Summary
The ability of cannabis to increase food consumption has been known for centuries. In addition to producing hyperphagia, cannabinoids can amplify existing preferences for calorically dense, palatable food sources, a phenomenon called hedonic amplification of feeding. These effects result from the action of plant-derived cannabinoids that mimic endogenous ligands called endocannabinoids. The high degree of conservation of cannabinoid signaling at the molecular level across the animal kingdom suggests hedonic feeding may also be widely conserved. Here we show that exposure of C. elegans to anandamide, an endocannabinoid common to nematodes and mammals, shifts both appetitive and consummatory responses toward nutritionally superior food, an effect analogous to hedonic feeding. We find that anandamide’s effect on feeding requires the C. elegans cannabinoid receptor NPR-19 but can also be mediated by the human CB1 cannabinoid receptor, indicating functional conservation between the nematode and mammalian endocannabinoid systems for regulation of food preferences. Furthermore, anandamide has reciprocal effects on appetitive and consummatory responses to food, increasing and decreasing responses to inferior and superior food respectively. Anandamide’s behavioral effects require the AWC chemosensory neurons, and anandamide renders these neurons more sensitive to superior food and less sensitive to inferior food, mirroring the reciprocal effects seen at the behavioral level. Our findings reveal a surprising degree of functional conservation in the effects of endocannabinoids on hedonic feeding across species and establish a new system to investigate the cellular and molecular basis of endocannabinoid system function in the regulation of food choice.
eTOC
The ability of cannabis to stimulate appetite for highly palatable food in humans has been known for centuries. Levichev et al. show that exposure of the nematode C. elegans to an endogenous cannabinoid specifically increases consumption of favored food, revealing an unexpected degree of conservation in cannabinoid effects on appetite.
Introduction
It has been known for centuries that smoking or ingesting preparations of the plant Cannabis sativa stimulates appetite.1,2 Users report persistent hunger while intoxicated, even if previously satiated. This feeling of hunger is often accompanied by a specific desire for foods that are sweet or high in fat content, a phenomenon colloquially known as “the munchies.”3–8 The effects of cannabinoids on appetite result mainly from Δ9-tetrahydrobannabinol (THC), a plant-derived cannabinoid. THC acts at cannabinoid receptors in the brain, mimicking endogenous ligands called endocannabinoids, which include anandamide (N-arachidonoylethanolamine, AEA) and 2-arachidonoylglycerol (2-AG). AEA and 2-AG are the best studied signaling molecules of the mammalian endocannabinoid system, which comprises the cannabinoid receptors CB1 and CB2, metabolic enzymes for synthesis and degradation of the endocannabinoids, and ancillary proteins involved in receptor trafficking and modulation.9–17
Numerous studies in laboratory animals have established a strong link between endocannabinoid signaling and energy homeostasis, defined as the precise matching of caloric intake with energy expenditure.18 Food deprivation increases endocannabinoid levels in the nucleus accumbens and hypothalamus, brain regions that express CB1 receptors and contribute to appetitive regulation.19 Systemic administration of THC or endogenous cannabinoids increases feeding.20 Micro-injection of cannabinoid receptor agonists or endocannabinoids directly into the nucleus accumbens also increases feeding.21,22 Thus, the endocannabinoid system can be viewed as a short-latency effector system for restoring energy homeostasis under conditions of food deprivation.18,23–25
To respond effectively to an energy deficit, an animal should be driven to seek food (appetitive behavior) and, once food is encountered, to maximize caloric intake (consummatory behavior). The endocannabinoid system is capable of orchestrating both aspects of this response. With respect to appetitive behavior, CB1 agonists reduce the latency to feed26–32 and induce animals to expend more effort to obtain a food or liquid reward,30,31,33,34 whereas CB1 antagonists have the opposite effects.26–32 As for consummatory behavior, rodent studies show that administration of THC or endocannabinoids not only increases consumption, but also alters food preferences in favor of palatable, calorically dense foods, such as those laden with sugars and fats. For example, THC causes rats to consume larger quantities of chocolate cake batter without affecting consumption of concurrently available laboratory pellets.35 It also causes them to consume larger quantities of sugar water than plain water, and of dry pellets rather than watered-down pellet mash, which is calorically dilute.36 Administration of endocannabinoids, systemically or directly into the nucleus accumbens, has similar effects, which can be blocked by administration of CB1 antagonists.22,37,38 Conversely, CB1 antagonists, administered alone, specifically suppress consumption of sweet and fatty foods in rats39–41 and primates,42 indicating that basal CB1 activation can be regulated up or down to alter consumption.
There is experimental support for the hypothesis that cannabinoids amplify the pleasurable or rewarding aspects of calorically dense foods. This phenomenon has been termed hedonic amplification,21,43 whereas the food-specific increase in consumption it engenders has been termed hedonic feeding.44 Although inferences about the subjective experience of animals can be difficult to establish, cannabinoids have been shown to increase overt expressions of pleasure during feeding. In rats, for example, both THC and AEA increase the vigor of licking at spouts delivering sweet fluids.45,46 Further, the frequency of orofacial movements associated with highly palatable foods is increased or decreased by injection of THC or a CB1 antagonist respectively, suggesting that pleasure may be increased by cannabinoid administration.47,48
The effects of cannabinoids on hedonic responses may be partially chemosensory in origin, involving both taste (gustation) and smell (olfaction). With respect to gustation, a majority of sweet-sensitive taste cells in the mouse tongue are immunoreactive to CB1, and a similar proportion shows heightened responses to saccharin, sucrose, and glucose following endocannabinoid administration.49,50 These effects are recapitulated in afferent nerves carrying gustatory signals from the tongue,49 as administration of AEA or 2-AG specifically increases chorda tympani responses to sweeteners rather than NaCl (salt), HCl (sour), quinine (bitter), or monosodium glutamate (umami). As for olfaction, CB1 receptors expressed in the olfactory bulb are required for post-fasting hyperphagia in mice, and THC decreases the threshold for food-odor detection during exploratory behavior.51
The high degree of evolutionary conservation of the endocannabinoid system at the molecular level is well established.52 Although CB1 and CB2 receptors are unique to chordates, there are numerous candidates for cannabinoid receptors in most animals. Furthermore, orthologs of the enzymes involved in synthesis and degradation of endocannabinoids occur throughout the animal kingdom. This degree of molecular conservation, coupled with the universal need in organisms to regulate energy balance, suggests the hypothesis that hedonic amplification and hedonic feeding are also widely conserved, but studies in animals other than rodents and primates appear to be lacking.
The present study tests the hypothesis that the hedonic effects of cannabinoids are conserved in the nematode C. elegans. This organism diverged from the lineage leading to mammals more than 500 million years ago.53 Nevertheless, C. elegans has a fully elaborated endocannabinoid signaling system including:54 (i) functionally validated endocannabinoid receptors NPR-19, which most closely resembles the mammalian CB1 receptor, and OCTR-1, and putative receptors encoded by npr-32, osm-9, and trp-4;55–57 (ii) the endocannabinoids AEA and 2-AG, which it shares with mammals,45,58–60 (iii) orthologs of the mammalian endocannabinoid synthesis enzymes NAPE-PLD and DAGL,61 and (iv) orthologs of the endocannabinoid degradative enzymes FAAH and MAGL (Y97E10AL.2 in worms).55 Endocannabinoid signaling in C. elegans is currently known to contribute to six main phenotypes: (i) axon navigation during regeneration,56,62 (ii) lifespan regulation related to dietary restriction,61,63 (iii) progression through developmental stages,61,64 (iv) suppression of nociceptive withdrawal responses,55 (v) inhibition of feeding rate,55 and (vi) inhibition of locomotion.55,57
The feeding ecology of C. elegans supports the possibility of hedonic feeding in this organism. C. elegans feeds on bacteria in decaying plant matter.65 It finds bacteria through chemotaxis guided by a combination of gustatory and olfactory cues.66,67 Bacteria are ingested through the worm’s pharynx, a rhythmically active muscular pump that constitutes the animal’s throat. Although C. elegans is an omnivorous bacterivore, different species of bacteria have a characteristic nutritional quality as a food source defined by the growth rate of individual worms feeding on that species.68 Hatchlings are naïve to food quality but in a matter of hours begin to exhibit a preference for nutritionally superior species (henceforth superior food) over nutritionally inferior species (henceforth inferior food).69
Here we show that exposure of C. elegans to the endocannabinoid AEA biases both consummatory and appetitive responses toward superior food. With respect to consummatory behavior, animals exposed to AEA increase their feeding rate on superior food and decrease their feeding rate on inferior food. As for appetitive behavior, the fraction of worms approaching and dwelling on patches of superior food increases whereas the fraction approaching and dwelling on inferior food decreases. Taken together, the consummatory and appetitive manifestations of cannabinoid exposure in C. elegans imply increased consumption of superior food characteristic of hedonic feeding on calorically dense foods by mammals. We also find that AEA’s effects require the NPR-19 cannabinoid receptor. Further, AEA’s effects persist when npr-19 gene is replaced by the human CB1 receptor-gene CNR1, indicating a high degree of conservation between the nematode and mammalian endocannabinoid systems. At the neuronal level, we find that under the influence of AEA, AWC, an olfactory neuron required for chemotaxis to food, becomes more sensitive to superior food and less sensitive to inferior food. Together, our findings indicate that the hedonic effects of endocannabinoids may be conserved in C. elegans.
Results
AEA exposure increases consumption of superior food
In mammals, cannabinoids can selectively increase consumption of foods that are nutritionally superior in the sense that they are calorically dense.35,36 We asked whether cannabinoids can selectively increase consumption of nutritionally superior food in C. elegans, where nutritional quality is defined in terms of the growth rate of individual worms.68 C. elegans swallows bacteria by rhythmically contracting its pharynx; each contraction is called a pump. To quantify consumption, we recorded pumping rate electrically in individual worms restrained in a microfluidic channel containing a single type of food (OD600 0.8; Figure 1A).70,71 We first tested the effect of AEA exposure on consumption of the bacterial strain OP50, a classical laboratory food source. As previously reported,72 AEA exposure decreased consumption of OP50 (Figure 1A, 1B; Table S1, line 1). We then tested the effect of AEA exposure on consumption of five bacteria strains for which objective quality as a food source has been measured.68 Baseline food consumption in unexposed worms did not correlate with nutritional quality. Nevertheless, AEA exposure increased the consumption of superior food, decreased the consumption of inferior food, and had no effect on food of intermediate quality (Figure 1C; Table S1, lines 2–6).72 We conclude that AEA induces hedonic feeding in C. elegans. Furthermore, its effects on feeding are reciprocal, increasing and decreasing consumption of superior and inferior food, respectively.
Figure 1. AEA-mediated hedonic feeding: consummatory behavior.
A. Electrical recordings of pharyngeal pumping in two individual worms under the conditions shown. Each spike is the electrical correlate of one pharyngeal pump. Traces are representative of the median pumping frequency under each condition. B. Effect of AEA on mean pumping frequency in OP50 (OD 0.8). C. Effect of AEA on mean pumping frequency in five different bacteria species (OD 0.8). Pairs of bars are ordered (left to right) according to growth rate of C. elegans, defined as the inverse of the number of days to grow from L1 to adult, when cultivated on the corresponding bacteria; each growth rate value is the mean of four test conditions in previously published work. Gray bars, AEA–. Black bars, AEA+. Red lines, median pumping frequency. For details of statistics see Table S1. Symbols, *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant. Error bars, 95% confidence interval. Number of recorded worms is shown in parentheses.
AEA exposure increases appetitive responses to superior food
In mammals, cannabinoids cause a shift toward nutritionally superior food not only in consummatory behavior but also appetitive behavior, defined as the tendency to seek a particular food. We asked whether cannabinoids can selectively increase appetitive responses to nutritionally superior food in C. elegans, measured in terms of chemotaxis preference. We began by assessing changes in relative preference for the superior food DA1877 and the inferior food DA1885. Preference was measured by placing a small population of worms at the start of a T-maze (Figure 2A) baited with patches of the two bacteria strains at equal concentration (OD600 1). The T-maze assay is analogous to mammalian studies in which both palatable and standard food options are simultaneously available.22,35–38 The number of worms in each food patch was counted at 15-minute intervals for one hour. Preference index I at each time point was quantified as I = (nS − nI)/(nS + nI), where nS and nI are the number of worms on superior and inferior food, respectively; I = 0 indicates indifference between the two food types. We pre-exposed well-fed worms from the reference strain N2 to 100 μM AEA for 20 min in foodless M9 buffer. We found that AEA exposure increased preference for superior food (Figure 2B, C; Table S2, line 2). This effect lasted at least 60 minutes without significant decrement (Figure 2B; Table S2, lines 3–4) despite the absence of AEA on the assay plates.
Figure 2. AEA-mediated hedonic feeding: appetitive behavior.
A. Food preference assay. T-maze arms were baited with patches of superior (blue) and inferior (orange) bacteria. B. Mean preference index (I) versus time for AEA-exposed animals (AEA+) and unexposed controls (AEA–), where I > 0 is preference for superior food, I < 0 is preference for inferior food, and I = 0 is indifference (dashed line). Superior food, DA1877, OD600 1; inferior food, DA1885, OD600 1. C. Summary of the data in B. Each dot is mean preference over time in a single T-maze assay. Dot color indicates preference index according to the color scale on the right. D, E. Effect of AEA on preference when baseline preference is statistically indistinguishable from the indifference point (symbols and color scale as in C). For preference time courses, see Figure S1A, B. In D: Superior food, DA1877, OD600 0.5; inferior food, DA1885, OD600 3. In E: superior food, DA1877, OD600 0.5; inferior food, DA1885, OD600 8. F. Effect of AEA on preference for a different pair of superior and inferior bacteria. Superior food, HB101, OD600 0.5; inferior food, DA837, OD600 2.2 (symbols and color scale as in C). For preference time course, see Figure S1C. G. Effect of AEA on fraction of worms in superior and inferior food patches versus time. Same experiment as in panels B, C. For statistics see Table S2. Symbols: *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant. Error bars, 95% confidence interval.
One interpretation of the data in Figure 2B–C is that AEA exposure specifically increases the attractiveness of superior food relative to inferior food. However, an alternative interpretation is that AEA promotes the attractiveness of whichever food is already preferred under the baseline conditions of the experiment (AEA–). To distinguish between these possibilities, we titrated the densities of superior and inferior food until, under baseline conditions, neither food was preferred (I ≈ 0; Figure 2D, E; Figure S1A, B). Under these conditions, AEA still increased the preference for superior food (Table S2, lines 6, 10). This finding suggests that AEA differentially affects accumulation based on food identity. We also tested the effect of AEA on preference for a second pair of superior and inferior bacteria, HB101 and DA837, for which the difference in nutritional quality is smaller than in the previous pair (Figure 2F; Figure S1C); as before, the baseline preference was titrated approximately to zero. Once again, AEA caused increased preference for superior food (Table S2, line 14). Taken together, the data in Figure 2B–F show that AEA’s ability to increase preference for superior food is limited neither to a particular pair of foods nor their relative concentrations.
Because worms in the T-maze could occupy foodless regions between the food patches, the increase in preference index could represent increased attraction to superior food, decreased attraction to inferior food, or both. An increase in the preference index that resulted only from decreased attraction to inferior food would not be evidence of increased appetitive responses to superior food. However, further analysis revealed that AEA exposure increased the fraction of worms on superior food (Figure 2G; Table S2, line 18), and decreased the fraction of animals on inferior food (Figure 2G; Table S2, line 22). Thus, AEA-induced changes in appetitive responses to superior and inferior food that result in increased accumulation on superior food are consistent with the increased appetitive responses to superior food. We conclude that AEA has reciprocal actions on both appetitive and consummatory responses.
Chemosensory correlates of hedonic feeding
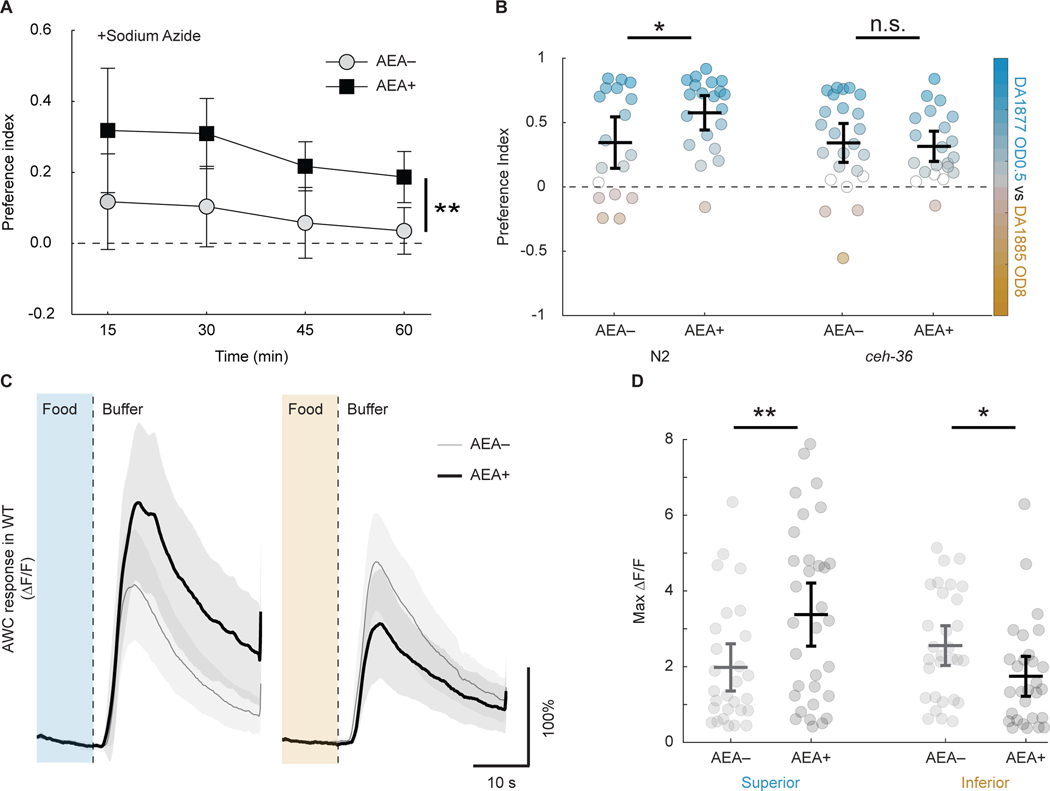
Accumulation in a food patch is determined by only two factors: the rates of food-patch entry and exit. AEA could modulate appetitive responses by acting on either or both rates. Chemotaxis toward food patches is driven mainly by olfactory neurons responding to distal cues.66,67 Thus, changes in entry rate as a function of AEA exposure would imply changes in the function of olfactory neurons. We therefore added a paralytic agent73 to both food patches in the T-maze, thereby setting exit rate to zero. We found that AEA still produced a marked increase in preference for superior food (Figure 3A; Table S3, line 2), showing that it differentially affects patch entry rates.
Figure 3. Chemosensory correlate of hedonic feeding.
A. Mean preference index (I) versus time for AEA-exposed animals (AEA+) and unexposed controls (AEA–) when sodium azide was added to food patches. Superior food, DA1877, OD600 0.5; inferior food, DA1885, OD600 3. B. Effect of AEA on preference in N2 and ceh-36 mutants. Superior food, DA1877, OD600 0.5; inferior DA1885, OD600 8. Each dot is mean preference in a single T-maze assay. For preference time course, see Figure S2A, B. C. Effect of AEA on the amplitude of AWC calcium transients in response to the removal of superior or inferior food in N2 worms. Each trace is average normalized fluorescence change (ΔF⁄F) versus time. Superior food (blue), DA1877, OD600 1; inferior food (orange), DA1885, OD600 1. D. Summary of the data in C, showing mean peak ΔF⁄F. For statistics in A-D, see Table S3. Symbols: *, p < 0.05; **, p < 0.01; n.s., not significant. Error bars and shading, 95% confidence interval.
We next considered the possibility that AEA acts on specific olfactory neurons to produce the appetitive component of hedonic feeding. C. elegans senses food-related odors by 11 classes of chemosensory neurons (two neurons/class).67,74 We focused on AWC, a class of olfactory neurons that respond directly to many volatile odors75 and are required for chemotaxis to them.67 We measured AEA’s effect on preference in ceh-36 mutants, in which AWC function is impaired. The gene ceh-36 is expressed by AWC and encodes a homeodomain transcription factor required for expression of genes essential for chemosensory transduction.76,77 Accordingly, ceh-36 mutants are strongly defective in chemotaxis toward food-related odors sensed by AWC.77 ceh-36 is also expressed in one other chemosensory neuron class, ASE, but as ASE neurons inherit their sensitivity to odorants via peptidergic signaling from AWC, loss of appetitive responses to food in ceh-36 mutants would nevertheless be attributable to loss of AWC function. In T-maze assays comparing appetitive responses in ceh-36 mutants and N2 worms, we found a modest strain by AEA interaction (p = 0.08), and a significant effect of AEA in N2 animals that was absent in the mutants (Figure 3B; Figure S2A, B; Table S3, lines 6, 10–11, 13). This finding indicates that AWC is required for the appetitive component of hedonic feeding.
AWC is activated by decreases in the concentration of food or food-related odors.74,78,79 AWC can nevertheless promote attraction to food patches because its activation truncates locomotory head bends away from the odor source, thereby steering the animal toward it. Additionally, its activation causes the animal to stop moving forward, reverse, and resume locomotion in a new direction better aligned with the source, a behavioral motif known as a pirouette.80 To test whether AEA alters AWC sensitivity to superior and inferior foods, we compared AWC calcium transients in response to the removal of food in N2 worms exposed to AEA, and in unexposed controls. In unexposed animals, AWC neurons responded equally to the removal of either food (Figure 3C, D, Table S3, line 21). However, exposure to AEA caused a reciprocal change in food sensitivity, increasing AWC’s response to the removal of superior food and decreasing its response to the removal of inferior food (Figure 3C, D, Table S3, lines 17, 19–20, 22). These reciprocal effects mirror AEA’s effect on both the consummatory and appetitive aspects of hedonic feeding (Figures 1 and 2) and are consistent with a model in which hedonic feeding is triggered, at least in part, by modulation of chemosensation in AWC neurons.
Dissection of signaling pathways required for hedonic feeding
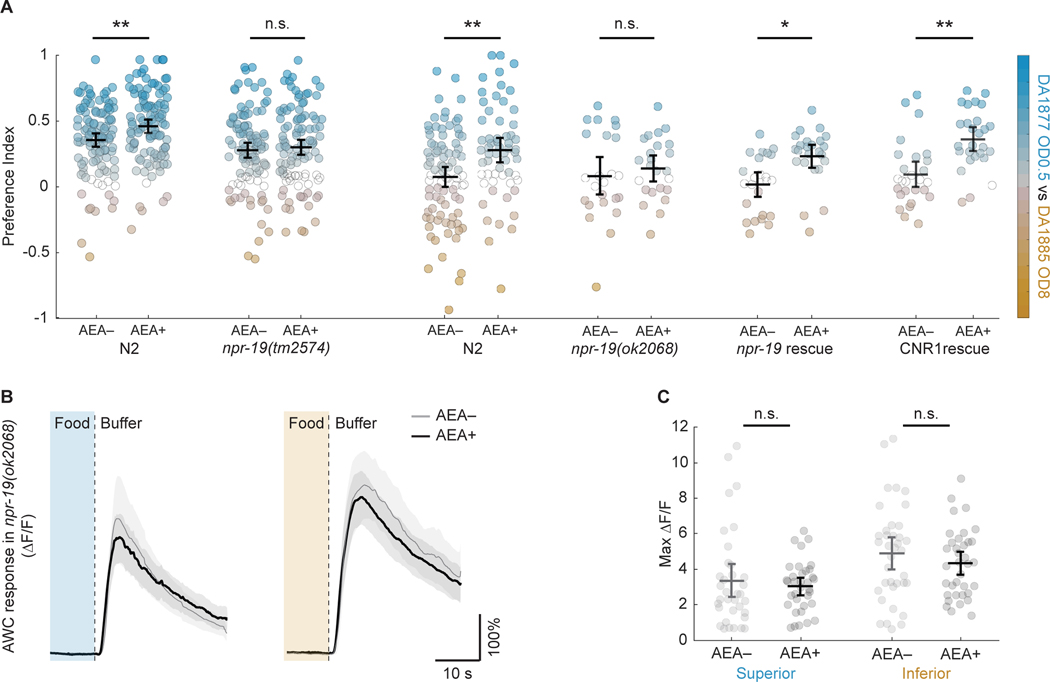
The G-protein coupled receptor NPR-19, encoded by the C. elegans gene npr-19, has been shown to be required for AEA-mediated suppression of withdrawal responses and feeding rate.55 To test whether npr-19 is required for hedonic feeding, we measured food preference in two deletion alleles of npr-19 following exposure to AEA. Mutant worms failed to exhibit increased preference for superior food (Figure 4A; Figure S2C–F; Table S4, lines 6–7, 10–11). This defect was rescued by over-expressing npr-19 under control of the native npr-19 promoter (Figure 4A; Figure S2E, F, G; Table S4, lines 19–20, 22). We conclude that npr-19 is required for the appetitive component of hedonic feeding. This defect was also rescued by over-expressing the human cannabinoid receptor gene CNR1 (Figure 4A; Figure S2E, F, H; Table S4, lines 28–29, 31). This finding indicates a remarkable degree of conservation between the nematode and human endocannabinoid systems, as previously reported.55
Figure 4. Requirement of NPR-19 for hedonic feeding and chemosensory modulation.
A. Effect of AEA on preference in N2 worms and the indicated genetic background. Separate N2 control groups were used for npr-19(tm2574) vs and npr-19(ok2068) and rescue strains as these two sets of experiments were not contemporaneous. Superior food, DA1877, OD600 0.5; inferior food, DA1885, OD600 8. Each dot is mean preference over time in a single T-maze assay. Dot color indicates preference index according to the color scale on the right. For preference time course, see Figure S2. B. Effect of AEA on amplitude of AWC calcium transients in response to the removal of superior or inferior food in npr-19(ok2068). Each trace is average normalized fluorescence change (ΔF⁄F) versus time. Superior food (blue), DA1877, OD600 1; inferior food (orange), DA1885, OD600 1. C. Summary of the data in B, showing mean peak ΔF⁄F. See also Figure S3 and Table S5. For statistics in A-C, see Table S4. Symbols: *, p < 0.05; **, p < 0.01; n.s., not significant. Error bars and shading, 95% confidence interval.
The foregoing results suggest a model of hedonic feeding in C. elegans in which activation of the NPR-19 receptor by AEA triggers reciprocal changes in AWC’s food sensitivity to induce the appetitive component of hedonic feeding. We therefore tested whether npr-19 is required for AEA’s effects on AWC. The effect of AEA on AWC’s response to food was abolished in npr-19 mutants (Figure 4B, C, Table S4, lines 34, 37–38, 43, 46–47). This phenotype was partially rescued by over-expression of npr-19 under control of the native npr-19 promoter (Figure S3A, B, Table S5, lines 3, 6–7,12, 15–16), and by over-expression of human cannabinoid receptor gene CNR1 (Figure S3C, D, Table S5, lines 22, 25–26, 31, 34–35). We conclude that the appetitive component of AEA-induced hedonic feeding requires both the NPR-19 receptor and AWC neurons, and that activation of the NPR-19 receptor by AEA triggers reciprocal changes in AWC’s food sensitivity, contributing to increased preference for superior food.
The simplest explanation for AEA’s effect on AWC would be that NPR-19 is expressed in AWC, and its activation by AEA produces the observed reciprocal modulation of AWC sensitivity to superior and inferior foods. To characterize the npr-19 expression pattern, we expressed a pnpr-19::GFP transgene together with either pcho-1::mCherry or peat-4::mCherry, two markers whose neuronal expression pattern is known completely.81,82 We observed expression of npr-19 in approximately 29 neuron somata in the head and 8 in the tail (Figure 5A, Table S6). Using positional cues in addition to the markers, we positively identified 28 of the GFP-positive somata, which fell into 15 neuron classes (Table 1). These classes could be organized into four functional groups: sensory neurons (URX, ASG, AWA, and PHC), interneurons (RIA, RIM, and LUA), motor neurons (URA and PDA), and pharyngeal neurons (M1, M3, MI, MC, I2, and I4). Although AWC could be identified in every worm by its characteristic position in the peat-4::mCherry expressing strain, co-expression of pnpr-19::GFP transgene was not observed in this neuron class. Our expression data, together with the absence of significant npr-19 expression in AWC in RNA sequencing experiments based on the C. elegans Neuronal Gene Expression Map & Network (CeNGEN) consortium,83 suggests that AWC does not express npr-19. These findings are inconsistent with a direct model of action of AEA on AWC neurons mediated by the NPR-19 receptor.
Figure 5. Genetic pathways underlying AEA-mediated AWC modulation.
A. Expression pattern of npr-19 in head and tail neurons. npr-19 expression is shown in green. Left, eat-4 expression, labeling glutamatergic neurons, is shown in magenta. Dashed circle, the soma of AWC, which is glutamatergic. Right, cho-1 expression, labeling cholinergic neurons, is shown in magenta. Top, bottom, head and tail expression, respectively. B. Effect of AEA on amplitude of AWC calcium transients in response to the removal of superior or inferior food in unc-13 null mutants. Each trace is average normalized fluorescence change (ΔF⁄F) versus time. Superior food (blue), DA1877, OD600 1; inferior food (orange), DA1885, OD600 1. C. Summary of the data in B, showing mean peak ΔF⁄F. D. Effect of AEA on amplitude of AWC calcium transients in response to the removal of superior or inferior food in unc-31 null mutants. Each trace is average normalized fluorescence change (ΔF⁄F) versus time. Superior food (blue), DA1877, OD600 1; inferior food (orange), DA1885, OD600 1. E. Summary of the data in D, showing mean peak ΔF⁄F. See also Table S6. For statistics in B-E, see Table S7. Symbols: *, p < 0.05; **, p < 0.01; n.s., not significant. Error bars and shading, 95% confidence interval.
Table 1. npr-19-expressing neurons.
The npr-19 expression pattern was characterized by expressing a pnpr-19::GFP transgene together with either pcho-1::mCherry or peat-4::mCherry, labeling cholinergic and glutamatergic neurons, respectively.81,82 GFP-positive neurons that expressed neither of the markers were identified by position and morphology, and confirmed by cross-reference to CeNGEN expression data showing npr-19. Neurotransmitter identity and unc-31 expression of each identified neuron class are shown for comparison.83,85 See also Table S6.
| Function | Identity of npr-19::GFP+ neurons | eat-4::mCherry expression | cho-1::mCherry expression | Cell body position and | CenGen npr-19 expression | Transmitters | unc-31 expression |
|---|---|---|---|---|---|---|---|
| Pharyngeal | M3 L/R | * | Glu, FLP-18, NLP-3 | ||||
| MI | * | * | Glu | * | |||
| MC L/R | * | * | Ach, FLP-21 | * | |||
| I2 L/R | * | Glu, NLP-3, NLP-8 | * | ||||
| I4 | * | * | NLP-3, NLP-13 | * | |||
| M1 | * | * | Ach, NLP-3 | * | |||
| Sensory | PHC L/R | * | * | Glu | |||
| URX L/R | * | * | Ach, FLP-8, FLP-10, FLP-11, FLP-19 | * | |||
| ASG L/R | * | * | Glu, 5HT, FLP-6, FLP-13, FLP-22, INS-1 | * | |||
| AWA L/R | * | * | INS-1 | * | |||
| Interneuron | RIA L/R | * | Glu | * | |||
| RIM L/R | * | * | Glu, Tyr | ||||
| LUA L/R | * | * | Glu, NLP-13, PDF-1 | ||||
| Motor | URA D/V L/R |
* | * | ACh | * | ||
| PDA | * | * | ACh | * |
The npr-19 expression pattern supports two possible indirect models of AEA’s effect on AWC. In the first model, AWC inherits its sensitivity to AEA from AEA-sensitive synaptic pathways that involve classical neurotransmitters; this mechanism is plausible because cannabinoid signaling is known to inhibit release of classical neurotransmitters in mammals.84 In the second model, AWC inherits its sensitivity from AEA-sensitive signaling pathways that involve neuromodulators85. To test whether classical synaptic pathways render AWC sensitive to AEA, we imaged AWC activity in worms with a null mutation in unc-13, the C. elegans homolog of Munc13, which is required for exocytosis of the clear-core synaptic vesicles that contain classical neurotransmitters.86 In unc-13 mutants, exposure to AEA caused a reciprocal change in food sensitivity, just as in N2. (Figure 5B, C; Table S7, lines 3, 6–7, 9, 13, 15–16, 18). This result makes it unlikely that AWC inherits its AEA sensitivity from synaptic pathways that involve classical neurotransmitters.
We next investigated the model in which AEA causes the release of neuromodulators that might act on AWC. Most neuromodulatory substances, such as neuropeptides and biogenic amines, are released by exocytosis of dense-core vesicles.87,88 Gene expression data83 indicate that most of the npr-19-expressing neurons also express unc-31 (11 out of 15, Table 1),89 the C. elegans ortholog of human CADPS/CAPS, a gene required for exocytosis of dense-core vesicles. This correspondence provides an anatomical substrate for cannabinoid-mediated release of neuromodulators. We therefore recorded from AWC in an unc-31 deletion mutant. If AEA’s effect on AWC were solely the result of neuromodulation mediated by unc-31, one would expect this mutation to phenocopy npr-19 null, exhibiting no AEA effects on AWC responses. This appeared to be the case for the response to superior food, in which there was no effect of AEA (Figure 5D, E; Table S7, lines 21, 24–25, 27). Although AWC responses to inferior food were still modulated by AEA, they were increased rather than decreased (Figure 5D, E; Table S7, lines 31, 33, 36). We conclude that AEA’s modulation of AWC food sensitivity is severely disrupted in unc-31 mutants. We cannot rule out the possibility that overall disruption of neuromodulation in unc-31 mutants results in non-specific developmental or functional disruption in AWC physiology. Nevertheless, the phenotypes of unc-13 and unc-31 taken together support a model in which NPR-19 receptors activated by AEA promote the release of dense-core vesicles containing modulatory substances that act on AWC (Figure 6).
Figure 6. A model for AEA-induced hedonic feeding.
AEA binds to NPR-19 on a neuron upstream of AWC, releasing dense-core vesicle release containing neuromodulators. These neuromodulators increase AWC’s activation in response to superior food removal and, conversely, decreases AWC’s activation in response to inferior food removal. As AWC causes worm attraction to, and retention in food patches, this bidirectional modulation leads to increased aggregation of worms on superior food and decreased aggregation on inferior food.
Discussion
In mammals, administration of THC or endocannabinoids induces hedonic feeding. The present study provides two converging lines of evidence supporting the hypothesis that cannabinoids induce hedonic feeding in C. elegans. First, in the five bacteria strains for which food quality has previously been characterized,68 AEA reciprocally altered food consumption, causing worms to feed at higher and lower rates on superior food and inferior food, respectively (Figure 1C), with no effect on a food of intermediate quality. We found that this trend extends to a sixth strain, OP50, whose quality as a food source was not previously characterized but is likely to be an inferior food,90,91 as its consumption was suppressed by AEA, as previously reported (Figure 1B).55 In the second line of evidence, AEA can differentially alter appetitive behavior. AEA exposure causes increased preference for superior food, which can be traced to a larger proportion of worms accumulating on superior food and smaller proportion accumulating on inferior food (Figure 2G). In the T-maze assay, individual worms are capable of exiting one patch and entering the other multiple times over the duration of the experiments.69 Thus, the proportions of worms accumulating in each patch are mathematically equivalent to the average fraction of time that an individual worm spends feeding in each patch. Therefore, even if worms were feeding at the same rate in the two patches, consumption of superior food would be increased under the influence of AEA. We can therefore infer that the effect of AEA on accumulation is further evidence of increased consumption of superior food. Together, these findings support the conclusion that AEA induces hedonic feeding in C. elegans.
Our findings confirm and extend previous investigations concerning the role of the endocannabinoid system in regulating feeding in C. elegans. We confirmed expression of npr-19 in the inhibitory pharyngeal motor neuron M3 and the sensory neuron URX.55 We extended these results by identifying 13 additional npr-19 expressing neurons, including sensory neurons, interneurons, and motor neurons. Of particular interest is the detection of npr-19 expression in five additional pharyngeal neurons. Thus, 6 of the 20 neurons comprising the pharyngeal nervous system are potential sites for endocannabinoid mediated regulation of pumping rate. Interestingly, these 6 neurons include the motor neuron MC, the pacemaker regulating pharyngeal pumping frequency,92,93 and M3, which regulates pump duration.94 It will now be illuminating to investigate the neuronal mechanism underlying reciprocal modulation of pumping rate in response to superior and inferior foods.
To date, only a small number of studies have examined the effects of cannabinoids on feeding and food preference in invertebrates. Early in evolution, the predominant effect may have been feeding inhibition. For example, cannabinoid exposure shortens bouts of feeding in Hydra95 and larvae of the tobacco hornworm moth Manduca sexta prefer to eat leaves containing lower rather than higher concentrations of the phytocannabinoid cannabidiol.96 In adult fruit flies (Drosophila melanogaster), exposure to phyto- or endocannabinoids (AEA and 2-AG) for several days before testing reduces consumption of standard food.97 On the other hand, in side-by-side tests of sugar-yeast solutions with and without added phyto- or endocannabinoids, flies prefer the cannabinoid-spiked option. The picture that emerges is that whereas the original response to cannabinoids may have been feeding suppression, through evolution the opposite effect arose, sometimes in the same organism. As we have shown, C. elegans exhibits both increases and decreases in consummatory and appetitive responses under the influence of cannabinoids.
Although administration of cannabinoids causes hedonic feeding in C. elegans and mammals, there are notable differences in how it is expressed. One experimental design commonly used in mammalian studies is to measure consumption of a single test food, which is either standard laboratory food or calorically dense food. In such experiments, consumption of both types of food is increased following cannabinoid system activation.20,98,99 The analogous experiment in the present study is the experiment of Figure 1, in which consumption was measured in response to different foods presented alone, ranging from nutritionally superior to inferior. We found that consumption of superior food increases as in mammalian studies whereas, in contrast, consumption of inferior food decreases. A second experimental design commonly used in mammalian studies is to measure consumption of standard and calorically dense foods when both options are simultaneously available. In this type of experiment, cannabinoids increase consumption of calorically dense food, but consumption of standard food is unchanged.22,35–38 Cannabinoid receptor antagonists produce the complementary effect: reduced consumption of calorically dense food with little or no change in consumption of standard food.40,41 The analogous experiments in the present study are the T-maze assays in which maze arms are baited with superior and inferior food. We find that following cannabinoid administration, consumption of superior food increases whereas consumption of inferior food decreases.
Considering both experimental designs, cannabinoids in C. elegans have reciprocal effects on consumption, whereas in mammals this appears not to be the case. It is conceivable that reciprocal responses are energetically advantageous in that they produce a stronger bias in favor of superior food than a unidirectional response, raising the question of why reciprocal responses have not been reported in mammals. There are, of course, considerable differences in the feeding ecology of nematodes and mammals, possibly making unidirectional responses a better strategy in mammals. On the other hand, differences in experimental procedures may explain the absence of reciprocal responses in mammals. In mammalian studies in which the two foods are presented together, standard and calorically dense foods are placed in close proximity such that there is essentially no cost in terms of physical effort for the animal to switch between feeding locations. It is conceivable that increasing the switching cost100 could lead to a differential effect on consumption in mammals.
We propose the following model of differential accumulation on food leading to hedonic feeding in C. elegans (Figure 6). The model focuses on the olfactory neuron AWC, which is necessary and sufficient for navigation to the source of food-related odors101 and whose responses exhibit reciprocal modulation by AEA. In mammals, cannabinoids have been observed to modify chemosensitivity in the periphery and brain. Both AEA and 2-AG amplify the response of primary chemosensory cells, such as sweet-taste cells in the tongue,49,50 which might explain increased consumption of sweet foods and liquids. Cannabinoids can also increase the sensitivity of the mammalian central olfactory system during food-odor exploration.51,102,103 We found that AEA alters the sensitivity of the olfactory neuron AWC. In unexposed worms, AWC is equally sensitive to superior and inferior food, suggesting it cannot detect a difference in the odors released by the two food types.
However, in remarkable alignment with the reciprocal changes we observed in consumption, AEA makes this neuron more sensitive and less sensitive to superior food and inferior food, respectively. Previous studies have demonstrated that activating AWC, by decreasing attractant concentration or by exogenous activation, triggers reorientation toward attractants.101,104–106 The increased response of AWC to removal of superior food triggered by AEA likely enhances reorientation toward such food; conversely, the decreased response to removal of inferior food likely weakens reorientation to such food. The requirement for ceh-36 in rendering C. elegans food preferences sensitive to AEA suggests that AWC neurons provide a link between AEA and hedonic feeding. However, we do not exclude the possibility of contributions from other chemosensory neurons. Of particular interest are the two chemosensory neurons AWA and ASG, both of which express npr-19 and are required for chemotaxis.66,67 It will now be important to map cannabinoid sensitivity across the entire population of food-sensitive neurons to understand how cannabinoids alter the overall chemosensory representation of superior and inferior foods.
AEA’s effect on AWC appears to be indirect. Our results are consistent with a model in which AEA activates NPR-19 receptors to promote release of dense-core vesicles containing neuromodulators that act on AWC. This model is supported by evidence in C. elegans that 2-AG, which activates NPR-19, stimulates widespread release of serotonin;55,57 therefore, NPR-19 activation seems capable of promoting dense-core vesicle release. Additionally, AWC expresses receptors for biogenic amines and responds to neuropeptides released by neighboring neurons,107,108 suggesting that it has postsynaptic mechanisms for responding to various neuromodulators. Identification of one or more neuromodulators responsible for AEA’s effect on AWC, together with their associated receptors, will help answer the question of how AEA causes reciprocal changes in food-odor sensitivity.
Our results establish a new role for endocannabinoids in C. elegans: the induction of hedonic feeding. The endocannabinoid system and its molecular constituents offer significant prospects for pharmacological management of health, including eating disorders and substance abuse.109 Clear parallels between the behavioral, neuronal, and genetic basis of hedonic feeding in C. elegans and mammals establish the utility of this organism as a new genetic model for the investigation of molecular and cellular bases of these and related disorders.
STAR methods
Resource availability
Lead contact
Requests for strains, information or datasets should be directed to the lead contact, Shawn R. Lockery (shawn@uoregon.edu).
Materials availability
Strains and plasmids generated in this study are available upon request to the lead contact.
Data and code availability
All datasets will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Strains.
Animals were cultivated under standard conditions111 using E. coli OP50 as a food source. Young adults of the following strains were used in all experiments: (see Key Resources Table for details): N2, Bristol (Reference strain), FK311, RB1668, C02H7.2(tm2574), XL324, XL325 (Preference and feeding assays), XL322, XL327, XL326, XL346, XL348 (Calcium imaging), XL334, XL335 (npr-19 expression pattern).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |
|---|---|---|---|
| Bacterial strains | |||
| OP50 | C. elegans Genetic Center (CGC) | RRID:WB-STRAIN:WBStrain00041969 | |
| DA1877 | CGC | RRID:WB-STRAIN:WBStrain00040995 | |
| DA1885 | CGC | RRID:WB-STRAIN:WBStrain00040997 | |
| DA837 | CGC | RRID:WB-STRAIN:WBStrain00040994 | |
| HB101 | CGC | RRID:WB-STRAIN:WBStrain00041075 | |
| DA1881 (S13) | Raizen lab DOI: 10.1242/jeb.00433 | ||
| C. elegans strains | |||
| (genotype) | |||
| N2, Bristol | Wild type | CGC | RRID:WB-STRAIN:WBStrain00000001 |
| FK311 | CGC | RRID:WB-STRAIN:WBStrain00007515 | |
| ceh-36(ks86) | |||
| RB1668 | CGC | RRID:WB-STRAIN:WBStrain00032361 | |
| npr-19(ok2068) | |||
| C02H7.2(tm2574) | National Bioresource Project for the Experimental Animal “Nematode C. elegans” | ||
| npr-19(tm2574) | |||
| XL324 | This manuscript | ||
| ntIS1701[npr-19::CNR1::gfp-npr-19(1.1);unc-122::RFP] | |||
| XL325 | This manuscript | ||
| ntIS1702[npr-19::npr-19::gfp-npr-19(1.1)] | |||
| XL322 | This manuscript | ||
| ntIS1703[str-2::GCaMP6::wCherry;unc-122::dsRed] | |||
| XL327 | This manuscript | ||
| unc-13(e51);ntIs1703[str-2::GCaMP6::wCherry;unc-122::dsRed] | |||
| XL326 | This manuscript | ||
| unc-31(e928);ntIs1703[str-2::GCaMP6::wCherry;unc-122::dsRed] | |||
| XL346 | This manuscript | ||
| npr-19(ok2068);ntIs1912[str-2::GCaMP6::wCherry;unc-122::dsRed] | |||
| XL348 | This manuscript | ||
| npr-19(ok2068);ntIs2301[npr-19::npr-19,str-2::GCamp6+wormcherry,unc-122::dsred] | |||
| XL334 | This manuscript | ||
| otIs544[cho-1::SL2::mCherry::H2B+pha-1(+)];ntIS19114[npr-19::GFP1.1;unc122::dsred] | |||
| XL335 | This manuscript | ||
| ntIS19114[npr-19::GFP1.1;unc-122::dsred];otIs518[eat-4::SL2::mCherry::H2B+pha-1(+)] | |||
| Software | |||
| MATLAB | MathWorks https://www.mathworks.com | R2022a (9.12) | |
| Igor Pro | Wavemetrics https://www.wavemetrics.com/ | Version 9.01 | |
| R studio | R Core Team https://www.rproject.org/ |
Version 2022.12.0+353 | |
Bacteria.
The following bacterial strains were used in this study (see Key Resources Table for details): DA1885 (Bacillus simplex), DA1877 (Comamonas sp.), E. Coli HB101, E. Coli DA837, E. Coli OP50 and DA1881 (S13, Bacillus cereus). Bacteria were grown overnight at 37°C (in presence of 50 mg/mL streptomycin for streptomycin-resistant strains: DA1877, DA1885, HB101).
Method details
Bacteria preparation.
Bacteria were concentrated by centrifugation, rinsed three times with either M9 buffer (for EPG experiments) or A0 buffer (for behavioral/imaging experiments; MgSO4 1 mM, CaCl2 1 mM, HEPES 10 mM, glycerol to 350 mOsm, pH 7.1), and resuspended to their final concentration. Concentration was defined as optical density at 600 nm (OD600), as measured with a DSM cell density meter (Laxco, Bothell, WA, USA). All measurements were performed on samples diluted into the linear range of the instrument (OD600 0.1–1). Previous experiments determined that OD600 1 corresponds to approximately 2.35 × 109 and 2.00 × 109 colony forming units/mL of Comamonas and B. Simplex, respectively (Katzen et al., 2021).
Animal preparation.
Worms were washed five times in M9 buffer for EPG experiments or A0 buffer (see above) for behavioral/imaging experiments. Worms were then incubated for 20 minutes with either buffer alone (A0 for behavioral/imaging experiments, M9 for EPG pumping-rate assays) or buffer + 300 μM (pumping-rate assays) or 100 μM (behavioral/imaging experiments) Arachidonoylethanolamide (AEA, Cayman Chemical, Ann Arbor, MI, USA). The incubation time and relatively high concentration reflects the low permeability of the C. elegans cuticle to many exogenous molecules.112,113 We based our AEA concentrations on a previous study that shows the concentration dependence of AEA effects on pharyngeal pumping rate.55 In pilot experiments for pumping-rate and T-maze assays, we used lower AEA doses (100 μM and 50 μM, respectively). As these experiments revealed small, variable effects, we chose the higher concentration given above, which are still within the effective range.55
Behavioral assays.
Freshly poured NGM agar plates were dried in a dehydrator for 45 minutes at 45°C. A maze cut from foam sheets (Darice, Strongsville, OH, USA) using a laser cutter or a cutting machine (Cricut, South Jordan, UT, USA) was placed on each plate (Figure 2A). Maze arms were seeded with 4.5 μL of bacteria. Animals were deposited at the starting point of the maze by liquid transfer and a transparent plastic disc was placed over the maze to eliminate air currents; 12 plates were placed on a flatbed scanner and simultaneously imaged every 15 minutes.114,115 The number of worms in the two patches of food and the region between them was counted manually and a preference index I calculated as: I = (nS − nI)/(nS + nI), where nS is the number of worms in the superior food patch, and nI is the number of worms in the inferior food patch. Worms that did not leave the starting point were excluded. For experiments involving mutants, a cohort of N2 animals was run in parallel on the same day. In some experiments, a paralytic agent (sodium azide, NaN3, 3 μL at 20 mM), was added to each food patch to prevent animals from leaving the patch of food after reaching it. Sodium azide diffuses through the agar over time and its action is not instantaneous. These two characteristics resulted in some worms becoming paralyzed around rather than in the patch of food, as they stop short of the patch or escape the patch briefly before becoming paralyzed. To account for these effects all worms within 5 mm of the end of the maze’s arm, rather than on food, were used when calculating preference index.
Pumping rate assays.
Pharyngeal pumping was measured electrophysiologically using a ScreenChip microfluidic system (InVivo Biosystems, Eugene, OR, USA).71 Following pre-incubation as described above (Animal preparation), worms mixed with bacterial food (OD600 0.8) ±AEA 300 μM were loaded into the worm reservoir of a microfluidic device; this food density was chosen to reduce possible ceiling effects on pumping rate modulation by AEA. Individual worms were resident in the reservoir for 5–55 min. prior to being recorded; they were presumably feeding, and gaining food experience, during this time. To record voltage transients associated with pharyngeal pumping,70 worms were transferred one at a time from the reservoir to the recording channel. Worms were given three minutes to acclimate to the channel before being recorded for one minute. Mean pumping frequency was extracted using custom code written in Igor Pro (Wavemetrics, Lake Oswego, OR, USA).
Calcium imaging.
After pre-incubation with buffer (A0) or buffer +AEA (Animal preparation), worms were immobilized in a custom microfluidic chip and presented with alternating 30-second epochs of buffer and bacteria (either B. Simplex or Comamonas sp. at OD600 1, at a flow rate of 100 μL/min) for 3 minutes. Optical recordings of GCaMP6-expressing AWC neurons were performed on a Zeiss Axiovert 135, using a Zeiss Plan-Apochromat 40X oil, 1.4 NA objective, a X-Cite 120Q illuminator, a 470/40 excitation filter, and a 560/40 emission filter. Neurons were imaged at 3–10 Hz on an ORCA-ERA camera (Hamamatsu Photonics). Images were analyzed using custom code written in MATLAB: the change in fluorescence in a hand-drawn region of interest that contained only the soma and neurite. Data were normalized to the average fluorescence Fo computed over the 15 second interval before the first food stimulus. We computed normalized fluorescence change as ΔF(𝑡)/Fo, where ΔF(𝑡) = F(𝑡) − Fo; following convention, we refer to this measure as “ΔF/F.” For comparison of treatment groups, we used the peak amplitude of post-stimulus ΔF/F. In some animals, AWC appeared not to respond to the food stimulus, regardless of treatment group. To classify particular AWC neurons as responsive or non-responsive, we obtained the distribution of peak ΔF/F values in control experiments in which the stimulus channel contained no food; responsive neurons were defined as those whose peak ΔF/F value exceeded the 90th percentile of this distribution. Critically, the percentage of non-responders did not vary between AEA-treated and non-treated animals (25.46% vs 22.49% respectively; χ2(1,759) = 0.699, p = 0.4031).
Expression profile for npr-19.
Worms were immobilized with 10 mM sodium azide (NaN3) and mounted on 5% agarose pads formed on glass slides. Image stacks (30–80 images) were acquired using a Zeiss confocal microscope (LSM800, ZEN software) at 40X magnification. Identification of neurons was done based on published expression profiles of the pcho-1::mCherry82 and peat-4::mCherry81 transgenes in C. elegans. Individual neurons were identified by mCherry expression and the relative positions of their cell bodies; npr-19 expression was visualized using a pnpr-19::GFP transgene. Co-expression of GFP and mCherry was assessed by visual inspection using 3D image analysis software Imaris (Oxford Instruments). Representative images (Figure 5) are maximum intensity projections of 30–80 frames computed using ImageJ software.116 Expression of the NPR-19 receptor was widespread in body wall muscles but restricted to 29 neurons in the head (27 – 31, 95% confidence interval, n = 20 worms imaged) and 8 neurons in the tail (7.8 – 8.5, 95% confidence interval, n = 22 worms imaged) (Table S7). Overall, 28 of the npr-19-expressing neurons co-localized with either cho-1 or eat-4, whereas ∼9 did not co-localize with either marker. The identity of cells that did not co-localize with cho-1 or eat-4 was ascertained based on cell body position and morphology, and verified by npr-19 expression (threshold = 2) as reported in the C. elegans Neuronal Gene Expression Map & Network (CeNGEN) consortium database.83
Quantification and statistical analysis
A detailed description of statistical tests used and their results is presented in Tables S1–S6. Data were checked for normality with a Kolmogorov-Smirnov test.
Number of replicates.
The minimal sample size for the T-maze assays were based on pilot experiments which demonstrated the ability to detect moderate to small effect sizes with ~10–30 replicates per experimental condition. Previously published EPG data showed that mutants/treatments could be distinguished with ~10 replicates. In order to ensure detection of small effect size across experimental conditions, ~70 to 120 replicates were performed in EPG experiments. Similarly, the minimal number of replicates for imaging experiments were based on previously published data.
Effect sizes.
Effect sizes were computed as follow: Cohen’s d for t-tests, partial eta-squared for ANOVAs, and for Mann-Whitney test, where is the Z-score and is the number of observations.
Behavioral experiments (T-mazes).
Preference indices were analyzed using a two-factor ANOVA with repeated measures (effect of AEA by effect of time, with time as a repeated measure). For easier presentation, an average index across the four time-points was calculated and displayed (Figure 2C–F, 3B, 4A). All timeseries are nonetheless available for inspection in Figure 2, 3A and Figure S1 and S2. The effect of AEA was deemed significant if main effect of AEA was significant in the ANOVA. Averaging the four time points in a series would only be problematic if there was a non-ordinal interaction AEA by time. Inspection of ANOVA results and time series reveal that the only AEA by time interactions are ordinal and minimal (Figure S1, S2). In cases where the effect of time was important (Figure 3A) or the interaction AEA by time was meaningful (Figure 2G) the time series of preference indices was presented. The comparison of preference indices between N2 and mutants relied on a two-factor ANOVA (effect of strain by effect of AEA). The average preference index across the four time-points was used for the comparison. In addition to an ANOVA, planned comparisons were incorporated in the experimental design using t-tests and focusing on four scientifically relevant contrasts: (1) mutants, AEA– vs AEA+; (2) N2, AEA– vs AEA+; (3) AEA–, mutants vs N2; (4) AEA+, mutants vs N2.
Pumping rate assay.
As the data were not normally distributed in most of the cohorts, a non-parametric test (Mann-Whitney) was used to compared pumping frequencies between strains/treatments.
Calcium imaging.
Peak ΔF/F was used as the primary measure. A two-factor ANOVA (effect of AEA by effect of bacteria type) was used to assess the effect of AEA on AWC responses. Planned t-tests were focused on four contrasts: (1) superior food, AEA– vs AEA+; (2) inferior food, AEA– vs AEA+; (3) AEA-, superior food vs inferior food; (4) AEA+, superior food vs inferior food. For comparisons between N2 and mutants, a two-factor ANOVAs (effect of AEA by effect of strain) was performed for each of the bacteria type (superior and inferior) and followed by four contrasts (t-tests): (1) mutants, AEA– vs AEA+; (2) N2, AEA– vs AEA+; (3) AEA–, mutants vs N2; (4) AEA+, mutants vs N2.
Multiple comparisons.
No correction for multiple comparisons was applied in t-tests used in pair-wise comparisons of means in multifactor experiments as the experimental design in this study relied on a small number (3 per condition) of planned (a priori), rather than unplanned (a posteriori), scientifically relevant contrasts.117
Supplementary Material
Highlights.
AEA reciprocally alters consumption of high and low quality food in C. elegans
Reciprocity is evident in both feeding rate and chemotaxis preference
Deletion of the native cannabinoid receptor npr-19 is rescued by the human CNR1 gene
AEA reciprocally alters olfactory neuron sensitivity to high and low quality foods
Acknowledgements
We thank Richard Komuniecki for the npr-19(ok2068) and rescue strains, and David Raizen for providing the DA1881 (B. cereus) bacterial strain. The unc-13, unc-31, ceh-36, cho-1, and eat-4 worm strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Oliver Hobert, Jonathan Millet, and Jon Pierce for discussion, and Leon Avery and Matthew Smear for comments on the manuscript. We thank Chris Doe for use of a Zeiss LSM800 confocal microscope for imaging. Funding for this project was provided by NIDA (DA047645) and NIGM (GM129576).
Footnotes
Inclusion and diversity statement
We support inclusive, diverse, and equitable conduct of research.
Declaration of Interests
Shawn R. Lockery is co-founder and Chief Technology Officer of InVivo Biosystems, Inc., which manufactures instrumentation for electrophysiological recording of pumping rate in nematodes and is the author of the patent Electropharyngeogram arrays and methods of use (US-9723817-B2). The other authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abel EL (1971). Effects of marijuana on the solution of anagrams, memory and appetite. Nature 231, 260–261. 10.1038/231260b0. [DOI] [PubMed] [Google Scholar]
- 2.Kirkham TC, and Williams CM (2001). Endogenous cannabinoids and appetite. Nutr. Res. Rev 14, 65–86. 10.1079/NRR200118. [DOI] [PubMed] [Google Scholar]
- 3.Foltin RW, Brady JV, and Fischman MW (1986). Behavioral analysis of marijuana effects on food intake in humans. Pharmacol. Biochem. Behav 25, 577–582. 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Abel EL (1975). Cannabis: Effects on hunger and thirst. Behav. Biol 15, 255–281. 10.1016/S0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- 5.Tart CT (1970). Marijuana Intoxication : Common Experiences. Nat. 1970 2265247 226, 701–704. 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- 6.Halikas J, Goodwin D, and Guze S. (1971). Marihuana effects. A survey of regular users. JAMA 217, 692–694. 10.1001/JAMA.217.5.692. [DOI] [PubMed] [Google Scholar]
- 7.Hollister LE (1971). Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clin. Pharmacol. Ther 12, 44–49. 10.1002/CPT197112144. [DOI] [PubMed] [Google Scholar]
- 8.Foltin RW, Fischman MW, and Byrne MF (1988). Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 11, 1–14. 10.1016/S0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 9.Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, Poetz O, Pluschke G, and Gertsch J. (2012). Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem 287, 36944–36967. 10.1074/JBC.M112.382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, et al. (2011). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci 15, 64–69. 10.1038/NN.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaczocha M, Glaser ST, and Deutsch DG (2009). Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci 106, 6375–6380. 10.1073/PNAS.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, Rapino C, Finazzi-Agrò A, and Maccarrone M. (2009). Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem. Biol 16, 624–632. 10.1016/J.CHEMBIOL.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Kaczocha M, Vivieca S, Sun J, Glaser ST, and Deutsch DG (2012). Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J. Biol. Chem 287, 3415–3424. 10.1074/JBC.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liedhegner ES, Vogt CD, Sem DS, Cunningham CW, and Hillard CJ (2014). Sterol carrier protein-2: binding protein for endocannabinoids. Mol. Neurobiol 50, 149–158. 10.1007/S12035-014-8651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, and Whistler JL (2007). Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 21, 802–811. 10.1096/FJ.06-7132COM. [DOI] [PubMed] [Google Scholar]
- 16.Rozenfeld R, and Devi LA (2008). Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 22, 2311–2322. 10.1096/FJ.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, and Mackie K. (1999). Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci 19, 3773–3780. 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristino L, Becker T, and Di Marzo V. (2014). Endocannabinoids and energy homeostasis: An update. BioFactors 40, 389–397. 10.1002/BIOF.1168. [DOI] [PubMed] [Google Scholar]
- 19.Kirkham TC, Williams CM, Fezza F, and Di Marzo V. (2002). Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol 136, 550. 10.1038/SJ.BJP.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CM, and Kirkham TC (1999). Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 143, 315–317. 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 21.Mahler SV, Smith KS, and Berridge KC (2007). Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking’ of a Sweet Reward. Neuropsychopharmacology 32, 2267–2278. 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh RR, and Sharma PL (2012). Stimulation of accumbens shell cannabinoid CB1 receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol. Res 66, 276–282. 10.1016/J.PHRS.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Parker L. (2017). Cannabinoids and the Brain (The MIT Press; ). [Google Scholar]
- 24.Munro S, Thomas KL, and Abu-Shaar M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 25.Devane WA, Dysarz FA, Johnson MR, Melvin LS, and Howlett AC (1988). Determination and characterization of a cannabinoid receptor in rat brain - PubMed. Mol Pharmacol 34, 605–613. [PubMed] [Google Scholar]
- 26.Maccioni P, Pes D, Carai MAM, Gessa GL, and Colombo G. (2008). Suppression by the cannabinoid CB1 receptor antagonist, rimonabant, of the reinforcing and motivational properties of a chocolate-flavoured beverage in rats. Behav. Pharmacol 19, 197–209. 10.1097/FBP.0B013E3282FE8888. [DOI] [PubMed] [Google Scholar]
- 27.Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, and Parker LA (2007). Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol. Behav 91, 383–388. 10.1016/J.PHYSBEH.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton-Jones ZD, Vickers SP, and Clifton PG (2005). The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl). 179, 452–460. 10.1007/S00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, and Salamone JD (2003). The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav. Pharmacol 14, 583–588. 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Freedland CS, Poston JS, and Porrino LJ (2000). Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol. Biochem. Behav 67, 265–270. 10.1016/S0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 31.Gallate JE, Saharov T, Mallet PE, and McGregor IS (1999). Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur. J. Pharmacol 370, 233–240. 10.1016/S0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 32.Gallate JE, and McGregor IS (1999). The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl). 142, 302–308. 10.1007/S002130050893. [DOI] [PubMed] [Google Scholar]
- 33.Guegan T, Cutando L, Ayuso E, Santini E, Fisone G, Bosch F, Martinez A, Valjent E, Maldonado R, and Martin M. (2013). Operant behavior to obtain palatable food modifies neuronal plasticity in the brain reward circuit. Eur. Neuropsychopharmacol 23, 146–159. 10.1016/J.EURONEURO.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Barbano MF, Castañé A, Martín-García E, and Maldonado R. (2009). Delta-9-tetrahydrocannabinol enhances food reinforcement in a mouse operant conflict test. Psychopharmacology (Berl). 205, 475–487. 10.1007/s00213-009-1557-9. [DOI] [PubMed] [Google Scholar]
- 35.Koch JE, and Matthews SM (2001). Delta9-tetrahydrocannabinol stimulates palatable food intake in Lewis rats: effects of peripheral and central administration. Nutr. Neurosci 4, 179–187. [DOI] [PubMed] [Google Scholar]
- 36.Brown JE, Kassouny M, and Cross JK (1977). Kinetic studies of food intake and sucrose solution preference by rats treated with low doses of delta9-tetrahydrocannabinol. Behav. Biol 20, 104–110. [DOI] [PubMed] [Google Scholar]
- 37.Escartín-Pérez RE, Cendejas-Trejo NM, Cruz-Martínez AM, González-Hernández B, Mancilla-Díaz JM, and Florán-Garduño B. (2009). Role of cannabinoid CB1 receptors on macronutrient selection and satiety in rats. Physiol. Behav 96, 646–650. 10.1016/J.PHYSBEH.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara Y, Inui T, Yamamoto T, and Shimura T. (2009). Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport 20, 1382–1385. 10.1097/WNR.0B013E3283318010. [DOI] [PubMed] [Google Scholar]
- 39.Mathes CM, Ferrara M, and Rowland NE (2008). Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, R67. 10.1152/AJPREGU.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gessa GL, Orrù A, Lai P, Maccioni P, Lecca R, Lobina C, Carai MAM, and Colombo G. (2006). Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology (Berl). 185, 248–254. 10.1007/S00213-006-0327-1/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- 41.Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, and Le Fur G. (1997). Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 132, 104–106. [DOI] [PubMed] [Google Scholar]
- 42.Simiand J, Keane M, Keane PE, and Soubrié P. (1998). SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol 9, 179–181. [PubMed] [Google Scholar]
- 43.Castro DC, and Berridge KC (2017). Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl. Acad. Sci. U. S. A 114, E9125–E9134. 10.1073/PNAS.1705753114/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards A, and Abizaid A. (2016). Driving the need to feed: Insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci. Biobehav. Rev 66, 33–53. 10.1016/J.NEUBIOREV.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Higgs S, Williams CM, and Kirkham TC (2003). Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after Δ9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl). 165, 370–377. 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- 46.Davis JD, and Smith GP (1992). Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions - PubMed. Behav Neurosci 106, 217–228. [PubMed] [Google Scholar]
- 47.Grill HJ, and Norgren R. (1978). The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143, 263–279. 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 48.Jarrett MM, Limebeer CL, and Parker LA (2005). Effect of Δ9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol. Behav 86, 475–479. 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, et al. (2010). Endocannabinoids selectively enhance sweet taste. Proc. Natl. Acad. Sci. U. S. A 107, 935–939. 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, and Ninomiya Y. (2013). Modulation of sweet responses of taste receptor cells. Semin. Cell Dev. Biol 24, 226–231. 10.1016/J.SEMCDB.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, Ruehle S, Remmers F, Desprez T, Matias I, et al. (2014). The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci 17, 407–415. 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 52.Elphick MR (2012). The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci 367, 3201–3215. 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raible F, and Arendt D. (2004). Metazoan Evolution: Some Animals Are More Equal than Others. Curr. Biol 14, R106–R108. 10.1016/j.cub.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Estrada-Valencia R, de Lima ME, Colonnello A, Rangel-López E, Saraiva NR, de Ávila DS, Aschner M, and Santamaría A. (2021). The Endocannabinoid System in Caenorhabditis elegans. 1–31. 10.1007/112_2021_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oakes M, Law WJ, Clark T, Bamber B, and Komuniecki R. (2017). Cannabinoids activate monoaminergic signaling to modulate key C. elegans behaviors. J. Neurosci 37, 2859–2869. 10.1523/JNEUROSCI.3151-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastuhov SI, Matsumoto K, and Hisamoto N. (2016). Endocannabinoid signaling regulates regenerative axon navigation in Caenorhabditis elegans via the GPCRs NPR-19 and NPR-32. Genes to Cells 21, 696–705. 10.1111/gtc.12377. [DOI] [PubMed] [Google Scholar]
- 57.Oakes M, Law WJ, and Komuniecki R. (2019). Cannabinoids stimulate the TRP channel-dependent release of both serotonin and dopamine to modulate behavior in C. elegans. J. Neurosci 39, 4142–4152. 10.1523/JNEUROSCI.2371-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehtonen M, Reisner K, Auriola S, Wong G, and Callaway JC (2008). Mass-Spectrometric Identification of Anandamide and 2-Arachidonoylglycerol in Nematodes. Chem. Biodivers 5, 2431–2441. 10.1002/cbdv.200890208. [DOI] [PubMed] [Google Scholar]
- 59.Lehtonen M, Storvik M, Malinen H, Hyytiä P, Lakso M, Auriola S, Wong G, and Callaway JC (2011). Determination of endocannabinoids in nematodes and human brain tissue by liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. B 879, 677–694. 10.1016/J.JCHROMB.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, and Waku K. (1995). 2-Arachidonoylgylcerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun 215, 89–97. 10.1006/BBRC.1995.2437. [DOI] [PubMed] [Google Scholar]
- 61.Harrison N, Lone MA, Kaul TK, Reis Rodrigues P, Ogungbe IV, and Gill MS (2014). Characterization of N-Acyl Phosphatidylethanolamine-Specific Phospholipase-D Isoforms in the Nematode Caenorhabditis elegans. PLoS One 9, e113007. 10.1371/journal.pone.0113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastuhov SI, Fujiki K, Nix P, Kanao S, Bastiani M, Matsumoto K, and Hisamoto N. (2012). Endocannabinoid-Goα signalling inhibits axon regeneration in Caenorhabditis elegans by antagonizing Gqα-PKC-JNK signalling. Nat. Commun 3, 1136. 10.1038/ncomms2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, and Gill MS (2011). N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 473, 226–229. 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reis-Rodrigues P, Kaul TK, Ho J-H, Lucanic M, Burkewitz K, Mair WB, Held JM, Bohn LM, and Gill MS (2016). Synthetic Ligands of Cannabinoid Receptors Affect Dauer Formation in the Nematode Caenorhabditis elegans. G3 (Bethesda) 6, 1695–1705. 10.1534/g3.116.026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frézal L, and Félix MA (2015). The Natural History of Model Organisms: C. elegans outside the Petri dish. Elife 2015. 10.7554/ELIFE.05849.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bargmann CI, and Horvitz HR (1991). Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729–742. 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 67.Bargmann CI, Hartwieg E, and Horvitz HR (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. 10.1016/0092-8674(93)80053-H. [DOI] [PubMed] [Google Scholar]
- 68.Avery L, and Shtonda BB (2003). Food transport in the C. elegans pharynx. J. Exp. Biol 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shtonda BB (2006). Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raizen DM, and Avery L. (1994). Electrical Activity and Behavior in the Pharynx of Caenorhabditis elegans. Neuron 12, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lockery SR, Hulme SE, Roberts WM, Robinson KJ, Laromaine A, Lindsay TH, Whitesides GM, and Weeks JC (2012). A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab Chip 12, 2211–2220. 10.1039/c2lc00001f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oakes MD, Law WJ, Clark T, Bamber BA, and Komuniecki R. (2017). Cannabinoids Activate Monoaminergic Signaling to Modulate KeyC. elegansBehaviors. J. Neurosci 37, 2859–2869. 10.1523/JNEUROSCI.3151-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hart AC (2006). Behavior. In WormBook (The C. elegans Research Community). 10.1895/WORMBOOK.1.87.1. [DOI] [Google Scholar]
- 74.Zaslaver A, Liani I, Shtangel O, Ginzburg S, Yee L, and Sternberg PW (2015). Hierarchical sparse coding in the sensory system of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 112, 1185–1189. 10.1073/pnas.1423656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leinwand SG, Yang CJ, Bazopoulou D, Chronis N, Srinivasan J, and Chalasani SH (2015). Circuit mechanisms encoding odors and driving aging-associated behavioral declines in Caenorhabditis elegans. Elife 4, 1–26. 10.7554/eLife.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koga M, and Ohshima Y. (2004). The C . elegans ceh-36 Gene Encodes a Putative Homemodomain Transcription Factor Involved in Chemosensory Functions of ASE and AWC Neurons. J. Mol. Biol 336, 579–587. 10.1016/j.jmb.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 77.Lanjuin A, Vanhoven MK, Bargmann CI, Thompson JK, and Sengupta P. (2003). Otx / otd Homeobox Genes Specify Distinct Sensory Neuron Identities in C . elegans. Dev. Cell 5, 621–633. [DOI] [PubMed] [Google Scholar]
- 78.Calhoun AJ, Tong A, Pokala N, Fitzpatrick JAJ, Sharpee TO, and Chalasani SH (2015). Neural mechanisms for evaluating environmental variability in caenorhabditis elegans. Neuron 86, 428–441. 10.1016/j.neuron.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, and Bargmann CI (2007). Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 80.Pierce-Shimomura JT, Morse TM, and Lockery SR (1999). The Fundamental Role of Pirouettes in Caenorhabditis elegans Chemotaxis. J. Neurosci 19, 9557–9569. 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serrano-Saiz E, Poole RJ, Felton T, Zhang F, De La Cruz ED, and Hobert O. (2013). Modular Control of Glutamatergic Neuronal Identity in C. elegans by Distinct Homeodomain Proteins. Cell 155, 659–673. 10.1016/J.CELL.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, White JG, LeBoeuf B, Garcia LR, Alon U, et al. (2015). A cellular and regulatory map of the cholinergic nervous system of C. elegans. Elife 4, e12432. 10.7554/eLife.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammarlund M, Hobert O, Miller DM, and Sestan N. (2018). The CeNGEN Project: The Complete Gene Expression Map of an Entire Nervous System. Neuron 99, 430–433. 10.1016/j.neuron.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Good CH (2007). Endocannabinoid-dependent regulation of feedforward inhibition in cerebellar purkinje cells. J. Neurosci 27, 1–3. 10.1523/JNEUROSCI.4842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wormatlas. https://www.wormatlas.org.
- 86.Richmond JE, Davis WS, and Jorgensen EM (1999). UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci 2, 959–964. 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Probert L, De Mey J, and Polak JM (1983). Ultrastructural localization of four different neuropeptides within separate populations of p-type nerves in the guinea pig colon. Gastroenterology 85, 1094–1104. 10.1016/S0016-5085(83)80077-8. [DOI] [PubMed] [Google Scholar]
- 88.Devine CE, and Simpson FO (1968). Localization of tritiated norepinephrine in vascular sympathetic axons of the rat intestine and mesentery by electron microscope radioautography. J. Cell Biol 38, 184–192. 10.1083/JCB.38.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, and Martin TFJ (2007). UNC-31 (CAPS) Is Required for Dense-Core Vesicle But Not Synaptic Vesicle Exocytosis in Caenorhabditis elegans. J. Neurosci 27, 6150–6162. 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sowa JN, Mutlu AS, Xia F, and Wang MC (2015). Olfaction Modulates Reproductive Plasticity through Neuroendocrine Signaling in Caenorhabditis elegans. Curr. Biol 25, 2284–2289. 10.1016/j.cub.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.So S, Miyahara K, and Ohshima Y. (2011). Control of body size in C. elegans dependent on food and insulin/IGF-1 signal. Genes to Cells 16, 639–651. 10.1111/j.1365-2443.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 92.Avery L, and Horvitzt HR (1989). Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron 3, 473–485. 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- 93.Raizen DM, Lee RY, and Avery L. (1995). Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141, 1365–1382. 10.1093/GENETICS/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avery L. (1993). Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol 175, 283–297. 10.1242/JEB.175.1.283. [DOI] [PubMed] [Google Scholar]
- 95.De Petrocellis L, Melck D, Bisogno T, Milone A, and Di Marzo V. (1999). Finding of the endocannabinoid signalling system in Hydra, a very primitive organism: possible role in the feeding response. Neuroscience 92, 377–387. 10.1016/S0306-4522(98)00749-0. [DOI] [PubMed] [Google Scholar]
- 96.Park SH, Staples SK, Gostin EL, Smith JP, Vigil JJ, Seifried D, Kinney C, Pauli CS, and Heuvel BDV (2019). Contrasting Roles of Cannabidiol as an Insecticide and Rescuing Agent for Ethanol-induced Death in the Tobacco Hornworm Manduca sexta. Sci. Rep 9. 10.1038/S41598-019-47017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He J, Tan AMX, Ng SY, Rui M, and Yu F. (2021). Cannabinoids modulate food preference and consumption in Drosophila melanogaster. Sci. Rep 11, 1–13. 10.1038/s41598-021-84180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DiPatrizio NV, and Simansky KJ (2008). Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J. Neurosci 28, 9702–9709. 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williams CM, Rogers PJ, and Kirkham TC (1998). Hyperphagia in pre-fed rats following oral δ9-THC. Physiol. Behav 65, 343–346. 10.1016/S0031-9384(98)00170-X. [DOI] [PubMed] [Google Scholar]
- 100.Salamone JD, Cousins MS, and Bucher S. (1994). Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav. Brain Res 65, 221–229. 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 101.Kocabas A, Shen CH, Guo ZV, and Ramanathan S. (2012). Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature 490, 273–277. 10.1038/nature11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinbockel T, and Straiker A. (2021). Cannabinoids Regulate Sensory Processing in Early Olfactory and Visual Neural Circuits. Front. Neural Circuits 15. 10.3389/FNCIR.2021.662349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nogi Y, Ahasan MM, Murata Y, Taniguchi M, Sha MFR, Ijichi C, and Yamaguchi M. (2020). Expression of feeding-related neuromodulatory signalling molecules in the mouse central olfactory system. Sci. Rep 10. 10.1038/S41598-020-57605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fraenkel GS, and Gunn DL (1961). The orientation of animals: Kineses, taxes and compass reactions. - PsycNET (Dover). [Google Scholar]
- 105.Gordus A, Pokala N, Levy S, Flavell SW, and Bargmann CI (2015). Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161, 215–227. 10.1016/j.cell.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gray JM, Hill JJ, and Bargmann CI (2005). A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. PNAS 90, 2227–2231. 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, and Bargmann CI (2010). Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat. Neurosci 13, 615–621. 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leinwand SG, and Chalasani SH (2013). Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat. Neurosci 16, 1461–1467. 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parsons LH, and Hurd YL (2015). Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci 16, 579–594. 10.1038/NRN4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.WormAtlas. https://www.wormatlas.org/.
- 111.Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rand JB, and Johnson CD (1995). Genetic pharmacology: interactions between drugs and gene products in Caenorhabditis elegans. Methods Cell Biol. 48, 187–204. 10.1016/S0091-679X(08)61388-6. [DOI] [PubMed] [Google Scholar]
- 113.Sandhu A, Badal D, Sheokand R, Tyagi S, and Singh V. (2021). Specific collagens maintain the cuticle permeability barrier in Caenorhabditis elegans. Genetics 217. 10.1093/GENETICS/IYAA047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mathew MD, Mathew ND, and Ebert PR (2012). WormScan: A technique for high-throughput phenotypic analysis of Caenorhabditis elegans. PLoS One 7, e33483. 10.1371/journal.pone.0033483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stroustrup N, Ulmschneider BE, Nash ZM, López-Moyado IF, Apfeld J, and Fontana W. (2013). The caenorhabditis elegans lifespan machine. Nat. Methods 10, 665–670. 10.1038/nmeth.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Collins TJ (2007). ImageJ for microscopy. Biotechniques 43, S25–S30. 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 117.Keppel G, and Zedeck S. (1989). Data analysis for research designs: analysis-of-variance and multiple regression/correlation approaches. 594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.