Abstract
Innate defense against microbial infection requires the action of neutrophils, which have cytoplasmic granules replete with antibiotic proteins and peptides. Bactericidal/permeability-increasing protein (BPI) is found in the primary granules of adult neutrophils, has a high affinity for lipopolysaccharides (or “endotoxins”), and exerts selective cytotoxic, antiendotoxic, and opsonic activity against gram-negative bacteria. We have previously reported that neutrophils derived from newborn cord blood are deficient in BPI (O. Levy et al., Pediatrics 104:1327–1333, 1999). The relative deficiency in BPI of newborns raised the possibility that supplementing the levels of BPI in plasma might enhance newborn antibacterial defense. Here we determined the effects of addition of recombinant 21-kDa N-terminal BPI fragment (rBPI21) on the growth and tumor necrosis factor (TNF)-inducing activity of representative gram-negative clinical isolates. Bacteria were tested in citrated newborn cord blood or adult peripheral blood. Bacterial viability was assessed by plating assay, and TNF-α release was measured by enzyme-linked immunosorbent assay. Whereas adult blood limited the growth of all isolates except Klebsiella pneumoniae, cord blood also allowed logarithmic growth of Escherichia coli K1/r and Citrobacter koseri. Bacteria varied in their susceptibility to rBPI21's bactericidal action: E. coli K1/r was relatively susceptible (50% inhibitory concentration [IC50], ∼10 nM), C. koseri was intermediate (IC50, ∼1,000 nM), Klebsiella pneumoniae was resistant (IC50, ∼10,000 nM), and Enterobacter cloacae and Serratia marcescens were highly resistant (IC50, >10,000 nM). All isolates were potent inducers of TNF-α activity in both adult and newborn cord blood. In contrast to its variable antibacterial activity, rBPI21 consistently inhibited the TNF-inducing activity of all strains tested (IC50, 1 to 1,000 nM). The antibacterial effects of rBPI21 were additive with those of a combination of conventional antibiotics typically used to treat bacteremic newborns (ampicillin and gentamicin). Whereas ampicillin and gentamicin demonstrated little inhibition of bacterially induced TNF release, addition of rBPI21 either alone or together with ampicillin and gentamicin profoundly inhibited release of this cytokine. Thus, supplementing newborn cord blood with rBPI21 potently inhibited the TNF-inducing activity of a variety of gram-negative bacterial clinical pathogens and, in some cases, enhanced bactericidal activity. These results suggest that administration of rBPI21 may be of clinical benefit to neonates suffering from gram-negative bacterial infection and/or endotoxemia.
Host defense against bacterial invasion requires an innate immune system with the ability to respond to infection independent of prior exposure to the pathogen (16). As evidenced by the increased frequency and severity of infections in patients who are neutropenic, neutrophils are important cellular effectors of the innate immune system. Neutrophils exert microbicidal activity by at least three mechanisms: (i) generation of oxygen radicals by the phagocyte oxidase (1), (ii) generation of nitric oxide (36), and (iii) mobilization of antibiotic proteins and peptides which are stored in neutrophil cytosolic granules (14, 20).
Neutrophil microbicidal activity is diminished in a variety of clinical conditions. Chronic granulomatous disease is caused by mutations in the genes encoding components of the leukocyte oxidase enzyme (22). Specific granule deficiency is associated with decreased defensin content, but this disorder is associated with pleiotropic neutrophil abnormalities, leaving it uncertain as to whether an increased rate of infection is solely related to deficiency of these broadly cytotoxic peptides (13, 26).
The neutrophils of newborns, who are particularly susceptible to invasive bacterial infections, have also been found to function suboptimally (6, 29, 38). Although gram-positive bacteria (particularly group B streptococcus) cause the majority of bacterial infections in newborns, gram-negative bacteria account for up to 20 to 40% of newborn bacterial infection (2), are associated with a relatively high mortality rate (∼40% [31]), and have been increasing in incidence at some medical centers (30).
We have recently demonstrated that newborns are selectively deficient in a neutrophil antibiotic protein with selective activity against gram-negative bacteria: bactericidal/permeability-increasing protein (BPI). BPI is a 55-kDa protein found in primary (azurophilic) granules with high affinity for the lipid A moiety of gram-negative bacterial lipopolysaccharides (LPSs) (21). BPI exerts selective cytotoxic, antiendotoxic, and opsonic activities against gram-negative bacteria (12). As predicted by their lower mean BPI content, newborn neutrophils have relatively low bactericidal activity against the encapsulated serum-resistant pathogen Escherichia coli K1/r (21). This result suggested that the relatively low BPI content of newborn neutrophils may contribute to the increased risk to newborns of gram-negative sepsis. Moreover, this study raised the possibility that supplementing newborns with exogenous BPI might enhance their antibacterial and antiendotoxic activities. Importantly, BPI's action in blood is greatly enhanced by synergy with the membrane attack complex of the complement system (33), whose function is impaired in newborns by virtue of markedly lower levels of C3 as well as C8 and C9 (37). Thus, it is unknown whether the effects of BPI that have been demonstrated in adult whole blood (33) would also be manifested in newborn blood.
With these considerations in mind, we undertook the current study to determine the effect of addition of exogenous BPI on the survival and cytokine-inducing activity of gram-negative bacteria isolated from newborns with bacteremia clinically associated with sepsis syndrome. We decided to measure tumor necrosis factor alpha (TNF-α) as a marker of endotoxin-induced cytokine release, because this cytokine is known to be elevated in newborns with bacterial sepsis (3) and is believed to contribute to the pathophysiology of septic shock by damaging neonatal tissues (4, 10, 23). Exogenous BPI was provided in the form of rBPI21, a recombinant modified N-terminal fragment which carries the antibacterial and antiendotoxic activities of the holoprotein (17, 24) and is currently being evaluated for potential clinical utility in meningococcemia (15) and other applications (12).
MATERIALS AND METHODS
Reagents.
Trypticase soy broth (TSB) was purchased from Becton Dickinson and Co. (Cockeysville, Md.). Nutrient broth and Bacto agar were obtained from Difco Laboratories (Detroit, Mich.). Sterile saline was bought from Baxter (Deerfield, Ill.). Ampicillin was obtained from Apothecon (Princeton, N.J.), and gentamicin was obtained from American Pharmaceutical Partners (Los Angeles, Calif.). Slick Seal Eppendorf tubes were supplied by Research Products International (Mount Prospect, Ill.). Hank's balanced salts solution (HBSS) and RPMI medium were obtained from GIBCO BRL (Grand Island, N.Y.). Sterile buffered sodium citrate (0.129 M [3.8%]) tubes were obtained from Becton Dickinson (Franklin Lakes, N.J.). The Quantikine TNF-α enzyme-linked immunosorbent assay (ELISA) kit was purchased from R&D Systems (Minneapolis, Minn.).
rBPI21.
rBPI21 was provided by XOMA (US) LLC (Berkeley, Calif.). It is a recombinant 21-kDa protein derived from a genetic construct encoding the N-terminal 193 amino acids of human BPI with an alanine-for-cysteine substitution at position 132. rBPI21 was expressed in CHO-K1 cells and purified by cation-exchange chromatography as previously described (17).
Bacterial strains.
E. coli K1/r, a K1-encapsulated rough LPS chemotype strain, is a bacteremic isolate provided by Alan Cross, Department of Bacterial Diseases, Walter Reed Army Medical Center, Washington, D.C. (34). Citrobacter koseri was isolated from the blood and cerebrospinal fluid of a 14-day-old male with meningitis and was provided by the Bacteriology Laboratory at Baystate Medical Center (Springfield, Mass.). Klebsiella pneumoniae, Enterobacter agglomerans, Enterobacter cloacae, and Serratia marcescens were isolated from blood cultures of newborns (7 to 27 days old) with congenital cardiac defects requiring invasive surgery at Children's Hospital of Boston. In order to prepare frozen stocks of these bacterial isolates, bacteria were grown in TSB and sterile glycerol was added to 15% (vol/vol) prior to freezing at −80°C.
Blood.
After Institutional Review Board approval at Brigham & Women's Hospital, cord blood samples were collected immediately after vaginal delivery (n = 17) or cesarean section (n = 26) into sterile tubes anticoagulated with sodium citrate (for whole blood and plasma experiments; final concentration, 129 mM citrate) or into sterile tubes without additives (for serum experiments). Newborns receiving perinatal antibiotics were excluded. The average gestational age was 38 weeks, with a range of 33 3/7 to 40 3/7 weeks. All samples were labeled numerically, and the results were kept anonymous. Samples were kept at room temperature and tested within 30 to 60 min of collection. Similar results were obtained with cord blood derived from vaginal and C-section delivery. Adult peripheral blood was obtained by venous phlebotomy of non-patient adult volunteers.
Bactericidal assays.
For bactericidal assays, subcultures of bacterial stocks were prepared by inoculating a loopful into 20 ml of TSB and incubating at 37°C with shaking for ∼4 h (late logarithmic phase). The bacterial concentration was determined by measuring the optical density at 550 nm in a spectrophotometer. Subcultures were harvested by centrifugation and resuspended in sterile physiologic saline to the desired concentration.
Antibacterial assays were conducted in Eppendorf tubes in a total volume of 100 μl. Samples contained 80 μl of blood or buffered saline (20 mM sodium phosphate [pH 7.4], 0.9% NaCl) as control, 10 μl of rBPI21 (or buffered saline), and 10 μl of bacteria (added last; final concentration, 104/ml). Samples were incubated with shaking at 37°C. At the indicated time points, 10 μl of each sample was plated on a petri dish and dispersed with ∼9 ml of molten (∼50°C) Bacto agar containing 0.8% (wt/vol) nutrient broth and 0.5% (wt/vol) NaCl. The agar was allowed to solidify at room temperature, and bacterial viability was measured as the number of colonies formed after incubation of plates at 37°C for 18 to 24 h. Bacterial viability was expressed in terms of CFU as a percentage of that of the buffered saline control sample. Experiments employing ampicillin and gentamicin were conducted similarly, except that samples contained 70 μl of blood (or buffered saline) and ampicillin-gentamicin (premixed in saline at a 20:1 mass ratio based on the clinically relevant dosages) dilutions were added to sample tubes in a 10-μl volume.
To compare the effects of rBPI21 in the presence and absence of host cells, antibacterial assays were also conducted with both whole blood and plasma derived from the same newborns (n = 2). Plasma was generated by collection of blood into citrate tubes and centrifugation (1,200 × g for 5 min) prior to recovery of the supernatant (i.e., plasma).
In order to measure the antibacterial activity of rBPI21 under physiologic divalent cation concentrations, bactericidal assays were conducted in serum diluted to 10% (vol/vol) with HBSS containing 1.26 mM calcium and 0.9 mM magnesium. Serum was prepared by collection of newborn cord blood (n = 4) and adult peripheral blood (n = 3) into sterile tubes devoid of any additives and with samples allowed to clot at room temperature for 20 min prior to centrifugation (1,200 × g for 5 min) and recovery of the supernatant (i.e., serum). All serum samples were prepared fresh and tested the same day.
Measurement of TNF-α release.
In order to measure the ability of bacteria to induce TNF-α release in blood, the bacteria were incubated in blood for 5 h. Blood was diluted fivefold with RPMI and then centrifuged at 1,000 × g for 5 min to collect the extracellular fluid (diluted plasma). Samples were stored frozen at −80°C prior to measurement of TNF-α by using a specific ELISA according to the manufacturer's instructions (R&D Systems).
RESULTS
Antibacterial activity of rBPI21 in adult and newborn cord blood.
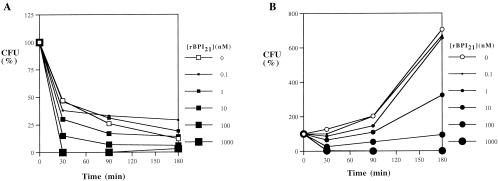
In order to assess whether supplementing newborn blood with exogenous BPI might enhance its antibacterial activity against clinically relevant pathogens, we made use of an assay system which employs anticoagulated (citrated) blood (33). We first tested E. coli K1/r, an encapsulated serum-resistant clinical isolate which has been shown to be sensitive to BPI-mediated killing in both artificial medium (34) and whole adult blood ex vivo (33). Whereas the growth of E. coli K1/r was inhibited by adult blood (Fig. 1A), newborn cord blood served as a growth medium for this organism, which grew logarithmically over several hours (Fig. 1B). Nevertheless, rBPI21 was able to markedly diminish growth of this organism in both adult and newborn cord blood with a 50% inhibitory concentration (IC50) of 10 nM (Fig. 1).
FIG. 1.
Effect of added rBPI21 on the survival of E. coli K1/r in adult and newborn cord blood. E. coli K1/r cells (104 bacteria/ml) were incubated in citrated adult blood (A) or newborn cord blood (B) with increasing concentrations of rBPI21. Samples were periodically plated to assess bacterial survival (CFU) as a percentage of that of a control sample incubated in buffered saline alone. The results shown represent the mean of 2 to 12 independent determinations (error bars omitted to enhance figure clarity).
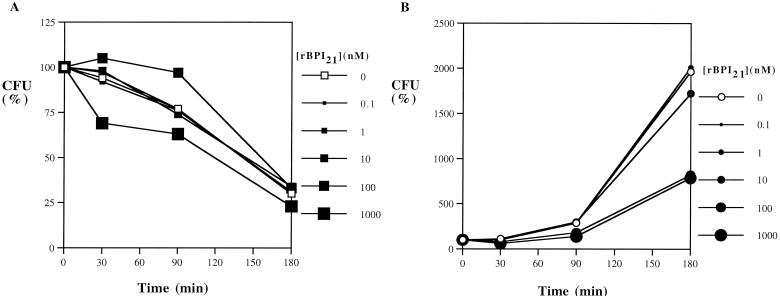
Similarly, Citrobacter koseri was inhibited by adult blood, but grew logarithmically in newborn cord blood (Fig. 2). This isolate was intermediate in sensitivity to rBPI21, requiring 1,000 nM to reduce the number of CFU by 50% in newborn cord blood (Fig. 2B). K. pneumoniae was relatively BPI resistant, with ∼10,000 nM rBPI21 required to inhibit growth by 50% (Table 1). E. cloacae and S. marcescens were highly resistant to the antibacterial activity of rBPI21 (IC50, >10,000 nM; Table 1). E. agglomerans was rapidly killed by both adult and newborn cord blood (not shown), precluding analysis of rBPI21's antibacterial activity against this organism. In order to determine the combined effects of rBPI21 with conventional antibiotics, assays were performed in which rBPI21 was added to whole blood together with a combination of ampicillin and gentamicin, which are frequently used in many nurseries and neonatal intensive care units to treat newborns with presumed bacterial sepsis. Based on their clinical dosages, ampicillin and gentamicin were added at a 20:1 mass ratio (0.1 to 1,000 μg of ampicillin per ml together with 0.005 to 50 μg of gentamicin per ml). When tested against E. coli K1/r, C. koseri, and K. pneumoniae, the antibacterial effects of rBPI21 were found to be additive with those of ampicillin and gentamicin; no antagonism or synergy was noted (results not shown).
FIG. 2.
Effect of added rBPI21 on the survival of C. koseri in adult and newborn cord blood. C. koseri cells (104 bacteria/ml) were incubated in citrated adult (A) or newborn cord blood (B) with increasing concentrations of rBPI21. The results represent the mean of three to four independent determinations (error bars omitted to enhance figure clarity).
TABLE 1.
Antibacterial activity of rBPI21 against gram-negative bacterial isolates
| Bacterial strain | IC50 (nM)
|
|
|---|---|---|
| Adult blood | Newborn cord blood | |
| E. coli K1/r | 10 | 10 |
| C. koseri | 1,000 | 1,000 |
| K. pneumoniae | ∼10,000 | ∼10,000 |
| E. cloacae | >10,000 | >10,000 |
| S. marcescens | >10,000 | >10,000 |
In order to compare the bactericidal activities of rBPI21 in the presence and absence of host cells, antibacterial assays were also conducted in whole blood and plasma derived from the same newborns (n = 2). The activities against E. coli K1/r were similar in both whole blood and plasma (IC50, ∼10 nM [data not shown]), suggesting that rBPI21 activity was not dependent on the presence of host cells.
The use of the divalent cation chelator citrate as an anticoagulant in our whole blood and plasma assay systems raised the issue of whether this additive might have enhanced the antibacterial activity of BPI by removing stabilizing divalent cations (i.e., calcium and magnesium) from the outer surface of the bacterial membrane. In order to determine rBPI21 bactericidal activity in the absence of citrate (and in the presence of divalent cations), antibacterial activity was measured in newborn (n = 4) and adult (n = 5) serum diluted to 10% (vol/vol) with HBSS containing calcium and magnesium. The IC50 of rBPI21 against E. coli K1/r was ∼10 to 100 nM in both newborn and adult 10% serum (not shown), indicating that rBPI21 is able to exert antibacterial activity in the absence of citrate (and in the presence of divalent cations) as well.
Antiendotoxic activity of rBPI21 in adult and newborn cord blood.
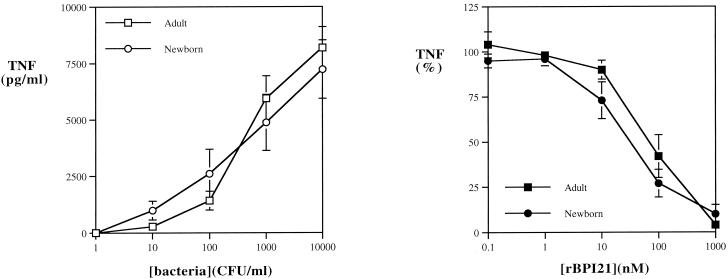
In order to assess the ability of added rBPI21 to inhibit the endotoxic activity of bacteria in blood, bacteria were added to citrated blood and incubated for 5 h to allow accumulation of TNF-α, which was then measured by ELISA. The release of TNF-α in newborn cord blood in response to E. coli K1/r was at least as great as that in adult blood (Fig. 3A). rBPI21 was able to inhibit bacterially induced TNF-α release with similar potencies in both adult and newborn cord blood (IC50, 10 to 100 nM) (Fig. 3B).
FIG. 3.
Effect of added rBPI21 on TNF-α induction by E. coli K1/r in adult and newborn cord blood. (A) E. coli K1/r (101 to 104 bacteria/ml) were incubated in citrated adult or newborn cord blood for 5 h, at which point samples were diluted with RPMI and the extracellular fluid was collected by centrifugation. TNF-α release was measured by ELISA. In order to determine the effects of rBPI21 on bacterially induced TNF release, E. coli K1/r cells (104 bacteria/ml) were incubated in citrated adult or newborn cord blood with increasing concentrations of rBPI21. (B) TNF release is expressed as a percentage of that of a control sample incubated without rBPI21. Results represent the mean ± standard error of two to four determinations.
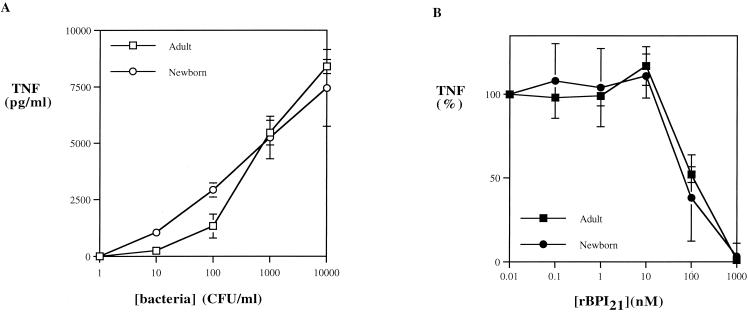
C. koseri, K. pneumoniae, E. cloacae, E. agglomerans, and S. marcescens also induced substantial TNF-α release in both adult and newborn cord blood. Of note, the overall average levels of TNF-α release induced by all six of the gram-negative isolates tested were closely similar in both adult and newborn cord blood (Fig. 4A). In contrast to its variable antibacterial activity (Table 1), rBPI21 was able to inhibit induction of TNF-α release by all of the species tested (IC50, 1 to 1,000 nM) (Fig. 4B and Table 2).
FIG. 4.
Gram-negative bacterial strains are potent inducers of TNF-α in both adult and newborn cord blood, as shown by inhibition by rBPI21. (A) Data points represent averages of TNF-α release induced at each bacterial concentration by the six species tested. (B) Average percentage of TNF release induced by six bacterial species incubated in the presence of increasing concentrations of rBPI21. Results represent the mean ± standard error of 22 to 25 independent determinations.
TABLE 2.
Antiendotoxic activity of rBPI21 shown by inhibition of TNF-α release
| Bacterial strain | IC50 (nM)
|
|
|---|---|---|
| Adult blood | Newborn cord blood | |
| E. coli K1/r | 10–100 | 10–100 |
| C. koseri | 100 | 100 |
| K. pneumoniae | 100–1,000 | 10–100 |
| E. cloacae | 100–1,000 | 100 |
| E. agglomerans | 10–100 | 10–100 |
| S. marcescens | 10–100 | 1–10 |
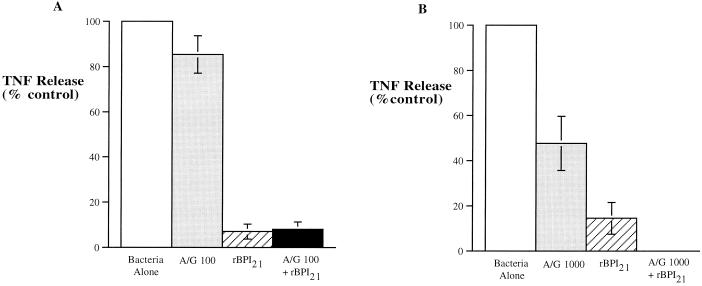
Of note, although ampicillin and gentamicin had little effect on the TNF-inducing activity of E. coli K1/r or K. pneumoniae, addition of rBPI21 either alone or together with ampicillin and gentamicin profoundly inhibited TNF release induced by these bacteria (Fig. 5).
FIG. 5.
Combined effects of ampicillin-gentamicin (A/G) and rBPI21 on bacterially induced TNF-α release. E. coli K1/r (A) and K. pneumoniae (B) were incubated at a concentration of 104/ml in newborn cord blood either alone (white bar), with a bactericidal concentration of ampicillin and gentamicin (100 μg of ampicillin and 5 μg of gentamicin per ml in panel A and 1,000 μg of ampicillin and 50 μg of gentamicin per ml in panel B [grey bar]), with an endotoxin-neutralizing concentration of rBPI21 alone (100 nM in panel A and 1,000 nM in panel B [striped bar]), or with a combination of ampicillin-gentamicin and rBPI21 (black bar). Results represent the mean ± standard error of three independent determinations.
DISCUSSION
Newborns are at increased risk of bacterial infection. Although gram-positive bacterial infections, especially group B streptococcus infections, are more frequent, gram-negative infections remain an important cause of morbidity and mortality in this age group (2, 30, 31). In this study, we show that newborn cord blood serves as a rich growth medium for E. coli K1/r and C. koseri (Fig. 1 and 2, respectively), as well as K. pneumoniae (data not shown). These results are consistent with those of other investigators who have shown inefficient killing of E. coli by serum of human neonates (19), which may be related to relatively low complement activity and, possibly, decreased immunoglobulin M activity (37). Despite the relatively low baseline antibacterial activity of newborn cord blood, we have demonstrated that augmentation of BPI levels in the form of rBPI21 enhances the antibacterial activity of newborn cord blood against E. coli K1/r and C. koseri (Fig. 1 and 2). Given the important role of the complement system in enhancing BPI activity in biologic fluids (34), we speculate that the relatively low level of complement activity in newborn cord blood is sufficient to support BPI activity against these isolates.
In contrast, K. pneumoniae was relatively resistant (IC50, ∼10,000 nM) and E. cloacae and S. marcescens were highly resistant (IC50, >10,000 nM) to rBPI21 (Table 1). Gram-negative bacteria are known to vary in their susceptibility to BPI's bactericidal action. For example, strains with long-chain LPS are relatively BPI resistant, presumably secondary to steric hindrance impairing BPI's access to its lipid A target (8). Our results do not speak to the factors that may confer increased BPI resistance to the K. pneumoniae, E. cloacae, and S. marcescens isolates tested, an important topic that is beyond the scope of this study. The effects of rBPI21 against all species tested were, however, additive with the combination of ampicillin and gentamicin. Although no synergy was observed against these pathogens, antagonism was not observed either, indicating that rBPI21 does not interfere with the action of these conventional antibiotics and raising the possibility that addition of rBPI21 might contribute to antibacterial activity in settings in which concentrations of ampicillin and gentamicin are limiting.
Some studies have documented reduced responses of newborn leukocytes to endotoxin (27, 28, 32). In contrast, our study, which employs a whole-blood assay system using whole, live bacteria, demonstrates that newborn cord blood releases quantities of the cytokine TNF-α similar to those of adult blood (Fig. 4). Our study does not address the reasons for the relatively high levels of cytokine release in cord blood stimulated with gram-negative bacteria. We speculate that multiple factors might account for this result, including the presence of plasma and other blood-derived cofactors, as well as a more pathophysiologically relevant presentation of endotoxin in the context of whole live bacteria which has been previously shown to be markedly more stimulatory than purified endotoxin (18). Since our assay system employs a biologic fluid ex vivo (i.e., blood), our results suggest that gram-negative bacteria may induce high levels of TNF-α release in newborns in vivo. Moreover, significant bacterially induced TNF release occurred even in the presence of ampicillin and gentamicin, but addition of rBPI21 effectively neutralized such endotoxic activity in this context as well (Fig. 5). Excessive TNF release has been implicated in the pathophysiology of neonatal septic shock (23), and agents that reduce TNF-α release have been shown to have beneficial effects in newborn animal models of gram-negative infection in vivo (9, 25). In contrast, the tendency to have low ex vivo TNF production in response to endotoxin challenge increases the risk of fatal bacterial infection (35). Although the complexity of inflammatory cytokine networks and antiinflammatory counterregulation makes it difficult to predict the clinical effects of cytokine modulation, it is possible that inhibition of bacterially induced cytokine release (especially in the context of bacterial growth inhibition) might have a beneficial impact on newborns with gram-negative bacterial septic shock.
We have argued that newborns are likely to have limited amounts of BPI at inflammatory sites because (i) they have limited marrow reserve and are thus prone to neutropenia (5), (ii) newborn neutrophils demonstrate impaired migration and chemotaxis (11), and (iii) newborn neutrophils are relatively deficient in BPI (21). Studies of neutrophil (granulocyte) transfusion have shown some promise (7), but such therapy is complicated by the need to prepare large numbers of histocompatible cells and can be complicated by transfusion reactions. Of note in this regard is that a pure formulation of rBPI21 has been safely administered to over 1,000 human subjects and is currently being assessed as a potential therapeutic agent in pediatric meningococcemia (15) and for other applications. Because the antibacterial and antiendotoxic effects of rBPI21 or gram-negative bacteria tested in newborn cord blood ex vivo are manifested at concentrations achievable by exogenous administration of this agent in vivo (15), our current study suggests that supplementing BPI may be of clinical benefit to newborns with gram-negative bacterial infection and/or endotoxemia. Further studies to assess the potential utility of rBPI21 in newborns at high risk for or with gram-negative bacterial infection are therefore indicated.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Center for Research Resources/General Clinical Research Center grant M01RR02172, an American Academy of Pediatrics Resident Research Award, and a grant from XOMA (US) LLC.
We acknowledge the support and help of the following groups and individuals: from Children's Hospital, Philip Pizzo, Frederick Lovejoy, and Joseph Majzoub for advice and encouragement; Dixon Yun for assistance with computer graphics; Cheryl Sweeney and Eileen Gorss for administrative support; and Irena Clark, Pamela Sale, and the technical staff of the Bacteriology Lab; from The Brigham & Woman's Hospital, the nursing, midwife, and obstetrical staff for assistance with cord blood collection; and from XOMA (US) LLC, Stephen Carroll and Patrick Scannon for advice and encouragement.
REFERENCES
- 1.Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Beck-Sague C, Azimi P, Fonseca S, Baltimore R, Powell D, Bland L, Ardino M, McAllister S, Huberman R, Sinkowitz R. Bloodstream infections in neonatal intensive care unit patients: results of a multicenter study. Pediatr Infect Dis J. 1994;13:1110–1116. [PubMed] [Google Scholar]
- 3.Berner R, Niemeyer C, Leititis J, Funke A, Schwab C, Rau U, Richter K, Tawfeek M, Clad A, Brandis M. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-α, interleukin-1b, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–477. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan I, Leib S, Bergeron M, Chow L, Tauber M. Tumor necrosis factor-α contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 5.Bracho F, Goldman S, Cairo M. Potential use of granulocyte colony-stimulating factor in neonates. Curr Opin Hematol. 1998;5:215–220. doi: 10.1097/00062752-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Cairo M. Neonatal neutrophil host defense. Prospects for immunologic enhancement during neonatal sepsis. Am J Dis Child. 1989;143:40–46. doi: 10.1001/archpedi.1989.02150130050014. [DOI] [PubMed] [Google Scholar]
- 7.Cairo M, Rucker R, Bennetts G, Hicks D, Worcester C, Amlie R, Johnson S, Katz J. Improved survival of newborns receiving leukocyte transfusions for sepsis. Pediatrics. 1984;74:887–892. [PubMed] [Google Scholar]
- 8.Capodici C, Chen S, Sidorczyk Z, Elsbach P, Weiss J. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect Immun. 1994;62:259–265. doi: 10.1128/iai.62.1.259-265.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochran J, Genovese F, Romeo C, Guyton K, Teti G, Cook J. The effect of a tyrosine kinase inhibitor on endotoxin mortality and splenocyte mediator production in the neonatal rat. Shock. 1999;11:35–38. doi: 10.1097/00024382-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Dinauer M. The phagocyte system and disorders of granulopoiesis and granulocyte function. In: Nathan D, Orkin S, editors. Hematology of infancy and childhood. 5th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1998. pp. 889–967. [Google Scholar]
- 12.Elsbach P. The bactericidal/permeability-increasing protein (BPI) in antibacterial host defense. J Leukoc Biol. 1998;64:14–18. doi: 10.1002/jlb.64.1.14. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Metcalf J, Gallin J, Boxer L, Lehrer R. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and “specific” granule deficiency. J Clin Investig. 1988;82:552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 15.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N J, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman J, Kafatos F, Janeway C, Ezekowitz R. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz A H, Leigh S D, Abrahamson S, Gazzano-Santoro H, Liu P S, Williams R E, Carroll S F, Theofan G. Expression and characterization of cysteine-modified variants of an amino-terminal fragment of bactericidal/permeability-increasing protein. Protein Expr Purif. 1996;8:28–40. doi: 10.1006/prep.1996.0071. [DOI] [PubMed] [Google Scholar]
- 18.Katz S S, Chen K, Chen S, Doerfler M E, Elsbach P, Weiss J. Potent CD14-mediated signalling of human leukocytes by Escherichia coli can be mediated by interaction of whole bacteria and host cells without extensive prior release of endotoxin. Infect Immun. 1996;64:3592–3600. doi: 10.1128/iai.64.9.3592-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassiter H, Tanner J, Miller R D. Inefficient bacteriolysis of Escherichia coli by serum from human neonates. J Infect Dis. 1992;165:290–298. doi: 10.1093/infdis/165.2.290. [DOI] [PubMed] [Google Scholar]
- 20.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 21.Levy O, Martin S, Eichenwald E, Ganz T, Valore E, Carroll S, Lee K, Goldmann D, Thorne G. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein (BPI) Pediatrics. 1999;104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 22.Meischl C, Roos D. The molecular basis of chronic granulomatous disease. Springer Semin Immunopathol. 1998;19:417–434. doi: 10.1007/BF00792600. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, Zimny-Arndt U, He C, Darmer D, Jungblut P, Stadler J, Holtz J, Werdan K. Tumor necrosis factor alpha (TNF-α) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–2923. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 24.Ooi C E, Weiss J, Doerfler M E, Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55–60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991;174:649–655. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park W, Chang Y, Ko S, Kang M, Han J, Lee M. Efficacy of anti-tumor necrosis factor-α antibody as an adjunctive therapy in experimental Escherichia coli meningitis in the newborn piglet. Biol Neonate. 1999;75:377–387. doi: 10.1159/000014118. [DOI] [PubMed] [Google Scholar]
- 26.Parmley R, Gilbert C, Boxer L. Abnormal peroxidase-positive granules in “specific granule” deficiency. Blood. 1989;73:838–844. [PubMed] [Google Scholar]
- 27.Peters A, Bertram P, Gahr M, Speer C. Reduced secretion of interleukin-1 and tumor necrosis factor-α by neonatal monocytes. Biol Neonate. 1993;63:157–162. doi: 10.1159/000243926. [DOI] [PubMed] [Google Scholar]
- 28.Pillay V, Savage N, Laburn H. Circulating cytokine concentrations and cytokine production by monocytes from newborn babies and adults. Eur J Physiol. 1994;428:197–201. doi: 10.1007/BF00724497. [DOI] [PubMed] [Google Scholar]
- 29.Schelonka R, Infante A. Neonatal immunology. Semin Perinatol. 1998;22:2–14. doi: 10.1016/s0146-0005(98)80003-7. [DOI] [PubMed] [Google Scholar]
- 30.Shah S, Ehrenkranz R, Gallagher P. Increasing incidence of Gram-negative rod bacteremia in a newborn intensive care unit. Pediatr Infect Dis J. 1999;18:591–595. doi: 10.1097/00006454-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Stoll B, Gordon T, Korones S, Shankkaran S, Tyson J, Bauer C, Fanaroff A, Lemons J, Donovan E, Oh W, Stevenson D, Ehrenkranz R, Papile L, Verter J, Wright L. Late-onset sepsis in very low birthweight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:63–71. doi: 10.1016/s0022-3476(96)70191-9. [DOI] [PubMed] [Google Scholar]
- 32.Weatherstone K, Rich E. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989;25:342–346. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, Theofan G. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Investig. 1992;90:1122–1130. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss J, Victor M, Cross A S, Elsbach P. Sensitivity of K1-encapsulated Escherichia coli to killing by the bactericidal/permeability-increasing protein of rabbit and human neutrophils. Infect Immun. 1982;38:1149–1153. doi: 10.1128/iai.38.3.1149-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westendorp R, Langermans J, Huizinga T, Elouali A, Verweij C, Boomsma D, Vandenbroucke J. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler M A, Smith S D, Garcia-Cardena G, Nathan C F, Weiss R M, Sessa W C. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Investig. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolach B, Dolfin T, Regev R, Gilboa S, Schlesinger M. The development of the complement system after 28 weeks' gestation. Acta Pediatr. 1997;86:523–527. doi: 10.1111/j.1651-2227.1997.tb08924.x. [DOI] [PubMed] [Google Scholar]
- 38.Wright W J, Ank B, Herbert J, Stiehm E. Decreased bactericidal activity of leukocytes of stressed newborn infants. Pediatrics. 1975;56:579–584. [PubMed] [Google Scholar]