Abstract
Investigating the co-activation of hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenomedullary (SAM) responses to acute stress can provide insight into how risk might become biologically embedded during early adolescence and improve understanding of what distinguishes physiological dysregulation from normative/expected physiological responses to stress. Evidence has thus far been mixed as to whether symmetric or asymmetric co-activation patterns are associated with higher exposure to chronic stress and poorer mental health outcomes during adolescence. This study expands on a prior multisystem, person-centered analysis of lower-risk, racially homogenous youth by focusing on HPA–SAM co-activation patterns in a higher-risk, racially diverse sample of early adolescents from low-income families (N=119, Mage=11.79 years, 55.5% female, 52.7% mono-racial Black). The present study was conducted by performing secondary analysis of data from the baseline assessment of an intervention efficacy trial. Participants and caregivers completed questionnaires; youth also completed the Trier Social Stress Test-Modified (TSST–M) and provided six saliva samples. Multitrajectory modeling (MTM) of salivary cortisol and alpha-amylase levels identified four HPA–SAM co-activation profiles. In accordance with the asymmetric-risk model, youth exhibiting Low HPA–High SAM (n=46) and High HPA–Low SAM (n=28) profiles experienced more stressful life events, posttraumatic stress, and emotional and behavioral problems relative to Low HPA–Low SAM (n=30) and High HPA–High SAM (n=15) youth. Findings highlight potential differences in biological embedding of risk during early adolescence based on individuals’ exposure to chronic stress and illustrate the utility of multisystem and person-centered approaches in understanding how risk might get “underneath the skin” across systems.
Keywords: adolescence, cortisol, alpha-amylase, multisystem, multitrajectory modeling
Evidence increasingly suggests that exposure to chronic stressors can get “underneath the skin” and overtax regulatory physiological systems involved in stress responsivity (Danese & McEwen, 2012). This biological “wear and tear” is also related to increased risk for psychopathology (Guidi et al., 2021). Better understanding of physiological dysregulation as a potential mechanism linking chronic stressors and mental health outcomes is crucial for preventing and alleviating their individual and public health costs (Bellis et al., 2019).
A Multisystem Approach to Examining Biological Embedding of Risk
Many studies on the biological embedding of risk focus on either the hypothalamic-pituitary-adrenal (HPA) axis or the sympathetic-adrenomedullary (SAM) pathway in isolation (Ali & Nater, 2020; Hellhammer et al., 2009). Exposure to childhood adversity is associated with both HPA hyper- and hyporesponsivity (Joos et al., 2019; Young et al., 2020), which are in turn associated with emotional and behavioral problems (Hartman et al., 2013; Lopez-Duran et al., 2015). SAM response variations are also predicted by exposure to chronic stressors (Kuras et al., 2017; Young-Southward et al., 2020) and linked to psychopathology (Schumacher et al., 2013).
While evidence from these single-system studies inform our understanding of physiological manifestations of potential risk, they do not illustrate the interrelated nature of the two systems. In theory, the HPA axis and SAM pathway work in tandem to help with stress coping. After exposure to a stressor, a typical SAM response is initiated by the hypothalamus’s relatively quick activation (e.g., within seconds) of the sympathetic nervous system (Bauer et al., 2002). At the same time, the HPA response, a relatively slower process (e.g., up to half an hour after initial stressor exposure), is triggered and results in the production and release of glucocorticoids into the body (Engel & Gunnar, 2020; Smith & Vale, 2006). During a well-coordinated HPA–SAM response, HPA-produced cortisol can downregulate the axis itself in a negative feedback loop as well as turn down the SAM response’s reflexive processes (Munck et al., 1984; Sapolsky et al., 2000). In contrast, a dysregulated HPA–SAM response may compromise either system’s ability to upregulate in response to a stressor and/or downregulate once the stressor has passed (McEwen, 1998). Although mostly studied in isolation, concurrent examination of these systems may provide a closer approximation of their inter-related nature and possibly improve understanding of how risk can become biologically embedded (Jones et al., 2020; Wadsworth et al., 2019).
Given evidence that the HPA axis and SAM pathway are structurally and functionally linked (Mueller et al., 2022), a multisystem framework for examining both systems has thus far focused on their co-activation in predicting psychopathology (Bauer et al., 2002). One theoretical model suggests symmetrical HPA–SAM responses to a stressor, regardless of whether both systems are relatively hyper- or hyporesponsive, is indicative of higher risk for psychopathology (Kagan et al., 1998). A second model instead implicates HPA–SAM asymmetry in predicting increased psychopathology (Munck et al., 1984; Sapolsky et al., 2000). There has been empirical evidence in support of both models (for reviews, see Jones et al., 2020; Wadsworth et al., 2019).
Multitrajectory Modeling as a Candidate Multisystem Approach
Though many studies have found that HPA–SAM co-activation in general is linked to psychopathology, there are still numerous knowledge gaps remaining. First, HPA–SAM symmetry and asymmetry have both been associated with emotional and behavioral problems, demonstrating conflicting findings and support for two opposing theoretical models. For instance, internalizing problems has been associated with hyperresponsive symmetric (Bendezú & Wadsworth, 2018; El-Sheikh et al., 2008; Wadsworth et al., 2019), hyporesponsive symmetric (Bae et al., 2015), and asymmetric HPA–SAM patterns (Allwood et al., 2011). Individuals’ contexts may contribute to conflicting findings. In an investigation of youth living in low versus high conflict homes, a link between hyporesponsive symmetry and internalizing problems was significant only for youth exposed to low levels of marital conflict (Koss et al., 2014).
A second plausible explanation for conflicting findings may pertain to how HPA–SAM co-activation is assessed. Studies often utilize summative stress response indices (e.g., averaging levels across time, area-under-the-curve increase (AUCi), percent increase). These approaches do not take advantage of multi-time point stress response data for illustrating multiple aspects of the HPA–SAM response (e.g., basal levels, reactivity, recovery), which collectively may help differentiate between well-orchestrated and dysregulated dual-system function. Additionally, HPA–SAM studies often utilize variable-centered approaches (e.g., multiple regression, growth curve modeling) (Bae et al., 2015; Koss et al., 2014; Wadsworth et al., 2019) that assume population homogeneity (e.g., youth on average exhibit the same HPA–SAM co-activation patterns) and are thus limited in their ability to illustrate individual differences in HPA–SAM co-activation, particularly in samples where population heterogeneity (e.g., youth exhibit qualitatively distinct HPA–SAM co-activation patterns) may be more the rule than the exception (e.g., early adolescence, youth at risk for psychopathology due to chronic stress exposure).
Third, “blunted responses” and “normative non-responses” are often observed but not distinguished from each other in either the single-system or multisystem literatures. For instance, low HPA responsivity displayed by youth believed to be at risk for psychopathology due to their exposure to chronic stressors is commonly referred to as blunted HPA response (Bunea et al., 2017). However, low HPA responsivity is also expected of early adolescence, with normative HPA non-response thought to protect developing brains from potential neurotoxic cortisol overexposure effects (Shansky & Lipps, 2013). Differentiating between blunted HPA response and normative HPA non-response might be achieved by considering concurrent SAM activation. A blunted HPA response might be accompanied by elevated SAM basal levels, exaggerated reactivity, and protracted recovery, reflecting compromised cross-system feedback processes and asymmetrical activation. A normative HPA non-response then might be accompanied by low SAM basal levels, more moderate reactivity, and efficient recovery reflective of well-coordinated HPA–SAM functioning and symmetrical activation.
To address these points, we used a multisystem, person-centered approach (multitrajectory modeling; Nagin et al., 2018) to identify latent HPA–SAM co-activation profiles based on the extent to which subgroups exhibited similar within-person patterns of cortisol and alpha-amylase response (e.g., intercepts, reactivity, recovery) to a standardized psychosocial stressor. We hoped to provide a more nuanced, fine-grained approximation of HPA–SAM co-activation to help clarify inconsistencies in the summative stress response and variable-centered literature, identify links between stressful life events, mental health outcomes, and select co-activation patterns, and potentially illustrate distinctions between blunted HPA response and normative HPA non-response within the HPA–SAM co-activation framework.
Importance of Early Adolescence
Early adolescence is a key developmental stage for calibrations of both the HPA axis and SAM pathway (Engel & Gunnar, 2020; Harteveld et al., 2021; Mulkey & du Plessis, 2019), with implications for mental health outcomes throughout the lifespan. Both pathways undergo significant environment-dependent structural and functional changes during early adolescence (DePasquale et al., 2021; Wade et al., 2020). These changes occur against the backdrop of expected and normative increased physiological stress responses (e.g., increased cortisol and alpha-amylase levels) from childhood to adolescence (Gunnar et al., 2009; Stroud et al., 2009; Sumter et al., 2010). These pieces of evidence point to early adolescence as a potential inflection point during which HPA–SAM links can become biologically embedded for better or worse. In the present study, we sought to understand emerging stress- and outcome-linked physiological co-activation patterns during early adolescence and their implications for mental health.
Present Study
Our study aimed to 1) identify within-person profiles of HPA–SAM co-activation and 2) link those profiles to indices of stress exposure and psychopathology. We applied multitrajectory modeling to cortisol and alpha-amylase data collected from a sample of early adolescents from low-income homes who underwent a standardized laboratory stressor. For Aim 1, we hypothesized that four HPA–SAM profiles would emerge based on previous variable-centered (e.g., Allwood et al., 2011; Bae et al., 2015; El-Sheikh et al., 2008; Gordis et al., 2006) and person-centered (Bendezú & Wadsworth, 2018) findings: Low HPA–Low SAM, High HPA–High SAM, Low HPA–High SAM, High HPA–Low SAM. To characterize the profiles, HPA and SAM trajectories were distinguished by cortisol and alpha-amylase baseline levels, reactivity patterns, and length of time observed for levels to return to baseline (i.e., recovery).
For Aim 2, we sought to identify if stress exposure and mental health outcomes further distinguished the profiles. Given a) the dearth of studies with youth samples similarly considered high risk for psychopathology based on economic disadvantage relative to our study sample and b) inconsistencies in support of the symmetric and asymmetric risk models, we were reluctant to make strong predictions regarding linkages between profiles and maladjustment. Stemming from an anticipated multisystem, person-centered distinction between blunted HPA response and normative HPA non-response, we speculated that youth demonstrating Low HPA–High SAM (relative to Low HPA–Low SAM youth) would experience a greater number of stressful life events and exhibit higher levels of posttraumatic stress and emotional and behavior problems.
Method
Participants
One hundred twenty-nine parent-child dyads from low-income families participated in an efficacy trial of a preventive intervention. Participants were recruited for and participated in the trial in eight cohorts. Ten parent-child dyads withdrew from the study. The remaining 119 youth (55.5% female) completed the TSST–M at baseline and were examined in the present study. Most youth were ages 11 or 12 years (Mage= 11.79, SD=0.56). Fifty percent of youth identified as mono-racial Black, 12% identified as mono-racial White, 16% identified as biracial, 3% identified as mono-racial Native American, and 19% identified as Other. Forty-four percent of youth identified as Hispanic/Latine. Average household size was 5.12 (SD=1.94). Nearly 40% of families received public assistance, and 63% reported food insecurity. Twenty-nine percent of participating parents did not complete high school, 30% had a high school diploma/GED, 22% received education beyond high school but did not receive a degree, 15% received professional or associate degrees from technical or academic programs, and 4% had either a bachelor’s or master’s degree. Forty-one percent of parents were unemployed, and 24% reported received social security benefits in the 12 months prior to study enrollment.
The youth intervention, Building a Strong Identity and Coping Skills (BaSICS), was developed to alleviate the negative effects of poverty-related stress in early adolescents through targeting individual- and community-level coping. Baseline data from the trial was used in the present study. For further details on the efficacy trial, see Wadsworth et al. (2018).
Recruitment and Procedures
Participants were recruited from two low-income communities situated in Harrisburg, Pennsylvania. Families with an eligible child enrolled in school districts serving these two communities were recruited through in-person contact with recruitment staff at community and school events as well as through community partners. Interested parent-child dyads were screened over the phone or in person by trained staff. Youth had to be ages 10–12 years at the time of baseline and fluent in English to be eligible for the study. Other eligibility criteria included: parent fluency in English or Spanish and family income at or below 200% of the federal poverty level during the year of enrollment. Exclusion criteria were parent report of lifetime diagnosis of child autism spectrum disorder and/or intellectual disability; parent report of child current depression or anxiety symptoms meeting clinical cut-off criteria on the Children’s Depression Inventory, 2nd edition (Kovacs, 2015) and the Beck Anxiety Inventory (Beck et al., 1988), respectively; and child enrollment in special education services for over half of the school day. Families excluded due to youth clinical-level depression and/or anxiety symptoms were referred to mental health service providers in the area.
Eligible dyads were consented and scheduled for a baseline assessment between 3 and 5 pm. The three-hour assessment consisted of parent and child completion of questionnaires in separate rooms. Additionally, youth completed the Trier Social Stress Test-Modified (TSST–M; Yim et al., 2010). The TSST–M is a standardized laboratory stressor that has been shown to elicit an increased cortisol response in typically developing youth. In front of two confederate judges, youth prepared and delivered a 5-minute speech and completed a 5-minute oral serial subtraction task. Six saliva samples were collected before and after the TSST–M from participants. Between the third and fourth sample, youth were instructed to sit alone in the assessment room and allowed to choose from a variety of activities (e.g., drawing, playing music) to participate in while their performance on the TSST–M “was being scored.” Between the fourth and fifth sample, youth responded to questions about how they coped with the TSST–M. Between the fifth and sixth sample, youth listened to a 10-minute audio recording guiding them through progressive muscle relaxation exercises. Youth were debriefed on the TSST–M after the sixth sample. The participant dyads received $40 following completion of the assessment. All procedures were approved by the university Institutional Review Board.
Measures
Cortisol and Alpha-Amylase
Each saliva sample was collected by passive drool into a 5 mililiter tube. Youth participants were instructed to not eat a large meal or brush their teeth one hour before and to not have dairy or a sugary/acidic snack twenty minutes before their assessment. Due to the length of the assessment, youth were given a light and non-sugary snack to eat and a small amount of water to drink upon arrival. Forty minutes after the beginning of the assessment and immediately before the TSST–M, participants provided the first saliva sample. The second sample was collected immediately after the TSST–M. The remaining four samples were subsequently collected at 10-minute intervals. Participant wake time on the day of assessment and times of each sample collection were recorded. Samples were stored in a −80°C freezer until they were sent to be assayed in duplicate at The Pennsylvania State University’s Core Biomarker Lab. Cortisol and alpha-amylase means for each sample were used in analyses. The mean intra-assay and inter-assay coefficients of variation for cortisol are 4.60% and 6.00%, respectively. For alpha-amylase, intra-assay and inter-assay coefficients of variation are 5.47% and 4.70%, respectively.
Stressful Life Events
Parents completed the Multicultural Events Scale for Adolescents (MESA; Gonzales et al., 1995). Parents indicated whether their child had experienced any of 86 family-, peer-, and community-level stressors (e.g., “A close family member or someone you live with had serious emotional problems.”, “You saw someone threatened with a knife or gun.”) in the last six months. The total number of stressful life events endorsed was used in analyses.
Posttraumatic Stress
Youth trauma-specific symptoms were assessed through parent report on the Trauma Symptom Checklist for Young Children (TSCYC; Briere et al., 2001). The measure consists of 90 items assessing how often the child experienced each symptom (0=“Never” to 2=“Almost all of the time”). Posttraumatic stress total scores (i.e., sum of intrusion, avoidance, and arousal subscale scores) were sex-normed, standardized, and used in analyses (α=.89).
Emotional and Behavior Problems
Parents completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), a 113-item scale of youth behavior problems rated on a 3-point Likert scale (0=“Never true” to 2=“Very often true”). The CBCL’s Total Problems score was used in a priori analyses. Internalizing and externalizing problems were examined separately in our post hoc analyses. Cronbach alphas on the CBCL range from .90 (Internalizing Problems) to .97 (Total Problems).
Covariates
Parent-child dyads reported on demographics such as child age and sex, which were included as primary study covariates in Aim 2 analyses. An additional secondary set of variables that have been linked to HPA and SAM system functioning were considered as potential covariates in Aim 2 analyses. Parents reported on their child’s pubertal status on the Pubertal Development Scale (PDS; Petersen et al., 1988). Boys’ progression on height, body hair, facial hair, and voice deepening were each rated on a 4-point Likert scale with higher scores indicating more advanced pubertal development. Girls’ development on height, body hair, and breast development were rated on a similar scale; additionally, whether girls had started menstruation (1=“no; 2=“yes”) were reported. Within-sex pubertal staging scores were calculated by averaging PDS items for boys (M=1.71, SD=0.43) and girls (M=2.53, SD=0.63). A saliva sample timing variable was computed by subtracting wake time from the initial saliva sample collection time (M=9.50 h, SD=1.96). This approach to saliva sample timing accounts for variation in diurnal cortisol and alpha-amylase rhythms, given that wake time is highly variable in adolescents (e.g., Calhoun et al., 2012, 2014; Johnson et al., 2019; Owens et al., 2019; Slavich et al., 2020; Young et al., 2021). Parents also reported on child medication use and medications known to impact cortisol, alpha-amylase, or saliva assessment were coded and considered (Granger et al., 2009; Rohleder & Nater, 2009). The efficacy trial’s first five cohorts completed all assessments prior to the start of the COVID-19 pandemic whereas the last three cohorts completed some assessments after the pandemic started. Due to potential pandemic-related effects on the present study’s measures of interest, cohort number was also considered as a potential covariate.
Data Preparation and Preprocessing
Cortisol and Alpha-Amylase Data
Seventeen cortisol values were above three standard deviations from the grand mean: T1 (n=2), T2 (n=3), T3 (n=3), T4 (n=3), T5 (n=3), T6 (n=3). Eight alpha-amylase values were above three standard deviations from the grand mean: T1 (n=2), T2 (n=1), T3 (n=1), T4 (n=1), T5 (n=2), T6 (n=1). As recommended (Felt et al., 2017; Miller & Plessow, 2013), a fourth root transformation was applied and successfully normalized the observed positive skew.
Analysis Plan
Aim 1
Following Bendezú and Wadsworth (2018), multitrajectory modeling (MTM; Nagin et al., 2018) was used to explore potential within-person profiles of HPA–SAM co-activation. Specifically, adolescents were grouped based on the extent to which they exhibited similar cortisol and alpha-amylase stress response trajectories (e.g., intercept and reactivity polynomial parameter estimates). As described elsewhere (Bendezú, Calhoun, et al., 2022), the PROC TRAJ procedure (SAS 9.4) with the MULTGROUPS option employed was utilized. A nonsignificant Little’s (1988) MCAR test, Χ2 (308)=302.33, p>.25, supported our use of Full-Information-Maximum likelihood (FIML) to handle missing data. In the current study, baseline levels for each trajectory are operationalized by their respective intercept estimates while reactivity patterning for each trajectory is operationalized by their respective polynomial parameter estimates. To specify the best fitting model, quadratic and quartic functions were estimated for cortisol and alpha-amylase, respectively,1 at each model specification step (e.g., comparing the two-profile solution to the one-profile solution, comparing the three-profile solution to the two-profile solution, etc.). Before proceeding to the next step in model specification, non-significant highest order polynomial functions for both cortisol and alpha-amylase were trimmed from each trajectory equation. The model was then re-run and the trimming process repeated until a solution containing only significant polynomial parameter estimates for each trajectory in each profile was obtained. The log Bayes factor approximation [2loge(B10)] was utilized at each step as a fit index, with a value above ten providing evidence in support of the more complex solution (e.g., two-profile solution over one-profile solution). Given our sample size (N=119) and recommendations from PROC TRAJ procedure developers (N>100; Nagin, 2005), we limited model specification to four profiles. Following specification, we utilized average posterior probability (AvePPj>0.70), odds of correct classification (OCCj>5.00), and ratio of the probability of subgroup assignment to the proportion of adolescents assigned to subgroups ([Probj/Propj]≈1) as statistical guideposts for evaluating the overall adequacy of the final MTM (Nagin et al., 2018).
After adequacy evaluation, Wald tests comparing different aspects of the identified trajectories were used to distinguish the profiles and inform our labeling conventions. For example, a significant Wald test comparing intercept estimates for two profiles’ cortisol trajectories would indicate that cortisol baseline values were significantly different from one another (e.g., two profiles with relatively lower and higher cortisol baseline levels). A significant Wald test comparing highest order polynomial estimates for two profiles’ alpha-amylase trajectories would indicate that the magnitude of alpha-amylase reactivity significantly differed across profiles (e.g., two profiles with relatively more and less pronounced alpha-amylase reactivity). At times, trajectory distinction analyses can reveal two profiles’ trajectories to be similar in some aspects (e.g., baseline) and distinct in others (e.g., reactivity). In the current study, the differing aspect was used to label the profiles (e.g., lowest, low, high, highest).
Wald tests can also be used to examine aspects of each trajectory that might strengthen inference pertaining to well-regulated and dysregulated HPA–SAM co-activation. To elaborate, Wald tests comparing baseline (e.g., T1) and reactivity (e.g., T3–T6) levels can be used to examine recovery efficiency (i.e., length of time observed for biomarker levels to return to baseline) for each trajectory in each profile. For example, more efficient recovery can be indexed by nonsignificant positive differences between baseline and reactivity levels (i.e., return to baseline) that are identified at earlier reactivity time points (e.g., T3, T4). Alternatively, more protracted recovery can be indexed by nonsignificant positive differences between baseline and reactivity levels at later reactivity time points (e.g., T5, T6) or the absence of a nonsignificant positive difference between baseline and reactivity levels (i.e., failure to return to baseline).
Aim 2
A series of chi-square tests and one-way ANOVA models were utilized to examine potential profiles differences in demographics (e.g., child age (years), sex (0=male, 1=female), cohort (0=pre-COVID cohorts 1–5, 1=post-COVID cohorts 6–8) as well as variables known to impact HPA axis and SAM system function and its assessment (e.g., pubertal status, medication use (0=no, 1=yes), saliva sample timing). Then, MANCOVA was used to examine subgroup membership and correlate associations (e.g., stressful life events, posttraumatic stress, total problem behaviors). Demographic or neuroendocrine function variables that significantly differed across profiles were included as covariates in MANCOVA analyses. Given their association with psychopathology during childhood and adolescence (Sayed et al., 2015; Zahn-Waxler et al., 2015), child age and sex were controlled for in all MANCOVA analyses.
Post Hoc Analyses
Given that the extant literature has focused on how HPA–SAM co-activation is related to specific types of mental health outcomes (e.g., Bae et al., 2015; El-Sheikh et al., 2008; Wadsworth et al., 2019), we performed post hoc analyses to explore if profiles demonstrated unique linkages to different forms of problem behavior: internalizing problems, externalizing problems. Given the exploratory nature of these post hoc analyses, we were reticent to make strong predictions. However, and because youth exhibiting Low HPA–High SAM co-activation were expected to display more total problem behavior relative to Low HPA–Low SAM, we anticipated that this profile might potentially exhibit linkages to a) either more internalizing problems, b) more externalizing problems, or c) more internalizing and externalizing problems.
Results
Descriptive statistics and bivariate correlations for our key study variables are provided in Table 1. Over the course of the TSST–M, cortisol levels were positively correlated (r=.36–.95) as were alpha-amylase levels (r=.61–.84). However, significant between-person associations for cortisol and alpha-amylase did not emerge (r=−.16–.13), which supported our use of a within-person approach for exploring potential cross-system co-activation patterns. Additionally, neither stressful life events, posttraumatic stress, nor total problem behaviors were associated with cortisol or alpha-amylase levels independently. These correlates were also associated with one another in the moderate range (r=.50–.58), which, together, supported our use of MANCOVA to explore potential correlates of identified HPA-SAM co-activation patterns.
Table 1.
Descriptives and Bivariate Correlations for Key Study Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. sC +0 min TSST start | — | ||||||||||||||
| 2. sC +15 min TSST start | .95* | — | |||||||||||||
| 3. sC +25 min TSST start | .51* | .54* | — | ||||||||||||
| 4. sC +35 min TSST start | .41* | .44* | .83* | — | |||||||||||
| 5. sC +45 min TSST start | .36* | .42* | .82* | .93* | — | ||||||||||
| 6. sC +55 min TSST start | .45* | .50* | .92* | .84* | .84* | — | |||||||||
| 7. sAA +0 min TSST start | .13 | .11 | −.09 | .01 | −.03 | −.07 | — | ||||||||
| 8. sAA +15 min TSST start | −.03 | −.01 | −.03 | −.03 | −.02 | −.03 | .61* | — | |||||||
| 9. sAA +25 min TSST start | −.03 | −.02 | −.08 | −.11 | −.11 | −.06 | .64* | .82* | — | ||||||
| 10. sAA +35 min TSST start | −.02 | −.01 | −.10 | −.12 | −.07 | −.09 | .69* | .75* | .80* | — | |||||
| 11. sAA +45 min TSST start | −.06 | −.04 | −.10 | −.03 | −.03 | −.09 | .71* | .66* | .74* | .84* | — | ||||
| 12. sAA +55 min TSST start | −.06 | −.04 | −.05 | −.04 | .01 | −.04 | .71* | .68* | .76* | .83* | .83* | — | |||
| 13. Stressful life events (MESA) | −.11 | −.06 | −.08 | −.11 | −.13 | −.11 | .18 | .16 | .12 | .12 | .04 | .07 | — | ||
| 14. Post-traumatic stress (TSCYC) | −.01 | −.03 | −.08 | −.08 | −.10 | −.11 | .02 | −.16 | −.12 | −.06 | −.17 | −.12 | .58* | — | |
| 15. Total problems (CBCL) | .02 | .05 | −.03 | −.16 | −.15 | −.09 | −.02 | −.12 | −.05 | .02 | −.09 | −.04 | .50* | .61* | — |
| M | 0.09 | 0.08 | 0.08 | 0.09 | 0.07 | 0.06 | 108.9 | 175.3 | 117.8 | 117.7 | 104.7 | 105.9 | 10.14 | 52.06 | 56.10 |
| SD | 0.11 | 0.11 | 0.08 | 0.11 | 0.08 | 0.06 | 83.21 | 118.4 | 78.22 | 85.70 | 84.87 | 73.61 | 8.23 | 10.55 | 12.63 |
| Min | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 2.92 | 23.60 | 12.76 | 5.64 | 10.43 | 5.48 | 0.00 | 40.00 | 32.00 |
| Max | 0.87 | 0.95 | 0.57 | 0.82 | 0.65 | 0.48 | 498.0 | 640.5 | 358.6 | 547.8 | 565.3 | 465.6 | 44.00 | 99.00 | 90.00 |
Note. sC = salivary cortisol. sAA = salivary alpha-amylase. TSST = Trier Social Stress Test. MESA = Multicultural Events Schedule for Adolescents. TSCYC = Trauma Symptoms Checklist for Young Children. CBCL = Child Behavior Checklist.
p < .05.
Within-Person Profiles of HPA-SAM Co-activation (Aim 1)
MTM parameter estimates, adequacy indices, and trajectory distinction analysis results (i.e., differing subscripts) are shown in Table 2. As expected, results obtained from MTM specification supported a four-profile solution (Figure 1): two- to one-profile comparison [2loge(B10)=317.66], three- to two-profile comparison [2loge(B10)=274.04], four- to three-profile comparison [2loge(B10)=48.72]. As per Nagin (2005), a systematic examination of model adequacy indices suggested that the final four-profile solution fit the data well. Our trajectory distinction analyses revealed significant differences that helped characterize the profiles and went on to inform our labeling conventions (Table 2). Consistent with results from Bendezú and Wadsworth (2018), Wald tests revealed that alpha-amylase trajectories for each profile were for the most part distinct with respect to intercept, but not polynomial, estimates. Thus, our alpha-amylase labels reflect differences in baseline levels as opposed to reactivity.
Table 2.
Parameter Estimates (Standard Errors) and Model Adequacy Indices for Final Multitrajectory Modeling for Four-Group Solution
| Salivary Cortisol | Salivary Alpha-Amylase | AvePPj | OCCj | Probj | Propj | Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Low HPA-Low SAM (n=30) | .966 | 86.107 | .253 | .252 | 1.004 | |||
| Intercept | 0.460* (0.010) A | 2.813* (0.081) A,T3 | ||||||
| Linear | −0.001* (0.001) a | 0.087* (0.031) | ||||||
| Quadratic | −0.007* (0.003) | |||||||
| Cubic | 0.001* (0.001) | |||||||
| Quartic | −0.001* (0.001) a | |||||||
| High HPA-High SAM (n=15) | .996 | 745.354 | .127 | .126 | 1.008 | |||
| Intercept | 0.666* (0.016) C, 15 | 3.118* (0.107) B 14 | ||||||
| Linear | 0.002* (0.001) | 0.108* (0.043) | ||||||
| Quadratic | −0.001* (0.001) b | −0.008* (0.004) | ||||||
| Cubic | 0.001* (0.001) | |||||||
| Quartic | −0.001† (0.001) a | |||||||
| Low HPA-High SAM (n=46) | .962 | 76.746 | .381 | .387 | 0.984 | |||
| Intercept | 0.499* (0.008) B | 3.505* (0.063) C T6 | ||||||
| Linear | −0.001* (0.001) a | 0.106* (0.025) | ||||||
| Quadratic | −0.007* (0.002) | |||||||
| Cubic | 0.001* (0.001) | |||||||
| Quartic | −0.001* (0.001) a | |||||||
| High HPA-Low SAM (n=28) | ||||||||
| Intercept | 0.515* (0.012) B, T6 | 2.675* (0.084) A, T5 | .925 | 37.120 | .239 | .235 | 1.017 | |
| Linear | 0.002* (0.001) | 0.049* (0.013) | ||||||
| Quadratic | −0.001* (0.001) b | −0.002* (0.001) | ||||||
| Cubic | 0.001* (0.001) | |||||||
Note. AvePPj = Average posterior probability; OCCj = Odds of correct classification; Probj = Probability of group assignment; Propj = Proportion of children assigned to each group; Ratio = Ratio of Probj to Propj; T3 = Third saliva sample; T4 = Fourth saliva sample; T5 = Fifth saliva sample; T6 = Sixth saliva sample. Upper-case superscripts denote significant differences in intercept estimates within the biological index. Lower-case superscripts denote significant differences in polynomial parameter estimates within the same biological index. Saliva sample time (e.g., T5) superscripts denote the first assessment point at which baseline and reactivity levels did not significantly differ from one another (i.e., recovery achieved).
p = .08.
p < .05.
Figure 1.
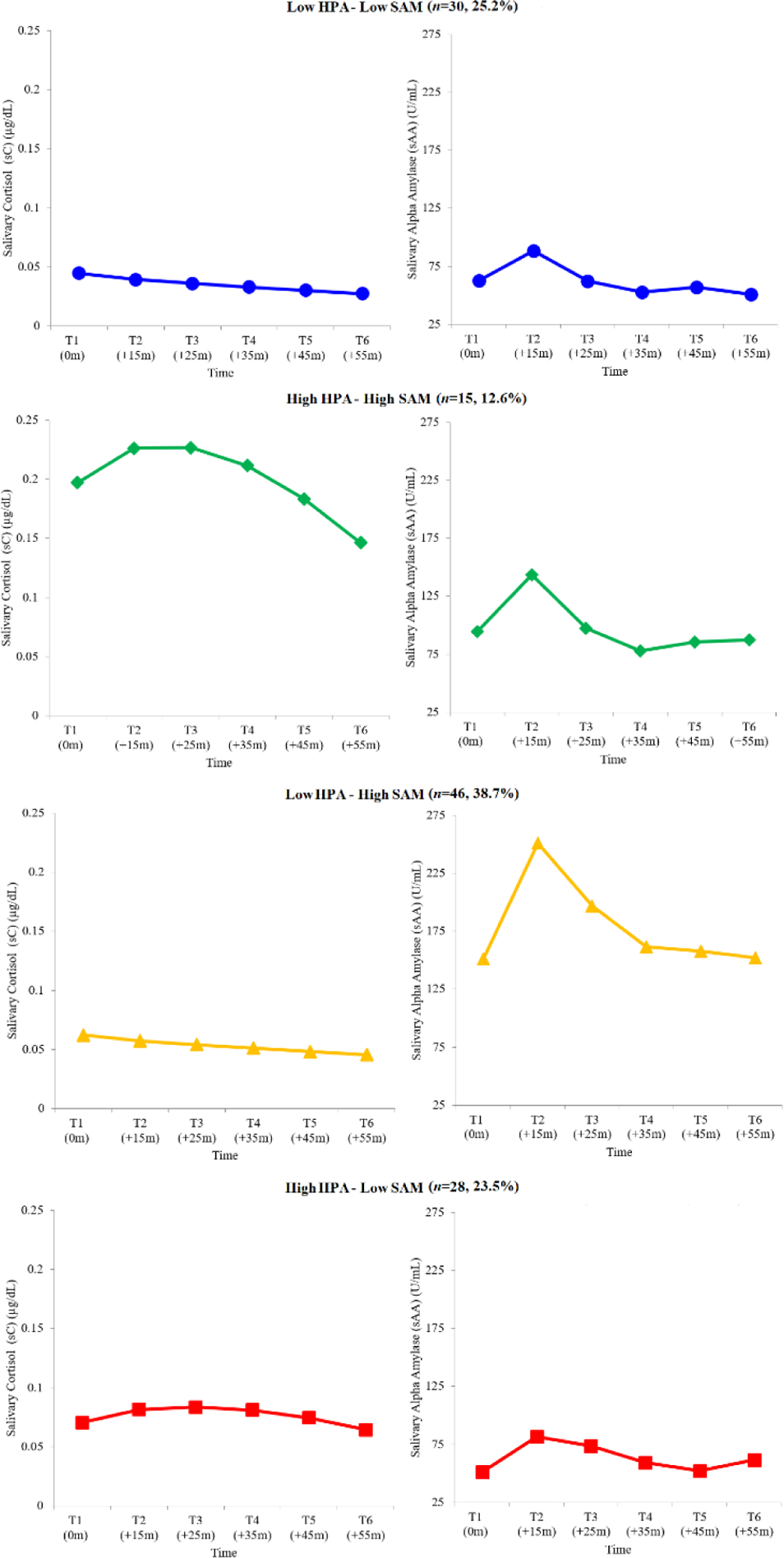
Salivary Cortisol and Salivary Alpha-Amylase Trajectories for the Final Four-Group Multitrajectory Modeling Solution
Note. Reverse fourth root transformed estimated mean values presented for ease of interpretation and cross-study communication. Title values in parentheses reflect the number and percentage of children from the full sample assigned to each group.
As expected, two profiles emerged whose HPA–SAM trajectories reflected symmetric co-activation patterns consistent with those identified in prior MTM studies (Bendezú & Wadsworth, 2018). The Low HPA–Low SAM profile (n=30) displayed trajectories characterized by the lowest cortisol baseline levels in the sample and lack of cortisol reactivity (i.e., linear declining slope). This profile also displayed relatively low alpha-amylase baseline levels and alpha-amylase reactivity similar to that observed in other profiles (i.e., quartic slope). Of note, alpha-amylase reactivity levels for youth exhibiting this profile returned to baseline at T3, indicating efficient recovery. The High HPA–High SAM profile (n=15) displayed trajectories characterized by the relatively highest cortisol baseline levels in the sample and pronounced cortisol reactivity (i.e., quadratic slope). This profile also displayed relatively high alpha-amylase baseline levels and alpha-amylase reactivity similar to that observed in other profiles (i.e., quartic slope). Of note, cortisol and alpha-amylase reactivity levels for youth exhibiting this profile returned to baseline at T5 and T3, respectively, indicating protracted recovery of cortisol levels and efficient recovery of alpha-amylase levels.
As expected, two profiles emerged whose HPA–SAM trajectories reflected asymmetric co-activation patterns consistent with those identified in prior MTM studies. The Low HPA–High SAM profile (n=46) displayed trajectories characterized by relatively low cortisol baseline levels and lack of cortisol reactivity (i.e., linear declining slope). This profile also displayed the relatively highest alpha-amylase baseline levels in the sample and alpha-amylase reactivity similar to that observed in other profiles (i.e., quartic slope). Of note, alpha-amylase reactivity levels for youth exhibiting this profile returned to baseline at T6, indicating protracted recovery. The High HPA–Low SAM profile (n=28) displayed trajectories characterized by relatively low cortisol baseline levels, but also pronounced cortisol reactivity (i.e., quadratic slope). This profile also displayed relatively low alpha-amylase baseline levels and alpha-amylase reactivity similar to that observed in other profiles (i.e., cubic slope). Of note, cortisol and alpha-amylase reactivity levels for youth exhibiting this profile returned to baseline at T6 and T5, respectively, indicating protracted recovery across biological indices.
Correlates of HPA-SAM Co-Activation Profiles (Aim 2)
There were no significant differences among the four profiles with respect to demographics or variables known to impact HPA axis or SAM system function and its assessment: child age (F(3,106)=0.93, p>.25), sex (χ2(3)=4.22, p=.24), cohort (χ2(3)=3.914, p>.25), pubertal status (F(3,90)=0.50, p>.25), medication use (χ2(3)=1.23, p>.25), and saliva sample timing (F(3,105)=1.41, p=.24). Child age and sex were retained and controlled for in all MANCOVA analyses. Two outlier values for both stressful life events and posttraumatic stress were observed and winsorized to 3 standard deviations from the mean in order to meet normality assumptions. Covariance matrices between subgroups of youth were assumed equal for the purposes of MANCOVA (Box’s M=31.07, p=.05).
The association between subgroup membership and our focal correlates was significant; Wilk’s Lambda=0.823, F(9,207.02)=1.93, p=.05. A series of Levene’s F tests suggested that the homogeneity of variance assumption was satisfied: stressful life events (F(3,89)=4.08, p=.01),2 posttraumatic stress (F(3,89)=1.41, p=.12), total problem behavior (F(3,89)=0.58, p>.25). Follow-up ANCOVAs revealed a significant association between subgroup membership and each of our three focal correlates: stressful life events (F(3,87)=3.24, p=.03), posttraumatic stress (F(3,87)=2.79, p=.04), total problem behavior (F(3,87)=3.61, p=.01).
Estimated marginal means, standard errors bars, and the results of Fisher’s LSD tests comparing subgroup mean estimates are depicted in Figure 2. Relative to those with symmetric HPA–SAM profiles (i.e., Low HPA–Low SAM, High HPA–High SAM), youth with asymmetric HPA–SAM profiles (i.e., Low HPA–High SAM, High HPA–Low SAM) generally presented with a) exposure to greater number of stressful life events, b) greater experience of posttraumatic stress, and c) greater total problem behavior.
Figure 2.
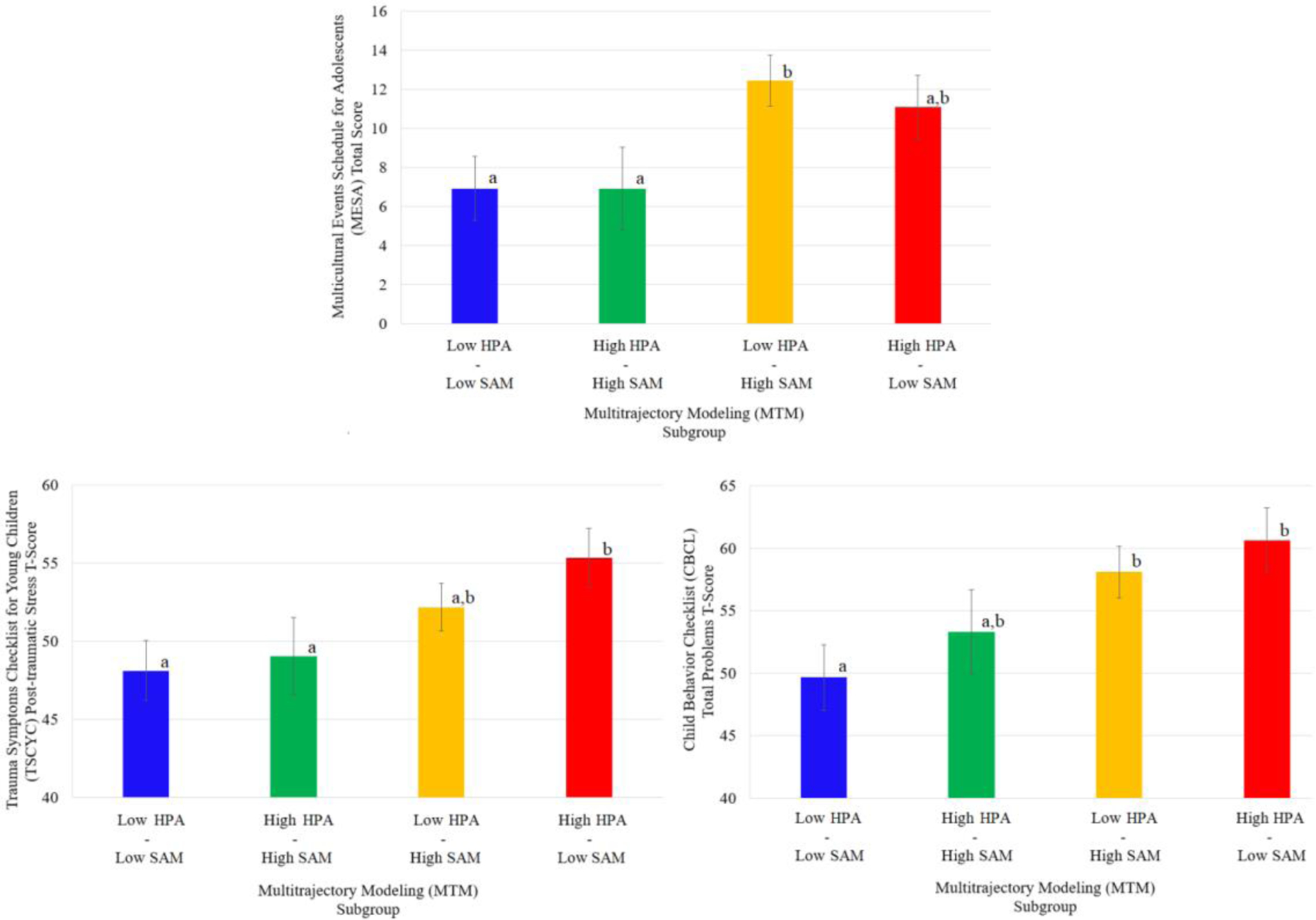
Estimated Means and Standard Error Bars for Risk and Mental Health Indices by Multitrajectory Modeling Subgroup
Note. Differing superscripts denote significant differences between groups.
Post Hoc Analyses
Estimated marginal means, standard errors bars, and the results of Fisher’s LSD tests comparing subgroup mean estimates on internalizing and externalizing problems are depicted in Figure 3. Post-hoc ANCOVAs revealed a significant association between subgroup membership and externalizing problems (F(3,87)=2.99, p=.03), but not internalizing problems (F(3,87)=1.83, p=.14). Fisher’s LSD tests comparing subgroup mean estimates revealed an overall pattern of findings for internalizing and externalizing problems similar to that of total problem behavior.
Figure 3.
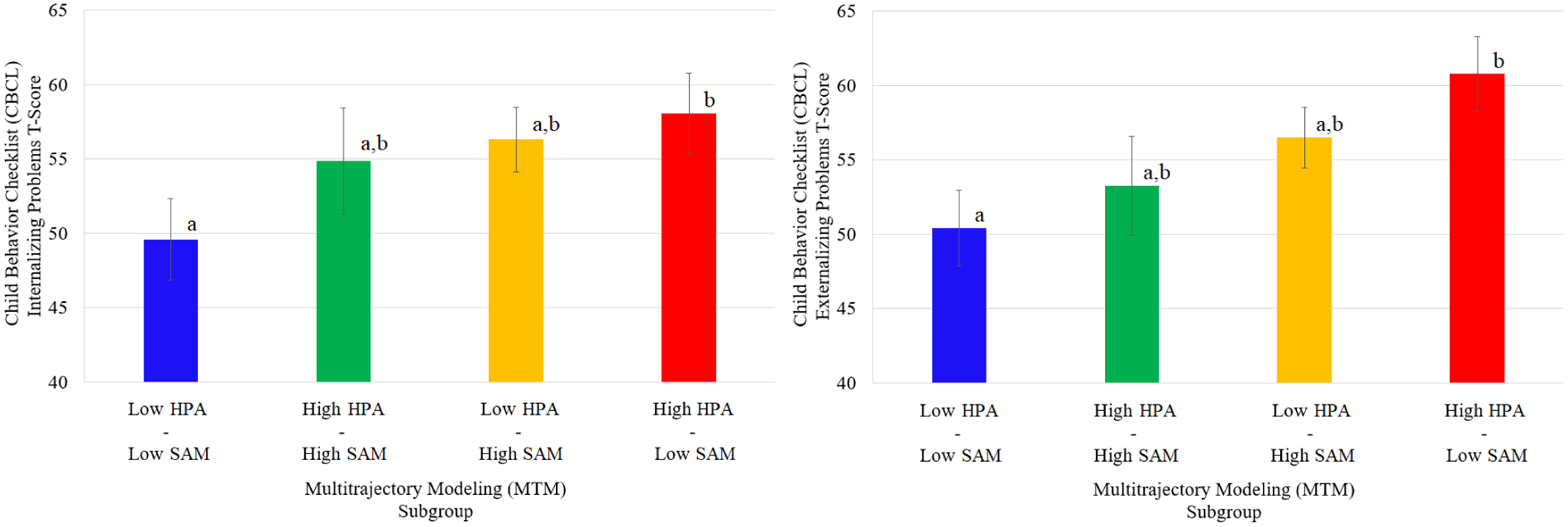
Estimated Means and Standard Error Bars for Internalizing and Externalizing Parameters by Multitrajectory Modeling Subgroup
Note. Differing superscripts denote significant differences between groups.
Discussion
This study identified within-person profiles of HPA–SAM co-activation in a sample of racially diverse, economically disadvantaged early adolescents. Salivary cortisol and alpha-amylase levels collected prior to and following exposure to the TSST–M were modeled simultaneously to generate concurrent physiological stress response trajectories using a novel multisystem, person-centered approach. Multitrajectory modeling (MTM) identified four profiles: Low HPA–Low SAM, High HPA–High SAM, Low HPA–High SAM, and High HPA–Low SAM. This study also linked the identified profiles with measures of parent-reported exposure to stress and mental health and found that youth exhibiting profiles characterized by asymmetric co-activation (i.e., Low HPA–High SAM, High HPA–Low SAM) had higher number of stressful life events, greater posttraumatic stress, and more total behavior problems compared to youth exhibiting symmetric co-activation profiles (i.e., Low HPA–Low SAM, High HPA–High SAM). Of note, profile–maladjustment linkages did not appear to vary when specific type of problem behavior was examined (i.e., internalizing, externalizing), suggesting that our significant total problem results perhaps reflect a severity effect (e.g., Essex et al., 2006). If so, it is possible HPA–SAM co-activation as a putative risk factor is associated with overall symptom severity regardless of symptom type. Longitudinal studies are needed to adjudicate this claim.
The present sample’s profiles largely replicate those identified in Bendezú and Wadsworth (2018), despite major differences in participant attributes and differences in the experimental protocols (e.g., experimental coping manipulation introduced between T2 and T3; Bendezú & Wadsworth, 2018). Person-centered analysis of co-occurring stress response patterns can potentially provide insight into how the interplay between multiple stress-sensitive biological systems is associated with exposure to stress and mental health outcomes. As with any person-centered analysis or approach, there are legitimate concerns as to whether profiles identified with these techniques are in fact a) statistical artifacts (e.g., groups reflecting measurement error), b) limited to a specific sample, or c) reflect methodological nuance (e.g., unique to a specific study design). Our replication of these profiles across studies perhaps helps to allay those concerns.
Profile links to stress exposure and psychopathology support the asymmetric-risk model and with previous studies demonstrating that HPA and SAM stress response mismatches are associated with higher risk for mental health problems (Allwood et al., 2011; Bae et al., 2015; Bendezú & Wadsworth, 2018; Gordis et al., 2006). Prior findings that specific forms of asymmetry are differentially associated with higher (e.g., Low HPA–High SAM) and lower (e.g., High HPA–Low SAM) forms of risk (Bendezú & Wadsworth, 2018; Gordis et al., 2006) run contrary to the present findings showing stress exposure and psychopathology associated with either form of asymmetry. However, the current study included only youth from low-income households, whereas Bendezú and Wadsworth’s sample was composed of early adolescents from a wide range of SES backgrounds and Gordis and colleagues compared maltreated youth with a normative comparison group without consideration of SES. Sample differences in demographic composition and stress exposure might have contributed to these disparate findings. It is possible that chronic exposure to stressors, such as the poverty-related ones that economically disadvantaged youth in the present study’s sample face daily, disrupt HPA–SAM co-activation and contribute to biological embedding of risk in ways not observed in lower-risk samples.
Benefits of Multitrajectory Modeling in Characterizing HPA–SAM Co-Activation
Though the present study’s characterization of HPA–SAM co-activation is in terms of symmetry versus asymmetry (an intentional effort to keep in line with extant physiological risk models (Bauer et al., 2002)), this characterization can be reductive. Our utilization of MTM to capture HPA–SAM symmetry and asymmetry allowed for examination of different aspects of cross-system response trajectories (e.g., baseline levels, reactivity patterning, length of time for biomarker levels to return to baseline) that help to distinguish healthy and aberrant co-activation patterns. This person-centered approach captures nuance in theoretically meaningful ways that may encourage researchers to move beyond classifications based on “high” or “low” summative levels in favor of characterizations that incorporate more aspects of dynamic stress responsivity.
For example, youth exhibiting Low HPA–Low SAM co-activation displayed low cortisol and alpha-amylase baseline levels, which is consistent with prior variable-centered characterizations incorporating basal levels. However, our examination of cross-system reactivity patterns and recovery helped strengthen inference as to whether this co-activation pattern reflected well-regulated or dysregulated HPA–SAM stress response function. Specifically, this trajectory was characterized by a modest increase in alpha-amylase which quickly returned to baseline and concomitant linear decline in cortisol levels. For youth exhibiting this profile, an abbreviated SAM response may have been sufficient to quickly neutralize socio-evaluative threat and efficiently navigate the demands of the TSST–M, thereby circumventing activation of the HPA and mobilization of physiologic resources for longer-term stressor management. That youth exhibiting this pattern of co-activation had relatively fewer parent-reported stressful life events, posttraumatic stress experiences, and problem behaviors suggests that this pattern might be indicative of well-regulated HPA–SAM stress response functioning. This would align with extant theory to further strengthen inferences that abbreviated SAM response minimizes over taxation of cardiovascular, immunologic, and central nervous system resources while a cortisol non-response limits neurotoxic effects of cortisol overexposure (Ábrahám et al., 2001; Chrousos & Gold, 1992; Sapolsky et al., 1986).
Attending to reactivity patterns and recovery for youth exhibiting High HPA–High SAM responsivity was similarly informative. Despite exhibiting pronounced increases in both cortisol and alpha-amylase levels following the TSST–M, these youths’ alpha-amylase levels decreased quickly back to baseline while a more prolonged pattern of recovery was observed with cortisol. One possibility is that High HPA–High SAM youth exhibited successful termination of the physiologically taxing SAM response, healthy signaling towards the HPA axis, and effective glucocorticoid-mediated mobilization of longer-term resources for managing stress. That these youths presented with fewer stressful life events, less posttraumatic stress, and fewer emotional and behavior problems further supports the idea that this symmetric co-activation pattern reflects well-orchestrated HPA–SAM stress response function and potentially lower risk.
Closer examination of different trajectory aspects also helped to strengthen inference about whether asymmetrical profiles reflect dysregulated co-activation. Asymmetrical HPA–SAM co-activation might be indicative of difficulties with cross-system reactivity and recovery. For example, Low HPA–High SAM trajectories were characterized by a pronounced increase in alpha-amylase levels followed by a protracted return to baseline, as well as a concomitant linear decline in cortisol levels. For these youth, the physiologically taxing SAM system may have failed to terminate efficiently, potentially signaling poor communication with the HPA axis, and difficulty mounting an appropriate cortisol response. As a further illustration, High HPA–Low SAM trajectories were characterized by a pronounced increase in both cortisol and alpha-amylase levels that were each followed by a protracted return to baseline. This suggests that High HPA–Low SAM youth were successful at initially recruiting resources to address the stressor but had difficulties effectively managing and shutting down their physiological responses when the stressor was no longer present. Such a pattern might be indicative of reduced cortisol suppression of the SAM system activation and dysregulated negative feedback loop processes (Munck et al., 1984; Sapolsky et al., 2000), which potentially contributes to over taxation of the body and cortisol neurotoxicity (Ábrahám et al., 2001; Chen & Baram, 2016; Chrousos & Gold, 1992). These findings highlight the potential utility of a multisystem, person-centered approach in mapping out dysregulated reactivity patterns and recovery that may further unpack how symmetric and asymmetric HPA–SAM co-activation might contribute to risk.
Normative HPA Non-Response, Blunted HPA Response: Is There a Difference?
This person-centered exploration of HPA–SAM co-activation helps build on prior single biomarker stress vulnerability research by possibly clarifying weak or inconsistent cortisol–maladjustment linkages. Single-system-identified low cortisol responses may be unknowingly comprised of both a low-risk normative HPA non-response and high-risk blunted HPA response. The present study’s two low HPA subgroups helps to illustrate this possibility. Though youth in both subgroups exhibited cortisol trajectories that were quantitatively indistinguishable from one another (e.g., statistically nonsignificant differences in intercept and polynomial parameter estimates), connections with stress exposure and maladjustment indices suggested that subgroup trajectories were qualitatively distinct. One possibility may be that Low HPA–Low SAM youth exhibited cortisol trajectories consistent with what has been termed normative HPA non-response in the single-system literature. In contrast, Low HPA–High SAM youth may have exhibited cortisol trajectories consistent with what has been referred to as blunted HPA response.
In a similar manner, youth in both high HPA subgroups displayed cortisol hyperresponsivity that might otherwise not have been distinguished from each other using single-system approaches. By examining HPA and SAM responsivity together, our findings revealed that these subgroups differed on stressful life event exposure. It is possible that High HPA–High SAM youth demonstrated what has been referred to as a low-risk normative HPA response whereas High HPA–Low SAM youth exhibited what has been often characterized as a high-risk hyper HPA response. That the former group presented with lower stress exposure than the latter group supports the utility of more descriptive nomenclatures that account for the nuances of multisystem responses to stress and should encourage researchers to consider such approaches.
Limitations and Future Directions
The present study extends the existing literature by demonstrating how HPA–SAM co-activation profiles among racially diverse, economically disadvantaged early adolescent youth are differentially linked to stress exposure and mental health problems. That said, it is important to note that our sample size was relatively small for a person-centered design. It is possible that sample size restricted the number of profiles identified and, as such, other HPA–SAM co-activation profiles could exist. Replication in larger samples is necessary to adjudicate this claim.
We examined pubertal staging as a potential influence on HPA–SAM subgroups but found that puberty did not play a significant role. Regardless, identification of similar HPA–SAM co-activation groups in different age groups and at different stages of puberty as well as stability in group membership across puberty would help test whether the patterns we found are better explained by normative development or by risk factors (i.e., exposure to stressful life events, mental health problems) as is suggested by the present study.
Longitudinal examination of HPA–SAM co-activation profiles would not only allow for the parsing of normative versus risk-driven development but also would be a good opportunity to explore the stability of co-activation patterns. If HPA–SAM cross-system activity is impacted by risk, it would be important to know if the nature of the link between the two systems changes if the individual continues to be exposed to stressful life events or if mental health problems increase in severity. Additionally, the introduction of protective factors might impact HPA–SAM co-activation. For the latter claim, randomized clinical trials could fill this gap by demonstrating whether interventions modify previously established patterns of cross-system dysregulation.
Our findings illustrate how HPA and SAM responses to stressors could be examined simultaneously to uncover important individual differences. Future investigations might benefit from continued adoption of multisystem approaches. First, examining diurnal fluctuations of the HPA axis and SNS would help support the phenomenon of physiological dysregulation versus normative and/or expected regulation observed in the stress response literature. To date, only one study has explicitly examined diurnal cortisol and alpha-amylase using a multisystem approach (Frost et al., 2021). Linking findings on diurnal HPA–SNS co-activation with stress HPA–SAM co-activation will further understanding about how risk impacts physiology.
Second, other physiological systems previously linked to risk would be natural additions to HPA–SAM studies. Increasing interest has been placed on the immune system’s role in risk. One study examined adolescent girls’ inflammatory and HPA stress responses using the same multitrajectory method used in the present study and found that Low HPA–High inflammation predicted higher likelihood of experiencing peer-related stressors (Bendezú, Calhoun, et al., 2022). Low HPA–High inflammation patterns have also been linked to higher risk in adults (Lucas et al., 2017). Future studies integrating HPA, SAM, and inflammatory stress responses will aid in the uncovering of multisystem risk mechanisms.
Third, many studies assess subjective mood ratings when administering laboratory stressor tasks, but these ratings are rarely reported. When they are, it is usually to demonstrate that the laboratory stressor was effective in increasing subjective stress. It is rare that these subjective measures are treated as a potential system in their own right. Three investigations that did examine participant self-report and behavioral observations in tandem with salivary cortisol found that youth who had low HPA responsivity but reported being and were observed by study confederates to be highly stressed in response to the TSST were more at risk for emotional and behavioral problems compared to youth with more consistent stress responses across the subjective, objective, and neuroendocrine stress measures (Bendezú, Thai, et al., 2022; Carosella et al., under review; Wiglesworth et al., under review). Studies that incorporate subjective ratings would be important especially if the adolescent stress response literature were to move from beyond single-system characterizations towards more complete descriptions of if and how individuals respond to stress and at what levels of analysis can this be observed.
Conclusions
The present study identified multiple subgroups of early adolescents that were distinguished by HPA–SAM co-activation, stress exposure, and mental health outcomes. These findings simultaneously highlight potential differences in biological embedding of risk during early adolescence based on individuals’ exposure to chronic stress and illustrate the utility of multisystem and person-centered approaches in understanding how risk might get “underneath the skin” across systems. That the subgroups found in this sample of youth exposed to significant economic hardship mirror those found in samples containing significantly less economic hardship and fewer risk factors suggests that this multisystem approach could help identify reliable patterns of stress response that portend better and worse developmental trajectories, and which may have utility for tailored intervention approaches. Further, the findings support that taking a multisystem approach may be necessary for identifying meaningful variances in stress responsivity when singular system investigations are unable to parse apart individual differences in “low” physiological responsivity. Hence, future multisystem and person-centered work incorporating risk factors, stress physiology, and health outcomes across the lifespan will continue to expand the field’s understanding of biological embedding of risk across individuals.
Highlights.
HPA–SAM co-activation in response to stress was examined in early adolescents
Two subgroups had asymmetric profiles (i.e., Low HPA–High SAM, High HPA–Low SAM)
Two subgroups had symmetric profiles (i.e., Low HPA–Low SAM, High HPA–High SAM)
Asymmetric subgroups reported higher levels of risk factors than symmetric subgroups
Acknowledgments
This work was supported by funding from the National Institute of Mental Health (R21 MH107631 and R33MH107631) awarded to the third author, M. Wadsworth (PI) and by the Prevention and Methodology Training Program (T32 DA017629; MPIs: J. Maggs & S. Lanza) with funding from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This decision was informed by examining sample average plots of cortisol and alpha-amylase data prior to and following the TSST–M, but also motivated by unique subgroups and distinct co-activation patterns that have been identified in prior studies (e.g., Bendezú & Wadsworth, 2018).
As per Howell (2012), no standard deviation value was four times larger than the smallest standard deviation value, suggesting that follow-up ANCOVAs conducted were robust to potential violations of the homogeneity of variance assumption indicated by Levene’s F test.
References
- Ábrahám IM, Harkany T, Horvath KM, & Luiten PGM (2001). Action of glucocorticoids on survival of nerve cells: Promoting neurodegeneration or neuroprotection? Journal of Neuroendocrinology, 13(9), 749–760. 10.1046/j.1365-2826.2001.00705.x [DOI] [PubMed] [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Ali N, & Nater UM (2020). Salivary alpha-amylase as a biomarker of stress in behavioral medicine. International Journal of Behavioral Medicine, 27(3), 337–342. 10.1007/s12529-019-09843-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, & Stroud LR (2011). Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology, 88(1), 57–64. 10.1016/j.biopsycho.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YJ, Stadelmann S, Klein AM, Jaeger S, Hiemisch A, Kiess W, Ceglarek U, Gaudl A, Schaab M, von Klitzing K, Thiery J, Kratzsch J, & Döhnert M (2015). The hyporeactivity of salivary cortisol at stress test (TSST-C) in children with internalizing or externalizing disorders is contrastively associated with α-amylase. Journal of Psychiatric Research, 71, 78–88. 10.1016/j.jpsychires.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, & Boyce WT (2002). Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics, 23(2), 102–113. 10.1097/00004703-200204000-00007 [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, & Passmore J (2019). Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: A systematic review and meta-analysis. The Lancet Public Health, 4(10), e517–e528. 10.1016/S2468-2667(19)30145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú JJ, Calhoun CD, Vinograd M, Patterson MW, Rudolph KD, Giletta M, Hastings P, Nock MK, Slavich GM, & Prinstein MJ (2022). Exploring joint HPA–inflammatory stress response profiles in adolescent girls: Implications for developmental models of neuroendocrine dysregulation. Developmental Psychobiology, 64(3), 1–18. 10.1002/dev.22247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú JJ, Thai M, Wiglesworth A, Cullen KR, & Klimes-Dougan B (2022). Adolescent stress experience–expression–physiology correspondence: Links to depression, self-injurious thoughts and behaviors, and frontolimbic neural circuity. Journal of Affective Disorders, 300(December 2021), 269–279. 10.1016/j.jad.2021.12.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú JJ, & Wadsworth ME (2018). Person-centered examination of salivary cortisol and alpha-amylase responses to psychosocial stress: Links to preadolescent behavioral functioning and coping. Biological Psychology, 132(December 2017), 143–153. 10.1016/j.biopsycho.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Johnson K, Bissada A, Damon L, Crouch J, Gil E, Hanson R, & Ernst V (2001). The Trauma Symptom Checklist for Young Children (TSCYC): Reliability and association with abuse exposure in a multi-site study. Child Abuse & Neglect, 25(8), 1001–1014. 10.1016/S0145-2134(01)00253-8 [DOI] [PubMed] [Google Scholar]
- Bunea IM, Szentágotai-Tǎtar A, & Miu AC (2017). Early-life adversity and cortisol response to social stress: A meta-analysis. Translational Psychiatry, 7(12). 10.1038/s41398-017-0032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun CD, Franklin JC, Adelman CB, Guerry JD, Hastings PD, Nock MK, & Prinstein MJ (2012). Biological and cognitive responses to an in vivo interpersonal stressor: Longitudinal associations with adolescent depression. International Journal of Cognitive Therapy, 5(3), 283–299. 10.1521/ijct.2012.5.3.283 [DOI] [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, & Prinstein MJ (2014). Relational victimization, friendship, and adolescents’ hypothalamic–pituitary–adrenal axis responses to an in vivo social stressor. Development and Psychopathology, 26(3), 605–618. 10.1017/S0954579414000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosella KA, Wiglesworth A, Brower R, Mirza SA, Mueller BA, Bendezú JJ, Cullen KR, & Klimes-Dougan B (n.d.). How do patterns of experience, expression, and physiology of stress relate to psychopathology in adolescents? A person-centered approach. [DOI] [PMC free article] [PubMed]
- Chen Y, & Baram TZ (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology, 41(1), 197–206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, & Gold PW (1992). The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA: The Journal of the American Medical Association, 267(9), 1244–1252. 10.1001/jama.1992.03480090092034 [DOI] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology and Behavior, 106(1), 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- DePasquale CE, Herzberg MP, & Gunnar MR (2021). The pubertal stress recalibration hypothesis: Potential neural and behavioral consequences. Child Development Perspectives, 1–8. 10.1111/cdep.12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, & Mize J (2008). Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology, 36(4), 601–611. 10.1007/s10802-007-9204-6 [DOI] [PubMed] [Google Scholar]
- Engel ML, & Gunnar MR (2020). The development of stress reactivity and regulation during human development. In International Review of Neurobiology (1st ed., Vol. 150). Elsevier Inc. 10.1016/bs.irn.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Felt JM, Depaoli S, & Tiemensma J (2017). Latent growth curve models for biomarkers of the stress response. Frontiers in Neuroscience, 11. 10.3389/fnins.2017.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Rodriguez M, Imrisek S, Dash A, & Bernard K (2021). Externalizing behavior and stress system functioning in infants exposed to early adversity: A multi-system exploration. Developmental Psychobiology, 63(5), 1255–1265. 10.1002/dev.22091 [DOI] [PubMed] [Google Scholar]
- Gonzales NA, Gunnoe ML, Jackson KM, & Samaniego RY (1995). Validation of a multicultural events scale for urban adolescents. Biennial Conference of the Society for Community Research and Action. [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, & Trickett PK (2006). Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31(8), 976–987. 10.1016/j.psyneuen.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Guidi J, Lucente M, Sonino N, & Fava GA (2021). Allostatic load and its impact on health: A systematic review. Psychotherapy and Psychosomatics, 90(1), 11–27. 10.1159/000510696 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, & Griggs C (2009). Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology, 21(1), 69–85. 10.1017/S0954579409000054.Developmental [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteveld LM, Nederend I, Ten Harkel ADJ, Schutte NM, de Rooij SR, Vrijkotte TGM, Oldenhof H, Popma A, Jansen LMC, Suurland J, Swaab H, & de Geus EJC (2021). Maturation of the cardiac autonomic nervous system activity in children and adolescents. Journal of the American Heart Association, 10(4), 1–22. 10.1161/JAHA.120.017405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, & Ormel J (2013). Self- or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: A systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypo. Biological Psychology, 94(1), 175–184. 10.1016/j.biopsycho.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, & Kudielka BM (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34(2), 163–171. 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Howell DC (2012). Statistical Methods for Psychology. Cengage Learning. [Google Scholar]
- Johnson AE, Perry NB, Hostinar CE, & Gunnar MR (2019). Cognitive–affective strategies and cortisol stress reactivity in children and adolescents: Normative development and effects of early life stress. Developmental Psychobiology, 61(7), 999–1013. 10.1002/dev.21849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Rohleder N, & Schreier HMC (2020). Neuroendocrine coordination and youth behavior problems: A review of studies assessing sympathetic nervous system and hypothalamic-pituitary adrenal axis activity using salivary alpha amylase and salivary cortisol. Hormones and Behavior, 122(December 2019), 104750. 10.1016/j.yhbeh.2020.104750 [DOI] [PubMed] [Google Scholar]
- Joos CM, McDonald A, & Wadsworth ME (2019). Extending the toxic stress model into adolescence: Profiles of cortisol reactivity. Psychoneuroendocrinology, 107(August 2018), 46–58. 10.1016/j.psyneuen.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Arcus D, & Reznick JS (1998). Galen’s Prophecy: Temperament in Human Nature. Routledge. 10.4324/9780429500282 [DOI] [Google Scholar]
- Koss KJ, George MRW, Cummings EM, Davies PT, El-Sheikh M, & Cicchetti D (2014). Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology, 56(4), 836–849. 10.1002/dev.21156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (2015). Children’s Depression Inventory (CDI and CDI 2). In The Encyclopedia of Clinical Psychology (pp. 1–5). John Wiley & Sons, Inc. 10.1002/9781118625392.wbecp419 [DOI] [Google Scholar]
- Kuras YI, McInnis CM, Thoma MV, Chen X, Hanlin L, Gianferante D, & Rohleder N (2017). Increased alpha-amylase response to an acute psychosocial stress challenge in healthy adults with childhood adversity. Developmental Psychobiology, 59(1), 91–98. 10.1002/dev.21470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 1198–1202. 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- Lopez-Duran NL, McGinnis E, Kuhlman K, Geiss E, Vargas I, & Mayer S (2015). HPA-axis stress reactivity in youth depression: Evidence of impaired regulatory processes in depressed boys. Stress, 18(5), 545–553. 10.3109/10253890.2015.1053455.HPA-axis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79(3), 293–305. 10.1097/PSY.0000000000000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Miller R, & Plessow F (2013). Transformation techniques for cross-sectional and longitudinal endocrine data: Application to salivary cortisol concentrations. Psychoneuroendocrinology, 38(6), 941–946. 10.1016/j.psyneuen.2012.09.013 [DOI] [PubMed] [Google Scholar]
- Mueller B, Figueroa A, & Robinson-Papp J (2022). Structural and functional connections between the autonomic nervous system, hypothalamic–pituitary–adrenal axis, and the immune system: A context and time dependent stress response network. Neurological Sciences, 43(2), 951–960. 10.1007/s10072-021-05810-1 [DOI] [PubMed] [Google Scholar]
- Mulkey SB, & du Plessis AJ (2019). Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatric Research, 85(2), 120–126. 10.1038/s41390-018-0155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, & Holbrook NJ (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews, 5(1), 25–44. 10.1210/edrv-5-1-25 [DOI] [PubMed] [Google Scholar]
- Nagin DS (2005). Group-Based Modeling of Development. Harvard University Press. [Google Scholar]
- Nagin DS, Jones BL, Passos VL, & Tremblay RE (2018). Group-based multitrajectory modeling. Statistical Methods in Medical Research, 27(7), 2015–2023. 10.1177/0962280216673085 [DOI] [PubMed] [Google Scholar]
- Owens SA, Helms SW, Rudolph KD, Hastings PD, Nock MK, & Prinstein MJ (2019). Interpersonal stress severity longitudinally predicts adolescent girls’ depressive symptoms: The moderating role of subjective and HPA axis stress responses. Journal of Abnormal Child Psychology, 47(5), 895–905. 10.1007/s10802-018-0483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Rohleder N, & Nater UM (2009). Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology, 34(4), 469–485. 10.1016/j.psyneuen.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, & McEwen BS (1986). The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews, 7(3), 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. 10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Sayed S, Iacoviello BM, & Charney DS (2015). Risk factors for the development of psychopathology following trauma. Current Psychiatry Reports, 17(8), 70. 10.1007/s11920-015-0612-y [DOI] [PubMed] [Google Scholar]
- Schumacher S, Kirschbaum C, Fydrich T, & Ströhle A (2013). Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?-A review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology, 38(6), 729–743. 10.1016/j.psyneuen.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Shansky RM, & Lipps J (2013). Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Giletta M, Helms SW, Hastings PD, Rudolph KD, Nock MK, & Prinstein MJ (2020). Interpersonal life stress, inflammation, and depression in adolescence: Testing Social Signal Transduction Theory of Depression. Depression and Anxiety, 37(2), 179–193. 10.1002/da.22987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, & Vale WW (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8(4), 383–395. 10.31887/dcns.2006.8.4/ssmith [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68. 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, & Westenberg PM (2010). Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology, 35(10), 1510–1516. 10.1016/j.psyneuen.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Wade M, Sheridan MA, Zeanah CH, Fox NA, Nelson CA, & McLaughlin KA (2020). Environmental determinants of physiological reactivity to stress: The interacting effects of early life deprivation, caregiving quality, and stressful life events. Development and Psychopathology, 32(5), 1732–1742. 10.1017/S0954579420001327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Ahlkvist JA, McDonald A, & Tilghman-Osborne EM (2018). Future directions in research and intervention with youths in poverty. Journal of Clinical Child & Adolescent Psychology, 47(6), 1023–1038. 10.1080/15374416.2018.1485108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Broderick AV, Loughlin-Presnal JE, Bendezu JJ, Joos CM, Ahlkvist JA, Perzow SED, & McDonald A (2019). Co-activation of SAM and HPA responses to acute stress: A review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Developmental Psychobiology, 61(7), 1079–1093. 10.1002/dev.21866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiglesworth A, Mirza S, Carosella KA, Papke V, Bendezú JJ, Klimes-Dougan B, & Cullen KR (n.d.). Stress system concordance as a marker of adolescent resilience: Longitudinal implications for psychopathology and wellbeing over time. [DOI] [PMC free article] [PubMed]
- Yim IS, Quas JA, Cahill L, & Hayakawa CM (2010). Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology, 35(2), 241–248. 10.1016/j.psyneuen.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Young-Southward G, Svelnys C, Gajwani R, Bosquet Enlow M, & Minnis H (2020). Child maltreatment, autonomic nervous system responsivity, and psychopathology: Current state of the literature and future directions. Child Maltreatment, 25(1), 3–19. 10.1177/1077559519848497 [DOI] [PubMed] [Google Scholar]
- Young ES, Doom JR, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, & Simpson JA (2020). Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Development and Psychopathology, March. 10.1017/S0954579419001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ES, Doom JR, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, & Simpson JA (2021). Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Development and Psychopathology, 33(1), 301–312. 10.1017/S0954579419001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Crick NR, Shirtcliff EA, & Woods KE (2015). The origins and development of psychopathology in females and males. In Developmental Psychopathology (pp. 76–138). John Wiley & Sons, Inc. 10.1002/9780470939383.ch4 [DOI] [Google Scholar]


