Abstract
Antibodies against the Plasmodium falciparum P0 ribosomal phosphoprotein (PfP0) have been detected exclusively but extensively in malaria-immune persons. Polyclonal rabbit and mice sera were raised against two recombinant polypeptides of P. falciparum P0 protein, PfP0N and PfP0C, covering amino acids 17 to 61 and the remaining amino acids 61 to 316, respectively. Sera against both these domains detected a 35-kDa protein from Plasmodium yoelii subsp. yoelii, a rodent malarial parasite, and stained the surface of merozoites in immunofluorescence assays. Total immunoglobulin G (IgG) purified from rabbit and mouse anti-PfP0 sera by ammonium sulfate and DEAE-cellulose chromatography was used for passive transfer experiments in mice. Mice passively immunized with both anti-PfP0N and anti-PfP0C showed distinctly lower levels of parasitemia than control mice. With immunizations on days −1, 0, 1, 3, and 5, about 50% of both sets of mice receiving anti-PfP0N and anti-PfP0C cleared the lethal 17XL strain of P. yoelii and revived by day 25. All the control mice died by day 10. By extending the immunization schedule, the survival period of the mice could be extended for every mouse that received anti-PfP0 IgG. These data demonstrate the cross-protection of the anti-PfP0 IgG and establish parasite P0 protein as a target for invasion-blocking antibodies.
It has been documented that people living in areas of malaria endemicity become immune to malaria after repeated infections. Low levels of parasitemia in adults living in areas of malaria endemicity often accompany this clinical immunity (2). Passive transfer of gammaglobulins from such malaria-immune adults into patients has resulted in clearance of parasites in these patients (10, 12, 25, 29). These experiments worked across geographic borders, as West African sera could cure East African (12, 25) as well as Thai (29) malaria patients. It has been proposed that the immunoglobulin G (IgG) subtype (5) and monocytes (6) play important roles in such protection. Pooled IgG from immune African donors, which could control Plasmodium falciparum in Thai patients, was also able to control the parasite in Saimiri monkeys (6).
In order to elucidate the protective immunoglobulins and their targets, a differential immunoscreening of an erythrocyte stage-specific cDNA expression library of P. falciparum has been performed earlier in our laboratory, using malaria-immune and acute patient sera (22). This resulted in the identification of several novel cDNA clones, which reacted exclusively and yet extensively with immune serum samples (22). Clone λPf4, which reacted with the largest number of immune sera (80 of 92), has been cloned and sequenced (15). This was found to be the P. falciparum gene homologue of the ribosomal phosphoprotein P0 (PfP0). Further characterization showed that antibodies raised specifically to PfP0 inhibited the growth of P. falciparum in vitro and reacted to the surface of merozoites (8, 16).
Ribosomal phosphoprotein P0 is a neutral protein, related to the family of the acidic ribosomal phosphoproteins P1 and P2 because of the highly homologous carboxyl-terminal domain (26). There is evidence that P0 functions though the formation of a (P1)2-P0-(P2)2 protein complex, which interacts with the 28S large ribosomal subunit in eukaryotes (30, 34). A role for the P proteins in the assembly of the GTPase binding site in the large subunit of the ribosomes has been demonstrated in rat tissue (35). Through gene disruption studies, it has been documented that P0 protein is absolutely required for cell viability in Saccharomyces cerevisiae (31). The P0 protein has also been implicated in roles other than ribosomal, such as an apurinic-apyramidinic endonuclease in the nuclei in Drosophila melanogaster (36). It has also been shown to play a regulatory role in Drosophila (14), and the level of P0 protein is shown to be regulated during apoptosis and carcinogenesis in mammalian cells (7, 21).
To test whether the IgG raised against PfP0 protein domains has any effect on parasite growth in vivo, passive-transfer experiments were performed in mice. In this paper we show that mice passively immunized with IgG purified from sera raised in rabbits and mice against the human malarial parasite P. falciparum P0 protein domains were protected against challenge with the lethal 17XL variant of the rodent malarial parasite Plasmodium yoelii. Normally protection at the asexual stages has been documented to apply across a limited number of strains, and certainly not across different plasmodial species (11, 23, 28). This is the first instance of passive protection documented across species of Plasmodium.
MATERIALS AND METHODS
Expression of domains of P0 as GST fusion proteins.
A glutathione S-transferase (GST) reporter-based vector was used as an expression vector as described earlier (8, 16). The amino-terminal 45-amino-acid (a.a.) domain (a.a. 17 to 61) and the 256-amino-acid carboxy-terminal region (a.a. 61 to 316) of PfP0 were expressed as fusion proteins designated PfP0N and PfP0C, respectively. The induction and lysis of the recombinant Escherichia coli cells containing the PfP0N and PfP0C proteins were performed as described earlier (8). The cell lysates were run on denaturing sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue to check the fusion proteins. Both fusion proteins were obtained as insoluble proteins.
Immunization of rabbits and mice with PfP0N and PfP0C domains.
Immunizations were carried out with fusion proteins eluted from the polyacrylamide gel, as described earlier (8). Ten Swiss inbred mice were immunized intraperitoneally with 50 μg of protein emulsified in Freund's complete adjuvant. Booster doses were given every 3 weeks with 15 μg of the protein preparation emulsified with incomplete Freund's adjuvant, and sera were collected every 2 weeks. To obtain sera from rabbits, approximately 200 μg of PfP0N and PfP0C protein preparations was injected subcutaneously into two New Zealand White rabbits for each protein, using standard protocols (18). Subsequent boosts were given with 100 μg of protein at 3-week intervals, and sera were collected 2 weeks after each booster dose. The rabbit and mouse sera were checked for specific antibody against the fusion proteins by enzyme-linked immunosorbent assay (ELISA) and then pooled for IgG purification.
ELISA.
Wells of microtiter plates (Nunc, Roskilde, Denmark) were coated with 1 μg of the fusion protein in 200 μl of phosphate-buffered saline (PBS) overnight at 4°C. Plates were then washed with PBS containing 0.05% Tween 20 (PBST) and incubated with 400 μl of 5% milk in PBS for 1 h. After repeated washes with PBST, plates were incubated with the dilutions of the different sera or IgG. The binding of antibodies was detected by treatment with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (Organon Teknika, Copenhagen, Denmark) and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)]. The color was allowed to develop at room temperature, and the absorbance values were read at 405 nm. To determine the titers of the sera, absorbance values above the GST values were considered, with a cutoff at a reading of 0.10.
Western blotting and solution IFA.
To prepare parasite protein for immunoblotting and merozoites for the immunofluorescence assay (IFA), mice infected with P. yoelii strain 17XL were allowed to develop to about 50 to 60% parasitemia. Merozoites were prepared from infected blood which was incubated for 2 h at 37°C in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (2). The liberated merozoites were harvested at 4°C, washed, and resuspended in complete RPMI 1640 medium at a concentration of 107 merozoites/ml. Intracellular parasites were liberated from infected erythrocytes by saponin lysis (16). The total intracellular parasite pellet and the merozoite pellet were solubilized with 10 volumes of solubilization buffer (20 mM Tris-HCl [pH 7.8], 50 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1 mM iodoacetic acid, 0.1% sodium azide) at 4°C for 1 h. This was then spun at 12,000 rpm for 20 min, and the supernatant containing the solubilized protein suspension was used for Western blot analysis. The immunoblots were treated with the preimmune and anti-PfP0C sera after clearing off the anti-GST antibodies by incubation with nitrocellulose filters coated with lysates of E. coli cells expressing GST protein.
For the solution IFA (SIFA), merozoites were double-labeled with rabbit anti-PfP0 antibodies and the monoclonal anti-MSP1 antibody Mab302 (23) for 30 min on ice. All the subsequent steps were also carried out on ice. Anti-PfP0 IgG (1 mg/ml) was diluted 1:50 in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum, while Mab302 culture medium was used directly. Merozoites were washed three times with incomplete RPMI and resuspended at a 1:100 dilution of secondary antibody for 30 min. The secondary antibody solution consisted of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and rhodamine-conjugated goat anti-rabbit IgG (Boehringer, Mannheim, Germany). Merozoites were washed and mounted under glass coverslips in 5 μl of Citifluor mountant medium (Ted Pella Inc., Redding, Calif.) on glass slides. The slides were observed under a Nikon microscope using a ×100 phase-contrast objective.
IgG purification from polyclonal anti-PfP0 rabbit and mice sera.
IgG was prepared from 25 ml of pooled rabbit sera and 20 ml of mouse sera (pooled after different bleeds), using standard protocols (18). After 50% ammonium sulfate precipitation, the IgG was purified through a DEAE-cellulose column. The fractions containing IgG were pooled and concentrated by lyophilization. The IgG was run on SDS-PAGE to test for purity, and ELISAs were performed to test the activity of the IgG against PfP0N and PfP0C proteins.
Passive-transfer experiments.
The challenges were performed with the 17XL variant of P. yoelii. Frozen stock parasites were injected into mice, and parasites from the second round of passage (at about 10 to 15% parasitemia) were used for the experiments. The parasite count used for challenge was determined by enumerating the number of red blood cells on a hemocytometer and by estimating the percent parasitemia by microscopic examination of a thin slide smear stained with Giemsa. For each of the passive-transfer experiments, parasites were introduced on day 0, and parasitemia was monitored daily by preparing slides from tail bleeds.
(i) Experiments I and II.
The first two experiments were performed on sets of six BALB/c mice for each treatment, with the injections of rabbit anti-PfP0N and anti-PfP0C IgG given intraperitoneally. Specific preimmune sera obtained from the same rabbits prior to immunizations with PfP0N and PfP0C were used as the source of preimmune IgG P. yoelii 17XL parasites were used for the challenge through the orbital route. For experiment I, 0.6 mg of IgG per mouse per day was injected on days −1, 0, and 1, followed by 0.5 mg per mouse per day on days 3 and 5, and 104 parasites per mouse were used for challenge. For experiment II, 1 mg of IgG per mouse per day was introduced on days −1, 0, and 1, followed by 0.5 mg per mouse per day on each day from days 2 to 7. In this experiment, 3 × 104 parasites were used for challenge.
(ii) Experiment III.
Experiment III was performed with groups of six Swiss inbred mice, with the intraperitoneal route for IgG and intravenous tail injections for the parasite. IgG purified from anti-PfP0N and anti-PfP0C mouse sera were used in this experiment. One milligram of IgG per mouse per day was injected on days −1, 0, and 1, followed by 0.5 mg of IgG per mouse per day on days 4 and 5, and each mouse was challenged with 6 × 104 parasites of P. yoelii 17XL.
RESULTS
Detection of P. yoelii P0 with anti-PfP0 sera.
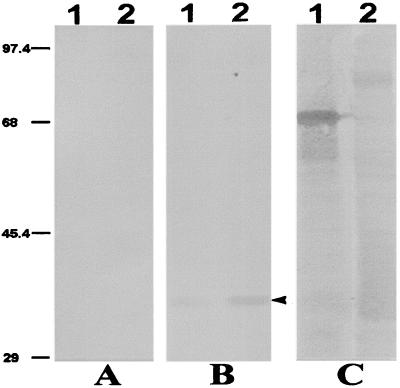
To establish that anti-PfP0 antibodies raised against P. falciparum P0 protein recognize the murine malarial parasite P0, Western blot analysis was carried out. Figure 1 shows the reaction of protein extracted from P. yoelii strain XL17 with rabbit anti-PfP0C antibodies. All eukaryotic P0 proteins are approximately 35 to 38 kDa in size (15). A 35-kDa band was observed with merozoites as well as asynchronous asexual-stage parasite extracts of P. yoelii, while no reaction was observed with the preimmune sera. The cross-reactivity was faint but distinct, with no other protein bands reacting. This demonstrated the absence of any other cross-reactive proteins of P. yoelii. The amount of P0 protein is higher in the intraerythrocytic stages than in merozoites. P0 plays a role in protein synthesis, and trophozoites are the stage where maximum protein synthesis occurs. We have demonstrated earlier that the amount of P0 gene transcript is greater during the trophozoite stages (18). Merozoites are known to be a stage when no protein synthesis occurs (9), and this may be a reason why the level of P0 is lower in merozoites. The levels of P0 have recently been shown to be modulated in mammalian cells (7, 21). The monoclonal Mab302 is known to react to the carboxy-terminal domain of the MSP1 protein and has been shown to immunoprecipitate 230-kDa along with 67- and 36-kDa fragments of MSP1 protein (23). It interacts only with the 67-kDa fragment in the merozoite stage on a denaturing gel.
FIG. 1.
Western blot analysis of P. yoelii protein extract. Lane 1, 5 μg of merozoite protein extract; lane 2, 10 μg of total asexual parasite extract. Samples were run on SDS–10% PAGE under reducing conditions. Immunoblotting was performed using preimmune sera (1:50 dilution) (A), rabbit anti-PfP0C sera (1:50 dilution) (B), or anti-MSP1 monoclonal antibody Mab302 (ascites fluid used at 1:100 dilution) (C). The arrowhead shows the 35-kDa P0 protein. Sizes are shown on the left (in kilodaltons).
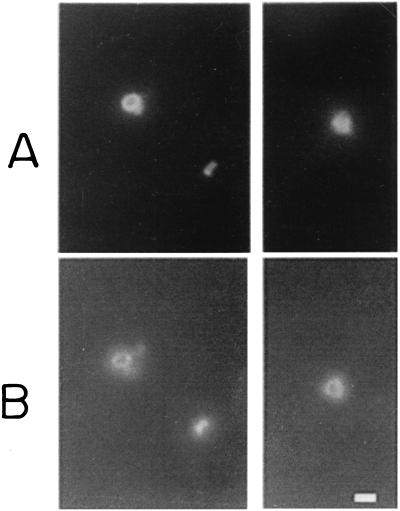
The P0 ribosomal protein is expected to be an internal protein, with a role in ribosomal assembly. However, a surface localization of PfP0 on asexual and sexual stages of P. falciparum has been documented recently (8). To determine whether there may be surface localization of P0 domains on P. yoelii merozoites, SIFA was performed using anti-PfP0 sera. Figure 2 shows double labeling of P. yoelii merozoites using the monoclonal antibody Mab302 and anti-PfP0N antibodies. Anti-PfP0N antibodies and anti-MSP1 antibodies gave identical staining patterns, with complete colocalization on every merozoite. Anti-PfP0C antibodies also showed the same results (data not shown). These results show that the antibodies against P. falciparum P0 protein do recognize the 35-kDa P. yoelii P0 protein and that anti-PfP0 antibodies stain the surface of P. yoelii merozoites.
FIG. 2.
IFA analysis of P. yoelii merozoites using double-staining technique. Merozoites in two different fields are shown in the left and right panels. (A) Mab302 culture supernatant, used directly and visualized with FITC-labeled anti-mouse IgG. (B) Purified rabbit anti-PfP0N IgG solution, 1 mg/ml, used at a 1:50 dilution and visualized with rhodamine-labeled anti-rabbit IgG. Bar, 1 μm.
Passive-transfer experiments.
The titer of purified rabbit anti-PfP0 IgG was determined by ELISA, by observing for specific responses for PfP0N and PfP0C domains above the GST activity. The titers of anti-PfP0N and anti-PfP0C IgG were about 3,200 and >10,000, respectively. Antisera raised in mice against PfP0 domains showed lower titers of 800 and 1,600 for anti-PfP0N and anti-PfP0C proteins, respectively (data not shown).
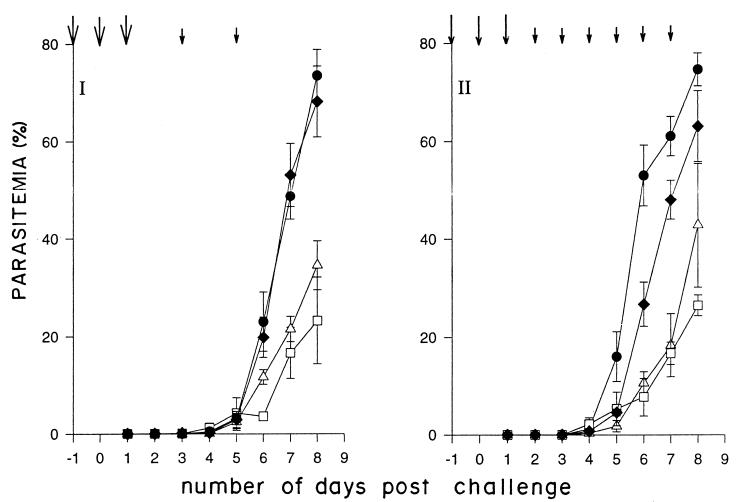
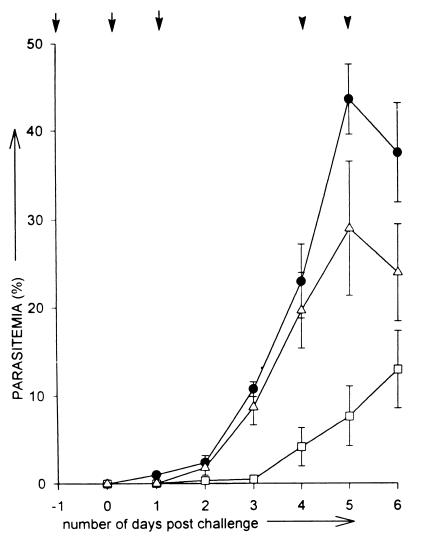
Figure 3 shows the average parasitemia profile in the immunized mice over the first 8 days. With injections on days −1, 0, 1, 3, and 5 and a challenge dose of 104 parasites, a significant reduction in average parasitemia was observed. With extended inoculations with anti-PfP0 IgG (Fig. 3II), it was observed that the average parasitemia could be kept lower for slightly longer periods. The parasitemia was kept low (<20%) in every mouse over the first 7 days, while that in the control mice had reached an average of 50%. On the average, anti-PfP0N was more effective in restricting the parasitemia than anti-PfP0C. In each of these experiments, mice injected with IgG prepared from preimmune sera showed no significant difference in parasitemia compared to control mice (Fig. 3 and 4). Mice immunized with IgG prepared from rabbit anti-GST sera also showed no difference in parasitemia compared to mice receiving no IgG (data not shown). It has been documented earlier that administration of anti-GST IgG has no effect on the growth of the malarial parasite either in vitro or in vivo (16, 23).
FIG. 3.
Average parasitemia as a function of time. Experiments I and II were performed with challenges of 104 and 3 × 104 P. yoelii 17XL parasites, respectively. Mice were not immunized (● [passive control]) or were passively immunized with preimmune IgG (⧫), rabbit anti-PfP0N IgG (□), or rabbit anti-PfP0C IgG (▵). All parasite challenges were made on day 0. The arrows indicate the IgG injection schedule. Large arrows, 0.6 mg/mouse (experiment I) and 1 mg/mouse (experiment II); small arrows, 0.5 mg/mouse (experiments I and II).
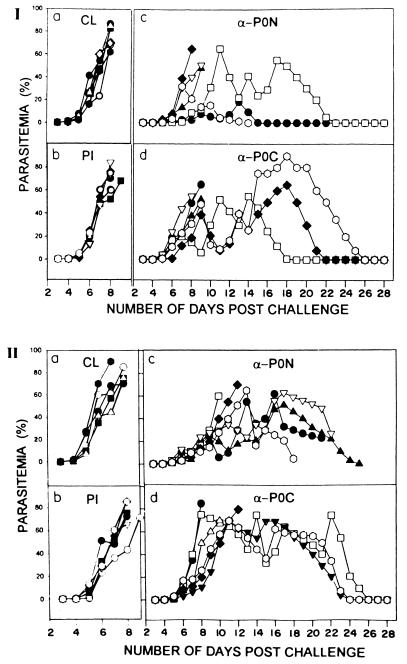
FIG. 4.
Parasitemia profile of each mouse in passive transfer experiments I and II over a period of 28 days. Details of the experiments are given in the legend to Fig. 3. Each symbol represents an individual mouse. Immunization: (a) none (control) (CL); (b) preimmune IgG (PI); (c) rabbit anti-PfP0N IgG (α-P0N); (d) rabbit anti-PfP0C IgG (α-P0C).
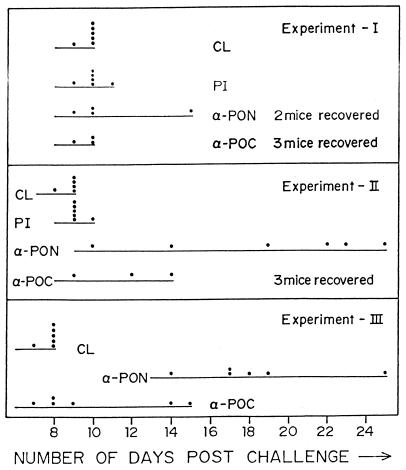
Figure 4 shows the parasitemia profile of each mouse over a period of 28 days for experiments I and II. All the control mice (with parasites but no IgG injections) died by day 10, and the profile was very similar for mice injected with preimmune IgG (Fig. 5). With anti-PfP0 IgG, however, about 50% of the mice cleared their parasites subsequently. In experiment I, three of six mice, and in experiment II, two of six mice injected with anti-PfP0N IgG controlled the parasitemia to <2%, though only two of these recovered completely (Fig. 5). Of the six mice injected with anti-PfP0C IgG, three in each set cleared the parasitemia and recovered completely (Fig. 5). The parasitemia varied in different mice but generally was more controlled in mice treated with anti-PfP0N IgG (Fig. 4). In experiment I, the highest average parasitemia was observed to be 28% on day 11 for mice injected with anti-PfP0N IgG, while the highest average parasitemia was 53% on day 15 for anti-PfP0C. In experiment II, these values were 48% on day 16 and 68% on day 12, respectively. In experiment II, where additional doses of IgG were injected, the initial parasitemia was better controlled in every mouse (Fig. 4), and the survival period for each mouse receiving anti-PfP0 IgG was increased (Fig. 5).
FIG. 5.
Survival period of mice subjected to passive-transfer experiments. Each dot represents the time point of the death of a mouse in that set. The number of mice that recovered completely is shown next to the corresponding treatment. See the legend to Fig. 3 for details.
A similar profile was obtained for passive-transfer experiments (III) performed on mice with IgG prepared from mouse sera (Fig. 6). As observed with IgG prepared from rabbit sera, anti-PfP0N showed a greater reduction in average parasitemia than anti-PfP0C IgG (Fig. 3 and 6). Virtually all the mice injected with anti-PfP0N IgG kept the parasitemia in check (<10% parasitemia) until day 5, while control mice had reached >40% parasitemia (Fig. 6). All the control mice died by day 8, but mice injected with anti-PfP0N IgG survived for 14 to 25 days (Fig. 5). The effect of anti-PfP0C IgG was weaker than that of anti-PfP0N, but it did help in lengthening the survival period in some of the mice (Fig. 5). The weaker effects of anti-PfP0 IgG from mouse sera may be due to the lower titers of mouse anti-PfP0 IgG.
FIG. 6.
Average parasitemia as a function of time. No immunization (●) or passive immunization with mouse anti-PfP0N IgG (□) or mouse anti-PfP0C (▵). IgG. A total of 6 × 104 P. yoelii 17XL parasites were used for challenge on day 0. The arrows indicate the IgG injection schedule: vertical arrow, 1 mg/mouse; arrowhead, 0.5 mg/mouse.
DISCUSSION
We have recently reported the characterization of the amino-terminal domain of the P. falciparum P0 ribosomal phosphoprotein PfP0N (16), which was isolated in a differential immunoscreen using malaria-immune and patient sera (22). Subsequent analysis showed that the PfP0C fusion protein also reacted extensively with the immune serum samples and that IgG from sera raised against both these domains inhibited the growth of P. falciparum in vitro in a concentration-dependent manner (8). The in vitro inhibition data showed that the block occurs at the red cell invasion step (16). This indicated merozoites as the target for anti-PfP0 IgG. The surface reactivity of both anti-PfP0N and anti-PfP0C antibodies on P. falciparum merozoites, gametocytes, and gametes has been documented recently (8). Neither the P0 gene nor the P0 protein has yet been identified for the rodent malarial parasite P. yoelii. In this paper, we have shown that anti-PfP0 antibodies raised against P. falciparum P0 protein cross-react with the P. yoelii P0 protein and stain the surface of P. yoelii merozoites. The P0 protein has been shown to play diverse roles (14, 35, 36), and it is likely that P0 plays a role in such diverse functions through interactions with other protein complexes. In binding to ribosomes, P0 has been demonstrated to participate as a complex in the membrane fractions of the endoplasmic reticulum (30). The surface localization of P0 protein, which does not appear to have consensus signal or transmembrane stretches, may occur through interactions with other membrane proteins. In this paper we also show that mice which received IgG purified from sera raised against PfP0N and PfP0C polypeptides could be protected against a challenge with the lethal strain 17XL of P. yoelii. The control on the initial level of parasitemia and the survival period of the mice were proportional to the titer and frequency of anti-PfP0 IgG administered. These observations indicate an important function(s) played by conserved P0 protein domains on the surface of the parasites, which presumably constitute the target for anti-PfP0 IgG.
The in vivo protection of mice through the administration of anti-PfP0 IgG can perhaps be divided into two main steps, the initial delay in the rise of parasitemia followed by the ability of the mice to resolve the infection. This is analogous to the resolution of infections in mice injected with nonlethal strains of P. yoelii. With a challenge of 106 parasites, mice infected with the nonlethal 17X strain of P. yoelii can resolve the infection in about 16 to 26 days, depending on the inbred strain of mice (20, 23). The parasitemia in such mice remains <10% for about 8 to 12 days before rising further. However, even with a challenge of 104 parasites of the lethal strain 17XL, the parasitemia rises rapidly to levels >10% within 5 days of challenge and continues to rise exponentially, resulting in the death of mice within 10 days (23; this study). Passive immunization with anti-PfP0 antibodies restrains the initial level of parasitemia in the mice and appears to change the course of infection from lethal to nonlethal. The ability of mice to finally resolve the infection and recover completely depends on a large number of genetic properties (17) as well as the individual constitutions of the mice. Several passive immunizations have been performed using anti-PfP0 IgG, and it was consistently observed that anti-PfP0N IgG was more effective in restraining the initial parasitemia. However, more mice recovered completely, despite prolonged high parasitemia, among the mice injected with anti-PfP0C IgG. The implications of this difference in the effects of anti-PfP0N and anti-PfP0C are not clear, and the use of clonal reagents against different domains of PfP0 may help in the resolution of this observation.
Most of the Plasmodium peptide domains belonging to the erythrocytic stages, which are currently considered protective, confer protection mainly upon challenge with homologous strains but have no effect on heterologous strains or species (11, 23, 28). This is the first report of a cross-species passive protection using antibodies raised against P. falciparum protein protecting against challenges with P. yoelii. Passive immunizations have also been carried out using anti-PfP0 IgG against Plasmodium berghei parasite challenge, and a considerable increase in the survival period of the immunized mice was observed, once again indicating cross-species protection (Chatterjee et al., unpublished results).
Active vaccination studies with mice immunized with PfP0 domains yield results similar to passive immunizations (Sohoni et al., unpublished results). Significant reductions in the initial levels of parasitemia were recorded, and there was a considerable lengthening of the survival period, but eventually all the mice succumbed to the infection and died. P0 is a conserved protein, and autoantibodies against the conserved carboxy-terminal domain of the human P proteins have been detected in 10 to 15% of patients with the autoimmune disorder systemic lupus erythematosus (SLE) (13). It was therefore envisaged that vaccination attempts with large PfP0 constructs may have the risk of generating activated B cells that may produce host cross-reactive anti-P0 antibodies, whose role in lupus disease progression and pathology is controversial (24, 32, 33). For vaccination studies, smaller stretches of P0 protein need to be identified with no conformational homology to host P0 protein, to rule out possible long-term adverse effects. Passive immunizations, on the other hand, have been performed earlier on malaria patients with preparations of IgG obtained from immune adults, and the recipients have shown no ill effects from such treatments (10, 12, 25, 29). The sera of the eight mice that recovered completely in our passive-transfer experiments were tested for SLE parameters, such as reactivity with anti-double-stranded or anti-single-stranded DNA and antinuclear antibodies. No positive reactions were observed (data not shown), indicating a lack of SLE-like symptoms.
It has been reported earlier that IgG from malaria-immune adults, which conferred protection in malaria patients, did not inhibit parasite growth on their own and did so only in the presence of monocytes (4). However, the anti-PfP0 IgG was found to inhibit the growth of P. falciparum independent of monocytes (16). In studies using the merozoite surface protein 1 (MSP-1) of Plasmodium, it has been demonstrated that protection can be correlated with antigen-specific antibody titer and not with CD4+ T cells (19). Passive transfer of P. yoelii hyperimmune sera resulted in resistance to a lethal challenge of the parasite even in FcR gamma chain-deficient mice (27), showing that protection is directly mediated by antibodies and does not require the participation of Fc receptors. Thus, there is evidence that IgG on its own may play important roles in blocking crucial events in parasite biology. A comparison between in vitro and in vivo protection of neutralizing antibodies in infection with vesicular stomatitis virus has been performed (1). It was shown that in vitro the neutralizing activity correlated with the measure of on rate, but in vivo the only determinants for controlling the disease were a minimum avidity threshold and serum concentration of the neutralizing antibodies. With these results, we establish the potential for the use of specific anti-PfP0 IgG in therapeutic and prophylactic control of malarial parasites. The therapeutic usage will be beneficial in acute cases of cerebral malaria, especially in regions of drug-resistant P. falciparum. P0 is a crucial protein, as established by the knockout studies performed in S. cerevisiae (31) as well as our in vitro growth inhibition assays in P. falciparum culture (8, 16). It is therefore unlikely to undergo deletion or a significant degree of polymorphism under therapeutic pressure. The mapping of the important inhibitory B-cell epitopes of PfP0 will help in the design of specific anti-PfP0 IgG as well as in elucidation of the diverse functions of this multifunctional protein.
ACKNOWLEDGMENTS
This work was supported by a grant from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
We thank A. D. Ingle, Yang Kang, and Thomas Daly for their help with immunizing the mice. We also acknowledge the contributions of Radhika Nair in the preliminary rounds of passive immunization experiments.
REFERENCES
- 1.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartnee H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 2.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 3.Blackman M J. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. In: Rusell D G, editor. Methods in cell biology: microbes as tools for cell biology. Vol. 45. London, United Kingdom: Academic Press; 1994. p. 213. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druihle P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in co-operation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Druihle P. P. falciparum malaria: evidence for an isotype imbalance which may be responsible for the delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druihle P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockstedt E, Rickers A, Kostka S, Laubersheimer A, Dorken B, Wittmann-Liebold B, Bommert K, Otto A. Identification of apoptosis-associated proteins in a human Burkitt lymphoma cell line. Cleavage of heterogeneous nuclear ribonucleoprotein A1 by caspase 3. J Biol Chem. 1998;273:28057–28064. doi: 10.1074/jbc.273.43.28057. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Singh S, Sohoni R, Kattige V, Deshpande C, Chiplunkar S, Kumar N, Sharma S. Characterization of domains of the phosphoriboprotein P0 of Plasmodium falciparum. Mol Biochem Parasitol. 2000;107:143–154. doi: 10.1016/s0166-6851(99)00226-1. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Butcher G A, Crandall R B. Action of malarial antibody in vitro. Nature. 1969;223:368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Mcgregor I A, Carrington S P. Gammaglobulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 11.Crewther P E, Matthew M L S M, Flegg R H, Anders R F. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. 1996;64:3310–3317. doi: 10.1128/iai.64.8.3310-3317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edozien J C, Gilles H M, Udeozo I O K. Adult and cord-blood gamma-globulin and immunity to malaria in Nigerians. Lancet. 1962;ii:951–955. [Google Scholar]
- 13.Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83:7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frolov M V, Birchler J A. Mutation in P0, a dual function ribosomal protein/apurinic/apyrimidinic endonuclease, modifies gene expression and position effect variegation in Drosophila. Genetics. 1998;150:1487–1495. doi: 10.1093/genetics/150.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goswami A, Chatterjee S, Sharma S. Cloning of a ribosomal phosphoprotein P0 gene homologue from Plasmodium falciparum. Mol Biochem Parasitol. 1996;82:117–120. doi: 10.1016/0166-6851(96)02717-x. [DOI] [PubMed] [Google Scholar]
- 16.Goswami A, Singh S, Redkar V D, Sharma S. Characterization of P0, a ribosomal phosphoprotein of Plasmodium falciparum. J Biol Chem. 1997;272:12138–12143. doi: 10.1074/jbc.272.18.12138. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg J E, Nadel M, Coatney R. Differences in survival of several strains of mice and their hybrids infected with Plasmodium berghei. J Infect Dis. 1954;95:114–116. doi: 10.1093/infdis/95.1.114. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies—a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Hirunpetcharat C, Tian J H, Kaslow D C, van Rooijen N, Kumar S, Berzofsky J A, Miller L H, Good M F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 20.Hoffmann E J, Weidanz W P, Long C A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984;43:981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, Goseki N, Matsubara O, Takenaka K, Shichita M, Tanaka K, Shuda M, Yamamoto M. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- 22.Lobo C A, Kar S K, Ravindran B, Kabilan L, Sharma S. Novel proteins of Plasmodium falciparum identified by differential immunoscreening using immune and patient sera. Infect Immun. 1994;62:651–656. doi: 10.1128/iai.62.2.651-656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majarian W R, Daly T M, Weidanz W P, Long C A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984;132:3131–3137. [PubMed] [Google Scholar]
- 24.Martin A L, Reichlin M. Fluctuations of antibody to ribosomal P proteins correlate with appearance and remission of nephritis in SLE. Lupus. 1996;5:22–29. doi: 10.1177/096120339600500106. [DOI] [PubMed] [Google Scholar]
- 25.McGregor I A, Carrington S P, Cohen S. Treatment of East African P. falciparum malaria with West African human gamma-globulin. Trans R Soc Trop Med Hyg. 1963;57:170–175. [Google Scholar]
- 26.Rich B E, Steitz J A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987;7:4065–74. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotman H L, Daly T M, Clynes R, Long C A. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. Immunology. 1998;161:1908–1912. [PubMed] [Google Scholar]
- 28.Rotman H L, Daly T M, Long C A. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp Parasitol. 1999;91:78–85. doi: 10.1006/expr.1999.4357. [DOI] [PubMed] [Google Scholar]
- 29.Sabchareon A, Burnouf T, Quattara D, Attanath T, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druihle P. Parasitological and clinical response to immunoglobulin administration in P. falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 30.Saenz-Robles M T, Remacha M, Vilella M D, Zinker S, Ballesta J P G. The acidic ribosomal proteins as regulators of the eukaryotic ribosomal activity. Biochim Biophys Acta. 1990;1050:51–55. doi: 10.1016/0167-4781(90)90140-w. [DOI] [PubMed] [Google Scholar]
- 31.Santos C, Ballesta J P G. Ribosomal protein P0, contrary to phosphoproteins P1 and P2, is required for ribosome activity and Saccharomyces cerevisiae viability. J Biol Chem. 1994;269:15689–15696. [PubMed] [Google Scholar]
- 32.Schneebaum A B, Singleton J D, West S G, Blodgett J K, Alley L G, Cheronis J C, Kotzin B L. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90:54–62. doi: 10.1016/0002-9343(91)90506-s. [DOI] [PubMed] [Google Scholar]
- 33.Teh L S, Bedwell A E, Isenberg D A, Gordon C, Emery P, Charles P J, Harper M, Amos N, Williams B D. Antibodies to protein P in systemic lupus erythematosus. Ann Rheum Dis. 1992;51:489–494. doi: 10.1136/ard.51.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiumi T, Wahha A J, Traut R R. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by cross linking. Proc Natl Acad Sci USA. 1987;84:5580–5584. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchiumi T, Kominami R. Direct evidence for interaction of the conserved GTPase domain within 28S RNA with mammalian ribosomal acidic phosphoproteins and L12. J Biol Chem. 1992;267:19179–19185. [PubMed] [Google Scholar]
- 36.Yacoub A, Kelley M R, Deutsch W A. Drosophila ribosomal protein P0 contains apurinic/apyrimidinic endonuclease activity. Nucleic Acids Res. 1996;24:4298–4303. doi: 10.1093/nar/24.21.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]