Abstract
This review is a synopsis of the main points from the opening presentation by the authors in the Resolution of Inflammation session at the 8th European Workshop on Lipid Mediators held at the Karolinska Institute, Stockholm, Sweden, June 29th, 2022. Specialized pro-resolving mediators (SPM) promote tissue regeneration, control infections and resolution of inflammation. These include resolvins, protectins, maresins and the newly identified conjugates in tissue regeneration (CTRs). We reported mechanisms of CTRs in activating primordial regeneration pathways in planaria using RNA-sequencing. Also, the 4S,5S-epoxy-resolvin intermediate in the biosynthesis of resolvin D3 and resolvin D4 was prepared by total organic synthesis. Human neutrophils convert this to resolvin D3 and resolvin D4, while human M2 macrophages transformed this labile epoxide intermediate to resolvin D4 and a novel cysteinyl-resolvin that is a potent isomer of RCTR1. The novel cysteinyl-resolvin significantly accelerates tissue regeneration with planaria and inhibits human granuloma formation.
Keywords: human leukocytes, neutrophils, M2 macrophages, wound healing
Introduction
Excessive or uncontrolled inflammation is now widely recognized as a key process underlying the pathological feature for many diseases including cancer, arthritis, metabolic syndrome, chronic pain, periodontal, cardiovascular and neurological diseases, as well as bacterial and viral infections, such as Coronavirus Disease 2019 (COVID-19) [reviewed in 1, 2]. The acute inflammatory response is normally a protective mechanism that should ideally be self-limited. This physiologic response enables repair of injured tissues and the elimination of invading organisms and/or toxic materials, thus leading to complete resolution of the leukocyte infiltrates and clearance of cellular debris and microbes enabling homeostasis [1, 3]. Focusing on fundamental mechanisms in the resolution responses, the Serhan laboratory uncovered several novel families of pro-resolving lipid mediators (LMs) of inflammation from self-resolving inflammatory exudates that demonstrated potent bioactions in key cellular systems known to be involved in the resolution phase of the acute inflammatory response (Figure 1). These new compounds, born in Boston, MA beginning in 2000 [4] and first presented at the Florence meeting on eicosanoids in Italy that same year, are biosynthesized from the essential polyunsaturated fatty acid precursors, e.g. eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and n-3 docosapentaenoic acid (DPA) (Figure 2). Given their potent stereoselective actions with isolated human neutrophils and in vivo in mouse inflammation models promoting resolution, these potent endogenous bioactive molecules were named resolvins (Rv), protectins (PD), their aspirin-triggered (AT) epimers, and maresins (MaR); they together constitute a superfamily coined specialized pro-resolving mediators (SPMs) because of their highly specialized cellular functions in the resolution of inflammation and tissue injury, microbial clearance and reducing pain.
Figure 1. Overall strategy in the SPM elucidation of structures and functions.
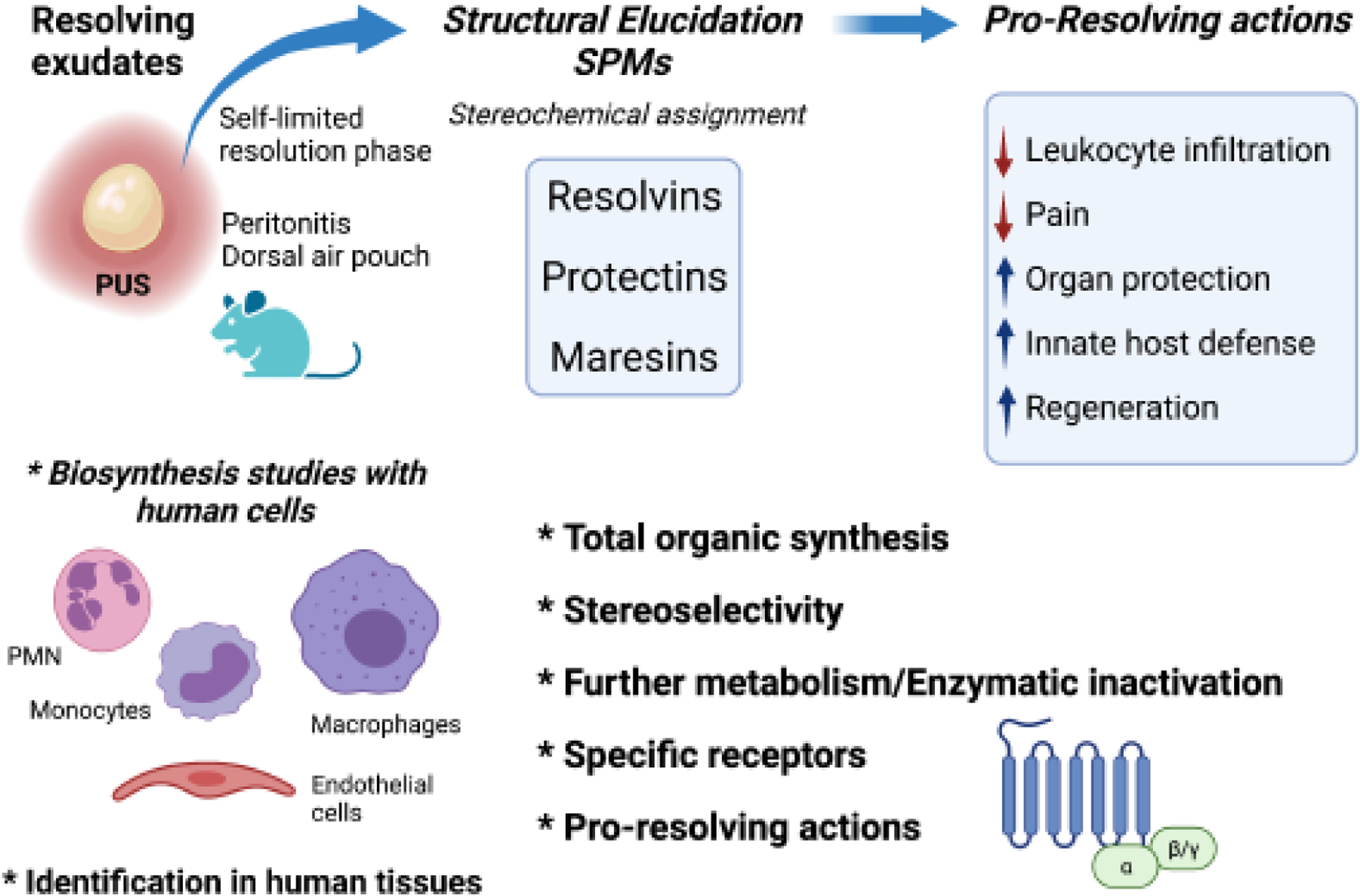
The first SPM, resolvin E1, was identified from resolving exudates in the Serhan lab in Boston, MA nearly 20 years ago [4], and SPM structure elucidation systematically carried out using a systems approach. With our Program Project team, we achieved complete stereochemical assignment of each bioactive SPM. In parallel, biosynthesis of SPMs was established with human cells, and their production in human tissues was documented. Following structural elucidation, total organic synthesis was achieved, stereoselectivity and further metabolism profile for each SPM were determined. Specific SPM cell surface receptors were identified, and SPM-receptor mediated potent pro-resolving actions were confirmed, such as reducing leukocyte infiltration and pain, promoting organ protection.
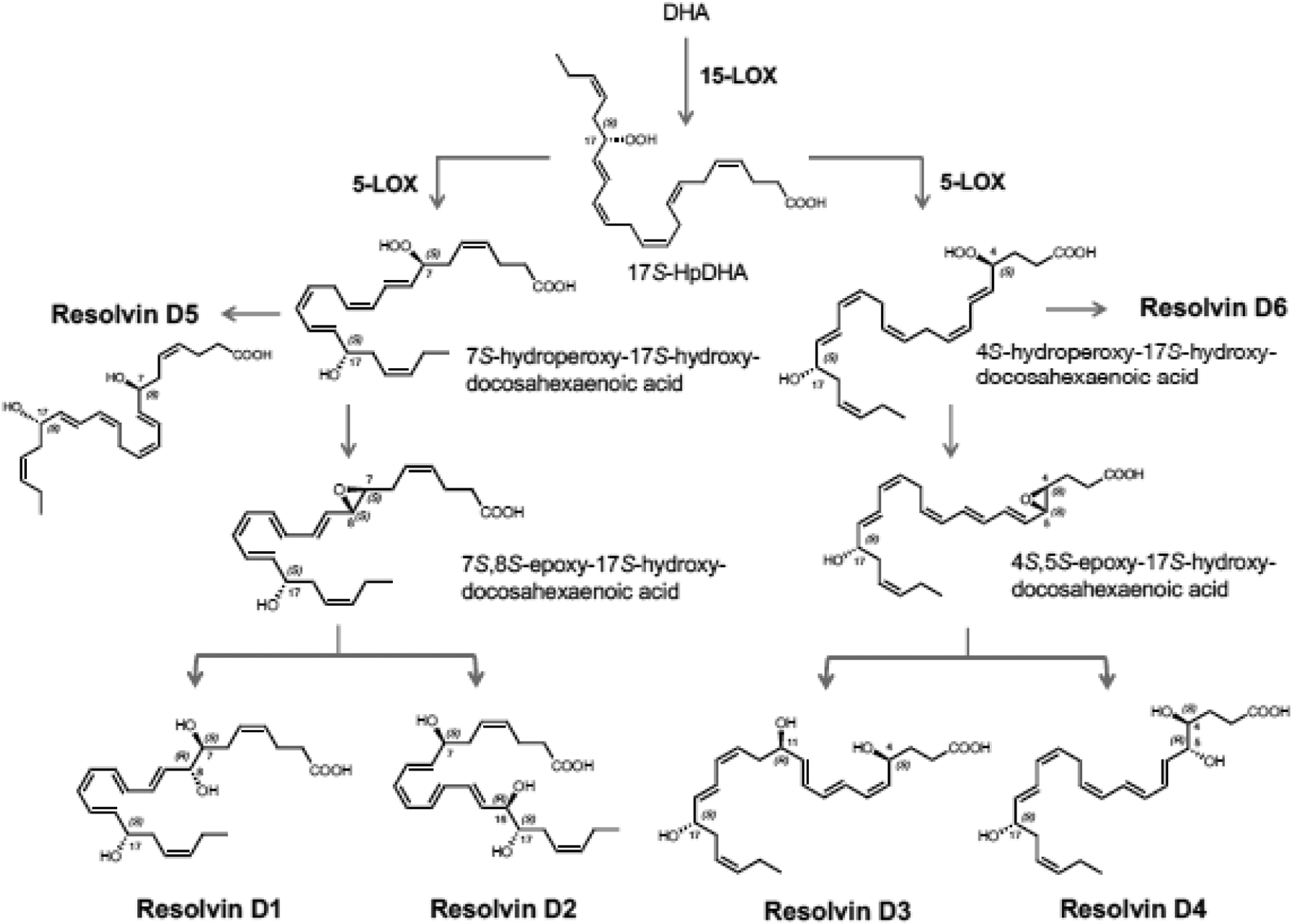
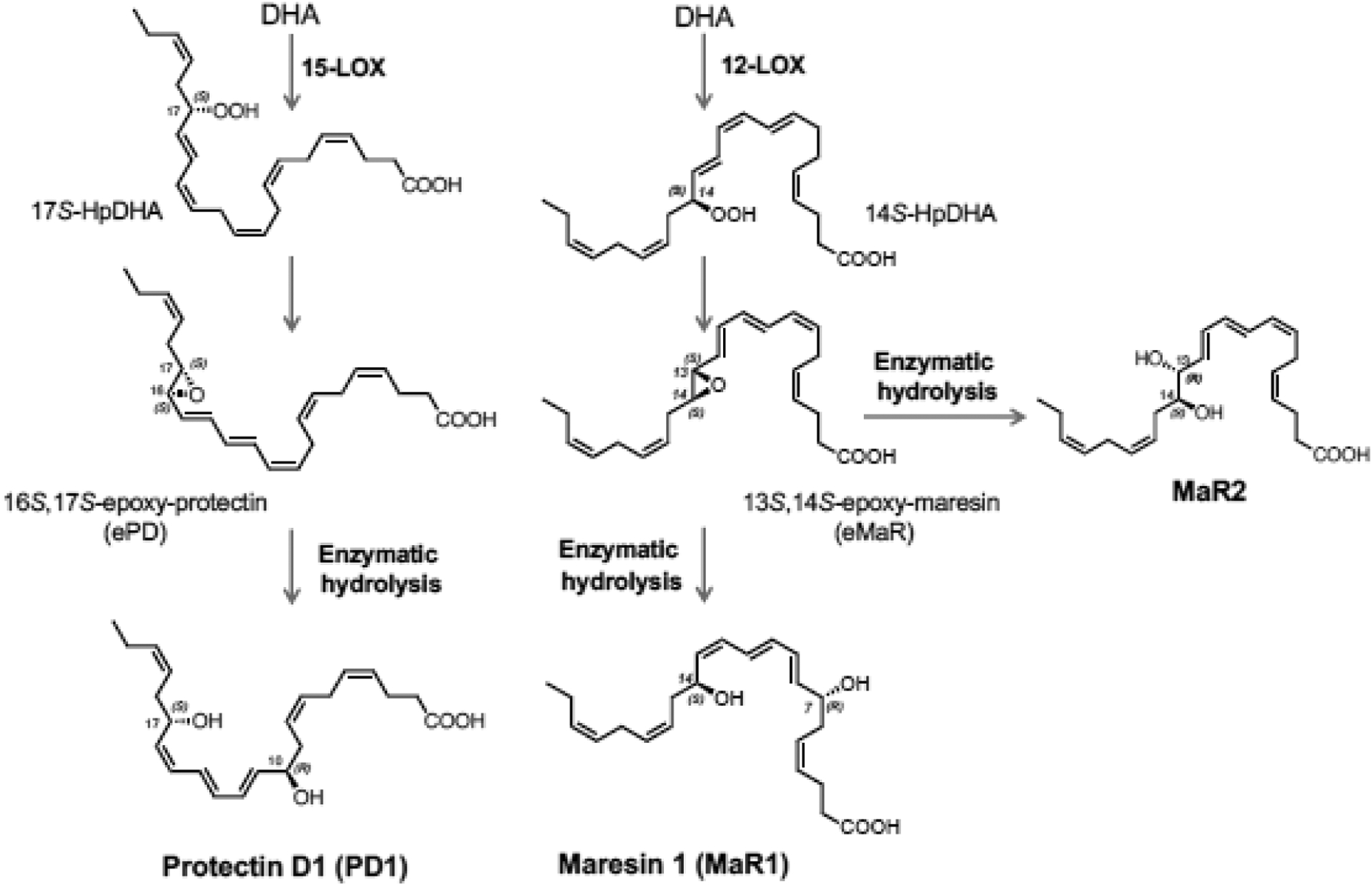
Figure 2. Biosynthesis of resolvins, protectins and maresins.
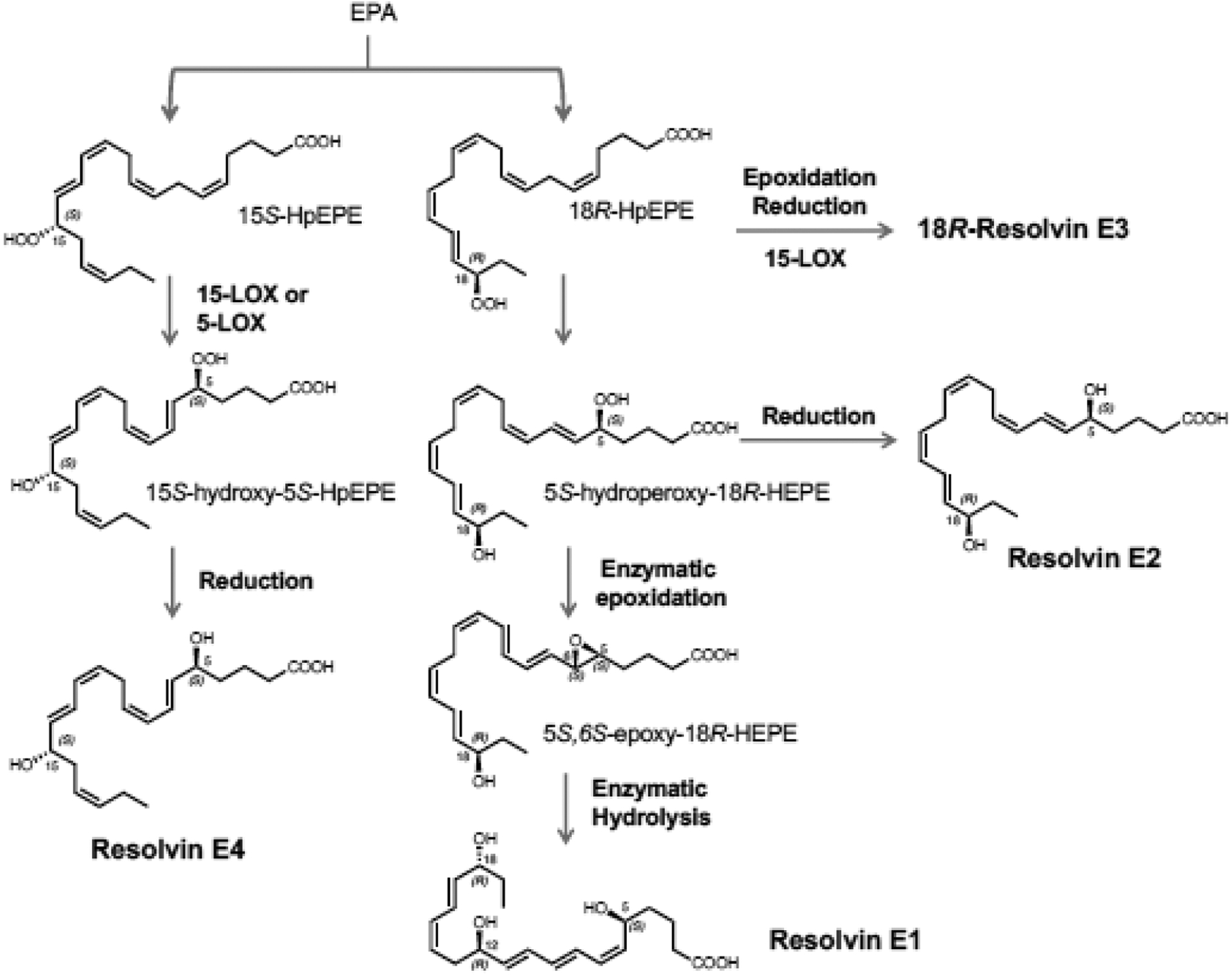
(A) Biosynthesis of E-series resolvins is initiated via lipoxygenation of EPA at carbon-18 position to form 18-HpEPE, which is converted to bioactive E-series members resolvin E1, resolvin E2 and resolvin E3. A new bioactive member, termed Resolvin E4, was recently elucidated and stereochemistry established.
(B) Biosynthesis of D-series resolvins is initiated via 17-lipoxygenation of DHA to form 17S-HpDHA, which is converted to resolvin-epoxide intermediates by the leukocyte 5-lipoxygenase to resolvins D1-D6.
(C) 17S-HpDHA is also converted to 16S,17S-epoxide-protectin intermediate that is further transformed to protectin D1/neuroprotectin D1 and related protectins. Maresins are produced by macrophages via initial lipoxygenation at carbon-14 position by lipoxygenation to produce a 13S,14S-epoxide-maresin intermediate that is enzymatically converted to maresin family members. For original reports, total organic synthesis and stereochemical assignments, see text and cited recent reviews with original citations for details.
SPMs function as potent local resolution agonists, providing the first evidence that resolution is actively ‘turned on’ and not simply a passive process. The complete structural elucidations of most SPMs are established [reviewed in 1] and total organic synthesis achieved, which have enabled the confirmation of their potent bioactions and pro-resolving mechanisms at picomolar to low nanomolar concentrations in many cell types, and picogram to nanogram range in pre-clinical in vivo disease systems by researchers worldwide [1], since they were rapidly made commercially available for academic use by several companies. This review focuses on the EPA- and DHA-derived SPMs. For an overview of the n-3 DPA-derived SPMs, please see the recent review and references within [5].
The real scoop on the SPMs: as presented for the Workshop participants
Each family of SPMs was isolated from biologic fractions of resolving inflammatory exudates, and basic structures of the bioactive material were determined using chemical degradation methods, derivatives and physical methods; for example, see [4].
E-series resolvins: EPA-derived resolvins
- RvE1 –
- First identified SPM from self-resolving murine exudates with modeled biosynthesized via human PMN-endothelial cell-cell interactions during co-incubations. We used several bioassays to study the resolution of inflammation [4]. Results from these studies demonstrated that RvE1 reduced PMN trans-endothelial migration, cleared acute inflammation in the mouse air pouch, and partially competed for 3H-LTB4 binding with recombinant human BLT1 cell surface receptor.
- Complete stereochemistry of RvE1 was established next, and a second RvE1 receptor identified, i.e., human ChemR23 [6].
- A synthetic RvE1 stable analog mimetic, i.e., 19-(p-fluorophenoxy)-RvE1, that resists rapid metabolic inactivation, retains biological activity reducing PMN infiltration and pro-inflammatory cytokine/chemokine production in vivo, was prepared, demonstrating potent pro-resolving activity in mice in vivo [7].
- >275 publications confirming RvE1 production and functions are in PubMed.gov to date.
- RvE2 –
- RvE4 –
- Identified during studies of macrophage-PMN interactions in physiologic hypoxia conditions and stimulates efferocytosis [14].
- Total organic synthesis of RvE4 confirmed the actions of proposed biogenically-prepared structure, complete stereochemistry assignment and function [15].
- The second total organic synthesis, achieved with Professor Trond Hansen‟s team in Oslo, was also reported for RvE4, which physical properties (UV-Vis and LC-MS/MS) match those obtained from biological materials [16].
- RvE4 is produced in humans; its levels are lower in cerebrospinal fluid from patients with Alzheimer’s disease compared to those with subjective cognitive impairment [17].
D-series resolvins: DHA-derived resolvins RvD1-RvD6, identified from self-resolving murine exudates, biosynthesized via human endothelial-PMN interactions under hypoxia conditions, and reduces microglial cell cytokine expression as well as in vivo dermal inflammation and peritonitis [18, 19]. As of February 2023, there are >1,590 publications using the search term “resolvin” on PubMed (https://pubmed.ncbi.nlm.nih.gov/?term=resolvin&sort=date) confirming the potent pro-resolving actions of resolvins and their in vivo biosynthesis.
- RvD1 –
- Total organic synthesis of RvD1 was used to establish its complete stereochemistry and confirm potent bioactions of RvD1 in vitro and in vivo [20].
- A metabolically stable analog 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester was prepared, that reduces lung vascular permeability, PMN and inflammatory cytokines in immune complex-induced lung injury [21].
- >560 publications confirm RvD1 biosynthesis and functions from many independent laboratories around the globe (see PubMed.gov).
- RvD3 –
- Complete stereochemistry of RvD3 was established; synthetic RvD3 potently regulates murine peritonitis and dermal inflammation, as well as leukocyte-directed actions with human cells [23].
- RvD4 –
- Total synthesis of RvD4 established its absolute stereochemical configuration; synthetic RvD4 reduces PMN infiltration in vivo and enhances uptake of apoptotic PMN by human dermal fibroblasts [24], the main processes in the resolution response phase of the acute inflammatory response in vivo.
- RvD4 also reduces the severity of deep-vein thrombosis in vivo and improves thrombus resolution [25].
- RvD5 –
- Controls murine E. coli and S. aureus infections in an anti-phlogistic manner, enhancing containment by the host of the invading microbes and lowers antibiotic requirements for bacterial clearance [26].
- RvD6 –
- RvD6 isomer (RR-RvD6) promotes corneal wound healing and nerve regeneration [27].
Protectins/Neuroprotectins: When of neural origin, neuroprotectin (NPD) is used, and Protectin (PD) is used to denote its peripheral actions, a tissue address.
- PD1 –
- Identified in blood, leukocytes, brain, and glial cells, reduces murine PMN in vivo and human glial cell cytokine production [19].
- Neuroprotectin D1 was identified in neural stem cells, and promotes neuronal and cardiac differentiation [30].
- >250 publications confirm PD1/NPD1 biosynthesis and potent functions (PubMed.gov).
Maresins: macrophage mediator in resolving inflammation
- MaR1 –
- First identified in the later resolution phase of self-resolving inflammatory exudates when macrophages enter, carries potent anti-inflammatory and pro-resolving activity [34].
- MaR1 stereochemistry was established using several isomers prepared by total organic synthesis; synthetic MaR1 confirmed its potent defining bioactions; namely, MaR1 stimulating efferocytosis, tissue regeneration and reducing pain [35].
- ~450 publications on PubMed for the maresins, of which ~270 publications confirm both MaR1 production and its potent functions.
- MaR2 –
- Human macrophages produce MaR2, biosynthesized via 12-LOX and soluble epoxide hydrolase; MaR2 is the second potent bioactive member of the Maresins, reduces PMN infiltration in mouse peritonitis and enhances human macrophage phagocytosis [36].
- Brown adipose tissues produce MaR2, which reduces inflammation in obesity in part by targeting macrophages in the liver [37].
Human Resolution Metabolome
Production of SPMs in human tissues has been documented using mass spectrometry-based profiling approaches in more than 45 human trials using the current NIH definition of clinical trial (Table 1A). For example, human vagus nerves ex vivo produce SPMs, e.g., RvE1, NPD1/PD1, MaR1, upon electrical stimulation suggesting that these vagus-SPM circuits contribute to a new pro-resolving vagal reflex [39]. In the recent WARRIOR Trial, women with coronary microvascular dysfunction (CMD) had significantly lower plasma concentrations of RvD1 and MaR1 than the reference subject group [40]. Also, several randomized clinical trials demonstrate that omega-3 or marine oil supplementation increases SPMs in vivo in humans (Table 1B).
Table 1(A).
Endogenous SPM production in human tissues: Identification and quantification by mass spectrometry*,^
| Tissue/organ | SPM | Quantities | Reference |
|---|---|---|---|
| Serum | Tuberculosis and Type 2 diabetes | [104] | |
| Lymph nodes | MCTR1–3, PCTR1–3 | 1–5 pg/500 mg protein | [76] |
| Spleen | MCTR1, MCTR2, PCTR1, | 1–5 pg/500 mg protein | [76] |
| RCTR1–3 | 60–400 pg/500 mg protein | ||
| Brain | MCTRs, PCTRs, RCTRs | 1–5 pg/500 mg protein | [76] |
| Plasma | RvDn-3 DPA | 10–30 pg/ml | [105] |
| RvE1 RvD1, RvD5 | 2–22 pg/ml | [101] | |
| Cerebrospinal fluid | RvT2, RvT4 (Tuberculous meningitis) | 1–2 pg/ml | [107] |
| Synovial fluid | PD1, RvD1, RvD2, RvD5, MaR1 (RA & OA) | ~5 pmol/ml | [109] |
| Bone marrow | RvD4 | [110] | |
| RCTR1–3 | 24–180 pg/4 ml | ||
| Blister | RvD1, RvD3 | 10–15 pg/ml | [111] |
| Vagus nerve (Electric stimulation) | RvE1–3, RvD3–6, NPD1/PD1, MaR1 | 1–40 pg/3.5 cm of tissue | [39] |
| Metabolic syndrome (weight loss program) | RvE1, RvE3, RvD2, MaR1 (Neutrophils ex vivo) | 26–1340 pg/4.5 × 106 PMN | [112] |
| Obesity | RvD1–6, MaR1, MaR2, PD1, RvEs (Plasma and leukocytes) | 0.2–200 pg/ml plasma, 0.1–2 pg/3×106 cells | [113] |
| Stenotic aortic valves | RvE1, RvD3 | ~500 – 3500 pg/g tissue | [114] |
| Sputum (Cystic fibrosis) | RvD1 | ~200 pg/ml | [115] |
| Nonobstructive coronary artery disease (WARRIOR) trial | RvD1, RvD2, RvD3, RvD5, RvE1, MaR1, 18-HEPE | [40] | |
| Chronic rhinosinusitis | RvD1, RvD2, LXA4 | [116] | |
| Bariatric surgery | RvD3, PD1, MaR1, 17-HDHA, 14-HDHA | [117] |
Table 1(B).
Omega-3 supplementation increases SPMs in humans
| Diseases/conditions | Doses and regimen | SPM present | SPM that are increased by supplementation | Reference |
|---|---|---|---|---|
| Chronic kidney disease | n-3 PUFA; 4 g/day; 8 wks | RvE1, RvE2, RvE3, RvD5 (plasma) | RvE1, RvE2, RvE3, RvD5 | [118] |
| Effect of n-3 in pregnancy on offspring | n-3 PUFA; 3.7 g/day; from 20 wks gestation until delivery | 18-HEPE, 17-HDHA RvE1, RvE2, RvE3, RvD1, 17R-RvD1, RvD2 (cord blood) | 18-HEPE, 17-HDHA | [119] |
| Healthy individuals (Serum & plasma) | ω-3 ethyl esters; 4 g/day; 8–12 wks | RvE1, RvD1, 17-HDHA, 18-HEPE (plasma and serum) | RvE1, RvD1, 17-HDHA, 18-HEPE | [120] |
| Peripheral artery disease (OMEGA-PAD II trial) | n-3 PUFA; 4.4 g/day; 3 months | RvE1, RvE2, RvE3 (plasma) | RvE3 | [121] |
| Peripheral artery disease with marine oil supplementation | PUFA with EPA (≈46%), n-3 DPA (≈18%), and DHA (≈33%); 1.5, 3, and 4.5 g/day; 5 days | RvEs, RvDs, PD, MaR, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA (plasma) | MaR | [122] |
| Healthy individuals (marine oil supplementation) | PUFA with EPA (≈46%), n-3 DPA (≈18%), and DHA (≈33%); 1.5, 3, and 4.5 g/day; 2 wks | RvEs, RvDs, PD1, MaR1, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA (plasma) | RvEs, RvDs, PD1, MaR1, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA | [123] |
| Arthritis | Microalgae oil (Schizochytrium sp); 2.1 g DHA/day; 10 wks | 14-HDHA, 17-HDHA (plasma) | 14-HDHA, 17-HDHA | [124] |
| Coronary artery disease | EPA and DHA, 3.36 g daily | RvE1, MaR1, 18-HEPE | [125] | |
| Pregnant women (Umbilical cord blood) | EPA-rich fish oil (1060 mg EPA plus 274 mg DHA), or DHA-rich fish oil (900 mg DHA plus 180 mg EPA) | 17-HDHA, 14-HDHA | [126] |
These tables report publications in the period of 2017–2021 that confirm and extend original findings (reviewed and references in [5]).
For human trial publications on resolvins, please see https://pubmed.ncbi.nlm.nih.gov/?term=Resolvin&filter=pubt.clinicaltrial&sort=date
SPM-receptor pro-resolving axes
Each EPA- and DHA-derived SPM, including RvE1, RvD1, RvD2, PD1 and MaR1, exhibits potent stereoselective actions (pico- to low nanomolar concentrations) via activation of their specific G protein-coupled receptors (GPCR) on phagocytes and additional select cell types (Figure 3). The bioactive concentration ranges of SPMs are in the picomolar to low nanomolar ranges, consistent with the affinities of SPMs for their respective GPCRs (i.e., Kd values) (Figure 3). These GPCRs contribute to SPM functions as demonstrated in animal systems and select cell types in vivo and in vitro using transgenic and/or knockout mice, gene silencing, blocking antibodies and/or receptor antagonists (Table 2).
Figure 3. SPM receptors.
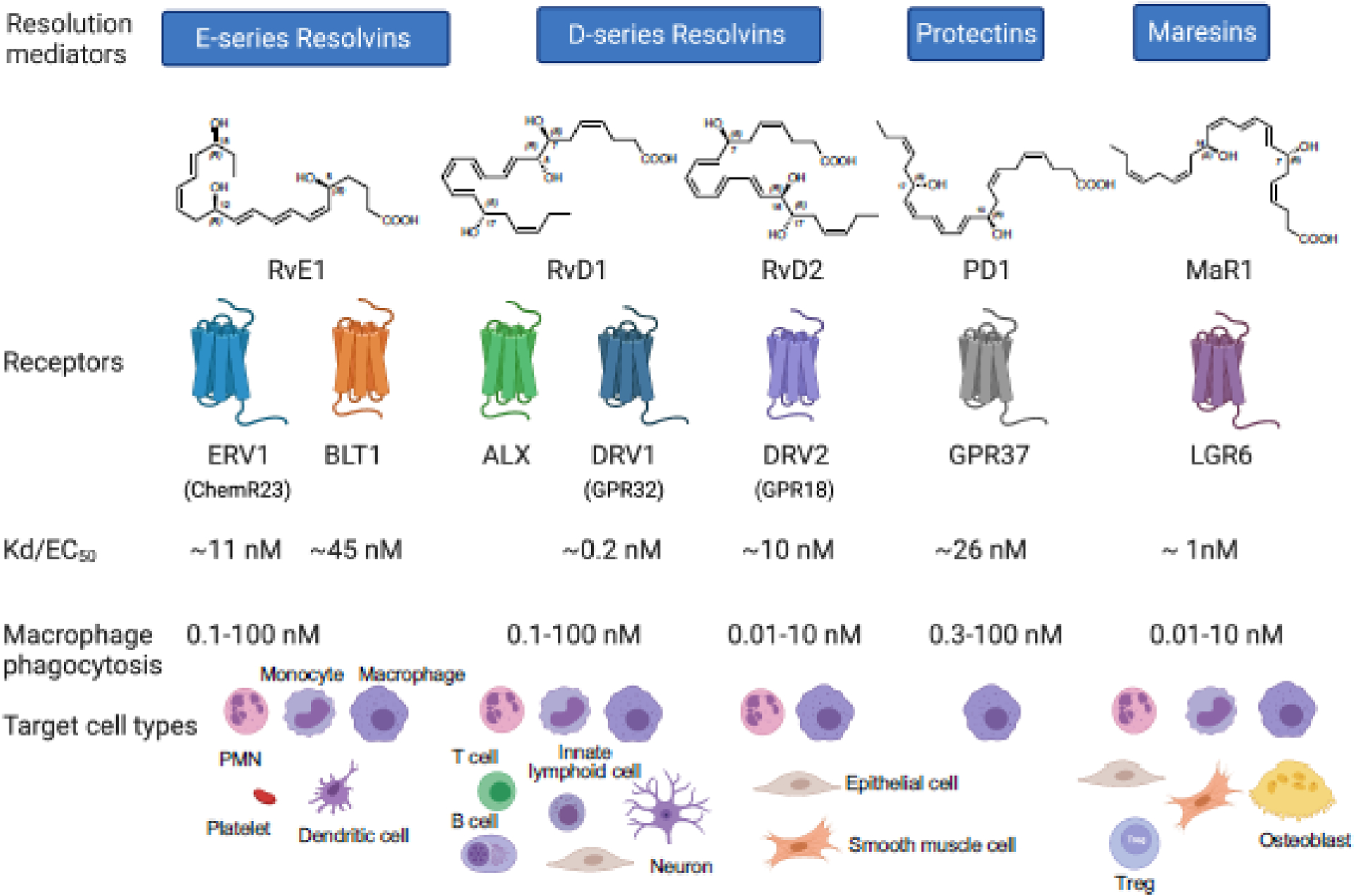
Each SPM demonstrates stereoselective activation of its cognate GPCR on select cell types, leading to intracellular signals, pathways, and pro-resolving functions. The affinities of SPMs for their respective recombinant GPCRs (i.e., Kd or EC50 values) are consistent with their bioactive concentration ranges, e.g., macrophage phagocytosis (picomolar to low nanomolar) in vitro and dose ranges (picograms to low nanograms) in vivo. The in vivo functions of these SPM receptors were demonstrated using transgenic and/or knock-out mice, as well as specific blockage of the receptor, e.g., siRNA, antibodies, or receptor antagonists (see Table 2).
Table 2.
SPM receptor-dependent functions
| SPM Receptor | Genetic modification or pharmacological intervention | Reference | |
|---|---|---|---|
| RvE1 receptor [ChemR23] | Transgenic mice | ↑ RvE1 action limiting PMN ↑ Phagocytosis of P. gingivalis ↓ Bone loss |
[127] |
| ↓ RvE1 control of hepatic transcriptional profile related to glucose homeostasis, insulin sensitivity, and inflammation | [129] | ||
| Leukotriene B4 receptor [BLT1] | Knockout mice | ↓ RvE1 regulation of PMN | [130] |
| BLT1 antagonist | ↓ RvE1-stimulated epithelial wound healing | [131] | |
| RvD1 receptor [ALX] | Transgenic mice | ↑ RvD1 action regulating PMN, miRNAs and cytokines | [132] |
| ↓ RvD1 protection of post-transplant lung ischemia-reperfusion injury | [134] | ||
| ↓ RvD1 pro-resolving actions in primary human macrophages | [136] | ||
| Transgenic/Knock-in | ↑ AT-RvD1 functions in macrophage phagocytosis and intracellular signaling | [137] | |
| Myeloid KO | ↑ Steatosis and hepatic fibrosis ↓ RvD2-enhanced phagocytosis with bone marrow macrophages |
[143] | |
| siRNA | ↓ RvD2’s ability to increase myotube fusion and growth | [142] | |
| ↓ RvD2-enhanced macrophage phagocytosis | |||
| MaR1 receptor [LGR6] | siRNA silencing of LGR6 | ↓ MaR1 functions in increasing cAMP, macrophage phagocytosis and efferocytosis, CREB phosphorylation | [148] |
| ↓ MaR1 protection of post-transplant lung ischemia-reperfusion injury | [134] | ||
| Overexpression | ↑ MaR1-increased cAMP in osteoblasts | [152] | |
| Knockout mice | ↓ Osteoblast proliferation, differentiation, and mineralization | [152] | |
| PD1 receptor [GPR37] | Knockout | Delayed resolution of inflammatory pain | [154] |
| ↓ PD1 protective actions in sepsis induced by LPS and Listeria | [155] | ||
Receptor mimetics. Since SPMs are subject to rapid local metabolism in or near the site of inflammation via enzymatic inactivation, metabolically stable analogs that resist enzymatic inactivation were designed and synthesized [7]. Also, using a high-throughput screening of small-molecule libraries (>45,000 compounds), several chemotypes were identified to activate the RvD1 receptor (DRV1/GPR32) and stimulate macrophage E. coli phagocytosis in DRV1-dependent manner [41]. Further, different synthetic approaches have been employed for structural modifications of SPMs [reviewed in 42]. Together, these molecules act as receptor mimetics, offering potentially more cost-effective synthesis to facilitate clinical development of therapeutics that stimulate resolution pathways.
Where is resolution of inflammation important? What is the evidence?
Why do we study endogenous resolution mechanism of acute inflammation and pro-resolving mediators? The currently available pharmacopeia for treating inflammatory diseases consists mainly of enzyme inhibitors and receptor antagonists, which could lead to many unwanted side effects [1]. For example, non-steroidal anti-inflammatory drugs could cause prolonged neuropathic and inflammatory pain [43]. COX-2 inhibitors are known to increase incidence of thrombosis and myocardial infarction [44, 45], and anti-TNFα therapy increases risk of infections [46]. Therefore, there is urgent need for new approaches in controlling excessive inflammation, e.g., SPMs as resolution agonists, that could spare these severe side effects. In this regard, the author (CNS) pointed out with the discovery of the first resolvin that the resolution phase of inflammation offers a new terrain to control the endogenous cellular mechanisms of inflammation in many diseases [47–49]. To pinpoint the actions in inflammation-resolution, we introduced quantitative indices (i.e., resolution indices, Ri) that demonstrate accelerated or delayed resolution [47–49].
SPMs are endogenous mediators that exhibit potent pro-resolving functions, limiting PMN and pro-inflammatory cytokines, while stimulating macrophage phagocytosis and efferocytosis [reviewed in 1] as well as activating autophagy [50]. In experimental in vivo systems, SPMs reduce collateral tissue damage, promoting tissue repair and regeneration as well as controlling pain [1]. These include neuroinflammation, pain, arthritis, periodontitis, cardiovascular diseases, lung inflammation and metabolic syndromes, to name just a few. These are the areas where resolution of inflammation is essential, and the goals of resolution pharmacology [51, 52] might be achieved in part via personalized profiling of resolution metabolomes and following specific SPM treatment to provide precision medicine to promote the natural endogenous active resolution pathway [47, 49].
- Neuroinflammation
- In Alzheimer disease (AD), resolution of inflammation is impaired in the brain. NPD1 repressed Abeta42-triggered activation of proinflammatory genes while upregulating the antiapoptotic genes [53].
- Intranasal delivery of a panel of SPMs rescues memory and gamma oscillation deficits as well as reduces microglial activation in AD mice [54].
- Pain
- In inflammatory pain models: RvE1 reduces inflammatory pain behaviors induced by formalin, carrageenan and complete Freund’s adjuvant [55].
- Periodontitis and Arthritis
- Periodontitis and arthritis are well-appreciated example of leukocyte-mediated inflammation and bone destruction. Failure of active resolution of inflammation pathways is implicated in disease pathogenesis.
- Overexpression of 15-LOX, a key biosynthesis enzyme for many of the SPMs and LXs, in a large animal model is associated with dampened PMN-mediated tissue degradation and reduced bone loss [58], suggesting that SPMs/LXs can be targets for novel approaches to diseases, e.g., periodontitis and arthritis.
- Topical application of RvE1 in rabbit periodontitis protects against inflammation-induced tissue and bone loss [59].
- In a recent randomized trial with 127 individuals, daily use of an oral rinse containing a SPM stable analog and LXA4 mimetic methyl ester-benzolipoxin A4 (BLXA4) reduces local inflammation and increases abundance of pro-resolution molecules systemically [62].
- Cardiovascular diseases
- Atherosclerosis results from a failure in the resolution of local inflammation. Overexpression of 12/15-LOX protects mice against atherosclerosis via its role in the local biosynthesis of SPMs [63].
- Administration of RvD1 to Ldlr−/− mice during plaque progression promotes plaque stability and improves lesional efferocytosis, and thickens fibrous caps [64].
- Lung inflammation
- In a murine model of asthma, a stable analog of LXA4 blocks both airway hyper-responsiveness and pulmonary inflammation [65].
- In E. coli lung infection, 17R-RvD1 restores TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation [66].
- In lung infections, Cys-SPMs MCTR1–3 decrease lung inflammation and bacterial load in Influenza A virus infections followed by secondary bacterial pneumonia [67].
- Metabolic syndromes
- Obesity and obesity-related disorders are linked to a chronic state of low-grade inflammation in adipose tissue [68].
- With inflamed obese adipose tissue, RvD1 and RvD2 each rescues impaired expression of adiponectin and decreases proinflammatory adipokine production, as well as reduces monocyte adhesion to adipocytes and their trans-adipose migration [69].
- In obese-diabetic mice, RvD1 decreases adipose tissue macrophages and improves insulin sensitivity in part via increasing adipose tissue AMP-activated protein kinase (AMPK) phosphorylation [70].
- RvD1 directs pro-resolving metabolic programs in macrophages, promoting fatty acid oxidation and oxidative phosphorylation, in part via activation of AMPK phosphorylation [71].
- Depression
- There is an unmet need for novel rapid-acting antidepressants with fewer side effects than currently available monoamine-based antidepressants.
- Resolvins (RvD1, RvD2, RvE1, RvE2 and RvE3) exert antidepressant effects in a rodent model of depression via mTORC1 activation [74].
Stereochemical assignment and biosynthesis of cys-SPMs
While interrogating self-resolving infectious exudates, the Serhan Lab uncovered three new series of conserved bioactive chemical signals that are peptide-lipid molecules, collectively termed cysteinyl-SPMs (cys-SPMs). These include three series, i.e., maresin conjugates in tissue regeneration (MCTRs), protectin-CTR (PCTRs) and resolvin-CTR (RCTRs) based on their DHA backbone and family structures [75] (Figure 4A). Each series contains three bioactive members, i.e., MCTR1–3, PCTR1–3 and RCTR1–3. Cys-SPMs exhibit pro-regenerative actions with freshwater Planaria, and also demonstrated classic pro-resolving functions such as limiting polymorphonuclear neutrophil (PMN) infiltration and enhancing MΦ phagocytosis and efferocytosis [75, 76].
Figure 4. Biosynthesis of cysteinyl-SPMs.
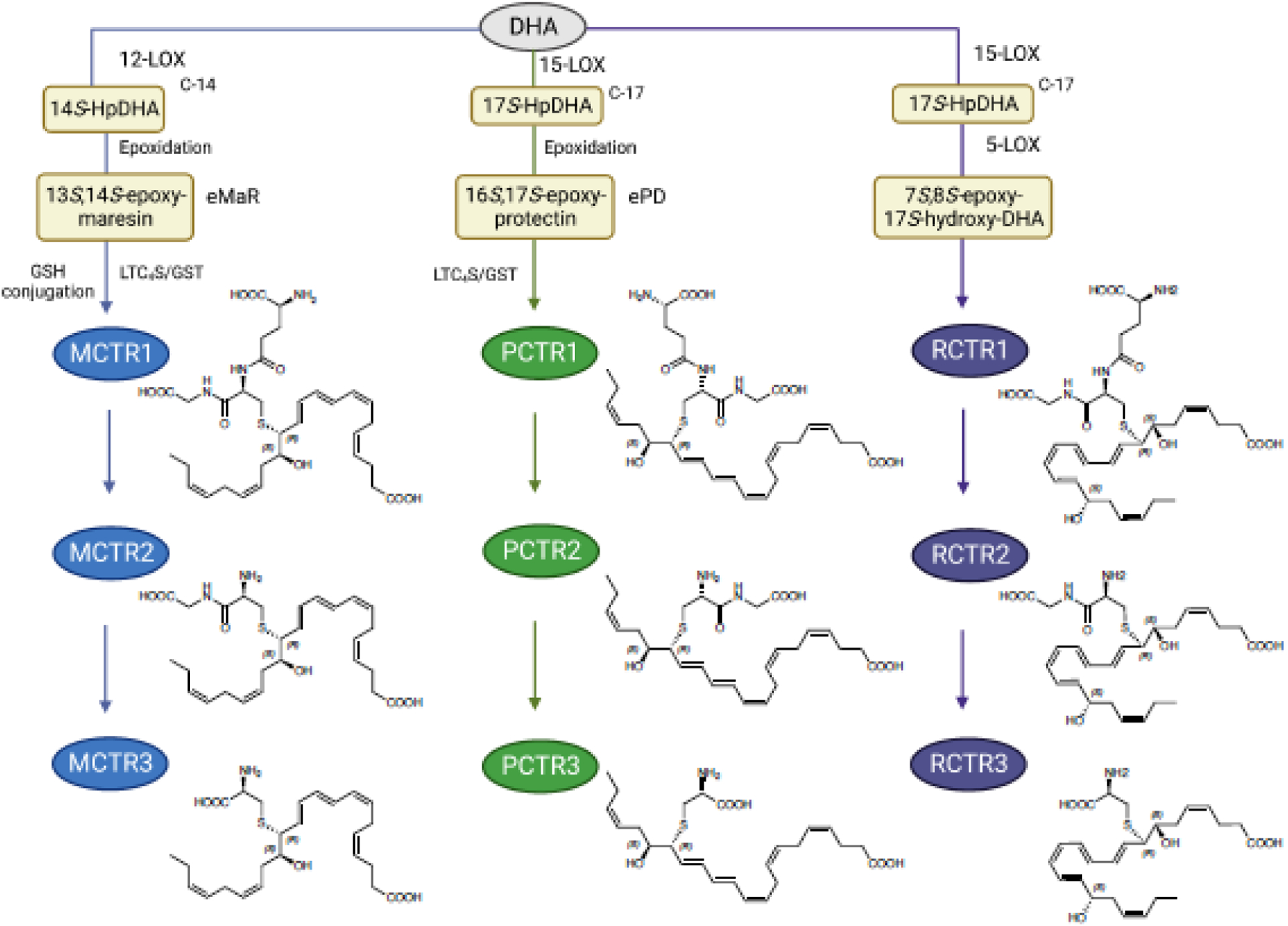
(A) Biosynthesis of MCTRs, PCTRs and RCTRs (see text for details).
(B) Formation of cysteinyl-SPMs by conjugation of the epoxides with L-glutathione via stereoselective enzymatic mechanism.
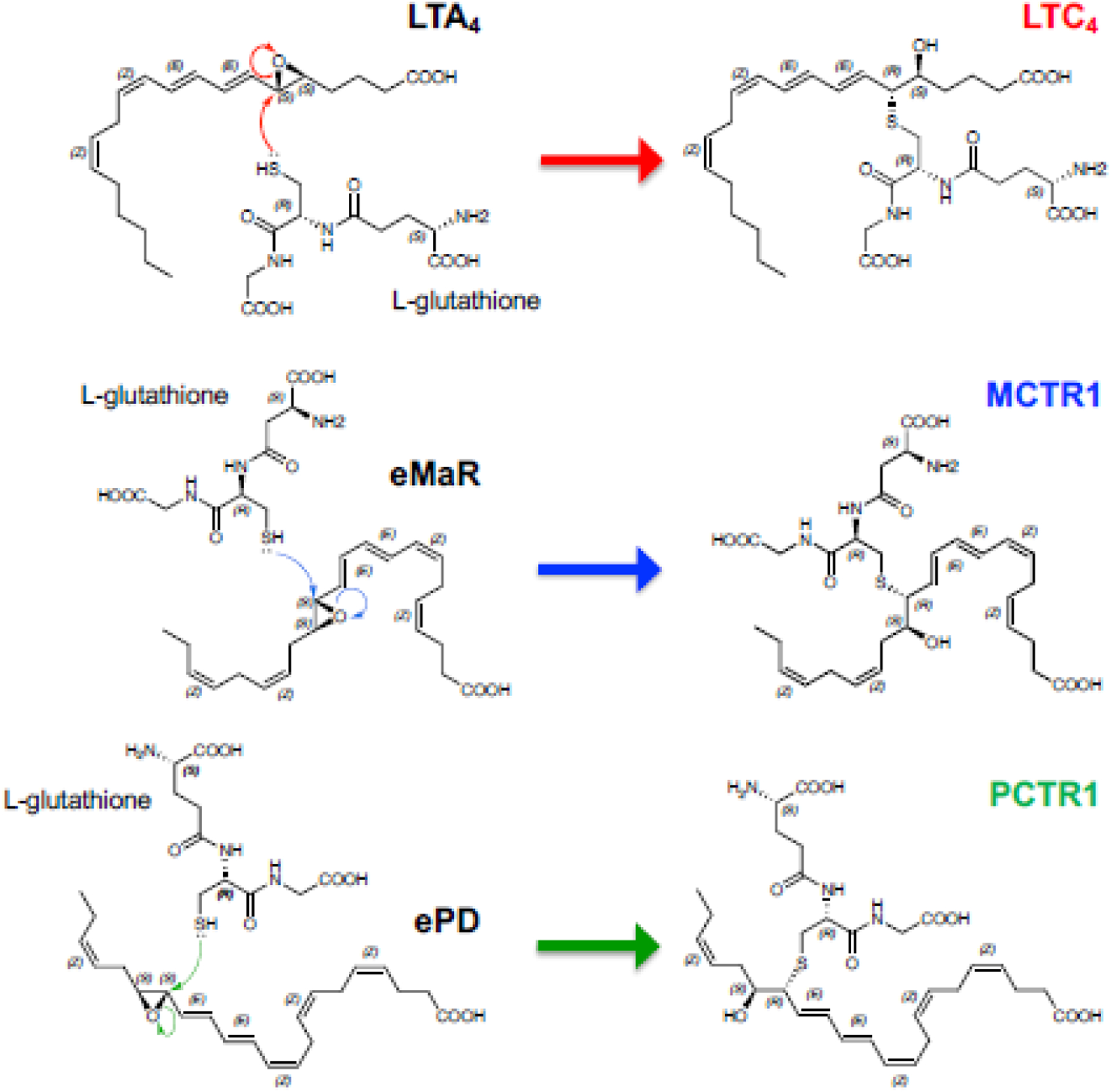
The complete stereochemical assignments of these nine potent cys-SPMs, i.e., MCTR1–2, PCTR1–3 and RCTR1–3 were established, and total organic synthesis achieved [75, 76], permitting demonstrations of their potent actions. See Table 3 for the complete stereochemical names and identifiers on Lipid Maps and PubChem. Biosynthesis metabolomes of MCTRs and PCTRs were established, and enzymes involved in their biosynthesis determined. During self-limited E. coli peritonitis in mice, endogenous MCTRs, PCTRs and RCTRs are produced time-dependently in infectious peritoneal exudates and distal spleens [33]. With human recombinant enzymes, PCTR1 and MCTR1 are each produced by leukotriene (LT) C4 synthase (LTC4S) and glutathione S-transferases (GSTs) [microsomal GST (mGST)2, mGST3, and GST-μ (GSTM)4] from their epoxide precursors [16S,17S-epoxy-PD (ePD) and 13S,14S-epoxy-MaR (eMaR)] (Figure 4B). Human M2-like macrophages express LTC4S, mGST2, mGST3, and GSTM4 and produce cys-SPMs [33]. These results demonstrate CTR biosynthesis in mouse tissues and human macrophages, as well as identified key enzymes in these pathways.
Table 3.
Stereochemical Assignment names and IDs of cys-SPMs
| cys-SPMs | Chemical names | Reference | *Lipid Maps LM ID | **PubChem CID |
|---|---|---|---|---|
| MCTR1 | 13R-glutathionyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | [156] | LMFA04050005 | 122368871 |
| MCTR2 | 13R-cysteinylglycinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | [156] | LMFA04050006 | 122368872 |
| MCTR3 | 13R-cysteinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | [156] | LMFA04050007 | 122368873 |
| PCTR1 | 16R-glutathionyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | [157] | LMFA04040004 | 132472316 |
| PCTR2 | 16R-cysteinylglycinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | [158] | LMFA04040005 | 132472317 |
| PCTR3 | 16R-cysteinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | [158] | LMFA04040006 | 132472318 |
| RCTR1 | 8R-glutathionyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | [76] | LMFA04030014 | 132472320 |
| RCTR2 | 8R-cysteinylglycinyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | [76] | LMFA04030015 | 132472321 |
| RCTR3 | 8R-cysteinyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | [76] | LMFA04030016 | 132472322 |
This table is an update from [5]
cys-SPM production and functions
cys-SPMs are evolutionarily conserved molecules from planaria, mouse to human. They were originally isolated in planaria and mouse infectious exudates [75]. cys-SPMs were also identified in numerous human tissues and cells, such as serum, plasma, lymph nodes, brain, bone marrow [75], macrophages [33] and platelets [77]. Recently, periodontal stem cells were found to biosynthesize MCTR3 [78]. See Table 4 for further details.
Table 4.
Endogenous cys-SPM production from planaria, mouse to human
| Species | Cellular or in vivo systems | Cys-SPMs | Reference |
|---|---|---|---|
| Planaria | Regeneration | MCTR1, MCTR2 | [75] |
| Mouse | Peritoneal exudates (E. coli) | PCTR1, PCTR3 | [33] |
| Skin (S. aureus infection) | PCTR1 | [83] | |
| Lung (RSV infection) | PCTR1 | [84] | |
| Pig | Periodontal ligament stem cells | MCTR3 | [78] |
| Human | Brain | MCTR1–3, PCTR1–3, RCTR3 | [76] |
| Lymph node | MCTR1–3, PCTR1–3, RCTR3 | ||
| Lungs | MCTR1–3 | [80] | |
| M2 macrophages | PCTRs | [77] | |
| Joints (arthritis) | MCTR1–3 | [159] |
In additional to the pro-regenerative actions of cys-SPMs in primordial organism freshwater planaria, cys-SPMs exhibit potent actions in experimental mammalian systems controlling inflammation and infection and are organ protective (Table 5). The first identified cys-SPM series MCTRs protect cardiovascular systems in mice and in primordial sea squirt, as well as counter LTD4-initiated signals and vascular response [79]. Each MCTR (10–100 nM) significantly reduced LTD4-initiated signaling via the recombinant human cysteinyl leukotriene receptor-1 (cysLT1) [79]. In murine allergic airway inflammation, MCTRs block LTD4-induced airway contraction [80, 81]. Most recently, MCTR3‟s protective actions in airway hyperresponsiveness were demonstrated to be cysLT1-dependent using cysLT1 deficient mice [82].
Table 5.
cys-SPM functions: independent confirmations
| Species | In vitro or in vivo | Cys-SPMs | Actions | Reference |
|---|---|---|---|---|
Dugesia japonica
|
Head regeneration | MCTRs, PCTRs, RCTRs | Accelerate head regeneration | [75] |
| MCTR3, PCTR3, RCTR3 | Gene regulation | [86] | ||
Ciona intestinalis
|
Heartbeats | MCTRs | Block LTD4-stimulated negative inotropic action | [79] |
Mus musculus
|
E. coli peritonitis | Accelerate resolution of infection | [75] | |
| [157] | ||||
| RCTRs | [76] | |||
|
Hind-limb | MCTR1, MCTR2 | Reduce lung tissue damages | [75] |
| Allergic airway inflammation | MCTRs | Promote resolution of allergic airway responses | ||
| [82] | ||||
| Respiratory Syncytial Virus pneumonia | PCTR1 | Decrease viral load and leukocytes | [84] | |
| Bacteria pneumonia and viral Infection | MCTRs | Decrease lung inflammation and bacterial load | [67] | |
| Acute lung injury | [160, 161] | |||
| [162, 163] | ||||
| MCTR3 | Reduces leukocytes and edema | [164] | ||
| ARDS | PCTR1 | Improves pulmonary edema fluid clearance | [165] | |
| Lung fibrosis | Attenuates lung inflammatory and fibrotic response | [166] | ||
|
Infectious skin wound | PCTR1 | Stimulates wound closure. Reduces bacterial titers. | [83] |
|
Septic Kidney injury | MCTR1 | Suppresses ferroptosis | [167] |
|
Arthritis | MCTR3 | Pro-resolving and tissue protective | [159] |
|
Cardiac injury | MCTR1 | Improves mitochondrial biogenesis Reduces IL-17A, enhances cardiac function | [168, 169] |
|
Hepatic ischemia-reperfusion | MCTR1 | Inhibits ferroptosis by promoting Nrf2 | [170] |
Sus domesticus
|
Periodontal stem cells | MCTR3 | Reduces proinflammatory cytokines | [78] |
Homo sapiens
|
Macrophages
|
|||
| MCTR3, PCTR3, RCTR3 | Increase TRAF3, IL-10, cAMP | [86] | ||
| Macrophages and monocytes | PCTR1 | Enhances chemotaxis and adhesion | [75] | |
PMN
|
[75, 157] | |||
| RCTRs | Reduces chemotaxis and adhesion | [76] | ||
| Keratinocyte |
PCTR1 | Promote migration | [83] | |
| Precision-cut lung slices | MCTR3 | blocks LTD4-initiated airway contraction | [80] | |
| bronchial epithelial cells | PCTR1 | Increases IFN-lambda expression | [84] |
Following our initial identification and structural elucidation of cys-SPMs as well as demonstration of their pro-regenerative and pro-resolving actions, their potent actions have been extended to many experimental disease systems. For example, cys-SPMs control both bacterial and viral infections. In S. aureus infectious skin wound, PCTR1 stimulates wound closure and reduces bacterial titers [83]. In a Respiratory Syncytial Virus (RSV, which is currently widely in the news given the very high number of infections worldwide) pneumonia model, PCTR1 decreases viral load [84]. In bacterial pneumonia and viral Infection, MCTRs decrease lung inflammation and bacterial titers [67]. MCTRs are also organ protective in acute kidney and cardiac injury, hepatic ischemia-reperfusion and arthritis (see Table 5 for details and references).
With human cells, cys-SPMs demonstrate cell type-specific pro-resolving functions. Each of the nine cys-SPMs stimulates human macrophage phagocytosis and efferocytosis. In addition, RCTRs reduce PMN chemotaxis and adhesion, and PCTR1 promotes keratinocyte migration [83] and Increases IFN-lambda in bronchial epithelial cells [84]. Thus, the organ-protective actions of cys-SPMs are evolutionarily conserved across phyla, from primordial lower-phylum species such as Planaria and sea squirt to mice and humans (Table 5).
cys-SPMs activate resolution-regeneration pathways and effectors
In order to assess the regenerative capacity of cys-SPMs, we used freshwater planaria because planaria are known to undergo robust regeneration via primordial pathways [85]. Each of the cys-SPMs promotes planaria head regeneration [76], permitting us to evaluate molecules and pathways activated by each cys-SPM. RNA-sequencing (RNA-seq) was carried out with surgically injured planaria and cys-SPMs; see [86] and Figure 5. The third member in each cys-SPM biosynthetic pathway was selected for these studies, namely MCTR3 (13R-cysteinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid), PCTR3 (16R-cysteinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid) and RCTR3 (8R-cysteinyl-7S,17Sdihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid), because each potently enhances regeneration. Following planaria (D. japonica) head resection, MCTR3, PCTR3, RCTR3 separately at 10 nM each or control was administered in water. Planaria were collected at 48h and mRNA was isolated for RNA-seq. Transcript abundance was obtained by generating gene counts using paired-end sequences [87], and reads were assembled using the Trinity program with Dj transcript sequences [88]. All coding sequences were translated into deduced peptide sequences, and genome-wide functional annotation was obtained via orthology assignment by eggNOG-mapper [89].
Figure 5. Strategy to identify resolution-regeneration effectors.
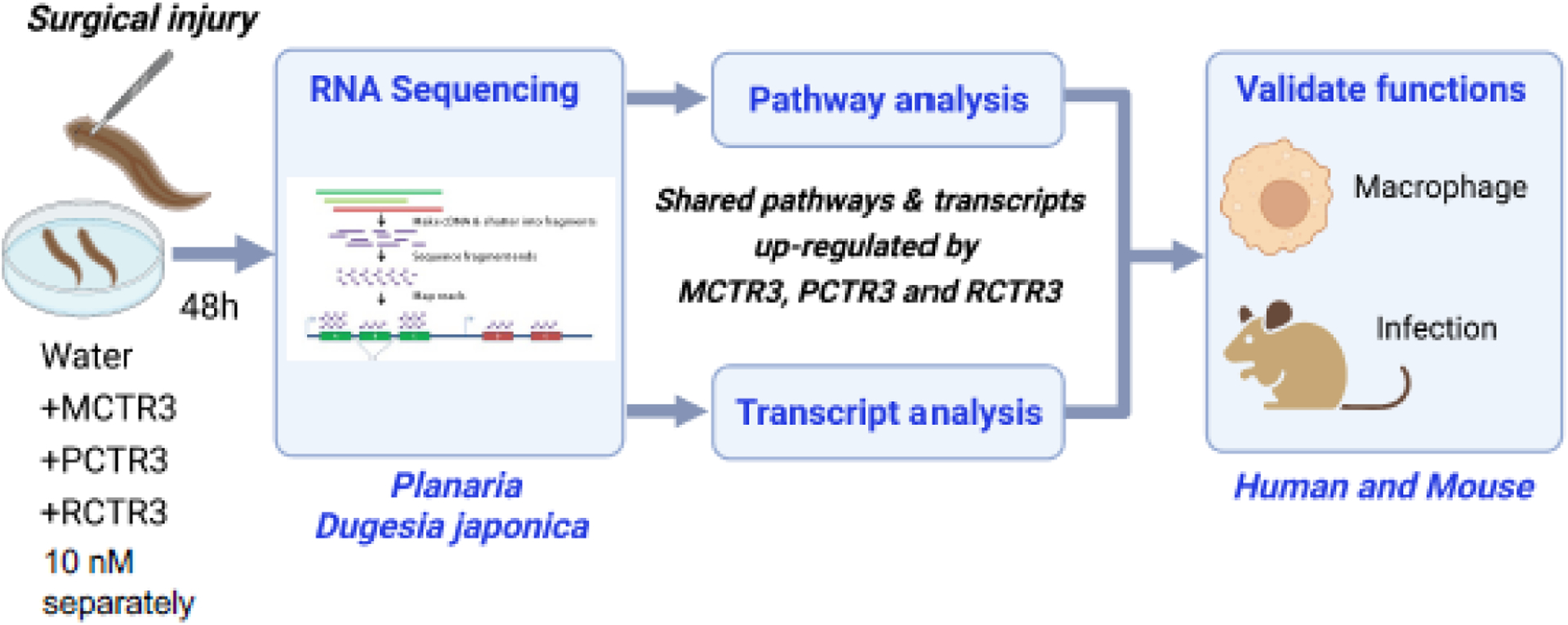
Following planaria (D. japonica) head resection, MCTR3, PCTR3, RCTR3 separately (10 nM each) or vehicle control was administered in water. Planaria were collected at 48h and mRNA isolated for RNA-seq. Transcript and pathway analysis were carried out, and we focused on the shared transcripts and pathways increased by the 3 cys-SPMs, and validated their functions in mammalian systems using human macrophages and mouse in vivo infection.
To identify gene and pathways significantly regulated by each of the bioactive cys-SPM, a Wald test was carried out using the R package sleuth to compare all groups to obtain the P-value, false discovery rate (FDR), i.e. adjusted P-value using Benjamini-Hochberg method), and the ‘beta’ value (analogous to fold change). The „beta‟ value (analogous to fold change) was obtained by comparing control (injured planaria with vehicle) vs. MCTR3, PCTR3 or RCTR3 using the transcript abundance (log2TPM) for each transcript. Pathway enrichment was assessed with the mean-rank gene set test (geneSetTest function in the R package limma) against the Canonical Pathways (CP) and Gene Ontology (GO) pathway databases. Interested readers are referred to [86] for further details. Among the total of 33,511 transcripts obtained, we focused on those shared transcripts increased by the 3 cys-SPMs; they up-regulated 175 known transcripts with predicted functions (FC>1.25). Further pathway and transcript analyses converged on TRAF3 [86], a “gatekeeper” controlling TLR and TNFR signaling pathways [90]. Therefore, we next focused on TRAF3 network analysis using Cytoscape and BioGrid interaction. Within this network, the up-regulated transcripts TRAF5, ABCC, BIRC2 and USO1 and down-regulated transcripts USP7 and OTUD5 by cys-SPMs could potentially interact with TRAF3 [86].
For human translation, we examined the functions of cys-SPMs and TRAF3 with human macrophages since macrophages are essential for tissue resolution, repair and regeneration [91]. MCTR3, PCTR3 and RCTR3 each selectively enhances transcript and protein levels of TRAF3 via a G protein-dependent cAMP-PKA pathway in human MΦ during microbial challenge and stimulated phagocytosis of E. coli. A downstream effector of TRAF3, i.e., IL-10, is also up-regulated during phagocytosis. In addition, overexpression of TRAF3 in human macrophages increases phagocytosis via an IL-10-STAT3 pathway. In vivo in mice, TRAF3 silencing hampers phagocyte functions and resolution of E. coli infection. These results with mice and human MΦ demonstrate a cys-SPM-TRAF3-IL10 pro-resolving axis (Figure 6).
Figure 6. cys-SPMs activate resolution-regeneration pathways and effectors.
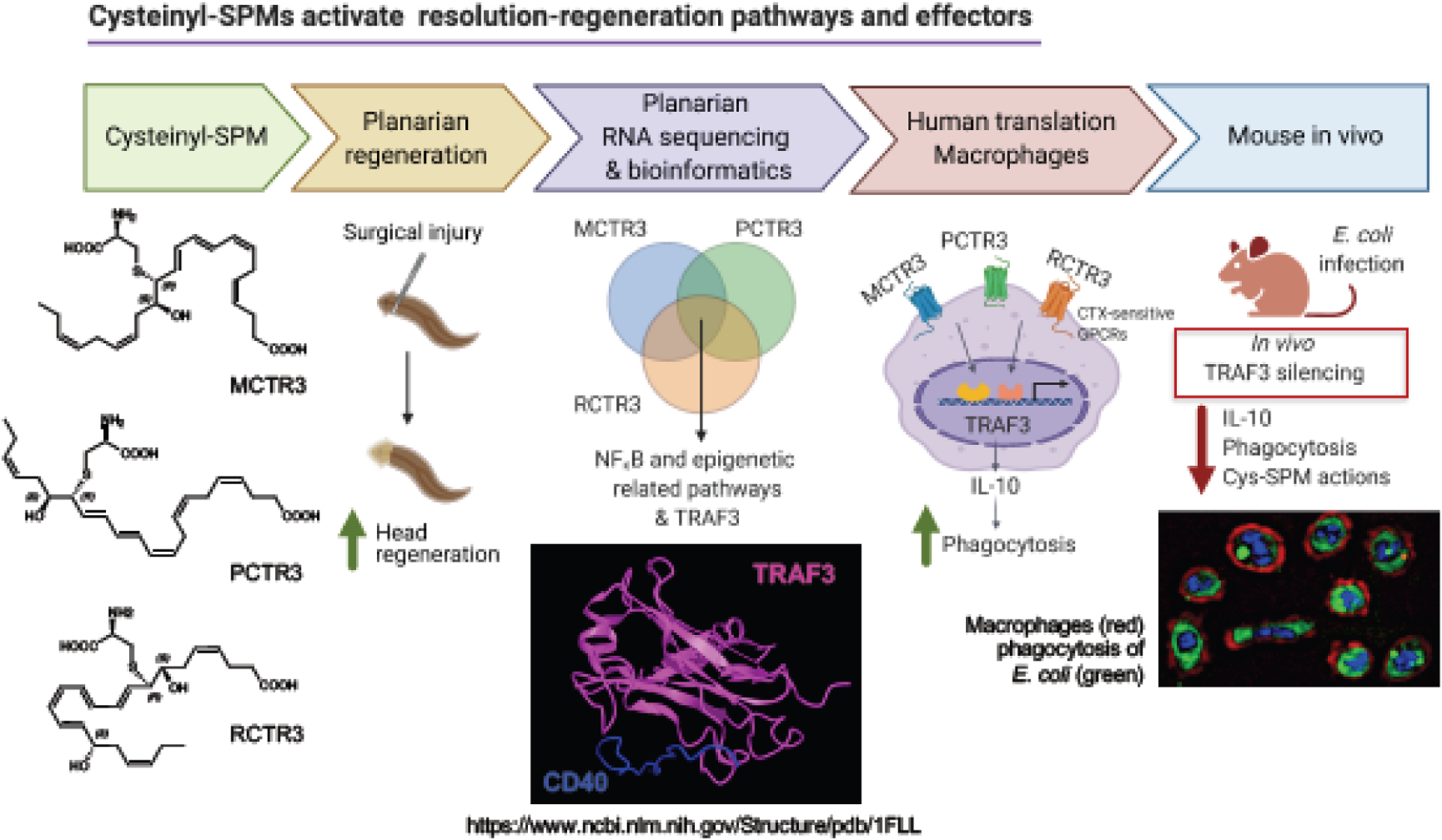
Using planaria as a model organism with RNA-seq, cys-SPM-activated pathways were identified during regeneration including TRAF3, which contributes to cys-SPM’s pro-resolving programs in human macrophage and in mouse E. coli infection.
TRAF3 contributes to cys-SPM’s pro-resolving programs, governing planaria regeneration, immune response with human phagocytes and in mice. Besides its functions in controlling TLR and TNFR signaling pathways in innate immunity, TRAF3 is also a negative regulator of platelet activation and thrombosis [92] and promotes antiviral signaling and type I interferon production upon RNA virus infections [93, 94]. Along these lines, resolvins reduce thrombosis [25], control influenza virus infections [reviewed in 5], limit SARS-CoV-2 Spike protein-induced cytokine storm [95], and attenuate NETs [96]. These protective actions of SPMs and TRAF3 are of interest and may be of clinical interest considering the SARS-CoV-2 pandemic, where cytokine storms and increased coagulopathies are associated with the disease pathologies [97, 98]. We presented new results, not reviewed herein in detail, on human neutrophil extracellular traps (NETs) that play a critical role in bacterial and viral infections, as in SARS-CoV-2 infections. We also showed in this presentation on the newly identified RvTs (13-series resolvins) regulating NET formation. Using microfluidic devices capturing NETs with human whole blood, resolvin D2 and the RvTs each potently (RvT1-RvT4; 2.5 nM) reduces NETs; interested readers are referred to [96] for further details. These new results provide evidence that resolvins reduce NETosis and enhance macrophage NET clearance to promote resolution.
By using planaria as a model organism/system for temporal tissue regeneration with RNA-seq, we identified the cys-SPM-activated pathways during regeneration including TRAF3. With human macrophages, cys-SPMs each increases TRAF3, which regulates phagocyte functions and promotes resolution of infection (Figure 6). We also presented at the workshop the total organic synthesis of the proposed 4S,5S-epoxy-resolvin intermediate, deduced from acid methanol trapping [18, 20], in the biosynthesis of RvD3 and RvD4. Human neutrophils convert this synthetic intermediate to RvD3 and RvD4, whereas human M2 macrophages transform this labile epoxide intermediate to RvD4 and a novel cysteinyl-resolvin that proved to be a positional isomer of RCTR1 [99]. This short-lived intermediate in the biosynthesis pathway, 4S,5S-epoxy-resolvin, was prepared by total organic synthesis [100] and subject to non-enzymatic aqueous hydrolysis to produce four distinct non-enzymatic products; please see [99] and supplement within for mechanistic details. These studies are reported with Professor Jesper Haeggström and Prof. Bengt Samuelsson of the Karolinska (Stockholm, Sweden). This novel cysteinyl-resolvin, termed 4,5-RCTR1, was recently synthesized via total organic synthesis. Its complete stereochemistry and bioactions are reported in1.
M2 macrophages play key roles in the resolution of inflammation and wound healing. Human M2 macrophages also converted leukotriene A4 to lipoxins; see [99] and supplement within. The novel cysteinyl-resolvin, 4,5-RCTR1, significantly accelerated tissue regeneration of surgically injured planaria. In a model of human granuloma formation, this novel cysteinyl-resolvin isomer significantly inhibited granuloma development by human peripheral blood leukocytes. These results provide evidence for human cell type-specific role of 4S,5S-epoxy-resolvin in the biosynthesis of RvD3 by human neutrophils, RvD4 by both human M2 macrophages and neutrophils, and a novel cysteinyl-resolvin positional isomer produced by M2 macrophages that processes potent activities in granuloma formation, resolution, and tissue regeneration. In comparison, biosynthesis of RvD1 and RvD2 is via a 7S,8S-epoxy-resolvin intermediate [20]. Targeting cys-SPM network could provide approaches for controlling infections, protecting from organ injury, wound healing, and inflammatory diseases where tissue regeneration and control of unresolved inflammation are critically needed.
Perspective
SPM are now proven to have a wide range of actions that open a new area for resolution physiology and pharmacology, where the mediators and their precursors are vital in supplying chemical signals for catabasis to homeostasis. These findings from our and other laboratories worldwide raise additional questions to be addressed. For example, regarding molecular and cellular mechanisms, are there overlapped and/or distinct signaling pathways in the SPM-receptor networks? Also, are there specific target cells in addition to phagocytes that express SPM receptors with cell-type dependency? Regarding SPM clinical development, the endogenous human formation of SPMs, their efficacy and optimal levels needed to promote timely resolution are of interest. Can we further develop personalized nutrition and supplementation to boost SPM production in men, women, children and the elderly? In this context, EPA supplementation dose-dependently (1, 2 and 4g/day) increases 18-HEPE in human circulation, which inversely associates with both systemic inflammation (plasma HPR levels) and symptoms of depression [103]. The field of resolution needs more clinical studies like this. Since SPM spatial-temporal resolution-metabolomes in humans are under intensive investigation (Table 1), and SPM-receptor networks with their structure-function relationships have been studied in many experimental systems (Table 2), then we can expect that more evidence will be published in the near future to support the pro-resolving functions of SPMs in human wellness and disease.
Conclusion/Summary:
In this invited review, we give a synopsis of the pro-resolving mediators, their discovery and their new roles in tissue regeneration, as presented at the workshop in Stockholm in the summer of 2022. The rigorous structural elucidation of the novel resolution phase of inflammation mediators, their defining cellular actions and biosynthesis from the Serhan laboratory are confirmed by many independent laboratories worldwide as reported in PubMed.gov (>1594 publications for resolvins, >2380 for lipoxins and >457 for maresins). These publications use available synthetic SPMs that are well-defined with complete stereochemistry, and extend the biologic impact and systems originally reported from the Serhan lab and cited herein. The evidence should be clear to all serious investigators that the SPMs are potent pro-resolving molecules, confirmed by many independent laboratories. Recent publications on the identification and SPMs in human and animal tissues as in [101,106,112] (see Table 1) should be taken seriously by the lipid mediator community, as well as the many other independent studies that report confirmation, extend and improve the SPM LC-MS-MS-based profiling and functions we introduced in [102]. At this workshop, CNS proclaimed our research mission in the Serhan Lab is to “uncover new approaches to control excessive inflammation that will not harm individuals and will lead to endogenous resolution of inflammation and tissue repair”. This is urgently needed because of the many diseases now widely recognized to have uncontrolled excessive inflammation, a major component in disease pathologies. As with all discoveries and new fields of research that emerge from them, there also rise “critics”. Here, we call on the famous quote of Professor Max Planck2 that addresses this very situation with his own research in quantum physics. There can be no doubt, today that the study of SPMs and their potent pro-resolving properties can offer new directions to help in many maladies and infections (both bacterial and viral). Can we really afford in these pandemic times not to dig deeper and do our very best for humanity as biomedical scientists?
Exciting research now opens as we await the next EU workshop. At the recent Eicosanoid Research Conference, November 2022 in New Orleans USA, of the 225 presentations at this international meeting, a substantial number of presentations showed exciting new results on the SPMs for resolvins, protectins and maresins from investigators around the world. Thus, the chapter on SPMs in human biology and medicine is open and continues to evolve, onward and upward. Please keep in mind that in the case of the prostaglandins, SPM forefathers, the first prostaglandins were identified in the 1930s with their complete structures and biosynthesis established in the 1960’s and 1970’s. Their exciting biology is still unfolding and remains widely studied today. As with prostaglandin research, some issues remain for the SPMs.
Highlights.
Resolution of inflammation is governed by specialized pro-resolving mediators (SPM).
SPM structural elucidation and complete stereochemistry are established.
Mass spectrometry-based profiling document SPMs in human and animal systems.
SPMs are pro-resolving, organ protective, control pain and infections.
SPMs evoke pro-resolving functions via specific GPCRs in nanomolar ranges.
Acknowledgements
We thank Mary H. Small for expert assistance in manuscript preparations. Figures were prepared using ChemDraw Level Professional version 20.1.0.112 (PerkinElmer, Waltham, MA) and https://biorender.com/. We also thank our coauthors in the original studies from the Serhan lab reviewed in this lecture presentation and here in this synopsis, as well as our colleagues and their own teams for their original contributions to this growing field of resolution of inflammation research. CNS’s research is supported by National Institutes of Health USA (grant number R35GM139430).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors declare no competing financial interests related to the preparation of this invited review or its content.
Robert Nshimiyimana, Stephania Libreros, Melissa Simard, Nan Chiang, Ana R. Rodriguez, Bernd W. Spur and Charles N. Serhan. Phagocyte functions and total organic synthesis of 4S,5R-RCTR1 in the resolution of inflammation (submitted for publication)
“A scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is familiar with it.” Max Planck.
References
- [1].Serhan CN, Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology, Mol. Aspects Med 58 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simopoulos AP, Serhan CN, Bazinet RP, The need for precision nutrition, genetic variation and resolution in Covid-19 patients, Mol. Aspects Med 77 (2021) 100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cotran RS, Kumar V, Collins T, Robbins Pathologic Basis of Disease, W.B. Saunders Co., Philadelphia, 1999, pp. Chap. 3, p. 78. [Google Scholar]
- [4].Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K, Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing, J. Exp. Med 192 (2000) 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chiang N, Serhan CN, Specialized pro-resolving mediator network: an update on production and actions, Essays Biochem. 64 (2020) 443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN, Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1, J. Exp. Med 201 (2005) 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arita M, Oh S, Chonan T, Hong S, Elangovan S, Sun Y-P, Uddin J, Petasis NA, Serhan CN, Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions, J. Biol. Chem 281 (2006) 22847–22854. [DOI] [PubMed] [Google Scholar]
- [8].Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN, Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis, Chem. Biol 13 (2006) 1193–1202. [DOI] [PubMed] [Google Scholar]
- [9].Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN, Resolvin E2 formation and impact in inflammation resolution, J. Immunol 188 (2012) 4527–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serhan CN, Libreros S, Nshimiyimana R, E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition, Semin. Immunol 59 (2022) 101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murakami Y, Fukuda H, Muromoto R, Hirashima K, Ishimura K, Fujiwara K, Ishihara J, Matsuda T, Watanabe M, Shuto S, Design and Synthesis of Benzene Congeners of Resolvin E2, a Proresolving Lipid Mediator, as Its Stable Equivalents, ACS Med Chem Lett 11(4) (2020) 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H, Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid, J. Biol. Chem 287(13) (2012) 10525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K, Inoue M, Arai H, Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3, J Biochem 153 (2013) 355–360. [DOI] [PubMed] [Google Scholar]
- [14].Norris PC, Libreros S, Serhan CN, Resolution metabolomes activated by hypoxic environment, Sci. Adv 5 (2019) eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Libreros S, Shay AE, Nshimiyimana R, Fichtner D, Martin MJ, Wourms N, Serhan CN, A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution, Front Immunol 11 (2021) 631319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reinertsen AF, Primdahl KG, Shay AE, Serhan CN, Hansen TV, Aursnes M, Stereoselective Synthesis and Structural Confirmation of the Specialized Pro-Resolving Mediator Resolvin E4, J. Org. Chem 86(4) (2021) 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Do KV, Hjorth E, Wang Y, Jun B, Kautzmann MI, Ohshima M, Eriksdotter M, Schultzberg M, Bazan NG, Cerebrospinal Fluid Profile of Lipid Mediators in Alzheimer’s Disease, Cell. Mol. Neurobiol doi: 10.1007/s10571-022-01216-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L, Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals, J. Exp. Med 196 (2002) 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN, Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation, J. Biol. Chem 278 (2003) 14677–14687. [DOI] [PubMed] [Google Scholar]
- [20].Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN, Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation, J. Biol. Chem 282 (2007) 9323–34. [DOI] [PubMed] [Google Scholar]
- [21].Tang H, Liu Y, Yan C, Petasis NA, Serhan CN, Gao H, Protective actions of aspirin-triggered (17R) resolvin D1 and its analogue, 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester, in C5a-dependent IgG immune complex-induced inflammation and lung injury, J. Immunol 193(7) (2014) 3769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN, Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis, Nature 461 (2009) 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, Petasis NA, Serhan CN, Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents, Chem. Biol 20 (2013) 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, Petasis NA, Serhan CN, Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance, Sci Rep 6 (2016) 18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cherpokova D, Jouvene CC, Libreros S, DeRoo E, Chu L, de la Rosa X, Norris P, Wagner DD, Serhan CN, Resolvin D4 attenuates the severity of pathological thrombosis in mice, Blood 134 (2019) 1458–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, Schmidt BA, Serhan CN, Infection regulates pro-resolving mediators that lower antibiotic requirements, Nature 484 (2012) 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pham TL, Kakazu AH, He J, Jun B, Bazan NG, Bazan HEP, Novel RvD6 stereoisomer induces corneal nerve regeneration and wound healing post-injury by modulating trigeminal transcriptomic signature, Sci Rep 10(1) (2020) 4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA, Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes, J. Immunol 176 (2006) 1848–1859. [DOI] [PubMed] [Google Scholar]
- [29].Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N, Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome, Biochim. Biophys. Acta 1851 (2015) 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yanes O, Clark J, Wong DM, Patti GG, Sánchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G, Metabolic oxidation regulates embryonic stem cell differentiation, Nat. Chem. Biol 6(6) (2010) 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aursnes M, Tungen JE, Colas RA, Vlasakov I, Dalli J, Serhan CN, Hansen TV, Synthesis of the 16S,17S-Epoxyprotectin Intermediate in the Biosynthesis of Protectins by Human Macrophages, J. Nat. Prod 78 (2015) 2924–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vidar Hansen T, Serhan CN, Protectins: Their biosynthesis, metabolism and structure-functions, Biochem. Pharmacol 206 (2022) 115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jouvene CC, Shay AE, Soens MA, Norris PC, Haeggstrom JZ, Serhan CN, Biosynthetic metabolomes of cysteinyl-containing immunoresolvents, FASEB J. 33 (2019) 13794–13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M, Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions, J. Exp. Med 206 (2009) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA, Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain, FASEB J. 26 (2012) 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN, Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages, PLoS One 9(7) (2014) e102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sugimoto S, Mena HA, Sansbury BE, Kobayashi S, Tsuji T, Wang CH, Yin X, Huang TL, Kusuyama J, Kodani SD, Darcy J, Profeta G, Pereira N, Tanzi RE, Zhang C, Serwold T, Kokkotou E, Goodyear LJ, Cypess AM, Leiria LO, Spite M, Tseng YH, Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation, Nat Metab 4(6) (2022) 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN, The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype, FASEB J. 27 (2013) 2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Serhan CN, De la Rosa X, Jouvene CC, Cutting Edge: Human vagus produces specialized pro-resolving mediators of inflammation with electrical stimulation reducing pro-inflammatory eicosanoids, J. Immunol 201 (2018) 3161–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Keeley EC, Li HJ, Cogle CR, Handberg EM, Merz CNB, Pepine CJ, Specialized Proresolving Mediators in Symptomatic Women With Coronary Microvascular Dysfunction (from the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD [WARRIOR] Trial), Am. J. Cardiol 162 (2022) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chiang N, Barnaeva E, Hu X, Marugan J, Southall N, Ferrer M, Serhan CN, Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions, Cell Chem Biol 26(2) (2019) 244–254.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Daly K, O’Sullivan K, O’Sullivan TP, Major structure-activity relationships of resolvins, protectins, maresins and their analogues, Future Med Chem doi: 10.4155/fmc-2022-0206 (2022). [DOI] [PubMed] [Google Scholar]
- [43].Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, Huising J, Verma V, Meloto CB, Silva JR, Dutra GGS, Markova T, Dang H, Tessier PA, Slade GD, Nackley AG, Ghasemlou N, Mogil JS, Allegri M, Diatchenko L, Acute inflammatory response via neutrophil activation protects against the development of chronic pain, Sci Transl Med 14(644) (2022) eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Strand V, Hochberg MC, The risk of cardiovascular thrombotic events with selective cyclooxygenase-2 inhibitors, Arthritis Rheum. 47(4) (2002) 349–55. [DOI] [PubMed] [Google Scholar]
- [45].Curfman GD, Morrissey S, Drazen JM, Expression of concern: Bombardier et al., “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis,” N Engl J Med 2000;343:1520–8, N. Engl. J. Med 353(26) (2005) 2813–4. [DOI] [PubMed] [Google Scholar]
- [46].Shah ED, Farida JP, Siegel CA, Chong K, Melmed GY, Risk for Overall Infection with Anti-TNF and Anti-integrin Agents Used in IBD: A Systematic Review and Meta-analysis, Inflamm. Bowel Dis 23(4) (2017) 570–577. [DOI] [PubMed] [Google Scholar]
- [47].Schwab JM, Chiang N, Arita M, Serhan CN, Resolvin E1 and protectin D1 activate inflammation-resolution programmes, Nature 447 (2007) 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL, Resolution of inflammation: state of the art, definitions and terms, FASEB J. 21 (2007) 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN, Molecular circuits of resolution: formation and actions of resolvins and protectins, J. Immunol 174(7) (2005) 4345–4355. [DOI] [PubMed] [Google Scholar]
- [50].Prieto P, Rosales-Mendoza CE, Terron V, Toledano V, Cuadrado A, Lopez-Collazo E, Bannenberg G, Martin-Sanz P, Fernandez-Velasco M, Bosca L, Activation of autophagy in macrophages by pro-resolving lipid mediators, Autophagy 11(10) (2015) 1729–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510(7503) (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Perretti M, Dalli J, Resolution Pharmacology: Focus on Pro-Resolving Annexin A1 and Lipid Mediators for Therapeutic Innovation in Inflammation, Annu. Rev. Pharmacol. Toxicol doi: 10.1146/annurev-pharmtox-051821-042743 (2022). [DOI] [PubMed] [Google Scholar]
- [53].Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG, A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease, J. Clin. Invest 115 (2005) 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Emre C, Arroyo-García LE, Do KV, Jun B, Ohshima M, Alcalde SG, Cothern ML, Maioli S, Nilsson P, Hjorth E, Fisahn A, Bazan NG, Schultzberg M, Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in App(NL-G-F/NL-G-F) mice, Commun Biol 5(1) (2022) 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xu Z-Z, Zhang L, Liu T, Park J-Y, Berta T, Yang R, Serhan CN, Ji R-R, Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions, Nat. Med 16 (2010) 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR, Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain, Neuron 100(6) (2018) 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Luo X, Gu Y, Tao X, Serhan CN, Ji RR, Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy, Front Pharmacol 10 (2019) 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE, Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous antiinflammatory lipid mediators, J. Immunol 171 (2003) 6856–6865. [DOI] [PubMed] [Google Scholar]
- [59].Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE, RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis, FASEB J. 20 (2006) 401–403. [DOI] [PubMed] [Google Scholar]
- [60].Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M, Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis, JCI Insight 1(5) (2016) e85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M, Serhan CN, Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation, J. Immunol 197(6) (2016) 2362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hasturk H, Schulte F, Martins M, Sherzai H, Floros C, Cugini M, Chiu CJ, Hardt M, Van Dyke T, Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation, Front Immunol 12 (2021) 704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Merched A, Ko K, Gotlinger KH, Serhan CN, Chan L, Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators, FASEB J. 22 (2008) 3595–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I, An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques, Nat Commun 7 (2016) 12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN, Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4, Nat. Med 8 (2002) 1018–1023. [DOI] [PubMed] [Google Scholar]
- [66].Sekheri M, El Kebir D, Edner N, Filep JG, 15-Epi-LXA(4) and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation, Proc. Natl. Acad. Sci. U. S. A 117(14) (2020) 7971–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tavares LP, Brüggemann TR, Rezende RM, Machado MG, Cagnina RE, Shay AE, Garcia CC, Nijmeh J, Teixeira MM, Levy BD, Cysteinyl Maresins Reprogram Macrophages to Protect Mice from Streptococcus pneumoniae after Influenza A Virus Infection, mBio 13(4) (2022) e0126722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brennan E, Kantharidis P, Cooper ME, Godson C, Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function, Nat Rev Nephrol 17(11) (2021) 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN, Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat, J. Immunol 189 (2012) 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M, Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice, FASEB J. 25 (2011) 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hosseini Z, Marinello M, Decker C, Sansbury BE, Sadhu S, Gerlach BD, Bossardi Ramos R, Adam AP, Spite M, Fredman G, Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation, Arterioscler. Thromb. Vasc. Biol 41(3) (2021) 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN, Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines, J. Immunol 193(8) (2014) 4235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, Maloney J, MacNamara KC, Spite M, Fredman G, Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage, FASEB J. 34(1) (2020) 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Deyama S, Kaneda K, Minami M, Resolution of depression: antidepressant actions of resolvins, Neurosci. Res doi: 10.1016/j.neures.2022.10.006 (2022). [DOI] [PubMed] [Google Scholar]
- [75].Serhan CN, Chiang N, Dalli J, New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration, Mol. Aspects Med 64 (2018) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].de la Rosa X, Norris PC, Chiang N, Rodriguez AR, Spur BW, Serhan CN, Identification and complete stereochemical assignments of the new Resolvin Conjugates in Tissue Regeneration (RCTR) in human tissues that stimulate proresolving phagocyte functions and tissue regeneration, Am J Pathol. 188 (2018) 950–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liening S, Romp E, Werz O, Scriba GKE, Garscha U, Liquid chromatography-coupled mass spectrometry analysis of glutathione conjugates of oxygenated polyunsaturated fatty acids, Prostaglandins Other Lipid Mediat. 144 (2019) 106350. [DOI] [PubMed] [Google Scholar]
- [78].Rakian A, Rakian R, Shay AE, Serhan CN, Van Dyke TE, Periodontal Stem Cells Synthesize Maresin Conjugate in Tissue Regeneration 3, J. Dent. Res 101(10) (2022) 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chiang N, Riley IR, Dalli J, Rodriguez AR, Spur BW, Serhan CN, New maresin conjugates in tissue regeneration pathway counters leukotriene D4-stimulated vascular responses, FASEB J. 32(7) (2018) 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Levy BD, Abdulnour RE, Tavares A, Brüggemann TR, Norris PC, Bai Y, Ai X, Serhan CN, Cysteinyl maresins regulate the prophlogistic lung actions of cysteinyl leukotrienes, J. Allergy Clin. Immunol 145(1) (2020) 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Godson C, Balancing the Effect of Leukotrienes in Asthma, N. Engl. J. Med 382(15) (2020) 1472–1475. [DOI] [PubMed] [Google Scholar]
- [82].Säfholm J, Abma W, Bankova LG, Boyce JA, Al-Ameri M, Orre AC, Wheelock CE, Dahlén SE, Adner M, Cysteinyl-maresin 3 inhibits IL-13 induced airway hyperresponsiveness through alternative activation of the CysLT(1) receptor, Eur. J. Pharmacol 934 (2022) 175257. [DOI] [PubMed] [Google Scholar]
- [83].Sansbury BE, Li X, Wong B, Riley CO, Shay AE, Nshimiyimana R, Petasis NA, Serhan CN, Spite M, PCTR1 Enhances Repair and Bacterial Clearance in Skin Wounds, Am. J. Pathol 191(6) (2021) 1049–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Walker KH, Krishnamoorthy N, Brüggemann TR, Shay AE, Serhan CN, Levy BD, Protectins PCTR1 and PD1 Reduce Viral Load and Lung Inflammation During Respiratory Syncytial Virus Infection in Mice, Front Immunol 12 (2021) 704427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sanchez Alvarado A, Planarian regeneration: its end is its beginning, Cell 124(2) (2006) 241–5. [DOI] [PubMed] [Google Scholar]
- [86].Chiang N, de la Rosa X, Libreros S, Pan H, Dreyfuss JM, Serhan CN, Cysteinyl-specialized proresolving mediators link resolution of infectious inflammation and tissue regeneration via TRAF3 activation, Proc. Natl. Acad. Sci. U. S. A 118(10) (2021) e2013374118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li YY, Chung GT, Lui VW, To KF, Ma BB, Chow C, Woo JK, Yip KY, Seo J, Hui EP, Mak MK, Rusan M, Chau NG, Or YY, Law MH, Law PP, Liu ZW, Ngan HL, Hau PM, Verhoeft KR, Poon PH, Yoo SK, Shin JY, Lee SD, Lun SW, Jia L, Chan AW, Chan JY, Lai PB, Fung CY, Hung ST, Wang L, Chang AM, Chiosea SI, Hedberg ML, Tsao SW, van Hasselt AC, Chan AT, Grandis JR, Hammerman PS, Lo KW, Exome and genome sequencing of nasopharynx cancer identifies NF-kappaB pathway activating mutations, Nat Commun 8 (2017) 14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chan JD, Zhang D, Liu X, Zarowiecki MZ, Berriman M, Marchant JS, Dataset for a Dugesia japonica de novo transcriptome assembly, utilized for defining the voltage-gated like ion channel superfamily, Data Brief 9 (2016) 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Huerta-Cepas J, Szklarczyk D, Forslund K, Cook H, Heller D, Walter MC, Rattei T, Mende DR, Sunagawa S, Kuhn M, Jensen LJ, von Mering C, Bork P, eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences, Nucleic Acids Res 44(D1) (2016) D286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Häcker H, Tseng PH, Karin M, Expanding TRAF function: TRAF3 as a tri-faced immune regulator, Nat Rev Immunol 11(7) (2011) 457–68. [DOI] [PubMed] [Google Scholar]
- [91].Okabe Y, Medzhitov R, Tissue biology perspective on macrophages, Nat Immunol 17(1) (2016) 9–17. [DOI] [PubMed] [Google Scholar]
- [92].Zhang R, Zhang G, Xiang B, Chen X, Tang L, Shi S, Liu Y, Ai X, Xie P, Li Z, TRAF3 negatively regulates platelet activation and thrombosis, Sci Rep 7(1) (2017) 17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Beachboard DC, Park M, Vijayan M, Snider DL, Fernando DJ, Williams GD, Stanley S, McFadden MJ, Horner SM, The small GTPase RAB1B promotes antiviral innate immunity by interacting with TNF receptor-associated factor 3 (TRAF3), J. Biol. Chem 294(39) (2019) 14231–14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhu W, Li J, Zhang R, Cai Y, Wang C, Qi S, Chen S, Liang X, Qi N, Hou F, TRAF3IP3 mediates the recruitment of TRAF3 to MAVS for antiviral innate immunity, EMBO J. 38(18) (2019) e102075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Recchiuti A, Patruno S, Mattoscio D, Isopi E, Pomilio A, Lamolinara A, Iezzi M, Pecce R, Romano M, Resolvin D1 and D2 reduce SARS-CoV-2-induced inflammatory responses in cystic fibrosis macrophages, FASEB J. 35(4) (2021) e21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chiang N, Sakuma M, Rodriguez AR, Spur BW, Irimia D, Serhan CN, Resolvin T-series reduce neutrophil extracellular traps, Blood 139(8) (2022) 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nathan C, Neutrophils and COVID-19: Nots, NETs, and knots, J. Exp. Med 217(9) (2020) e20201439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Panigrahy D, Gilligan MM, Huang S, Gartung A, Cortés-Puch I, Sime PJ, Phipps RP, Serhan CN, Hammock BD, Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19?, Cancer Metastasis Rev. 39 (2020) 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shay AE, Nshimiyimana R, Petasis NA, Haeggstrom JZ, Serhan CN, Human leukocytes selectively convert 4S,5S-epoxy-Resolvin to Resolvin D3, Resolvin D4, and a cys-Resolvin isomer, Proc. Natl. Acad. Sci. USA 118 (2021) e2116559118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nshimiyimana R, Lam TF, Aggarwal S, Serhan CN, Petasis NA, First stereoselective total synthesis of 4(S),5(S)-oxido-17(S)-hydroxy-6(E),8(E),10(Z),13(Z),15(E),19(Z)-docosahexaenoic acid, the biosynthetic precursor of resolvins D3 and D4, RSC Adv 12(19) (2022) 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fu X, Yin HH, Wu MJ, He X, Jiang Q, Zhang LT, Liu JY, High Sensitivity and Wide Linearity LC-MS/MS Method for Oxylipin Quantification in Multiple Biological Samples, J. Lipid Res 63(12) (2022) 100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN, Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue, Am J Physiol Cell Physiol 307 (2014) C39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lamon-Fava S, Liu M, Dunlop BW, Kinkead B, Schettler PJ, Felger JC, Ziegler TR, Fava M, Mischoulon D, Rapaport MH, Clinical response to EPA supplementation in patients with major depressive disorder is associated with higher plasma concentrations of pro-resolving lipid mediators, Neuropsychopharmacology doi: 10.1038/s41386-022-01527-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shivakoti R, Dalli J, Kadam D, Gaikwad S, Barthwal M, Colas RA, Mazzacuva F, Lokhande R, Dharmshale S, Bharadwaj R, Kagal A, Pradhan N, Deshmukh S, Atre S, Sahasrabudhe T, Kakrani A, Kulkarni V, Raskar S, Suryavanshi N, Chon S, Gupte A, Gupta A, Gupte N, Arriaga MB, Fukutani KF, Andrade BB, Golub JE, Mave V, Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes, Prostaglandins Other Lipid Mediat. 147 (2020) 106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, Marques RM, Dalli J, Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease, Circ. Res 122(6) (2018) 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hartling I, Cremonesi A, Osuna E, Lou PH, Lucchinetti E, Zaugg M, Hersberger M, Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS, Clin. Chem. Lab. Med 59 (2021) 1811–1823. [DOI] [PubMed] [Google Scholar]
- [107].Colas RA, Nhat LTH, Thuong NTT, Gomez EA, Ly L, Thanh HH, Mai NTH, Phu NH, Thwaites GE, Dalli J, Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis, FASEB J. 33 (2019) 13028–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van Het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiù V, Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction, Haematologica 105 (2020) 2056–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sano Y, Toyoshima S, Miki Y, Taketomi Y, Ito M, Lee H, Saito S, Murakami M, Okayama Y, Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis, Asia Pac Allergy 10(2) (2020) e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Winkler JW, Libreros S, De La Rosa X, Sansbury BE, Norris PC, Chiang N, Fichtner D, Keyes GS, Wourms N, Spite M, Serhan CN, Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation, J. Leukoc. Biol 103 (2018) 995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Motwani MP, Bennett F, Norris PC, Maini AA, George MJ, Newson J, Henderson A, Hobbs AJ, Tepper M, White B, Serhan CN, MacAllister R, Gilroy DW, Potent anti-inflammatory and pro-resolving effects of anabasum in a human model of self-resolving acute inflammation, Clin. Pharmacol. Ther 104 (2018) 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Barden A, Shinde S, Tsai IJ, Croft KD, Beilin LJ, Puddey IB, Mori TA, Effect of weight loss on neutrophil resolvins in the metabolic syndrome, Prostaglandins Leukot. Essent. Fatty Acids 148 (2019) 25–29. [DOI] [PubMed] [Google Scholar]
- [113].López-Vicario C, Titos E, Walker ME, Alcaraz-Quiles J, Casulleras M, Durán-Güell M, Flores-Costa R, Pérez-Romero N, Forné M, Dalli J, Clària J, Leukocytes from obese individuals exhibit an impaired SPM signature, FASEB J. 33(6) (2019) 7072–7083. [DOI] [PubMed] [Google Scholar]
- [114].Artiach G, Carracedo M, Plunde O, Wheelock CE, Thul S, Sjövall P, Franco-Cereceda A, Laguna-Fernandez A, Arnardottir H, Bäck M, Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease through the Resolvin E1 and ChemR23 Axis, Circulation 142 (2020) 776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Isopi E, Mattoscio D, Codagnone M, Mari VC, Lamolinara A, Patruno S, D’Aurora M, Cianci E, Nespoli A, Franchi S, Gatta V, Dubourdeau M, Moretti P, Di Sabatino M, Iezzi M, Romano M, Recchiuti A, Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis, Front Immunol 11 (2020) 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Vickery TW, Armstrong M, Kofonow JM, Robertson CE, Kroehl ME, Reisdorph NA, Ramakrishnan VR, Frank DN, Altered tissue specialized pro-resolving mediators in chronic rhinosinusitis, Prostaglandins Leukot. Essent. Fatty Acids 164 (2021) 102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Schulte F, Asbeutah AA, Benotti PN, Wood GC, Still C, Bistrian BR, Hardt M, Welty FK, The relationship between specialized pro-resolving lipid mediators, morbid obesity and weight loss after bariatric surgery, Sci Rep 10(1) (2020) 20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Barden AE, Shinde S, Burke V, Puddey IB, Beilin LJ, Irish AB, Watts GF, Mori TA, The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease, Prostaglandins Other Lipid Mediat. 136 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [119].See VHL, Mas E, Prescott SL, Beilin LJ, Burrows S, Barden AE, Huang RC, Mori TA, Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: a double-blind, randomised controlled clinical trial, Br. J. Nutr 118 (2017) 971–980. [DOI] [PubMed] [Google Scholar]
- [120].Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, Serhan CN, Maddipati KR, Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation, Sci Rep 8(1) (2018) 18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ramirez JL, Gasper WJ, Khetani SA, Zahner GJ, Hills NK, Mitchell PT, Sansbury BE, Conte MS, Spite M, Grenon SM, Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial), J. Surg. Res 238 (2019) 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schaller MS, Chen M, Colas RA, Sorrentino TA, Lazar AA, Grenon SM, Dalli J, Conte MS, Treatment with a marine oil supplement alters lipid mediators and leukocyte phenotype in healthy subjects and those with peripheral arterial disease, J. Am. Heart Assoc 9 (2020) e016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ, Dalli J, Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study, Circ. Res 126 (2020) 75–90. [DOI] [PubMed] [Google Scholar]
- [124].Dawczynski C, Dittrich M, Neumann T, Goetze K, Welzel A, Oelzner P, Volker S, Schaible AM, Troisi F, Thomas L, Pace S, Koeberle A, Werz O, Schlattmann P, Lorkowski S, Jahreis G, Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil, Clin. Nutr 37 (2018) 494–504. [DOI] [PubMed] [Google Scholar]
- [125].Welty FK, Schulte F, Alfaddagh A, Elajami TK, Bistrian BR, Hardt M, Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B(4), FASEB J. 35(4) (2021) e21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mozurkewich EL, Greenwood M, Clinton C, Berman D, Romero V, Djuric Z, Qualls C, Gronert K, Pathway Markers for Pro-resolving Lipid Mediators in Maternal and Umbilical Cord Blood: A Secondary Analysis of the Mothers, Omega-3, and Mental Health Study, Front Pharmacol 7 (2016) 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R, Resolvin E1 and Chemokine-like Receptor 1 Mediate Bone Preservation, J. Immunol 190 (2013) 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, Forteza MJ, Jiang X, Andonova T, Walker ME, Dalli J, Arnardottir H, Gistera A, Thul S, Wheelock CE, Paulsson-Berne G, Ketelhuth DFJ, Hansson GK, Back M, ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages, Circulation 138(16) (2018) 1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Al-Shaer AE, Pal A, Shaikh SR, Resolvin E1-ChemR23 Axis Regulates the Hepatic Metabolic and Inflammatory Transcriptional Landscape in Obesity at the Whole Genome and Exon Level, Front Nutr 8 (2021) 799492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN, Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation, J. Immunol 178 (2007) 3912–3917. [DOI] [PubMed] [Google Scholar]
- [131].Hayashi S, Muraleedharan CK, Oku M, Tomar S, Hogan SP, Quiros M, Parkos CA, Nusrat A, Intestinal epithelial BLT1 promotes mucosal repair, JCI Insight 7(23) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN, Resolvin D1 receptor stereoselectivity and regulation of inflammation and pro-resolving microRNAs, Am. J. Pathol 180 (2012) 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sansbury BE, Li X, Wong B, Patsalos A, Giannakis N, Zhang MJ, Nagy L, Spite M, Myeloid ALX/FPR2 regulates vascularization following tissue injury, Proc. Natl. Acad. Sci. U. S. A (2020) doi: 10.1073/pnas.1918163117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Leroy V, Cai J, Tu Z, McQuiston A, Sharma S, Emtiazjoo A, Atkinson C, Upchurch GR Jr., Sharma AK, Resolution of post-lung transplant ischemia-reperfusion injury is modulated via Resolvin D1-FPR2 and Maresin 1-LGR6 signaling, J. Heart Lung Transplant doi: 10.1016/j.healun.2022.12.013 (2022). [DOI] [PubMed] [Google Scholar]
- [135].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, Petasis NA, Serhan CN, Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Schmid M, Gemperle C, Rimann N, Hersberger M, Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32, J. Immunol 196(8) (2016) 3429–37. [DOI] [PubMed] [Google Scholar]
- [137].Arnardottir H, Thul S, Pawelzik SC, Karadimou G, Artiach G, Gallina AL, Mysdotter V, Carracedo M, Tarnawski L, Caravaca AS, Baumgartner R, Ketelhuth DF, Olofsson PS, Paulsson-Berne G, Hansson GK, Bäck M, The resolvin D1 receptor GPR32 transduces inflammation resolution and atheroprotection, J. Clin. Invest 131(24) (2021) e142883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chiang N, Dalli J, Colas RA, Serhan CN, Identification of resolvin D2 receptor mediating resolution of infections and organ protection, J. Exp. Med 212(8) (2015) 1203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Chiang N, de la Rosa X, Libreros S, Serhan CN, Novel Resolvin D2 Receptor Axis in Infectious Inflammation, J. Immunol 198(2) (2017) 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hellmann J, Sansbury BE, Wong B, Li X, Singh M, Nuutila K, Chiang N, Eriksson E, Serhan CN, Spite M, Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair, J. Invest. Dermatol 138 (2018) 2051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, Lehner KA, Bielenberg DR, Schmidt B, Dalli J, Greene ER, Gus-Brautbar Y, Piwowarski J, Mammoto T, Zurakowski D, Perretti M, Sukhatme VP, Kaipainen A, Kieran MW, Huang S, Panigrahy D, Resolvins suppress tumor growth and enhance cancer therapy, J. Exp. Med 215(1) (2018) 115–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Dort J, Orfi Z, Fabre P, Molina T, Conte TC, Greffard K, Pellerito O, Bilodeau JF, Dumont NA, Resolvin-D2 targets myogenic cells and improves muscle regeneration in Duchenne muscular dystrophy, Nat Commun 12(1) (2021) 6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Fitzgerald H, Bonin JL, Sadhu S, Lipscomb M, Biswas N, Decker C, Nabage M, Bossardi R, Marinello M, Mena AH, Gilliard K, Spite M, Adam A, MacNamara KC, Fredman G, The Resolvin D2-GPR18 Axis Enhances Bone Marrow Function and Limits Hepatic Fibrosis in Aging, bioRxiv doi: 10.1101/2023.01.05.522881 (2023). [DOI] [Google Scholar]
- [144].Zuo G, Zhang D, Mu R, Shen H, Li X, Wang Z, Li H, Chen G, Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats, Mol Brain 11(1) (2018) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Perna E, Aguilera-Lizarraga J, Florens MV, Jain P, Theofanous SA, Hanning N, De Man JG, Berg M, De Winter B, Alpizar YA, Talavera K, Vanden Berghe P, Wouters M, Boeckxstaens G, Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS, Gut 70(7) (2021) 1275–1286. [DOI] [PubMed] [Google Scholar]
- [146].Lu Q, Yang Y, Zhang H, Chen C, Zhao J, Yang Z, Fan Y, Li L, Feng H, Zhu J, Yi S, Activation of GPR18 by Resolvin D2 Relieves Pain and Improves Bladder Function in Cyclophosphamide-Induced Cystitis Through Inhibiting TRPV1, Drug Des Devel Ther 15 (2021) 4687–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Bardin M, Pawelzik SC, Lagrange J, Mahdi A, Arnardottir H, Regnault V, Fève B, Lacolley P, Michel JB, Mercier N, Bäck M, The resolvin D2 - GPR18 axis is expressed in human coronary atherosclerosis and transduces atheroprotection in apolipoprotein E deficient mice, Biochem. Pharmacol 201 (2022) 115075. [DOI] [PubMed] [Google Scholar]
- [148].Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN, Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions, J. Clin. Invest 129 (2019) 5294–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Elder CT, Filiberto AC, Su G, Ladd Z, Leroy V, Pruitt EY, Lu G, Jiang Z, Sharma AK, Upchurch GR Jr., Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation, FASEB J. 35(8) (2021) e21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Li X, Xu B, Wu J, Pu Y, Wan S, Zeng Y, Wang M, Luo L, Zhang F, Jiang Z, Xu Y, Maresin 1 Alleviates Diabetic Kidney Disease via LGR6-Mediated cAMP-SOD2-ROS Pathway, Oxid Med Cell Longev 2022 (2022) 7177889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li H, Li X, Hao Y, Wu C, Fu Y, Su N, Chen H, Ying B, Wang H, Su L, Cai H, He Q, Cai M, Sun J, Lin J, Scott A, Smith F, Huang X, Jin S, Maresin 1 intervention reverses experimental pulmonary arterial hypertension in mice, Br. J. Pharmacol 179(22) (2022) 5132–5147. [DOI] [PubMed] [Google Scholar]
- [152].Khedgikar V, Charles JF, Lehoczky JA, Mouse LGR6 regulates osteogenesis in vitro and in vivo through differential ligand use, Bone 155 (2022) 116267. [DOI] [PubMed] [Google Scholar]
- [153].Krishnamoorthy N, Walker KH, Brüggemann TR, Tavares LP, Smith EW, Nijmeh J, Bai Y, Ai X, Cagnina RE, Duvall MG, Lehoczky JA, Levy BD, The Maresin 1-LGR6 axis decreases respiratory syncytial virus-induced lung inflammation, Proc. Natl. Acad. Sci. U. S. A 120(2) (2023) e2206480120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR, GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain, J. Clin. Invest 128(8) (2018) 3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bang S, Donnelly CR, Luo X, Toro-Moreno M, Tao X, Wang Z, Chandra S, Bortsov AV, Derbyshire ER, Ji RR, Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice, Nat Commun 12(1) (2021) 1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Dalli J, Sanger JM, Rodriguez AR, Chiang N, Spur BW, Serhan CN, Identification and Actions of a Novel Third Maresin Conjugate in Tissue Regeneration: MCTR3, PLoS One 11(2) (2016) e0149319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ramon S, Dalli J, Sanger JM, Winkler JW, Aursnes M, Tungen JE, Hansen TV, Serhan CN, The Protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation Am. J. Pathol 186 (2016) 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rodriguez AR, Spur BW, Total synthesis of pro-resolving and tissue-regenerative Protectin sulfido-conjugates, Tetrahedron Lett. 56 (2015) 5811–5815. [Google Scholar]
- [159].Pistorius K, Ly L, Souza PR, Gomez EA, Koenis DS, Rodriguez AR, Foster J, Sosabowski J, Hopkinson M, Rajeeve V, Spur BW, Pitsillides A, Pitzalis C, Dalli J, MCTR3 reprograms arthritic monocytes to upregulate Arginase-1 and exert pro-resolving and tissue-protective functions in experimental arthritis, EBioMedicine 79 (2022) 103974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Han J, Li H, Bhandari S, Cao F, Wang XY, Tian C, Li XY, Zhang PH, Liu YJ, Wu CH, Smith FG, Jin SW, Hao Y, Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury, J Cell Mol Med 24(8) (2020) 4736–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Yang Q, Xu HR, Xiang SY, Zhang C, Ye Y, Shen CX, Mei HX, Zhang PH, Ma HY, Zheng SX, Smith FG, Jin SW, Wang Q, Resolvin Conjugates in Tissue Regeneration 1 Promote Alveolar Fluid Clearance by Activating Alveolar Epithelial Sodium Channels and Na, K-ATPase in Lipopolysaccharide-Induced Acute Lung Injury, J. Pharmacol. Exp. Ther 379(2) (2021) 156–165. [DOI] [PubMed] [Google Scholar]
- [162].Li H, Hao Y, Yang LL, Wang XY, Li XY, Bhandari S, Han J, Liu YJ, Gong YQ, Scott A, Smith FG, Jin SW, MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx, J. Cell. Physiol 235(10) (2020) 7283–7294. [DOI] [PubMed] [Google Scholar]
- [163].Wang XY, Li XY, Wu CH, Hao Y, Fu PH, Mei HX, Chen F, Gong YQ, Jin SW, Li H, Protectin conjugates in tissue regeneration 1 restores lipopolysaccharide-induced pulmonary endothelial glycocalyx loss via ALX/SIRT1/NF-kappa B axis, Respir Res 22(1) (2021) 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zhuang R, Yang X, Cai W, Xu R, Lv L, Sun Y, Guo Y, Ni J, Zhao G, Lu Z, MCTR3 reduces LPS-induced acute lung injury in mice via the ALX/PINK1 signaling pathway, Int Immunopharmacol 90 (2021) 107142. [DOI] [PubMed] [Google Scholar]
- [165].Zhang PH, Han J, Cao F, Liu YJ, Tian C, Wu CH, Smith FG, Hao Y, Jin SW, PCTR1 improves pulmonary edema fluid clearance through activating the sodium channel and lymphatic drainage in lipopolysaccharide-induced ARDS, J. Cell. Physiol 235 (2020) 9510–9523. [DOI] [PubMed] [Google Scholar]
- [166].Pan J, Li X, Wang X, Yang L, Chen H, Su N, Wu C, Hao Y, Jin S, Li H, MCTR1 Intervention Reverses Experimental Lung Fibrosis in Mice, J Inflamm Res 14 (2021) 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Xiao J, Yang Q, Zhang Y, Xu H, Ye Y, Li L, Yang Y, Jin S, Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury, Cell Biosci 11(1) (2021) 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yang Y, Zhu Y, Xiao J, Tian Y, Ma M, Li X, Li L, Zhang P, Li M, Wang J, Jin S, Maresin conjugates in tissue regeneration 1 prevents lipopolysaccharide-induced cardiac dysfunction through improvement of mitochondrial biogenesis and function, Biochem. Pharmacol 177 (2020) 114005. [DOI] [PubMed] [Google Scholar]
- [169].Yang Y, Li XY, Li LC, Xiao J, Zhu YM, Tian Y, Sheng YM, Chen Y, Wang JG, Jin SW, γδ T/Interleukin-17A Contributes to the Effect of Maresin Conjugates in Tissue Regeneration 1 on Lipopolysaccharide-Induced Cardiac Injury, Front Immunol 12 (2021) 674542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ye J, Peng J, Liu K, Zhang T, Huang W, MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury, Am J Physiol Gastrointest Liver Physiol 323(3) (2022) G283–G293. [DOI] [PubMed] [Google Scholar]


