Abstract
Introduction:
Puberty substantially alters the body’s mechanical properties, neuromuscular control, and sex differences therein, likely contributing to increased, sex-biased knee injury risk during adolescence. Female adolescents have higher risk for knee injuries than male adolescents of similar age engaging in similar physical activities, and much research has investigated sex differences in mechanical risk factors. However, few studies address the considerable variation in pubertal growth (timing, pace), knee mechanics, and injury susceptibility within sexes, or the impact of such growth variation on mechanical injury risk.
Objectives:
The present study tested for effects of variation in pubertal growth on established mechanical knee injury risk factors, examining relationships between and within sexes.
Methods:
Pubertal growth indices describing variation in the timing and rate of pubertal growth were developed using principal component analysis and auxological data from serial stature measurements. Linear mixed models were applied to evaluate relationships between these indices and knee mechanics during walking in a sample of adolescents.
Results:
Later developing female adolescents with slower pubertal growth had higher extension moments throughout stance, whereas earlier developers had higher valgus knee angles and moments. In male adolescents, faster and later growth were related to higher extension moments throughout gait. In both sexes, faster growers had higher internal rotation moments at foot-strike.
Conclusions:
Pubertal growth variation has important effects on mechanical knee injury risk in adolescence, affecting females and males differently. Earlier developing females exhibit greater injury risk via frontal plane factors, whereas later/faster developing males have elevated risk via sagittal plane mechanisms.
Keywords: puberty, walking gait, biomechanics, growth, knee injuries
1. INTRODUCTION
Puberty is a life history period characterized by widespread changes in the body’s spatial dimensions, including increases in stature, limb segment lengths, and proportionality. Pubertal accrual of muscle and fat tissue also alters body composition, body mass, and mass distribution, while modifying force production capacity. Moreover, puberty marks the onset of divergent male and female growth trajectories, resulting in average sex differences in postpubertal body size, shape, and composition, as well as muscular strength, power, and fatigability (Courtright et al., 2013; Hunter, 2009). Together, these shifts mean that pubertal growth has important consequences for the body’s mechanical properties, which occur in the additional context of ongoing nervous system maturation related to coordination, control, and cognitive components of movement planning and execution. These many interacting changes result in pubertal neuromuscular adaptations (Quatman et al., 2006) and modified kinematic solutions to motor tasks such as walking (Alderson et al., 2019; Corporaal et al., 2016; Froehle et al., 2013; Kraan et al., 2017), running (Davies & Rose, 2000; Taylor-Haas et al., 2022), jumping (Davies & Rose, 2000; Hewett et al., 2015), and throwing (Davies & Rose, 2000).
Understanding processes and patterns of variation in pubertal development between and within sexes is of interest to practitioners in various fields, attracting considerable attention in particular from clinical researchers studying musculoskeletal injury risk. This interest follows from the observation that modified movement strategies arising during puberty appear to be related to elevated musculoskeletal injury risk during adolescence and young adulthood, especially involving the knee (Beck et al., 2017; Csintalan et al., 2008; Dugan, 2005; Hewett et al., 2016; Knowles, 2010; Murphy et al., 2003; Myer et al., 2010; Seil et al., 2016; Taunton et al., 2002). From a behavioral perspective, injury exposure increases naturally along with a tendency for greater participation in organized, competitive sports during late childhood and early adolescence in many cultures, but this alone is unlikely to fully explain increased injury rates. Instead, multiple aspects of pubertal growth, maturation, and development are linked to the emergence or exacerbation of several key injury risk factors.
The observation that knee injury risk increases during and immediately following puberty is perhaps unsurprising. The joint lacks bony stability, yet is subjected to very high loads as it joins the two longest levers in the body, both of which lengthen substantially during puberty. Furthermore, under conditions of single-limb support common to a variety of motions, the knee bears the entire body’s weight, the center of which becomes more distant, and more top-heavy, as stature increases during puberty. The knee is also subject to loading by the large, strong muscle groups of the thigh, which grow and change in terms of relative strength across puberty (Myer et al., 2008). As a result, pubertal mechanical changes have been implicated in risk for various knee injuries and overuse conditions, including non-contact injury of the anterior cruciate ligament (ACL) (e.g., Hewett et al., 2015), patellofemoral pain syndrome (PFPS) (Myer et al., 2010, 2015), iliotibial band syndrome (ITBS) (Taylor-Haas et al., 2022), and tibial tuberosity traction apophysitis (i.e., Osgood Schlatter disease; OSD) (Circi et al., 2017; Enomoto et al., 2019), each associated with multiple negative short- and long-term sequelae (Campbell et al., 2014; Dugan, 2005; Hewett et al., 2012; Lohmander et al., 2004, 2017; Mather et al., 2013; Natri et al., 1998; Noehren et al., 2013; Schmale et al., 2014; Taunton et al., 2002).
Importantly, risk for knee injuries and their consequences is not evenly distributed across all individuals. Sex has been established as one of the key factors biasing risk, with studies frequently reporting higher rates of knee injuries in female athletes compared to male athletes engaging in the same physical activities at similar intensity levels (Beck et al., 2017; Dugan, 2005; Ford, Shapiro, et al., 2010; Knowles, 2010; Murphy et al., 2003; Taunton et al., 2002; Voskanian, 2013; Wild et al., 2012). Furthermore, as with most secondary sex differences, these disparities arise during puberty and are well-established by adolescence (Andrish, 2001; Gallagher et al., 2011; Hewett et al., 2004, 2015; Myer et al., 2010; Quatman et al., 2006). The suite of high-risk traits generally associated with post-pubertal females, but not males, is thus thought to be a product of diverging pubertal developmental trajectories (Ford, Shapiro, et al., 2010; Hass et al., 2005; Hewett et al., 2015; Holden et al., 2016; Kim & Lim, 2014; Quatman et al., 2008; Quatman-Yates et al., 2013; Shelbourne & Kerr, 2001; Wild et al., 2016). Sex, however, is simply a relatively-easy-to-assess proxy for what are likely multiple underlying risk factors, including variation in knee mechanics. Thus, in the interest of reducing injury rates and developing cost-effective, timely preventive interventions, substantial research effort has been devoted to identifying pubertal changes in knee mechanics that likely underlie sex-specific risk (Campbell et al., 2014; Foss et al., 2018; Hewett et al., 2012, 2015; Martinez et al., 2017; Myer et al., 2013; Powers, 2010).
Most of the relevant data come from clinical studies of sex disparities in ACL injuries and related mechanics during various movements, including jumping/landing, sidestep cutting, and running. Such studies have shown that on average across puberty, females develop increased sagittal plane stiffness (Chia et al., 2021; Ford, Myer, et al., 2010; Wild et al., 2016) along with higher peak knee abduction angles and moments (Chia et al., 2021; Ferber et al., 2003; Ford et al., 2005; Ford, Shapiro, et al., 2010; Hass et al., 2005; Hewett et al., 2015; Holden et al., 2016; Jacobs et al., 2007; Kim & Lim, 2014; S. McLean et al., 2004; Nakagawa et al., 2012; Wild et al., 2016). These mechanical patterns are likely related to pubertal shifts in neuromuscular control of the knee, including increases in extensor (quadriceps) strength without corresponding strengthening of flexors (hamstrings) (Quatman-Yates et al., 2013), reductions in hip abductor strength (Quatman-Yates et al., 2013), and medio-lateral imbalances in preparatory co-contraction of the vastus muscles and hamstrings (Palmieri-Smith et al., 2008, 2009; Wild et al., 2016). Collectively, these changes increase tensile loading in the sagittal plane while also elevating shear stress in the frontal plane, which the ACL is ill-equipped to withstand (Fukuda et al., 2003; Hewett et al., 2005; Kanamori et al., 2000; Kiapour et al., 2015; Lloyd & Buchanan, 2001; Markolf et al., 1995). As a result, “valgus collapse” is the most commonly observed pattern of non-contact ACL injury in female adolescents and young women (Hewett et al., 2005; Quatman & Hewett, 2009; but see Krosshaug et al., 2016 for an alternative view).
Male adolescents similarly exhibit an average sagittal plane stiffening during puberty (Ford, Myer, et al., 2010). In the frontal plane, however, males maintain stability or even show reductions in dynamic valgus kinematics and loading (Chia et al., 2021; Ford, Shapiro, et al., 2010; Hewett et al., 2015; Wild et al., 2016), accompanied by balanced development of quadriceps and hamstrings strength, and roughly equal medio-lateral activation (Palmieri-Smith et al., 2009). These tendencies have been interpreted as being protective against knee overuse conditions and injuries in male adolescents and young men, thus partially explaining their lower relative injury rates (Hewett et al., 2015; Wild et al., 2012). However, many male adolescents and young men do still experience knee injuries, and it appears that sagittal plane factors are central to male injury risk. Tensile loading of the ACL in the sagittal plane can be quite high during a variety of movements, particularly when the quadriceps rapidly produces large amounts of tension, and within 30 degrees of full extension (Demorat et al., 2017; Markolf et al., 1995; Yu & Garrett, 2007). Data show that males tend to maintain stiffer, less flexed knees than females during a drop vertical jump task, and previously injured males land more stiffly than uninjured males (Lam & Valovich McLeod, 2014). Furthermore, when males experience non-contact ACL injuries, their knees tend to be significantly more extended at the time of injury than in females (Krosshaug et al., 2017).
This body of past work has substantially improved our understanding of broad trends in the effects of sex and pubertal development on mechanical risk factors for knee injury. There are a number of limitations, however, that collectively, restrict interpretation of the results. The overarching issue is that no one study directly compares males and females by pairing measurements of growth with mechanical data, while also addressing the considerable interindividual variation that occurs in all such variables. For example, many previous studies are limited by cross-sectional designs, but even those measuring serial data tend not to directly measure or analyze growth (with the exception of Hewett et al., 2015), instead using secondary sexual characteristics as a proxy for maturation (e.g., Tanner stages and related methods). The limitation of this approach is that it assumes sexual maturity has the same effect on the body’s mechanical properties for all individuals, which is likely inaccurate.
Further, by not investigating interindividual variation within sexes, interpretations of the effects of puberty and sex can become reductive, in some cases leading to relatively simplistic, often binary constructions (i.e., males do this, females do that; see, e.g., Sokolove, 2009). Examining coefficients of variation (CV) reveals a great deal of interindividual variability in pubertal timing (CV: 6–13%) and change in stature (CV: 40–50%), as well as in muscle strength (15–50%) and knee mechanical variables (20–180%) (Froehle et al., 2020; Froehle et al., 2017; Hewett et al., 2015; Quatman-Yates et al., 2013; Steffensmeier et al., 2020). Moreover, the degree of variability in some of these factors is not the same in males and females, or differs between pubertal and postpubertal subjects, so that even the degree of variation varies with sex and maturity. Generally, CVs in this range are considered to indicate a high level of variation and low consistency between individual measurements (Gilchrist et al., 2016), and it is reasonable to assume that this variation is also related to variation in injury risk. For the most part, this assumption remains uninvestigated. Although some studies have examined variation within females to derive mechanical thresholds for injury risk (Bates et al., 2020; Hewett et al., 2005; Myer et al., 2015), none have connected the mechanical data to variation in the timing or pace of pubertal growth.
Our own recent work has attempted to address some of these limitations, examining interindividual variation within sexes in pubertal development, injury outcomes, and mechanics. That work has uncovered relationships between variation in pubertal timing and outcomes related to knee injuries, with earlier menarche in female adolescents related to higher valgus angles and loading during walking (Froehle et al., 2017), and a higher incidence of ACL injuries among collegiate athletes (Froehle et al., 2020). In young adult males, a later end to pubertal growth was related to stiffer landings during a drop vertical jump task (Steffensmeier et al., 2020). However, despite focusing on interindividual variation, our studies suffer from some of the same limitations as previous research, namely cross-sectional study designs, use of recall for growth and maturation data, and not comparing males and females directly using the same movement task. Thus, additional research is needed to more fully understand the influences of sex and variability in pubertal growth on interindividual variation in knee mechanical factors related to injury risk.
To that end, the present study investigates the impact of sex and variation in pubertal growth on knee mechanics during a common motor task, namely, walking. While the moderate task of walking is not typically associated with risk for knee injury (as opposed to jumping, landing, and cutting tasks used in most previous research), we assert that it can nonetheless provide valuable information on relationships between pubertal growth and neuromotor maturation. Although most children achieve proficient walking gait by age 7–8 y (Sutherland, 1997), walking continues to mature during puberty alongside ongoing growth and neuromuscular maturation (Froehle et al., 2013). It is also reasonable to expect that individualized joint mechanical patterns developed during a task as fundamental to human locomotion as walking are at least moderately correlated with mechanical patterns in more vigorous movements. In other words, aspects of skeletal alignment, muscle strength, and neuromuscular coordination underlying individual mechanical outcomes should apply similarly under multiple movement conditions. Although published data speaking directly to this assumption are limited, a recent study showed remarkable consistency in knee frontal plane angles between static alignment, walking, running, and a single leg drop-landing task (Barrios et al., 2016), suggesting that mechanical patterns observed in walking carry over to other more vigorous movements. Further, walking gait assessment is relatively simple to perform, is a task familiar to most people (vs., e.g., a drop vertical jump task, which can present difficulty in terms of correct performance), and low-risk compared to more vigorous tasks. Gait may thus be appealing as a more widely applicable screening tool for understanding and evaluating injury risk. Studying relationships between variability in pubertal growth and walking mechanics can thus provide a window into the ways in which growth relates to neuromotor control more broadly.
The dataset used in the present study was available as part of a larger, long-running longitudinal study on growth, development, and aging (see methods for details), which includes serial measurements of stature used to derive growth curves and related auxological variables across childhood and adolescence. Other data included additional anthropometric measures, measures of maturation including skeletal age and ages at key developmental milestones (e.g., menarche, adult stature), as well as health history and physical activity questionnaire responses. Finally, each subject participated in at least one walking gait assessment, in which three-dimensional quantitative methods were used to collect detailed kinematic and kinetic data. Thus, this dataset provides a powerful tool to test the present study’s hypotheses related to pubertal growth patterns and injury-risk-related knee mechanics. We define mechanical risk as a tendency toward stiffer, more extended knees in the sagittal plane, and toward higher valgus angles and loading in the frontal plane. On the basis of our previous findings, we hypothesize that earlier maturing females will exhibit riskier mechanics, while in males we hypothesize that later maturation is associated with riskier mechanics.
2. METHODS
2.1. Fels Longitudinal Study Participants
Samples for the present analysis were drawn from existing data on participants in the Fels Longitudinal Study, which at the time of data collection (2002–2009) was the world’s largest and longest-running longitudinal study of growth, body composition, and physical function (Sherwood & Duren, 2013). The study included over 1,200 serial participants, who were neither recruited into or excluded from the study on the basis of any specific trait or condition. This recruitment process generated a community-based study of normal population variation, albeit in a demographically constrained group of participants. Fels participants were overwhelmingly of European descent, and the majority were born in, and living in or near, southwest Ohio at the time of data collection. For the present study we utilized data from N=342 study participants to develop growth indices. Gait biomechanics data were then taken from a subset of this sample (N=52) for analysis. All study procedures were approved by the Wright State University Institutional Review Board at the time of data collection and analysis.
2.2. Anthropometry and Auxology
Childhood and pubertal growth curves rely on serial measurements of stature, which were taken at set intervals in Fels participants. Children and adolescents from ages 1–18 years (y) were measured every six months, and every 2 y in early adulthood (18–24 y). During data collection, participants wore light athletic clothing (shorts and tank top) and were unshod. Stature was measured to the nearest 0.1 cm with a stadiometer, as was sitting height using a stool of known height. Subischial leg length was calculated as (stature–sitting height). Body mass was measured to the nearest 0.1 kg using a digital scale, and body mass index (BMI; kg/m2) was calculated as (body mass/stature2). Bicristal pelvic breadth was measured to the nearest 0.1 cm using an anthropometer (Lohman et al., 1988). Marker and joint center spatial coordinates from static trials prior to walking gait motion capture (see below) were used to calculate Euclidean segment lengths for the thigh (hip joint center to knee joint center) and shank (knee joint center to ankle joint center), standing knee varus/valgus alignment (frontal plane angle between thigh segment and shank segment), and inter-anterior superior iliac spine (inter-ASIS) pelvic breadth. Crural index was calculated as (shank segment length∙100/thigh segment length).
Age at reaching mature stature was determined using serial stature data and a two-component piecewise linear regression model, in which the flexion point identified the age when stature ceased increasing (see Froehle et al., 2013 for similar modeling). Mature values for stature, sitting height, subischial leg length, and bicristal breadth were derived from the first visit after this age. For each serial visit preceding age at mature stature, the age-specific percent of mature stature achieved was calculated as (current stature/mature stature). Skeletal age at each childhood and adolescent visit was calculated from hand-wrist radiographs until achieving skeletal maturity (i.e. skeletal age=18 y), using the FELS method (Chumela et al., 1989; Nahhas et al., 2013). For visits prior to skeletal maturity, relative skeletal age was calculated as (skeletal age–chronological age), such that negative values indicate delayed skeletal maturity, and positive values indicate accelerated skeletal maturity.
Growth velocity curves were fit to serial stature data using a triple logistic Bock-Thissen-du Toit [BTT (Bock et al., 2003) model in AUXAL 3.1 software, Scientific Software International, Inc.]. Growth periods were defined as follows: early childhood (2 to <6 y), middle childhood (6 to <10 y), puberty (10 to <14 y), and late adolescence (14–18 y). Auxological variables included middle childhood maximum growth velocity (cm/y), age at middle childhood maximum growth velocity (y), middle childhood slope (i.e., rate of growth acceleration during middle childhood, in cm/y2), growth velocity at takeoff (i.e. onset of the pubertal growth spurt; cm/y), age at takeoff (y), peak height velocity (PHV; cm/y), age at PHV (y), adolescent slope (cm/y2), and adolescent increment (i.e., total contribution of adolescent growth to mature stature; cm). Additional details on auxological methods in this sample have been published previously (Towne et al., 2008), and [FIGURE 1] provides a visual display of the variables discussed above.
Fig. 1:
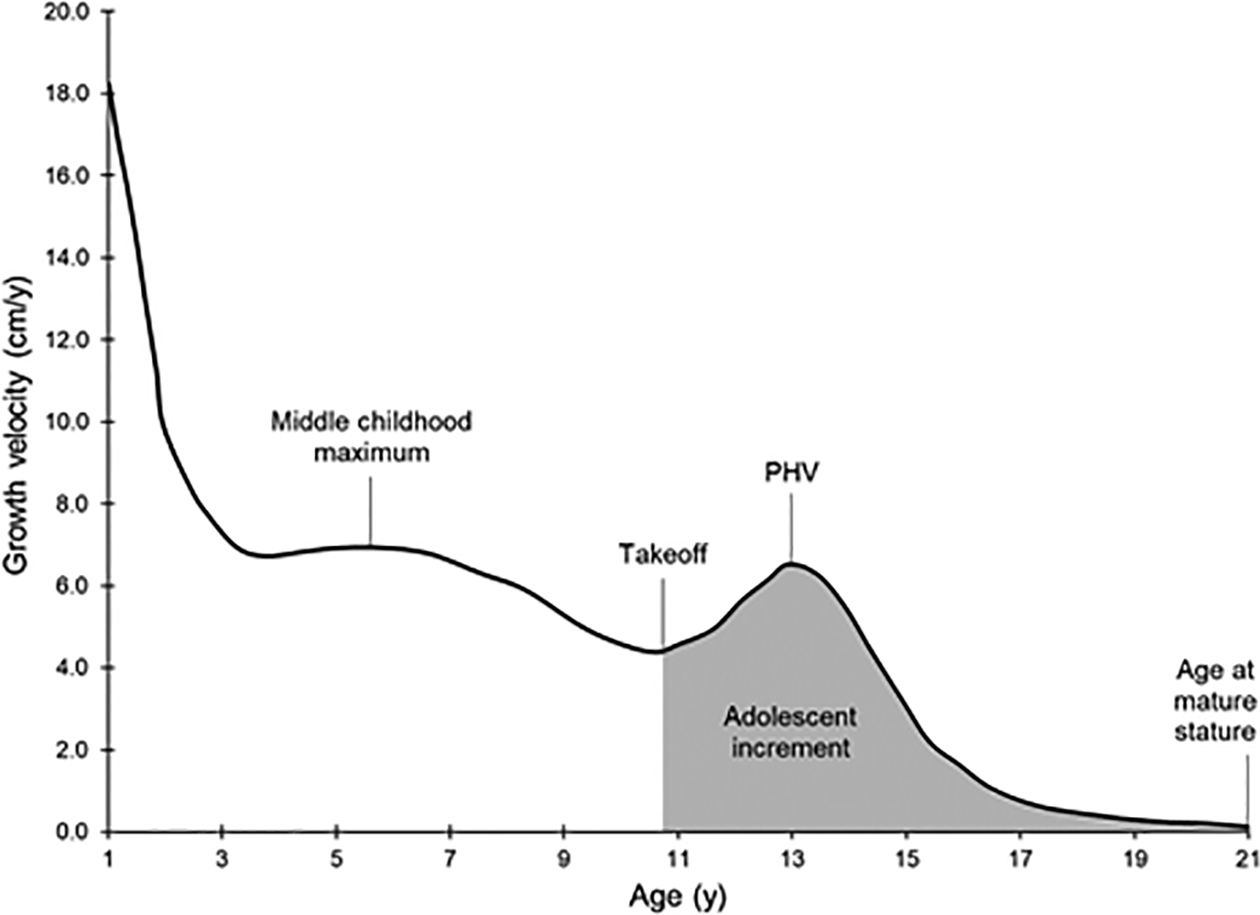
Representation of a typical stature growth velocity curve, plotting growth velocity (cm/y) on the vertical axis vs. age (y) on the horizontal axis. Key growth events and other derived auxological variables are labeled.
For female participants, age at menarche was also included in the dataset, assessed by menstrual status questionnaires administered at each six-month visit beginning at age 9 y (Demerath et al., 2004). All participants also completed questionnaires on health history and physical activity (PA). The former was used to screen participants in the present study in order to exclude for conditions affecting gait (see below). The latter was designed specifically for use in children and includes a sports PA index, which was used here to determine if sex differences in study outcomes were related to differences in PA. This sports index has been shown to be valid and reliable, especially in high school students (Treuth et al., 2005).
2.3. Growth Index Principal Component Analysis
Principal component analysis (PCA) is a multivariate statistical technique which generates linear combinations of interrelated variables (principal components) summarizing underlying, salient relationships among those variables (Hatcher, 1994). Each component provides a unique index of variation, and an individual’s score for a given index describes their relative, standardized position in multivariate space, and thus their particular expression of the underlying phenotypic vector encompassed by the index. These dimensionless data can then be analyzed to determine the influence of the latent vectors on other phenotypic outcomes. In the present study, we used PCA to reduce the set of nine auxological variables to a smaller set of growth indices, which we then analyzed for effects on knee mechanics during walking.
All Fels participants with serial stature measurements were eligible for inclusion in the PCA sample. Participants were excluded if they had incomplete auxological data or were obese at any time during childhood or adolescence (Freedman et al., 2005), resulting in a total eligible sample of n=348 (males: n=235; females: n=113). This sample size was sufficient for the study’s purposes, since the general rule of thumb is to have a minimum of five observations for each variable included in the PCA (Hatcher, 1994). With ten variables (nine auxological variables listed above, plus age at mature stature), the sample of n=348 provided nearly 35 observations per variable.
The PCA was run using SAS 9.4 PROC FACTOR (SAS Institute, Cary, NC). The analysis was performed on the covariance matrix, which allows for differences in variance between the variables and can be used when the variables are on similar scales and thus do not need to be standardized (Hatcher, 1994). Principal components were retained if they had an eigenvalue ≥1, were positioned to the left of the first break in the scree plot, and accounted for ≥5% of the total variance in the sample (Hatcher, 1994; Khattree & Naik, 2000). All output was preliminarily rotated to an oblique solution using the promax method, since growth-related components are expected to be correlated with one another; however, if component correlation values were ≤0.32 then varimax orthogonal rotation was used to generate the final model (Tabachnick & Fidell, 2019). Rotated factor loadings were interpreted qualitatively using the criteria that meaningful loadings were those that were ≥|0.35|. The rotated model coefficients were then used to calculate growth index values in the PCA sample, as well as in the gait sample (see below). The outcomes of these procedures are described below in the Results section.
It is important to note that males and females were ultimately pooled into a single sample for PCA, following a preliminary sex-specific analysis that found only marginal differences in factor loading magnitudes and directions between males and females. This should not be interpreted to mean that males and females did not differ for pubertal growth variables—indeed they did, as expected, particularly for variables related to timing (see Table 1). What it does suggest, however, is that the overall underlying domains of variation in pubertal growth are similar between sexes, as is the manner in which each individual variable contributes to those domains. Thus, although there are well-established sex differences in especially the timing of pubertal growth, these differences can be captured in indices derived from PCA while also describing variation within and between sexes continuously rather than dichotomously.
Table 1:
Anthropometric and Auxological Traits in the Gait Sample
| Variable | Male adolescents | Female adolescents | P-valuea |
|---|---|---|---|
| (n=25) | (n=27) | ||
| Mean ± SD | Mean ± SD | ||
| Values at gait analysis visit | |||
| Age (y) | 15.3 ± 2.1 | 16.7 ± 1.1 | 0.005 |
| Skeletal age (y) | 15.6 ± 2.2 | 17.5 ± 1.0 | <0.001 |
| Relative skeletal age (y) | 0.3 ± 1.1 | 1.0 ± 0.8 | 0.009 |
| Stature (cm) | 169.7 ± 12.3 | 162.7 ± 6.8 | 0.015 |
| Percent of mature stature (%) | 94.2 ± 6.2 | 99.6 ± 0.5 | <0.001 |
| Sitting height (cm) | 87.2 ± 6.6 | 86.4 ± 3.7 | 0.622 |
| Subischial leg length (cm) | 82.5 ± 6.3 | 76.2 ± 4.2 | <0.001 |
| Thigh segment length (cm) | 46.1 ± 3.0 | 42.0 ± 3.2 | <0.001 |
| Shank segment length (cm) | 40.3 ± 3.8 | 37.5 ± 2.4 | 0.003 |
| Crural index | 88.5 ± 8.0 | 89.6 ± 6.4 | 0.587 |
| Static knee varus/valgus alignment (°) | −1.5 ± 3.1 | −2.7 ± 2.5 | 0.167 |
| Bicristal breadth (cm) | 26.8 ± 2.4 | 27.5 ± 1.3 | 0.181 |
| Inter-ASIS breadth (cm) | 23.9 ± 2.5 | 24.7 ± 2.1 | 0.178 |
| Body mass (kg) | 60.6 ± 13.5 | 60.3 ± 8.4 | 0.934 |
| Body mass index (kg ∙ m-2) | 20.8 ± 3.2 | 22.9 ± 3.3 | 0.027 |
| Sport physical activity index | 3.0 ± 0.6 | 2.8 ± 0.6 | 0.310 |
| Age at menarche (y) | --- | 12.5 ± 0.9 | --- |
| Values at maturity | |||
| Mature stature (cm) | 180.1 ± 5.2 | 163.4 ± 7.1 | <0.001 |
| Mature sitting height (cm) | 93.9 ± 2.8 | 87.3 ± 3.9 | <0.001 |
| Mature subischial leg length (cm) | 86.2 ± 3.7 | 76 ± 4.3 | <0.001 |
| Mature bicristal breadth (cm) | 29.5 ± 2.1 | 28.7 ± 2.1 | 0.180 |
| Auxological variables | |||
| Age at mature stature (y) | 16.3 ± 1.0 | 13.5 ± 0.9 | <0.001 |
| Middle childhood maximum growth velocity (cm/y) | 6.7 ± 0.4 | 6.8 ± 0.5 | 0.323 |
| Age at middle childhood maximum growth velocity (y) | 6.4 ± 0.4 | 5.2 ± 0.4 | <0.001 |
| Middle childhood slope (cm/y2) | 6.5 ± 0.4 | 6.5 ± 0.6 | 0.960 |
| Growth velocity at takeoff (cm/y) | 5.0 ± 0.8 | 5.6 ± 0.5 | <0.001 |
| Age at takeoff (y) | 10.9 ± 1.0 | 8.3 ± 0.8 | <0.001 |
| PHV (cm/y) | 8.7 ± 1.3 | 8.1 ± 0.8 | 0.043 |
| Age at PHV (y) | 14.0 ± 1.0 | 11.2 ± 0.8 | <0.001 |
| Adolescent slope (cm/y2) | 9.4 ± 1.5 | 8.8 ± 0.9 | 0.105 |
| Adolescent increment (cm) | 36.5 ± 5.5 | 36.1 ± 3.7 | 0.777 |
P-value for independent samples t-tests comparing female and male adolescents.
On the basis of this latent similarity, we decided that using pooled PCAs in subsequent analyses of gait mechanics would provide several analytical and interpretive advantages. First, the pooled analysis would allow for growth variation and its relationships to gait mechanics to be described along the same axes in both sexes, and according to the same general principles of variability in growth timing and pace. This approach also allowed us to test specifically for the independent effects of sex on growth-gait mechanics relationships by including sex as an independent variable in subsequent statistical analyses (see below). Finally, the pooled analysis provided additional statistical power over the alternative, i.e. running two separate sex-specific analyses in smaller sub-samples. As an additional safeguard, we ran a sensitivity analysis of gait mechanics using the sex-specific PCAs to determine if growth-gait outcomes differed from the pooled PCA analysis. The results of the sensitivity analysis did not differ substantively from the pooled analysis, and thus only the latter results are presented below.
2.4. Walking Gait
Analysis of walking gait at self-selected speed was performed from 2002–2009 on all Fels participants over age 8 y who were able to walk. Data were collected using a three-dimensional motion analysis system consisting of six high-speed (120 Hz) digital cameras (Hawk, Motion Analysis Corp., Santa Rosa, CA) synced with three force plates (one Kistler Type 9281B11, Kistler Instruments, Winterthur, Switzerland; two AMTI OR6-7-1000, Advanced Mechanical Technology, Inc., Watertown, MA) embedded in a 15 m walkway. Cameras captured the motion of external retroreflective markers placed at anatomical landmarks following the Helen Hayes marker system (Kadaba et al., 1990), using Cortex software (Motion Analysis Corp., Santa Rosa, CA) to process and record data. Prior to walking trials, each participant completed a static trial, standing with their feet at shoulder width, knees fully extended, and arms outstretched laterally. Participants were then asked to walk at their preferred speed from one end of the walkway to the other, with no other instructions. Walking trials were repeated until the participant had naturally (i.e. without reaching or altering gait) completed at least five trials with one clean force plate strike per foot (foot-strike to toe-off, single foot on any one force plate at a time).
The three best trials (high-fidelity marker recognition, clean force plate strikes) were selected for further post-processing in Cortex, which included smoothing marker data using a 6 Hz low-pass Butterworth filter and interpolating data to percentages of the gait cycle. OrthoTrak software (Motion Analysis Corp., Santa Rosa, CA) was used to extract gait parameters for analysis. Spatiotemporal properties of gait included in this study’s analyses were forward velocity (m/s) and step width (cm). Base of support, which normalizes step width for body size, was calculated as (bicristal breadth/step width) (Sutherland et al., 1980). Residual base of support, an indicator of gait maturity in the frontal plane during late childhood/early adolescence was calculated using previously published equations (Froehle et al., 2013).
Knee joint osteokinematic motion was modeled as motion of the shank segment relative to the thigh segment, with three rotational degrees of freedom: flexion/extension (sagittal plane), varus/valgus (frontal plane), and internal/external rotation (IR/ER; transverse plane). Segment longitudinal axes were defined using joint center estimates from static trials for the hip (three-dimensional linear offsets from the mid-ASIS point and positions of the two ASIS and single mid-sacral markers, following Harrington et al., 2007), knee (mid-point between knee medial and lateral epicondylar markers), and ankle/talocrural (mid-point between the medial and lateral malleolar markers). The thigh segment longitudinal axis was defined as the line from the hip joint center to the knee joint center, and the shank segment longitudinal axis was defined as the line from the knee joint center to the ankle joint center. Ground reaction forces collected from the force plates were used to calculate peak vertical ground reaction force normalized to body weight (vGRF/body mass∙g). Net external knee joint moments, adjusted for body mass (Nm/kg), were calculated using measured values of ground reaction forces, force moment arms, segment velocities and accelerations, along with calculated segment inertial properties (following Dempster, 1955).
Knee kinematic and kinetic variables analyzed in this study included flexion/extension, varus/valgus, and internal/external rotation angles and moments at foot-strike, as well as peak stance phase values for flexion, extension, varus, valgus, internal rotation, and external rotation angles and moments. Directional signing conventions for each rotational axis are as follows: positive=flexion, varus, and internal rotation; negative=extension, valgus, and external rotation. Right and left side data for each kinetic and kinematic variable were compared using paired t-tests, which found no significant inter-limb asymmetry for any variable (for each, P≥0.081). Thus, the averages of the right and left sides for each variable were used in all subsequent analyses.
For inclusion in the walking gait sample, participants had to have had at least one visit with gait analysis under the age of 19 y at which they had achieved ≥90% of mature stature (i.e. past age at PHV—see Sanders et al., 2017). Inclusion also required each participant to have had a sufficient number of childhood and adolescent stature measurements to derive a growth velocity curve and related auxological variables, as these were needed to calculate growth index values. Participants were excluded if they were obese at the time of gait data collection (Freedman et al., 2005), if they were toe-walkers, wore prescription shoe inserts, reported a chronic gait-related neurological or musculoskeletal condition, reported sustaining any significant musculoskeletal, tendon, ligament, or joint injury to the spine, pelvic girdle, or lower extremity within 6 months prior to the selected gait visit, or reported a history of lower-extremity overuse injuries prior to the gait visit, such as ITBS, PFPS, OSD, or acute soft tissue injuries such as ACL tears. However, participants excluded for a knee overuse condition or injury were retained separately for a subanalysis comparing them to the main sample for aspects of pubertal growth. Female participants were also excluded for premature menarche (<10 y), as this is often associated with other developmental abnormalities (McLean et al., 2015).
The dataset for gait analysis included a single visit from each eligible participant. Where a participant had multiple qualifying visits, the visit at the oldest available age was selected if none of the visits occurred after the age at achieving mature stature. Where one or more qualifying visits occurred past the age of achieving mature stature, the visit closest to and past the age at mature stature was selected. Screening led to a final sample of N=52 (male adolescents: n=25; female adolescents: n=27).
2.5. Statistical Analysis
All statistical analysis was performed in SAS 9.4 (SAS Institute, Cary, NC) with significance set to α=0.05. Independent samples t-tests were used to determine if sex-specific mean values for each growth index in the gait sample differed from the PCA sample (the “population”), to gain an understanding of how representative the gait sample was. Relationships between growth indices and anthropometric traits were evaluated using Pearson correlation coefficients, and sex differences in growth index means were analyzed using independent samples t-tests. Independent samples t-tests were also used to test for sex differences in anthropometric traits, growth variables, and gait mechanics. A multiple linear mixed models approach was used to test for the effects of growth indices and sex on gait mechanical variables, initially testing for the effect of the interaction between sex and all growth indices. Where those interactions were not significant, they were removed from the models, and the two-way interactions between sex and each growth index were tested. Finally, for gait variables where none of the interaction effects were significant, the independent main effects of sex and each growth index were analyzed.
3. RESULTS
3.1. Growth Indices
The PCA produced nine principal components (PCs), two of which were retained: PC1 and PC2. These PCs explained 75.0% and 19.2% of the sample variance, respectively (cumulative explained variance =94.2%; eigenvalues: PC1=19.94; PC2=5.10). A discernible break in the slope of the scree plot occurred at the position of PC3 (both PC1 and PC2 were positioned to the left of this break), and PC3 explained just 3.1% of the variance with an eigenvalue of 0.83. We therefore removed PC3 and all subsequent PCs from further analysis. The retained PCs 1 and 2 were initially rotated using the promax approach, but due to a correlation between the PCs of 0.06, the final model was rotated to an orthogonal solution using the varimax approach. See [FIGURE 2] for rotated factor loadings in each auxological variable in the retained components.
Fig. 2:
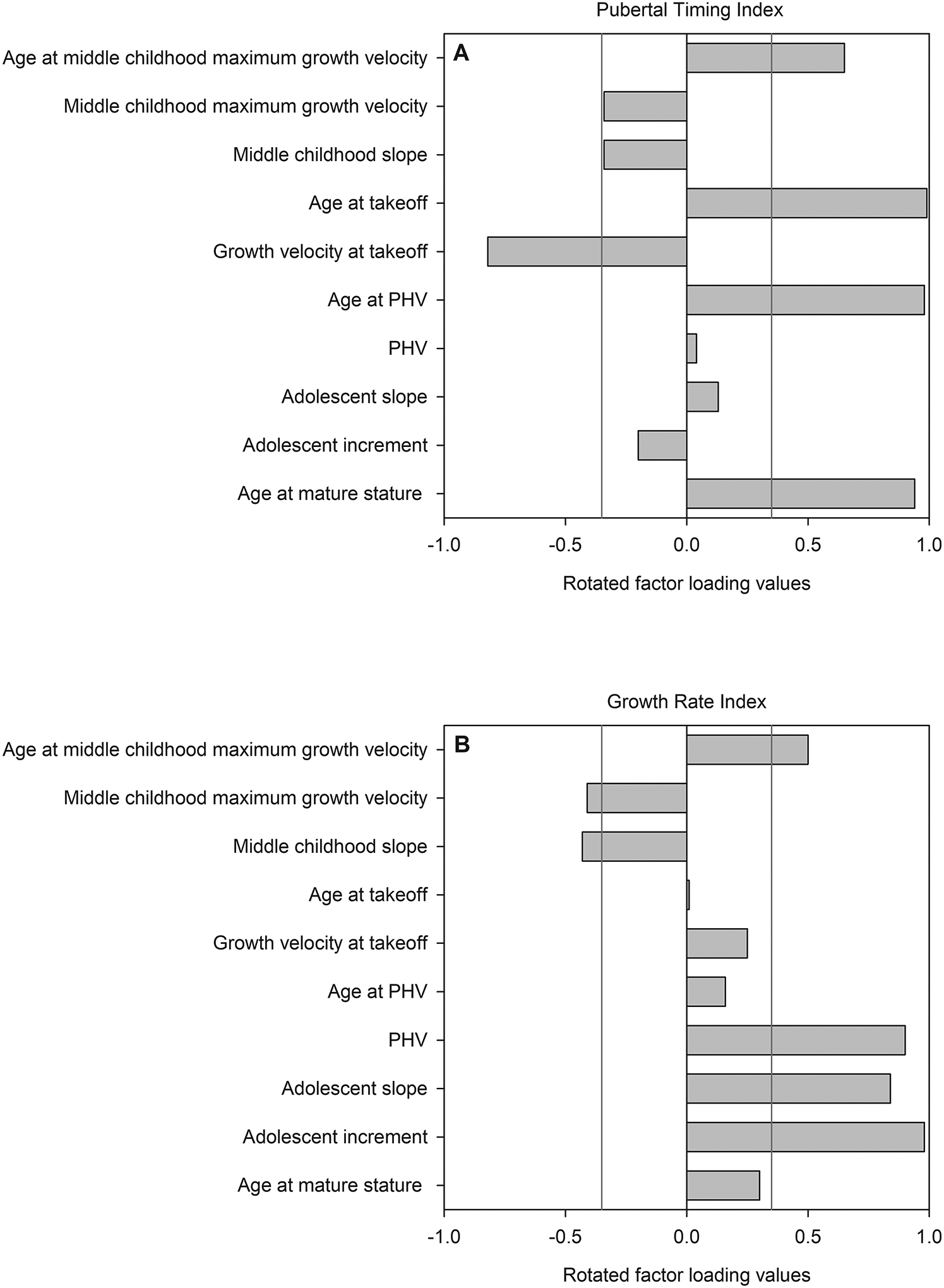
Rotated factor loadings for each auxological variable on each retained component (A: PC1/Pubertal Timing Index; B: PC2/Growth Rate Index). Vertical lines represent loading values of 0.35 and −0.35, which we set as the thresholds for meaningful loadings. Variables with bars extending past either threshold for a given index load meaningfully on that index. Other variables with bars not extending past the thresholds are still used in calculating each index and thus have a minor influence on index scores, but this influence is not typically considered to be meaningful.
Meaningful factor loadings (loading value ≥|0.35|) and their directions on PC1 included age at middle childhood maximum growth velocity (+0.65), growth velocity at takeoff (−0.82), age at takeoff (+0.99), age at PHV (+0.98), and age at mature stature (+0.94). Ages at key growth events all had strong positive effects on PC1, such that later ages were associated with higher, more positive values of the index, and earlier ages were associated with more negative values of the index. There was an inverse relationship between growth timing and growth velocity at takeoff, such that higher index values were also related to slower velocities at the onset of the pubertal growth spurt.
Qualitatively interpreting these factor loadings as a collective whole, we named PC1 the Pubertal Timing Index. Higher values of this index indicated later ages at the onset of pubertal growth (age at takeoff), its peak (age at PHV), and its end (age at mature stature), with faster childhood growth, but a slower initial rate of growth at the onset of pubertal growth. In other words, higher positive values of this index were associated with later developers who may have grown faster during middle childhood, but who started pubertal growth slowly. On the other hand, lower and negative values were associated with earlier developers who grew more slowly during childhood, but began to grow rapidly at the onset of the pubertal growth spurt. The association between slower childhood growth and an earlier, faster onset of pubertal growth, has been previously demonstrated, but generally in the context of adolescent catch-up growth in response to chronic childhood undernutrition (Campisi et al., 2018), which is unlikely to apply to this study sample. Still, the observed associations between these variables are of interest. With respect to sex differences in pubertal timing, this index clearly separated males and females into later and earlier developing clusters, respectively (see below), while also delineating an axis of early-to-late development within each sex.
For PC2, meaningful factor loadings (loading value ≥|0.35|) and their directions included middle childhood maximum growth velocity (−0.41), age at middle childhood maximum growth velocity (+0.50), middle childhood slope (−0.43), PHV (+0.90), adolescent slope (+0.84), and the adolescent increment (+0.98). Aside from age at middle childhood maximum growth velocity, all of the remaining meaningful loadings were related to the rate of growth and accumulated increases in stature during defined time periods.
Qualitatively interpreting these factor loadings as a collective whole, we named PC2 the Growth Rate Index. As in PC1, the loading directions for PC2 described an apparent tradeoff between childhood growth vs. pubertal growth. In this case, higher positive values of the index indicated faster rates of pubertal growth and greater adolescent stature gain, but slower childhood growth. In contrast, lower and negative values were associated with more rapid childhood growth, and slower adolescent growth with less adolescent addition of stature. Slower childhood growth was also related to a later age at middle childhood maximum growth velocity. Again, this pattern is consistent with catch-up growth, but not in the context of stunting or chronic undernutrition. Instead, it appears to describe normal variation in terms of the percentage of adult height achieved during childhood vs. during puberty. Higher values of this index identify individuals who gain a relatively larger proportion of their adult stature during puberty, whereas lower index values identify individuals who gain a relatively smaller proportion of their ultimate adult stature during the pubertal growth spurt.
Within the gait sample, mean (±SD) calculated index values in male and female adolescents, respectively, were 0.67±0.90 vs. −1.42±0.66 for the Pubertal Timing Index (P<0.001), and 0.15±1.23 vs. −0.35±0.81 for the Growth Rate Index (P=0.093). Males in the gait sample did not differ significantly from the PCA population for either index (Pubertal Timing Index: P=0.253; Growth Rate Index: P=0.580). Females in the gait sample had a significantly more negative mean Pubertal Timing Index compared to the population (P=0.006), but did not differ for the Growth Rate index (P=0.374). See [FIGURE 3] for index score distributions within these groups.
Fig. 3:
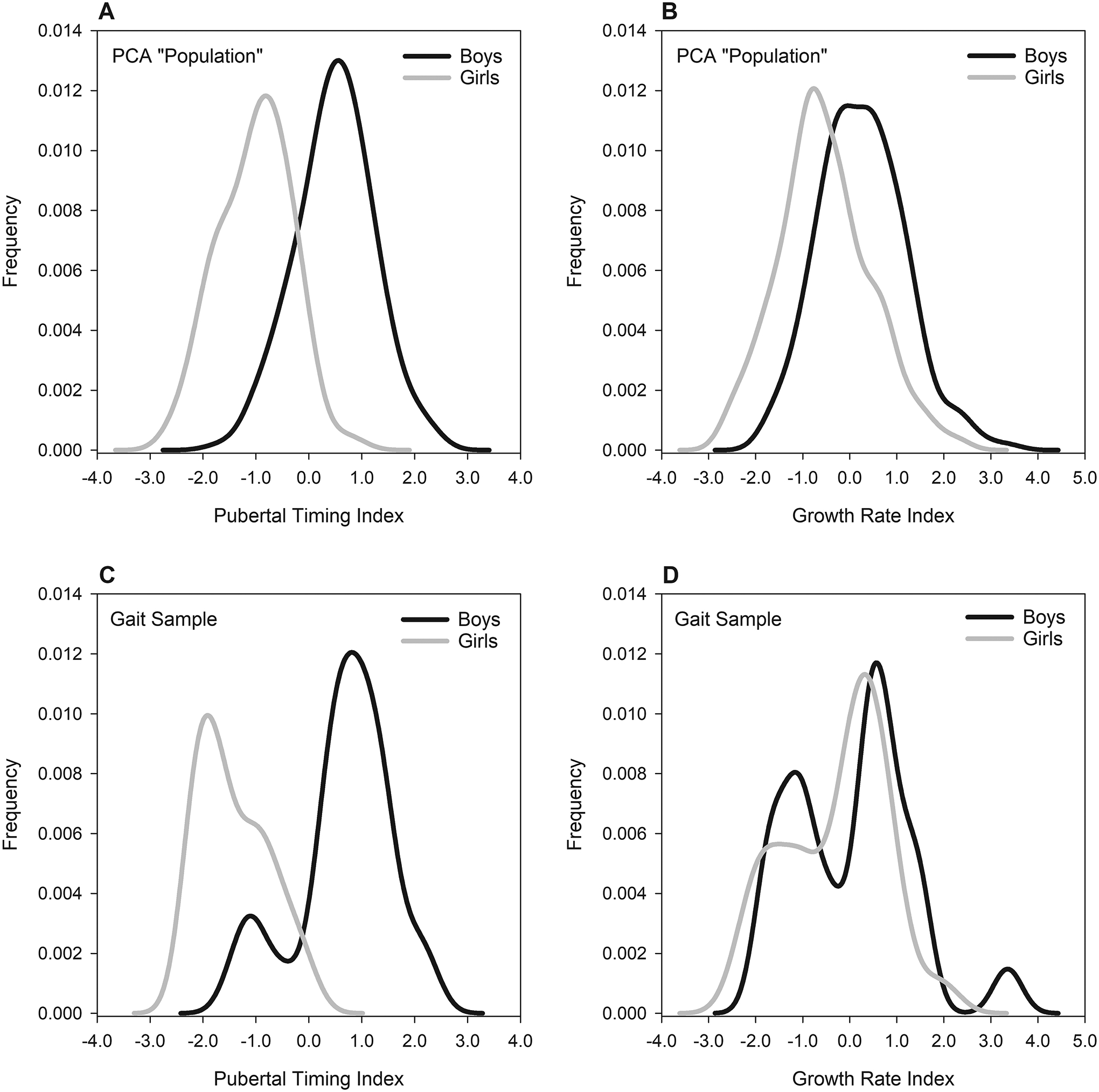
Distributions of principal component scores for each index, within each sex (males: black lines; females: gray lines), and within the two groups of participants: the “population” of n=348 (235 males, 113 females) on which the PCA was run (panels A and B), and the smaller gait sample of n=25 males adolescents and n=27 female adolescents (panels C and D). Within the PCA population, sex-specific means (±SD) in males and females, respectively, were 0.49±0.73 vs. −1.02±0.67 for the Pubertal Timing Index, and 0.26±0.91 vs. −0.53± 0.97 for the Growth Rate Index. See the text for gait sample means and statistical results for between-groups comparisons.
3.2. Gait Sample Anthropometric and Auxological Traits
Complete statistical results for analysis of sex differences in anthropometric traits and auxological variables in the gait sample are presented in [TABLE 1]. Of the variables measured at the gait analysis visit, significant sex differences were found for age, skeletal age, relative skeletal age, stature, percent of mature stature, subischial leg length, thigh segment length, shank segment length, and BMI (for each, P≤0.027). There were no significant sex differences in sitting height, crural index, static knee varus/valgus alignment, bicristal breadth, inter-ASIS breadth, body mass, or the sport physical activity index (for each, P≥0.167).
Correlations between anthropometric variables at the time of the gait visit and each index are presented in [TABLE 2]. The Pubertal Timing Index was significantly negatively correlated with skeletal age, relative skeletal age, percent of mature stature, bicristal breadth, and body mass index (for each, P≤0.040), and significantly positively correlated with subischial leg length, thigh segment length, shank segment length, and, within females, age at menarche (for each, P≤0.037). Collectively, these results indicate that in this sample later developers tended to be less skeletally mature at the gait visit, had narrower pelves, had lower weight for stature, and had longer lower limb segments. Broadly speaking, these observed relationships correspond to average sex differences in the timing of skeletal maturity, pelvic breadth, BMI, and lower limb length, as well as in the Pubertal Timing Index. Within each sex, none of these correlations were statistically significant (for each, P≥0.157), with the exception of relative skeletal age, which was significantly negatively correlated with Pubertal Timing Index scores in both male (r=−0.73, P<0.001) and female (r=−0.39, P=0.042) adolescents. The Growth Rate Index was significantly negatively correlated with age, skeletal age, and body mass index at the gait analysis visit (for each, P≤0.020). These results indicate a weak trend toward faster adolescence growers being younger, less skeletally mature, and lighter for their stature at the gait visit, compared to subjects who grew more slowly during adolescence. None of the other correlations concerning variables at the gait analysis visit were statistically significant (for each, P≥0.063).
Table 2:
Correlations between Growth Indices and Anthropometric Variables at the Gait Visit.
| Variable | Pubertal Timing Index ra | Growth Rate Index ra |
|---|---|---|
| Age (y) | −0.18 | −0.34 * |
| Skeletal age (y) | −0.42 ** | −0.32 * |
| Relative skeletal age (y) | −0.63 ** | −0.03 |
| Stature (cm) | 0.24 | 0.14 |
| Percent of mature stature (%) | −0.48 ** | −0.26 |
| Sitting height (cm) | 0.01 | 0.07 |
| Subischial leg length (cm) | 0.40 ** | 0.17 |
| Thigh segment length (cm) | 0.55 ** | 0.02 |
| Shank segment length (cm) | 0.29 * | 0.14 |
| Crural index | −0.19 | 0.09 |
| Static knee varus/valgus alignment (°) | 0.13 | 0.10 |
| Bicristal breadth (cm) | −0.29 * | −0.08 |
| Inter-ASIS breadth (cm) | −0.22 | −0.14 |
| Body mass (kg) | −0.06 | −0.22 |
| Body mass index (kg ∙ m-2) | −0.30 * | −0.40 ** |
| Sport physical activity index | 0.13 | 0.13 |
| Age at menarche (y)b | 0.77 ** | −0.27 |
Pearson’s r-values for correlations between each growth index and anthropometric variables (sexes pooled).
P<0.05;
P<0.01.
Only females included in analysis.
Upon reaching maturity, stature, sitting height, and subischial leg length all differed significantly between the sexes (for each, P<0.001), and each variable was significantly positively correlated with both growth indices (for each, P≤0.040). Bicristal breadth neither differed between sexes (P=0.180), nor was it correlated with either growth index (for each, P≥0.625). In terms of auxological variables, age at mature stature, age at middle childhood maximum growth velocity, age at takeoff, age at PHV, growth velocity at takeoff, and PHV all differed significantly between the sexes (for each, P≤0.043). No significant sex differences were found for middle childhood maximum growth velocity, middle childhood slope, adolescent slope, or the adolescent increment (for each, P≥0.105).
3.3. Relationships between Growth Indices, Knee Mechanics, and Sex
Sex-specific mean values for knee kinematic and kinetic variables are presented in [TABLE 3] along with P-values for the effect of sex. The effect of the interaction between sex, the Pubertal Timing Index, and the Growth Rate Index was significant for flexion/extension moment at foot-strike (P=0.027) and peak stance phase extension moment (P=0.045) [FIGURE 4], but not for any other variable (for each, P≥0.173). None of the interaction effects between the Pubertal Timing Index and the Growth Rate Index were statistically significant (for each, P≥0.240), nor were any interaction effects between sex and the Growth Rate Index (for each, P≥0.238). The effect of the interaction between sex and the Pubertal Timing Index was significant for peak stance phase valgus angle (P=0.030), and approached significance for peak stance phase varus angle (P=0.055) [FIGURE 5]. None of the other effects of the sex*Pubertal Timing Index interaction were significant (for each, P=0.205). The main effect of the Pubertal Timing Index was significant for varus/valgus moment at foot-strike (P=0.048), and for peak flexion angle during support (P=0.024) [FIGURE 6]. None of the remaining main effects of the Pubertal Timing Index were statistically significant (for each, P≥0.078). The main effect of the Growth Rate Index was significant for internal/external rotation moment at foot-strike (P=0.030) [FIGURE 6], but not for any other outcome (for each, P≥0.229).
Table 3.
Gait Kinematic and Kinetic Variables
| Variable | Male adolescents (N = 25) | Female adolescents (N = 27) | P-value a |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Forward velocity (m/s) | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.497 |
| Step width (cm) | 11.7 ± 1.8 | 9.5 ± 2.0 | < 0.001 |
| Base of support | 2.3 ± 0.4 | 3.0 ± 0.6 | < 0.001 |
| Residual base of support | −0.2 ± 0.4 | 0.1 ± 0.6 | 0.033 |
| Flexion/extension angle at foot-strike (°) | 3.8 ± 4.3 | 7.9 ± 4.3 | 0.001 |
| Varus/valgus angle at foot-strike (°) | −1.3 ± 2.3 | −2.9 ± 2.4 | 0.020 |
| IR/ER angle at foot-strike (°) | −5.5 ± 8.5 | −1.1 ± 13.2 | 0.167 |
| Flexion/extension moment at foot-strike (Nm∙kg-1) | −0.252 ± 0.083 | −0.236 ± 0.107 | 0.578 |
| Varus/valgus moment at foot-strike (Nm∙kg-1) | 0.050 ± 0.062 | 0.004 ± 0.159 | 0.202 |
| IR/ER moment at foot-strike (Nm∙kg-1) | −0.004 ± 0.007 | −0.001 ± 0.019 | 0.885 |
| Peak stance phase flexion angle (°) | 29.3 ± 5.5 | 27.2 ± 6.0 | 0.201 |
| Peak stance phase extension angle (°) | 2.7 ± 3.7 | 2.3 ± 5.9 | 0.747 |
| Peak stance phase varus angle (°) | 2.2 ± 2.3 | 0.9 ± 2.7 | 0.082 |
| Peak stance phase valgus angle (°) | −3.0 ± 1.9 | −4.6 ± 3.1 | 0.033 |
| Peak stance phase IR angle (°) | −0.3 ± 7.5 | 3.2 ± 11.4 | 0.200 |
| Peak stance phase ER angle (°) | −15.8 ± 8.2 | −16.6 ± 8.6 | 0.732 |
| Peak stance phase flexion moment (Nm∙kg-1) | 0.575 ± 0.258 | 0.574 ± 0.218 | 0.992 |
| Peak stance phase extension moment (Nm∙kg-1) | −0.388 ± 0.171 | −0.471 ± 0.229 | 0.134 |
| Peak stance phase varus moment (Nm∙kg-1) | 0.511 ± 0.204 | 0.762 ± 0.248 | <0.001 |
| Peak stance phase valgus moment (Nm∙kg-1) | −0.095 ± 0.122 | −0.474 ± 0.214 | < 0.001 |
| Peak stance phase IR moment (Nm∙kg-1) | 0.028 ± 0.025 | 0.059 ± 0.050 | 0.005 |
| Peak stance phase ER moment (Nm∙kg-1) | −0.173 ± 0.059 | −0.189 ± 0.063 | 0.294 |
| Peak vGRF (body weight) | 1.15 ± 0.1 | 1.14 ± 0.05 | 0.885 |
P-values for sex differences reflect sex main effects from linear mixed models analysis.
Fig. 4:
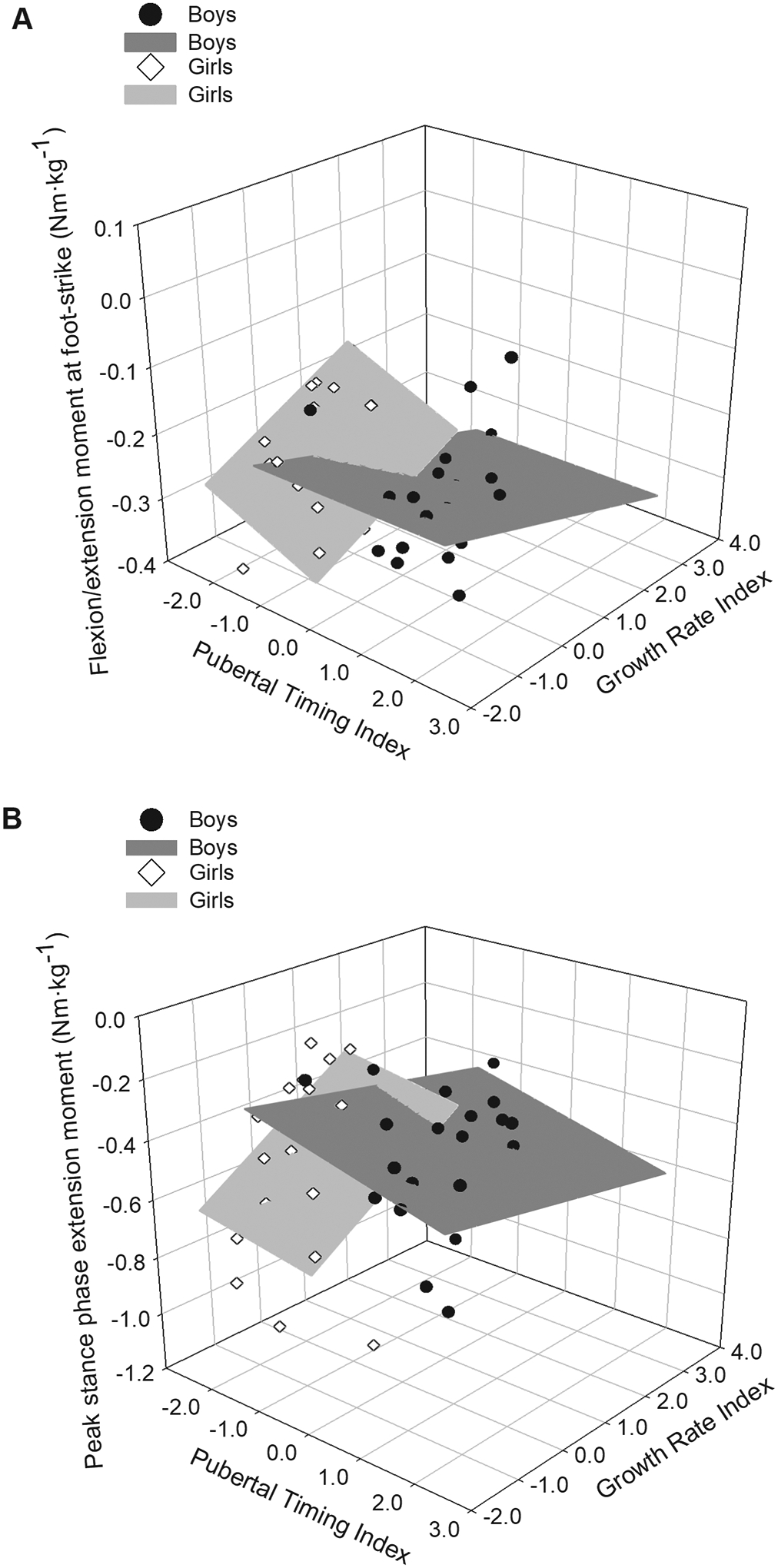
Three-dimensional plot of sagittal plane moments in males (dark gray planes and black circles) and females (light gray planes and white diamonds) relative to the Pubertal Timing and Growth Rate indices. The three-way sex*Pubertal Timing Index*Growth Rate Index was statistically significant for the displayed outcome variables (for each, P≤0.045). A: Flexion/extension moment at foot-strike. B: Peak stance phase extension moment. See text for additional statistical results and descriptions of the relationships between sagittal plane moments, sex, and pubertal growth.
Fig. 5:
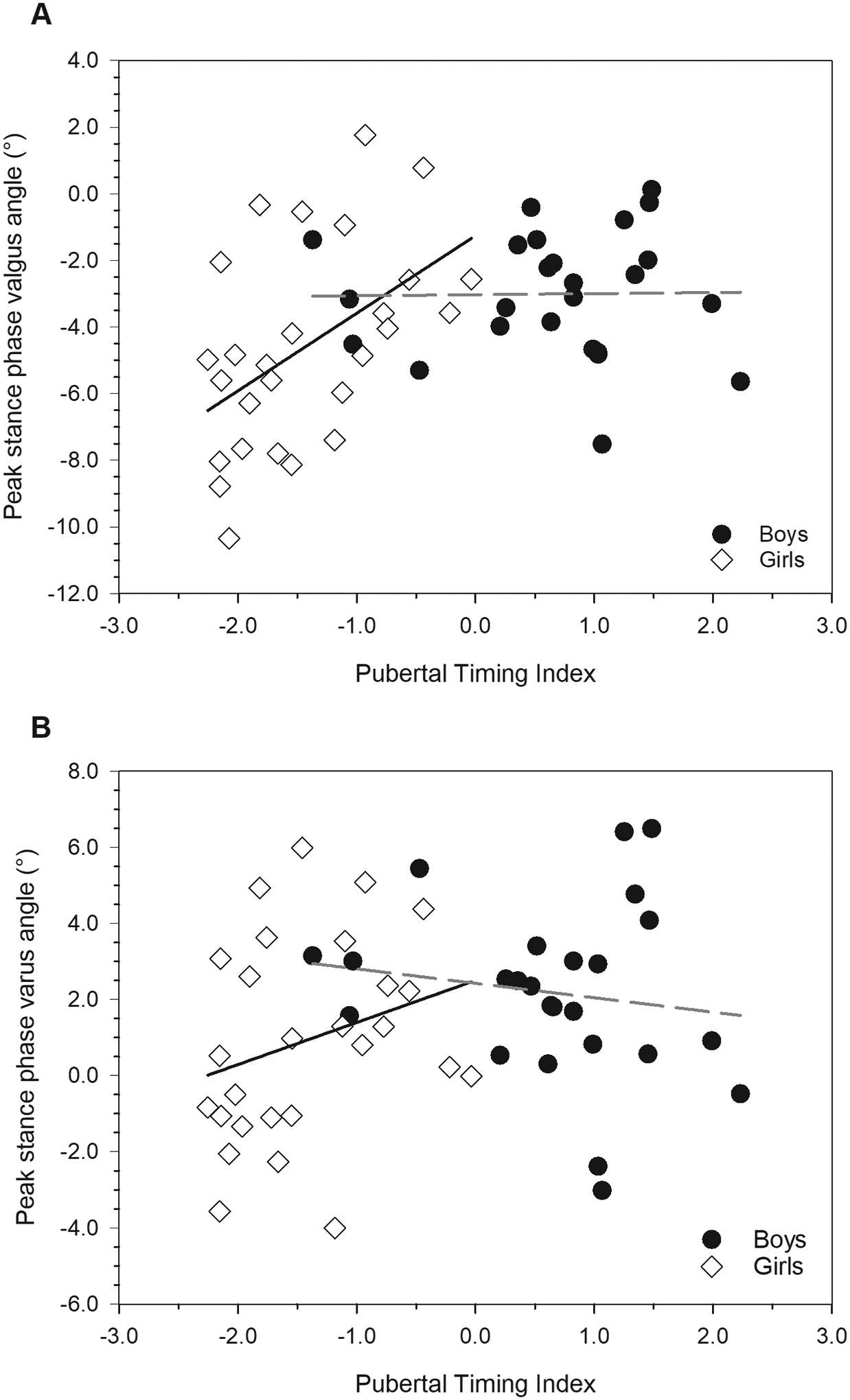
Relationships between frontal plane angles and the Pubertal Timing Index in males (black circles) and females (white diamonds). The sex*Pubertal Timing Index was statistically significant for peak stance phase valgus angle (P=0.030, panel A), and approached significance for peak stance phase varus angle (P=0.055, panel B). See text for additional statistical results and descriptions of the relationships between frontal plane angles, sex, and pubertal timing.
Fig. 6:
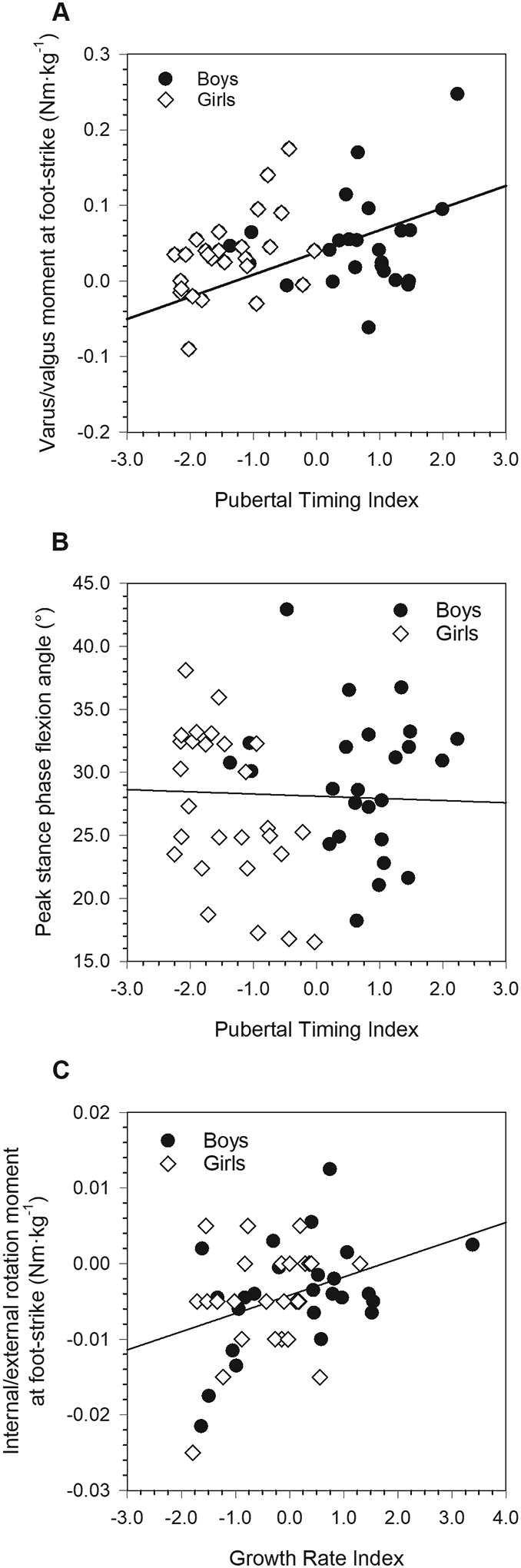
Relationships between pubertal growth indices and mechanical variables where one of the pubertal index main effects was statistically significant (for each, P≤0.048; males: black circles; females: white diamonds). A: Varus/valgus moment at foot-strike. B: Peak stance phase flexion angle. C: Internal/external rotation moment at foot-strike. See text for additional statistical results and descriptions of the relationships between these outcomes and the relevant pubertal indices.
3.4. Subanalysis Comparing Excluded Injured Participants to Healthy Participants
A total of 10 participants (6 male adolescents, 4 female adolescents) were excluded from the walking gait sample for a knee injury in their health history (6 ACL ruptures, 1 PFPS, 1 OSD, 2 meniscal damage). Values of the Pubertal Timing and Growth Rate indices were calculated for these individuals, and they were plotted against sex-specific 95% confidence ellipses for the healthy walking gait sample [see FIGURE 7]. For each sex, all but one member fell within their respective 95% confidence ellipses. The two that did not both sustained ACL injuries. Generally speaking, the excluded males clustered tightly in the upper right corner of their ellipse, indicating relatively high values of the Growth Rate Index and generally average-to-higher-than-average values of the Pubertal Timing Index. This positioning indicates a tendency among the injured males toward faster pubertal growth and later ages at key growth events. Females were less tightly clustered, but 3 of the 4 had below-average Growth Rate Index values. Similarly, 3 of the 4 females had average-to-below-average Pubertal Timing Index values. Collectively, then, females excluded for injury or overuse conditions tended to have slower pubertal growth and experienced key pubertal events at younger ages.
Fig. 7:
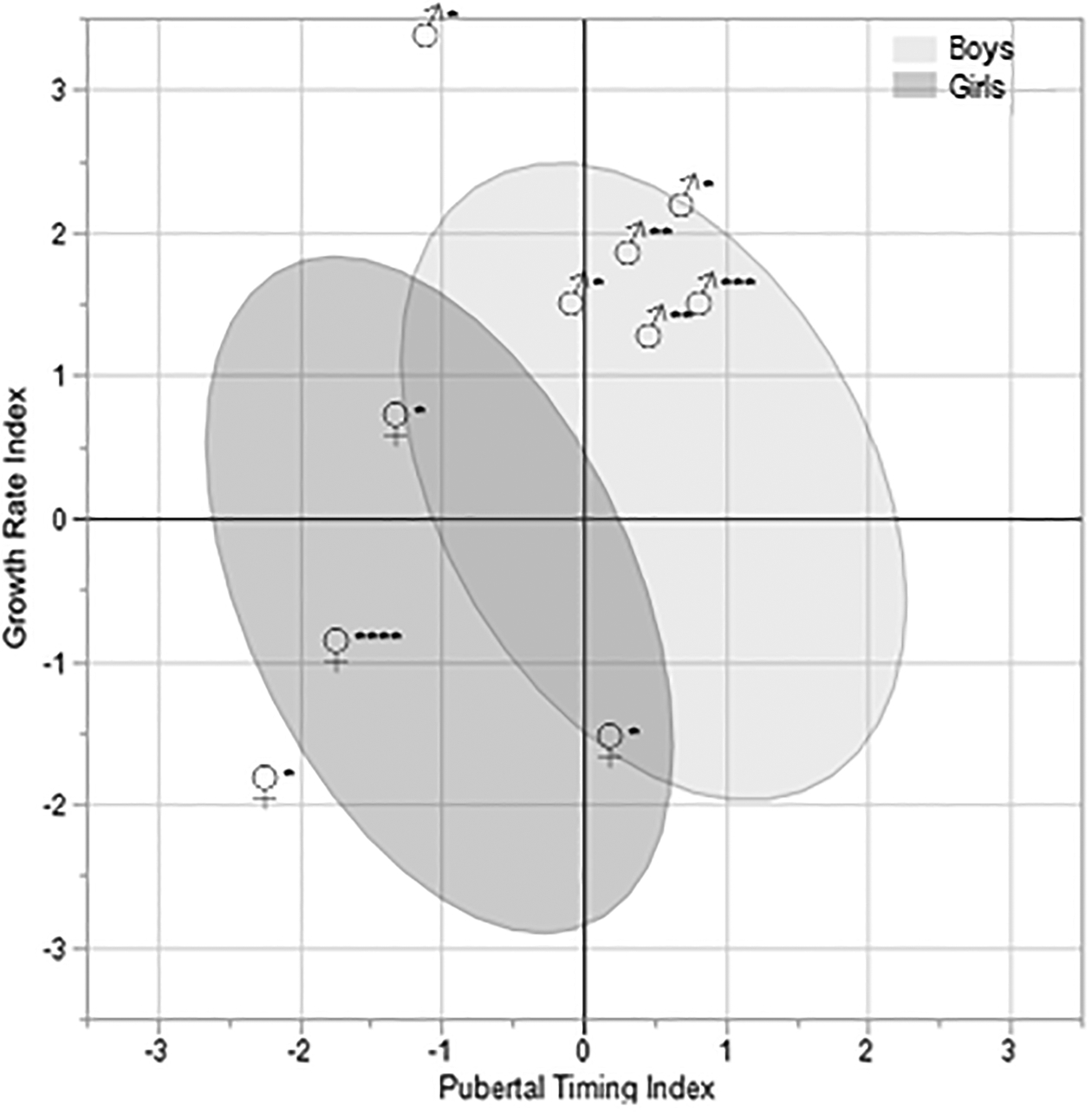
Male adolescents (♂) and female adolescents (♀) excluded for injuries or overuse conditions, plotted against sex-specific 95% confidence ellipses (males: light gray; females: dark gray) for the pubertal growth indices in uninjured, healthy participants. Asterisks next to symbols indicate the type of injury or condition: *ACL, **meniscal damage, ***OSD, ****PFPS.
4. DISCUSSION
The results of the present study contribute to a more complete understanding of the manner in which variation in pubertal growth affects knee mechanics, within and between sexes, with implications for risk for knee-related injury and overuse condition risk. The study generated two indices of growth during childhood and adolescence, and analyzed their relationships with key knee kinematic and kinetic parameters during normal walking. Broadly, the results show significant effects of variation in pubertal growth, both in relation to and independent of sex, on knee mechanics during walking in the sagittal, frontal, and transverse planes.
Variation between males and females is expected for both gait and pubertal timing. In the present study, sex differences were abundant across all categories of variables, including in the Pubertal Growth Index. The strong influence of ages at important growth events on this index suggest that it largely describes the effects of sex on growth, which is consistent with the large proportion of variance it accounts for. In contrast, overlap between the sexes for the Growth Rate Index was much greater, aligning with the established concept that although sex has a major influence on growth timing, variation in the pace of growth is less sex-dependent (Malina et al., 2004). Comparisons between the sexes for individual auxological variables localized these differences specifically to earlier ages at growth landmarks in females vs. males, with females showing faster growth velocity at takeoff, but slower PHV relative to males. The effects of sex-differences in growth were manifested in anthropometric traits at the gait visit, as well as upon reaching adult stature, where males were significantly taller with longer lower limbs and lower limb segments, despite being slightly younger and less skeletally mature.
Negative correlations between the Pubertal Timing Index and indicators of skeletal maturity (skeletal age, relative skeletal age, and percent of mature stature) at the gait visit were also expected, since later developers should be relatively less skeletally mature than earlier developers when measured cross-sectionally in adolescence. Similarly, higher values of the Growth Rate Index, which indicate slower growth during childhood, were related to less skeletal maturity at the gait visit as expected. The Pubertal Timing Index’s negative correlations with bicristal breadth and BMI, coupled with its positive correlations with overall lower limb length and segmental lengths, together suggest that earlier developers tend to be shorter and stockier, whereas later developers tend to be taller and more slender. Lower BMI was also related to slower childhood growth and greater/faster adolescent growth, as indicated by higher values of the Growth Rate Index. These findings are consistent with previous research in both sexes (Froehle et al., 2017; He & Karlberg, 2001; Widén et al., 2012).
Sex differences in gait mechanics largely corresponded to differences observed under higher-intensity task conditions in previous research. Despite walking at similar self-selected speeds, female adolescents maintained a narrower base of support, exhibited a more flexed and more valgus knee at foot-strike, and, during stance phase, had greater peak frontal plane angles and moments, as well as greater peak internal rotation moments than males. The manner in which mechanical variables were related to growth indices in females reveals a mixed set of growth-related injury risk profiles depending on the plane analyzed. In the sagittal plane, higher values of the Pubertal Timing Index and lower values of the Growth Rate Index were related to a tendency for a greater extension moment at foot-strike, as well as a higher peak extension moment during stance. Higher values of the Pubertal Timing Index were also related to lower peak flexion during stance. Thus, later maturing females who experienced slower pubertal growth and faster childhood growth tended to have higher extension moments throughout ground contact. Higher extension moments in movement tasks more strenuous than walking have been shown to be predictive of knee injury (Myer et al., 2011). On the other hand, in the frontal plane in females, lower values of the Pubertal Timing Index were associated with a greater peak valgus angle and maintenance of a valgus knee throughout stance, as well as a tendency toward a greater valgus moment at foot-strike. In other words, earlier developing females exhibited frontal plane mechanics that are characteristic of elevated risk for knee injuries. This pattern is consistent with our previous findings on relationships between age at menarche and knee mechanics during walking (Froehle et al., 2017), as well as our finding that female collegiate athletes with ACL injuries tended to have earlier ages at menarche than their uninjured peers (Froehle et al., 2020). A higher internal rotation moment at foot-strike, also associated with injury under conditions of higher loading (Pappas et al., 2015; Shultz et al., 2012), was related to higher values of the Growth Rate Index, and thus faster adolescent growth/slower childhood growth.
In male adolescents, while there was little variation in extension moment at foot-strike related to the Pubertal Timing Index, this moment was negatively related to the Growth Rate Index, such that faster adolescent growth was associated with a higher extension moment at foot-strike. Peak stance phase extension moment was also negatively related to the Growth Rate Index, as well as to the Pubertal Timing Index, such that later overall developers and faster adolescent growth were associated with higher peak extension moments. Thus, later developing males who experienced faster adolescent growth exhibited riskier sagittal plane mechanics. This finding is consistent with our previous research (Steffensmeier et al., 2020). In the frontal plane, the Pubertal Timing Index did not covary with males’ peak valgus and varus angles during stance, but was positively related to the frontal plane moment at foot-strike, such that earlier developers tended to have a slightly less varus foot-strike moment than later developers. Similar to females, males with a higher Growth Rate Index and thus faster adolescent growth also tended to exhibit a higher internal rotation moment at foot-strike.
Taken together, the results suggest that there are significant impacts of variation in the timing and pace of pubertal growth on knee mechanics in adolescence, in both sexes, with potential implications for injury risk. In female adolescents, later developers with slower pubertal growth maintained less flexed, stiffer knees in the sagittal plane, whereas earlier developers with faster pubertal growth tended to exhibit greater valgus angulation and loading in the frontal plane, along with higher internal rotation moments. Given that frontal plane mechanisms are most often implicated in female knee injuries, it may be that earlier developers are at greater risk, but the effect of later development on sagittal plane factors should not be ignored. In males, later developers, and especially those that experienced faster adolescent growth, tended to maintain stiffer knees with greater extension loading, as well as higher internal rotation moments, all of which are associated with increased injury risk in males. The subanalysis of participants excluded for knee injuries further illustrates the relationship between pubertal growth and mechanical risk for injury [see FIGURE 6].
Although our results generally support our hypotheses and demonstrate significant relationships between variation in pubertal growth and knee mechanics, there are limitations that should be taken into consideration when interpreting the results. First, due to the demographics and small geographical range of Fels participants, the generalizability of these results to other populations is unknown. Second, female adolescents in the gait sample tended to develop earlier on average than the wider sample from which the pubertal growth indices were derived. Thus, the data on females may skew towards earlier development, potentially further limiting generalizability. However, even if the female gait sample is biased toward earlier average development, it still includes the full range of developmental variation seen in the wider pubertal growth sample, and thus likely achieves a reasonable approximation of population-level variability. Another limitation is the absence of serial walking gait data. Although multiple gait visits were present for some individuals, most had only one, such that analysis of the effects of pubertal variation on changes in gait mechanics was not possible. Instead, the present study was limited to examining the effects of pubertal growth on a single instance of walking gait at or near the age of reaching adult stature. The results thus do not address intraindividual pubertal changes in neuromuscular control or gait mechanics per se, and cannot speak to additional postpubertal changes that may affect subsequent injury risk in later adolescence and young adulthood. A related limitation is that subjects were not all sampled at the same skeletal age, meaning that gait data were collected on different individuals at slightly different levels of skeletal maturity. More specifically, later developers tended to be less skeletally mature at their gait visits than earlier developers. It is unknown what effect this may have had on mechanical outcomes, but none of the kinematic or kinetic variables was significantly correlated with skeletal age in this sample (for each, P≥0.132). Nevertheless, it is important for future work in this area to try to control for this factor.
It is unclear if mechanical patterns observed during walking carry over into higher-intensity activities with higher levels of joint loading, and thus elevated injury risk. There is some indication that underlying individual mechanical habits are expressed similarly in different tasks (see Barrios et al., 2016). Furthermore, the concept that mechanical patterns from one task (i.e., training tasks) carry over to other tasks (i.e., a suite of sport-specific in-game movements) is foundational to neuromuscular retraining interventions, which can be effective in reducing injury risk (Noyes & Barber-Westin, 2018). Our data suggest that even if the loading thresholds for injury are not surpassed during walking, the underlying kinematic risk factors may be present, creating a situation ripe for injury when higher intensity tasks are undertaken. Bates and colleagues (Bates et al., 2020) identified kinematic thresholds for ACL injury using valgus angle at initial contact and peak valgus angle during contact during a drop vertical jump task, along with injury surveillance data. At initial contact and at peak valgus during contact, their injury threshold values are only 1.7° more valgus than the means at foot-strike and during stance in our female participants. In earlier developing females (below sex-specific-average Pubertal Timing Index values), frontal plane kinematics during walking came even closer to these thresholds (within 0.8° at foot-strike, and within 0.3° at peak valgus during stance). If during more rapid, forceful movement, control over frontal plane kinematics is reduced, then it appears that earlier developing females could easily cross these thresholds while also experiencing higher knee loading, thus creating injury-prone conditions. If so, then earlier developmental timing in females may indicate elevated risk (with the same reasoning applying to later and faster developing males).
5. CONCLUSIONS
This study identified relationships between variation in the timing and pace of pubertal growth and walking gait mechanics during adolescence. Although key sex differences exist, the study’s findings also point to variation within sexes that potentially results in variation in mechanical risk for injury. Furthermore, the results suggest that variation in pubertal growth affects male and female adolescents differently, such that earlier developing females appear to be at greater risk for frontal plane injury factors, whereas later developing males who experience faster adolescent growth appear to be at elevated risk for sagittal plane injury mechanisms.
ACKNOWLEDGEMENTS:
The authors acknowledge study participants, who gave their time and energy to this project, and the staff at the Lifespan Health Research Center (Wright State University) who assisted with data collection, entry, and curation for this project.
FUNDING:
This work was supported by grants from the Wright State University Boonshoft School of Medicine (gait data collection and analysis), and the National Institutes of Health (NIH R01HD012252; anthropometry and questionnaires). Funding sources had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of this manuscript; or in the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST: None of the authors has any conflicts of interest to report.
DATA AVAILABILITY:
Research data for this study are not available for sharing.
REFERENCES
- Alderson LM, Joksaite SX, Kemp J, Main E, Watson T, Platt FM, & Cortina-Borja M (2019). Age-related gait standards for healthy children and young people: The GOS-ICH paediatric gait centiles. Archives of Disease in Childhood, 104(8), 755–760. 10.1136/archdischild-2018-316311 [DOI] [PubMed] [Google Scholar]
- Andrish J (2001). Anterior cruciate ligament injuries in the skeletally immature patient. American Journal of Orthopedics (Belle Mead, N.J.), 30(2), 103–110. https://europepmc.org/article/med/11234936 [PubMed] [Google Scholar]
- Barrios JA, Heitkamp CA, Smith BP, Sturgeon MM, Suckow DW, & Sutton CR (2016). Three-dimensional hip and knee kinematics during walking, running, and single-limb drop landing in females with and without genu valgum. Clinical Biomechanics, 31, 7–11. 10.1016/J.CLINBIOMECH.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Bates NA, Myer GD, Hale RF, Schilaty ND, & Hewett TE (2020). Prospective Frontal Plane Angles Used to Predict ACL Strain and Identify Those at High Risk for Sports-Related ACL Injury. Orthopaedic Journal of Sports Medicine, 8(10), 1–10. 10.1177/2325967120957646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck NA, Lawrence JTR, Nordin JD, DeFor TA, & Tompkins M (2017). ACL Tears in School-Aged Children and Adolescents Over 20 Years. Pediatrics, 139(3). 10.1542/PEDS.2016-1877 [DOI] [PubMed] [Google Scholar]
- Bock RD, du Toit SHC, & Thomas CDL (2003). AUXAL: Auxological analysis of longitudinal measurements of human stature, Version 3. Scientific Software International. [Google Scholar]
- Campbell CJ, Carson JD, Diaconescu ED, Celebrini R, Rizzardo MR, Godbout V, Fletcher JA, McCormack R, Outerbridge R, Taylor T, Constantini N, & Cote M (2014). Canadian academy of sport and exercise medicine position statement: Neuromuscular training programs can decrease anterior cruciate ligament injuries in youth soccer players. Clinical Journal of Sport Medicine, 24(3), 263–267. 10.1097/JSM.0000000000000068 [DOI] [PubMed] [Google Scholar]
- Campisi SC, Carducci B, Söder O, & Bhutta ZA (2018). The Intricate Relationship between Chronic Undernutrition, Impaired Linear Growth and Delayed Puberty : Is ‘ catch-up ‘ growth possible during adolescence ? Office of Research - Innocenti Working Paper, WP-2018–12(July). [Google Scholar]
- Chia L, Myer GD, Hewett TE, McKay MJ, Sullivan J, Ford KR, & Pappas E (2021). When puberty strikes: Longitudinal changes in cutting kinematics in 172 high-school female athletes. Journal of Science and Medicine in Sport. 10.1016/J.JSAMS.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumela WC, Roche AF, & Thissen D (1989). The FELS method of assessing the skeletal maturity of the hand‐wrist. American Journal of Human Biology, 1(2). 10.1002/ajhb.1310010206 [DOI] [PubMed] [Google Scholar]
- Circi E, Atalay Y, & Beyzadeoglu T (2017). Treatment of Osgood-Schlatter disease: review of the literature. Musculoskeletal Surgery, 101, 195–200. 10.1007/s12306-017-0479-7 [DOI] [PubMed] [Google Scholar]
- Corporaal SHA, Swinnen SP, Duysens J, Bruijn SM, & Swinnen SP (2016). Slow maturation of planning in obstacle avoidance in humans. J Neurophysiol, 115, 404–412. 10.1152/jn.00701.2015.-Complex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright SH, McCormick BW, Postlethwaite BE, Reeves CJ, & Mount MK (2013). A meta-analysis of sex differences in physical ability: Revised estimates and strategies for reducing differences in selection contexts. Journal of Applied Psychology, 98(4), 623. 10.1037/A0033144 [DOI] [PubMed] [Google Scholar]
- Csintalan RP, Inacio MCS, & Funahashi TT (2008). Incidence Rate of Anterior Cruciate Ligament Reconstructions. The Permanente Journal, 12(3), 17. 10.7812/TPP/07-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, & Rose JD (2000). Motor skills of typically developing adolescents: awkwardness or improvement? Physical & Occupational Therapy in Pediatrics, 20(1), 19–42. 10.1080/J006V20N01_03 [DOI] [PubMed] [Google Scholar]
- Demerath EW, Towne B, Chumlea WC, Sun SS, Czerwinski SA, Remsberg KE, & Siervogel RM (2004). Recent decline in age at menarche: The fels longitudinal study. American Journal of Human Biology, 16(4), 453–457. [DOI] [PubMed] [Google Scholar]
- Demorat G, Weinhold P, Blackburn T, Chudik S, & Garrett W (2017). Aggressive Quadriceps Loading Can Induce Noncontact Anterior Cruciate Ligament Injury: 10.1177/0363546503258928, 32(2), 477–483. 10.1177/0363546503258928 [DOI] [PubMed] [Google Scholar]
- Dempster WT (1955). Space requirements of the seated operator: Geometrical, kinematic, and mechanical aspects of the body with special reference to the limbs (WADC Technical Report 55–159). Wright Air Development Center. Wright-Patterson Air Force Base, Dayton, OH. [Google Scholar]
- Dugan SA (2005). Sports-related knee injuries in female athletes - What gives? American Journal of Physical Medicine & Rehabilitation, 84(2), 122–130. [DOI] [PubMed] [Google Scholar]
- Enomoto S, Tsushima A, Oda T, & Kaga M (2019). The characteristics of the muscle‐tendon unit in children affected by Osgood‐Schlatter disease. Translational Sports Medicine, 2(4), 196–202. 10.1002/TSM2.79 [DOI] [Google Scholar]
- Ferber R, Davis IMC, & Williams DS (2003). Gender differences in lower extremity mechanics during running. Clinical Biomechanics, 18(4), 350–357. 10.1016/S0268-0033(03)00025-1 [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, & Hewett TE (2010). Longitudinal Effects of Maturation on Lower Extremity Joint Stiffness in Adolescent Athletes: 10.1177/0363546510367425, 38(9), 1829–1837. 10.1177/0363546510367425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Toms HE, & Hewett TE (2005). Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc, 37(1), 124–129. [PubMed] [Google Scholar]
- Ford KR, Shapiro R, Myer GD, van den Bogert AJ, & Hewett TE (2010). Longitudinal Sex Differences during Landing in Knee Abduction in Young Athletes. Medicine and Science in Sports and Exercise, 42(10), 1923. 10.1249/MSS.0B013E3181DC99B1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss KDB, Thomas S, Khoury JC, Myer GD, & Hewett TE (2018). A School-Based Neuromuscular Training Program and Sport-Related Injury Incidence: A Prospective Randomized Controlled Clinical Trial. Journal of Athletic Training, 53(1), 20–28. 10.4085/1062-6050-173-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, & Horlick M (2005). Relation of BMI to fat and fat-free mass among children and adolescents. International Journal of Obesity, 29(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Froehle A, Cox J, May J, Grannis K, & Duren D (2020). Effect of Age at Menarche on Anterior Cruciate Ligament Injury Incidence and Anterior Knee Laxity in Collegiate Athletes. Journal of Sports Medicine and Allied Health Sciences: Official Journal of the Ohio Athletic Trainers Association, 6(2), 3. 10.25035/jsmahs.06.02.03 [DOI] [Google Scholar]
- Froehle AW, Grannis KA, Sherwood RJ, & Duren DL (2017). Relationships Between Age at Menarche, Walking Gait Base of Support, and Stance Phase Frontal Plane Knee Biomechanics in Adolescent Girls. PM&R, 9(5), 444–454. 10.1016/J.PMRJ.2016.07.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehle AW, Nahhas RW, Sherwood RJ, & Duren DL (2013). Age-related changes in spatiotemporal characteristics of gait accompany ongoing lower limb linear growth in late childhood and early adolescence. Gait Posture, 38(1), 14–19. https://doi.org/S0966-6362(12)00377-3 [pii]; 10.1016/j.gaitpost.2012.10.005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Woo SL-Y, Loh JC, Tsuda E, Tang P, McMahon PJ, & Debski RE (2003). A quantitative analysis of valgus torque on the ACL: A human cadaveric study. Journal of Orthopaedic Research, 21(6), 1107–1112. 10.1016/S0736-0266(03)00084-6 [DOI] [PubMed] [Google Scholar]
- Gallagher SS, Finison K, Guyer B, & Goodenough S (2011). The incidence of injuries among 87,000 Massachusetts children and adolescents: results of the 1980–81 Statewide Childhood Injury Prevention Program Surveillance System. 10.2105/AJPH.74.12.1340, 74(12), 1340–1347. 10.2105/AJPH.74.12.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist I, Moglo K, Storr M, & Pelland L (2016). Effects of head flexion posture on the multidirectional static force capacity of the neck. Clinical Biomechanics, 37, 44–52. 10.1016/J.CLINBIOMECH.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Harrington ME, Zavatsky AB, Lawson SE, Yuan Z, & Theologis TN (2007). Prediction of the hip joint centre in adults, children, and patients with cerebral palsy based on magnetic resonance imaging. J Biomech, 40(3), 595–602. https://doi.org/S0021-9290(06)00058-3 [pii]; 10.1016/j.jbiomech.2006.02.003 [doi] [DOI] [PubMed] [Google Scholar]
- Hass CJ, Schick EA, Tillman MD, Chow JW, Brunt D, Cauraugh JH, Schick EA, Tillman MD, Chow JW, Brunt D, & Cauraugh JH (2005). Knee Biomechanics during Landings: Comparison of Pre-and Postpubescent Females. Med. Sci. Sports Exerc, 37(1), 100–107. 10.1249/01.MSS.0000150085.07169.73 [DOI] [PubMed] [Google Scholar]
- Hatcher L (1994). A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling. SAS Institute Inc. [Google Scholar]
- He Q, & Karlberg J (2001). BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatric Research, 49(2). 10.1203/00006450-200102000-00019 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, & Ford KR (2004). Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am, 86-A(8), 1601–1608. http://www.ncbi.nlm.nih.gov/pubmed/15292405 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS Jr., Colosimo AJ, Mclean SG, van den Bogert AJ, Paterno M. v, & Succop P (2005). Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am.J Sports Med, 33(4), 492–501. https://doi.org/0363546504269591 [pii]; 10.1177/0363546504269591 [doi] [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Paterno M. v., & Quatman CE (2012). The 2012 ABJS Nicolas Andry Award: The Sequence of Prevention: A Systematic Approach to Prevent Anterior Cruciate Ligament Injury. Clinical Orthopaedics and Related Research® 2012 470:10, 470(10), 2930–2940. 10.1007/S11999-012-2440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Paterno M. v., & Quatman CE (2016). Mechanisms, prediction, and prevention of ACL injuries: Cut risk with three sharpened and validated tools. Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society, 34(11), 1843–1855. 10.1002/JOR.23414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Kiefer AW, & Ford KR (2015). Longitudinal Increases in Knee Abduction Moments in Females during Adolescent Growth. Medicine and Science in Sports and Exercise, 47(12), 2579. 10.1249/MSS.0000000000000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden S, Boreham, • Colin, Delahunt E, & Boreham C (2016). Sex Differences in Landing Biomechanics and Postural Stability During Adolescence: A Systematic Review with Meta-Analyses. Sports Medicine, 46, 241–253. 10.1007/s40279-015-0416-6 [DOI] [PubMed] [Google Scholar]
- Hunter SK (2009). Sex Differences and Mechanisms of Task-Specific Muscle Fatigue. Exerc Sport Sci Rev, 37(3), 113–122. 10.1097/JES.0b013e3181aa63e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs CA, Uhl TL, Mattacola CG, Shapiro R, & Rayens WS (2007). Hip Abductor Function and Lower Extremity Landing Kinematics: Sex Differences. Journal of Athletic Training, 42(1), 76. [PMC free article] [PubMed] [Google Scholar]
- Kadaba MP, Ramakrishnan HK, & Wootten ME (1990). Measurement of lower extremity kinematics during level walking. J Orthop.Res, 8(3), 383–392. 10.1002/jor.1100080310 [doi] [DOI] [PubMed] [Google Scholar]
- Kanamori A, Woo SLY, Ma CB, Zeminski J, Rudy TW, Li G, & Livesay GA (2000). The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 16(6), 633–639. 10.1053/JARS.2000.7682 [DOI] [PubMed] [Google Scholar]
- Khattree R, & Naik DN (2000). Multivariate Data Reduction and Discrimination with SAS® Software. SAS Institute. [Google Scholar]
- Kiapour AM, Kiapour A, Goel VK, Quatman CE, Wordeman SC, Hewett TE, & Demetropoulos CK (2015). Uni-directional coupling between tibiofemoral frontal and axial plane rotation supports valgus collapse mechanism of ACL injury. Journal of Biomechanics, 48(10), 1745–1751. 10.1016/J.JBIOMECH.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, & Lim BO (2014). Effects of menarcheal age on the anterior cruciate ligament injury risk factors during single-legged drop landing in female artistic elite gymnasts. Arch Orthop Trauma Surg, 134(11), 1565–1571. 10.1007/s00402-014-2055-z [DOI] [PubMed] [Google Scholar]
- Knowles SB (2010). Is there an injury epidemic in girls’ sports? British Journal of Sports Medicine, 44(1), 38–44. [DOI] [PubMed] [Google Scholar]
- Kraan CM, Tan AHJ, & Cornish KM (2017). The developmental dynamics of gait maturation with a focus on spatiotemporal measures. In Gait and Posture (Vol. 51, pp. 208–217). Elsevier B.V. 10.1016/j.gaitpost.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, & Bahr R (2017). Mechanisms of Anterior Cruciate Ligament Injury in Basketball: Video Analysis of 39 Cases. 10.1177/0363546506293899, 35(3), 359–367. 10.1177/0363546506293899 [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Steffen K, Kristianslund E, Nilstad A, Mok K-M, Myklebust G, Andersen TE, Holme I, Engebretsen L, & Bahr R (2016). The Vertical Drop Jump Is a Poor Screening Test for ACL Injuries in Female Elite Soccer and Handball Players: A Prospective Cohort Study of 710 Athletes. 10.1177/0363546515625048, 44(4), 874–883. 10.1177/0363546515625048 [DOI] [PubMed] [Google Scholar]
- Lam KC, & Valovich McLeod TC (2014). The impact of sex and knee injury history on jump-landing patterns in collegiate athletes: A clinical evaluation. Clinical Journal of Sport Medicine, 24(5), 373–379. 10.1097/JSM.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Lloyd DG, & Buchanan TS (2001). Strategies of muscular support of varus and valgus isometric loads at the human knee. Journal of Biomechanics, 34(10), 1257–1267. 10.1016/S0021-9290(01)00095-1 [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, & Roos EM (2017). The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. 10.1177/0363546507307396, 35(10), 1756–1769. 10.1177/0363546507307396 [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, & Roos H (2004). High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis and Rheumatism, 50(10), 3145–3152. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R, & (Eds.). (1988). Anthropometric Standardization Reference Manual. Human Kinetics Publishers. [Google Scholar]
- Malina RM, Bouchard C, & Bar-Or O (2004). Growth, Maturation, and Physical Activity (2nd ed.). Human Kinetics. [Google Scholar]
- Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GAM, & Slauterbeck JL (1995). Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research, 13(6), 930–935. 10.1002/JOR.1100130618 [DOI] [PubMed] [Google Scholar]
- Martinez JC, Mazerolle SM, Denegar CR, Joseph MF, Pagnotta KD, Trojian TH, & DiStefano LJ (2017). Female adolescent athletes’ attitudes and perspectives on injury prevention programs. Journal of Science and Medicine in Sport, 20(2), 146–151. 10.1016/J.JSAMS.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Mather RC III, Koenig L, Kocher MS, Dall TM, Gallo P, Scott DJ, Bach BR Jr., Group, the M. K., & Spindler KP (2013). Societal and Economic Impact of Anterior Cruciate Ligament Tears. The Journal of Bone and Joint Surgery. American Volume, 95(19), 1751. 10.2106/JBJS.L.01705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Davis AJ, & Reindollar RH (2015). Abnormalities of Female Pubertal Development. Endotext. https://www.ncbi.nlm.nih.gov/books/NBK278950/ [Google Scholar]
- McLean S, Lipfert S, & van den Bogert A (2004). Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Medicine and Science in Sports and Exercise, 36(6), 1008–1016. 10.1249/01.MSS.0000128180.51443.83 [DOI] [PubMed] [Google Scholar]
- Murphy DF, Connolly DAJ, & Beynnon BD (2003). Risk factors for lower extremity injury: a review of the literature. British Journal of Sports Medicine, 37(1), 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Chu DA, Brent JL, & Hewett TE (2008). Trunk and Hip Control Neuromuscular Training for the Prevention of Knee Joint Injury. Clinics in Sports Medicine, 27(3), 425. 10.1016/J.CSM.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Barber Foss KD, Goodman A, Ceasar A, Rauh MJ, Divine JG, & Hewett TE (2010). The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clinical Biomechanics, 25(7), 700–707. 10.1016/J.CLINBIOMECH.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, & Hewett TE (2011). Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. British Journal of Sports Medicine, 45(4), 245–252. 10.1136/BJSM.2009.069351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, di Stasi SL, Foss KDB, Micheli LJ, & Hewett TE (2015). High knee abduction moments are common risk factors for patellofemoral pain (PFP) and anterior cruciate ligament (ACL) injury in girls: Is PFP itself a predictor for subsequent ACL injury? British Journal of Sports Medicine, 49(2), 118–122. 10.1136/BJSPORTS-2013-092536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Sugimoto D, Thomas S, & Hewett TE (2013). The Influence of Age on the Effectiveness of Neuromuscular Training to Reduce Anterior Cruciate Ligament Injury in Female Athletes. American Journal of Sports Medicine, 41(1), 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahhas RW, Sherwood RJ, Chumlea WC, & Duren DL (2013). An update of the statistical methods underlying the FELS method of skeletal maturity assessment. Annals of Human Biology, 40(6). 10.3109/03014460.2013.806591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Moriya E, Maciel C, & Serrão A (2012). Frontal plane biomechanics in males and females with and without patellofemoral pain. Medicine and Science in Sports and Exercise, 44(9), 1747–1755. 10.1249/MSS.0B013E318256903A [DOI] [PubMed] [Google Scholar]
- Natri A, Kannus P, & Järvinen M (1998). Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Medicine and Science in Sports and Exercise, 30(11), 1572–1577. 10.1097/00005768-199811000-00003 [DOI] [PubMed] [Google Scholar]
- Noehren B, Wilson H, Miller C, & Lattermann C (2013). Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci.Sports Exerc, 45(7), 1340–1347. 10.1249/MSS.0b013e318285c6b6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes FR, & Barber-Westin S (Eds.). (2018). ACL Injuries in the Female Athlete: Causes, impacts, and conditioning programs (2nd ed.). Springer. [Google Scholar]
- Palmieri-Smith RM, Mclean SG, Ashton-Miller JA, & Wojtys EM (2009). Association of quadriceps and hamstrings cocontraction patterns with knee joint loading. J Athl.Train, 44(3), 256–263. 10.4085/1062-6050-44.3.256 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri-Smith RM, Wojtys EM, & Ashton-Miller JA (2008). Association between preparatory muscle activation and peak valgus knee angle. Journal of Electromyography and Kinesiology : Official Journal of the International Society of Electrophysiological Kinesiology, 18(6), 973–979. 10.1016/J.JELEKIN.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Pappas E, Nightingale EJ, Simic M, Ford KR, Hewett TE, & Myer GD (2015). Do exercises used in injury prevention programmes modify cutting task biomechanics? A systematic review with meta-analysis. British Journal of Sports Medicine, 49, 673–680. 10.1136/bjsports-2014-093796 [DOI] [PubMed] [Google Scholar]
- Powers CM (2010). The Influence of Abnormal Hip Mechanics on Knee Injury: A Biomechanical Perspective. 10.2519/Jospt.2010.3337, 40(2), 42–51. [DOI] [PubMed] [Google Scholar]
- Quatman CE, Ford KR, Myer GD, & Hewett TE (2006). Maturation Leads to Gender Differences in Landing Force and Vertical Jump Performance: A Longitudinal Study. 10.1177/0363546505281916, 34(5), 806–813. 10.1177/0363546505281916 [DOI] [PubMed] [Google Scholar]
- Quatman CE, Ford KR, Myer GD, Paterno M. v., & Hewett TE (2008). The effects of gender and pubertal status on generalized joint laxity in young athletes. Journal of Science and Medicine in Sport, 11(3), 257–263. 10.1016/J.JSAMS.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatman CE, & Hewett TE (2009). The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? British Journal of Sports Medicine, 43(5), 328–335. 10.1136/BJSM.2009.059139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatman-Yates CC, Myer GD, Ford KR, & Hewett TE (2013). A longitudinal evaluation of maturational effects on lower extremity strength in female adolescent athletes. Pediatr.Phys Ther, 25(3), 271–276. 10.1097/PEP.0b013e31828e1e9d [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JO, Qiu X, Lu X, Duren DL, Liu RW, Dang D, Menendez ME, Hans SD, Weber DR, & Cooperman DR (2017). The Uniform Pattern of Growth and Skeletal Maturation during the Human Adolescent Growth Spurt OPEN. Nature Scientific Reports, 7, 16705. 10.1038/s41598-017-16996-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale GA, Kweon C, Larson RV, & Bompadre V (2014). High Satisfaction Yet Decreased Activity 4 Years After Transphyseal ACL Reconstruction. Clin Orthop.Relat Res, 472(7), 2168–2174. 10.1007/s11999-014-3561-6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seil R, Mouton C, Lion A, Nührenbörger C, Pape D, & Theisen D (2016). There is no such thing like a single ACL injury: Profiles of ACL-injured patients. Orthopaedics & Traumatology: Surgery & Research, 102(1), 105–110. 10.1016/J.OTSR.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Shelbourne K, & Kerr B (2001). The relationship of femoral intercondylar notch width to height, weight, and sex in patients with intact anterior cruciate ligaments. The American Journal of Knee Surgery, 14(2), 92–96. https://europepmc.org/article/med/11401176 [PubMed] [Google Scholar]
- Sherwood RJ, & Duren DL (2013). Growth of a Species, an Association, a Science: 80 Years of Growth and Development Research. American Journal of Physical Anthropology, 150(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SJ, Schmitz RJ, Benjaminse A, Chaudhari AM, Collins M, & Padua DA (2012). ACL Research Retreat VI: An Update on ACL Injury Risk and PreventionMarch 22–24, 2012; Greensboro, NC. Journal of Athletic Training, 47(5), 591–603. 10.4085/1062-6050-47.5.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove M (2009). Warrior Girls: Protecting our daughters against the injury epidemic in women’s sports. Simon & Schuster. [Google Scholar]
- Steffensmeier AM, Lamont SM, Metoyer G, DiPaolo Z, & Froehle AW (2020). Relationship Between Age at Adult Height and Knee Mechanics During a Drop Vertical Jump in Men: 10.1177/2325967120944912, 8(8). 10.1177/2325967120944912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D (1997). The development of mature gait. Gait & Posture, 6(2), 163–170. [Google Scholar]
- Sutherland DH, Olshen R, Cooper L, & Woo SLY (1980). The Development of Mature Gait. Journal of Bone and Joint Surgery-American Volume, 62(3), 336–353. wos:A1980JP63800003 [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2019). Using Multivariate Statistics (7th ed.). Pearson. [Google Scholar]
- Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, & Zumbo BD (2002). A retrospective case-control analysis of 2002 running injuries. British Journal of Sports Medicine, 36(2), 95–101. 10.1136/BJSM.36.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Haas JA, Long JT, Garcia MC, Rauh MJ, Paterno M. v., Brindle RA, Bazett-Jones DM, & Ford KR (2022). The influence of maturation and sex on pelvis and hip kinematics in youth distance runners. Journal of Science and Medicine in Sport, 25(3), 272–278. 10.1016/J.JSAMS.2021.09.193 [DOI] [PubMed] [Google Scholar]
- Towne B, Williams KD, Blangero J, Czerwinski SA, Demerath EW, Nahhas RW, Dyer TD, Cole SA, Lee M, Choh AC, Duren DL, Sherwood RJ, Chumlea WC, & Siervogel RM (2008). Presentation, Heritability, and Genome-Wide Linkage Analysis of the Midchildhood Growth Spurt in Healthy Children from the Fels Longitudinal Study. Human Biology, 80(6), 623. 10.3378/1534-6617-80.6.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuth MS, Hou NQ, Young DR, & Maynard LM (2005). Validity and reliability of the Fels Physical Activity Questionnaire for children. Medicine and Science in Sports and Exercise, 37(3), 488–495. [DOI] [PubMed] [Google Scholar]
- Voskanian N (2013). ACL Injury prevention in female athletes: review of the literature and practical considerations in implementing an ACL prevention program. Current Reviews in Musculoskeletal Medicine 2013 6:2, 6(2), 158–163. 10.1007/S12178-013-9158-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widén E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen AL, Laitinen J, Pouta A, Kaprio J, Järvelin MR, Peltonen L, & Palotie A (2012). Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care, 35(4). 10.2337/dc11-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CY, Munro BJ, & Steele JR (2016). How Young Girls Change Their Landing Technique Throughout the Adolescent Growth Spurt: 10.1177/0363546516629419, 44(5), 1116–1123. 10.1177/0363546516629419 [DOI] [PubMed] [Google Scholar]
- Wild CY, Steele JR, & Munro BJ (2012). Why Do Girls Sustain More Anterior Cruciate Ligament Injuries Than Boys? Sports Medicine 2012 42:9, 42(9), 733–749. 10.1007/BF03262292 [DOI] [PubMed] [Google Scholar]
- Yu B, & Garrett WE (2007). Mechanisms of non-contact ACL injuries. British Journal of Sports Medicine, 41(suppl 1), i47–i51. 10.1136/BJSM.2007.037192 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data for this study are not available for sharing.


