reported by others using a similar method (Wen et al., 2017). Correlations among sleep metrics and covariates are presented in Table 2, created using scipub package (Pagliaccio, 2021). In addition, on average, participants had no preference for morning or evening (M=25.83, SD=5.42, range 15–39). Of 84 participants who completed the CMEP, 21 were identified as evening types, 22 were identified as morning types, and 41 were considered neither type. Circadian preference types were based on Díaz-Morales et al.’s (2007) suggested cutoffs at the 20th and 80th percentiles.
Table 2.
Correlation Table of Continuous Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Tanner Stage | ||||||||||||
| 2. Age | .34** | |||||||||||
| 3. Morningness | −0.15 | −0.20 | ||||||||||
| 4. Obj Efficiency | 0.13 | 0.05 | −0.16 | |||||||||
| 5. Obj Sleep Duration | −0.06 | −0.18 | −0.19 | .43*** | ||||||||
| 6. Subj Satisfaction | −.26* | −0.05 | .37*** | −0.12 | 0.03 | |||||||
| 7. Subj Sleep Duration | −0.13 | −0.20 | .28* | −0.13 | .35*** | .46*** | ||||||
| 8. BMI | .28* | 0.03 | 0.12 | 0.03 | −0.07 | −0.04 | −0.07 | |||||
| 9. Household Inc | −0.08 | −0.20 | −0.05 | 0.13 | 0.13 | 0.14 | 0.14 | −0.21 | ||||
| 10. sleep RR | −0.17 | 0.21 | 0.10 | −.21* | −0.01 | 0.01 | .25* | 0.00 | −0.01 | |||
| 11. ELS Severity | 0.08 | 0.03 | 0.08 | 0.03 | −0.10 | −0.10 | −.28** | 0.09 | −.37*** | −0.09 | ||
| 12. Perceived Stress | .21* | −0.01 | −.38*** | 0.17 | 0.03 | −.28** | −.22* | 0.04 | 0.06 | −.23* | .26* |
Note. This table presents Pearson correlation coefficients with pairwise deletion. Obj=Objective; Subj=Subjective; BMI=Body Mass Index; Household Inc=Household Income; sleep RR=sleep EMA response rate; ELS=early life stress. N=5 missing Morningness. N=11 missing BMI. N=4 missing Household Inc. N=1 missing ELS Severity. N=2 missing Perceived Stress.
p<.05,
p<.01,
p<.001
Aim 1: Subjective sleep and pubertal stage
On a scale from 1 (not at all) to 100 (very), participants reported that, on average, their sleep was closer to “very restful/satisfying” than to “not at restful/satisfying” (M=59.81; SD=17.28; median=62.43; range=19.78–100). Participants also reported sleeping an average of 7.29 hours per night (SD=0.86; median=7.40; range=5.14–9+ hours). No participants had outlying mean sleep satisfaction scores based on our criteria; one participant had an outlying mean sleep duration score and was excluded from analyses modeling sleep duration. Sleep satisfaction was not associated with any continuous covariates except for perceived stress and circadian preference (see Table 2). Sleep satisfaction was also not associated with sex (β=−0.25, p=0.270), or with having been assessed during COVID-19 (β=−0.09, p=0.680).
Tanner stage was negatively associated with mean sleep satisfaction (β[95%CI]=−0.29 [−0.47, −0.10], p=0.007, BF= 5.88) and age was not associated with mean sleep satisfaction (β[95%CI]=−0.12[−0.32, 0.10], p=0.290, BF=0.37) (see Fig. 3). Tanner stage was not associated with subjective sleep duration (β[95% CI]=−0.11 [−0.32, 0.11], p=0.330, BF=0.34); consequently, we did not proceed to test the model of subjective sleep duration with covariates.
Fig. 3.
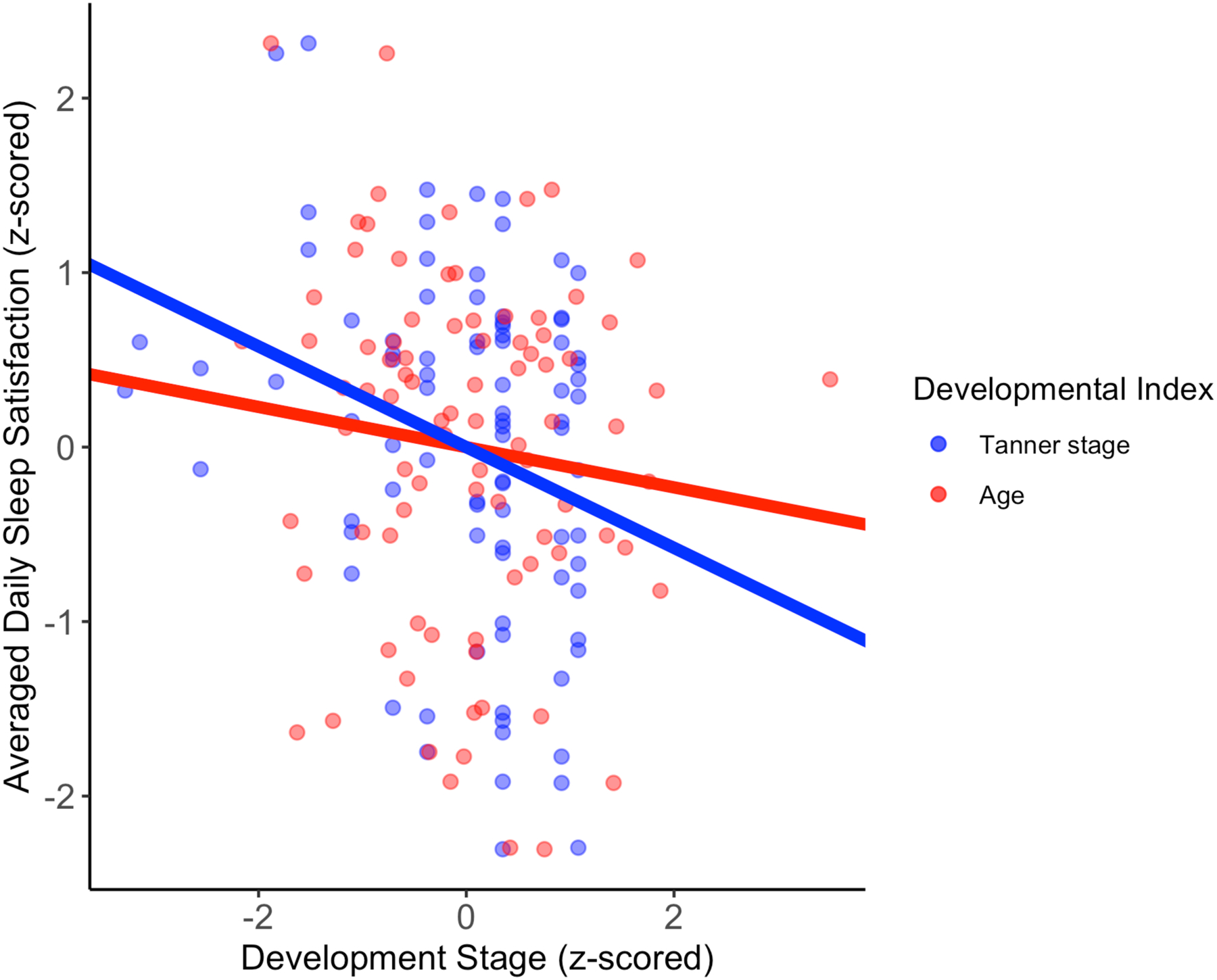
Developmental Stage and Averaged Daily Sleep Satisfaction
Adjusting for perceived stress severity and circadian preference yielded a reduced effect of Tanner stage in relation to averaged daily sleep satisfaction (β[95% CI]=−0.22 [−0.41, −0.02], p=0.041, BF= 6.04). The effect of perceived stress severity was no longer significant β[95% CI]=−0.12 [−0.33, 0.10], p=0.310, BF= 3.69); but the effect of circadian preference β[95% CI]=0.30 [0.11, 0.49], p=0.009, BF= 49.61) was still associated with averaged daily sleep satisfaction. Retaining the two participants post-high-school yielded slightly reduced effects, but directionally remained consistent with these findings (see Supplement, page S8).
Aim 1: Objective sleep and pubertal stage
On average, participants sleep efficiency was 81.73% (SD=5.42, median=82.51, range=67.75% to 93.39%). In addition, participants objectively slept for an average of 6.82 hours per night (SD=0.81, median=6.80, range=4.55–8.65). One participant had outlying mean sleep efficiency and duration scores.
Contrary to our hypothesis, Tanner stage was not associated with averaged daily objective sleep efficiency (β[95% CI]=0.19[−0.04, 0.39], p=0.086, BF=0.83) or with objective sleep duration (β[95% CI]=0.01[−0.30, 0.10], p=0.910, BF=0.23); therefore, we did not proceed to test the model covariates. Age was also not associated with averaged daily objective sleep efficiency (β[95% CI]=0.13[−0.06, 0.33], p=0.230, BF=0.42) or objective sleep duration (β[95% CI]=−0.01[−0.31, 0.10], p=0.34, BF=0.34).
Aim 2 and 3: Daily sleep and affect by pubertal stage
Our results from Aim 1 indicated that subjective sleep satisfaction was the only sleep metric associated with pubertal stage. Thus, we tested whether pubertal stage moderates the longitudinal association between sleep satisfaction and each type of affect. After visualizing the distributions of NA and PA, we square root transformed NA scores due to the significant positive skew of the data. The scores of PA were normally distributed. As presented in Table 3, created using the bruceR (Bao, 2022) and sjPlot (Lüdecke, 2021) packages, both NA and PA increased over the course of the day; and across days, NA increased, whereas PA remained stable. As expected, higher between-person (i.e., trait-level) sleep satisfaction was associated with lower NA and higher PA. Consistent with our hypothesis, higher within-person (i.e., state-level) sleep satisfaction was associated with lower NA. However, contrary to hypotheses, within-person sleep satisfaction was not related to PA. Contrary to our hypotheses, pubertal stage did not moderate associations between either level of sleep satisfaction (state- or trait-level) and NA or PA.
Table 3.
Longitudinal Association between Sleep and Affect by Pubertal Stage
| NA | PA | ||||
|---|---|---|---|---|---|
| Level | Predictors | β | CI 95% | β | CI 95% |
| L1 | time-of-day | 0.05*** | 0.02 – 0.07 | 0.15*** | 0.11 – 0.19 |
| L1 | PA.State | −0.16*** | −0.18 – −0.14 | ||
| L1 | NA.State | −0.20*** | −0.23 – −0.17 | ||
| L2 | day | 0.03* | 0.00 – 0.06 | −0.00 | −0.04 – 0.03 |
| L2 | DailySleepSat.State | −0.06** | −0.10 – −0.03 | 0.04 | −0.00 – 0.09 |
| L3 | DailySleepSat.Trait.c | −0.29** | −0.47 – −0.11 | 0.31*** | 0.15 – 0.47 |
| L3 | PA.Trait | −0.10 | −0.28 – 0.08 | ||
| L3 | NA.Trait | −0.08 | −0.24 – 0.09 | ||
| L3 | TannerStage | 0.10 | −0.09 – 0.30 | 0.08 | −0.09 – 0.25 |
| L1*L2 | time-of-day*DailySleepSat.State | 0.03 | −0.00 – 0.07 | −0.03 | −0.06 – 0.01 |
| L1*L3 | time-of-day*TannerStage | 0.01 | −0.03 – 0.05 | −0.00 | −0.06 – 0.06 |
| L2*L3 | DailySleepSat.State*TannerStage | 0.02 | −0.02 – 0.06 | 0.04 | −0.01 – 0.08 |
| L3*L3 | DailySleepSat.Trait.c*TannerStage | 0.13 | −0.03 – 0.28 | −0.07 | −0.22 – 0.07 |
| L1*L2*L3 | time-of-day*DailySleepSat.State*TannerStage | 0.02 | −0.01 – 0.05 | −0.01 | −0.05 – 0.03 |
| Random Effects | σ2 | 0.50 | 105.03 | ||
| τ00 | 0.60 day:ID | 111.58 day:ID | |||
| 2.02 ID | 209.02 ID | ||||
| τ11 | 0.19 day:ID.time-of-day | 30.71 day:ID.time-of-day | |||
| 0.00 ID.time-of-day | 11.44 ID.time-of-day | ||||
| ρ01 | −0.58 day:ID | −0.66 day:ID | |||
| −0.77 ID | −0.11 ID | ||||
| ICC | 0.83 | 0.74 | |||
| Observations | 2327 | 2327 | |||
| Marginal R2/Conditional R2 | 0.184 / 0.861 | 0.171 / 0.788 | |||
Note. Intercept reflects affect in the morning. TOD=time-of-day; NA=Negative Affect (square root transformed); PA=Positive Affect; DailySleepSat.State=within-person sleep satisfaction; DailySleepSat.Trait=between-person sleep satisfaction; NA.State=within-person negative affect; NA.Trait=between-person negative affect; PA.State=within-person positive affect; PA.Trait=between-person positive affect; σ2=residual variance; τ00=between-subject variance; τ11=random-slope variance; ρ01=random-slope-intercept-correlation; ICC=intraclass-correlation coefficient.
p<.05,
p<.01,
p<.001
Discussion
Over the past several decades, investigators have examined associations between puberty and sleep health, and between sleep health and affect. Importantly, research has aimed to disentangle the effects of pubertal stage and age in relation to subjective (Holm et al., 2009) and objective sleep quality and duration (Becker et al., 2019; Sadeh et al., 2009; Short et al., 2012; Zhang et al., 2014). The distinction between age and puberty is important because chronological and biological maturation do not occur at the same rate (Pfeifer & Allen, 2020). Thus, we first aimed replicate and extend findings of associations between pubertal stage and sleep duration and quality including daily objective (i.e., actigraphy) and subjective (i.e., EMA) measurements over a period of two weeks in a sample of early- to post-pubertal adolescents. Relatedly, there is a strong link between sleep and affect (Egbert et al., 2020; Forbes et al., 2004; Joinson et al., 2012; Klipker et al., 2017) across the pubertal transition. Few reports have examined within- and between-person effects of sleep quality on next-morning affect. Accordingly, our second aim was to separate between- and within-person effects of sleep health in relation to next day affect. Moreover, it is unclear whether pubertal stage impacts the association between daily sleep health and affect. Thus, in our third aim, we examined whether our puberty-related sleep findings were associated with EMA-measured negative and positive affect.
We first tested associations among our measures of sleep health (subjective sleep satisfaction, subjective sleep duration, objective sleep duration, objective sleep efficiency). Perhaps not surprisingly, the subjective measures were moderately intercorrelated, as were the objective measures. Further, as expected, the association between subjective sleep satisfaction and sleep efficiency was not significant, replicating findings of discrepancies between objectively and subjectively assessed sleep quality (efficiency and satisfaction) (Short et al., 2012). It is likely that subjective and objective measures of sleep provide different, complementary information about sleep quality. Although our subjective measure of sleep duration lacked precision, there was a significant association between objective and subjective sleep duration, which is consistent with findings from studies of other non-clinical samples of adolescents (Arora et al., 2013).
Aim 1: Associations between subjective and objective sleep and pubertal stage.
Next, we tested whether pubertal stage and age were associated with sleep health outcomes. As expected, we found that the association between pubertal stage and subjective sleep satisfaction was significant; pubertally advanced individuals had lower sleep satisfaction. However, age was not associated with sleep satisfaction. Interestingly, in our supplementary analysis including participants who were post-high school, the effect of pubertal stage was slightly reduced. Our findings are consistent with previous reports of an association between dissatisfaction with sleep and more mature pubertal development, independent of age (Knutson, 2005; Lustig et al., 2021). This association has also been found for self-reported sleep duration (Knutson, 2005); however, we did not find an association between pubertal stage and subjective or objective ratings of sleep duration in our study. Our null finding for the associations between pubertal stage and subjective and objective sleep duration is discrepant from previous reports of a negative relation between sleep duration and pubertal development (Knutson, 2005; Rutters et al., 2010). However, our null finding of the association between objective sleep duration and pubertal stage is consistent with results of other studies (Malone et al., 2016). It is possible that our sample did not experience enough sleep deprivation to allow us to detect associations of sleep duration with pubertal stage. In addition, given that California implemented a law in 2019 that prohibits high school classes to start before 8:30am (Pupil Attendance: School Start Time, 2019), our sample may be experiencing better sleep health than is the average adolescent in the United States.
Aims 2 and 3: Longitudinal association between sleep health and affect: moderation by pubertal stage
The second aim of this investigation was to replicate findings of associations between sleep and affect. First, we observed that NA and PA both increase within days. As expected, we found that between-person and within-person sleep satisfaction was associated with lower NA. Contrary to our expectations, we found that only between-person sleep satisfaction, but not within-person sleep satisfaction, was associated with PA. Our findings of sleep quality in relation to NA are consistent with previous within- (van Zundert et al., 2015) and between-person effects (Shen et al., 2022) of sleep quality in relation to NA. Consistent with Shen et al., 2022, we did not find within-person effects of sleep quality related to PA. However, this differs from van Zundert et al. (2015) who showed within-person effects of sleep quality related to PA. Overall levels and within-person fluctuations of sleep quality may affect NA, whereas only overall levels of sleep quality affect PA. Given that items that comprised our measure of PA were mainly high-arousal positive emotions (e.g., excited, energetic), is possible that we may see different effects with low arousal items.
The third aim of this investigation was to examine whether pubertal stage moderates the longitudinal association between sleep quality and affect. We did not find evidence of puberty moderating the association between subjective sleep satisfaction and affect; nor was pubertal stage associated with trajectories of NA or PA. Although this finding is consistent with another report of null associations between average levels of PA and pubertal stage (Forbes et al., 2010), it stands in contrast to more recent findings of a positive association between sensitivity to reward – an aspect of PA – and pubertal development. It may be that pubertal development is more specific to sensation seeking than to PA more broadly. In addition, our null effect of pubertal stage in relation to trajectories of NA stands in contrast with many reports linking advanced pubertal stage with internalizing symptoms (Barendse et al., 2021; Marceau et al., 2011). It is possible that overall low levels of NA in our sample did not allow us to detect an effect of pubertal stage in relation to trajectories of NA.
We should note three limitations of this study. First, we cannot make strong inferences about the onset of puberty and within-individual change over puberty. For instance, although we identified an association between pubertal stage and sleep quality (specifically, sleep satisfaction), it is not necessarily the case that those who entered puberty earlier would continue to be advanced in pubertal stage relative to their peers throughout development. Addressing this issue will require a larger longitudinal study in which measures of sleep, pubertal stage, and affect are obtained repeatedly, ideally with pre- to post-pubertal adolescents. As a related point, most of the participants in this study were in late puberty; greater variability in pubertal stage may have yielded significant associations with our other sleep metrics. Second, it is possible that pubertal stage is associated with variability in sleep onset and offset times during the weekend and during the week (social jetlag). We did not obtain data from a sufficient number of weekend days to examine this possibility. Third, although we obtained a moderately strong (r=.46) correlation between subjective and objective sleep duration, a finer-grained response selection (e.g., increments of 30 minutes) for subjective sleep duration may have yielded a stronger association with pubertal stage.
Despite these limitations, the present study is important in obtaining multimodal measurements of sleep, a critical consideration given that sleep measurement and sleep parameters can affect empirical results (Slavish et al., 2021). We also assessed daily sleep and affect in adolescents’ natural environments in order to increase ecological validity. We demonstrated that although puberty and age are developmentally linked, pubertal stage shows a stronger effect than does age in relation to sleep satisfaction during adolescence. In addition, sleep satisfaction is associated with adolescents’ NA above and beyond their mean levels of NA, underscoring the important effects of sleep satisfaction on a day-to-day basis. In future research investigators should extend these findings by examining the effects of early pubertal timing (i.e., the onset of puberty) on a wider range of sleep health metrics.
Supplementary Material
Acknowledgments
The authors thank the participants and their families for participating in this study.
Funding and Disclosure
This research was supported by the National Sciences Foundation (Graduate Research Fellowship to J.S.K.), National Institutes of Health (NIH; R37MH101495 to I.H.G.), and the Stanford University Precision Health and Integrated Diagnostics Center (PHIND to J.S.K., I.H.G., and R.M.).
Footnotes
Conflicts of Interest: All authors report no financial interests or conflicts of interest.
Data Availability Statement
The data underlying this article will be shared upon request to the corresponding author. Code is publicly available at https://github.com/jackie-schwartz/sleep_puberty_affect_associations.
References
- Arora T, Broglia E, Pushpakumar D, Lodhi T, & Taheri S (2013). An Investigation into the Strength of the Association and Agreement Levels between Subjective and Objective Sleep Duration in Adolescents. PLoS ONE, 8(8), e72406. 10.1371/journal.pone.0072406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H.-W.-S. (Bruce). (2022). BruceR: BRoadly Useful Convenient and Efficient R functions (0.8.6) [R]. https://psychbruce.github.io/bruceR/
- Barendse MEA, Byrne ML, Flournoy JC, McNeilly EA, Guazzelli Williamson V, Barrett A-MY, Chavez SJ, Shirtcliff EA, Allen NB, & Pfeifer JH (2021). Multimethod assessment of pubertal timing and associations with internalizing psychopathology in early adolescent girls. Journal of Psychopathology and Clinical Science, 131(1), 14. 10.1037/abn0000721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Dvorsky MR, Breaux R, Cusick CN, Taylor KP, & Langberg JM (2021). Prospective examination of adolescent sleep patterns and behaviors before and during COVID-19. Sleep, 44(8), 1–11. 10.1093/sleep/zsab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Langberg JM, Eadeh H-M, Isaacson PA, & Bourchtein E (2019). Sleep and daytime sleepiness in adolescents with and without ADHD: Differences across ratings, daily diary, and actigraphy. Journal of Child Psychology and Psychiatry, 60(9), 1021–1031. 10.1111/jcpp.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, & Hellman L (1972). Synchronization of Augmented Luteinizing Hormone Secretion with Sleep During Puberty. New England Journal of Medicine, 287(12), 582–586. 10.1056/NEJM197209212871203 [DOI] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep Health: Can We Define It? Does It Matter? Sleep, 37(1), 9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a Standard Research Assessment of Insomnia. Sleep, 29(9), 1155–1173. 10.1093/sleep/29.9.1155 [DOI] [PubMed] [Google Scholar]
- Byrne DG, Davenport SC, & Mazanov J (2007). Profiles of adolescent stress: The development of the adolescent stress questionnaire (ASQ). Journal of Adolescence, 30(3), 393–416. 10.1016/j.adolescence.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, & Seifer R (1997). An Approach to Studying Circadian Rhythms of Adolescent Humans. Journal of Biological Rhythms, 12(3), 278–289. 10.1177/074873049701200309 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, & Acebo C (1993). Association between Puberty and Delayed Phase Preference. Sleep, 16(3), 258–262. 10.1093/sleep/16.3.258 [DOI] [PubMed] [Google Scholar]
- Carvalho-Mendes RP, Dunster GP, de la Iglesia HO, & Menna-Barreto L (2020). Afternoon School Start Times Are Associated with a Lack of Both Social Jetlag and Sleep Deprivation in Adolescents. Journal of Biological Rhythms, 35(4), 377–390. 10.1177/0748730420927603 [DOI] [PubMed] [Google Scholar]
- Chambers JM (1992). Linear models. In: Chambers JM, Hastie TJ (Eds.). In Statistical models in S (1st ed., pp. 95–144). Routledge. [Google Scholar]
- Chan NY, Zhang J, Tsang CC, Li AM, Chan JWY, Wing YK, & Li SX (2020). The associations of insomnia symptoms and chronotype with daytime sleepiness, mood symptoms and suicide risk in adolescents. Sleep Medicine, 74, 124–131. 10.1016/j.sleep.2020.05.035 [DOI] [PubMed] [Google Scholar]
- Choi SJ, Kang M, Sung MJ, & Joo EY (2017). Discordant sleep parameters among actigraphy, polysomnography, and perceived sleep in patients with sleep-disordered breathing in comparison with patients with chronic insomnia disorder. Sleep and Breathing, 21(4), 837–843. 10.1007/s11325-017-1514-5 [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Wolfson AR, Tarokh L, & Carskadon MA (2018). An update on adolescent sleep: New evidence informing the perfect storm model. Journal of Adolescence, 67, 55–65. 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, & Bögels SM (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews, 14(3), 179–189. 10.1016/j.smrv.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF, de León MCD, & Sorroche MG (2007). Validity of the Morningness-Eveningness Scale for Children among Spanish adolescents. Chronobiology International, 24(3), 435–447. 10.1080/07420520701420659 [DOI] [PubMed] [Google Scholar]
- Duarte LL, & Menna-Barreto L (2022). Chronotypes and circadian rhythms in university students. Biological Rhythm Research, 53(7), 1058–1072. 10.1080/09291016.2021.1903791 [DOI] [Google Scholar]
- Egbert AH, Haedt-Matt A, Smith KE, Culbert K, Engel S, & Goldschmidt AB (2020). Momentary associations between positive affect dimensions and dysregulated eating during puberty in a diverse sample of youth with overweight/obesity. International Journal of Eating Disorders, 53(10), 1667–1677. 10.1002/eat.23342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MA, Buysse DJ, Marsland AL, Wright AGC, Foust J, Carroll LW, Kohli N, Mehra R, Jasper A, Srinivasan S, & Hall MH (2021). Meta-analysis of age and actigraphy-assessed sleep characteristics across the lifespan. Sleep, 44(9), zsab088. 10.1093/sleep/zsab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, & Dahl RE (2010). Healthy Adolescents’ Neural Response to Reward: Associations With Puberty, Positive Affect, and Depressive Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 162–172.e5. 10.1016/j.jaac.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, & Dahl RE (2004). Positive and Negative Affect in Depression: Influence of Sex and Puberty. Annals of the New York Academy of Sciences, 1021(1), 341–347. 10.1196/annals.1308.042 [DOI] [PubMed] [Google Scholar]
- Galland BC, Short MA, Terrill P, Rigney G, Haszard JJ, Coussens S, Foster-Owens M, & Biggs SN (2018). Establishing normal values for pediatric nighttime sleep measured by actigraphy: A systematic review and meta-analysis. Sleep, 41(4), 1–16. 10.1093/sleep/zsy017 [DOI] [PubMed] [Google Scholar]
- Glozier N, Martiniuk A, Patton G, Ivers R, Li Q, Hickie I, Senserrick T, Woodward M, Norton R, & Stevenson M (2010). Short sleep duration in prevalent and persistent psychological distress in young adults: The DRIVE study. Sleep. 10.1093/sleep/33.9.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, & Carskadon MA (2009). Adolescent Changes in the Homeostatic and Circadian Regulation of Sleep. Developmental Neuroscience, 31(4), 276–284. 10.1159/000216538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, & Dahl RE (2009). Reward-Related Brain Function and Sleep in Pre/Early Pubertal and Mid/Late Pubertal Adolescents. Journal of Adolescent Health, 45(4), 326–334. 10.1016/j.jadohealth.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AL (2015). Leveraging Time-Varying Covariates to Test Within- and Between-Person Effects and Interactions in the Multilevel Linear Model. Emerging Adulthood, 3(6), 400–412. 10.1177/2167696815592726 [DOI] [Google Scholar]
- Jenni OG, Achermann P, & Carskadon MA (2005). Homeostatic Sleep Regulation in Adolescents. Sleep, 28(11), 1446–1454. 10.1093/sleep/28.11.1446 [DOI] [PubMed] [Google Scholar]
- Joinson C, Heron J, Araya R, Paus T, Croudace T, Rubin C, Marcus M, & Lewis G (2012). Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychological Medicine, 42(12), 2579–2589. 10.1017/S003329171200061X [DOI] [PubMed] [Google Scholar]
- Kajeepeta S, Gelaye B, Jackson CL, & Williams MA (2015). Adverse childhood experiences are associated with adult sleep disorders: A systematic review. Sleep Medicine, 16(3), 320–330. 10.1016/j.sleep.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, Ancoli-Israel S, Stone K, Friedman L, & Zeitzer JM (2017). When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biological Psychology, 123, 37–46. 10.1016/j.biopsycho.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, & Gotlib IH (2017). The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology, 77, 68–74. 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Dennis EL, Humphreys KL, Thompson PM, & Gotlib IH (2020). Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Developmental Cognitive Neuroscience, 44, 100796. 10.1016/j.dcn.2020.100796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Humphreys KL, Camacho MC, & Gotlib IH (2019). A person-centered approach to the assessment of early life stress: Associations with the volume of stress-sensitive brain regions in early adolescence. Development and Psychopathology, 31(02), 643–655. 10.1017/S0954579418000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipker K, Wrzus C, Rauers A, Boker SM, & Riediger M (2017). Within-person changes in salivary testosterone and physical characteristics of puberty predict boys’ daily affect. Hormones and Behavior, 95, 22–32. 10.1016/j.yhbeh.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Knutson KL (2005). The association between pubertal status and sleep duration and quality among a nationally representative sample of U. S. Adolescents. American Journal of Human Biology, 17(4), 418–424. 10.1002/ajhb.20405 [DOI] [PubMed] [Google Scholar]
- Konjarski M, Murray G, Lee VV, & Jackson ML (2018). Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Medicine Reviews, 42, 47–58. 10.1016/j.smrv.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Kruschke JK, & Liddell TM (2018). Bayesian data analysis for newcomers. Psychonomic Bulletin & Review, 25(1), 155–177. 10.3758/s13423-017-1272-1 [DOI] [PubMed] [Google Scholar]
- Krystal A, & Edinger JD (2008). Measuring sleep quality. Sleep Medicine, 9, S10–S17. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest Package: Tests in Linear Mixed Effects Models (3.1–3) [R]. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lüdecke D (2021). sjPlot: Data Visualization for Statistics in Social Science (2.8.9). https://CRAN.R-project.org/package=sjPlot
- Lustig KA, Cote KA, & Willoughby T (2021). The role of pubertal status and sleep satisfaction in emotion reactivity and regulation in children and adolescents. SLEEP Advances, 2(1), zpab003. 10.1093/sleepadvances/zpab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SK, Zemel B, Compher C, Souders M, Chittams J, Thompson AL, & Lipman TH (2016). Characteristics Associated With Sleep Duration, Chronotype, and Social Jet Lag in Adolescents. The Journal of School Nursing, 32(2), 120–131. 10.1177/1059840515603454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, & Susman EJ (2011). Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology, 47(5), 1389–1409. 10.1037/a0023838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RD, & Rouder JN (2018). BayesFactor: Computation of Bayes Factors for Common Designs (R package version 0.9.12–4.2). https://CRAN.R-project.org/package=BayesFactor
- Morris NM, & Udry JR (1980). Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence, 9(3), 271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. (2020). Teens and Sleep. https://www.sleepfoundation.org/articles/teens-and-sleep
- Pagliaccio D (2021). scipub: Summarize Data for Scientific Publication (R package version 1.2.2). https://CRAN.R-project.org/package=scipub
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Malow BA, Maski K, Nichols C, Quan SF, Rosen CL, Troester MM, & Wise MS (2016). Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine, 12(6), 785–786. 10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena EA, & Slate EH (2019). Global Validation of Linear Models Assumptions (1.0.0.3) [CRAN]. [DOI] [PMC free article] [PubMed]
- Pfeifer JH, & Allen NB (2020). Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biological Psychiatry, 89(2), 99–108. 10.1016/j.biopsych.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupil attendance: School start time, no. SB-328, Senate (2019). https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB328
- Ribbe D (1996). Psychometric review of Traumatic Events Screening Inventory for Children (TESI-C). In Stamm BH (Ed.),Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran, 386–387. [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, & Merrow M (2004). A marker for the end of adolescence. Current Biology, 14(24), R1038–R1039. 10.1016/j.cub.2004.11.039 [DOI] [PubMed] [Google Scholar]
- Rutters F, Gerver WJ, Nieuwenhuizen AG, Verhoef SPM, & Westerterp-Plantenga MS (2010). Sleep duration and body-weight development during puberty in a Dutch children cohort. International Journal of Obesity, 34(10), 1508–1514. 10.1038/ijo.2010.161 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, & Rosenblat-Stein S (2009). Sleep and the Transition to Adolescence: A Longitudinal Study. Sleep, 32(12), 1602–1609. 10.1093/sleep/32.12.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Wiley JF, & Bei B (2022). Sleep and affect in adolescents: Bidirectional daily associations over 28-day ecological momentary assessment. Journal of Sleep Research, 31(2), e13491. 10.1111/jsr.13491 [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal Development: Correspondence Between Hormonal and Physical Development: Hormonal Correlates of Pubertal Stage. Child Development, 80(2), 327–337. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Arora T, Gradisar M, Taheri S, & Carskadon MA (2017). How many sleep diary entries are needed to reliably estimate adolescent sleep? Sleep: Journal of Sleep and Sleep Disorders Research, 40(3), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright H, & Carskadon MA (2012). The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Medicine, 13(4), 378–384. 10.1016/j.sleep.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Slavish DC, Asbee J, Veeramachaneni K, Messman BA, Scott B, Sin NL, Taylor DJ, & Dietch JR (2021). The Cycle of Daily Stress and Sleep: Sleep Measurement Matters. Annals of Behavioral Medicine, 55(5), 413–423. 10.1093/abm/kaaa053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, & Roberts RE (2008). Short Sleep Duration across Income, Education and Race/Ethnic Groups: Population Prevalence and Growing Disparities over 34 Years of Follow-Up. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner DL (2016). Control or overcontrol for covariates? Evidence Based Mental Health, 19(1), 4–5. 10.1136/eb-2015-102294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brink M, Lee HY, Manber R, Yeager DS, & Gross JJ (2021). Stress, Sleep, and Coping Self-Efficacy in Adolescents. Journal of Youth and Adolescence, 50(3), 485–505. 10.1007/s10964-020-01337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert RMP, van Roekel E, Engels RCME, & Scholte RHJ (2015). Reciprocal Associations Between Adolescents’ Night-Time Sleep and Daytime Affect and the Role of Gender and Depressive Symptoms. Journal of Youth and Adolescence, 44(2), 556–569. 10.1007/s10964-013-0009-3 [DOI] [PubMed] [Google Scholar]
- Wen CKF, Schneider S, Stone AA, & Spruijt-Metz D (2017). Compliance With Mobile Ecological Momentary Assessment Protocols in Children and Adolescents: A Systematic Review and Meta-Analysis. Journal of Medical Internet Research, 19(4), e6641. 10.2196/jmir.6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lam S-P, Li SX, Ma RCW, Kong APS, Chan MHM, Ho C-S, Li AM, & Wing Y-K (2014). A Community-Based Study on the Association Between Insomnia and Hypothalamic-Pituitary-Adrenal Axis: Sex and Pubertal Influences. The Journal of Clinical Endocrinology & Metabolism, 99(6), 2277–2287. 10.1210/jc.2013-3728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon request to the corresponding author. Code is publicly available at https://github.com/jackie-schwartz/sleep_puberty_affect_associations.


