Abstract
Purpose
The cost implications of limb reconstruction techniques have not been adequately investigated. Aim of this pilot study was to compare the direct medical cost of tibial bone defects managed with distraction osteogenesis–Ilizarov method (ILF), or with Masquelet technique (MIF).
Methods
Data of 20 random patients treated in a single centre were analysed. Inclusion criteria included acute tibial defects, or post-debridement of nonunions with complete follow-up and successful union. The endpoint of clinical efficacy was the time-to-defect union. Comparisons were made between equally sized subgroups (ILF vs. MIF).
Results
The average defect length was 5.6 cm (2.6–9.6 cm). The overall cost of 20 cases reached £452,974 (mean £22,339, range £13,459–£36,274). Statistically significant differences favoring the MIF were found regarding the average time-to-union; number of surgeries, of admissions and follow-up visits, as well as the mean intraoperative cost (£8857 vs. £14,087). These differences lead to significant differences of the mean cost of the overall treatment (MIF £18,131 vs. ILF £26,126). Power analysis based on these data indicated that 35 patients on each group would allow detection of a 25% difference, with an alpha value of 0.05 and probability (power) of 0.9.
Conclusions
The results and analysis presented highlight factors affecting the high financial burden, even in a best-case scenario, this type of surgery entails. Larger pivotal studies should follow to improve the cost efficiency of clinical practice.
Keywords: Bone defect, Cost analysis, Tibia, Masquelet technique, Distraction osteogenesis, Ilizarov circular frame
Introduction
Successful management of bone defects remains a significant clinical challenge. Whether attributed to acute bone loss (occurring in 11.4% of severe open fractures) [1], resections for bone tumors, nonunions, or infections [2], they require considerable surgical expertise, patient compliance, multidisciplinary pathways, and consume significant resources [3, 4]. The most common site of bone loss is the tibia with a number of clinical series reporting different management strategies and outcomes [2, 5, 6].
Contemporary treatments include distraction osteogenesis (using circular fine wire fixators, monolateral external devices, or lengthening nails), vascularized bone grafts, the Masquelet technique, the use of titanium cages or even amputation under certain circumstances [2, 3, 5–7]. The relative rarity of this clinical problem and the complexity of its management have led to centralization of this work to specialized limb reconstruction groups. Having most of these techniques readily available, it offers flexibility, efficiency, individualized care, and theoretically limits the associated socioeconomic burden [5, 7–9].
The cost implications of these different techniques have not been adequately explored [10–12]. Under the current strenuous health economic climate [13], and the increasing complexity of medical care, sustainable provision of limb reconstruction services dictates appropriate reimbursement strategies based on reliable cost analyses [10, 14, 15].
The primary aim of this study was to produce a pilot cost analysis on tibial bone defects, to show the feasibility of collecting the data for conducting robust and detailed cost analysis and inform future evaluations of costs and effectiveness. Secondary endpoints were a) to compare the direct cost between bone transport using a fine wire circular fixator (ILF) and the Masquelet technique using internal fixation (MIF) and b) to compare the direct cost between acute tibial bone loss of open fractures vs. cases with secondary bone loss generated during the treatment of tibial nonunions.
Patients and methods
Prospectively collected data from a single centre acting as a level 1 trauma centre and regional referral unit for limb reconstruction were analysed. Exclusion criteria included patients below 18 years of age, tibial defects of different causation (tumor or otherwise), patients treated with other techniques, or who did not heal their defect or lost to follow up. The method that each of the patients was treated was decided at the time based on the consensus reached during departmental clinical governance meetings, and individual patient’s preference during the informed consent process. Patients with adverse outcomes were excluded, because we wished to assess the cost of both techniques in a best-case scenario. We felt that managing treatment failure in these patients will significantly increase cost and this would need investigation in a larger patient group to be meaningful.
To reduce selection bias, the first five patients in alphabetic order of their surname, that received treatment at the acute (ILFa–MIFa) or nonunion (ILFn–MIFn) settings with either technique were included for further assessment of their direct costs till completion of follow-up.
Data collected included demographics, comorbidities (Charlson’s score [16]), surgical risk (ASA score [17]), severity of trauma (ISS [18]), fracture type (AO/OTA [19]) and Gustilo–Anderson systems [20, 21] for the open fractures. The size of all defects was recorded at the operative notes of the final debridement, and further classified using the Solomin–Slongo system [22]. The duration of surgery, the implants used, administered blood products, laboratory tests performed, imaging investigations, length-of-hospital stay (LOS), visits to the outpatient clinics, time-to-union and time-to-discharge from further follow-up were collected in an excel database. Time-to-union was defined as the time till the first mention of a healed defect by the treating surgeon to the patient’s records and verified by the radiology reports.
To define the direct medical costs, we utilised the financial records of several clinical service units. These included the records of trauma-related specialties, operating theatres, blood bank, outpatient clinic and patient transport departments. Data from the 2019/20 National Tariff [23], the BNF (British National Formulary) [24], as well as from the price list of all devices and implants from industry partners were collected. All costs were adjusted for inflation to the United Kingdom’s 2020 consumer price index at a rate of 2.2% [25]. The detailed template of the exact prices per item are presented in Tables 1 and 2.
Table 1.
Direct medical costs (inhospital and outpatient stay, OR procedures, medications)
| Phase | Items | Cost | Source | Phase | Items | Cost | Source |
|---|---|---|---|---|---|---|---|
| Inhospital stay | Standard ward hospital stay per day | £241.00 | TRS CSU | Intraoperative costs | OR trauma (per minute) | £319.00 | NHS England. 2019/20 National Tariff Payment System |
| High observations ward hospital stay per day | £412.00 | ACC CSU | Consultant time in OR (per minute) | £49.00 | |||
| Laboratory tests | Full blood count (FBC) | £2.65 | NHS England. 2019/20 National Tariff Payment System | Registrar time in OR (per minute) | £30.00 | ||
| Biochemical tests (U&Es) | £2.12 | Sterilisation cost per kit | £75.00 | OR THEATRES CSU | |||
| Clotting tests | £3.83 | Outpatient clinics costs | Fup f2f visit | £104.00 | OPC CSU | ||
| group and save | £8.00 | First visit | £128.00 | ||||
| cRBC transfusion | £781.00 | BLOOD BANK | Transport W1–2 | £85.00 | |||
| Imaging tests | plain X-ray | £25.00 | NHS England. 2019/20 National Tariff Payment System | Transport T1–2 | £77.00 | ||
| CT scan | £118.00 | Transport SC | £43.00 | ||||
| MRI scan | £157.00 | Antibiotic therapies | Vancomycin iv 1gr vial | £12.50 | BNF 2020 NICE ORG UK27 | ||
| Intraoperative costs | OR Trauma (per minute) | £319.00 | NHS England. 2019/20 National Tariff Payment System | Gentamycin iv 40 mg/1 ml ampoule | £1.20 | ||
| Consultant time in OR (per minute) | £49.00 | Flucloxacillin iv 500 mg vial | £1.25 | ||||
| Registrar time in OR (per minute) | £30.00 | Flucloxacillin p.os 500 mg capsule | £0.25 | ||||
| Sterilisation cost per kit | £75.00 | Operating Theatres CSU | Co-amoxiclav iv 1.2 g vial | £1.60 | |||
| Palacos® MV G cement × 1 mix | £44.03 | Teicoplanin iv 400 mg vial | £12.00 | ||||
| Preoperative antibiotic prophylaxis at induction (open fractures) | £26.40 | Ciprofloxacin p.os 750 mg tablet | £0.80 | ||||
| Preoperative antibiotic prophylaxis at induction (other) | £8.40 | BNF 2020 NICE ORG UK27 | Ciprofloxacin iv 400 mg/200 ml vial | £19.92 | |||
| Piperacillin/tazobactam 2gr/250 mg vial | £9.95 | ||||||
| VTE prophylaxis | Tinzaparin 4500units prefilled disposable sc injection | £3.56 | BNF 2020 NICE ORG UK27 | Linezolid p.os 500 mg tablet | £31.24 | ||
| Painkillers | Codeine phosphate 30 mg tablet | £1.23 | BNF 2020 NICE ORG UK27 | Rifampicin p.os 150 mg capsule | £54.65 | ||
| Paracetamol 500 mg tablet | £0.20 | Clindamycin p.os 75 mg capsule | £0.31 |
ACC = acute critical care; BNF = British National Formulary; cRBC = concentrated red blood cell transfusion; CSU = Clinical Service Unit; g = gram; G = gentamicin; iv = intravenous administration route; LTHT = Leeds Teaching Hospitals Trust; mg = milligram; MRI = magnetic resonance imaging; MV = medium viscosity; NICE = national institute of health and care excellence; OR = operation room; p.os = oral administration route; SC = saloon care transport vehicle; T1/2 = tail lift transport vehicle; TRS = trauma-related services; UK = United Kingdom; VTE = venous thromboembolic events; W1/2 = wheelchair transport vehicle
Table 2.
Direct medical cost of implants and bone graft substitutes
| Description | Cost | Source | Description | Cost | Source | Description | Cost | Source | Description | Cost | Source | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALPS® distal tibial anterolateral plate | £1065.00 | Zimmer biomet UK | Half ring 160 mm | £276.17 | Smith and nephew UK | Expert tibial nail PROTECT | £2024.00 | DePuy Synthes UK | Stimulan bone graft 10 ml | £490.00 | Biocomposites UK | |||||||||||
| ALPS® distal tibial medial plate | £942.00 | Thick Italian nut | £24.91 | Guide wire 3.2 mm | £29.92 | Cerament V/G 10 ml | £1500.00 | BoneSupport UK | ||||||||||||||
| ALPS® distal fibula anatomical plate | £628.00 | Thread rod 150 mm | £74.04 | Ball tip guide wire | £187.00 | Cerament V/G 5 ml | £1000.00 | |||||||||||||||
| Locking cortical screw | £74.00 | Wire with stopper 400 mm × 12 | £1028.96 | Locking screw L144 | £43.71 | Bonalive 10 ml | £467.40 | Bonalive biomaterials | ||||||||||||||
| Non-locking cortical screw | £24.00 | Wire fixed bolt slotted | £101.33 | Cancellous locking screw L50 | £63.14 | Bonalive 5 ml | £820.00 | |||||||||||||||
| Kwire 1.6 × 150 mm | £48.00 | Foot composite ring | £339.69 | End cap | £41.60 | BMAC biocue 60 ml | £466.00 | Zimmer biomet UK | ||||||||||||||
| Drill bit 2.5 mm | £88.00 | Kits | × 4 | Drill bit 3.2 mm calibr L340 | £156.17 | PRP recover 60 ml | £278.00 | |||||||||||||||
| Drill bit 2.7 mm | £114.00 | Average 5 ring construct | £4892.70 | Drill bit 4.2 mm calibr L135 | £120.97 | RIA system harvest average use | £1285.87 | Procurement department | ||||||||||||||
| Kits | × 3 | Average 6 ring construct | £5519.08 | Outer protection sleeve (suprapatellar) | £30.88 | |||||||||||||||||
| evos 2.7/3.5 mm P/A M-D plate | £514.00 | smith & nephew UK | average 7 ring construct | £6096.33 | stryker UK | kits | × 4 | zimmer biomet UK | VACPAC average use | £146.40 | 3 M KCI—TRS CSU LTHT | |||||||||||
| evos 2.7/3.5 mm L-D fibula PL plate | £474.30 | Hoffman 2 clamp rod to rod | £549.00 | Versanail tibia | £515.00 | |||||||||||||||||
| 3.5 mm locking screw | £95.10 | Hoffman 2 clamp pin to rod | £527.00 | 3.2 × 444 mm threaded guide pin | £54.00 | |||||||||||||||||
| 4.7 mm osteopenic screw | £19.70 | 8 mm rod | £74.00 | ball nose guide wire 100 cm | £146.00 | |||||||||||||||||
| 3.5 mm non-Locking cortical screw | £24.90 | Hoffman 3 clamp rod to rod | £632.00 | 4.4 mm x 286 mm drill bit | £226.00 | |||||||||||||||||
| 2.7 mm locking screw | £50.90 | Hoffman 3 clamp pin to rod | £606.00 | 3.8 mm x 150 mm drill bit | £230.00 | |||||||||||||||||
| 2.7 mm non locking screw | £29.90 | 11 mm rod | £85.00 | end cap | £39.00 | |||||||||||||||||
| drill bit 2.5 mm | £86.50 | 5 mm × 180 apex pin | £88.00 | 5.5 mm solid cortical screw | £41.00 | |||||||||||||||||
| drill bit 2.0 mm | £129.20 | 3.2 mm drill bit | £51.00 | 4.5 mm solid cortical screw | £26.00 | |||||||||||||||||
| kits | × 3 | kits | × 3 | kits | × 4 | |||||||||||||||||
ALPS® advanced locking plating system, BMAC bone marrow aspirate concentrate, mm millimetre, PRP platelet rich plasma
Descriptive statistical methods (two sample t test) have been utilised. Independent samples t tests were performed to compare the means in all parameters, following log transformation of the recorded values. We studied the complete follow-up period and also different timepoints for (a) ILF vs. MIF groups; (b) acute vs. nonunion defects. The alpha value of 0.05 was used as the cutoff for statistical significance. We considered adjusting for baseline clinical differences in our analysis of cost differences, but the small numbers of patients in each subgroup at this pilot study prevented us from doing so.
Results
Data from 20 patients were analysed: five patients of each of the groups (ILFa, ILFn, MIFa, and MIFn). The overall direct medical cost treating these 20 tibial bone defects [mean length of 5.6 cm (range 2.6–9.5)] was £452,974. Patient and defect characteristics are shown in Table 3.
Table 3.
Characteristics of the overall group of patients with tibial defects, with comparison between different treatment methods (distraction osteogenesis with an Ilizarov frame) or Masquelet internal fixation technique (induced membrane); as well as comparison between acute defects vs. nonunion or debridement due to fracture-related infection
| Parameters | Overall | Distraction osteogenesis protocol (ILF) | Induced membrane protocol (MIF) | Difference between means (ILF minus MIF) | p value | Acute defect | Nonunion defect | Difference between means (acute minus nonunion defects) | p value |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 20 | 10 | 10 | n/a | n/a | 10 | 10 | n/a | n/a |
| Gender ratio | 14/6 | 9/1 | 5/5 | n/a | < 0.001 | 7/3 | 7/3 | n/a | 1 |
| (M/F) | |||||||||
| Mean age | 39 years | 38 years | 40 years | - 2 years | 0.738 | 39 years | 38 years | 1 year | 0.867 |
| (SD, median, range) | (13.17, 34, 20–64) | (12.22, 38, 23 to 56) | (14.05, 34, 20 to 64) | (14.14, 38, 20 to 57) | (12, 33, 26 to 64) | ||||
| Mean ISS | 12 | 12 | 13 | -1 | 0.722 | 12 | n/a | n/a | n/a |
| (SD, median, range) | (6.23, 9, 9–27) | (5.2, 9, to 9 to 22) | (7.04, 9, 9 to 27) | (6.23, 9, 9 to 27) | |||||
| Mean Charlson's score | 0.53 | 0.1 | 1 | -0.9 | 0.025 | 0.5 | 0.56 | -0.06 | 0.880 |
| (SD, median, range) | (0.88, 0, 0–3) | (0.3, 0, 0 to 1) | (1.05, 1, 0 to 3) | (0.92, 0, 0 to 3) | (0.83, 0, 0 to 3) | ||||
| Mean ASA score | 1.37 | 1.2 | 1.56 | -0.36 | 0.170 | 1.5 | 1.22 | 0.28 | 0.280 |
| (SD, median, range) | (0.58, 1, 1–3) | (0.4, 1, 1 to 2) | (0.68, 1, 1 to 3) | (0.67, 1, 1 to 3) | (0.42, 1, 1 to 3) | ||||
| Mean length of defect | 5.59 cm | 5.96 cm | 5.18 cm | 0.78 cm | 0.189 | 5.08 cm | 6.15 cm | - 1.07 cm | 0.433 |
| (SD, median, range) | (1.9, 5.15, 2.6–9.52) | (1.7, 5.21, 3.3 to 8.3) | (2.02, 4.26, 2.6 to 9.52) | (1.31, 5.03, 2.6 to 7.36) | (2.25, 5.7, 3.3 to 9.52) | ||||
| Mean follow up period | 30.52 months | 33.18 months | 27.57 months | 5.61 months | 0.123 | 23.57 months | 38.25 months | - 14.68 months | 0.04 |
| (SD, median, range) | (17, 26.03, 12–74) | (17.01, 26.73, 14.97 to 74) | (16.5, 21.83, 12 to 62.43) | (17.59, 18.4, 12.6 to 74) | (12.38, 34.8, 12 to 62.43) | ||||
| Mean time to union | 12.91 months | 15.5 months | 10.03 months | 5.47 months | 0.003 | 11.2 months | 14.8 months | - 3.6 months | 0.048 |
| (SD, median, range) | (4.38, 12.4, 4.6–22.2) | (3.83, 15.55, 8.3 to 22.2) | (2.92, 9.73, 4.6 to 15.9) | (4.07, 9.22, 6.97 to 17.27) | (3.5, 13.07, 4.6 to 22.2) | ||||
| Mean Healing Index | 2.23 | 2.35 | 1.99 | -0.36 | 0.276 | 2.11 | 2.37 | − 0.26 | 0.418 |
| (SD, median, range) | (0.71, 2.03, 1.1–3.76) | (0.76, 2.11, 1.5 to 3.76) | (0.67, 1.71, 1.1 to 2.85) | (0.67, 1.89, 1.35 to 3.52) | (0.73, 2.42, 1.1 to 3.76) | ||||
| Mean no of surgeries | 3 | 4 | 3 | 1 | 0.049 | 3 | 4 | -1 | 0.102 |
| (SD, median, range) | (1.27, 3, 2–7) | (1.3, 4, 2 to 7) | (0.67, 3, 2 to 4) | (0.9, 3, 2 to 5) | (1.57, 3, 2 to 7) | ||||
| Mean no of admissions | 3 | 3 | 2 | 1 | 0.026 | 2 | 3 | -1 | 0.036 |
| (SD, median, range) | (1.03, 2, 1–5) | (1.17, 4, 1 to 5) | (0.32, 2, 2 to 3) | (0.92, 2, 1 to 4) | (1.05, 3, 2 to 5) | ||||
| Mean LOS till union | 20 days | 21 days | 19 days | 2 days | 0.872 | 18 days | 22 days | -4 days | 0.342 |
| (SD, median, range) | (7.69, 18, 8–36) | (8.92, 20, 8 to 36) | (5.48, 18, 10 to 28) | (4.96, 17, 10 to 28) | (9.19, 20, 8 to 36) | ||||
| Mean cost of inpatient stay | £7024 | £6693 | £7392 | − £699 | 0.467 | £7121 | £6916 | £205 | 0.749 |
| (SD, median, range) | (4017.88, 6248, 2010.74–19,349.86) | (3408.84, 6041, 2010.74 to 13,289.08) | (4573, 6248, 3499.45 to 19,349.86) | (4854.87, 5041, 3499.45 to 19,349.86) | (2805.31, 7851, 2010.74 to 12,640.33) | ||||
| Mean cost of procedures | £11,610 | £14,087 | £8857 | £5230 | 0.004 | £11,199 | £12,066 | − £867 | 0.597 |
| (SD, median, range) | (3891.68, 10,177, 6222.84–20,333.93) | (3595.78, 14,688, 8318.9 to 20,333.93) | (1790.75, 8736, 6222.84 to 15,991.72) | (2992.26, 10,892, 6222.84 to 15,598.08) | (4650.64, 9429, 7213.85 to 20,333.93) | ||||
| Mean cost of outpatient Fup | £3775 | £5240 | £2147 | £3093 | < 0.001 | £3066 | £4564 | − £1498 | 0.172 |
| (SD, median, range) | (2212.9, 3324, 821.2–8771.85) | (1954.71, 5094, 1947 to 8771.85) | (1027.97, 2034, 821.2 to 3930.45) | (2342.96, 2010, 821.2 to 8771.85) | (1748.49, 3930, 898 to 8035.16) | ||||
| mean Overall Cost | £22,339 | £26,126 | £18,131 | £7995 | 0.025 | £21,385 | £23,399 | − £2014 | 0.475 |
| (SD, median, range) | (7142.82, 20,469, 13,459.45–36,273.98) | (7128.58, 27,096, 14,763.77 to 36,273.98) | (4195.51, 16,788, 13,459.45 to 29,530.05) | (5796.33, 21,504, 13,459.45 to 29,618.24) | (8260.97, 18,208, 14,763.77 to 36,273.98) | ||||
| Mean cost per cm of defect | £4295 | £4612 | £3944 | £668 | 0.534 | £4174 | £4208 | − £34 | 0.865 |
| (SD, median, range) | (1423.47, 4300, 1736.96–7447.75) | (1423.58, 4362, 1974.88 to 7447.75) | (1338.15, 3972, 1736.96 to 7030.96) | (1114.61, 4288, 1959.75 to 6044.54) | (1687.96, 4424, 1736.96 to 7447.75) |
ASA American Society of Anaesthesiologists physical status score, Fup follow up, Healing index time to union in months/length of defect in cm, LOS length of stay, ISS injury severity score, No number, SD standard deviation
According to the Solomin–Slongo system, four defects were type C1, three B2, and one B3, D2 and D3 [22]. According to the AO/OTA system [19], there were eight 43.A3, four 42.B3, three 42.B2, two 42.A2, and one fracture for each of the 41.A3, 42.A3, and 42.C2 types. The ten patients with an acute defect (ILFa and MIFa) all had a type-III [21] open fractures. The ten nonunion defects (ILFn and MIFn) were proven infected in seven. Systemic antibiotic treatment ranged between 6 and 9 weeks. The overall cost of the antibiotic therapy reached £6058 with a mean of £865 (range £54–£1969).
Wound vacuum-assisted closure was utilised in five open type-III-B fractures. Microsurgical soft-tissue reconstruction was required in 7 with an acute (4 × ILFa and 3 × MIFa) and in 4 with a nonunion defect (2 × ILFn and 2 × MIFn). Definitive orthoplastic surgery at the same seating was performed in 50% of the cases (1 × ILFa, 2 × MIFa, × 1 ILFn, and 1 × MIFn). The other 50% had first a free flap, and on a different day their definitive fixation (3 × ILFa, × 1 ILFn and × 1 MIFn) after a mean of 11.4 days (range 9–15).
The Masquelet staged protocol that we followed has been previously described. [5, 26] The mean period between the two stages was 61 days (range 42–128). The polymethacrylate cement spacer was combined with antibiotics (vancomycin 2 g and 40 mg of gentamycin per mix). Internal fixation was used at the first stage in five cases in the form of two reamed nails and three plate fixations. In the other five MIF cases, an external fixator was placed at the first stage, which at the second stage was revised to plate fixation. The reamer irrigator aspiration system (RIA® of DePuy Synthes)[27] was utilised in nine patients to harvest bone graft at the second stage. In four MIF cases, composite grafts were utilised combining the RIA harvest with bone-marrow-aspirate-concentrate and platelet-rich-plasma. In one MIFa case, iliac-crest-autologous-graft (ICAG) was combined with BMP2. The mean time-to-union index (ratio of time from first debridement till the verification of defect union in months, divided by the length-of-defect in cm) was 1.8 (range 1.1–2.9).
In one of the MIFn patients, chronic donor-site pain developed at the trochanteric area. Another patient 2 years after completion of union of his defect developed a relapse of the infection, which was managed effectively with plate removal and pathogen specific antibiotic therapy.
The bone transport patients were operated according to the principles of the Ilizarov technique [28, 29]. The mean period between frame application and corticotomy (single-level percutaneous) was 24.8 days (range 0–94). Transport was initiated after a latent period of 10–12 days with a distraction rate of 0.5–1 mm/day. Prior to removal, the frame was dynamized to verify the mechanical stability of the regenerate. The mean healing index (ratio of time from frame application till the date of its removal in months, divided by the length-of-defect in cm) was 2.1 (range 1.5–3.8).
One patient required a second corticotomy due to premature consolidation, a further patient required minor frame revision due to broken wires. Three ILF patients experienced a docking site refracture following frame removal (at 3–5 month postremoval). Two were successfully managed with a Sarmiento cast, and one with additional surgery (nailing). Three patients had recurrent pin-track infections that settled with oral antibiotics. An additional patient developed a delayed pin-track infection post union which required debridement, local antibiotics and a local fasciocutaneous flap. Only one frame patient had his frame removed at the outpatient clinics under gas-and-air anaesthesia. The rest required a day-case admission. Persistent neuropathic pain and acceptance of a 2 cm shortening in one patient, ankle stiffness in two at final follow-up were also recorded as associated complications.
Between the ILF and the MIF groups, no statistically significant difference was noted in regard to their mean age, ISS, ASA-score, length-of-defect, associated soft-tissue reconstruction procedures, the overall LOS and follow-up, and the cost of in-hospital stay. Statistically significant difference favoring the ILF group was found to the comorbidity index (p = 0.02), as well as to the gender ratio (p < 0.001). Results favoring the MIF group were found in regard to the mean time-to-union (p = 0.03), the number of procedures (p = 0.049), of admissions (p = 0.026), the operative room (OR) cost (p = 0.004), the cost of outpatient follow-up (p < 0.001), and the cost of the overall treatment (p = 0.025), Table 3.
The total cost in the MIF was £192,711, compared to £260,263 of the ILF, or else 26% lower for the same number of random patients with successful eventual defect union. For the ILF patients, 54% of the overall cost was related to the OR, the 25% to the inpatient stay, and 21% to the outpatient follow-up. Respectively, for the MIF patients, the costs at these different stages were 49%, 41%, and 10%). There was statistically significant difference favoring the MIF group on the average cost at the OR (p = 0.004) and the outpatient(p < 0.001) phases, Table 3.
When comparing the acute vs. the nonunion defect groups, there were no statistically significant differences for the majority of parameters. Exceptions to this were the mean number of admissions(p = 0.036), the time-to-union(p = 0.048), and the follow-up period(p = 0.04), which were all higher for the nonunion defects, Table 3.
Further subgroup analysis (Table 4) identified no statistically significant difference between the direct medical costs when the Ilizarov technique was used for an acute or a nonunion tibial defect. When the Masquelet groups were compared, the mean time-to-union (8 vs. 13 months), as well as the overall follow-up period (15 vs. 44 months), and the cost of outpatient follow-up (£1368 vs. £3122) were significantly higher when the defect was associated with a nonunion.
Table 4.
Head-to-head comparison between the four subgroups (ILFa, ILFn, MIFa, and MIFn)
| Subgroups | Mean age | Mean Charlson's index | Mean ASA | CAUSE | Mean ISS | Mean defect size | Mean time to union | Mean healing Index | Mean fup | Mean LOS till union | Mean no of admissions | Mean no of procedures | Mean cost overall | Mean cost inpatient stay | Mean cost OR | Mean cost outpatient clinic fup | Mean cost per cm of defect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | (SD, median, range) | ||
| ILFa | 40 years | 0.2 | 1.4 | Motorcycle (× 3) Pedestrian (× 1) Car occupant (× 1) | 12 | 5.42 cm | 14.49 m | 2.41 | 32.45 m | 19 days | 3 | 4 | £25,364.00 | £6906.00 | £13,695.00 | £4763.00 | £4776.16 |
| (14.57, 42, 23 to 56) | (0.4, 0, 0 to 1) | (0.49, 1, 1 to 2) | (5.2, 9, 9 to 22) | (0.81, 5.15, 4.76 to 7) | (3.19, 15.77, 8.3 to 17.27) | (0.7, 2.03, 1.58 to 3.52) | (21.17, 23.67, 14.97 to 74) | (3.31, 20, 13 to 20) | (1.17, 3, 1 to 4) | (0.63, 4, 3 to 5) | (3695.96, 24,621, 20,468.6 to 29,618.24) | (3270.29, 5806, 4199.43 to 13,289.08) | (1640.32, 14,335, 11,606.84 to 15,598.08) | (2220.27, 4377, 1947 to 8771.85) | (959.43, 4300.13, 3517.22 to 6044.54) | ||
| MIFa | 38 years | 0.8 | 1.6 | Fall > 2 m (× 3) Pedestrian (× 1) Car occupant (× 1) | 13 | 4.74 cm | 7.91 m | 1.99 | 14.69 m | 17 days | 2 | 3 | £17,407.00 | £7336.00 | £8703.00 | £1368.00 | £3972.36 |
| (13.57, 34, 20 to 57) | (1.17, 0, 0 to 3) | (0.8, 1, 1 to 3) | (7.04, 9, 9 to 27) | (1.6, 4.26, 2.2 to 7.6) | (1.17, 7.1, 6.97 to 9.97) | (0.48, 1.94, 1.35 to 2.73) | (3.58, 13, 13 to 21.83) | (6.01, 16, 10 to 28) | (0, 2, 2 to 2) | (0.49, 3, 2 to 3) | (4677.6, 15,767, 13,459.45 to 26,461.9) | (6029.25, 4480, 3499.45 to 19,349.86) | (1660.28, 9813, 6222.84 to 10,177.25) | (532.89, 1109, 821.2 to 2034.42) | (1114.07, 3972.46, 1959.75 to 5176.71) | ||
| p value | 0.754 | 0.153 | 0.511 | N/A | 0.722 | 0.332 | 0.005 | 0.137 | 0.026 | 0.382 | 0.024 | 0.001 | 0.017 | 0.813 | 0.013 | 0.002 | 0.274 |
| ILFn | 35 years | 0 | 1 | FRI (× 3) NU (× 2) | N/A | 6.5 cm | 16.5 m | 2.3 | 33.92 m | 23 days | 4 | 4 | £26,889.00 | £6481.00 | £14,480.00 | £5717.00 | £4448.14 |
| (8.47, 33, 26 to 47) | 0 | (0, 1, 1 to 1) | (2.12, 8.1, 3.3 to 8.3) | (4.14, 15.33, 12.1 to 22.2) | (0.8. 2.2, 1.5 to 3.76) | (11.39, 27.6, 25.47 to 55.7) | (11.77, 30, 8 to 36) | (1.02, 4, 2 to 4) | (1.72, 4, 2 to 7) | (9317.18, 32,756, 14,763.77 to 36,273.98) | (3529.22, 8394, 2010.74 to 10,029.17) | (4781.22, 16,109, 8318.9 to 20,333.93) | (1502.36, 5608, 3323.77 to 8035.16) | (1754,67, 4423.66, 1974.88 to 7447.75) | |||
| MIFn | 43 years | 1.25 | 1.5 | FRI (× 1) NU (× 1) SNU (× 3) | N/A | 5.72 cm | 12.67 m | 2.45 | 43.67 m | 21 days | 2 | 3 | £19,037.00 | £7460.00 | £9049.00 | £3122.00 | £4532.18 |
| (14.11, 39, 29 to 64) | (0.5, 1.5, 0 to 3) | (0.5, 1.5, 1 to 2) | (2.34, 4.95, 3.47 to 9.52) | (2.22, 12.52, 4.6 to 15.9) | (0.63, 2.8, 1.1 to 2.85) | (11.39, 40.22, 12 to 62.43) | (3.7, 19, 15 5 to 27) | (0.43, 2, 2 to 3) | (0.83, 3, 2 to 4) | (3283.24, 17,498, 16,535.86 to 29,530.05) | (1266.66, 7115, 6247.81 to 12,740.33) | (1924.29, 8367, 7213.85 to 15,991.72) | (560.13, 3098, 898 to 3930.45) | (188,178, 4837.91, 1736.96 to 7030.96) | |||
| p value | 0.145 | < 0.001 | 0.012 | N/A | N/A | 0.435 | 0.092 | 0.647 | 0.866 | 0.775 | < 0.001 | 0.122 | 0.382 | 0.24 | 0.167 | 0.016 | 0.974 |
| ILFa | 40 years | 0.2 | 1.4 | Motorcycle (× 3) Pedestrian (× 1) Car occupant (× 1) | 12 | 5.42 cm | 14.49 m | 2.41 | 32.45 m | 19 days | 3 | 4 | £25,364.00 | £6906.00 | £13,695.00 | £4763.00 | £4776.16 |
| (14.57, 42, 23 to 56) | (0.4, 0, 0 to 1) | (0.49, 1, 1 to 2) | (5.2, 9, 9 to 22) | (0.81, 5.15, 4.76 to 7) | (3.19, 15.77, 8.3 to 17.27) | (0.7, 2.03, 1.58 to 3.52) | (21.17, 23.67, 14.97 to 74) | (3.31, 20, 13 to 20) | (1.17, 3, 1 to 4) | (0.63, 4, 3 to 5) | (3695.96, 24,621, 20,468.6 to 29,618.24) | (3270.29, 5806, 4199.43 to 13,289.08) | (1640.32, 14,335, 11,606.84 to 15,598.08) | (2220.27, 4377, 1947 to 8771.85) | (959.43, 4300.13, 3517.22 to 6044.54) | ||
| ILFn | 35 years | 0 | 1 | FRI (× 3) NU (× 2) | N/A | 6.5 cm | 16.5 m | 2.3 | 33.92 m | 23 days | 4 | 4 | £26,889.00 | £6481.00 | £14,480.00 | £5717.00 | £4448.14 |
| (8.47, 33, 26 to 47) | 0 | (0, 1, 1 to 1) | (2.12, 8.1, 3.3 to 8.3) | (4.14, 15.33, 12.1 to 22.2) | (0.8. 2.2, 1.5 to 3.76) | (11.39, 27.6, 25.47 to 55.7) | (11.77, 30, 8 to 36) | (1.02, 4, 2 to 4) | (1.72, 4, 2 to 7) | (9317.18, 32,756, 14,763.77 to 36,273.98) | (3529.22, 8394, 2010.74 to 10,029.17) | (4781.22, 16,109, 8318.9 to 20,333.93) | (1502.36, 5608, 3323.77 to 8035.16) | (1754.67, 4423.66, 1974.88 to 7447.75) | |||
| p value | 0.364 | 0.148 | 0.03 | N/A | N/A | 0.505 | 0.452 | 0.747 | 0.575 | 0.874 | 0.057 | 1 | 0.959 | 0.62 | 1 | 0.893 | 0.564 |
| MIFa | 38 years | 0.8 | 1.6 | Fall > 2 m (× 3) Pedestrian (× 1) Car occupant (× 1) | 13 | 4.74 cm | 7.91 m | 1.99 | 14.69 m | 17 days | 2 | 3 | £17,407.00 | £7336.00 | £8703.00 | £1368.00 | £3972.36 |
| (13.57, 34, 20 to 57) | (1.17, 0, 0 to 3) | (0.8, 1, 1 to 3) | (7.04, 9, 9 to 27) | (1.6, 4.26, 2.2 to 7.6) | (1.17, 7.1, 6.97 to 9.97) | (0.48, 1.94, 1.35 to 2.73) | (3.58, 13, 13 to 21.83) | (6.01, 16, 10 to 28) | (0, 2, 2 to 2) | (0.49, 3, 2 to 3) | (4677.6, 15,767, 13,459.45 to 26,461.9) | (6029.25, 4480, 3499.45 to 19,349.86) | (1660.28, 9813, 6222.84 to 10,177.25) | (532.89, 1109, 821.2 to 2034.42) | (1114.07, 3972.46, 1959.75 to 5176.71) | ||
| MIFn | 43 years | 1.25 | 1.5 | FRI (× 1) NU (× 1) SNU (× 3) | N/A | 5.72 cm | 12.67 m | 2.45 | 43.67 m | 21 days | 2 | 3 | £19,037.00 | £7460.00 | £9049.00 | £3122.00 | £4532.18 |
| (14.11, 39, 29 to 64) | (0.5, 1.5, 0 to 3) | (0.5, 1.5, 1 to 2) | (2.34, 4.95, 3.47 to 9.52) | (2.22, 12.52, 4.6 to 15.9) | (0.63, 2.8, 1.1 to 2.85) | (11.39, 40.22, 12 to 62.43) | (3.7, 19, 15 5 to 27) | (0.43, 2, 2 to 3) | (0.83, 3, 2 to 4) | (3283.24, 17,498, 16,535.86 to 29,530.05) | (1266.66, 7115, 6247.81 to 12,740.33) | (1924.29, 8367, 7213.85 to 15,991.72) | (560.13, 3098, 898 to 3930.45) | (1881.78, 4837.91, 1736.96 to 7030.96) | |||
| p value | 0.43 | 0.285 | 0.742 | N/A | N/A | 0.603 | 0.366 | 0.08 | 0.044 | 0.121 | 1 | 1 | 0.215 | 0.3 | 0.383 | 0.065 | 0.803 |
ASA American Society of Anaesthesiologists physical status score, cm centimetre, ISS injury severity score, FRI fracture-related infection, ILFa Ilizarov fixation of an acute defect, ILFn Ilizarov fixation of a nonunion defect, LOS length of stay, m months, MIFa Masquelet and internal fixation of an acute defect, MIFn Masquelet and internal fixation of a nonunion/FRI defect, N/A not applicable, NU nonunion, OR operation room, SD standard deviation, SNU septic nonunion
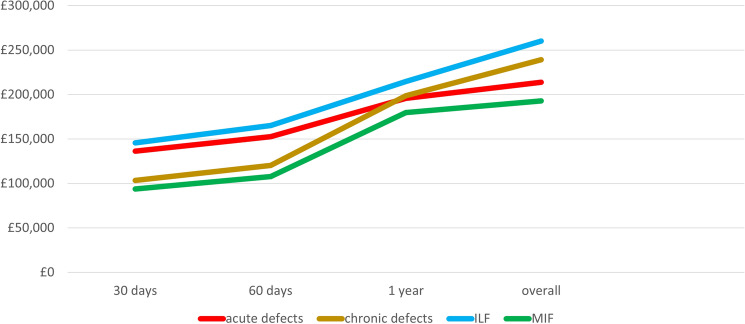
The evolution of the imposed costs per method and causative factor is presented in Fig. 1 and Table 5.
Fig. 1.
Evolution of the calculated costs between different time intervals (at 30 days, 2 months, and 12 months) from the primary debridement and defect relevant procedure between the different patient groups (acute, nonunion) and the two different methods of treatment (Ilizarov, Masquelet)
Table 5.
Evolution of the calculated costs between different time intervals from the primary debridement and defect relevant procedure between the different patient groups and the two different methods of treatment
| Costs | ||||
|---|---|---|---|---|
| Time period | Acute defects (× 10 patients) | Nonunion defects (× 10 patients) | Comparison of mean costs of acute vs. chronic p value |
|
| 30 days post admission | Mean, (SD) | £13,624, (£4542) | £10,070, (£4451) | 0.123 |
| Median, (range) | £13,104, (£5291–£20,940) | £8573, (£4368–£18,735) | ||
| Sum, (% to overall) | £136,242, (63.7%) | £103,263, (43.2%) | ||
| 60 days post admission | Mean, (SD) | £15,254, (£5028) | £11, 541, (£4633) | 0.195 |
| Median, (range) | £16,255, (£5704–£21,837) | £10,782, (£4834–£19,743) | ||
| Sum, (% to overall) | £152,541, (71.3%) | £120,235, (50.3%) | ||
| 1 year post admission | Mean, (SD) | £19,586, (£4372) | £18,933, (£5.343) | 0.934 |
| Median, (range) | £19,598, (£13,215–£26,216) | £16.936, (£12,419–£28,201) | ||
| Sum, (% to overall) | £195,862, (91.6%) | £198.598, (83.1%) | ||
| Till completion of follow-up | Mean, (SD) | £21,385, (£5796) | £23,399, (£8261) | 0.537 |
| Median, (range) | £21,504, (£13,459–£29,618) | £18,208, (£14,764–£36,274) | ||
| Sum, (% to overall) | £213,852, (100%) | £239,121, (100%) | ||
| Time period | Distraction osteogenesis protocol (ILF) (× 10 patients) | Induced membrane protocol (MIF) (× 10 patients) | Comparison of mean costs of ILF vs. MIF p value |
|
|---|---|---|---|---|
| 30 days post admission | Mean, (SD) | £14,561, (£4133) | £9390, (£3747) | 0.01 |
| Median, (range) | £15,192, (£8573–£20,940) | £9015, (£4368–£17,200) | ||
| Sum, (% to overall) | £145,607, (55.9%) | £93,898, (48.7%) | ||
| 60 days post admission | Mean, (SD) | £16,509, (£4220) | £10,769, (£4200) | 0.01 |
| median, (range) | £18,063, (£9261–£21,387) | £10,132, (£4833–£17,320) | ||
| Sum, (% to overall) | £165,087, (63.4%) | £107,689, (55.9%) | ||
| 1 year post admission | Mean, (SD) | £21,472, (£4482) | £17,974, (£5135) | 0.101 |
| Median, (range) | £22,715, (£12,897–£26,696) | £16,257, (£12,419–£28,201) | ||
| Sum, (% to overall) | £214,715, (82.5%) | £179,743, (93.3%) | ||
| Till completion of follow-up | Mean, (SD) | £26,126, (£7126) | £18,131, (£4196) | 0.024 |
| Median, (range) | £27,096, (£14,764–£36,274) | £16,788, (£13,459–£29,530) | ||
| Sum, (% to overall) | £260,263, (100%) | £192,711, (100%) | ||
ILF Ilizarov frame–distraction osteogenesis, MIF two-staged Masquelet protocol with internal fixation and grafting, SD standard deviation
Discussion
The complexity of managing large bone defects is well-described in the literature, as well as the various methods of treatment [2, 3], and their results. [30–32] However, evidence on the health economic aspect of their effective management is extremely scarce [33]. Theoretically, a complete health economic analysis includes direct, indirect, and intangible costs [4], whereas a cost effectiveness study should address both the societal and the health-care payer perspectives evaluating all relevant costs and benefits to the patient over their lifetime [34, 35].
Recently, Norris et al. [33] published a database analysis utilizing two different US-based sources including 904 patients with either the diagnosis of fracture/nonunion/osteomyelitis, treated with bone graft, cement spacer, or a frame fixator. Payer costs were analysed from the index admission to 12 months postoperatively. They concluded that patients with large defects require extended therapies, multiple hospital visits and admissions, representing a significant financial challenge.
Limb reconstruction procedures (ILF and MIF) are considered discrete episodes of care, associated with high up-front costs [33]. With this pilot cost analysis, we aimed to explore the differences of direct medical costs of the two main methods of managing acute or nonunion tibial defects in the best-case scenario of a successful union.
Within the limitations of our study, we recognise that we analysed a small number of patients (type II error). The size of our sample was not based on statistical power calculations, as the scope of this pilot study was to show the feasibility of collecting the data for conducting robust and detailed cost analysis and inform future evaluations of costs and effectiveness. The small number of patients in each subgroup prevented us from adjusting for clinical differences in terms of gender, Charlson’s score, etc. Since we studied a representative sample of patients with successful defect union (best-case scenario), our means and standard deviations may be artificially small, whilst we have compared values following their log transformation to address skewness. According to the power calculation based on the data herein, 35 patients from each group at a 1:1 ratio will be required to detect a 25% difference, with an alpha value of 0.05 and probability (power) of 0.9.
This study does refer to patients with complete data and a successful discharge following healing of their tibial defect. All possible direct medical costs during the initial treatment period, outpatient care, readmissions, and reoperations were measured. Exceptions were costs of outpatient rehabilitation, medications prescribed from primary care or purchased privately, and those of outpatient-parenteral-antibiotic-therapy services (OPAT), as well as productivity losses relevant to time off work. Noteworthy, the absence of health-related quality-of-life measures in this series, as well as the lack of adequate data in the literature, does not allow the comparison in QALY terms, but only into numerical figures of these direct medical costs.
The described clinical results in our series were found to be in accordance with other similar series for both the ILF [6, 36–39] and MIF [26, 30, 40, 41] methods. The demographics and bone defect size, the mean healing index of 2.1, and the incidence of complications per Paley classification [42] of the 5 ILFn patients in this study are consistent with those in the series of Krappinger et al. [38] Similarly, the baseline characteristics and overall outcome (mean healing index of 2.2 months/cm) reported by Mekhail et al.[39] were comparable to our subgroup of ILFa patients.
Main contributors on the cost differences noted (Tables 3, 4) were those related to the OR, and the more intense follow-up ILF patients require till defect union and consolidation of the regenerate bone. This is consistent with existing meta-analysis studies [30–32]. Selection bias between the groups is possible, as patients were not randomised preoperatively to receive either of the two methods.
The comparison between acute and nonunion/infected defects revealed lower number of admissions (p = 0.036), shorter follow-up (p = 0.04), and time-to-union for the acute defects (p = 0.048). No statistically significant differences were observed for the cost of infected cases (p = 0.537). This is perhaps attributable to the relatively low costs of the antibiotic therapies and the fact that it was not possible to capture costs incurred by primary care providers (including those of the OPAT service).
There is clear need for a pivotal health economic evaluation in this area, utilising the findings and some of the methodological aspects of the current study. The absolute need of using a health-related quality-of-life score as utility measures in future clinical series is also apparent to facilitate the translation of patient reported outcomes into effectiveness measures that are adequate to inform the optimal allocation of the scarce healthcare resources [34, 35].
Currently, in the NHS, limb reconstruction belongs to the specialist high-cost-tariff-excluded devices (HCTED) [43], attracting certain uplifts to their reimbursement. The generation of robust health economic evidence is expected to facilitate the update of such reimbursement arrangements, and their adoption into those managed with different techniques, as the Masquelet method.
This study does not report on the exact revenue of our unit, as this is influenced from the reimbursement arrangements of our hospital, and the reduced prices following the local implant tender. To provide more generalizable evidence, which could be relevant to different clinical groups, we based all our study on generic price lists and cost values, which do not take into account local negotiated prices.
The clinical need to have both methods available, together with others, is apparent from their widespread use globally. Each technique provides different features and advantages which make them preferable to certain scenarios. Bone transport (ILF) has many proven advantages in complex defects with associated deformities, allowing simultaneous tackling of all associated problems (bone defect reconstruction, realignment, infection control, mechanical stability, and immediate mobilisation) [6, 37, 44]. The more recently introduced Masquelet technique offers similar advantages and successful defect management independent of defect size. In addition, it requires less intense follow-up and probably is better suited for less compliant patients. [5, 40, 41]
Conclusion
This series of patients represents the routine experience of a large limb reconstruction trauma centre, utilising a variety of complementary methods to address the challenge of bone defect reconstruction. The results and analysis presented lead to some preliminary evidence on factors affecting the financial burden that such centres face. This highlights the need for further larger and more complete studies to aid decision makers and clinicians to improve contemporary reimbursement policies, ensuring that complex bone defect reconstruction is appropriately supported.
Data availability statement
Data supporting the reported results can be found to the Trauma-Related Services CSU clinical audit database of Leeds Teaching Hospitals NHS Trust.
Acknowledgements
To the following surgeons that have contributed to this series, as operating surgeons to the reported patients: Nikolaos Kanakaris, Peter Giannoudis, Paul Harwood, Martin Taylor, and Patrick Foster.
Author contributions
Conceptualization, NKK and PVG.; methodology, NKK, RMM, and PVG; validation, NKK, PJH, RMM, and PVG.; formal analysis, NKK and RMM.; investigation, NKK, GH, and GM.; data curation, NKK, GH, and GM; writing—original draft preparation, NKK.; writing—review and editing, NKK, RMM, PJH, and PVG. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Declarations
Conflict of interest
All the authors declare no conflict of interest.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Research and Innovation Review Board of Trauma-Related Services as a Quality Control—Clinical Audit study with protocol code 9015 and date of approval 04JAN2020). As such this study has been approved by the appropriate ethics—research and innovation committee and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent statement
Patient consent was waived due to the retrospective nature of fully anonymized data collection and analysis performed.
Contributor Information
Nikolaos K. Kanakaris, Email: nikolaoskanakaris@yahoo.co.uk
Paul J. Harwood, Email: paulharwood@nhs.net
Ruben Mujica-Mota, Email: r.e.mujica-mota@leeds.ac.uk.
Ganesh Mohrir, Email: ganesh.mohrir@nhs.net.
George Chloros, Email: gchlorosdoc@gmail.com.
Peter V. Giannoudis, Email: pgiannoudi@aol.com
References
- 1.Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J Bone Jt Surg Br. 2005;87(2):142–150. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 2.Bezstarosti H, Metsemakers WJ, van Lieshout EMM, Voskamp LW, Kortram K, McNally MA, et al. Management of critical-sized bone defects in the treatment of fracture-related infection: a systematic review and pooled analysis. Arch Orthop Trauma Surg. 2020 doi: 10.1007/s00402-020-03525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasanianos N, Kanakaris NK, Giannoudis PV. Current management of long bone large segmental defects. Orthop Trauma. 2009;24(2):149–163. doi: 10.1016/j.mporth.2009.10.003. [DOI] [Google Scholar]
- 4.Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38(Suppl 2):S77–84. doi: 10.1016/s0020-1383(07)80012-x. [DOI] [PubMed] [Google Scholar]
- 5.Masquelet A, Kanakaris NK, Obert L, Stafford P, Giannoudis PV. Bone repair using the masquelet technique. J Bone Jt Surg Am. 2019;101(11):1024–1036. doi: 10.2106/JBJS.18.00842. [DOI] [PubMed] [Google Scholar]
- 6.Papakostidis C, Bhandari M, Giannoudis PV Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Jt J. 2013;95-B(12):1673–80. 10.1302/0301-620X.95B12.32385. [DOI] [PubMed]
- 7.Summers S, Krkovic M. Bone transport with magnetic intramedullary nails in long bone defects. Eur J Orthop Surg Traumatol. 2020 doi: 10.1007/s00590-020-02854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan AN, Sayers R. Getting it right first time: what have we learnt? Surgery. 2020;38(10):627–631. doi: 10.1016/j.mpsur.2020.07.007. [DOI] [Google Scholar]
- 9.Briggs T, Perera JT, Picardo NE. Improving the quality of orthopaedic care within the National Health Service in England: "Getting it Right the First Time". 2012.
- 10.Kulkarni K, Shepherd S. Do we know the cost of orthopaedic care? Int J Health Plann Manag. 2019;34(1):71–86. doi: 10.1002/hpm.2571. [DOI] [PubMed] [Google Scholar]
- 11.Rupp M, Biehl C, Budak M, Thormann U, Heiss C, Alt V. Diaphyseal long bone nonunions—types, aetiology, economics, and treatment recommendations. Int Orthop. 2018;42(2):247–258. doi: 10.1007/s00264-017-3734-5. [DOI] [PubMed] [Google Scholar]
- 12.Coyle S, Kinsella S, Lenehan B, Queally JM. Cost-utility analysis in orthopaedic trauma; what pays? A systematic review. Injury. 2018;49(3):575–584. doi: 10.1016/j.injury.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Huff C. Talking treatment costs. Navigating financial discussions with patients. Exploring how the rising of healthcare affects the physician-patient relationship. Med Econ. 2015;92(8):38–40 (2). [PubMed]
- 14.Elliott DC, Rodriguez A. Cost effectiveness in trauma care. Surg Clin N Am. 1996;76(1):47–62. doi: 10.1016/s0039-6109(05)70421-7. [DOI] [PubMed] [Google Scholar]
- 15.Motta G, Hagler DD, McCooey AK. Reimbursement challenges and how to meet them. Ostomy Wound Manag. 1996;42(9):50–6 (8–9). [PubMed]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Doyle DJ, Goyal A, Bansal P, Garmon EH. American Society of Anesthesiologists Classification. StatPearls. Treasure Island (FL); 2020.
- 18.Association for the Advancement of Automotive Medicine. The Abbreviated Injury Scale: 2005 revision. Des Plaines, Ill.: 2005.
- 19.Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium-2018. J Orthop Trauma. 2018;32(Suppl 1):S1–S170. doi: 10.1097/BOT.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 20.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Jt Surg Am. 1976;58(4):453–458. doi: 10.2106/00004623-197658040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742–746. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Solomin L, Slongo T. Long bone defect classification: what it should be? J Bone Rep Recommend. 2016;2(1):1–2. doi: 10.4172/2469-6684.100016. [DOI] [Google Scholar]
- 23.NHS England and NHS Improvement. 2019/20 National Tariff Payment System. NHS England; 2019.
- 24.NICE (National Institute for Health and Care Excellence). BNF—British National Formulary. 2020.
- 25.Curtis L, Burns A. Unit Costs of Health and Social Care 2020. University of Kent, Canterbury.
- 26.Giannoudis PV, Harwood PJ, Tosounidis T, Kanakaris NK. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016;47(Suppl 6):S53–S61. doi: 10.1016/S0020-1383(16)30840-3. [DOI] [PubMed] [Google Scholar]
- 27.Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(Suppl 4):S28–34. doi: 10.1016/S0020-1383(11)70009-2. [DOI] [PubMed] [Google Scholar]
- 28.Paley D, Catagni MA, Argnani F, Villa A, Benedetti GB, Cattaneo R. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989;241:146–165. doi: 10.1097/00003086-198904000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Lowenberg DW, Randall RL. The Ilizarov method. Surg Technol Int. 1993;2:459–462. [PubMed] [Google Scholar]
- 30.Mi M, Papakostidis C, Wu X, Giannoudis PV. Mixed results with the Masquelet technique: a fact or a myth? Injury. 2020;51(2):132–135. doi: 10.1016/j.injury.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Xu C, Meng L, Dong P, Zhan H, Song S. Masquelet technique in treatment of infectious nonunion: a meta-analysis. Chin J Tissue Eng Res. 2021;25(15):2445–2452. [Google Scholar]
- 32.Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romano CL. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47(Suppl 6):S68–S76. doi: 10.1016/S0020-1383(16)30842-7. [DOI] [PubMed] [Google Scholar]
- 33.Norris BL, Vanderkarr M, Sparks C, Chitnis AS, Ray B, Holy CE. Treatments, cost and healthcare utilization of patients with segmental bone defects. Injury. 2021 doi: 10.1016/j.injury.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Rajan PV, Qudsi RA, Wolf LL, Losina E. Cost-effectiveness analyses in orthopaedic surgery: raising the bar. J Bone Jt Surg Am. 2017;99(13):e71. doi: 10.2106/JBJS.17.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders GD, Neumann PJ, Russell LB. Updated recommendations for cost-effectiveness studies-reply. JAMA. 2017;317(1):90. doi: 10.1001/jama.2016.17839. [DOI] [PubMed] [Google Scholar]
- 36.Battiston B, Santoro D, Baido RL, Pasquero F. Treatment of acute bone defects in severe lower limb trauma. Injury. 2019;50(Suppl 5):S40–S45. doi: 10.1016/j.injury.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Wei X, Liu P, Fu YH, Wang PF, Cong YX, et al. Quality of life and complications at the different stages of bone transport for treatment infected nonunion of the tibia. Medicine (Baltimore) 2017;96(45):e8569. doi: 10.1097/MD.0000000000008569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krappinger D, Irenberger A, Zegg M, Huber B. Treatment of large posttraumatic tibial bone defects using the Ilizarov method: a subjective outcome assessment. Arch Orthop Trauma Surg. 2013;133(6):789–795. doi: 10.1007/s00402-013-1712-y. [DOI] [PubMed] [Google Scholar]
- 39.Mekhail AO, Abraham E, Gruber B, Gonzalez M. Bone transport in the management of posttraumatic bone defects in the lower extremity. J Trauma. 2004;56(2):368–378. doi: 10.1097/01.TA.0000057234.48501.30. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu L, Durand M, Collombet JM, de Rousiers A, de l'Escalopier N, Masquelet AC. Induced membrane technique: a critical literature analysis and proposal for a failure classification scheme. Eur J Trauma Emerg Surg. 2020. 10.1007/s00068-020-01540-9. [DOI] [PubMed]
- 41.Zhao Z, Wang G, Zhang Y, Luo W, Liu S, Zeng Z, et al. Induced membrane technique combined with antibiotic-loaded calcium sulfate-calcium phosphate composite as bone graft expander for the treatment of large infected bone defects: preliminary results of 12 cases. Ann Transl Med. 2020;8(17):1081. doi: 10.21037/atm-20-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104. doi: 10.1097/00003086-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Commissioning N. National supply system for high-cost tariff-excluded devices. NHS England and NHS Improvement; 2020.
- 44.Salih S, Mills E, McGregor-Riley J, Dennison M, Royston S. Transverse debridement and acute shortening followed by distraction histogenesis in the treatment of open tibial fractures with bone and soft tissue loss. Strat Trauma Limb Reconstr. 2018;13(3):129–135. doi: 10.1007/s11751-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the reported results can be found to the Trauma-Related Services CSU clinical audit database of Leeds Teaching Hospitals NHS Trust.