Abstract
Type 2 diabetes (T2D) is a global health pandemic with significant humanitarian, economic, and societal implications, particularly for youth and young adults who are experiencing an exponential rise in incident disease. Youth-onset T2D has a more aggressive phenotype than adult-onset T2D and this translates to important differences in rates of progression of diabetic kidney disease (DKD). We hypothesize that youth-onset DKD due to T2D may exhibit morphometric, metabolic, and molecular characteristics that are distinct from adult-onset T2D and develop secondary to inherent differences in renal energy expenditure and substrate metabolism, resulting in a central metabolic imbalance. Kidney structural changes that are evident at the onset of puberty also serve to exacerbate the organ’s baseline high rates of energy expenditure. Additionally, the physiologic state of insulin resistance seen during puberty increases the risk for kidney disease and is exacerbated by both concurrent diabetes and obesity. A metabolic mismatch in renal energetics may represent a novel target for pharmacologic intervention, both for prevention and treatment of DKD. Further investigation into the underlying molecular mechanisms resulting in DKD in youth-onset T2D using metabolomics and RNA sequencing of kidney tissue obtained at biopsy is necessary to expand our understanding of early DKD and potential targets for therapeutic intervention. Furthermore, large scale clinical trials evaluating the duration of kidney protective effects of pharmacologic interventions that target a metabolic mismatch in kidney energy expenditure are needed to help mitigate the risk of DKD in youth-onset T2D.
Keywords: Youth, type 2 diabetes, diabetic kidney disease, albuminuria
Introduction:
Type 2 diabetes (T2D) is a growing health pandemic that is closely tied to multiple predisposing factors including central obesity, glucose intolerance, hypertension, and dyslipidemia, with a significant emphasis on insulin resistance (IR) as an underlying etiology for pathogenesis 1. The National Health and Nutrition Examination Survey (NHANES), a large cross-sectional survey of youth and adults in the United States, has demonstrated persistent rising rates of obesity in youth aged 12-19 years (16.0% in 1999-2002 to 20.9% in 2015-2018, p<0.001) 2, in conjunction with rising rates in adults >20 years of age (33.7% in 2007-2008 to 39.6% in 2015-2016, p<0.001) 3. In parallel with this drastic change in weight over time, adolescents in the United States are increasingly diagnosed with T2D and from 2002-2003 to 2014-2015 have demonstrated an annual increase of incident T2D of 4.8% 4. Race/ethnicity is also a significant risk factor for T2D diagnosis as the incidence of T2D among non-Hispanic Whites (0.6%) is lower than Hispanics (3.1%), non-Hispanic Blacks (6.3%), Asian or Pacific Islanders (8.5%), and Native Americans (8.9%) (p<0.05 for all comparisons) 5.
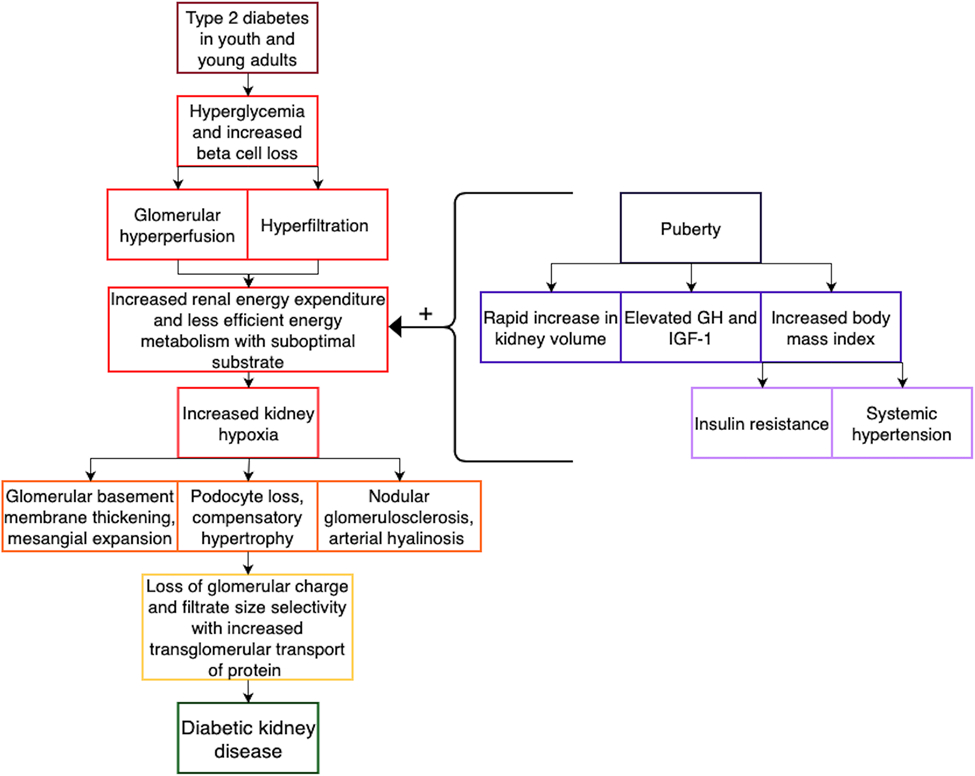
Youth-onset T2D displays a more extreme phenotype when compared to adult-onset T2D, including significantly greater IR and subsequent β-cell destruction 6,7, thus resulting in higher rates of dysglycemia and an increased risk for micro- and macro-vascular complications including retinopathy, neuropathy, cardiovascular disease, and diabetic kidney disease (DKD) 7-11. Differences in the age of presentation and ensuing characteristics of incident DKD are of particular interest as DKD remains a leading cause of morbidity and mortality in both youth and adults with T2D 12. Indeed, based on data from the UK Prospective Diabetes Study (UKPDS) Cohort, adolescents who develop T2D will have a life expectancy that is 15 years shorter than adolescents who do not develop T2D 13. We present a comprehensive review of the morphologic, metabolic, and molecular characteristics of DKD in both youth and adults with T2D, with a focus on further examination of potential underlying mechanisms for these age of onset-based differences (Figure 1).
Figure 1: Proposed Kidney Structural and Functional Changes Underlying the Development of Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes.
Abbreviations: GH – growth hormone; IGF-1 – insulin-like growth factor-1.
Epidemiology of DKD:
Diabetes affects 536.6 million adults (10.5%) aged 20-79 years globally 14 and represents a worldwide health burden that is only worsening in severity. Global diabetes-related costs were estimated to be 966 billion USD in 2021 and projections estimate an increase to 1,054 billion USD by 2045 14. DKD-associated complications contribute largely to the morbidity and mortality attributed to diabetes and the health-related costs. In 2019, there were 2.62 million incident cases of chronic kidney disease secondary to diabetes worldwide with a prevalence of 134.58 million affected individuals who experienced 13.09 million disability-adjusted life years and 405.99 thousand deaths 15. The societal impact of DKD continues to rise exponentially and presents an economic and humanistic burden to both youth and adults with diabetes, particularly as the average age of diabetes onset gradually declines. In the Treatment Options for Type 2 Diabetes in Adolescents and Youth Follow-Up Study (TODAY2), the 15-year cumulative incidence of DKD was >50% 12,16-19, a sobering statistic as DKD represents a severe and chronic health issue that has the potential to adversely impact an individual’s ability to live a productive and fulfilling life. Notably, DKD appears to differentially affect racial and ethnic minority populations and individuals from low to middle-income countries 15.
Prevalence of DKD in T1D vs. T2D:
DKD is consistently more aggressive in youth-onset T2D than T1D 20-24. However, whether this is secondary to an inherent difference in the progression of disease or other predisposing factors such as concurrent metabolic and/or vascular complications, a longer duration of diabetes, or a delay in diagnosis resulting in a prolonged period of hyperglycemia before diagnosis of T2D is unknown. The SEARCH for Diabetes in Youth study (SEARCH), a large, multi-center study involving youth diagnosed with either T1D (n=1,746) or T2D (n=272) before the age of 20 years and with a mean diabetes duration of 7.9 years, has demonstrated a significantly higher prevalence of DKD, as defined by either an estimated glomerular filtration rate ≤60 mL/min/1.73 m2 or a first morning urine albumin-to-creatinine ratio ≥30 mg/g, in youth with T2D (20%) than youth with T1D (6%) 8. While youth with T2D exhibited higher rates of other vascular complications including retinopathy, peripheral neuropathy, hypertension, and arterial stiffness in addition to DKD, when compared to youth with T1D, the odds of developing DKD in individuals with T2D were almost triple that seen in people with T1D, even after multivariable adjustment for age, sex, duration of time from diabetes diagnosis to onset of diabetes-related complication, clinical site, and body mass index 8. These findings suggest an inherent difference in the underlying mechanisms of DKD between T2D and T1D and warrants further study to help tailor future treatment strategies to mitigate the progression of chronic kidney disease in T2D.
High Risk Populations for the Development of DKD:
In large, multicenter studies throughout the United States, race/ethnicity plays a consistent role in the development and progression of DKD due to T2D 5,25. Populations at particular risk for youth-onset T2D include racial and ethnic minority groups such as non-Hispanic Blacks, Hispanics, and Indigenous populations 5. We have demonstrated that youth-onset T2D, as defined by absent/low T1D autoantibodies, absent maturity-onset diabetes of youth (MODY) loci, persistent insulin secretion, and lack of insulin dependence, continues to increase in prevalence in the Pima Indian population 26. Contributing factors include rising rates of youth-onset obesity resulting in significantly elevated rates of IR as well as a higher incidence of intrauterine exposure to diabetes 27. Gestational diabetes is often associated with a low birth weight which may result in a lower total number of nephrons 28. Additionally, the hyperglycemic milieu may also negatively affect the development of nephrons in the fetus 29. Each of these factors is likely associated with a younger average age of diagnosis of T2D and thus an earlier age of DKD development. Furthermore, Pima Indians with confirmed youth-onset T2D demonstrated a higher incidence of morbidity with a 5-fold increased risk of kidney failure secondary to diabetes in people 25-54 years of age, as well as increased mortality when compared to individuals with adult-onset T2D 30. While the Pima Indian population represents a particularly aggressive phenotype of youth-onset T2D, global rates of youth-onset T2D are rising 5 in parallel with consequent DKD.
Hemodynamics and Energetics of DKD:
T2D-associated alterations in kidney hemodynamic function are closely tied to rising rates of DKD and occur secondary to a multitude of metabolic factors that result in inflammatory changes, fibrosis, and hypertrophy of the kidneys 31,32. These factors include hyperglycemia, glomerular hyperperfusion, and hyperfiltration, complications that are further compounded by obesity, IR, and systemic hypertension 32,33.
In the kidneys, the complex physiologic combination of hematologic perfusion, filtration, and reabsorption results in a high metabolic rate and increased energy requirement 34. Diabetes then leads to increased glucose reabsorption from the proximal tubule via the sodium-glucose cotransporter 2 and decreased distal solute delivery, thereby resulting in decreased tubuloglomerular feedback and dilation of the afferent arteriole with increased glomerular perfusion 32,35. Elevated production of angiotensin II from the efferent arteriole also results in vasoconstriction and the combination of increased glomerular perfusion and efferent arteriolar vasoconstriction produces elevated intraglomerular pressure and glomerular hyperfiltration 32,35. Each of these diabetes-related complications consumes a greater amount of O2 and thereby increases the organ’s baseline energy requirement 36. When the kidneys are unable to compensate fully for the increased O2 consumption secondary to the negative effects of diabetes, IR, and hyperglycemic cellular toxicity, a progressive state of hypoxia and ischemic kidney injury ensues 37,38. IR is strongly correlated with hyperfiltration in youth with T2D in both the TODAY and Resistance to Insulin in Type 1 and Type 2 Diabetes (RESISTANT) cohorts 12,18, providing support for the more aggressive impact of T2D on DKD in youth than adults. Hyperfiltration predisposes the nephron to progressive damage by increasing glomerular pressure and leads to an increase in transcapillary convective flux of ultrafiltrate including the macromolecule albumin 39. Further kidney function decline is accelerated in remnant nephrons that are compensating for a reduction in total nephron mass through hyperfiltration which leads to increased urine albumin excretion in youth with T2D 40,41.
In the UKPDS Cohort, a randomized, non-blinded study of 5,102 adults aged 25-65 years with new onset T2D which investigated intensive blood glucose and blood pressure management on the progression of vascular complications in T2D from 1977 to 1997, the prevalence of microalbuminuria rose from 7.3% at diabetes diagnosis to 17.3% at 5 years, 24.9% at 10 years, and 28.0% at 15 years post-diagnosis 42. Microalbuminuria was found to transition to macroalbuminuria at a rate of 2.8% per year and then to kidney failure at a rate of 2.3% per year, with a loss of GFR occurring more rapidly in the setting of greater urine albumin excretion 42. Notably, both the Restoring Insulin Secretion (RISE) consortium, a study evaluating approaches to maintaining insulin secretion in children and adults with either prediabetes or newly-diagnosed T2D, and Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study demonstrated a more aggressive phenotypic pattern of dysglycemia and loss of β-cell function in youth vs. adult-onset T2D 6,7. This also translated to important differences in kidney physiology in youth and adult-onset T2D. Indeed, in the TODAY cohort of individuals with youth-onset T2D, the prevalence of elevations in urine albumin excretion increased from 6.3% at baseline to 16.6% at 3.9 years follow up and 18% at 5 years follow up 12,43. Rates of progression of DKD in youth-onset T2D, as evidenced by a higher cumulative prevalence of microalbuminuria, were significantly higher than those seen in both adult-onset T2D and adult-onset T1D 44.
Of note, adults with T2D also experience a higher rate of regression of microalbuminuria to normalbuminuria than similar populations of youth with T2D. Araki et al. demonstrated a 51% rate of remission and a 54% rate of regression of elevated urine albumin excretion over a 6 year follow up period in a population of 216 Japanese adults with type 2 diabetes and microalbuminuria 45. For comparison, Kyung Son et al. demonstrated a 37.5% rate of regression and a 20% rate of progression of microalbuminuria in a small pilot study of 18 adolescents with T2D 46. In the adults, multiple important factors were associated with increased rates of remission of microalbuminuria including shorter duration of microalbuminuria, use of renin-angiotensin aldosterone system blockade, lower hemoglobin A1c, and lower systolic blood pressure 45, elements that all warrant future consideration for optimization in youth and adults with T2D to help mitigate DKD progression.
Hemodynamics and Energetics of DKD in T1D vs. T2D:
DKD morphology is strongly impacted by both type of diabetes exposure and age of diabetes onset. T2D-associated pathological changes in kidney structure have demonstrated similarities to T1D, likely secondary to overlapping features including hyperglycemia exposure, IR, and duration of diabetes. Yet, the presentation of DKD in T2D displays a significantly higher degree of variability, potentially due to inherent differences in underlying diabetes pathology 47. In the Oxford Regional Prospective Study, a natural history study following youth with youth-onset T1D starting within 3 months of diabetes diagnosis, the cumulative prevalence of microalbuminuria in youth-onset T1D was 50.7% (95% CI: 40.5-60.9%) after 9.8 ± 3.8 years of diabetes duration which was significantly higher than a similar cohort of individuals with adult-onset T1D (33.6% [95% CI: 27.2-40.9%]) after 18 years of follow-up and similar glycemic exposure 44. SEARCH subsequently compared outcomes in youth with T1D vs. youth with T2D and individuals with youth-onset T2D demonstrated 3-fold odds of developing DKD vs. youth with youth-onset T1D 8. In the TODAY study, a prospective and longitudinal study of >500 youth with T2D of <2 years duration evaluating the time to treatment failure with either metformin, metformin plus rosiglitazone, or metformin plus lifestyle modification, DKD was demonstrated to have a much more aggressive and early-onset phenotype in youth with T2D, with >50% of study participants developing elevated urine albumin excretion by 15 years post-T2D diagnosis 16. Additionally, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, a large study of 10,251 adults aged 40-79 years with T2D and risk factors for cardiovascular disease, declines in estimated GFR were most rapid in both the youngest group of T2D-onset and the group with the longest duration of T2D 48. In individuals with similar diabetes duration, those diagnosed with T2D at <40 years of age demonstrated the fastest declines in estimated GFR of all groups 48. These data suggest that T2D age at onset and diabetes duration help determine future risk for DKD in people with T2D.
Structural lesions:
Pathological lesions in T2D-associated DKD are complex and warrant continued study with state-of-the-art kidney biopsy morphometrics to help expand our knowledge of their structural and functional associations. Known lesions in T2D include thickening of the glomerular basement membrane, mesangial expansion due to cellular enlargement and matrix secretion, podocyte loss with hypertrophy of the residual podocytes, nodular glomerulosclerosis, arterial hyalinosis, tubular epithelial atrophy, capillary rarefaction, and accumulation of inflammatory cells, atypical collagen, and activated myofibroblasts 49. While kidney structural lesions in T2D share many common features with lesions seen in T1D, they are significantly more heterogeneous and are less directly associated with the current clinical picture of T2D 50. The underlying causes of variation in kidney structural lesions in T2D have not been fully elucidated but may be secondary to inherent heterogeneity in diabetes pathology or the effects of comorbid metabolic conditions including systemic hypertension, atherosclerotic changes, and IR.
Arguably the most comprehensive evaluation of structural lesions associated with youth-onset DKD to date has come from studies involving kidney biopsies in the Pima Indian population with T2D. As with other race/ethnicities, the most common structural kidney injuries seen in Pima Indians with youth-onset T2D include glomerular basement membrane thickening and mesangial expansion, lesions that both show extensive associations with declining kidney function 51. Pima Indians demonstrate a unique form of DKD with relatively homogenous structural lesions that are largely present before the onset of clinically evident disease 51-53. These lesions are entirely attributable to T2D and represent a pattern that is not typically seen in other populations with T2D 54,55, making this population a prime target for DKD research. Indeed, Pima Indians with diabetes but normal GFR and no evidence of microalbuminuria have a greater glomerular basement membrane thickness and higher mesangial fraction volume than healthy donors without diabetes 52. Youth-onset T2D in Pima Indians is consistently associated with a more severe profile of kidney structural lesions than adult-onset T2D, and even mild lesions occur in conjunction with a higher median urine albumin to creatinine ratio and hemoglobin A1c 51. Indeed, in a cohort of Pima Indians including 52 individuals aged 39.1±9.9 years with youth-onset T2D and 109 people aged 51.4±10.2 years with adult-onset T2D of similar diabetes duration, mean urine albumin to creatinine ratio was higher in the youth-onset group (58 56 vs. 27 [25th-75th percentile 13-73], p=0.02) 56. Additionally, a multitude of structural lesions including glomerular basement membrane width, mesangial fraction volume, glomerular volume, and percentage of glomerular sclerosis were inversely associated with age at diabetes onset 56. Although Pima Indians represent a single racial and ethnic group, findings present in this population have been consistently demonstrated in other groups as well. Evidence strongly indicates that youth-onset T2D carries significantly greater risk of DKD than adult-onset T2D of a similar duration across populations 12,17,57.
Metabolic and Molecular Mechanisms of DKD:
Underlying causes for the increased severity of structural lesions in DKD associated with youth-onset T2D have not been fully explained; however, metabolic dysregulation has been repeatedly shown to have a long-term effect on programming future risk for DKD development. The developmental phenomenon known as “metabolic programming” plays an important role in promoting future DKD risk 58, as pediatric disease factors including brief periods of poor glycemic control in childhood can significantly increase future risk for DKD despite a prolonged period of excellent glycemic control as an adult 59. Furthermore, when combined with environmental factors, epigenetic mechanisms including DNA methylation, chromatin histone alterations, and non-coding RNAs mediate the persistent and long-term expression of DKD-related genes induced by historical glycemic exposure as a feature of metabolic memory 60. These features contribute to the early onset and aggressive evolution of DKD due to youth-onset T2D and subsequently places these individuals at higher risk of severe disease than people who present later in life with adult-onset disease.
Studies leveraging important advanced techniques including metabolomics and kidney tissue-level RNA sequencing will help us further understand the metabolic and molecular determinants of structural kidney lesions in youth-onset T2D and their worsened phenotype in comparison to adult-onset T2D. In fact, kidney-specific epigenome maps and tissue-specific expression quantitative trait loci maps are in development to help isolate kidney disease genome-wide association study (GWAS) loci to better identify and treat young persons at particularly high risk for DKD progression 61,62. Improvements in our understanding of the molecular and metabolic mechanisms underlying kidney disease may thus lead to the development of novel therapeutic strategies for mitigation of DKD in youth-onset T2D.
Additionally, gestational diabetes has been hypothesized to have an adverse effect on kidney morphogenesis in offspring. In mice models, maternal diabetes is associated with apoptosis of the glomerular podocytes, thus resulting in nephron collapse, dysmorphogenesis, and small kidneys in the offspring 63. Hyperglycemia in utero is also associated with persistent upregulation of angiotensinogen and renin messenger RNA, particularly in the proximal tubule, as well as upregulation of nuclear factor-κB, a key transcription factor upregulated in the setting of stress and generalized inflammation 63. Taken together, these factors place the kidneys of offspring of mothers with a history of gestational diabetes at higher risk for injury in the setting of a future diabetes diagnosis and warrant further mechanistic study.
Puberty may amplify risk of kidney injury in youth with obesity and diabetes:
A nation-wide screening program in Denmark found that urinary albumin excretion increased transiently during puberty in all adolescents, evidence that puberty places stress on the kidneys 64. However, the rise in albumin excretion was higher in those with elevated body mass index (BMI) and persisted after puberty in those with diabetes, arguing that puberty may induce irreversible kidney injury in youth with obesity and diabetes 64-66. Puberty has also been linked with increased biomarkers of oxidative stress and excessive kidney growth by ultrasound in youth with T1D 67-70 A correlation between growth hormone, which is physiologically elevated in puberty, and albuminuria has been observed in adolescents with T1D 71,72. Additionally, epidemiological data from Finland found that the cumulative risk of kidney failure was highest in youth with peripubertal onset of T1D compared to diagnoses of T1D in early childhood or after puberty 66. Finally, we and others have established that youth-onset T2D, which typically manifests during or shortly after puberty, carries significantly greater risk of DKD than T1D and adult-onset T2D of similar disease duration 12,17,73-76. Taken together, these data suggest that developing T2D during puberty amplifies the risk of kidney injury, yet the mechanisms by which reproductive maturation amplifies kidney disease remain elusive.
Puberty is associated with significant changes in physiology that may predispose to kidney hypoxia in individuals with obesity and/or diabetes. The kidneys are highly metabolically active and susceptible to oxidative stress and hypoxia 77,78. The high O2 demand is necessary to maintain adequate adenosine triphosphate (ATP) production for the Na+/K+-ATPase, as 95% of the ATP produced in the kidney is through aerobic metabolism 77,79. During puberty, the kidneys almost double in size (total kidney volume, TKV) 68, which likely increases the kidneys’ already high energy expenditure. Higher concentrations of growth hormone and insulin-like growth factor 1 (IGF-1) during puberty are also thought to exacerbate the tubular workload by increasing glomerular filtration rate (GFR) and the filtered Na+ load 80-83. The elevated GFR may be further magnified in pubertal adolescents with obesity and diabetes 12,17,70,74,84. Additionally, there is evidence that hyperinsulinism associated with obesity and T2D can stimulate Na+ reabsorption 85-92. Renin-angiotensin-aldosterone system (RAAS) and vasopressin, which are activated in diabetes, also stimulate Na+ reabsorption 93-102. In parallel, puberty is associated with IR that impairs substrate metabolism and ATP generation 89,103-105. The pubertal increase in IR is attributed to rises in fat mass, sex steroids, and growth factors 103-107. Although insulin sensitivity naturally decreases during puberty 89-91,108, the decline is accentuated by obesity and diabetes 91,92,108. IR shifts renal fuel utilization towards free fatty acid (FFA) oxidation 109-111, which has a lower ATP yield per O2 consumed compared to other substrates 112-114. Further, IR results in impaired oxidative phosphorylation and ATP synthesis by inhibition of adenosine monophosphate kinase 115,116, increased mechanistic target of rapamycin complex 1 (mTORC1) activity, and mitochondrial dysfunction 117-119. Accordingly, we posit a metabolic imbalance between renal energy expenditure and substrate metabolism during puberty in youth with obesity and/or diabetes. We postulate that the resultant metabolic mismatch predisposes to relative kidney hypoxia. In turn, the relative kidney hypoxia may result in loss of glomerular charge and size selectivity, with increased transglomerular transport of protein and kidney injury. Supporting these hypotheses, we recently published cross-sectional data showing that pubertal adolescents with T1D exhibit relative kidney hypoxia compared to controls without diabetes and that the degree of hypoxia was related to IR and BMI 70. The role puberty plays in the pathogenesis of DKD in youth with T2D, who exhibit a greater degree of IR and hyperfiltration than those with T1D, is unknown.
Therapeutic Strategies for Treatment of DKD:
Pharmacology:
Employing medical strategies to prevent and treat DKD in youth-onset T2D presents a significant challenge to diabetes providers due to a combination of pharmacologic and social factors. Adolescents and young adults exhibit a characteristic pattern of poor adherence to medication administration which is compounded by unique factors inherent to T2D including a high prevalence of diagnosis in racial/ethnic minority groups, a strong genetic influence and high prevalence of family history of diabetes, and low socioeconomic status 120. Additionally, youth with T2D exhibit a particularly suboptimal response to our available medical therapies for DKD 121 when compared to adults with T2D and this fact, in combination with a significantly lower number of U.S. Food and Drug Administration (FDA)-approved pharmacologic therapies for DKD in youth with T2D, results in a less successful treatment profile overall. Current medications for the management of DKD are largely targeted to strict glucose management (i.e., insulin, metformin, and select glucagon like peptide-1 receptor agonists [GLP-1RAs]) and renin-angiotensin-aldosterone system blockade through angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II receptor blockers (ARBs) 122. Sodium-glucose co-transporter 2 inhibitors (SGLT2is), medications which effectively block the sodium-glucose co-transported in the proximal tubule of the nephron from reabsorbing sodium and glucose, and GLP-1 RAs, agents aimed at increased insulin secretion and decreased glucagon secretion, serve as mainstay therapies for the mitigation of DKD, yet all SGLT2is and the majority of GLP-1RAs are not currently FDA approved in pediatrics 123-125.
Achieving approval to expand pharmacologic indications for medications beyond blood glucose or blood pressure control remains challenging in pediatrics for multiple reasons. First, achieving recruitment goals for clinical trials involving youth with T2D is difficult due to the relatively small population of youth who meet criteria for pancreatic autoantibody-negative diabetes, an over-representation of disadvantaged populations with confounding complications including psychiatric conditions requiring antipsychotic medications which may be diabetogenic, and strict requirements for pivotal trials which in some cases required drug-naïve participants 126. Requirement of drug-naïve participants for clinical trials is in direct contradiction to treatment guidelines by the American Academy of Pediatrics which currently recommends immediate treatment with metformin and/or insulin following a T2D diagnosis 127. Poor general adherence with treatment regimens and follow up visits for adolescents and young adults with T2D also compounds the issues surrounding completion of clinical trials in youth with T2D.
Medications targeting renal-specific pathophysiology beyond glycemia and blood pressure control may represent the future of advanced treatment for youth-onset DKD. We assert that a metabolic mismatch in renal oxygen availability and consumption may play a pivotal role in the development of youth-onset DKD, and targeted treatments may mitigate the progression of DKD in these individuals. One potential treatment mechanism to achieve this goal is through activation of mitochondrial-derived peptides, a type of peptide that targets the skeletal muscle which serves as a large disposal site for ingested glucose 128. Mitochondrial-derived peptides induce pentose phosphate pathway glucose uptake, improve insulin resistance, decrease liver gluconeogenesis and generalized inflammation, and promote adenosine monophosphate-activated protein kinase (AMPK) activation and glucose transporter type 4 (GLUT-4) expression in muscle tissue in preclinical trials 128-130, all mechanisms that may independently improve the risk for DKD. Activators of the sirtuin-1 (SIRT1)/AMPK pathway may serve as another potential mechanism for DKD mitigation due to downstream phosphorylation of proteins that positively regulate insulin sensitivity and increase fatty acid oxidation 131, factors that play an important role in kidney disease. Lastly, the mechanistic target of rapamycin pathway is a third potential targetable pathway for the treatment of DKD. Amino acids and growth factor stimulation of mTORC1 stimulates generalized cellular growth and proliferation 132 and activation of this pathway has a pivotal role in both physiologic and pathologic kidney hypertrophy 133, an initial kidney structural change with the development of DKD. Inhibition of this pathway may thus mitigate the risk of DKD progression. In conclusion, targeted therapies against factors contributing to a metabolic mismatch in kidney physiology may serve as optimal treatments for DKD in youth with T2D.
Metabolic Surgery:
One potential mechanism for future treatment of DKD in pediatrics that moves beyond pharmacotherapy and negates the challenges related to large-scale, randomized, placebo-controlled trials is bariatric surgery. Bariatric surgery is used extensively in adult populations for management of weight and refractory T2D, but emerging data demonstrates similar positive benefits in adolescent populations with obesity and T2D including weight reduction, remission of T2D, and improvements in insulin resistance and DKD risk marker profiles 134,135. In the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study, a longitudinal cohort of 22 obese youth and young adults without T2D undergoing laparoscopic Roux-en-Y gastric bypass surgery, a 38% reduction in BMI (61±12.3 kg/m2 to 39±8.0 kg/m2) with a concurrent 300% increase in the insulin sensitivity index and a 2-fold increase in the disposition index (both p<0.01) were demonstrated after a 1 year post-surgery follow up 135. In a subsequent multicenter study involving 5 U.S. weight-loss surgery centers involving 242 obese adolescents, Roux-en-Y gastric bypass surgery was associated with remission of T2D in 95% (95% CI: 85 to 100%) of participants and remission of abnormal kidney function as defined by low GFR or proteinuria in 86% (95% CI: 72 to 100%) of participants at 3 years post-procedure. When compared to medical management strategies employed in the TODAY study, the Teen-LABS cohort demonstrated significant improvements in both glycemia and markers of DKD. Teen-LABS participants exhibited a decrease in mean hemoglobin A1c from 6.8% (95% CI: 6.4-7.3%) to 5.5% (95% CIL 4.7-6.3%) at 2 years post-bariatric surgery follow up, while participants in the TODAY cohort who received pharmacologic management only demonstrated an increase in hemoglobin A1c from 6.4% (95% CI: 6.1-6.7%) to 7.8% (95% CI: 7.2-8.3%) 136. Similarly, albuminuria was present in 17% (39/230) of participants at baseline in the Teen-LABS cohort and this decreased to 11% (19/173) at 3 years post-bariatric surgery follow up (p<0.06) and individuals with albuminuria demonstrated a significant improvement in albumin to creatinine ratio over time (i.e., 74 mg/g [45-121 mg/g] to 17 mg/g [10-28 mg/g], p<0.0001) 137. In contrast, the prevalence of elevated albumin excretion in the TODAY cohort did not change over time 136. Further studies evaluating the effects of bariatric surgery vs. medical therapy on more advanced measures of kidney function including intraglomerular hemodynamic function, hyperfiltration, and renal oxygenation in youth and young adults with T2D are critical to elucidate the underlying treatment mechanisms and thereby optimize future surgical and non-surgical approaches for DKD management in youth with T2D.
Conclusion:
Current projections estimate that 50-70% of people with youth-onset T2D will develop DKD during adolescence and young adulthood. Data summarized in this review document that despite the high prevalence and severity of DKD in youth-onset T2D, the underlying mechanisms are poorly understood, and effective treatment options are lacking. Based on preliminary findings appraised in this review, we posit that youth-onset T2D may exhibit a distinct morphometric and molecular phenotype of DKD that stems from a metabolic imbalance between renal energy expenditure and substrate metabolism. We also contend that developing T2D during puberty amplifies the risk of kidney injury, as the kidneys almost double in size during sexual maturation, likely increasing the kidneys’ already high energy expenditure. In parallel, puberty is associated with physiologic IR, which is accentuated in obesity and diabetes.
If perturbed renal energetics plays an important role in the pathogenesis of DKD in youth-onset T2D, determinants of the metabolic mismatch could serve as promising therapeutic targets to mitigate their high burden of DKD (e.g., mitochondrial peptides and activators of AMPK and SIRT1, and mTORC1 inhibitors) 116,118,138,139. Additionally, increased uptake of bariatric surgery in the adolescent population with obesity and T2D may also result in significant improvements in weight and IR, remission of T2D, and reduction in DKD risk profiles. Even temporary efforts to correct the metabolic imbalance may translate to durable protection against DKD.
Acknowledgements:
K.L.T. receives salary and research support from the NIH/NHLBI (K23 HL159292), Children’s Hospital Colorado Research Institute Research Scholar Award, NIH/NIDDK (P30 DK116073), Ludeman Family Center for Women’s Health Research at the University of Colorado, ISPAD-JDRF Research Fellowship, and the Department of Pediatrics, Section of Endocrinology at the University of Colorado School of Medicine. J.K. receives salary and research support from NIDDK (R01 DK130255) and NIA (R21 AG068657). R.G.N. is supported by the Intramural Research Program of the NIDDK. P.B. receives salary and research support from NIDDK (R01 DK129211, R21 DK129720, K23 DK116720, UC DK114886, and P30 DK116073), JDRF (2-SRA-2019-845-S-B, 3-SRA-2017-424-M-B, 3-SRA-2022-1097-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine.
Footnotes
Disclosures:
K.L.T., J.K., and R.G.N. have no relationships relevant to the contents of this paper to disclose. P.B. has reported acting as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli-Lilly, Sanofi, Novo Nordisk and Horizon Pharma. P.B. has reported serving on the advisory boards of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX.
References
- 1.Shin JA, Lee JH, Lim SY, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. Jul 2013;4(4):334–43. doi: 10.1111/jdi.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Fryar CD, Martin CB, et al. Trends in Obesity Prevalence by Race and Hispanic Origin-1999-2000 to 2017-2018. JAMA. Sep 22 2020;324(12):1208–1210. doi: 10.1001/jama.2020.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA. Apr 24 2018;319(16):1723–1725. doi: 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao G, Jensen ET. Type 2 Diabetes in Youth. Glob Pediatr Health. 2020;7:2333794X20981343. doi: 10.1177/2333794X20981343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. Apr 13 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consortium R, Investigators RC. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes. Jun 9 2019;doi: 10.2337/db19-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consortium R. Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care. Aug 2018;41(8):1717–1725. doi: 10.2337/dc18-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. Feb 28 2017;317(8):825–835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Randomized Controlled Trial Research Support, N.I.H., Extramural. Diabetes Care. Jun 2013;36(6):1735–41. doi: 10.2337/dc12-2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care. May 2016;39(5):823–9. doi: 10.2337/dc15-0991 [DOI] [PubMed] [Google Scholar]
- 11.Consortium R. Lack of Durable Improvements in beta-Cell Function Following Withdrawal of Pharmacological Interventions in Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care. Sep 2019;42(9):1742–1751. doi: 10.2337/dc19-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornstad P, Nehus E, El Ghormli L, et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis. Jan 2018;71(1):65–74. doi: 10.1053/j.ajkd.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med. Apr 2012;29(4):453–63. doi: 10.1111/j.1464-5491.2011.03542.x [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. Jan 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Y, Li N, Wu Y, et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front Endocrinol (Lausanne). 2021;12:672350. doi: 10.3389/fendo.2021.672350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Caprio S, Goland R, et al. Incidence of Complications and Comorbidities in Young People with Type 2 Diabetes. N Engl J Med. 2021;In-press [Google Scholar]
- 17.Bjornstad P, Laffel L, Lynch J, et al. Elevated Serum Uric Acid Is Associated With Greater Risk for Hypertension and Diabetic Kidney Diseases in Obese Adolescents With Type 2 Diabetes: An Observational Analysis From the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care. Jun 2019;42(6):1120–1128. doi: 10.2337/dc18-2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjornstad P, Maahs DM, Cherney DZ, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care. Nov 2014;37(11):3033–9. doi: 10.2337/dc14-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seegmiller JC, Wolf BJ, Albtoush N, et al. Tubular Secretion Markers, Glomerular Filtration Rate, Effective Renal Plasma Flow, and Filtration Fraction in Healthy Adolescents. Kidney Medicine. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama H, Okudaira M, Otani T, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney international. Jul 2000;58(1):302–11. doi: 10.1046/j.1523-1755.2000.00166.x [DOI] [PubMed] [Google Scholar]
- 21.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. Jun 2006;29(6):1300–6. doi: 10.2337/dc05-2470 [DOI] [PubMed] [Google Scholar]
- 22.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. Dec 2013;36(12):3863–9. doi: 10.2337/dc12-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes care. Feb 2014;37(2):436–43. doi: 10.2337/dc13-0954 [DOI] [PubMed] [Google Scholar]
- 24.Amutha A, Anjana RM, Venkatesan U, et al. Incidence of complications in young-onset diabetes: Comparing type 2 with type 1 (the young diab study). Diabetes Res Clin Pract. Jan 2017;123:1–8. doi: 10.1016/j.diabres.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 25.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama. May 07 2014;311(17):1778–86. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson RG, Knowler WC, Kretzler M, et al. Pima Indian contributions to our understanding of diabetic kidney disease. Diabetes (in-press). 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanamas SK, Reddy SP, Chambers MA, et al. Effect of severe obesity in childhood and adolescence on risk of type 2 diabetes in youth and early adulthood in an American Indian population. Pediatr Diabetes. Jun 2018;19(4):622–629. doi: 10.1111/pedi.12627 [DOI] [PubMed] [Google Scholar]
- 28.Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. American journal of epidemiology. Oct 1 1998;148(7):650–6. doi: 10.1093/aje/148.7.650 [DOI] [PubMed] [Google Scholar]
- 29.Pavkov ME, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Nelson RG. Effect of intrauterine diabetes exposure on the incidence of end-stage renal disease in young adults with type 2 diabetes. Diabetes Care. Nov 2010;33(11):2396–8. doi: 10.2337/dc10-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. Jama. Jul 26 2006;296(4):421–6. doi: 10.1001/jama.296.4.421 [DOI] [PubMed] [Google Scholar]
- 31.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med. Jun 6 1991;324(23):1626–32. doi: 10.1056/NEJM199106063242304 [DOI] [PubMed] [Google Scholar]
- 32.Grabias BM, Konstantopoulos K. The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol. Mar 1 2014;306(5):F473–85. doi: 10.1152/ajprenal.00503.2013 [DOI] [PubMed] [Google Scholar]
- 33.Bjornstad P, Cherney DZ. Renal Hyperfiltration in Adolescents with Type 2 Diabetes: Physiology, Sex Differences, and Implications for Diabetic Kidney Disease. Curr Diab Rep. March 2018;18(5):22. doi: 10.1007/s11892-018-0996-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. Jul 2016;39(7):1115–22. doi: 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 35.Tuttle KR. Back to the Future: Glomerular Hyperfiltration and the Diabetic Kidney. Diabetes. Jan 2017;66(1):14–16. doi: 10.2337/dbi16-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Neill J, Fasching A, Pihl L, Patinha D, Franzen S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. Aug 1 2015;309(3):F227–34. doi: 10.1152/ajprenal.00689.2014 [DOI] [PubMed] [Google Scholar]
- 37.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. Apr 2008;4(4):216–26. doi: 10.1038/ncpneph0757 [DOI] [PubMed] [Google Scholar]
- 38.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. Jan 2006;17(1):17–25. doi: 10.1681/ASN.2005070757 [DOI] [PubMed] [Google Scholar]
- 39.Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol. Apr 2017;28(4):1023–1039. doi: 10.1681/ASN.2016060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. Jul 1981;241(1):F85–93. doi: 10.1152/ajprenal.1981.241.1.F85 [DOI] [PubMed] [Google Scholar]
- 41.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. Jun 1996;49(6):1774–7. doi: 10.1038/ki.1996.265 [DOI] [PubMed] [Google Scholar]
- 42.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. Jan 2003;63(1):225–32. doi: 10.1046/j.1523-1755.2003.00712.x [DOI] [PubMed] [Google Scholar]
- 43.Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. Sep 2016;39(9):1635–42. doi: 10.2337/dc16-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. Mar 2008;336(7646):697–701. doi: 10.1136/bmj.39478.378241.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araki S, Haneda M, Sugimoto T, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. Oct 2005;54(10):2983–7. doi: 10.2337/diabetes.54.10.2983 [DOI] [PubMed] [Google Scholar]
- 46.Son MK, Yoo HY, Kwak BO, et al. Regression and progression of microalbuminuria in adolescents with childhood onset diabetes mellitus. Ann Pediatr Endocrinol Metab. Mar 2015;20(1):13–20. doi: 10.6065/apem.2015.20.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. Dec 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buyadaa O, Salim A, Morton JI, Magliano DJ, Shaw JE. Rate of decline in kidney function and known age-of-onset or duration of type 2 diabetes. Sci Rep. Jul 19 2021;11(1):14705. doi: 10.1038/s41598-021-94099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. Jun 2014;124(6):2333–40. doi: 10.1172/JCI72271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia. Aug 2008;51(8):1347–55. doi: 10.1007/s00125-008-1051-7 [DOI] [PubMed] [Google Scholar]
- 51.Fufaa GD, Weil EJ, Lemley KV, et al. Structural Predictors of Loss of Renal Function in American Indians with Type 2 Diabetes. Clinical journal of the American Society of Nephrology : CJASN. Feb 05 2016;11(2):254–61. doi: 10.2215/cjn.05760515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. The Journal of clinical investigation. Jan 15 1997;99(2):342–8. doi: 10.1172/jci119163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Looker HC, Mauer M, Saulnier PJ, et al. Changes in Albuminuria But Not GFR are Associated with Early Changes in Kidney Structure in Type 2 Diabetes. J Am Soc Nephrol. Jun 2019;30(6):1049–1059. doi: 10.1681/ASN.2018111166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. Dec 1996;39(12):1569–76. doi: 10.1007/s001250050616 [DOI] [PubMed] [Google Scholar]
- 55.Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia. Oct 1993;36(10):1064–70. doi: 10.1007/bf02374500 [DOI] [PubMed] [Google Scholar]
- 56.Looker HC, Pyle L, Vigers T, et al. Structural Lesions on Kidney Biopsy in Youth-Onset and Adult-Onset Type 2 Diabetes. Diabetes Care. Feb 1 2022;45(2):436–443. doi: 10.2337/dc21-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The Association Between Age of Onset of Type 2 Diabetes and the Long-term Risk of End-Stage Kidney Disease: A National Registry Study. Diabetes Care. Aug 2020;43(8):1788–1795. doi: 10.2337/dc20-0352 [DOI] [PubMed] [Google Scholar]
- 58.Woroniecki R, Gaikwad AB, Susztak K. Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol. May 2011;26(5):705–11. doi: 10.1007/s00467-010-1714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. Mar 14 2011;171(5):412–20. doi: 10.1001/archinternmed.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. Jun 2019;15(6):327–345. doi: 10.1038/s41581-019-0135-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Susztak K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol. Jan 2014;25(1):10–7. doi: 10.1681/ASN.2013050461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ko YA, Yi H, Qiu C, et al. Genetic-Variation-Driven Gene-Expression Changes Highlight Genes with Important Functions for Kidney Disease. Am J Hum Genet. Jun 1 2017;100(6):940–953. doi: 10.1016/j.ajhg.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol. May 2008;19(5):943–52. doi: 10.1681/ASN.2007080864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mortensen HB, Marinelli K, Norgaard K, et al. A nation-wide cross-sectional study of urinary albumin excretion rate, arterial blood pressure and blood glucose control in Danish children with type 1 diabetes mellitus. Danish Study Group of Diabetes in Childhood. Diabet Med. Dec 1990;7(10):887–97. doi: 10.1111/j.1464-5491.1990.tb01324.x [DOI] [PubMed] [Google Scholar]
- 65.Ardissino G, Testa S, Dacco V, et al. Puberty is associated with increased deterioration of renal function in patients with CKD: data from the ItalKid Project. Arch Dis Child. Oct 2012;97(10):885–8. doi: 10.1136/archdischild-2011-300685 [DOI] [PubMed] [Google Scholar]
- 66.Helve J, Sund R, Arffman M, et al. Incidence of End-Stage Renal Disease in Patients With Type 1 Diabetes . Diabetes care. Mar 2018;41(3):434–439. doi: 10.2337/dc17-2364 [DOI] [PubMed] [Google Scholar]
- 67.Barkai L, Vamosi I, Lukacs K. Enhanced progression of urinary albumin excretion in IDDM during puberty. Diabetes Care. Jun 1998;21(6):1019–23. doi: 10.2337/diacare.21.6.1019 [DOI] [PubMed] [Google Scholar]
- 68.Lawson ML, Sochett EB, Chait PG, Balfe JW, Daneman D. Effect of puberty on markers of glomerular hypertrophy and hypertension in IDDM. Diabetes. Jan 1996;45(1):51–5. doi: 10.2337/diab.45.1.51 [DOI] [PubMed] [Google Scholar]
- 69.Elhadd TA, Khan F, Kirk G, et al. Influence of puberty on endothelial dysfunction and oxidative stress in young patients with type 1 diabetes. Diabetes Care. Nov 1998;21(11):1990–6. doi: 10.2337/diacare.21.11.1990 [DOI] [PubMed] [Google Scholar]
- 70.Vinovskis C, Li LP, Prasad P, et al. Relative Hypoxia and Early Diabetic Kidney Disease in Type 1 Diabetes. Diabetes. Jul 31 2020;doi: 10.2337/db20-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verrotti A, Cieri F, Petitti MT, Morgese G, Chiarelli F. Growth hormone and IGF-I in diabetic children with and without microalbuminuria. Diabetes Nutr Metab. Aug 1999;12(4):271–6. [PubMed] [Google Scholar]
- 72.Cummings EA, Sochett EB, Dekker MG, Lawson ML, Daneman D. Contribution of growth hormone and IGF-I to early diabetic nephropathy in type 1 diabetes. Diabetes. Aug 1998;47(8):1341–6. doi: 10.2337/diab.47.8.1341 [DOI] [PubMed] [Google Scholar]
- 73.Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The Association Between Age of Onset of Type 2 Diabetes With the Long-term Risk of End-Stage Kidney Disease: A National Registry Study. Diabetes Care. Jun 15 2020;doi: 10.2337/dc20-0352 [DOI] [PubMed] [Google Scholar]
- 74.Bjornstad P, Caprio S, Goland R, et al. Incidence of Complications and Comorbidities in Young People with Type 2 Diabetes. N Engl J Med. 2020;under review [Google Scholar]
- 75.Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. Jul 2009;58(7):1651–8. doi: 10.2337/db08-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. Nov 2010;53(11):2312–9. doi: 10.1007/s00125-010-1860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301 [DOI] [PubMed] [Google Scholar]
- 78.Hesp AC, Schaub JA, Prasad PV, et al. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. Sep 2020;98(3):579–589. doi: 10.1016/j.kint.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen JJ. Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am J Physiol. May 1979;236(5):F423–33. doi: 10.1152/ajprenal.1979.236.5.F423 [DOI] [PubMed] [Google Scholar]
- 80.Guler HP, Eckardt KU, Zapf J, Bauer C, Froesch ER. Insulin-like growth factor I increase glomerular filtration rate and renal plasma flow in man. Acta Endocrinol (Copenh). Jul 1989;121(1):101–6. doi: 10.1530/acta.0.1210101 [DOI] [PubMed] [Google Scholar]
- 81.Guler HP, Schmid C, Zapf J, Froesch ER. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. Apr 1989;86(8):2868–72. doi: 10.1073/pnas.86.8.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirschberg R, Rabb H, Bergamo R, Kopple JD. The delayed effect of growth hormone on renal function in humans. Kidney Int. Mar 1989;35(3):865–70. doi: 10.1038/ki.1989.65 [DOI] [PubMed] [Google Scholar]
- 83.Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol. Dec 1 2016;311(6):F1217–F1229. doi: 10.1152/ajprenal.00294.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cherney DZ, Miller JA, Scholey JW, et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care. Jun 2010;33(6):1344–6. doi: 10.2337/dc09-2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. Sep 1981;21(3):165–71. [DOI] [PubMed] [Google Scholar]
- 86.Brands MW, Manhiani MM. Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol. Dec 2012;303(11):R1101–9. doi: 10.1152/ajpregu.00390.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. May 2006;290(5):F1055–64. doi: 10.1152/ajprenal.00108.2005 [DOI] [PubMed] [Google Scholar]
- 88.Nakamura N, Matsui T, Ishibashi Y, Yamagishi S. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol Metab Syndr. 2015;7:48. doi: 10.1186/s13098-015-0044-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. Jul 24 1986;315(4):215–9. doi: 10.1056/NEJM198607243150402 [DOI] [PubMed] [Google Scholar]
- 90.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. Aug 2002;87(8):3814–8. doi: 10.1210/jcem.87.8.8765 [DOI] [PubMed] [Google Scholar]
- 91.Moran A, Jacobs DR Jr., Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. Oct 1999;48(10):2039–44. doi: 10.2337/diabetes.48.10.2039 [DOI] [PubMed] [Google Scholar]
- 92.Ball GD, Weigensberg MJ, Cruz ML, Shaibi GQ, Kobaissi HA, Goran MI. Insulin sensitivity, insulin secretion and beta-cell function during puberty in overweight Hispanic children with a family history of type 2 diabetes. Int J Obes (Lond). Dec 2005;29(12):1471–7. doi: 10.1038/sj.ijo.0803044 [DOI] [PubMed] [Google Scholar]
- 93.Lovshin JA, Boulet G, Lytvyn Y, et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI insight. Jan 11 2018;3(1)doi: 10.1172/jci.insight.96968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zaika O, Mamenko M, Staruschenko A, Pochynyuk O. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr Hypertens Rep. Feb 2013;15(1):17–24. doi: 10.1007/s11906-012-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jensen T, Bjornstad P, Johnson RJ, Sippl R, Rewers M, Snell-Bergeon JK. Copeptin and Estimated Insulin Sensitivity in Adults With and Without Type 1 Diabetes: The CACTI Study. Can J Diabetes. Mar 14 2018;doi: 10.1016/j.jcjd.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bjornstad P, Johnson RJ, Snell-Bergeon JK, et al. Albuminuria is associated with greater copeptin concentrations in men with type 1 diabetes: A brief report from the T1D exchange Biobank. J Diabetes Complications. Feb 2017;31(2):387–389. doi: 10.1016/j.jdiacomp.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bjornstad P, Maahs DM, Jensen T, et al. Elevated copeptin is associated with atherosclerosis and diabetic kidney disease in adults with type 1 diabetes. J Diabetes Complications. Aug 2016;30(6):1093–6. doi: 10.1016/j.jdiacomp.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol. Aug 15 2015;309(4):F280–99. doi: 10.1152/ajprenal.00093.2015 [DOI] [PubMed] [Google Scholar]
- 99.Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care. Mar 19 2013;17(2):221. doi: 10.1186/cc12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand. Sep 2009;53(8):1052–9. doi: 10.1111/j.1399-6576.2009.02037.x [DOI] [PubMed] [Google Scholar]
- 101.Bertuccio CA, Ibarra FR, Toledo JE, Arrizurieta EE, Martin RS. Endogenous vasopressin regulates Na-K-ATPase and Na(+)-K(+)-Cl(−) cotransporter rbsc-1 in rat outer medulla. Am J Physiol Renal Physiol. Feb 2002;282(2):F265–70. doi: 10.1152/ajprenal.00354.2000 [DOI] [PubMed] [Google Scholar]
- 102.Blot-Chabaud M, Djelidi S, Courtois-Coutry N, et al. Coordinate control of Na,K-atpase mRNA expression by aldosterone, vasopressin and cell sodium delivery in the cortical collecting duct. Cell Mol Biol (Noisy-le-grand). Mar 2001;47(2):247–53. [PubMed] [Google Scholar]
- 103.Cook JS, Hoffman RP, Stene MA, Hansen JR. Effects of maturational stage on insulin sensitivity during puberty. J Clin Endocrinol Metab. Sep 1993;77(3):725–30. doi: 10.1210/jcem.77.3.7690363 [DOI] [PubMed] [Google Scholar]
- 104.Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. May 2002;26(5):701–9. doi: 10.1038/sj.ijo.0801975 [DOI] [PubMed] [Google Scholar]
- 105.Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab. Jan 1995;80(1):172–8. doi: 10.1210/jcem.80.1.7829608 [DOI] [PubMed] [Google Scholar]
- 106.Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab. Mar 2003;88(3):1389–93. doi: 10.1210/jc.2002-020979 [DOI] [PubMed] [Google Scholar]
- 107.Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ. Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care. Mar 2012;35(3):536–41. doi: 10.2337/dc11-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelsey MM, Pyle L, Hilkin A, et al. The Impact of Obesity On Insulin Sensitivity and Secretion During Pubertal Progression: A Longitudinal Study. J Clin Endocrinol Metab. May 1 2020;105(5)doi: 10.1210/clinem/dgaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. May 2000;49(5):677–83. [DOI] [PubMed] [Google Scholar]
- 110.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. Sep 29 2015;14:121. doi: 10.1186/s12944-015-0123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. Dec 2010;5(12):2373–9. doi: 10.2215/CJN.08160910 [DOI] [PubMed] [Google Scholar]
- 112.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. Jan 7 2005;1706(1-2):1–11. doi: 10.1016/j.bbabio.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Math Biosci. Jul 2017;289:116–129. doi: 10.1016/j.mbs.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.P S, McDonough AA, THOMPSON SC. Metabolic basis of solute transport. . Brenner & Rector's the Kidney 10th Ed. 2016;Philadelphia, Elsevier:122–143. [Google Scholar]
- 115.Miyamoto S, Hsu CC, Hamm G, et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine. May 2016;7:121–34. doi: 10.1016/j.ebiom.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. Feb 2018;19(2):121–135. doi: 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cree-Green M, Gupta A, Coe GV, et al. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications. Jan 2017;31(1):141–148. doi: 10.1016/j.jdiacomp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morita M, Prudent J, Basu K, et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell. Sep 21 2017;67(6):922–935 e5. doi: 10.1016/j.molcel.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 119.Friederich M, Fasching A, Hansell P, Nordquist L, Palm F. Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim Biophys Acta. Jul-Aug 2008;1777(7-8):935–40. doi: 10.1016/j.bbabio.2008.03.030 [DOI] [PubMed] [Google Scholar]
- 120.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. Jan 2011;96(1):159–67. doi: 10.1210/jc.2010-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. Dec 2018;41(12):2648–2668. doi: 10.2337/dci18-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amatruda M, Gembillo G, Giuffrida AE, Santoro D, Conti G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina (Kaunas). Aug 25 2021;57(9)doi: 10.3390/medicina57090868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. Jun 13 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 124.Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. Aug 31 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011 [DOI] [PubMed] [Google Scholar]
- 125.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. Jul 28 2016;375(4):323–34. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 126.Tamborlane WV, Klingensmith G. Crisis in care: limited treatment options for type 2 diabetes in adolescents and youth. Diabetes Care. Jun 2013;36(6):1777–8. doi: 10.2337/dc13-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. Feb 2013;131(2):364–82. doi: 10.1542/peds.2012-3494 [DOI] [PubMed] [Google Scholar]
- 128.Kwon C, Sun JL, Jeong JH, Jung TW. Humanin attenuates palmitate-induced hepatic lipid accumulation and insulin resistance via AMPK-mediated suppression of the mTOR pathway. Biochem Biophys Res Commun. May 28 2020;526(2):539–545. doi: 10.1016/j.bbrc.2020.03.128 [DOI] [PubMed] [Google Scholar]
- 129.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee C, Zeng J, Drew BG, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. Mar 3 2015;21(3):443–54. doi: 10.1016/j.cmet.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Behrouz V, Dastkhosh A, Hedayati M, Sedaghat M, Sharafkhah M, Sohrab G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: a pilot study. Diabetol Metab Syndr. 2020;12:59. doi: 10.1186/s13098-020-00568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. Jul 26 2002;110(2):163–75. doi: 10.1016/s0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- 133.Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. May 2005;16(5):1384–91. doi: 10.1681/ASN.2004100894 [DOI] [PubMed] [Google Scholar]
- 134.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. Jan 14 2016;374(2):113–23. doi: 10.1056/NEJMoa1506699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inge TH, Prigeon RL, Elder DA, et al. Insulin Sensitivity and beta-Cell Function Improve after Gastric Bypass in Severely Obese Adolescents. J Pediatr. Nov 2015;167(5):1042–8 e1. doi: 10.1016/j.jpeds.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inge TH, Laffel LM, Jenkins TM, et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. May 1 2018;172(5):452–460. doi: 10.1001/jamapediatrics.2017.5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nehus EJ, Khoury JC, Inge TH, et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. February 2017;91(2):451–458. doi: 10.1016/j.kint.2016.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. Apr 2014;171(8):2017–28. doi: 10.1111/bph.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. Jun 2011;22(6):1041–52. doi: 10.1681/ASN.2010080808 [DOI] [PMC free article] [PubMed] [Google Scholar]