Abstract
Helicobacter pylori causes a common chronic infection of humans that leads to epithelial cell damage. Studies have shown that apoptosis of the gastric epithelium is increased during infection and this response is associated with an expansion of gastric T-helper type 1 (Th1) cells. We report that gastric T cells contribute to apoptosis of the epithelium by a Fas/Fas ligand (FasL) interaction. Fas receptor expression was detected on freshly isolated gastric epithelial cells by flow cytometry and immunohistochemistry, and this level of expression was increased during infection with H. pylori. The expression of Fas receptor on three gastric epithelial cell lines was increased by H. pylori, either alone or in combination with gamma interferon or tumor necrosis factor alpha. The role of Fas in apoptosis of gastric epithelial cell lines was evidenced by DNA fragmentation after cross-linking of Fas with specific antibodies. FasL expression was detected by immunohistochemistry on mononuclear cells in gastric biopsy specimens of infected but not uninfected subjects. Gastric T-cell lines were also shown to express FasL, as evidenced by reverse transcription-PCR and killing of target cells expressing Fas receptor. Moreover, these T-cell lines were capable of killing cultured gastric epithelial target cells and antibodies that block the interaction between Fas receptor and FasL inhibited this cytotoxic activity. These observations demonstrate that local Th1 cells may contribute to the pathogenesis of gastric disease during H. pylori infection by increasing the expression of Fas on gastric epithelial cells and inducing apoptosis through Fas/FasL interactions.
Helicobacter pylori causes a lifelong infection of the gastric antrum that affects more than 50% of humanity. Infection is associated with chronic antral gastritis, characterized by a mucosal infiltration of polymorphonuclear and mononuclear leukocytes (10, 14). Evidence for a pathogenic role of H. pylori infection in peptic ulcer is derived from clinical investigations showing that cure of H. pylori infection accelerates ulcer healing and prevents ulcer relapse (20). Besides ulcer disease, H. pylori infection has also been implicated as a cause of gastric lymphoma or carcinoma in some patients (7).
There are many reports that H. pylori infection interferes with the equilibrium between proliferation and apoptosis of the gastric epithelium (4, 7, 25, 32, 35, 49). Most of the in situ studies have shown that the number of apoptotic epithelial cells increases during H. pylori infection (25, 32, 35, 41). Numerous mechanisms could account for this, including the direct effects of the bacteria, as well as the inflammatory response elicited by the infection (13, 24, 41, 49).
Several independent approaches have suggested that T-helper type 1 (Th1) cells are selectively increased during infection (3, 9, 18, 26, 31). Th1 cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), can increase the release of proinflammatory cytokines, such as interleukin-8, from the epithelium (52), as well as Fas and Fas ligand (FasL) (6). Furthermore, these cytokines can also increase the expression of major histocompatibility complex class II molecules by gastric epithelial cells, thereby increasing the binding of H. pylori to the gastric epithelium (13). As Th1 cells are associated with cell-mediated immune responses, they may also play a role in damaging gastric tissues directly by triggering apoptosis in gastric epithelial cells.
Th1 cells can also express higher levels of FasL than Th2 cells (40, 46). This molecule belongs to the TNF family of proteins. The receptor of FasL, Fas (CD95), is a 45-kDa protein belonging to the TNF receptor family. Cross-linking of Fas with an agonistic immunoglobulin M (IgM) antibody or FasL will transduce the death signal to the cells, resulting in the induction of apoptosis. However, studies to date have not shown that gastric T cells express FasL and induce epithelial cell death by Fas/FasL interactions. The purpose of this study was to investigate the role of H. pylori in the modulation of lymphoepithelial interactions in the stomach that are mediated through Fas/FasL.
MATERIALS AND METHODS
Subjects.
Material from human tissue was obtained from consenting adults aged 20 to 55 years as approved by the respective institutional review boards at the National University of Ireland, the University of Göteborg, and the University of Texas Medical Branch. Individuals regularly using nonsteroidal anti-inflammatory drugs or antisecretory drugs were excluded from the study population. Biopsy specimens of the gastric antrum were obtained from consenting subjects undergoing gastroesophageal duodenoscopy for various clinical indications. Subjects were considered infected if H. pylori was detected either by a rapid urease test or by histopathology on biopsy specimens.
Preparation of freshly isolated gastric epithelium and T cells. (i) Gastric epithelium.
To evaluate the expression of Fas receptor, gastric epithelial cells were partially enriched using a modification of previously described techniques (13, 53). Briefly, biopsy specimens from subjects infected with H. pylori were collected into sterile collection medium (calcium- and magnesium-free Hanks balanced salt solution [HBSS] with 5% fetal calf serum [FCS] and penicillin plus streptomycin). Biopsy specimens were rinsed with HBSS medium containing 1 mM dithiothreitol and 1 mM EDTA (Sigma Chemical Co., St. Louis, Mo.). The specimens were agitated for 1 h at 37°C to obtain epithelial cells. The resulting cell suspensions were washed, and the viability of the gastric epithelial cells was determined by trypan blue exclusion. Cells were not used if viability did not exceed 80%. The yield from a single subject varied from 5 × 105 to 1 × 106, so further purification by density gradients was not possible. To determine purity, freshly isolated cells were stained for an epithelium-specific antigen using fluorescein isothiocyanate-conjugated monoclonal antibody (clone Ber-EP4; Dako, Flostrup, Denmark) as previously described (3, 17). Purity ranged from 50 to 80% with epithelial lymphocytes being the major contaminating cell type (3). Cells stained by the epithelial cell antigen were selected by electronic gating, and this population was examined by flow cytometry for the expression of Fas receptor.
(ii) Gastric T cells.
Gastric T cells were isolated using a modification of previously described techniques (3, 12). Briefly, biopsy specimens were collected into collection medium (calcium- and magnesium-free HBSS with 5% FCS and penicillin plus streptomycin). In some cases, biopsy specimens were stored at 4°C for up to 18 h prior to processing, this having previously been shown not to alter T-cell function. All manipulations were carried out using aseptic techniques. Biopsy specimens were rinsed with aqueous Betadine and immediately rinsed four times in collection medium before being placed in collection medium containing 1 mM dithiothreitol and 1 mM EDTA. The specimens were agitated for 1 h at 37°C to remove intraepithelial lymphocytes and epithelial cells before being placed in complete RPMI medium (RPMI 1640 medium with 10% FCS, penicillin, and streptomycin) and washed three times. Subsequently, lamina propria T cells were liberated by treatment with collagenase (30 U/ml; Sigma Chemical Co.) in complete RPMI medium for 3 h. Undigested biopsy material was removed, and the cells were washed three times with complete RPMI medium. The resulting cell suspensions were washed, and the viability of the mononuclear cells was determined by trypan blue exclusion. Cells were not used if viability did not exceed 90%.
Gastric T cells were cultured in RPMI 1640 medium containing 15% FCS, 10 mM HEPES, penicillin plus streptomycin, 10 U of recombinant interleukin-2 (ICN Pharmaceuticals Inc., Irvine, Calif.) per ml, and 10% phytohemagglutinin-stimulated lymphocyte supernatant. T cells were fed with 5 mg of phytohemagglutinin (Difco Laboratories, Detroit, Mich.) per ml and an irradiated (3,000 rads) allogeneic peripheral B-cell line every 2 weeks.
Immunohistochemistry. (i) Cytokines.
For cytokine detection studies, frozen sections were prepared from two biopsy specimens of the gastric antrum from infected and uninfected subjects. Two sections were examined from each biopsy. The slides were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 10 min, washed in PBS, air dried, and kept at −20°C until used. After rehydration, the slides were treated with 0.1% saponin (Sigma Chemical Co.) in PBS to permeabilize the cell membranes. Endogenous peroxidase and biotin activities were blocked with 1% H2O2 and 0.02% NaN3 in PBS and by use of an avidin-biotin blocking kit (Vector Laboratories, Burlingame, Calif.), respectively. The slides were then incubated overnight at 4°C with anti-TNF-α (MAB11; PharMingen, San Diego, Calif.) or anti-IFN-γ (DIK-1; Chromogenix, Molndal, Sweden) monoclonal antibodies at a concentration of 5 μg/ml. An irrelevant isotype-matched monoclonal antibody (Dako) was used as a control for nonspecific staining. After blocking with 1% normal goat serum, biotinylated goat anti-mouse IgG, absorbed against human IgG (Caltag Laboratories, San Francisco, Calif.), was added as a secondary antibody, and the mixture was incubated for 30 min at room temperature. The sections were then overlaid with an avidin-biotin-horseradish peroxidase complex (Vectastain ABC-HP kit; Vector Laboratories), followed by the substrate chromagen diaminobenzidine (Vector Laboratories), and finally counterstained with Mayer's hematoxylin.
(ii) FasL and FasR.
Paraffin-embedded sections of normal gastric tissues were deparaffinized in xylene and rehydrated prior to analysis. Slides were washed twice for 5 min each time in a wash buffer containing 50 mM Tris-HCl (pH 7.6), 50 mM NaCl, and 0.001% saponin. Endogenous peroxidase was quenched with 3.0% hydrogen peroxide in methanol for 5 min. Slides were washed as before, except that the wash buffer for this and all subsequent steps included 1% normal goat serum, and then blocked for 1 h in a wash buffer containing 5% normal goat serum. After washing and incubation overnight at 4°C with affinity-purified rabbit polyclonal anti-human FasL- or FasR-specific IgG (Santa Cruz Biotechnology, Santa Cruz, Calif.) at 0.1 μg/ml in wash buffer, antibody binding was localized using a biotinylated secondary antibody, avidin-conjugated horseradish peroxidase, and diaminobenzidine substrate (contained within the Vectastain ABC-HP kit [Vector Laboratories]). The appropriate immunizing peptide to which the antibody was raised (FasL amino acids 260 to 279 or FasR amino acids 316 to 335; Santa Cruz Biotechnology) was included at 1 μg/ml during primary antibody incubation as a direct internal competitive control for antibody specificity. Slides were counterstained with hematoxylin.
Bacteria and human cell lines.
H. pylori LC-11 is a cagA-bearing strain that was isolated from a child with duodenal ulceration as described previously (8, 11). To investigate the impact of the cag pathogenicity island (PAI) on the induction of Fas, strain 26695 and an isogenic mutant lacking the entire cag PAI, 8-1 (2), were used. Briefly, bacteria were grown on blood agar base (Becton Dickinson, San Jose, Calif.) at 37°C under microaerobic conditions and harvested on day 3 into PBS. After centrifugation at 2,500 × g for 15 min, bacteria were resuspended in sterile PBS (pH 7.4) to a concentration of 2 × 108/ml. Bacterial numbers were estimated by measuring the A530 using a DU-65 spectrophotometer (Beckman, Fullerton, Calif.). One unit of optical density at 530 nm (OD530) was equivalent to 2 × 108 bacteria per ml as determined by comparing the measured value to a standard curve generated by quantifying viable organisms from aliquots of bacteria at various concentrations that were also assessed for absorbance. Motility was confirmed by phase-contrast microscopy prior to experimental use.
Kato-III, N87, and AGS-NY2 are gastric epithelial cell lines, while Jurkat E6-1 is a leukemia T-cell line. Kato-III, N87, and Jurkat E6-1 cells were purchased from the American Type Culture Collection, Manassas, Va., while AGS-NY2 cells were generously provided by S. Moss. All cells were maintained in RPMI 1640 medium supplemented with 10% FCS as previously described (13).
Examination of Fas receptor expression by flow cytometry.
To measure Fas receptor expression, freshly isolated gastric epithelial cells were harvested, washed, and stained with an optimal amount of mouse anti-human Fas receptor (CD95) (DX2 at 1 μg/ml; PharMingen) or an appropriate isotype control. Subsequently, cells were washed with PBS with 0.1% bovine serum albumin and fixed in 1% paraformaldehyde in PBS before specific fluorescence was measured using a FACScan (Becton Dickinson) after correction for nonspecific fluorescence using the appropriate isotype controls. The results are presented as the mean fluorescence intensity (MFI) and percentage of positive cells.
In other experiments, resting or stimulated gastric epithelial cell lines were stained in an identical fashion for the evaluation of CD95 expression. Briefly, cells were exposed to the various strains of H. pylori (300 bacteria/epithelial cell), TNF-α (40 ng/ml; R&D Systems, Minneapolis, Minn.), or IFN-γ (100 U/ml; R&D Systems) either alone or in combination. These cytokine concentrations were based on a previous determination of optimal responses as reported by several laboratories (8, 13, 21, 24, 49). After incubation for 48 h, the cells were harvested and Fas expression was detected by flow cytometry. The results are presented as the relative MFI, which equals (MFI of Fas/MFI of isotype) × 100, to compare the changes in Fas expression.
To determine if functional Fas was expressed on gastric epithelial cells, Kato-III, AGS-NY2, and N87 cells were incubated with medium or various stimuli as described above for 6 h, followed by incubation with medium or 30 to 100 ng of IgM anti-Fas antibody CH11 (Kamiya Biomedical Company, Thousand Oaks, Calif.) per ml for 12 h. DNA fragmentation was evaluated by enzyme-linked immunosorbent assay (ELISA) and a DNA ladder assay as described below.
Detection of FasL mRNA. (i) Extraction of total RNA and reverse transcription (RT).
Total RNA was extracted from gastric biopsy specimens or cell lines using Trizol reagent (Life Technologies, Houston, Tex.) in accordance with the protocol provided by the manufacturer (8). Briefly, cells or tissues were lysed using Trizol reagent, followed by extraction with chloroform and precipitation with isopropanol, washed one time using 70% ethanol, and then diluted into diethylpyrocarbonate-treated distilled water. The purity and amount of the RNA were determined by measuring OD260 and OD280 using a DU-65 spectrophotometer (Beckman). cDNA was synthesized using Superscript II reverse transcriptase and oligo(dT)s primer (Life Technologies, Gaithersburg, Md.) at 42°C in accordance with the protocol provided by the manufacturer.
Amplification of FasL.
Primers for FasL were designed in accordance with the human gene sequences as described elsewhere (37). The length of the product is 344 bp. β-Actin primers were purchased from Clontech Laboratory (Palo Alto, Calif.); the length of the product is 838 bp. The sequences of FasL primers were as follows: sense primer, 5′-CAGCTCTTCCACCTACAGAAGG-3′; antisense primer, 5′-GAGAGACCAGTTAAAACTCCTTAGA-3′. Thermal cycling was done as follows: denaturation at 96°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 150 s; 40 cycles were performed. Primers were used at a final concentration of 0.1 μM each in a 50-μl volume, and the concentration of Mg2+ was 1.0 mM. PCR products were identified after separation by electrophoresis using 1.2% agarose gels, followed by staining with ethidium bromide.
Detection of DNA fragmentation. (i) JAM test.
Induction of apoptosis was detected using a cytotoxicity assay termed the JAM test (33). Briefly, target cells (T) were labeled with [3H]thymidine (ICN Pharmaceuticals Inc., Irvine, Calif.) at 10 μCi/ml for 12 h and seeded into 96 wells at a concentration of 2 × 104 cells per well. Effector cells (E) were cocultured with targets at various E/T ratios for 12 h. Subsequently, cells were collected with a cell harvester (Skatron Instruments Inc., Sterling, Va.) and radioactivity was determined using a liquid scintillation counter (Beckman). The degraded DNA was washed through the membrane, and the undegraded, high-molecular-weight DNA was captured on the membrane. Thus, killing (expressed as the percentage of killed cells) can be calculated as (S − E)/S × 100, where S is counts per minute in the medium control and E is the counts per minute of treated cells.
To study Fas/FasL interactions, gastric T cells expressing FasL were incubated with target cells expressing Fas (Jurkat T cells or gastric epithelial cells) in the presence or absence of 1 μg of anti-Fas IgG antibody (ZB4; Kamiya Biomedical Company) or the appropriate isotype control (37) per ml.
(ii) ELISA.
DNA fragmentation was also measured using a commercially available ELISA (Boehringer, Mannheim, Germany). This assay detects low-molecular-weight nucleosome fragments in the cytoplasmic fractions of affected cells that arise during apoptosis but not as a result of necrosis. A405 was measured by Titertek Multiskan MCC/340 (ICN Pharmaceuticals Inc.) and compared to that of the substrate solution used as a blank. The apoptotic index was calculated in accordance with the manufacturer's instructions by dividing the absorbance of stimulated cells by the absorbance of control cells (13).
Gel electrophoresis.
Agarose gel electrophoresis to detect DNA fragmentation was conducted in accordance with a standard procedure for assaying fragmentation in total genomic DNA (48). Briefly, cells under different culture conditions were harvested and pelleted by centrifugation at 400 × g for 5 min. Subsequently, 106 epithelial cells were washed with PBS, pelleted, and resuspended in sample buffer composed of Tris-buffered glycerol with ZnSO4 at 1.4 mg/ml, bromophenol blue, and RNase (Sigma) at 10 mg/ml. Nucleosomal ladders were visualized in 2% agarose gels in TBE buffer (0.09 M Tris, 0.09 mM boric acid, 0.24 M EDTA, pH 8.0). Cells were lysed by 2% sodium dodecyl sulfate and proteinase K (Sigma) at 53 μg/ml contained within a 1% agarose gel slice above the wells. The gel was run for 10 to 14 h at 30 V, stained with ethidium bromide at 2 μg/ml for 1 h, and washed overnight with a large volume of distilled water to remove excess stain. UV light was used to visualize DNA fragments in the gel that were photographed, and their migration was compared to that of the HaeIII-digested fX174 standard (Promega, Madison, Wis.).
Statistical analyses.
Results are expressed as the mean ± the standard error of the mean (SEM). Fas/FasL expression and killing rates were compared using a two-tailed Student t test, and differences were considered significant if P values were <0.05.
RESULTS
Gastric epithelial cells express Fas receptor in vivo.
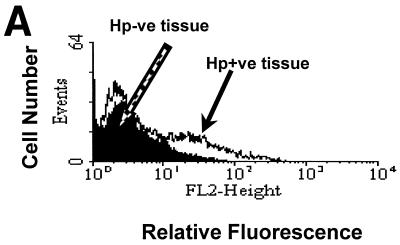
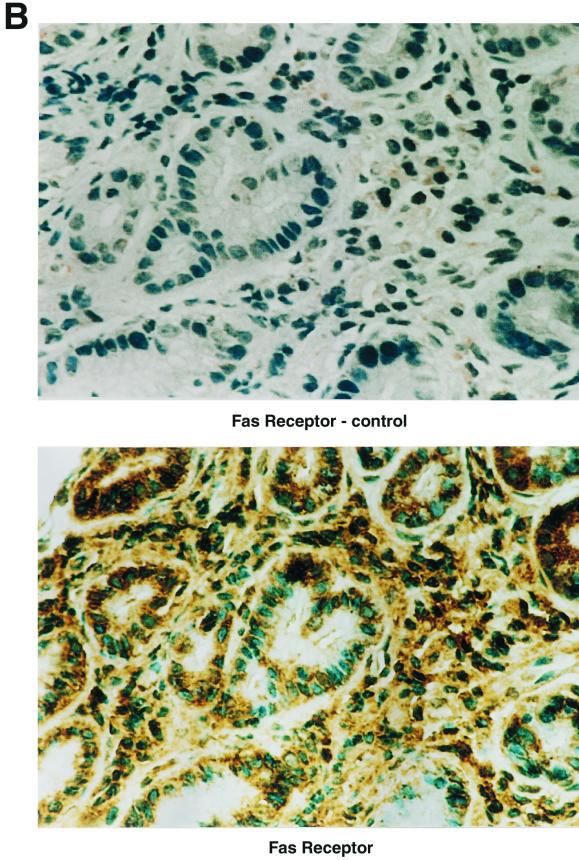
In order for gastric T cells to mediate epithelial cell death through Fas/FasL interactions, the target cell must express Fas. To confirm the expression of Fas by gastric epithelium and to compare the expression on gastric epithelial cells in the presence or absence of H. pylori infection, gastric biopsy specimens were collected from infected and uninfected subjects and isolated epithelial cells were examined by flow cytometry (Fig. 1A). After staining to detect surface Fas, the MFI was 19.0 in eight infected subjects versus 7.8 in eight uninfected subjects, while the mean percentage of Fas-positive cells was 11.3% versus 1.4%, respectively (P < 0.05). Since the expression of Fas was restricted to a small subset of the isolated epithelial cells from infected subjects, the distribution of Fas expression was examined by immunohistochemistry. Consistent with the findings obtained using flow cytometry, Fas expression was limited to an extremely small percentage of the gastric epithelial cells in normal, uninfected specimens (data not shown) whereas Fas expression by epithelial cells from infected tissues was much greater (Fig. 1B). Thus, Fas expression by gastric epithelial cells is increased during infection with H. pylori.
FIG. 1.
Fas receptor expression is increased in vivo during infection with H. pylori. (A) Epithelial cells were isolated from multiple biopsy specimens obtained from H. pylori-positive or -negative subjects, and Fas expression was detected by flow cytometry. Shown is a representative histogram in which Fas expression on epithelial cells from one H. pylori-infected subject (solid arrow) was higher than that on cells from an uninfected subject (open arrow). The mean percentage of positive cells expressing Fas ± SEM was 11.3% ± 5.6% for infected subjects versus 1.4% ± 0.3% for uninfected subjects (n = 8). Hp−ve, H. pylori negative; Hp+ve, H. pylori positive. (B) Fas expression on gastric epithelium was detected in paraffin-embedded gastric specimens from H. pylori-infected subjects using immunohistochemistry as described in Materials and Methods. This image shows that Fas was expressed on the gastric epithelium of infected subjects (n = 5) and the detection was blocked when Fas peptide at 1 μg/ml was added with the primary antibody (control). Expression in uninfected tissues was almost undetectable (n = 5; data not shown).
Th1 cytokine production adjacent to the gastric epithelium.
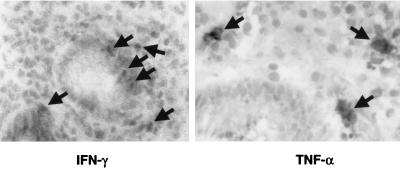
Since Fas expression on epithelial cells was increased and this can be achieved by cytokines from Th1 cells, the expression of IFN-γ and TNF-α adjacent to gastric epithelial cells was examined by immunohistochemistry. IFN-γ-positive cells were found in all but one of the H. pylori-infected subjects, whereas TNF-α was found in five of eight infected biopsy specimens. IFN-γ or TNF-α was never found in biopsy specimens from healthy uninfected subjects using this approach. As shown in Fig. 2, both cytokines were detected in mononuclear cells within, or adjacent to, the gastric epithelium. The median number of IFN-γ-stained intraepithelial lymphocytes was 1.7/mm2 of tissue area (interquartile range, 0.8 to 4), and the median number of positively stained lamina propria was 4.8 (interquartile range, 1.8 to 11). TNF-α-positive cells, median number, 1.2 (interquartile range, 0 to 2.9), were often located in the lamina propria surrounding the neck region of the gastric glands, but no intraepithelial lymphocytes were specifically stained for TNF-α. Interestingly, positive staining for both cytokines was occasionally also found in the epithelial cells themselves. The finding of IFN-γ and TNF-α-positive cells was consistent with the reports describing the cytokine production in gastric tissue during infection (3, 9, 26, 31) and suggested a potential role for Th1 cells in the regulation of Fas/FasL interactions.
FIG. 2.
Detection of Th1 cytokines adjacent to gastric epithelial cells during infection with H. pylori. Biopsy specimens from infected and uninfected subjects were stained with antibodies recognizing human IFN-γ or TNF-α as described in Materials and Methods. Whereas few positive cells were detected in uninfected subjects, cytokine-positive cells were observed within or adjacent to the gastric epithelium (arrows). These images are representative of eight different subjects.
Regulation of Fas expression on gastric epithelial cells by H. pylori and Th1 cytokines.
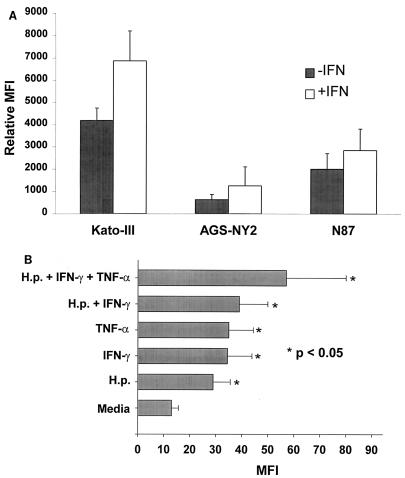
Since Th1 cytokines are present adjacent to the gastric epithelium in H. pylori-infected tissues, we determined if these factors might account for the observed increase in Fas expression by gastric epithelial cells. Gastric epithelial cell lines were stimulated with H. pylori in the presence or absence of various cytokines and examined by flow cytometry. Figure 3A shows that Fas expression on three separate gastric epithelial cell lines was increased by IFN-γ, with Kato-III being the most sensitive cell line. In another experiment, Fas expression on Kato-III cells was detected after incubation with H. pylori, IFN-γ, and TNF-α, either alone or in various combinations. Figure 3B shows that Fas expression on Kato-III cells was increased consistently by all of these stimuli alone while the combination of H. pylori, IFN-γ, and TNF-α yielded the greatest induction of Fas. To investigate the role of the cag PAI in the induction of Fas, Kato-III, AGS-NY2, and N87 cells were incubated with strain 26695 or the cag PAI-deficient isogenic mutant 8-1. As shown in Table 1, both strains induced Fas expression although the level of induction was greater with cag-bearing strain 26695.
FIG. 3.
Regulation of Fas receptor expression by H. pylori and Th1 cytokines. (A) Kato-III, AGS-NY2, and N87 gastric epithelial cells were treated with or without IFN-γ (100 U/ml) for 24 h, and Fas expression was detected by flow cytometry. It was found that IFN-γ was capable of increasing Fas expression in all of these epithelial cell lines (n = 3, P < 0.05), with Kato-III being the most sensitive cell line. (B) Gastric epithelial cells (Kato-III) were treated for 48 h with medium, H. pylori (H.p.), IFN-γ (100 U/ml), TNF-α (40 ng/ml), H. pylori and IFN-γ, or H. pylori, IFN-γ, and TNF-α. Subsequently, Fas expression was detected by flow cytometry. This graph shows that all of these stimuli, either alone or in combination, increased the expression of Fas compared to medium (∗, P < 0.05). These results were obtained from three separate experiments.
TABLE 1.
Relative expression of Fas by gastric epithelial cells after stimulation with strains of H. pylori that vary in the presence of the cag PAI
| Cell line | % of control MFI in H. pylori strain:
|
|
|---|---|---|
| 26695 | 8-1 | |
| Kato-III | 248 | 267 |
| AGS-NY2 | 322 | 233 |
| N87 | 545 | 339 |
Data are reported as averages of two assays detecting the MFI (expressed as a percentage relative to the control) for Fas expression as measured by flow cytometry in cell lines exposed to medium or strains of H. pylori with (26695) or without (8-1) the cag PAI.
Fas expressed by gastric epithelium is functional.
The function of Fas expressed on three separate gastric epithelial cell lines was examined after control or IFN-γ-treated cells were incubated with anti-Fas IgM antibody. DNA degradation products isolated from the cytosol of stimulated and control cells were evaluated by an ELISA to detect DNA fragments released from the nuclei of cells undergoing apoptosis. Figure 4A shows that the ELISA used was able to detect DNA fragmentation induced during apoptosis in as few as 10 cells. IFN-γ and anti-Fas antibody alone killed gastric epithelial cells; however, the combination of both markedly increased the apoptosis of gastric epithelial cells. Figure 4B shows the low-molecular-weight DNA isolated from Kato-III cells (106/ml) resolved on a 2% agarose gel after control or IFN-γ-treated cells were incubated with anti-Fas IgM antibody and not an isotype control antibody. Moreover, Fas was shown to be functional on other cell lines, including AGS-NY2 and N87 (Table 2). Significant augmentation of killing (P < 0.05) was observed in all lines treated with IFN-γ and in AGS-NY2 or N87 cells exposed to H. pylori 26695 but not under the other conditions tested. These findings indicate that innately expressed Fas and IFN-γ-induced Fas are functional in the transduction of the apoptotic signal.
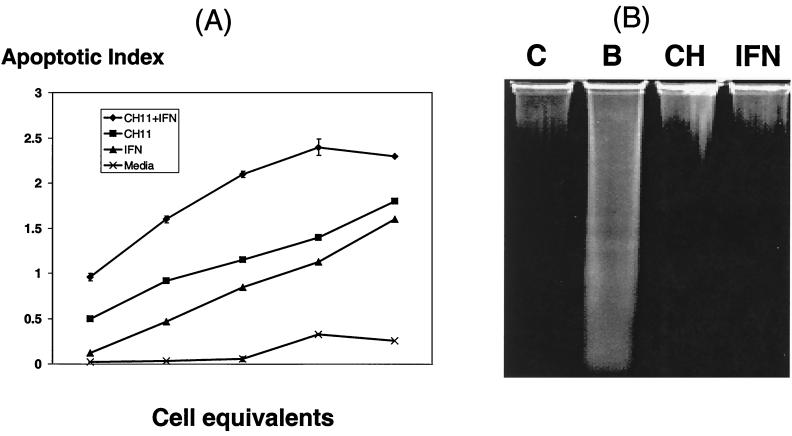
FIG. 4.
Induction of Fas-mediated apoptosis in gastric epithelial cells. Kato-III cells treated with medium or IFN-γ (100 U/ml, 48 h) were incubated with anti-Fas IgM antibody (CH11; 100 ng/ml) for 12 h. Subsequently, apoptosis was evaluated by measuring DNA degradation using the ELISA and the DNA ladder assay as described in Materials and Methods. (A) The apoptotic index of Kato-III cells after pretreatment with IFN-γ and CH11 was higher than that detected after exposure to IFN-γ or CH11 alone, although all of the stimuli induced greater DNA fragmentation than the medium control. (B) The sensitivity for the detection of DNA degradation using agarose gel electrophoresis was less than that of the ELISA, as evidence for apoptosis was only found in Kato-III cells treated with IFN-γ and CH11. Lanes: C, untreated control; B, both IFN-γ and CH11; CH, CH11; IFN, IFN-γ.
TABLE 2.
Relative sensitivities of different gastric epithelial cell lines to Fas-mediated apoptosis after induction of Fasa
| Cell line | Mean % specific killing ± SEM
|
|||
|---|---|---|---|---|
| Medium | H. pylori 26695 | H. pylori 8-1 | IFN-γ | |
| Kato-III | 9 ± 3 | 15 ± 1 | 12 ± 1 | 35 ± 2 |
| AGS-NY2 | 16 ± 3 | 32 ± 1 | 18 ± 2 | 28 ± 2 |
| N87 | 31 ± 8 | 55 ± 4 | 36 ± 4 | 61 ± 3 |
Fas expression was induced as described in Table 1, footnote a; the legend to Fig. 3; and Materials and Methods. Subsequently, Fas-mediated killing was detected using anti-Fas antibody CH11 at 50 ng/ml and assayed by the JAM test. Data represent the mean ± SEM of three observations measuring percent specific killing. Significant killing was detected under all of the conditions tested (P < 0.05), confirming the functional capacity of epithelial Fas.
FasL is expressed in gastric tissues.
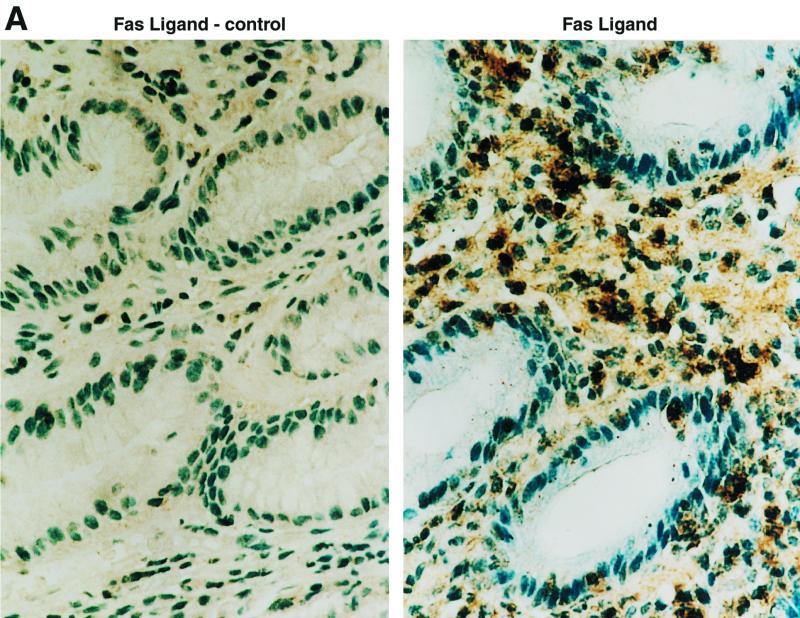
To determine if Fas-bearing epithelial cells would be exposed to effector cells expressing FasL, gastric biopsy specimens from infected or uninfected subjects were examined for FasL protein by immunohistochemistry. As shown in Fig. 5A, FasL protein was present on mononuclear cells in the gastric mucosa of infected individuals, whereas the expression in normal uninfected tissues was undetectable (M. Bennett et al., unpublished observations). To detect FasL mRNA, total RNA of gastric biopsy specimens with or without H. pylori infection was extracted and assayed using RT-PCR. As shown in Fig. 5B, all four infected gastric tissues contained mRNA for FasL while only one weakly positive band was detected in the three uninfected biopsy specimens.
FIG. 5.
Detection of FasL in gastric tissue. (A) FasL was assayed in gastric mucosa using immunohistochemistry. An intensely positive subset of infiltrating cells of lymphoid morphology was detected in tissue from five subjects infected with H. pylori, while tissue from uninfected subjects was negative. Binding of the anti-FasL antibody was entirely blocked when FasL peptide at 1 μg/ml was added with the primary antibody (control). (B) Total RNA of gastric biopsy specimens was extracted, and 10 μg of RNA was used to detect FasL mRNA by RT-PCR. All gastric samples from infected subjects (lanes 1 to 4) expressed FasL, while only one (lane 6) of the samples from uninfected subjects (lanes 5 to 7) expressed FasL mRNA and the level of expression was low. All samples contained comparable amounts of β-actin mRNA.
Functional FasL is expressed by gastric T cells.
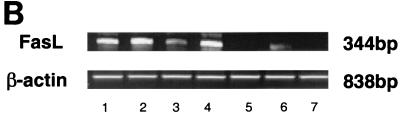
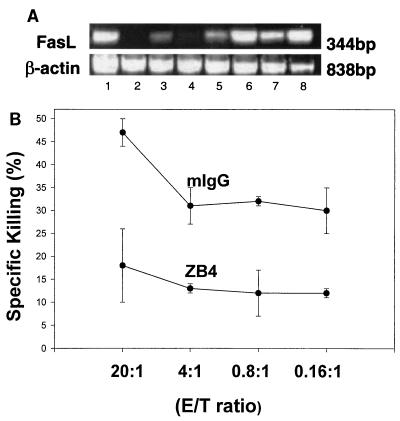
Gastric T cells were isolated from biopsy specimens and expanded in vitro in order to evaluate the expression and function of FasL. As shown in Fig. 6A, FasL mRNA was detected by RT-PCR in gastric T cells isolated from H. pylori-infected individuals. To evaluate the function of FasL, the T-cell lines were assayed using the JAM test to determine if they induced apoptosis in Jurkat T-cell line E6-1. The Jurkat E6.1 clone expressed a high level of Fas and was susceptible to IgM anti-Fas (CH11) antibody-mediated killing (data not shown). As shown in Fig. 6B, gastric T-cell lines could kill these target cells and this cytotoxicity was inhibited by antibody ZB4, which blocks Fas/FasL interactions while an isotype control, mouse IgG, did not (P < 0.05). These results imply that FasL expressed by T cells in the stomach during H. pylori infection was capable of mediating apoptosis in Fas-bearing cells.
FIG. 6.
Gastric T cells express functional FasL. T cells were isolated from gastric biopsy specimens and expanded in vitro. (A) Total RNA was extracted from gastric T-cell lines, and RT-PCR was used to detect mRNAs for FasL and β-actin. The mRNA for FasL was detected in T-cell lines regardless of whether they were obtained from infected or uninfected subjects, although products were more easily detected in T-cell lines from infected subjects. Lanes: 1, positive control; 2 to 4, gastric T-cell lines from uninfected subjects; 5 to 8, gastric T-cell lines from infected subjects. (B) Functional evidence for FasL expression on gastric T cells was obtained using the JAM test to detect DNA fragmentation of Fas-expressing target cells after exposure to T cells. Cytotoxicity was calculated by comparing the levels of radioactivity in cells that had or had not been exposed to the T cells. Murine anti-Fas IgG (ZB4; 500 ng/ml), but not isotype control antibody (mouse IgG [mIgG]), blocked the killing after being preincubated with target cells for 1 h (P < 0.05). These data are representative of three separate experiments.
To confirm the Fas/FasL interaction between T cells and epithelial cells, Kato-III cells were incubated with gastric T-cell lines and assayed for apoptosis using the JAM test. As shown in Fig. 7, gastric T cells killed gastric epithelial cells and this interaction was blocked by anti-Fas antibody ZB4 (P < 0.05 compared to the isotype control, mouse IgG). This observation further supports the hypothesis that Fas/FasL interactions are involved in the lymphoepithelial cell interactions that lead to epithelial cell damage during H. pylori infection.
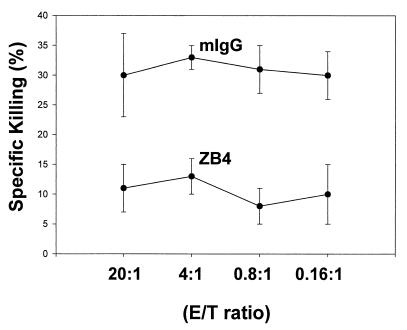
FIG. 7.
Killing of gastric epithelium by T cells through Fas/FasL interactions. Expanded gastric T cells were harvested and incubated with [3H]thymidine-labeled gastric epithelial (Kato-III) cells for 12 h at the indicated E/T ratios. Specific killing was calculated by comparing the levels of radioactivity of cells with or without exposure to T cells. Gastric T cells could kill Kato-III cells, and this cytotoxicity was blocked effectively by anti-Fas IgG antibody (ZB4; 500 ng/ml) but not by an isotype control (mouse IgG [mIgG]) (P < 0.05). These data are representative of three separate experiments.
DISCUSSION
Diseased tissue is often associated with cell death due to various processes, including necrosis and apoptosis. Apoptosis is a normal mechanism that controls epithelial cell turnover; however, the rate of apoptosis can be altered in response to infection and inflammation. Induction of apoptosis by H. pylori has been reported in subjects with duodenal ulcer and gastritis (25). Jones et al. found that the apoptotic index increased threefold in persons with H. pylori gastritis compared to noninflamed controls, and this change decreased following the eradication of H. pylori and resolution of gastritis (25). Other authors have described an increase in both the proliferation rate and the apoptotic index in H. pylori-infected stomachs (4, 29, 39). These observations imply that during infection with H. pylori, gastric epithelial apoptosis is induced and followed by an increase in the repair process. However, the mechanisms regulating cell death have only begun to be defined. Recently, it has been shown that H. pylori can induce apoptosis directly (13, 24, 41, 49). In addition, some cytokines, such as TNF-α or IFN-γ, can induce apoptosis directly, as well as augment the effects of the bacteria alone (13, 49). The results presented here provide evidence that T cells can contribute to epithelial cell death through Fas/FasL interactions.
IFN-γ-producing T cells, both CD4+ and CD8+, have been reported to be increased in the gastric mucosa of infected subjects (3, 17). Importantly, D'Elios and colleagues have shown that some gastric Th1 cell lines specifically recognize H. pylori antigens and produce both TNF-α and IFN-γ (9). More recent immunohistochemical evidence suggests that cytokine-producing Th1 cells predominate in the gastric antral mucosa during infection (31, 44). In addition, Th1 cells also dominate in the healthy uninfected gastric mucosa (17) or in a state of gastritis for other reasons (26). Thus, the predominance of Th1 cells suggests that local T cells contribute significantly to cell-mediated immunity targeted toward the epithelium during gastritis for various reasons.
One mechanism by which T cells kill their targets is expression of FasL and its binding to the Fas receptor. This mechanism of killing can be achieved by both CD8+ cytotoxic T lymphocytes, as well as CD4+ Th1 cells (18, 46, 51). T cells isolated from gastric tissues and expanded in vitro expressed FasL mRNA and were capable of inducing apoptosis in target cells bearing Fas. Unfortunately, too few T cells were isolated from gastric biopsy specimens to permit direct evaluation of FasL expression and function, therefore requiring the activation in vitro that may have induced FasL expression artificially. However, RT-PCR showed that FasL expression was increased in gastric tissue and immunohistochemistry confirmed the increase in FasL protein, including its expression on mononuclear cells adjacent to the epithelium. These data provide a human correlate of Fas/FasL-mediated epithelial cell damage that occurs in response to lymphoepithelial interactions in mice (16, 23, 30, 42, 45). Thus, epithelial cell damage may play a role in the pathogenesis of gastrointestinal disease in humans, as well as in the animal models studied to date.
In order for T cells bearing FasL to mediate apoptosis in gastric epithelial cells, the target must express the Fas receptor. Fas is expressed on T cells and is a key factor in the differentiation of T cells and prevention of autoimmune diseases. However, Fas can be expressed by a variety of cells that can be damaged by T cells expressing FasL and has therefore been associated with the development of some autoimmune diseases (5). For example, it has been found that Fas is involved in the development of murine autoimmune gastritis (36).
Previous reports have described the expression of Fas on gastric epithelial cell lines (19, 21, 24, 28). Moreover, Fas molecules on the surface of gastric epithelial cells can be stimulated to induce apoptosis (19, 22, 24). Rudi et al. recently reported that cagA+ H. pylori strains can upregulate the expression of Fas and FasL on gastric tissues, and this may contribute to the apoptosis during infection (41). In our study, gastric epithelial cells expressed Fas both in vivo and in vitro, verifying that they can serve as target cells in Fas-mediated cell death. Approximately 20% of the freshly isolated cells expressed Fas, as detected by fluorescence-activated cell sorter. Immunohistochemistry supported the fluorescence-activated cell sorter findings by demonstrating that Fas expression on gastric epithelial cells in gastric biopsy specimens from infected subjects was higher than that in uninfected tissues. Furthermore, the high levels of Fas expression were unevenly distributed, preferentially in the neck of the stomach. This is the region in which cytokine-producing T cells are enriched (3), suggesting that induction of Fas is greatest adjacent to cytokine-producing cells.
Th1 cytokines (52), including IFN-γ and TNF-α, and H. pylori infection (15, 27, 34, 43, 47) activate transcription factors in gastric epithelial cells that bind to the NF-κB and AP-1 regulatory sites. Since these regulatory sites are located in the 5′ region of the fas gene, it is possible that both can contribute to the induction of Fas expression. However, our results showed that H. pylori strain 8-1, which lacks the cag PAI, induced Fas expression, although to a lesser degree. Since the activation of NF-κB by H. pylori is dependent on the presence of the cag PAI (15, 52), AP-1 likely plays a role in the induction of Fas. Possibly, other transcription factors play a role but remain to be defined.
Three different cell lines expressing Fas were susceptible to apoptosis after being exposed to anti-Fas antibodies and/or T cells expressing FasL. Interestingly, the ability to detect apoptosis was dependent on the assay being used. Based on the detection of DNA fragmentation using electrophoresis, apoptosis could only be detected after pretreatment with IFN-γ to increase the expression of Fas. This is similar to another observation using intestinal epithelial cell lines (1). However, using the ELISA or the JAM test, apoptosis could be detected without boosting the expression of Fas. Therefore, cytokines can not only induce apoptosis directly but also increase the expression of Fas and enhance cell death by Fas/FasL interactions. It remains to be determined if some of the synergism is due to the effect of cytokines on signaling pathways that “arm” the cell to respond more efficiently subsequent to the engagement of the Fas receptor.
Further evidence in support of the notion that gastric T-cell cytokines collaborate in the regulation of epithelial cell death is found in the presence of IFN-γ- and TNF-α-producing cells within the gastric mucosa. IFN-γ and TNF-α are known to increase Fas expression on several types of cells, including renal endothelium (38), intestinal epithelium (50), and astrocytes (6). We found that these Th1 cytokines could act synergistically with H. pylori to increase Fas expression on all three of the gastric epithelial cell lines tested (AGS-NY2, Kato-III, and N87), which extends previous observations describing the regulation of Fas on gastric epithelial cells (24). Thus, Th1 cytokines can not only injure the gastric epithelium directly (13, 49) but also accelerate epithelial damage by modulating Fas/FasL interactions.
In conclusion, H. pylori infection induces the expansion of Th1-like cells that have the potential to express FasL. T cells with this phenotype can increase the expression of Fas on gastric epithelial cells and induce cell death through Fas/FasL interactions. Thus, apoptosis of the gastric epithelium can occur due to multiple mechanisms and thereby lead to a breach in the epithelial barrier that facilitates tissue damage due to luminal acid and pepsin.
ACKNOWLEDGMENTS
This work was supported by NIH grants DK 50669, DK 51577, and CHD 35741 and a John Sealy Memorial Endowment development grant. X.F. is a recipient of a McLaughlin Fellowship. F. Shanahan, M. Bennett, and J. O'Connell are supported in part by the Health Research Board of Ireland. J. O'Connoll is supported by the Wellcome Trust.
We thank Kim Palkowetz for her technical assistance with flow cytometry.
REFERENCES
- 1.Abreu-Martin M T, Vidrich A, Lynch D H, Targan S R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J Immunol. 1995;155:4147–4154. [PubMed] [Google Scholar]
- 2.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Burkanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 3.Bamford K B, Fan X J, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 4.Bechi P, Balzi M, Becciolini A, Maugeri A, Raggi C C, Amorosi A, Dei R. Helicobacter pylori and cell proliferation of the gastric mucosa: possible implications for gastric carcinogenesis. Am J Gastroenterol. 1996;91:271–276. [PubMed] [Google Scholar]
- 5.Brunner T, Mueller C. Is autoimmunity coming to a Fas(L) end? Nat Med. 1999;5:19–20. doi: 10.1038/4695. [DOI] [PubMed] [Google Scholar]
- 6.Choi C, Park J, Lee J, Lim J H, Shin E C, Ahn Y S, Kim C H, Kim S J, Kim J, Choi I S, Choi K L. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889–1895. [PubMed] [Google Scholar]
- 7.Correa P, Miller M J S. Carcinogenesis, apoptosis and cell proliferation. Br Med Bull. 1998;54:151–162. doi: 10.1093/oxfordjournals.bmb.a011665. [DOI] [PubMed] [Google Scholar]
- 8.Crowe S E, Alvarez L, Sherman P M, Jin Y, Dytoc M, Hunt R H, Patel J, Muller M J, Ernst P B. Expression of interleukin-8 and CD54 by human gastric epithelium after H. pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 9.D'Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 10.Dixon M F. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:7–10. [PubMed] [Google Scholar]
- 11.Dytoc M, Gold B, Louie M, Huesca M, Fedorko L, Crowe S, Lingwood C, Brunton J, Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1992;61:448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X J, Chua A, Shahi C N, McDevitt J, Keeling P W N, Kelleher D. Gastric T lymphocyte response to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X J, Crowe S E, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley W K, Ernst P B, Reyes V E. The effect of class II MHC expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for Th1 cell-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genta R M, Lew G M, Graham D Y. Changes in the gastric mucosa following eradication of Helicobacter pylori. Mod Pathol. 1993;6:281–289. [PubMed] [Google Scholar]
- 15.Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl H L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy-Grand D, DiSanto J P, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Haeberle H A, Kubin M, Bamford K B, Garofalo R, Graham D Y, El-Zaatari F, Karttunen R, Crowe S E, Reyes V E, Ernst P B. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi H, Tatebe S, Osaki M, Goto A, Suzuki Y, Ito H. Expression of Fas antigen and its mediation of apoptosis in human gastric cancer cell lines. Jpn J Cancer Res. 1997;88:49–55. doi: 10.1111/j.1349-7006.1997.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins R J, Girardi L S, Turney E A. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 21.Houghton J, Korah R M, Condon M R, Kim K H. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig Dis Sci. 1999;44:465–478. doi: 10.1023/a:1026628601284. [DOI] [PubMed] [Google Scholar]
- 22.Houghton J, Macera-Bloch L S, Harrison L, Kim K H, Korah R M. Tumor necrosis factor alpha and interleukin 1β up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189–1195. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki-Ohara K, Nishimura H, Sakai T, Lynch D H, Yoshikai Y. Potential for involvement of Fas antigen/Fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Lab Investig. 1997;77:421–429. [PubMed] [Google Scholar]
- 24.Jones N L, Day A S, Jennings H A, Sherman P M. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 26.Karttunen R, Karttunen T, Ekre H-P T, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keates S, Hitti Y S, Upton M, Kelly C P. Helicobacter pylori activate NFκB in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 28.Kume T, Oshima K, Yamashita Y, Shirakusa T, Kikuchi M. Relationship between Fas-ligand expression on carcinoma cell and cytotoxic T-lymphocyte response in lymphoepithelioma-like cancer of the stomach. Int J Cancer. 1999;84:339–343. doi: 10.1002/(sici)1097-0215(19990820)84:4<339::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Mellgard B, Helander I M. Inoculation of VacA- and CagA-Helicobacter pylori delays gastric ulcer healing in the rat. Scand J Gastroenterol. 1999;32:439–444. doi: 10.3109/00365529709025078. [DOI] [PubMed] [Google Scholar]
- 30.Lin T, Brunner T, Tietz B, Madsen J, Bonfoco E, Reaves M, Huflejt M, Green D R. Fas ligand-mediated killing by intestinal intraepithelial lymphocytes. Participation in intestinal graft-versus-host disease. J Clin Investig. 1998;101:570–577. doi: 10.1172/JCI896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm A-M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannick E E, Bravo L E, Zarama G, Realpe J L, Zhang X-J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 33.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 34.Meyer-Ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. Helicobacter pylori activates MAP kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem., in press. [DOI] [PubMed]
- 35.Moss S F, Calam J, Agarwal B, Wang S, Holt P G. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishio A, Katakura K, Toshima C, Kasakura S, Sakai M, Yonehara S, Suda T, Nagata S, Masuda T. A possible involvement of Fas-Fas ligand signaling in the pathogenesis of murine autoimmune gastritis. Gastroenterology. 1996;111:959–967. doi: 10.1016/s0016-5085(96)70063-x. [DOI] [PubMed] [Google Scholar]
- 37.O'Connell J, O'Sullivan G C, Collins J K, Shanahan F. The Fas counter-attack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz-Arduan A, Danoff T M, Kalluri R, Gonzalez-Cuadrado S, Karp S L, Elkon K, Egido J, Neilson E G. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol. 1996;271:F1193–1201. doi: 10.1152/ajprenal.1996.271.6.F1193. [DOI] [PubMed] [Google Scholar]
- 39.Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany B L. Induction of acute gastritis and epithelial apoptosis by Helicobacter pylori lipopolysaccharide. Scand J Gastroenterol. 1997;32:203–211. doi: 10.3109/00365529709000195. [DOI] [PubMed] [Google Scholar]
- 40.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 41.Rudi J, Kuck D, Strand S, Von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai T, Kimura Y, Inagaki-Ohara K, Kusugami K, Lynch D H, Yoshikai Y. Fas-mediated cytotoxicity by intestinal intraepithelial lymphocytes during graft-versus-host disease in mice. Gastroenterology. 1997;113:168–174. doi: 10.1016/s0016-5085(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 44.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E G, Zeus J, Röllinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuber E, Buschenfeld A, von Freier A, Arendt T, Folsch U R. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL-Fas interaction: effect of pentoxifylline on the development of mucosal atrophy. Gut. 1999;45:229–235. doi: 10.1136/gut.45.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 47.van Den Brink G R, ten Kate F J, Ponsioen C Y, Rive M M, Tytgat G N, van Deventer S J, Peppelenbosch M P. Expression and activation of NF-kappa B in the antrum of the human stomach. J Immunol. 2000;164:3353–3359. doi: 10.4049/jimmunol.164.6.3353. [DOI] [PubMed] [Google Scholar]
- 48.Van Houten N, Budd R C. Accelerated programmed cell death of MRL-lpr/lpr T lymphocytes. J Immunol. 1992;149:2513–2517. [PubMed] [Google Scholar]
- 49.Wagner S, Beil W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Fu X Y, Plate J, Chong A S. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1999;58:2832–2837. [PubMed] [Google Scholar]
- 51.Yagita H, Hanabuchi S, Asano Y, Tamura T, Nariuchi H, Okumura K. Fas-mediated cytotoxicity—a new immunoregulatory and pathogenic function of Th1 CD4+ T cells. Immunol Rev. 1995;146:223–239. doi: 10.1111/j.1600-065x.1995.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 52.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kappaB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 53.Ye G, Barrera C, Fan X J, Crowe S E, Ernst P B, Reyes V E. Gastric epithelial cells express the costimulatory molecules B7.1 and B7.2 during infection with Helicobacter pylori and activate CD4 T cells. J Clin Investig. 1997;99:1628–1636. doi: 10.1172/JCI119325. [DOI] [PMC free article] [PubMed] [Google Scholar]