Abstract
Social-emotional deficits in psychosis may be indexed by deviations in emotional scene processing, but event-related potential (ERP) studies indicate such deviations may not map cleanly to diagnostic categories. Neurobiologically defined psychosis subgroups offer an alternative that may better capture neurophysiological correlates of social-emotional deficits. The current study investigates emotional scene-elicited ERPs in Biotypes of psychosis in a large (N=622), well-characterized sample. Electroencephalography was recorded in healthy persons (N=129), Biotype-1 (N=195), Biotype-2 (N=131), and Biotype-3 (N=167) psychosis cases. ERPs were measured from posterior and centroparietal scalp locations. Neural responses to emotional scenes were compared between healthy and psychosis groups. Multivariate group discrimination analyses resulted in two composite variates that differentiated groups. The first variate displayed large differences between low-cognition (Biotype-1, Biotype-2) and intact-cognition groups (Biotype-3, healthy persons). The second indicated a small-to-moderate distinction of Biotypes-2 and −3 from Biotype-1 and healthy persons. Two multivariate correlations were identified indicating associations between 1) self-reported emotional experience and generalized cognition and 2) socio-occupational functioning and late-stage emotional processing. Psychosis Biotypes displayed emotional processing deficits not apparent in DSM psychosis subgroups. Future translational research may benefit from exploring emotional scene processing in such neurobiologically-defined psychosis groups.
Keywords: psychotic disorders, emotions, electroencephalography, biomarkers, social cognition, cognitive neuroscience
1. Introduction
Socioemotional deficits are core features of schizophrenia-spectrum disorders and are related to emotional processing impairments. These deficits may manifest as difficulties perceiving and responding to emotional signals from others, impairing social functioning. However, it is unclear if individuals with schizophrenia-spectrum disorders differ in their internal emotional experiences (Green et al., 2015). Internal emotion processing may be studied using neural correlates of complex emotional scenes which index rapid responses to naturalistic emotional stimuli. Our previous work with a large sample (N > 1000) showed that EEG correlates of emotional scene processing are only mildly deficient in schizophrenia and schizoaffective disorder and do not differ from healthy comparisons in bipolar disorder. However, we found a strong multivariate correlation across diagnostic groups (r=.44) between emotional-scene evoked ERPs and socio-cognitive deficit (Trotti et al., 2020, 2021). This finding indicates that individuals with severe cognitive and social difficulties, regardless of diagnosis, are more likely to experience reduced neural reactivity to emotional scene stimuli.
Despite largely null results regarding emotional scenes, other domains of emotional perception and recognition show abnormalities in clinically defined psychosis groups (Facial emotion: Addington et al., 2006; Rubin et al., 2021; Turetsky et al., 2007; Emotional prosody: Hoekert et al., 2007). Furthermore, psychiatric subtype analyses using various unsupervised machine learning methods have been growing in popularity (Voineskos et al., 2020). These analyses may be sensitive to individual variation in emotional processing abilities. For example, a hierarchical clustering analysis of self-reported emotion measures identified a schizophrenia subgroup with atypical emotional experience and emotionally relevant clinical features (Strauss and Herbener, 2011).
Similarly, the B-SNIP consortium described and replicated three bio-cognitive subtypes (“Biotypes”) of idiopathic psychosis. This process parsed the substantial biological heterogeneity present in schizophrenia-spectrum disorders to identify groups that are biologically and cognitively more homogenous than traditional DSM diagnoses. Biotypes may represent differing etiologies or responsiveness to particular treatments. Groups were identified by performing k-means clustering on auditory EEG, cognitive, and saccade variables (Clementz et al., 2022, 2016). Subsequent clinical characterization demonstrated that Biotypes have unique behavioral signatures, with differences in cognitive, social, and emotional features. Biotypes-1 and −2 display high levels of socio-cognitive disability and difficulties recognizing facial emotion while Biotype-3 has relatively intact abilities (Clementz et al., 2020; Rubin et al., 2021). These differences indicate that Biotypes-1 and −2 may capture emotional scene processing abnormalities within idiopathic psychosis not apparent in DSM categories.
Two well-studied EEG correlates of emotional scene processing are the early posterior negativity (EPN) and the late positive potential (LPP). The EPN occurs early (~150-300 ms) in occipito-temporal scalp regions and may arise from lateral occipital sources (Junghöfer et al., 2001; Schupp et al., 2006). The LPP occurs later (~400 ms onward) in centroparietal scalp regions (Hajcak et al., 2010; Schupp et al., 2000) and likely arises from widespread cortical and subcortical structures (Liu et al., 2012; Mini et al., 1996; Sabatinelli et al., 2013). Psychosis research most commonly focuses on these components because the EPN and LPP may capture problems with visual and emotional brain systems at early and late processing stages.
The current study investigated how psychosis Biotypes differ on EEG measures of emotional scene processing using the EPN and LPP. We hypothesized that Biotypes with low cognitive, social, and emotional functioning (Biotypes-1 and −2; Clementz et al., 2020; Rubin et al., 2021) will show significant, moderate-to-large reductions in emotional EPN and LPP amplitudes compared to healthy participants. We expect only mild differences between healthy and Biotype-3 groups, concordant with their mild social expressive deficit and negative symptoms (Clementz et al., 2020). Additionally, six other components of the emotional scene ERP response were evaluated in line with previous work. This approach has identified useful features for characterizing and distinguishing psychosis subgroups (Parker et al., 2021, 2020, 2019). While we expect to find a general pattern of the most severe deficits in Biotypes-1 and −2, this more comprehensive approach may also identify biomarkers unique to Biotype-3.
We employed multivariate methods to identify emotional biomarkers with promise for translation to clinical practice. Our prior work indicated that late-occurring emotional ERPs were associated with composite scores on the Brief Assessment of Cognition in Schizophrenia (BACS; Keefe et al., 2004) and Social Functioning Scales (Birchwood et al., 1990; Trotti et al., 2021). To further specify the nature of this association, we performed a multivariate correlation between ERP components and specific domains of social and cognitive function. We hypothesize a prominent relationship between emotional ERP amplitudes and subdomains of these scales most relevant to socio-emotional communication, particularly social engagement and activity, communication, and verbal fluency.
2. Methods
2.1. Participants
Researchers from five sites of the B-SNIP consortium collected EEG data from 129 healthy participants, 195 participants from Biotype-1 (BT-1), 131 from Biotype-2 (BT-2), and 167 from Biotype-3 (BT-3). All individuals assigned a Biotype were previously diagnosed with schizophrenia, schizoaffective disorder, or bipolar disorder with psychosis. Biotypes were derived from a biomarker panel of neurocognitive, saccade, and auditory EEG data. Briefly, participants completed the BACS (Keefe et al., 2004), stop-signal, pro- and anti-saccade, and auditory paired stimulus and oddball tasks. B-SNIP methods and findings within each task are documented in prior publications (Clementz et al., 2016; Ethridge et al., 2015, 2014; Gotra et al., 2020; Hamm et al., 2014; Hill et al., 2013; Huang et al., 2021; Parker et al., 2021, 2020; Reilly et al., 2014). Within each task, a principal components analysis was completed based on the full combined sample (healthy and psychosis), reducing the 2 stop-signal, 6 saccade, and 31 EEG variables to 9 composite “Biofactors.” Biofactor data were winsorized, standardized, and submitted to a k-means clustering algorithm. GAP statistics and other clustering validation tools were used to verify that a 3 cluster solution was optimal. These 3 clusters were termed “Biotypes.” Additional details about this process are documented in the supplement, with extensive details and discussion in Clementz et al., (2016) and (2022).
Healthy participants had no history of psychosis, mania, recurrent depression, or first-degree family history of psychosis. Across-site consistency was ensured with a human phantom system. Full study procedures and inclusion/exclusion criteria are detailed in Tamminga et al., (2013). Demographics are provided in Table 1, and full clinical details (medications, illness duration, clinical scales) are reported in Supplement Table S1.
Table 1.
Demographics
| HC | BT-1 | BT-2 | BT-3 | Statistic | p | |
|---|---|---|---|---|---|---|
| N | 129 | 195 | 131 | 167 | ||
| Mean age | 40 | 41 | 39 | 35 | F(3,613) = 7.64*** | <.001 |
| Age SD | 11.08 | 10.75 | 11.05 | 11.92 | ||
| Sex (% F/M) | 44/66 | 57/43 | 43/57 | 52/48 | x2(3) = 8.96* | .03 |
| N from each site | ||||||
| Boston | 24 | 16 | 21 | 23 | ||
| Chicago | 34 | 68 | 18 | 66 | ||
| Dallas | 16 | 14 | 16 | 9 | ||
| Georgia+ | 32 | 42 | 35 | 25 | ||
| Hartford | 23 | 55 | 41 | 44 | ||
Note. HC = Healthy comparisons, BT-1 = Biotype-1, BT-2 = Biotype-2, BT-3 = Biotype-3.
Participants recruited from Athens, GA and Augusta, GA.
All subjects provided written informed consent prior to participation after obtaining a complete description of study procedures. This project was approved by the institutional review board at all participating sites and procedures were in accordance with the Helsinki Declaration of 2013.
2.2. Procedures
2.2.1. Stimuli
Participants viewed 20 neutral, 20 pleasant, and 20 unpleasant grayscale scene stimuli in pseudorandom order during continuous 64-sensor EEG recording. Scenes were obtained through internet searches and consistent with the International Affective Picture System (IAPS; Lang et al., (1997)). Additional stimulus details can be found in Trotti et al., (2021). Participants viewed each scene three times during the experimental session and were instructed to passively view scenes with their eyes loosely fixed on the red central fixation point.
2.2.2. Data collection
Participants wore a 64-sensor EEG net plus mastoid and CB 1/2 sensors with nose reference and forehead ground (QuikCap, Compumedics Neuroscan, El Paso, TX). Impedances were kept below 10 kΩ and data were sampled at 1000 Hz with a bandpass filter of direct current (DC)-100 Hz. Participants viewed each image for 1000 ms, followed by 3.5 seconds of a black screen. After EEG recording, participants rated each scene according to experienced pleasantness and arousal using the Self-Assessment Manikin (Bradley and Lang, 1994).
2.2.3. Data preprocessing
Preprocessing followed previously published methods (Parker et al., 2021, 2020; Thomas et al., 2019; Troth et al., 2020, 2021). Raw data were inspected for bad sensor recordings and interpolated in BESA (MEGIS Software, Gräfelfing, Germany) with no more than 5% of channels interpolated per subject. Data were transformed into an average reference and digitally filtered from 0.1 (12 dB/oct, zero phase) to 100 Hz (48 dB/oct, zero phase) with a notch filter at 60 Hz and width of 2 Hz. Eye blinks, heart rate, and muscle tension artifacts were minimized using the ICA toolbox in EEGLAB (Delorme and Makeig, 2004) under Matlab (MathWorks, Natick, MA). No more than 5 of ICA artifacts were removed per subject. Data were downsampled to 500 Hz and epochs containing an amplitude greater than 120 μV at any sensor were excluded. No less than 25 trials were included in each subject’s ERP waveform average per scene content. Number of included trials did not differ between conditions (F(2,1242) = 21, p = .81), with an average of 56.9 trials included in neutral ERPs (SD = 4.31; range = 34-60), 56.96 (SD = 4.39; range = 28-60) in pleasant ERPs, and 56.93 (SD = 4.16; range = 34-60) in unpleasant ERPs. Continuous data were adjusted for effects of age by calculating age regression coefficients in the healthy group and removing these age-related effects from all groups’ data, as documented in Dukart et al., (2011).
2.2.4. Component extraction
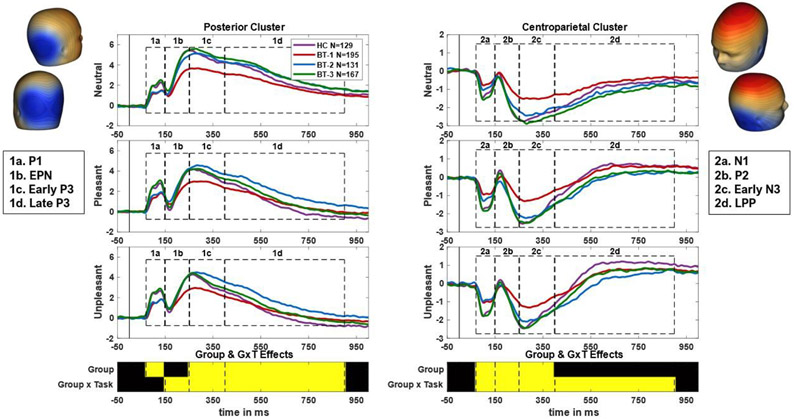
As in prior work (Trotti et al., 2020, 2021), 64-sensor data was downsampled to 2 sensor clusters where the emotional scene response is maximal: 1 cluster from 6 posterior sensors (P7, P8, PO7, PO8, CB1, CB2) and 1 from 5 centroparietal sensors (FCz, C1, Cz, C2, CPz). The EPN (150-250 ms) and LPP (400-900 ms) time ranges were chosen to correspond with previous publications. Additional components were selected according to visual inspection of waveforms. This resulted in 4 components extracted from each cluster: 70-150 ms, 150-250 ms, 250-400 ms, and 400-900 ms. Components from the posterior cluster were named P1, EPN, early P3, and late P3. Components from the centroparietal cluster were named N1, P2, early N3, and LPP. These names were chosen based on EEG convention and prior publications (Jessen and Kotz, 2011; Naumann et al., 2022; Tavakoli et al., 2021). This segmentation is illustrated in Figure 1.
Figure 1. ERP segmentation.
ERPs were constructed from 2 sensor clusters: a posterior cluster and a centroparietal cluster based on scalp distributions of the emotional difference wave (average of pleasant and unpleasant minus neutral response) at EPN and LPP time periods. Headplots of these difference waves are shown on the top left (EPN; negative maximal difference shown in blue at posterior region of scalp) and top right (LPP; positive maximal difference shown in red at centroparietal region of scalp). Components were chosen and named based on their peaks and timing. The P/N1 (1/2a) spans from 70-150 ms, the EPN/P2 (1/2b) spans from 150-250 ms, the early P/N3 (1/2c) spans from 250-400 ms, and the late P3/LPP (1/2d) spans from 400-900 ms. Components are visually separated using dotted lines, with ERPs from neutral, pleasant, and unpleasant scenes depicted in descending order for both clusters. Under the ERPs, a plot shows which components exhibited statistically significant effects of group and group X task interactions. Yellow indicates p<.00625 in omnibus ANOVAs.
2.2.5. Univariate analyses
To determine entry into subsequent multivariate analyses, individual variables (ERP components, pleasantness ratings, valence ratings) were first examined for effects of Biotype, valence, and sex in SPSS using mixed-design ANOVAs with a 3 (valence: neutral, pleasant, unpleasant) X 4 (group: HC, BT-1, BT-2, BT-3) X 2 (sex: male, female) design and Bonferroni correction (ERP α=.00625, self-report α=.025)1. Sex was included as a variable to address the NIH policy on sex as a biological variable (National Institutes of Health Office of Research on Women’s Health, 2023) and increase translational value of results. Tests violating Mauchley’s Test of Sphericity were performed using Greenhouse-Geisser corrections, though uncorrected degrees of freedom are reported in the text for ease of reading. In any case of group X valence interactions where the nature of the interaction was not apparent by main effects of group or valence, an emotional difference score was calculated (average pleasant/unpleasant response amplitudes minus neutral amplitude) and compared between groups. Individual variables demonstrating a significant main effect of group were then entered into subsequent multivariate analyses.
2.2.6. Group discrimination analysis
A canonical discriminant analysis (CDA) was conducted to identify the most promising candidate biomarkers and summarize overall data patterns since univariate methods can produce many variables that include redundant information. A CDA is a supervised factor analysis procedure similar to principal component analysis (PCA) but uses pooled within-group covariance matrices and pits group means as variables and measurements as observations (Kshirsagar, 1972; Lawley, 1959; Mardia et al., 1979). Resulting latent variables are uncorrelated and maximize group differences (Parker et al., 2019). Weights/loadings of individual variables indicate which single variables best differentiate groups. Measures displaying significant main effects of Biotype in univariate analyses were entered into the CDA. Consistent with prior studies (Parker et al., 2020; Thomas et al., 2019), Tukey’s B tests were conducted on resulting CDA latent variables (components) to compare groups while controlling for multiple comparisons.
2.2.7. Symptom associations
Our previously published findings indicated an association between emotional scene ERPs and socio-cognitive function. To specify which facets of social and cognitive functioning were most related to emotional scene processing, a canonical correlation analysis (CCA) was conducted using scores from subdomains of these 2 scales. CCA identifies the relationship between two sets of variables by forming linear combinations of each set that maximize the correlation between “predictor” and “criterion” variable sets. In this case the two sets were 1) EEG and self-reported emotion measures and 2) cognitive and social measures. CCA is most suitable when there are high intercorrelations within variable sets (Levine, 1977). Results of a CCA are correlated pairs of latent variates. Each pair is independent and composed of weighted sums of the predictor variables that maximally correlate with the weighted sums of the criterion variables. Interpretation of what the latent variates represent and how they are related to each other can be determined by the weighted sums (loadings) of individual measures on the latent structure (Rodrigue et al., 2018).
In this analysis, cognitive items included verbal memory, digit sequencing, token motor task, verbal fluency, symbol coding, and Tower of London (Keefe et al., 2004). Social functioning items include social engagement/withdrawal, interpersonal communication, independence-performance, independence-competence, recreation, prosocial activity, and occupation/employment (Birchwood et al., 1990). Emotional items include all variables demonstrating significant group differences, as in the CDA.
3. Results
3.1. ERP main effects
Effect sizes for all significant group differences are listed in Table 2. The EPN and LPP exhibited interaction effects (below), but not significant main effects of Biotype (F(3,614) = 3.16, 3.45; both p = .02). However, all other ERP measures displayed significant main effects of Biotype (all F(3,614) > 4.60, p < .004), suggesting they may index visual processing differences in psychosis. BT-1 displayed blunted response amplitudes at most measures (P/N1, centroparietal P2, and early P/N3). BT-2 displayed blunted P/N1 amplitudes but enhanced posterior late P3 amplitudes. BT-3 had intermediate blunting of the centroparietal N1. Main effects of valence were significant and in expected directions for all components (all F(2,1228) > 19.99, p < .001). Significant main effects of sex were also observed in the two early components at both sensor clusters (see Supplementary Table S2).
Table 2.
Univariate Effect Sizes
| BT-1 | BT-2 | BT-3 | |
|---|---|---|---|
| Average P1 | −0.45 | −0.43 | −0.15 |
| Neutral EPN | −0.40 | −0.09 | −0.02 |
| Early P3 Emotional Δ | 0.30 | 0.44 | 0.11 |
| Pleasant Late P3 | 0.11 | 0.52 | 0.20 |
| Unpleasant Late P3 | 0.14 | 0.48 | 0.10 |
| N1 Unpleasant Δ | 0.01 | 0.43 | 0.21 |
| Neutral P2 | 0.42 | 0.11 | 0.03 |
| Pleasant P2 | 0.29 | −0.04 | 0.00 |
| Early N3 Unpleasant Δ | −0.31 | −0.26 | 0.05 |
| Unpleasant LPP | −0.19 | −0.41 | −0.19 |
| Average Pleasantness | −0.28 | −0.36 | 0.06 |
| Neutral Arousal | −0.64 | −0.73 | −0.19 |
| Unpleasant Arousal | 0.42 | 0.48 | 0.11 |
Note. Glass’ Δ effect sizes relative to healthy group. Effect sizes also presented visually in supplement.
3.2. ERP interaction effects
All measures except the posterior P1 (F(6,1228) = 1.77, p = .10) displayed significant Biotype by valence interactions (all F(6,1228) > 3.4, p < .001). These interactions are described below. No significant valence by sex (all F(2,1228) < 5, p > .01), Biotype by sex (all F(3,614) < 1.6 p > .05), or Biotype by valence by sex interactions were observed (all F(6,1228) < 1.2, p > .05). These results indicate that effects of emotional content and Biotype group do not differ between males and females.
3.3. Nature of Biotype by valence interactions
3.3.1. Posterior sensors
In the traditional EPN measure, there was a significant main effect of Biotype on the neutral response (BT-1 < BT-2/BT-3/HC; F(3,618) = 6.94, p < .001), but not on emotional responses (F(3,618) = 2.85, 2.37; p = .04, .07). Effects suggest that Biotype 1 displays reduced neural responses during early visual appraisal of neutral scene content. In the early posterior P3 (250-400 ms), there was an effect of Biotype on the emotional difference score (average pleasant/unpleasant amplitude minus neutral amplitude; HC/BT-3 < BT-3/BT-1 < BT-1/BT-2; F(3,618) = 5.65, p < .001). This indicates that emotional modulation of this component was reduced (smaller absolute difference) in Biotype-2 with intermediate reductions in Biotype-1 and Biotype-3. In the late posterior P3 (400-900 ms), there was a significant effect of Biotype on the emotional responses (both pleasant and unpleasant: HC/BT-1/BT-3 < BT-2; F(3,618) = 5.63, 5.30; both p < .001), but not the neutral response (F(3,618) = 3.33, p = .019). This suggests that Biotype-2 experiences reduced (less negative amplitude) late-stage processing of emotional stimuli.
3.3.2. Centroparietal sensors
In the centroparietal N1 (70-150 ms), there was a significant effect of Biotype on the unpleasant emotional difference score (unpleasant – neutral amplitude; HC/BT-1/BT-3 < BT-3/BT-2; F(3,618) = 5.44, p = .001), but not the pleasant difference score (F(3,618) = 1.01, p = .39). This effect indicates reduced modulation of early scene processing by negative content in Biotype-2, with intermediate effects in Biotype-3. There was also a significant effect of Biotype on the early centroparietal N3 (250-400 ms; unpleasant difference: BT-1/BT-2 < HC/BT-3; F(3,618) = 5.73, p < .001, pleasant difference: F(3,618) = .78, p = .51). This indicates that Biotypes-1 and −2 also exhibit blunted effects of unpleasant content on mid-latency processing. In the centroparietal P2 (150-250 ms), there were significant effects of Biotype on neutral and pleasant responses (both HC/BT-3/BT-2 < BT-1; F(3,618) = 9.11, 5.33; p < .001, p = .001), but not unpleasant responses (F(3,618) = 2.29, p = .08). This indicates blunted early processing of neutral and pleasant scenes. Finally, in the LPP, there was a significant effect of Biotype on unpleasant responses (BT-2/BT-1/BT-3 < BT-1/BT-3/HC; F(3,618) = 4.17, p = .006), but not neutral and pleasant responses (F(3,618) = 3.76, 3.89; both p = .01). This suggests impaired late-stage processing of unpleasant scenes in Biotype-2 and intermediate effects in Biotype-1 and Biotype-3.
3.3.3. Within-subject results
Every group consistently exhibited significant effects of stimulus valence across every measure (all F > 6, p < .01) except for Biotype-2 and Biotype-3 at the centroparietal N1 (B2: F(2,260) = 4.35, p = .01; B3: F(2,332) = 3.35, p = .04). Aside from the N1, results indicate that even though groups with psychosis display some reduced emotion-sensitive ERP amplitudes or weakened emotional responses, neural sensitivity to emotional content in scenes is not entirely absent. Non-significant valence effects on the N1 may indicate that Biotype-2 and Biotype-3 have reduced early registration of emotional content in scenes.
3.4. Self-report analysis
Effect sizes for all significant group differences are listed in Table 2.
3.4.1. Pleasantness
Pleasantness ratings exhibited a significant main effect of Biotype (BT-2/BT-1/HC < BT-1/HC/BT-3; F(3,592) = 3.68, p = .01). Interactions of valence by Biotype (F(6,1184) = 2.54, p = .03), sex by Biotype (F(3,592) = .23, p = .88), and valence by sex by Biotype (F(6,1184) = .74, p = .60) were not significant. As expected, within-subject pleasantness ratings exhibited a main effect of valence in expected directions (unpleasant < neutral < pleasant; F(2,1184) = 1810.78, p < .001). Sex effects and interactions are reported in the supplement.
3.4.2. Arousal
Arousal ratings did not show a main effect of Biotype (F(3,592) = .56, p = .64), but there was a significant valence by Biotype interaction (F(6,1184) = 11.92, p < .001). This effect was explained by a main effect of Biotype on arousal ratings of neutral (BT-2/BT-1 < BT-3/HC; F(3,596) = 13.42, p < .001) and unpleasant scenes (HC/BT-3 < BT-1/BT-2; F(3,596) = 6.39, p < .001) but not pleasant scenes (F(3,596) = 2.18, p = .09). BT-1 and BT-2 reported enhanced arousal to neutral scenes and reduced arousal to unpleasant scenes relative to BT-3 and HC.
Also as expected, there was a significant within-subjects effect of valence on arousal ratings in expected directions (neutral < pleasant/unpleasant; F(2,1184) = 144.34, p < .001). Sex effects and interactions are reported in the supplement.
3.5. Canonical discriminant analysis (CDA)
The 13 variables differentiating groups (out of 30 possible variables) were used to identify canonical variates best capturing group differences: overall P1, neutral EPN, early P3 emotional difference score, pleasant and unpleasant late P3, N1 and early N3 unpleasant difference scores, neutral and pleasant P2, unpleasant LPP, overall pleasantness rating, and neutral and unpleasant arousal ratings.
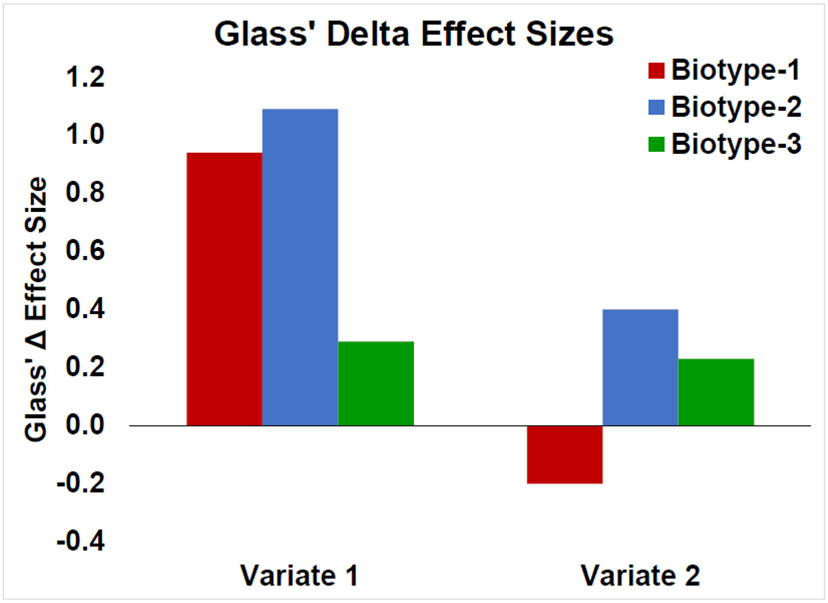
The CDA yielded two significant variants accounting for 75% and 20% of the variance respectively (variate-1: Wilks’ Lambda = .79, p < .001; variate-2: Wilks’ Lambda = .94, p = .045). Variate-1 formed a continuum of illness severity (Figure 2; HC < BT-3 < BT-1/BT-2; F(3,596) = 36.97, p < .001). Effect sizes were large for Biotypes-1 and −2 (BT-1: 0.94, BT-2: 1.09) and moderate for Biotype-3 (0.29). This variate was primarily driven by arousal ratings and early visual processing components, variables indexing complex cognitive appraisal and early sensory registration (Table 3). The second variate displayed an unexpected pattern of BT-1/HC < HC/BT-3 < BT-3/BT-2 (Figure 2; F(3,596) = 9.80, p < .001). Effects were small to moderate in size (BT-1: −0.20, BT-2: 0.40, BT-3: 0.23). This variate was largely defined by ERP components in the EPN and LPP time ranges at both sensor clusters (Table 3). Results from this variate indicate that Biotypes-2 and −3 may exhibit emotion-relevant neural differences from Biotype-1 and healthy persons. Glass’ Delta effect sizes for Biotypes on these variates are shown in Figure 2.
Figure 2. Glass’ Delta effect sizes of CDA variate scores.
The mean of the healthy group is the 0 point on the Y axis. Variate 1 (left) depicts a pattern of differences associated with psychosis & cognitive severity: HC < BT-3 < BT-1/BT-2. Variate 2 (right) separates BT-1 from BT-2 and BT-3, with HC intermediate. Error bars show +/− 1 standard error.
Table 3.
CDA Variate Weights
| Variate 1 | Variate 2 | |
|---|---|---|
| Neutral Arousal | −.60 | .02 |
| Average P1 | −.43 | .17 |
| Unpleasant Arousal | .42 | −.03 |
| Early P3 Emotional Δ | .39 | .17 |
| Neutral P2 | .33 | −.71 |
| N1 Unpleasant Δ | .14 | .68 |
| Pleasant P2 | .16 | −.67 |
| Neutral EPN | −.27 | .66 |
| Pleasant Late P3 | .24 | .61 |
| Unpleasant Late P3 | .28 | .47 |
| Unpleasant LPP | −.27 | −.37 |
| Average Pleasantness | −.30 | .05 |
| Early P3 Unpleasant Δ | −.35 | .20 |
Note. Color of variate weights indicate their direction, saturation indicates their intensity. Weights > .30 are considered to meaningfully contribute to variance in the data.
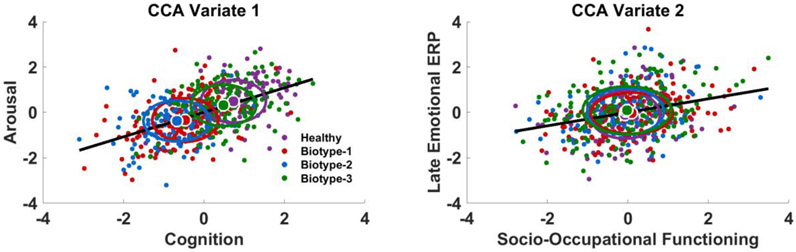
3.6. Canonical correlation analysis (CCA)
The CCA yielded two significant variates. Variate-1 had a correlation of r = .54 (F(169,4996) = 2.275, p < .001, eigenvalue = .41, Wilks’ Statistic = .50) and variate-2 had a correlation of r = .30 (F(144,4624) = 1.31, p = .01, eigenvalue = .10, Wilks’ Statistic = .71). Variate-1 depicted a relationship between general cognitive ability (except for motor skills [Token Motor task]) and self-reported arousal ratings (Table 4). Variate-2 depicted a relationship between socio-occupational functioning and late-occurring emotional ERP components (Table 4).
Table 4.
CCA Weights for Sets 1 and 2
| Set 1 | Variate 1: Cognition |
Variate 2: Socio- Occupational Functioning |
Set 2 | Variate 1: Arousal Ratings |
Variate 2: Late Emotional ERP |
|---|---|---|---|---|---|
| Social Engagement/Withdrawal | −.27 | −.28 | Average P1 | −.38 | .22 |
| Interpersonal Communication | −.49 | −.26 | Neutral EPN | −.37 | .08 |
| Independence-Performance | −.32 | .15 | Early P3 Emotional Δ | .16 | .45 |
| Independence-Competence | −.25 | −.11 | Pleasant Late P3 | .06 | .43 |
| Recreation | −.27 | .21 | Unpleasant Late P3 | .14 | .27 |
| Prosocial | −.61 | .05 | Early N3 Unpleasant Δ | −.38 | −.42 |
| Occupation/Employment | −.57 | .39 | N1 Unpleasant Δ | .07 | .10 |
| Verbal Memory | −.73 | −.10 | Neutral P2 | .37 | −.11 |
| Digit Sequencing | −.68 | .18 | Pleasant P2 | .24 | −.28 |
| Token Motor | −.37 | −.22 | Unpleasant LPP | −.25 | −.45 |
| Verbal Fluency | −.66 | .04 | Average Pleasantness | −.27 | .42 |
| Symbol Coding | −.72 | −.34 | Neutral Arousal | −.59 | −.08 |
| Tower of London | −.67 | .03 | Unpleasant Arousal | .65 | −.20 |
Note. Color of variate weights indicate their direction, saturation indicates their intensity. Weights > .30 are considered to meaningfully contribute to the correlation between sets. Set 1 (cognition and social function) is shown on the left. Set 2 (emotional ERPs and ratings) are shown on the right. Weights for each variate maximize the relationship between each set. 2 variates resulted from this analysis, indicating that there are 2 independent relationships/correlations between input variables.
By plotting CCAs by group identity (Figure 3), the first variate pair differentiates Biotype-1 and Biotype-2 from healthy participants and Biotype-3, with Biotypes-1 and −2 showing abnormal arousal (heightened to neutral stimuli, reduced to unpleasant) and cognitive deficit relative to healthy persons and Biotype-3. This is consistent with the pattern of cognitive abilities in each group. The second variate pair is unrelated to group membership and indicates a relationship between late ERP components and socio-occupational functioning irrespective of psychosis presence.
Figure 3. Canonical correlations.
The first CCA variate (left) depicts a moderate (r = .54) correlation between cognitive ability (set 1, x axis) and arousal ratings (set 2, y axis). Variate 2 (right) depicts a weak relationship (r = .30) between social-occupational functioning (set 1, x axis) and late emotional ERP components (set 2, y axis). In both panes, negative scores indicate deficit values/lower functioning and positive scores indicate intact values/higher functioning. Black sloped lines indicate the overall correlation. Dots represent individual scores on the two latent variables and are colored according to their group: purple dots are healthy participants, red are BT-1, blue are BT-2, and green are BT-3. Large circles are group means and ellipses indicate standard deviation of each group.
3.7. CDA for DSM groups
To provide a direct comparison between the Biotype and DSM models of psychosis subgroups, we performed a CDA using the same methods, variables, and sample as the Biotype analysis (above) to differentiate DSM groups (schizophrenia [SZ], schizoaffective disorder [SAD], bipolar disorder with psychosis [BD]). This analysis is like that performed in Trotti et al. (2021) which only tested the EPN and LPP and did not incorporate a CDA. Univariate analyses (Supplementary Tables S3 & S4) yielded 4 variables out of a possible 30 to include in the CDA: late posterior P3 and LPP responses to unpleasant scenes, and arousal ratings of neutral and unpleasant scenes. The CDA yielded one significant variate accounting for 91% of the variance (Wilks’ Lambda = .88, p < .001). This variate separated HC and bipolar disorder from schizophrenia and schizoaffective disorder (HC/BD < SAD/SZ, F(3,596) = 23.49, p < .001), consistent with prior findings (Trotti et al., 2021). Effect sizes were as follows: SZ = .87, SAD = .68, BD = .05. This variate was primarily driven by arousal ratings (loadings: unpleasant arousal = .70, neutral arousal = −.65, unpleasant LPP = −.35, unpleasant late P3 = .32).
Compared to the CDA differentiating Biotypes, this analysis indicates that fewer emotional scene processing variables substantially distinguish DSM categories from one another and healthy controls, and these variables distinguish only schizophrenia and schizoaffective disorder from healthy, not bipolar disorder or psychosis as a whole. Self-report measures primarily contribute to this relationship with little contribution from ERP variables. While effect sizes in schizophrenia and schizoaffective disorder are large, they are more notable in the first CDA variate distinguishing Biotype groups.
4. Discussion
This study examined emotional scene processing in neurocognitively defined psychosis subgroups using electrophysiology and self-report. Results support the hypothesis that emotional processing primarily differentiates Biotypes-1 and −2 from Biotype-3 and healthy persons, along a general cognitive functioning dimension. Additionally, some emotional ERP components from early (N1, P2/EPN) and late (late P3/LPP) time ranges may further distinguish Biotypes-2 and −3 from Biotype-1 and healthy persons. This finding warrants further evaluation, as few variables in our biomarker battery show abnormality in Biotype-3 (Tamminga et al., 2021). The association with cognitive ability displayed by multiple structural and functional neural markers (termed “BANCC,” or “BAsic Neuro-Cognitive Continuum” in Tamminga et al., (2021)) suggests that such biomarkers could be related to “a broad vulnerability to serious psychiatric syndromes,” rather than psychosis-specific features (McTeague et al., 2016; Tamminga et al., 2021).
Measures that deviate from this trend could be related to a “second hit,” whether environmental or genetic, that specifically affects the development of psychosis. Another measure that may deviate from this pattern is hippocampal structure (Guimond et al., 2021), which could affect emotional ERP amplitudes identified in the present study.
4.1. Multivariate relationships
As hypothesized, the largest differences in emotional processing were found between low-cognition Biotypes (Biotypes-1 and −2) and groups with higher levels of cognitive performance (Biotype-3, healthy comparisons). Further, general cognition shared the strongest relationship with emotional measures. This relationship was not domain-specific, supporting the presence of a basic neurocognitive continuum underlying neural liability for serious psychiatric syndromes. As such, the emotional measures that track with general cognition may only yield biomarker information that is overlapping with cognitive functioning and may not usefully supplement diagnosis and treatment selection in the clinic, although they could be beneficial for tracking practical functional outcomes.
In contrast, the second CDA variate separates Biotypes-2 and −3 from Biotype-1 and healthy persons, deviating from the pattern of a cognitive continuum. This variate emphasized impairments in Biotypes-2 and −3, primarily from emotion-sensitive ERP components in the EPN and LPP time ranges. Amplitude differences in these components suggest that Biotypes-2 and −3 could share emotional processing abnormalities in occipital and subcortical structures that give rise to these components. Functional studies using MRI and source analysis in high density EEG or MEG are necessary to test this hypothesis.
4.2. Contrast with DSM findings
In our prior publication assessing the EPN and LPP in DSM-defined psychosis subgroups, some significant differences between schizophrenia/schizoaffective disorder and healthy comparisons were found, but these differences were small in size. In the current report, more measures show significant effects of Biotype than of DSM diagnosis, particularly biological measures, and multivariate analyses show that effect sizes are larger in Biotypes-1 and −2 than in schizophrenia and schizoaffective disorder. These outcomes indicate that biomarkers of emotional scene processing have limited utility in symptom-defined categories but may be important and useful biomarkers of emotion differences in biologically defined groups. Future investigations could also employ biological or cognitive subtypes of psychosis to test other domains of emotion and aspects of scene processing.
4.3. Limitations
Biotypes were identified in a cross-sectional community sample of people with idiopathic psychosis. Participants were taking a wide range of psychoactive medications and had varying lengths and severity of illness. It is possible that some variance parsed by the Biotype clustering procedure is related to medication status, illness state, or other factors, although such a conclusion is not consistent with previous analyses (Clementz et al., 2022). Several studies are underway to determine the boundaries of these Biotype categories and whether biomarkers can be affected by targeted therapies.
The clear differences between Biotype groups on these emotional measures support the hypothesis that Biotypes are trait-based entities indicative of underlying neurobiology. It should be noted that Biotype groups were largely defined by ERP measurements from auditory, non-emotional tasks, so while the emotional scene-evoked ERPs analyzed in the present article are valid external validators of the Biotype model, they are not ideal. Behavioral variables, such as the emotional self-report variables, are more ideal for the purpose of model validation.
Finally, the sample assessed in this article was limited by symptom range. Most participants were clinically stable and not experiencing severe symptoms. It is possible that results could differ or be more prominent in an inpatient setting where symptoms are more acute. However, this study was primarily interested in cognitive and social variance, which had a wide range in the present sample and could be adequately related to neural processing variables.
4.4. Conclusions and future directions
One aim of the B-SNIP consortium is identifying biomarkers that could be used in clinical practice for diagnosis and treatment. Preliminary data from cross-sectional samples predict that B-SNIP psychosis Biotypes may differentially benefit from existing pharmaceutical, sensory, and early intervention treatments. If so, Biotype identification could be useful for treatment stratification. The current study tested measures that would be easily implemented in most clinical settings (EEG and self-report) and are readily translatable to clinical practice. Multivariate analysis shows that these measures can provide useful separation between groups to aid diagnosis or track treatment response, especially for interventions aimed at cognitive or emotional symptoms. Longitudinal intervention studies should use these measures to identify likely treatment responders and track biological changes during social, cognitive, and biological treatments. For example, Cognitive Enhancement Therapy (CET), has shown preliminary efficacy towards improving social cognition (Eack et al., 2007). Future CET studies could incorporate these emotional biomarkers to track neurobiological changes underlying sociocognitive improvements. Additionally, biological interventions using noninvasive brain stimulation could use emotional biomarkers as treatment targets and track neural engagement with stimulation (Yamada et al., 2022). We look to examine these possibilities in future studies to test the translational value of these findings.
Supplementary Material
Highlights.
Biological subtypes of psychosis display unique emotional processing deficits
Biotypes with impaired cognition displayed severe impairments in emotional ERPs
Some ERPs were impaired in a cognitively-intact Biotype
ERPs and self-reported emotion are primarily related to generalized cognition
Funding:
This work was supported by the National Institute of Mental Health (grant numbers MH096942, MH078113, MH096900, MH103366, MH096913, MH077851, MH096957, MH077945, MH103368) and the National Center for Advancing Translational Sciences (Georgia CTSA: grant numbers UL1TR002378, TL1TR002382) at the National Institutes of Health. The funding sources had no involvement in the study design, collection, analysis, or interpretation of data, the writing of the report, or the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Drs. Trotti, Parker, Sabatinelli, Keedy, Gershon, Pearlson, Hill, McDowell, and Clementz reported no potential conflicts of interest. Dr. Keshavan reports serving as an advisor to Alkermes, Takeda, and Vanna Inc. Dr. Tamminga reports the following financial disclosures: American Psychiatric Association – Deputy Editor; Astellas – Ad Hoc Consultant; Autifony – Ad Hoc Consultant; The Brain and Behavior Foundation – Council Member; Eli Lilly Pharmaceuticals – Ad Hoc Consultant; Intra-cellular Therapies (ITI, Inc.) – Advisory Board, drug development; Institute of Medicine – Council Member; National Academy of Medicine – Council Member; Pfizer – Ad Hoc Consultant; Sunovion – Investigator Initiated grant funding.
Figures depicting results for individual ERP variables found in Supplementary Figures S1-3.
References
- Addington I, Saeedi H, Addington D, 2006. Facial affect recognition: A mediator between cognitive and social functioning in psychosis? Schizophr Res 85, 142–150. 10.1016/j.schres.2006.03.028 [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith T, Cochrane R, Wetton S, Copestake S, 1990. The Social Functioning Scale: the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry 157, 853–859. 10.1192/bjp.157.6.853 [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, 1994. Measuring emotion: The Self-Assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Parker DA, Trotti RL, McDowell JE, Keedy SK, Keshavan MS, Pearlson GD, Gershon ES, Ivleva EI, Huang L-Y, Hill SK, Sweeney JA, Thomas O, Hudgens-Haney M, Gibbons RD, Tamminga CA, 2022. Psychosis Biotypes: Replication and Validation from the B-SNIP Consortium. Schizophr Bull 48, 56–68. 10.1093/schbul/sbab090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry 173, 373–384. 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Trotti RL, Pearlson GD, Keshavan MS, Gershon ES, Keedy SK, Ivleva EI, Mcdowell JE, Carol A, 2020. Testing Psychosis Phenotypes from B-SNIP for Clinical Application: Biotype Characteristics and Targets. Biol Psychiatry Cogn Neurosci Neuroimaging. 10.1016/j.bpsc.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods 134, 9–21. https://doi.org/ 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dukart J, Schroeter ML, Mueller K, The Alzheimer’s Disease Neuroimaging Initiative, 2011. Age correction in dementia - Matching to a healthy brain. PLoS One 6, 1–9. 10.1371/journal.pone.0022193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS, 2007. Cognitive enhancement therapy improves emotional intelligence in early courseschizophrenia: Preliminary effects. Schizophr Res 89, 308–311. 10.1016/j.schres.2006.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA, 2015. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry 77, 127–136. 10.1016/j.biopsych.2014.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill, S., Keefe RSE, Gershon ES, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, 2014. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res 159, 491–498. 10.1016/j.schres.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotra MY, Hill SK, Gershon ES, Tamminga CA, Ivleva EI, Pearlson GD, Keshavan MS, Clementz BA, McDowell JE, Buckley PF, Sweeney JA, Keedy SK, 2020. Distinguishing patterns of impairment on inhibitory control and general cognitive ability among bipolar with and without psychosis, schizophrenia, and schizoaffective disorder. Schizophr Res 223, 148–157. 10.1016/j.schres.2020.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J, 2015. Social cognition in schizophrenia. Nat Rev Neurosci 16, 620–631. 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- Guimond S, Gu F, Shannon H, Kelly S, Mike L, Devenyi GA, Chakravarty MM, Sweeney JA, Pearlson G, Clementz BA, Tamminga C, Keshavan M, 2021. A Diagnosis and Biotype Comparison Across the Psychosis Spectrum: Investigating Volume and Shape Amygdala-Hippocampal Differences from the B-SNIP Study. Schizophr Bull 47, 1706–1717. 10.1093/schbul/sbab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Macnamara A, Olvet DM, 2010. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev Neuropsychol 35, 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney JA, Pearlson GD, Tamminga CA, Clementz BA, 2014. Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology 51, 348–357. 10.1111/psyp.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KS, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, 2013. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. American Journal of Psychiatry 170, 1275–1284. 10.1176/appi.ajp.2013.12101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A, 2007. Impaired recognition and expression of emotional prosody in schizophrenia: Review and meta-analysis. Schizophr Res 96, 135–145. 10.1016/j.schres.2007.07.023 [DOI] [PubMed] [Google Scholar]
- Huang LY, Jackson BS, Rodrigue AL, Tamminga CA, Gershon ES, Pearlson GD, Keshavan MS, Keedy SS, Hill SK, Sweeney JA, Clementz BA, Mcdowell JE, 2021. Antisaccade error rates and gap effects in psychosis syndromes from bipolar-schizophrenia network for intermediate phenotypes 2 (B-SNIP2). Psychol Med. 10.1017/S003329172000478X [DOI] [PubMed] [Google Scholar]
- Jessen S, Kotz SA, 2011. The temporal dynamics of processing emotions from vocal, facial, and bodily expressions. Neuroimage 58, 665–674. 10.1016/j.neuroimage.2011.06.035 [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ, 2001. Fleeting images: A new look at early emotion discrimination. Psychophysiology 38, 175–178. [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L, 2004. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68, 283–297. 10.1016/j.schres.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Kshirsagar AM, 1972. Multivariate Analysis. MDekker. [Google Scholar]
- Lang PT, Bradley MM, Cuthbert BN, 1997. International Affective Picture System (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. [Google Scholar]
- Lawley DN, 1959. Tests of significance in canonical analysis. Biometrika 46, 59–66. 10.1093/biomet/46.1-2.59 [DOI] [Google Scholar]
- Levine MS, 1977. Canonical Analysis and Factor Comparison, 6th ed. Sage, Newbury Park, CA. [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M, 2012. Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience 32, 14563–14572. 10.1523/jneurosci.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardia KV, Kent JT, Bibby JM, 1979. Multivariate Analysis, in: Probability and Mathematical Statistics. Academic Press, London. [Google Scholar]
- McTeague LM, Goodkind MS, Etkin A, 2016. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 10.1016/j.jpsychires.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mini A, Palomba D, Angrilli A, Bravi S, 1996. Emotional information processing and visual evoked brain potentials. Percept Mot Skills 83, 143–152. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Office of Research on Women’s Health, 2023. NIH Policy on Sex as a Biological Variable [WWW Document], URL https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable (accessed 4.4.23).
- Naumann S, Bayer M, Dziobek I, 2022. Preschoolers’ Sensitivity to Negative and Positive Emotional Facial Expressions: An ERP Study. Front Psychol 13. 10.3389/fpsyg.2022.828066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DA, Hamm JP, McDowell JE, Keedy SK, Gershon ES, Ivleva EI, Pearlson GD, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA 2019. Auditory steady-state EEG response across the schizo-bipolar spectrum. Schizophr Res. 10.1016/j.schres.2019.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DA, Trotti RL, McDowell JE, Keedy SK, Gershon ES, Ivleva EI, Pearlson GD, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, 2020. A uditory paired-stimuli responses across the psychosis and bipolar spectrum and their relationship to clinical features. Biomark Neuropsychiatry 3, 100014. 10.1016/j.bionps.2020.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DA, Trotti RL, McDowell JE, Keedy SK, Hill SK, Gershon ES, Ivleva EI, Pearlson GD, Keshavan MS, Tamminga CA, Clementz BA, 2021. Auditory Oddball Responses Across the Schizophrenia-Bipolar Spectrum and Their Relationship to Cognitive and Clinical Features. Am J Psychiatry 178, 952–964. 10.1176/appi.ajp.2021.20071043 [DOI] [PubMed] [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS,Pearlson GD, Tamminga CA, Sweeney JA, 2014. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull 40, 1011–1021. 10.1093/schbul/sbt132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue AL, McDowell JE, Tandon N, Keshavan MS, Tamminga CA, Pearlson GD, Sweeney JA, Gibbons RD, Clementz BA, 2018. Multivariate relationships between cognition and brain anatomy across the psychosis spectrum. Biol Psychiatry CognNeurosci Neuroimaging 3, 992–1002. 10.1016/j.bpsc.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Han J, Coughlin JM, Hill SK, Bishop JR, Tamminga CA, Clementz BA, Pearlson GD, Keshavan MS, Gershon ES, Heilman KJ, Porges SW, Sweeney JA, Keedy S, 2021. Real-time facial emotion recognition deficits across the psychosis spectrum: A B-SNIP Study. Schizophr Res 243, 489–499. 10.1016/j.schres.2021.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, Lang PJ, 2013. Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol 92, 513–519. 10.1016/j.biopsycho.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cacioppo JT, Ito T, Lang PJ, Bradley MM, Cuthbert BN, 2000. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology 37, 257–261. 10.1111/1469-8986.3720257 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghofer M, 2006. Emotion and attention: event-related brain potential studies. Prog Brain Res 156, 31–51. 10.1016/S0079-6123(06)56002-9 [DOI] [PubMed] [Google Scholar]
- Strauss GP, Herbener ES, 2011. Patterns of emotional experience in schizophrenia: Differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. SchizophrRes 128, 117–123. 10.1016/j.schres.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Clementz BA, Pearlson G, Keshavan M, Gershon ES, Ivleva EI, McDowell J, Meda SA, Keedy S, Calhoun VD, Lizano P, Bishop JR, Hudgens-Haney M, Alliey-Rodriguez N, Asif H, Gibbons R, 2021. Biotyping in psychosis: using multiple computational approaches with one data set. Neuropsychopharmacology 46, 143–155. 10.1038/s41386-020-00849-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA, 2013. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). American Journal of Psychiatry 170, 1263–1274. 10.1176/appi.ajp.2013.12101339 [DOI] [PubMed] [Google Scholar]
- Tavakoli P, Boafo A, Jerome E, Campbell K, 2021. Active and Passive Attentional Processing in Adolescent Suicide Attempters: An Event-Related Potential Study. Clin EEG Neurosci 52, 29–37. 10.1177/1550059420933086 [DOI] [PubMed] [Google Scholar]
- Thomas O, Parker DA, Trotti RL, McDowell JE, Gershon ES, Sweeney JA,Keshavan MS, Keedy SK, Ivleva EI, Tamminga CA, Pearlson GD, Clementz BA, 2019. Intrinsic neural activity differences in psychosis biotypes: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. Biomark Neuropsychiatry 1, 100002. 10.1016/j.bionps.2019.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti RL, Abdelmageed S, Parker DA, Sabatinelli D, Tamminga CA, Gershon ES, Keedy SK, Keshavan MS, Pearlson GD, Sweeney JA, Mcdowell JE, Clementz BA, 2021. Neural processing of repeated emotional scenes in schizophrenia, schizoaffective disorder, and bipolar disorder. Schizophr Bull 1–9. 10.1093/schbul/sbab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti Rebekah L, Parker DA, Sabatinelli D, Tamminga CA, Gershon ES, Keedy SK, Keshavan MS, Pearlson GD, Sweeney JA, Mcdowell JE, Clementz BA, 2020. Electrophysiological correlates of emotional scene processing in bipolar disorder. J Psychiatr Res 120, 83–90. 10.1016/j.jpsychires.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti RL, Parker DA, Sabatinelli D, Tamminga CA, Gershon ES, Keedy SK, Keshavan MS, Pearlson GD, Sweeney JA, McDowell JE, Clementz BA, 2020. Electrophysiological correlates of emotional scene processing in bipolar disorder. J Psychiatr Res 120. 10.1016/j.jpsychires.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC, 2007. Facial emotion recognition in schizophrenia: When and why does it go awry? Schizophr Res 94, 253–263. 10.1016/j.schres.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Jacobs GR, Ameis SH, 2020. Neuroimaging Heterogeneity in Psychosis: Neurobiological Underpinnings and Opportunities for Prognostic andTherapeutic Innovation. Biol Psychiatry 88, 95–102. 10.1016/j.biopsych.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Inagawa T, Hirabayashi N, Sumiyoshi T, 2022. Emotion Recognition Deficits in Psychiatric Disorders as a Target of Non-invasive Neuromodulation: A Systematic Review. Clin EEG Neurosci 53, 506–512. 10.1177/1550059421991688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.