Abstract
Malaria is a human health hazard in the tropical and subtropical zones of the globe and poised to be eliminated by the year 2030. Despite a decrease in incidence in the last two decades, many endemic countries including India report cases regularly. Epidemiology of malaria in India stands unique due to several features of the Plasmodium parasites, Anopheles vectors, eco-epidemiological situations conducive for disease transmission and susceptible humans living in rural and forested areas. Limitations in public health reach and poor health seeking behaviour of vulnerable populations living in hard-to-reach areas add to the problem. We herewith have brought all these factors together in a comprehensive framework and opine that in spite of complexities, targeted elimination of malaria in India is achievable with planned programmatic approaches.
Keywords: Malaria, Anopheles, Plasmodium, Epidemiology, Elimination, India
Evolutionary epidemiology of malaria in the context of elimination
Malaria is a vector-borne infectious disease of the tropical and sub-tropical regions of the globe. Malaria has put threat to human lives both in term of mortality and morbidity in all its distribution zones.. As per the World Malaria Report, 2022 released by World Health Organization (WHO), 247 million malaria cases and about 0.619 million deaths were reported worldwide in the year 2021 [1]. Although incidences of malaria have been decreased over past few years [2], if history of malaria resurgence is to be believed, the future havoc of malaria is uncertain [3]. It is a well-known fact that epidemiological outcome of malaria depends on several factors: (i) the biology of parasites, (ii) adaptive behaviour of vectors, (iii) susceptibility of human host and (iv) influence of local eco-climatic situations [4]. Following the theory of Darwinian evolution where species populations undergo natural selection and thereby adapt to simultaneous changes in eco-climatic conditions, evidences of natural selection in genes involved in malaria of all the three different organisms in malaria triad (host-parasite-vector) have been detected [5]. For example, both the parasites and vectors have successfully adapted to antimalarials and to insecticides, respectively. This situation ultimately change epidemiological scenarios of malaria in local settings, posing new challenges to control the disease in almost all the endemic countries in the globe. It is therefore considered that malaria epidemiology is dynamic and more of ‘local and focal’ nature than ‘global’ [6], needing deep basic understanding on the determinants of such outcome of evolutionary epidemiology (see Glossary) at the local level. Any successful elimination strategy must therefore rely on such basic evolutionary epidemiological information on malaria in a local setting, and such knowledge must be kept in mind while intervention measures are developed [7].
To this extent, although much of the global malaria mortality and morbidity are contributed by the African countries, the southeast Asian and south American countries contribute substantially [8]. Over the past two decades, incidences of malaria have drastically gone down in the South East Asian countries (from 22.8 million in the year 2000 to about 5.4 million in 2021) with only 2% of the global malaria burden [1]. India accounted for about 79% malaria cases and about 82.4% of all malaria deaths in the WHO South-East Asia Region in the year 2021 [1]. It is argued that epidemiology of malaria in India is quite different from other endemic countries in the globe (including the African countries with high malaria morbidity and mortality) in term of variable complexities [9, 10].
Epidemiological complexities of malaria in India
Distribution, species composition and insecticide resistance of malaria vectors
About 58 morphologically identified species of the genus Anopheles are present in India; with six (An. culicifacies, An. fluviatilis, An. sundaicus, An. stephensi, An. baimai and An. minimus) serve as primary and four (An. annularis, An. subpictus, An. philippinensis and An. jeyporiensis) as secondary vectors of malaria [11]. Besides An. stephensi, other five primary malaria vectors display species complexes [12]. For example, An. culicifacies, that contributes to about 65% malaria transmission, form five morphologically indistinguishable species (species A to E). Interestingly, species A is considered to be the most efficient vector of malaria, while species B is a poor or non-vector [13]. Similarly, An. fluviatilis (that contributes to about 15% malaria transmission) comprises three sibling species; S, T and U [14], with the possible existence of one additional form in India, provisionally designated An. fluviatilis form X [15]. Likewise, the An. minimus species complex comprises three sibling species: A, C and E. The species A, formally recognized as An. minimus sensuo stricto is distributed in India [16, 17]. An. baimaii (a highly anthropophagic vector found in the north-eastern states of India) and An. elegans (distributed mainly in Shimoga hills of the Karnataka state) are two of the seven species of the An. dirus complexe that are found in India [18]. A new cytotype D has also been reported in An. sundaicus in Andaman and Nicobar Island [17, 18]. The distribution of malaria vectors along with members of species complexes in different states of India (Figure 1) indicates widespread and localized prevalence based on eco-climatic conditions. While An. culicifacies (responsible for about 65% malaria transmission) is prevalent in rural and peri-urban areas, An. fluviatilis (responsible for about 15% malaria transmission) is predominantly found in forest plains and foothills in India.
Figure 1. Map of India showing distribution of different species (and sibling species) of the malaria vector, Anopheles.
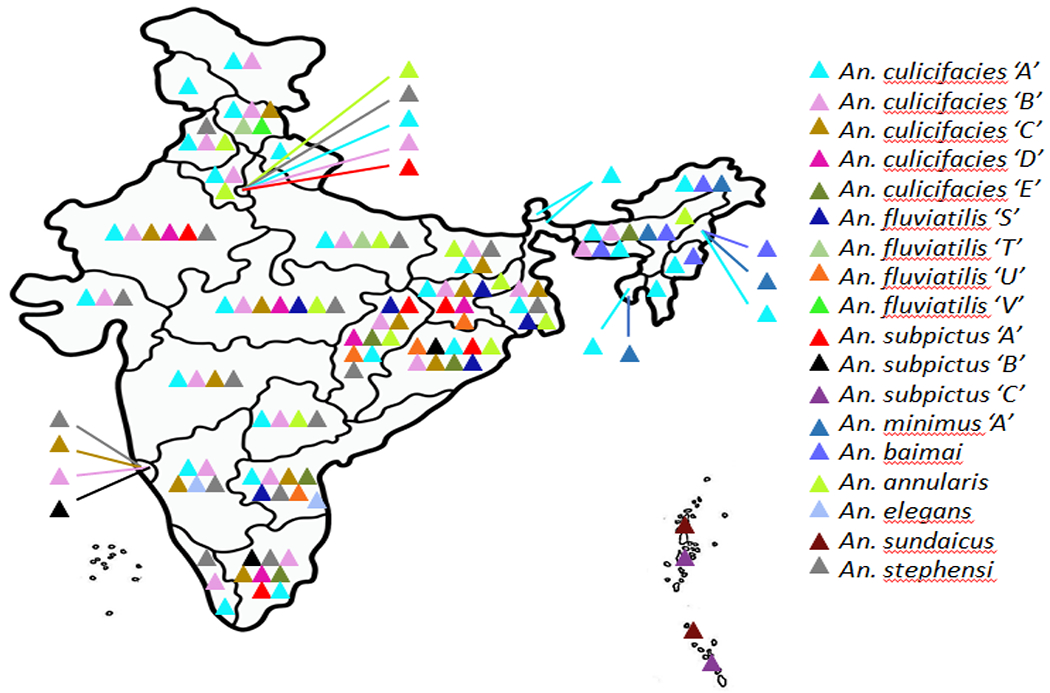
Triangles with different colours represent to a species of Anopheles (and sibling species, under inverted coma). Data source: [17].
Migration of different malaria vectors across nearby locations, ecological successions and colonization to new habitat through evolutionary adaptation to eco-climatic changes have been reported in many instances in India [17]. For example, An. stephensi that contributes to about 12-15% of malaria transmission, is widespread and majorly contribute to transmission of urban malaria in India. Interestingly, over the past 50 years, urbanization and associated water storage practices are considered to have helped in extensive range expansions of An. stephensi across wide swaths of Asia, into the Middle East, and more recently into Africa [19, 20]. In addition, changes in biting and resting behaviour, host preferences and breeding sites in the primary malaria vectors in India are some of the other complex behavioural features [21]. Change in vectorial capacity (from non-vector to secondary vector, as calculated by a formula according to the Ross-Macdonald model) [22] and territory expansion by An. subpictus [23] are examples of behavioural and eco-climatic adaptation of malaria vectors in India. Similarly, ecological succession of the principal malaria vector, An. culicifacies is also noteworthy. This species, which was very rarely found in the north-eastern states of India previously, now overshadows the known principal malaria vector, An. minimus and An. baimai [24]. Likewise, An. minimus, principally found in the north-eastern states had expanded its range to far places in the southern Indian states (Andhra Pradesh, Tamil Nadu, Kerala and Karnataka) and resurfaced in Uttar Pradesh and Odisha where it was found about three decades ago [25]. This possibly happened in parallel with change in resting behaviour of An. minimus (traditionally endophilic to exophilic) for adaptation to new habitat [25].
Considering controlling vector populations as the most effective measure of malaria control, insecticides are of common use for preventing man-mosquito contacts either by indoor residual spraying (IRS) or insecticide treated nets (ITNs). Long use of insecticides had created evolutionary pressure for adaption to the insecticides used in programmatic mode in India by the Anopheles mosquitoes. As a result, insecticide-resistant malaria vectors are widely populated in India. For example, An. culicifacies is reported to display complete resistance to the insecticide, dichloro-diphenyl trichloroethane (DDT), and also is mostly resistant to malathion. Resistance to multiple insecticides in a single An. Culicifacies mosquito had also been reported in India [26]. Similarly, the other three primary vectors of malaria; An. fluviatilis, An. sundaicus and An. stephensi are also resistant to DDT and malathion [27]. Distribution of different species of Anopheles with current status of resistance to different insecticides in different malaria endemic states of India is depicted in Table 1. Interestingly however, An. minimus, apart from some degree of tolerance to DDT [28] is still susceptible to almost all the insecticides, although cases of behavioural avoidance to some insecticides have been reported [29].
Table 1.
Status of resistance to different insecticides by different species of Anopheles that are vectors (both primary and secondary) of malaria parasites in India.
| Malaria vectors of Anopheles species | Resistance status of different insecticides in different Indian states | ||
|---|---|---|---|
| Organochlorine (DDT) | Organophosphate (Malathion) | Pyrethroid (Deltamethrin) | |
| An. culicifacies (Primary vector) | Andhra Pradesh, Assam, Chhattisgarh, Delhi, Gujarat, Haryana, Jharkhand, Maharashtra, Gujarat, Karnataka, Madhya Pradesh, Odisha, Rajasthan, Tamil Nadu, Telangana, Uttar Pradesh, West Bengal | West Bengal, Uttarakhand, Telangana, Andhra Pradesh, Odisha, Maharashtra, Madhya Pradesh, Karnataka, Jharkhand, Haryana, Gujarat, Chhattisgarh | Andhra Pradesh, Assam, Chhattisgarh, Gujarat, Madhya Pradesh, Odisha, Tamil Nadu, Telangana |
| An. fluviatilis (Primary vector) | Chhattisgarh, Himachal Pradesh, Jharkhand, Karnataka, Maharashtra, Tamil Nadu, Uttarakhand | Jharkhand, Maharashtra | Maharashtra |
| An. sundaicus (Primary vector) | Andaman and Nicobar Islands | No report available | No report available |
| An. minimus (Primary vector) | Assam, Odisha, Tripura | No report available | No report available |
| An. stephensi (Primary vector) | Delhi, Goa, Gujarat, Karnataka, Rajasthan, Maharashtra, Uttar Pradesh, West Bengal | Delhi, Goa, Gujarat, Karnataka, Rajasthan, West Bengal | Gujarat, Karnataka, Rajasthan |
| An. baimai (Primary vector) | No report available | No report available | No report available |
| An. subpictus (Secondary vector) | Gujarat, Rajasthan | Gujarat, Punjab, Rajasthan | Gujarat, Punjab, Rajasthan |
| An. annularis (Secondary vector) | Assam, Jharkhand, Maharashtra, Odisha, Rajasthan | Maharashtra, Rajasthan | Assam, Maharashtra |
| An. philippinensis (Secondary vector) | No report available | No report available | No report available |
| An. jeyporiensis (Secondary vector) | No report available | No report available | No report available |
Updated published literature have been used to gather information on the three insecticides that are used in the field in programmatic mode in India.
Distribution, pattern of infection and drug resistance in Indian malaria parasites
Complementing to the intricacies in malaria vectors, malaria parasites in India too pose high degree of complex features and are distinct in many respects in comparison to other endemic countries [10]. All the five known human malaria parasites of the genus Plasmodium (Plasmodium falciparum, P. vivax, P. ovale, P. malaria and P. knowlesi) are present either in single or in mixed infections (as inferred with PCR diagnosis) in different states of India (Figure 2) [10]. The two globally distributed major species of malaria parasites (P. vivax and P. falciparum) are distributed in almost all the endemic regions and the proportion of malaria caused by P. falciparum to P. vivax is about 49:51 [30]. Interestingly, population genetic structure of these two malaria parasites inferred with evolutionarily neutral genetic loci seem to be quite opposite. While local and geographically close populations are genetically identical in P. falciparum [31] (Figure 3A), genetic similarity in geographically distant populations are features of Indian P. vivax [32] (Figure 3B). Furthermore, human infections of hitherto neglected malaria parasites (P. ovale and P. malarie) are majorly confined to Odisha state that contribute highly to malaria incidences [33]. This fact has recently been confirmed with detection of infections with substantial frequencies (14.1% for P. ovale and 10.3% for P. malarie) in Odisha [34]. Similarly, P. knowlesi has so far been reported in five different Indian states/union territories, majorly in mixed infection with P. falciparum [35].
Figure 2. Distributional prevalence of the five human malaria parasites in India as revealed with PCR diagnosis.
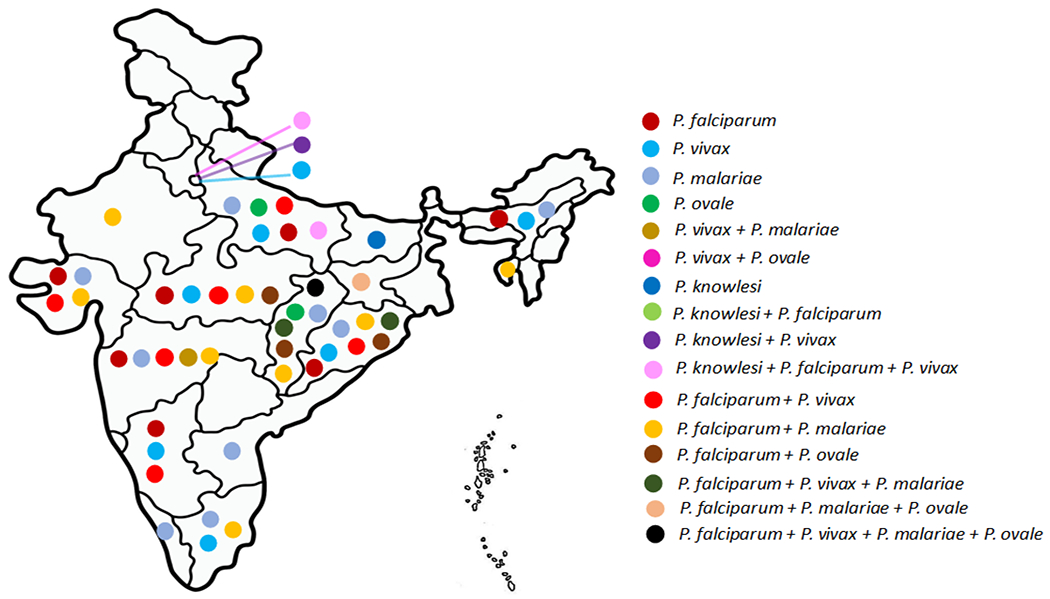
Dots of different colors depict different malaria parasites either in mono or mixed infections. Data source: [30, 35].
Figure 3. Differential population genetic structure of the two widely distributed malaria parasites (P. falciparum and P. vivax) in India inferred with neutral DNA sequence polymorphisms (A and B) and distributional prevalence of four different haplotypes of the Pfcrt associated with CQ resistance (C).
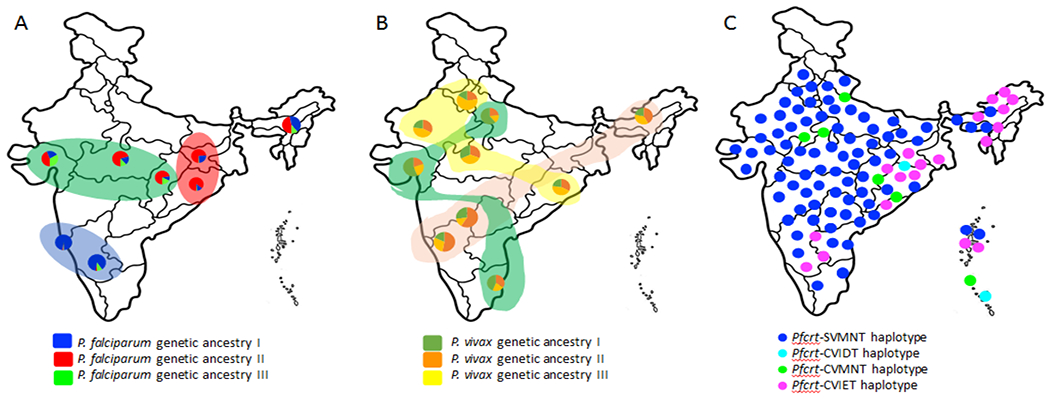
While P. falciparum (A) displays geographically structured populations (data source: [31]), no such structure could be seen in Indian P. vivax (data source: [32]). (B). The colors in the pie charts (both in A and B) refer to presence of different genetic ancestries of the malaria parasite P. falciparum (A) and in P. vivax (B) in different Indian populations. (C) Graphical representation on the distribution of four major haplotypes of the Pfcrt gene (SVMNT, CVIDT, CVMNT and CVIET) in different regions of Indian P. falciparum [10]. Data not on scale.
Malaria epidemiological intricacies are further convoluted by the recent finding on the presence of a novel P. falciparum-like parasite in Indians (PfIndia*) that is considered to be a missing-link during the course of host-switching of P. falciparum [10, 36]. Further, detection of P. falciparum in rhesus and bonnet monkeys of India [37] provides evidence on the ongoing exchange of P. falciparum isolates between Indians and Indian non-human primates. Interestingly, whereas malaria caused by P. vivax are considered to be benign with generally favourable treatment outcome, finding of (i) quick relapse pattern in P. vivax infections is suggested to be attributed to Chesson strain type [38]. Furthermore, with (ii) several reports of P. vivax infections displaying severe malaria outcome [39, 40], the future of P. vivax malaria in India remains concerning. On the other hand, P. falciparum seems to be evolving to demonstrate no acute clinical symptoms (asymptomatic/sub-microscopic infections), generally correlating with low density infection in high endemic areas [41], although some proportion of these asymptomatic infection may display symptoms and cause mortality and morbidity. Interestingly, prevalence of asymptomatic infection in the community has been found to be in the range of 5-50% in different malaria transmission settings in India [41, 42, 43]. Moreover, infections by P. falciparum and P. vivax in a single individual (mixed infections) that was thought to be negligible before, have come out to be as high as 13% [30], 30% [33] and 45% [44] at different timepoints and in different malaria epidemiological settings in India. Interestingly, the two neglected malaria parasites, P. ovale and P. malarie seem to use the infection pathways of either P. vivax and P. falciparum [33], and such incidences can be up to 75.8% for P. ovale [34] in India.
Malaria treatment relies on antimalarials; with chloroquine (CQ) as the principal first-line drug in the 20th century that had sustained for the longest duration. However, continuous and irrational use of CQ had led to evolution of drug-resistant malaria parasite, P. falciparum [45] that is now spread to all the P. falciparum malaria endemic regions of the globe [10]. Distributional prevalence and the patterns of drug-resistance in P. falciparum add further to complexity of malaria epidemiology in India. Field surveillance with molecular markers had provided evidence of absolute resistance to chloroquine (CQ) [10, 46] (Figure 3C) and great degree of resistance for sulfadoxine and pyrimethamine (SP) in P. falciparum isolates from almost all over India [47]. Similarly, large-scale resistance to SP in Indian P. vivax had also been reported [48]. Moreover, higher diversities in genes providing resistance to antimalarials (CQ and SP) in Indian P. falciparum than African isolates had been detected [49, 50]. Interestingly, except single reports each in eastern [51] and southern Indian isolates [52], Indian P. falciparum remains grossly susceptible to artemisinin. However, recently, emergence of reduced sensitivity to artesunate-sulfadoxine-pyrimethamine combination drug in central Indian P. falciparum isolates had been reported [53]. It had also been suggested that artemisinin-resistance in Indian P. falciparum might be kelch13-independent [53]. To this extent, P. vivax in India remains largely susceptible to CQ [54], although sporadic cases of resistance to this drug had been reported [55].
Do socio-behavioural and cultural aspects in communities influence malaria intervention plans?
Since local eco-climatic factors drive malaria transmission in a defined zone, malaria is considered as a ‘local and focal’ disease. As a result, socio-behavioural and cultural aspects of local communities contribute to the net malaria outcome in the concerned malaria endemic zone. Apart from the fact that malaria epidemiological outcome depends on the level of community awareness to follow preventive measures (e.g., minimizing man-mosquito contact), health seeking behaviour and treatment adherence are also considered equally important [56]. Availability of healthcare facility in locations that can easily accessed by the local communities also plays a major role. Furthermore, in tribal areas in India, where malaria is highly endemic, easy access and faith on the ‘traditional tribal healers’ also minimize access to public health facilities by the tribal communities [57]. Therefore, whether decisions taken by the government on malaria control/elimination are effectively implemented in the field due to various social, cultural, and behavioural factors associated with specific areas, to be regularly assessed and if required, to be set right [58]. To this extent, bringing public health facilities on malaria close to communities living in high malaria endemic regions and generating awareness among the local communities through counselling and advocacy for utilization of the public health facilities could help [59]. Such complexities due to socio-behavioural and cultural aspects put an additional layer of complexity to the malaria elimination plan in India. Social mobilization to shift ownership of health including malaria to the local communities [60] with public health support could be a sustainable solution in term of early screening, prompt treatment, drug adherence and prevention of malaria in India.
How might malaria complexities impact elimination in India?
Undoubtedly, there exists multifaceted epidemiological complexities of malaria in India (Box 1). This is reflected by several unique features of the malaria parasites, vectors and eco-climatic situations conducive for malaria. In this regard, (i) range expansion and disappearance of species of Anopheles with high transmission capacity is of high values in term of malaria elimination. Further, (ii) dominance of An. culicifacies that is responsible for 60-65% malaria transmission in India in the north-eastern states and (iii) range expansion of An. minimus to four south Indian states will surely put a challenge in malaria elimination efforts. Moreover, (iv) high degree of resistance to multiple insecticides in An. culicifacies will add the problem. Similarly, (v) change in vectorial capacity (from non-vector to vector) by An. subpictus will put further challenge. Elimination program in India should therefore consider these changes in vector behaviour and integrate appropriate measures to deal with these complexities while framing elimination strategies.
Box 1. The Center for the Study of Complex Malaria in India (CSCMi).
The Center for the Study of Complex Malaria in India (CSCMi; http://malariacenterindia.org) is one of ten International Centers of Excellence in Malaria Research (ICEMRs) funded by the United State National Institute of Allergy and Infectious Diseases/National Institutes of Health [70]. Active since 2010 and led by faculty at New York University and the London School of Hygiene and Tropical Medicine, the overall goal of the center is to address gaps in knowledge on the epidemiology, transmission and pathogenesis of malaria in India, and to help train the next generation of malariologists through capacity building and transfer of technology. The CSCMi is a close partnership between Indian and international researchers, clinicians and public health workers at a variety of private, public, and government institutions, including the Indian Council of Medical Research (ICMR)-National Institute for Research in Tribal Health, Indian Institute for Public Health-Shillong, Ispat General Hospital, Community Welfare Society Hospital, and ICMR-National Institute for Malaria Research.
The focus of CSCMi is to understand the complexity of malaria in India [71–72]. Considering the fact that research and surveillance of malaria in India must include studies on P. falciparum and P. vivax, less prevalent P. malariae and P. ovale, and ten different Anopheles species known to be vectors [10], studies have been conducted at six different field sites in the states of Meghalaya, Odisha, Gujarat, Tamil Nadu, Madhya Pradesh, and the National Capital Territory of Delhi (Figure I). Following are the key findings:
Malaria epidemiological studies in four Indian states revealed high burdens of asymptomatic and sub-microscopic P. falciparum infections that may act as a reservoir, with implications for malaria elimination [73–75].
Testing of novel surveillance tools, such as reactive case detection [76], and sero-epidemiological ones [74, 77].
Studies on severe P. vivax as well as severe P. falciparum malaria, informed first report of posterior reversible encephalopathy syndrome [78] in P. falciparum, and clinical characterization of P. vivax [79].
Generation of the first reference genomes of Indian P. vivax and P. falciparum that indicated greater genetic diversity in P. vivax than P. falciparum [80, 81].
Anopheles surveillance studies confirmed (i) decline in An. baimaii and An. minimus populations in the northeast India [73], (ii) shift in An. fluviatilis to more zoophilic behaviour warranting redirecting control efforts towards the zoophilic cycle [82], and (iii) identification of preference to cattle shed by An. stephensi over human dwellings [83].
Figure I. Map of the six sites involved in CSCMi studies since 2010 to till date.
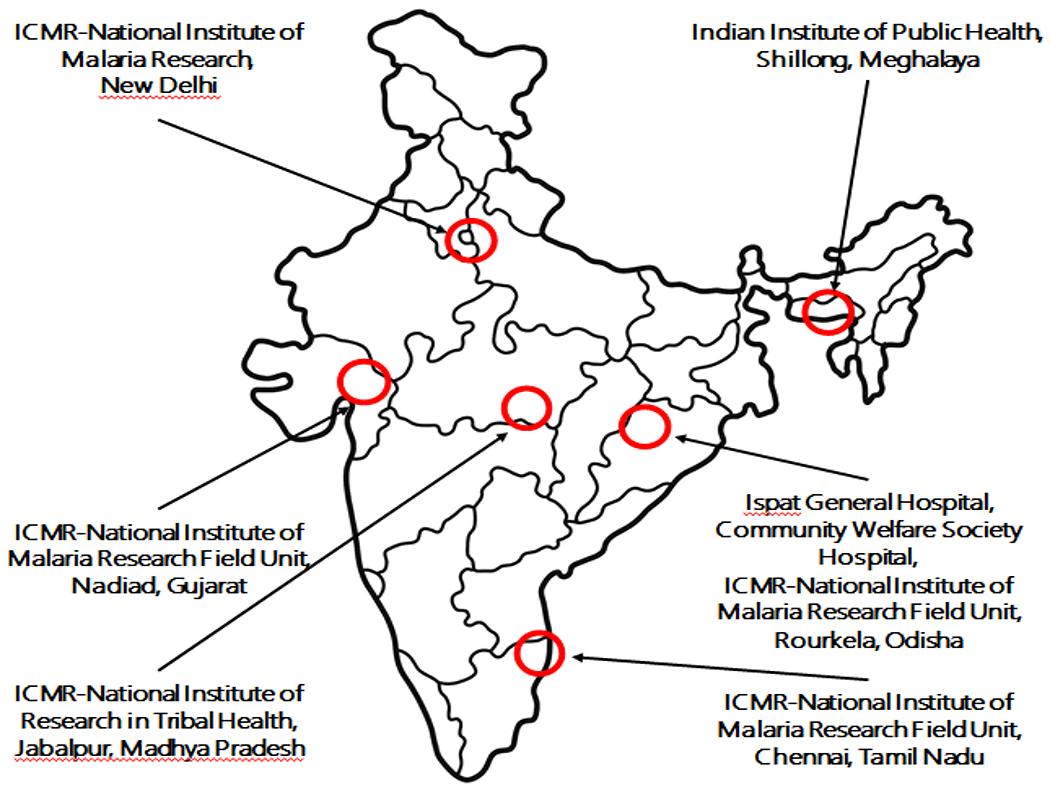
The sites are indicated with red circles and the names of the institutions involved in the study are indicated in addition.
Multidimensional complexities in malaria parasites and malaria epidemiological outcome in India can also put further challenges to malaria elimination efforts in India. Apart from distribution of all the five human malaria parasites in defined eco-climatic setups, infection of multiple parasites in a single individual as mixed infection might jeopardize treatment outcomes and will have far-reaching impact in elimination program. This is true, as both P. falciparum and P. vivax have undergone demographic expansion over the years and are currently growing in population size [31, 32]. Further, high genetic diversity in the drug-resistant genes in P. falciparum indicating genetic reconstruction [49] might attract natural selection for better adaptation to the existing drug and evolve resistance to new drugs as well [61]. To this respect, evolution of P. falciparum isolates to the working antimalarial artemisinin with involvement of some other genetic determinant for artemisinin resistance [53] is daunting. Furthermore, range expansion of the two neglected malaria parasites (P. ovale and P. malarie) [33], high asymptomatic infections of P. falciparum [42] might pose great hindrance in malaria elimination drive. This is because, some proportion of asymptomatic infection, with time, may become symptomatic causing mortality and morbidity. Moreover, evolutionary connection of P. falciparum with non-human primates [36, 37], reports of Chesson strain of P. vivax [38] might enhance zoonotic transmission of malaria to an unimaginable extent that have not been considered by the elimination plans. Apart from this, differential genetic structure displayed by P. vivax and P. falciparum in India (Figure 3) is quite intriguing. Observation of genetic similarities among physically close populations (in P. falciparum) that are quite genetically distinct from other such local populations (Figure 3A) indicates that one single elimination program might not be effective in whole of India. Alternatively, tailored-made, locally suitable, customized and sustainable management and elimination plans might work for that particular locality [62]. Therefore, India needs several such different local and focal P. falciparum malaria elimination plans to reach the goal by the year 2030. Conversely, P. vivax showing no population genetic structure (Figure 3B) with high genetic differentiation among local populations and high similarities with geographically distant population in India will require thorough genetic mapping for similarities/differences, on which basis further elimination strategies of P. vivax malaria can be built up.
Indian malaria elimination initiatives and lessons learnt
In order to eliminate malaria nationally and contribute to improved health, quality of life and alleviation of poverty in India, the Indian government has framed a guideline called the “National Framework for Malaria Elimination 2016-2030” (NFME) [63]. Developed in line with the WHO global technical strategy for malaria and the malaria elimination roadmap of Asia Pacific Leaders Malaria Alliance (APLMA), the primary aims of the NFME are to (i) eliminate malaria (zero indigenous cases) in the country by the year 2030, (ii) maintain malaria-free status where transmission has been interrupted and (iii) prevent reintroduction of malaria. In line of this, and to develop models for elimination of malaria in the most affected areas and populations (tribal populations and hard-to-reach areas), two independent malaria elimination initiatives had been undertaken in India in two highly endemic states with different malaria epidemiological scenarios. While (i) the Malaria Elimination Demonstration Project (MEDP) was undertaken in a tribal district (Mandla) in the state of Madhya Pradesh covering 1233 villages consisting of a population of >1.15 million under a public-private-partnership (PPP) mode [64], (ii) the Durgama Anchalre Malaria Nirakaran (DAMaN) (malaria elimination in hard-to-reach areas) project was undertaken by the government of Odisha state in difficult-to-access areas covering about 5000 different villages/hamlets of Odisha [65]. These two independent intervention projects followed close-to-similar strategies in many instances, but demand-based unique action plans were also followed in each. For example, both the projects were implemented in high malaria endemic settings and malaria interventions were provided in addition to the regular interventions through programmatic approach by the respective state governments. The MEDP relied upon (i) both active and passive case detection, (ii) mass screening and treatment (MSaT) to identify asymptomatic cases, (iii) monitoring and supervising the ongoing vector control activities, (iv) sentinel surveillance of cases treated by the private practitioners (v) providing information, education and communication (IEC) at communities and school levels and (vi) building capacities in health functionaries [66]. Robust accountability, reviews, monitoring and feedback at each level also constituted the backbone of MEDP. At the same time, the DAMaN initiative was taken up (i) to provide early diagnosis and complete treatment in a camp-based approach (with about 200 population in each camp). Simultaneously, (ii) intensive vector control approaches for blocking malaria transmission were also implemented. After about three years of project implementation, reduction of indigenous malaria cases was achieved about 91% in the MEDP and about 88% in the DAMaN initiatives [66]. These two implementation programs have not only yielded success in demonstrating malaria reduction in the respective endemic areas, but also opened new directions for similar, large-scale programs to be taken up for malaria elimination on programmatic modes. It appears that not only robust surveillance and management of malaria (early diagnosis, prompt treatment, regular treatment follow-up, etc.) and intervention with regard to vector control by long lasting insecticide treated nets (LLINs) and indoor residual spraying (IRS) were essential, but sensitization to the affected community through appropriate behaviour change communication (BCC) strategies were equally important [66]. The national program should not only take these learnings and implement in other similar endemic areas of India, but also ensure sustainability of successful demonstration of malaria elimination by MEDP and DAMaN initiatives.
Concluding remarks
Clearly, malaria epidemiology in India stands apart in term of complexity in comparison to other global endemic counties, including Africa. New malaria epidemiological studies are also adding up hitherto unknown epidemiological facts that further contribute to the complexities. Enormous diversity in term of species of malaria vectors, genetic structure and expanding parasite populations, high frequencies of mixed parasite infection, emergence of asymptomatic P. falciparum infections, and consistent evolution of resistance to insecticides by Anopheline mosquitoes and drug resistance by malaria parasites, etc., are some of the hallmarks to malaria outcome in India. Epidemiological complexities caused due to range expansions by the species of malaria vectors and parasites, human migration and porous political borders with endemic countries are supposed to further complicate the issues. Apart from epidemiological complexities, challenges pose by false-negative diagnosis of P. falciparum through rapid diagnosis test (RDT) due to deletion in the histidine-rich-protein-2 (hrp2) region, that is widely used in the field [67] are of primary concern in malaria public health in India. In addition, with no clear-cut treatment guidelines for mixed species malaria infection and especially of single P. malarie and/or P. ovale infection, and considering the fact that these two species have expanded range in recent times [33], management of malaria involving these two species in India will be under great threat in future. Also, there is a chance that unreported mixed malaria parasite species infection might increase P. vivax malaria [68]. Moreover, rising evidence of asymptomatic infection of P. falciparum in recent times in India [41] can contribute to silent malaria transmission in populations and increase hushed burden of malaria. In the absence of defined treatment for asymptomatic and mixed malaria infections, the epidemiological outcome of P. falciparum malaria might remain elusive in future. While high genetic diversity in drug-resistant genes provide evidence for massive genetic reconstructions [49], high diversity in functionally inert genes indicated demographic expansion in both P. falciparum and P. vivax in India [31, 32]. Considering malaria in India is principally confined to economically weak and underprivileged rural and tribal populations that remain inaccessible by public health system in major places, and the health seeking behaviour of these vulnerable populations is considered to be poor, measures must be in place to alleviate the underprivileged communities from malaria burden. More importantly, the NFME in India [63], developed following recommendations of WHO and of Southeast Asian countries seems to be quite limiting, as the epidemiological complexities, as discussed here have been grossly overlooked.
In addition to the research already conducted and knowledge generated on various aspects of malaria epidemiology in India, further research involving multidirectional and multidisciplinary approaches will help in devising implementable research towards elimination of malaria (see Outstanding Questions). To this extent, a six-point agenda has recently been suggested [69]. In addition, and in complementation to these suggestions, additional six points may be considered and composite plans of action to eliminate malaria in India be prepared. Since majorities of malaria burden comes from Indians living in hard-to-reach areas (rural, forested and tribal areas), where public healthcare facilities are scarce, (i) clear-cut and defined elimination plans for eliminating malaria in such specific vulnerable populations shall have to be prepared. This must include (ii) estimation of realistic malaria burden in defined habitat and communities (forest, rural, urban, industrial, tribal, etc.) that remained foci of malaria since time immemorial. Undoubtedly, this will help in unravelling hidden epidemiological complexities. Whereas (iii) intensified case detection through mass screening approaches followed by prompt treatment have been proved to reduce malaria burden significantly in the two malarial elimination initiatives (see above), (iv) sustainability of malaria reduction in these areas is to be ensured. Future elimination strategies (v) must integrate community participation under stakeholder model, not as beneficiaries. Moreover, in the absence of an effective vaccine for malaria, (vi) sensitization in local community to follow preventive measures against mosquito breeding and biting, promptness in getting tested in case malaria symptoms appear and strict drug compliance in patients under treatment, etc. could prove as boons in eliminating malaria from India (Figure 4, Key Figure). Such a ‘social vaccine’ could prevent malaria infection locally and ultimately contribute to a great extent in achieving the targeted elimination in India by the year 2030.
Outstanding questions.
Multiple species complexes in malaria vectors. Why malaria vectors present multiple species complexes? Does this due to high adaptabilities of Indian Anopheles to changing eco-climatic conditions, or largely due to resource partitioning to reduce competition?
Insecticide susceptibility in An. minimus. While almost all the Indian malaria vectors are resistant to insecticides in use, why An. minimus is still susceptible to all the insecticides in spite of regular exposure to different insecticides? In spite of its susceptibility to insecticides, it is still a successful species.
An. subpictus as an emerging vector of malaria. A malaria vector in Sri Lanka, Malaysia and Maldives, this species has emerged as an important vector of malaria transmission in Odisha and coastal areas of south India. How to prevent further spreading of this species to other places in India needs to be established.
Host-switching of P. falciparum. Preliminary results on the presence of common mitochondrial DNA mutations in P. falciparum-like malaria parasites in Indian rhesus monkey and P. falciparum infecting Indians suggest that P. falciparum might have switched its host from non-human parasites to human in India. What are the clinical and epidemiological relevance of this new parasite (PfIndia*) in malaria outcome in India?
Growing prevalence of asymptomatic P. falciparum infections. Why P. falciparum, known for severe malaria outcome, has evolved to ‘asymptomatic’ from and spreading rapidly in India? Is this species following a similar strategy as P. vivax? If so, what is the mechanism of its survival in a low density parasitemia in human host? Which kind of evolutionary changes did P. falciparum make in its genome?
Contrasting population genetic structures in P. vivax and P. falciparum. Despite living in sympatry in India by both the malaria parasites (P. falciparum and P. vivax), why local populations of the former species display genetic similarities, whereas geographically far away populations are genetically similar in the latter?
Figure 4, Key Figure. A model describing the process from evolution of malaria complexity to malaria elimination.
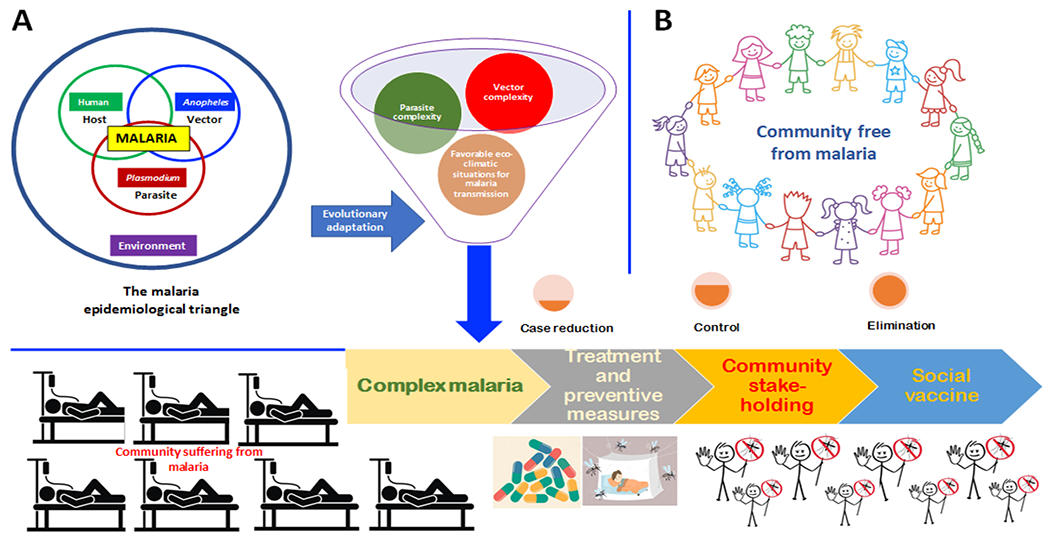
(A) the evolution of malaria complexity out of differential competition among host (human), vector (Anopheles) and parasite (Plasmodium) in the presence of changing environment that makes the community sick of malaria. (B) Malaria complexities can be tackled with treatment and preventive measures (case reduction) keeping community as stakeholders (control) and shifting the onus to the community as a part of social vaccine to bring back the community free from malaria.
Highlights.
Malaria epidemiology in India is quite complex due to wide distribution of all the five species of human malaria parasites of the genus Plasmodium and 10 different species of malaria vectors of the genus Anopheles.
Presence of species complexes in Anopheles vectors, mixed Plasmodium species infections in a single human, rising cases of asymptomatic P. falciparum cases and host-switching of P. falciparum from Indian rhesus monkey to Indians make malaria epidemiology further complex.
Wide distribution of drug-resistant P. falciparum parasite, insecticide-resistant Anopheles vectors and malaria susceptible humans create conducive environment for malaria transmission in India.
Two malaria elimination models were demonstrated in recent past in highly endemic tribal and inaccessible areas in India, indicating malaria can be eliminated with planned and concerted efforts.
Acknowledgments
The authors are thankful to Jane Carlton, Professor, New York University, USA for kind help in generating compiled information on the work conducted under the Centre for the Study of Complex Malaria in India (CSCMi) project. Comments and suggestions from three anonymous reviewers and the editor had immensely helped in improving the manuscript to a significant extent. NK is a Senior Research Fellow of Indian Council of Medical Research (ICMR). Both NK and AD are thankful to the Director General, ICMR, New Delhi for support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U19AI089676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, USA.
Glossary
- Asymptomatic/sub-microscopic infections
the presence of asexual parasites in blood, without symptoms of illness. In this case, malaria parasites are not identified by microscopy (microscopy negative) but con be found to be positive by PCR analysis.
- Demographic expansion
spatial expansion of population when a species spreads outward to fill vacant ecological space or to overthrow resident species, resulting in increased geographic range.
- Drug-resistant malaria parasite
genetic adaptation in the malaria parasite that reduces the effectiveness of an antimalarial used to treat malaria.
- Evolutionary epidemiology
study involving epidemiology and evolution of rapidly evolving infectious disease agents. Usually, such studies are performed conjointly to be properly understood. For example, during the course of an epidemic, mutations are generated de novo and can spread in the population, creating a reciprocal link between the polymorphisms of the pathogen and its propensity for onward transmission in the host population over time.
- Host-switching
an evolutionary change of the host specificity of a pathogen.
- Insecticide-resistant malaria vectors
genetic adaptation in the malaria vectors that reduces the effectiveness of an insecticide used to kill/suppress mosquito vectors.
- Malaria elimination
deliberate efforts with continued intervention resulting in reduction of the incidence of malaria to zero in a defined geographical area.
- Missing link
transitional morphologies, or forms. Any organism in possession of in-between evolutionary properties of both the ancestors’ original traits and the traits of the evolved descendants, showing a clear connection between the two.
- Population genetic structure
study of genetic variation in time and space. Assessment of population genetic structure informs on the dispersal of species, mating behaviours and the delimitation of species and population boundaries.
- Range expansion
events when a population expands into space that was previously unoccupied by that population.
- Social vaccine
refers to actions addressing social mobilizations in society, which can act as a precursor to the public health problem being addressed.
- Vectorial capacity
-
is a measure of transmission potential of a vector-pathogen system, classically known as the Ross-Macdonald model. The classical formula for calculating vectorial capacity (V) is (i) the parasite’s extrinsic incubation period (EIP, n days), (ii) the ratio of mosquitoes to humans (m), (iii) mosquito survival through one day (p) and (iv) human biting rates (a).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.World Health Organization (2022) World Malaria Report, World Health organization [Google Scholar]
- 2.Touré M et al. (2022) Trends in malaria epidemiological factors following the implementation of current control strategies in Dangassa, Mali. Malar. J 21, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JM et al. (2012) Malaria resurgence: a systematic review and assessment of its causes. Malar. J 11, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro MC (2017) Malaria Transmission and prospects for malaria eradication: The role of the environment. Cold Spring Harb Perspect. Med, 7: a025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucock MD (2022) A brief introduction to Darwinian medicine. Explor. Res. Hypothesis Med 7, 108–124 [Google Scholar]
- 6.Nelson CS et al. (2019) High-resolution micro-epidemiology of parasite spatial and temporal dynamics in a high malaria transmission setting in Kenya. Nat. Commun 10, 5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasir SMI et al. (2020) Prevention of re-establishment of malaria: historical perspective and future prospects. Malar. J 19, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss DJ et al. (2019) Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000-17: a spatial and temporal modelling study. Lancet 394: 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das A et al. (2012) Malaria in India: the center for the study of complex malaria in India. Acta Trop. 121: 267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A (2015) The distinctive features of Indian malaria parasites. Trends Parasitol. 31: 83–86 [DOI] [PubMed] [Google Scholar]
- 11.Singh V et al. (2009) Why is it important to study malaria epidemiology in India? Trends Parasitol. 25: 452–457 [DOI] [PubMed] [Google Scholar]
- 12.Subbarao SK et al. (2019) Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar. J 18, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami G et al. (2005) PCR–RFLP of mitochondrial cytochrome oxidase subunit II and ITS2 of ribosomal DNA: markers for the identification of members of the Anopheles culicifacies complex (Diptera: Culicidae). Acta Trop. 95, 92–99 [DOI] [PubMed] [Google Scholar]
- 14.Chen B et al. (2006) Molecular variation, systematics and distribution of the Anopheles fluviatilis complex in southern Asia. Med. Vet. Entomol 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 15.Naddaf SR et al. (2003) Molecular characterization of Anopheles fluviatilis species complex in the Islamic Republic of Iran. East. Mediterr. Health J 9, 257–265 [PubMed] [Google Scholar]
- 16.Harbach RE et al. (2006) Anopheles (Cellia) minimus Theobald (Diptera: Culicidae): neotype designation, characterization, and systematics. Proc. Entomol. Soc. Washington 108, 198–209 [Google Scholar]
- 17.Dev V and Sharma VP (2013) The dominant mosquito vectors of human malaria In India, In Manguin S (ed.), Anopheles mosquitoes - New insights into malaria vectors, IntechOpen, London [Google Scholar]
- 18.Nanda N et al. (2004) Cytogenetic characterization of Anopheles sundaicus (Diptera: Culicidae) population from Car Nicobar Island, India. Ann. Entomol. Soc. Am 97, 171–176 [Google Scholar]
- 19.Surendran SN, et al. (2019) Anthropogenic factors driving recent range expansion of the malaria vector Anopheles Stephensi. Front. Public Health 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mnzava A et al. (2022) Anopheles Stephensi in Africa requires a more integrated response. Malar. J 21, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kareemi TI et al. (2021) Population dynamics and insecticide susceptibility of Anopheles culicifacies in malaria endemic districts of Chhattisgarh, India. Insects 12, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald G (1952) The analysis of equilibrium in malaria Trop. Dis. Bull 49, 813–829 [PubMed] [Google Scholar]
- 23.Kumar A et al. (2016) Anopheles subpictus carry human malaria parasites in an urban area of Western India and may facilitate perennial malaria transmission. Malar. J 15, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhtar N et al. (2016) Role of Anopheles culicifacies as a vector of malaria in changing ecological scenario of North-eastern states of India. J. Vect. Borne Dis 53, 264–271 [PubMed] [Google Scholar]
- 25.Dev V and Manguin S (2016) Biology, distribution and control of Anopheles (Cellia) minimus in the context of malaria transmission in north-eastern India. Parasit. Vectors 9, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra AK et al. (2020) A study of malaria vector surveillance as part of the malaria elimination demonstration project in Mandla, Madhya Pradesh. Malar. J 19, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavendra K et al. (2017) Temporo-spatial distribution of insecticide-resistance in Indian malaria vectors in the last quarter-century: Need for regular resistance monitoring and management. J. Vect. Borne Dis 54, 111–130 [PubMed] [Google Scholar]
- 28.Das MK et al. (2021) Insecticide susceptibility status of malaria vectors, Anopheles culicifacies, Anopheles fluviatilis and Anopheles minimus in the tribal districts of Jharkhand state of India. J Vect. Borne Dis 58, 374–382 [DOI] [PubMed] [Google Scholar]
- 29.Riveron JM et al. (2018) Insecticide resistance in malaria vectors: An update at a global scale, in Manguin S, Dev V (eds.), Towards Malaria Elimination - A Leap Forward, IntechOpen, London [Google Scholar]
- 30.Siwal N et al. (2018) Malaria diagnosis by PCR revealed differential distribution of mono and mixed species infections by Plasmodium falciparum and P. vivax in India. PLoS One 13, e0193046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagi S and Das A (2015) Mitochondrial population genomic analyses reveal population structure and demography of Indian Plasmodium falciparum. Mitochondrion 24, 9–21 [DOI] [PubMed] [Google Scholar]
- 32.Gupta B et al. (2012) Inferring the evolutionary history of Indian Plasmodium vivax from population genetic analyses of multilocus nuclear DNA fragments. Mol. Ecol 21, 1597–1616 [DOI] [PubMed] [Google Scholar]
- 33.Singh US et al. (2017) Can mixed parasite infections thwart targeted malaria elimination program in India?. Biomed Res. Int 2017, 2847548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bal M et al. (2021) Neglected malaria parasites in hard-to-reach areas of Odisha, India: implications in elimination programme. Malar. J 20, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi R et al. (2020) Trends of neglected Plasmodium species infection in humans over the past century in India. One Health 11, 100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi S et al. (2014) New insights into the evolutionary history of Plasmodium falciparum from mitochondrial genome sequence analyses of Indian isolates. Mol. Ecol 23, 2975–2987 [DOI] [PubMed] [Google Scholar]
- 37.Dixit J et al. (2018) Reinvestigating the status of malaria parasite (Plasmodium sp.) in Indian non-human primates. PLoS Negl. Trop. Dis 12, e0006801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gogtay NJ et al. (2000) Relapse pattern of Plasmodium vivax in Mumbai: a study of 283 cases of vivax malaria. J. Assoc. Physicians India 48, 1085–1086 [PubMed] [Google Scholar]
- 39.Kochar DK et al. (2009) Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in north-western India. Am. J. Trop. Med. Hyg 80, 194–198 [PubMed] [Google Scholar]
- 40.Matlani M et al. (2020) Severe vivax malaria trends in the last two years: a study from a tertiary care centre, Delhi, India. Ann. Clin. Microbiol. Antimicrob 19, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A et al. (2021) Asymptomatic low-density Plasmodium falciparum infections: A challenge in malaria elimination in India. J. Infect. Public Health 14, 1600–1602 [DOI] [PubMed] [Google Scholar]
- 42.Deora N et al. (2022) A symptomatic review and meta-analysis on sub-microscopic Plasmodium infection in India: Different perspectives and global challenges. Lancet Reg. Health Southeast Asia 2, 1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankar H et al. (2021) Asymptomatic low-density Plasmodium infection during non-transmission season: a community-based cross-sectional study in two districts of north-eastern region, India. Trans. R. Soc. Trop. Med. Hyg 115, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 44.Gupta B et al. (2010) High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop. Med. Int. Health 15, 819–824 [DOI] [PubMed] [Google Scholar]
- 45.Plowe CV (2009) The evolution of drug-resistant malaria. Trans. R. Soc. Trop. Med. Hyg 103, S11–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozarkar A et al. (2021) Analysis of drug resistance marker genes of Plasmodium falciparum after implementation of artemisinin-based combination therapy in Pune district, India. J. Biosci 46, 77. [PubMed] [Google Scholar]
- 47.Kumar A et al. (2020) Comparative analysis of Plasmodium falciparum dihydrofolate-reductase gene sequences from different regions of India. Heliyon 6, e03715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dayananda KK et al. (2018) Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vect. Borne Dis 55, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das A and Dash AP (2007) Evolutionary paradigm of chloroquine-resistant malaria in India. Trends Parasitol. 23: 132–135 [DOI] [PubMed] [Google Scholar]
- 50.Kumar A et al. (2019) Genetic profiling of the Plasmodium falciparum parasite population in uncomplicated malaria from India. Malar. J 18, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das S et al. (2018) Evidence of artemisinin-resistant Plasmodium falciparum malaria in eastern India. N. Engl. J. Med 379, 1962–1964 [DOI] [PubMed] [Google Scholar]
- 52.Akunuri S et al. (2018) Suspected artesunate resistant malaria in south India. J. Glob. Infect. Dis 10, 26–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das S et al. (2021) Artemisinin combination therapy fails even in the absence of Plasmodium falciparum kelch13 gene polymorphism in Central India. Sci. Rep 11, 9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saravu K et al. (2016) Therapeutic assessment of chloroquine-primaquine combined regimen in adult cohort of Plasmodium vivax malaria from primary care centres in southwestern India. PLoS One 11, e0157666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh RK (2000) Emergence of chloroquine-resistant vivax malaria in south Bihar (India). Trans. R. Soc. Trop. Med. Hyg 94, 327. [PubMed] [Google Scholar]
- 56.Hasabo EA et al. (2022) Treatment-seeking behaviour, awareness and preventive practice toward malaria in Abu Ushar, Gezira state, Sudan: a household survey experience from a rural area. Malar. J 21, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saxena N et al. (2021) Connecting the unconnected: the way forward for public health to reach the unreached tribal communities in India. Curr. Sci 120, 24–26 [Google Scholar]
- 58.Dhiman S (2019) Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect. Dis. Poverty 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Awasthi KR et al. (2022) A qualitative study of knowledge, attitudes and perceptions towards malaria prevention among people living in rural upper river valleys of Nepal. PLoS ONE 17, e0265561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ingabire CM et al. (2014) Community mobilization for malaria elimination: application of an open space methodology in Ruhuha sector, Rwanda. Malar. J 13, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su XZ et al. (2019) Plasmodium genomics and genetics: New insights into malaria pathogenesis, drug resistance, epidemiology, and evolution. Clin Microbiol. Rev 32, e00019–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma S et al. (2022) What India can learn from globally successful malaria elimination programmes. BMJ Glob. Health 7, e008431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Vector Borne Disease Control Program (2016) National Framework for Malaria Elimination in India (2016-2030), Ministry of Health and Family Welfare, Government of India [Google Scholar]
- 64.Bharti PK et al. (2020) Demonstration of indigenous malaria elimination through Track-Test-Treat-Track (T4) strategy in a malaria elimination demonstration project in Mandla, Madhya Pradesh. Malar. J 19, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bal M et al. (2020) Assessment of effectiveness of DAMaN: A malaria intervention program initiated by Government of Odisha, India. PLoS One;15, e0238323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajvanshi H et al. (2021) Learnings from two independent malaria elimination demonstration projects in India. Trans. R. Soc. Trop. Med. Hyg 115, 1229–1233 [DOI] [PubMed] [Google Scholar]
- 67.Verma AK et al. (2018) HRP-2 deletion: a hole in the ship of malaria elimination. Lancet Infect. Dis 18, 826–827 [DOI] [PubMed] [Google Scholar]
- 68.Nema S et al. (2022) Malaria elimination in India: Bridging the gap between control and elimination. Indian Pediatr. 57, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahi M and Sharma A (2022) Malaria control initiatives that have the potential to be gamechangers in India’s quest for malaria elimination Lancet Reg. Health Southeast Asia 2, 100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao MR (2015) International centers of excellence for malaria research. Am. J. Trop. Med. Hyg 93, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlton JM et al. (2022) Advances in basic and translational research as part of the center for the study of complex malaria in India. Am. J. Trop. Med. Hyg 107, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlton JM et al. (2022) The impact, emerging needs, and new research questions arising from 12 years of the center for the study of complex malaria in India. Am. J. Trop. Med. Hyg 107, 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessler A et al. (2021) Spatial and temporal village-level prevalence of Plasmodium infection and associated risk factors in two districts of Meghalaya, India. Malar. J 20, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarkar R et al. (2021) Household and individual level risk factors associated with declining malaria incidence in Meghalaya, India: implications for malaria elimination in low-endemic settings. Malar. J 20, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Eijk AM et al. (2019) The burden of submicroscopic and asymptomatic malaria in India revealed from epidemiology studies at three varied transmission sites in India. Sci. Rep 9, 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Eijk AM et al. (2016) What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar. J 15, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uplekar S et al. (2017) Characterizing antibody responses to Plasmodium vivax and Plasmodium falciparum antigens in India using genome-scale protein microarrays. PLoS Negl. Trop. Dis 11, e0005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohanty S et al. (2017) Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere 2, e00193–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anvikar AR et al. (2020) Clinical and epidemiological characterization of severe Plasmodium vivax malaria in Gujarat, India. Virulence 11, 730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hupalo DN et al. (2016) Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat. Genet 48, 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neafsey DE et al. (2012) The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat. Genet 44, 1046–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waite JL et al. (2017) Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci. Rep 7, 40551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas S et al. (2017) Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar. J 6, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]


