Abstract
Caspase 1, formerly designated interleukin 1β (IL-1β)-converting enzyme, processes pro-IL-1β and pro-IL-18 to yield active cytokines that play a pivotal role in inflammation and cell activation. We show here the effect of caspase 1 deficiency on the inflammatory and adaptive immune responses to the fungus Candida albicans. Caspase 1 deficiency did not affect susceptibility to primary systemic infection with the fungus, as revealed by survival and fungal growth. However, Th1-mediated resistance to reinfection was greatly impaired in caspase 1-deficient mice, and this correlated with low-level production of IL-12 and gamma interferon. Early in infection, production of these cytokines and that of tumor necrosis factor alpha, IL-6, and, interestingly, IL-1β occurred normally in caspase 1-deficient mice, while that of IL-18 was severely impaired. Exogenous administration of IL-18, more than IL-12, restored the Th1-mediated resistance to the infection. We conclude that, while caspase 1 is not indispensable for release of mature IL-1β in candidiasis, the caspase 1-dependent production of IL-18 may represent an important and novel pathway for the expression of sustained Th1 reactivity to the fungus.
Caspases, an expanding family of cysteine proteases with a substrate specificity for aspartic acid, play pivotal roles in inflammation and mammalian apoptotic cell death (9, 11). The prototype, caspase 1, or interleukin 1β (IL-1β)-converting enzyme (8, 51), participates in the cellular export of some proinflammatory cytokines, thus having a prominent role in inflammation. Precursors of both IL-1β (8, 50) and IL-18 (16, 17, 23, 50) undergo proteolytic cleavage by caspase 1, permitting the activated cytokines to exit the cells. Peptide inhibitors of caspase 1 block IL-1β and IL-18 release from activated macrophages in vitro (17). Caspase 1-deficient mice fail to exhibit elevated levels of IL-1β, IL-18, or other proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1α, or IL-6, following lipopolysaccharide (LPS) challenge. They are also resistant to LPS toxicity (24). However, other enzymes, in addition to caspase 1, are able to cleave pro-IL-1β and generate biologically active molecules (13, 53), including bacterial (4, 19) and fungal (3) enzymes. Thus, it appears that caspase 1 is not always indispensable for release of active IL-1β, but it is necessary for the production of bioactive mature IL-18 (12, 13). Like IL-12, IL-18 promotes gamma interferon (IFN-γ) production by Th1 and natural killer cells in both mice and humans (reviewed in references 10 and 31) and increases Th1 resistance to infections (5, 20, 36, 52, 56). Unlike IL-12, however, IL-18 by itself is unable to induce IFN-γ (32) and to drive Th1 development (37).
Previous studies showed that IL-12-induced IFN-γ production is essential for resistance to Candida albicans (28, 35, 38, 39, 46), the most frequently isolated fungal pathogen of humans (30). In mucosal colonization and systemic infection of mice with the fungus, Th1 cells mediate phagocyte-dependent protection and are the principal mediators of acquired protective immunity. In contrast, production of inhibitory cytokines such as IL-4 and IL-10 by Th2 cells and high levels of immunoglobulin E are associated with disease progression (28, 35, 38, 39, 46). Th2-like reactivity is frequently observed in patients with Candida-related pathology, such as in symptomatic infections (14, 35) and allergy (2). Th1-type responses may thus characterize the carriage of saprophytic yeast and the resistance to disease seen in healthy humans, whereas Th2 responses may be associated predominantly with pathology.
In murine candidiasis, Th1 differentiation requires the combined effects of different cytokines in the relative absence of counterregulatory cytokines, such as IL-4 and IL-10, which are, per se, necessary and sufficient to drive Th2 polarization (25). Deficient IFN-γ (6), transforming growth factor β (48), IL-6 (43), and TNF-α (26) responses could each block the induction of protective immunity; however, only IL-12 was both required and prognostic for the development of protective Th1 responses to Candida (44, 45).
Because IL-18 synergizes with IL-12 for induction of Th1 cell development (37, 49), in the present study we used caspase 1-deficient mice to assess (i) the patterns of proinflammatory and Th cytokine production in C. albicans infection and (ii) the effect of exogenous IL-18 on IL-12 and IFN-γ production and resistance to the infection.
MATERIALS AND METHODS
Mice.
Caspase 1−/− mice were obtained as previously described (17). Briefly, chimeric mice were obtained by injection of embryonic stem cells, in which the caspase 1 gene was disrupted and replaced with a neomycin resistance cassette gene, into C57BL/6 blastocysts. The chimeric males were then mated with C57BL/6 mice. Homozygous mice with two copies of the disrupted caspase 1 gene were identified by Southern blotting of genomic DNA, and the absence of caspase 1 mRNA in caspase 1−/− mice was confirmed by reverse transcriptase PCR (RT-PCR) analysis. Homozygous mice were then interbred and used for the experiments. Animals were housed under specific-pathogen-free conditions at the breeding facilities of the University of Perugia, Perugia, Italy. C57BL/6 mice, 6 to 8 weeks old, were obtained from Charles River (Calco, Italy). (SV129 × C57BL/6)F1 mice, hereafter designated (SV129 × B6)F1, 6 to 8 weeks old, were obtained from the Jackson Laboratory (Bar Harbor, Maine). For each experiment, groups of mice were matched, as closely as possible, for sex and age. Procedures involving animals and their care were conducted in conformity with national and international laws and policies.
Yeasts, infections, in vivo analysis, and treatments.
The origin and characteristics of the C. albicans low-virulence, live vaccine strain PCA-2 and the high-virulence CA-6 strain used in this study have been described in detail previously (6, 25, 42). For infection, yeast cells were washed twice in saline and diluted to the desired density to be injected intravenously (i.v.) via the lateral tail vein in a volume of 0.5 ml/mouse as previously described (6, 25, 42). The viability of the cells was >95% on trypan blue dye exclusion test and quantitative cultures. Resistance to reinfection was assessed by injecting mice with 106 virulent Candida cells i.v. 14 days after primary infection. Mice succumbing to yeast challenge were routinely necropsied for histopathologic confirmation of disseminated candidiasis. Absolute numbers of neutrophils in peripheral blood were determined by total and differential white cell counts. Quantification of yeast in the organs of infected mice was performed by a plate dilution method, using Sabouraud dextrose agar. Results were expressed as CFU per organ (mean ± standard error [SE]). Recombinant murine IL-18 (rIL-18) (R&D Systems Inc., Minneapolis, Minn.) or rIL-12 (Genetics Institute, Cambridge, Mass.) was given intraperitoneally (i.p.) at the dose of 1 μg/injection or 10 ng/injection, respectively, on the day of primary or secondary infection and 1 and 3 days later.
Purification and culture of cells.
CD4+ T splenocytes were purified by using anti-mouse CD4-conjugated magnetic MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, 107 total spleen cells were incubated with 100 μl of magnetically activated cell-sorting CD4 MicroBeads for 15 min at 6°C, washed, and magnetically separated with a positive selection column (Miltenyi Biotec), according to the manufacturer's instructions. Splenic macrophages were obtained by 2-h plastic adherence as previously described (6, 27, 42). Peritoneal neutrophils were collected 18 h after i.p. inoculation of aged, endotoxin-free 10% thioglycolate solution (Difco, Detroit, Mich.) as previously described (40, 41). Unfractionated splenocytes or CD4+ cells (10 × 106/ml) were cultured in complete medium with 106 heat-inactivated C. albicans cells per ml for 48 h, before cytokine measurement in culture supernatants. Irradiated splenocytes (2,000 rad) were added (106/ml) to the CD4+ cultures as antigen-presenting cells. In selected experiments, rIL-18 (50 ng/ml) was added to the cultures of unfractionated splenocytes together with rIL-2 (100 U/ml) as described previously (18).
Cytokine assays.
The levels of TNF-α, IL-6, IFN-γ, and IL-12 in culture supernatants were determined by means of cytokine-specific enzyme-linked immunosorbent assay (ELISA), using pairs of anticytokine monoclonal antibodies as previously described (6, 27, 42). The monoclonal antibody pairs used were as follows, listed by capture-biotinylated detection: TNF-α, MP6-XT22–MP6-XT3; IL-6, MP5-20F3–MP5-32c11; and IFN-γ, R4-6A2–XMG1.2 (PharMingen, San Diego, Calif.). For IL-12p70 measurement, a modified antibody-capture bioassay was used (48). The levels of IL-1β and IL-18 were determined using the specific ELISA kit (R&D Systems).
Candidacidal assay and NO production.
For the candidacidal assay, 5 × 105 peritoneal neutrophils or splenic macrophages were incubated with 5 × 104 PCA-2 cells in 96-well flat-bottomed microtiter plates (Costar, Cambridge, Mass.) for 1 or 4 h, respectively, and the number of CFU was determined as described previously (7). The percentage of CFU inhibition (mean ± SE) was determined as a percentage of colony formation inhibition = 100 − (CFU for experimental group/CFU for control cultures) × 100. Nitrite concentration, a measure of nitric oxide (NO) synthesis, was assayed in culture supernatants by a standard Griess reaction adapted to microplates, as described previously (7). The data represent the means ± SEs of quadruplicate determinations and are expressed as micromolar concentrations of NO2− per 107 cells.
RT-PCR.
RNA extraction and amplification of synthesized cDNA from splenic adherent macrophages and purified CD4+ splenocytes were performed as previously described (6, 26, 27). For hypoxanthine-guanine phosphoribosyltransferase (HPRT), IL-12p40, IFN-γ, IL-4, and IL-12 receptor β2 (IL-12Rβ2), the primers and positive controls, cycles, and temperatures were as previously described (6, 26, 27). For IL-18R, the primers were synthesized using a 391 DNA synthesizer (PCR-MATE; Applied Biosystems, Foster City, Calif.). The sequences of 5′ sense primer and 3′ antisense primer were as follows: sense, 5′-ATGTTGTCGTCTCCTTCCTG-3′; antisense, 5′-ATGTTGTCGTCTCCTTCCTG-3′. Each cycle consisted of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The HPRT primers were used as a control for both reverse transcription and the PCR itself and also for comparing the amounts of products of samples obtained with the same primer. The PCR fragments were analyzed by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. PCR-assisted mRNA amplification was repeated at least twice for at least two separately prepared cDNA samples for each experiment. Data are representative of three different experiments.
Statistical analysis.
Survival data were analyzed using the Mann-Whitney U test. Student's t test for unpaired data was used to compare the fungal growth, the cytokine and NO production, and the candidacidal activity. Significance was defined as P ≤ 0.05. In vivo groups consisted of four to six animals. Unless otherwise specified, the data reported were pooled from three to five experiments.
RESULTS
Course of primary and secondary C. albicans infections in caspase 1-deficient mice.
Caspase 1−/− and C57BL/6 and (SV129 × B6)F1 mice, which have a genetic background comparable with that of the caspase 1−/− mice, were injected i.v. with 106 cells of low-virulence strain PCA-2 or 105 cells of highly virulent C. albicans strain CA-6. For secondary infection, 14 days after primary i.v. challenge, mice were i.v. injected with 106 cells of CA-6. The results (Table 1) show that survival of the primary systemic infection with either PCA-2 or CA-6 did not differ between caspase 1−/− mice and either C57BL/6 or (SV129 × B6)F1 mice, each group of mice having survived the PCA-2 infection while similarly succumbing to the CA-6 infection. However, upon reinfection of mice surviving PCA-2 infection, C57BL/6 and (SV129 × B6)F1 mice survived the infection, but caspase 1−/− mice did not. Quantification of fungal growth in organs in the course of the infection did not reveal major differences between mutant and wild-type mice (data not shown). Similarly, histopathological examination of the kidneys of PCA-2-infected mice revealed a slightly increased number of foci of inflammatory reaction throughout the kidney parenchyma in caspase 1−/− mice, compared to the few lesions observed in the cortex of kidneys from (SV129 × B6)F1 mice (data not shown). Therefore, these results suggest that effector mechanisms of resistance to primary C. albicans infection were not affected in caspase 1-deficient mice, which indeed efficiently oppose infectivity in the initial stage of infection. However, caspase 1 deficiency appears to impair the development of acquired resistance upon primary sublethal infection.
TABLE 1.
Susceptibility of caspase 1-deficient mice to primary and secondary C. albicans infections
| Mice | Value for infection:
|
|||||
|---|---|---|---|---|---|---|
| Primarya
|
Secondaryb
|
|||||
| C. albicans strain | Dose (cells) | MSTc | D/Td | MSTc | D/Td | |
| Caspase 1−/− | PCA-2 | 106 | >60 | 2/18 | 17 | 12/12 |
| CA-6 | 105 | 13 | 12/12 | |||
| C57BL/6 | PCA-2 | 106 | >60 | 0/18 | >60 | 0/12 |
| CA-6 | 105 | 14 | 12/12 | |||
| (SV129 × B6)F1 | PCA-2 | 106 | >60 | 3/18 | >60 | 0/12 |
| CA-6 | 105 | 16 | 12/12 | |||
Mice were infected i.v. with low-virulence PCA-2 or virulent CA-6 C. albicans.
CA-6 (106 cells) was given i.v., 14 days after primary infection.
MST, median survival time (days).
D/T, number of dead mice over total number of mice injected.
Antifungal effector functions are unimpaired in caspase 1-deficient mice.
To assess the antifungal phagocytic response in caspase 1-deficient mice upon primary infection, caspase 1−/− and C57BL/6 mice were infected i.v. with PCA-2 and the antifungal effector functions of splenic macrophages and peritoneal neutrophils were assessed 3 days after the infection. The results (Fig. 1) indicate that both types of cells were equally activated to a candidacidal state in mutant and wild-type mice. Similarly, neutrophils and, to a lesser extent, macrophages produced NO upon exposure to C. albicans in vitro. We also determined the number of circulating neutrophils 2 days after infection and found that neutrophil counts increased in both types of mice upon infection, being actually higher in caspase 1-deficient mice (from 361 ± 44 to 4,168 ± 380 in mutant mice and from 936 ± 89 to 2,980 ± 235 in wild-type mice). Therefore, innate antifungal effector functions are unimpaired in caspase 1-deficient mice.
FIG. 1.
Antifungal effector functions of macrophages and neutrophils from caspase 1−/− (solid bars) and caspase 1+/+ C57BL/6 (open bars) mice, uninfected or upon primary i.v. infection with C. albicans. Three days after infection, splenic adherent macrophages and elicited peritoneal neutrophils were assessed for their ability to kill yeast cells and to secrete NO in vitro. Assays were done as described in Materials and Methods. ∗, P < 0.05, caspase 1−/− versus caspase 1+/+ mice.
Protective anticandidal Th1 responses are inhibited in caspase 1-deficient mice.
Protective acquired resistance to C. albicans correlates with the induction of CD4+ Th1 cells, producing IFN-γ and expressing the IL-12Rβ2 (6, 26, 42). To assess the pattern of Th1 (IL-12 and IFN-γ) and Th2 (IL-4) cytokine and IL-12R expression in caspase 1-deficient mice, mice were infected i.v. with PCA-2 and reinfected with CA-6 14 days later. Three days after reinfection, the expression of IL-12p40 (splenic macrophages), IFN-γ, IL-4, and IL-12Rβ2 (CD4+ T-lymphocyte) genes was assessed by RT-PCR for mutant and wild-type mice. Messages for IFN-γ, IL-12p40, and IL-12Rβ2 were poorly (IFN-γ) or not (IL-12p40 and IL-12Rβ2) detected in caspase 1-deficient mice upon infection, as opposed to what was observed for wild-type mice (Fig. 2). Moreover, the IL-4 mRNA was detected in CD4+ cells from mutant but not wild-type mice. Because the IL-18R was found to be selectively expressed on Th1 but not Th2 cells (54), we also looked for the IL-18R message in CD4+ splenocytes. No differences were found in the expression of the message between mutant and wild-type mice. These results indicate that susceptibility of caspase 1−/− mice to secondary C. albicans infection correlates with the failure to induce the activation of Th1 cells and the occurrence of IL-4-producing CD4+ Th2 cells.
FIG. 2.
Cytokine and cytokine receptor gene expression in caspase 1−/− and caspase 1+/+ C57BL/6 mice upon C. albicans infection. Mice were either uninfected (lanes 1) or reinfected (lanes 2) with virulent CA-6, 14 days after the primary infection with PCA-2. Levels of IFN-γ, IL-4, IL-12Rβ2, and IL-18R mRNA (in CD4+ T splenocytes) and of IL-12p40 (in splenic macrophages) were determined by RT-PCR, 3 days after reinfection. C, HPRT- or cytokine- or cytokine receptor-specific control. N, no DNA added to the amplification mix during PCR. Mφ, macrophages.
Production of IL-18 is impaired in caspase 1-deficient mice infected with C. albicans.
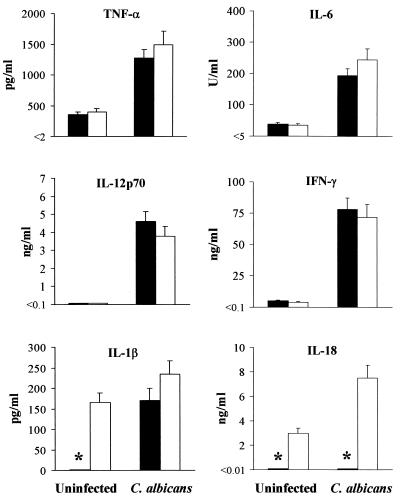
As the release of some proinflammatory cytokines could be impaired in caspase 1-deficient mice (16, 17, 23, 24) and production of TNF-α (26), IL-6 (43), IFN-γ (6), and IL-12 (27) is required for the generation of anticandidal Th1 cell responses in vivo, we assessed levels of these cytokines, together with those of IL-1β and IL-18, in mutant and wild-type mice upon infection. As early as 3 days after PCA-2 infection, the production of TNF-α, IL-6, IL-12, and IFN-γ was observed in caspase 1-deficient mice at levels similar to those observed for wild-type mice (Fig. 3). IL-1β was produced in wild-type mice and, interestingly, even in mutant mice. However, IL-18 could not be detected in the latter mice as opposed to wild-type mice (Fig. 3), despite the presence of the IL-18 message (data not shown). Both cytokines were detected in culture supernatants of purified neutrophils and macrophages from wild-type infected mice upon stimulation with IFN-γ and LPS in vitro (data not shown). These results suggest that, early in infection, production of proinflammatory cytokines, including IL-12 and IFN-γ, was not affected in caspase 1-deficient mice upon C. albicans infection. In contrast, a defective production of IL-18 was observed.
FIG. 3.
Production of proinflammatory cytokines in caspase 1−/− (solid bars) and caspase 1+/+ (open bars) mice uninfected or infected with C. albicans. Mice were i.v. infected with 106 PCA-2 cells and assessed 3 days later for cytokine production in culture supernatants of antigen-stimulated splenocytes. Levels of cytokines were determined by means of cytokine-specific ELISA. Cytokine levels in culture supernatants of unstimulated responder cells were below the detection limit of the assay, indicated by < in the y axis. ∗, P < 0.05, caspase 1−/− versus caspase 1+/+ mice.
Exogenous IL-18 restores antifungal resistance and IFN-γ and IL-12p70 production in caspase 1-deficient mice.
To assess whether IL-18 deficiency is responsible for the impaired anticandidal Th1 reactivity in caspase 1-deficient mice, exogenous rIL-18 was administered to mutant mice infected with PCA-2. The cytokine was given either at the time of the primary infection with PCA-2 or at the time of the secondary infection with CA-6. For comparison, exogenous rIL-12 was similarly administered to infected mice. Mice were monitored for resistance to the secondary infection, in terms of fungal growth in the kidneys and production of IFN-γ and IL-12. The results (Table 2) show that the fungal load was significantly decreased in mice treated with rIL-18, at the time of primary or secondary infection. Moreover, the production of IFN-γ by CD4+ T cells and that of IL-12p70 by splenocytes were significantly increased in treated, compared to untreated, mice. Similar results, although to a lesser extent, were obtained upon treatment with rIL-12 at the time of the secondary infection. The failure of earlier treatment with rIL-12 to increase Th1-mediated resistance to reinfection is a finding in line with previous results (44). In vitro, rIL-18 also increased IFN-γ and IL-12 production by antigen-activated splenocytes from caspase 1-deficient mice upon reinfection, particularly in the presence of IL-2. Such an increase was not observed upon antigen activation of splenocytes from nonvaccinated mice, upon infection with virulent CA-6 cells (Table 3). These results suggest that rIL-18 restores the Th1-mediated resistance of caspase 1-deficient mice to C. albicans infection, an activity that cannot be fully compensated for by exogenous rIL-12. In addition, it appears that memory rather than naive Th cells are more susceptible to the ability of IL-18 to promote IFN-γ production. Therefore, IL-18 plays an important role in maintaining sustained IFN-γ and IL-12 production in mice with C. albicans infection.
TABLE 2.
Effect of rIL-18 on Th1-mediated resistance of caspase 1−/− mice to candidiasis
| Treatment | 103 CFU/kidneysa | Cytokine productionb
|
|
|---|---|---|---|
| IFN-γ | IL-12p70 | ||
| None | 4,532 ± 754 | 81 ± 27 | <0.1 |
| rIL-18c | 1,785 ± 540e | 168 ± 34e | 5.89 ± 1.0e |
| rIL-18d | 1,207 ± 365e | 149 ± 38e | 6.52 ± 0.8e |
| rIL-12c | 4,032 ± 540 | 94 ± 12 | <0.1 |
| rIL-12d | 2,671 ± 481e | 159 ± 27e | 2.01 ± 0.8e |
Caspase 1−/− mice were infected i.v. with 106 cells of PCA-2 and reinfected with 106 cells of CA-6 C. albicans, 14 days later. Data are shown as CFU (means ± SEs) in the kidneys, at 2 weeks after reinfection (n = 12).
Cytokine content (nanograms per milliliter) in the culture supernatants of antigen-stimulated CD4+ splenocytes (IFN-γ) or in the blood (IL-12p70), at 2 weeks after reinfection, as assessed by cytokine-specific ELISA (n = 12).
Murine rIL-18, 1 μg/injection, or rIL-12, 10 ng/injection, was administered i.p. on the day of PCA-2 infection and 1 and 3 days later.
rIL-18, 1 μg/dose, or rIL-12, 10 ng/dose, was administered i.p. on the day of reinfection with CA-6 and 1 and 3 days later.
P < 0.05 (cytokine-treated versus untreated mice).
TABLE 3.
Effect of rIL-18 on IFN-γ and IL-12 production by C. albicans-stimulated cells from naive or vaccinated mice
| Cell typea | rIL-18 | Cytokine productionb
|
|
|---|---|---|---|
| IFN-γ | IL-12p70 | ||
| Naive | − | 58 ± 12 | 2.04 ± 1.1 |
| + | 61 ± 10 | 1.91 ± 0.8 | |
| Vaccinated | − | 95 ± 24 | 0.81 ± 0.4 |
| + | 214 ± 31c | 4.24 ± 0.7c | |
Spleen cells were taken from naive or vaccinated caspase 1−/− mice after 3 days of infection with 106 CA-6 cells. Cells were cultured in vitro with heat-inactivated C. albicans and 100 U of rIL-2 per ml in the presence (+) or absence (−) of 50 ng of rIL-18 per ml for 48 h. Vaccinated mice received 106 cells of low-virulence PCA-2, 14 days before reinfection.
Cytokine content (nanograms per milliliter) as assessed by cytokine-specific ELISA.
P < 0.05, rIL-18 (+) versus rIL-18 (−) cultures.
DISCUSSION
In the present study, the use of caspase 1-deficient mice has provided us with new insights into the cytokine-dependent regulation of immunity to C. albicans. The major findings are, firstly, that production of IL-18 is required for sustained expression of Th1 protective immunity to the fungus and, secondly, that caspase 1 activity is not necessary for the production of mature IL-1β, as it is for mature IL-18.
Studies performed on caspase 1 (12, 13, 17, 23, 24)- or IL-18 (49, 52)-deficient mice have revealed an essential role for IL-18 in IFN-γ production in models of infection and inflammation. Despite normal (13) or even higher (52) levels of IL-12 production, reduced levels of IFN-γ were observed in caspase 1-deficient mice after stimulation with LPS (10, 12, 13) or in IL-18-deficient mice upon infection with intracellular or extracellular pathogens (52). Although IL-18 and IL-12 exerted a synergistic effect on IFN-γ production by Th1 cells (1, 55), IL-18 also acted as an IL-12-independent regulator of IFN-γ production (21) and of cell proliferation induced by microbial stimuli (13). Indeed, in the absence of IL-18, IL-12 alone was insufficient for the induction of Th1 cell expansion in vivo (49, 52). Furthermore, it has recently been demonstrated that IL-18R is selectively expressed on murine Th1, but not Th2, cells (54, 55). Therefore, although IL-18, unlike IL-12, was unable to drive Th1 cell expansion in vitro (37), these results clearly indicate that IL-18 has a direct and profound effect on the activation and development of Th1 cells in vivo. The results of the present study confirm this notion, by clearly showing that IL-18 is required for sustained production of IFN-γ and IL-12 in C. albicans infection. Acquired immunity to the fungus relied on the induction of protective Th1 cells producing IFN-γ and expressing the IL-12Rβ2. Although caspase 1-dependent IL-18 production was not required for IFN-γ production after concanavalin A stimulation (13), IL-18 sustained the expression of the IL-12Rβ2 mRNA (54). Thus, IL-18R may transmit signals that maintain antifungal Th1 development through the IL-12R complex. As in turn IL-12 up-regulates the expression of the IL-18R (55), the synergistic effect of IL-12 and IL-18 on Th1 development may rely on the reciprocal regulation of their receptors. However, the expression of the IL-18R gene was not impaired in caspase 1-deficient mice upon C. albicans infection, nor in IL-12-deficient mice after infection (data not shown). These findings indicate that factors other than IL-12 may regulate the IL-18R mRNA in C. albicans infection.
Production of proinflammatory cytokines, including IFN-γ and IL-12, occurred normally in caspase 1-deficient mice early in infection, a finding suggesting that the early cytokine response in C. albicans infection is relatively independent of caspase 1 processing of pro-IL-18. This observation is apparently at variance with what was observed in an experimental model of cryptococcosis, in which the protective efficacy of IL-18 alone (20) or combined with IL-12 (36) was seen early on but not at 3 to 6 weeks after infection and was dependent on IFN-γ production by stimulated NK cells (56). It appears that differences in the relative contributions of various effector mechanisms in the host defense against each fungal pathogen may determine the outcome of treatment with IL-18.
One important observation of the present study is that production of IL-1β was observed in caspase 1-deficient mice infected with C. albicans. That caspase 1 is not always required for release of active IL-1β and that the requirement for caspase 1 in IL-1β processing is stimulus dependent has already been reported (13, 53). In particular, the finding that IL-1β is produced in caspase 1-deficient mice after C. albicans infection suggests that proteinases secreted by C. albicans may play an important role in IL-1β processing, as already demonstrated (3). In this regard, it is worth mentioning that proteinases are produced by the PCA-2 C. albicans strain used in the present study, particularly during infection (F. de Bernardis, personal communication).
It has been suggested that C. albicans proteinases may contribute to the inflammatory nature of mucosal candidiasis by local activation of inflammatory IL-1β (3). In our study, caspase 1−/− mice were not overtly more susceptible than wild-type mice to gastrointestinal C. albicans infection (data not shown). This finding would suggest a nonessential role of IL-1β in the pathogenesis of mucosal candidiasis, even though local production of IL-1β was not measured in caspase 1-deficient mice with gastrointestinal infection. Instead, production of IL-1β was observed in the course of disseminated infection with low-virulence C. albicans, a finding that confirms the protective effect that IL-1β may have in infection (34).
Given the involvement of caspase 1 and IL-18 in inflammatory (15, 29) and noninflammatory (47) disease and the growing importance of IL-18 in the induction of optimal host immune defenses against pathogens (22, 31) and tumors (33), the present study provided us with important insights into the caspase 1-dependent IL-18 production in mice with candidiasis. As the experimental model adopted in the present study closely mimics the state of long-lived commensalism with the fungus and the ensuing immunity to it (35, 46), it appears that IL-18 meets the requirement of a candidate cytokine which is required for sustained expression of anticandidal Th1 immunity in self-limiting infection and saprophytism.
ACKNOWLEDGMENTS
This study was supported by the National Research Project on AIDS, contract 50B.33, “Opportunistic Infections and Tuberculosis,” Italy.
We thank Jo-Anne Rowe for editorial assistance. The murine rIL-12 was supplied by the Bioanalytical Sciences Department of Genetics Institute Inc., Cambridge, Mass.
REFERENCES
- 1.Ahn H, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J Immunol. 1997;159:2135–2141. [PubMed] [Google Scholar]
- 2.Akiyama K, Shida T, Yasueda H, Yanagihara Y, Hasegawa M, Maeda Y, Yamamoto T, Takesako K, Yamaguchi H. Allergenicity of acid protease secreted by Candida albicans. Allergy. 1996;51:887–892. doi: 10.1111/j.1398-9995.1996.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 3.Beauséjour A, Grenier D, Goulet J P, Deslauriers N. Proteolytic activation of the interleukin-1β precursor by Candida albicans. Infect Immun. 1998;66:676–681. doi: 10.1128/iai.66.2.676-681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R A, Kronheim S R, Cantrell M, Deeley M C, March C J, Prickett K S, Wignall J, Conlan P J, Cosman D, Hopp T P, Mocnizuki D Y. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- 5.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heeseman J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 6.Cenci E, Mencacci A, Del Sero G, d'Ostiani C F, Mosci P, Kopf M, Romani L. IFN-γ is required for IL-12 responsiveness in mice with Candida albicans infection. J Immunol. 1998;161:3543–3550. [PubMed] [Google Scholar]
- 7.Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 8.Cerretti D P, Kozlosky C J, Mosley B, Nelson N, Van Ness K, Greenstreet T A, March C J, Kronheim S R, Druck T, Cannizzaro L A, Heubner K, Black R A. Molecular cloning of the interleukin-1β-converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello C A, Novick D, Puren A J, Fantuzzi G, Shapiro L, Mühl H, Yoon D-Y, Reznikov L L, Kim S-H, Rubinstein M. Overview of interleukin-18: more than an interferon-γ inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 11.Elkon K B. Caspases: multifunctional proteases. J Exp Med. 1999;190:1725–1728. doi: 10.1084/jem.190.12.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantuzzi G, Ku G, Harding M W, Livingston D J, Sipe J D, Kuida K, Flavell R A, Dinarello C A. Response to local inflammation of IL-1β-converting enzyme deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 13.Fantuzzi G, Puren A J, Harding M W, Livingston D J, Dinarello C A. Interleukin-18 regulation of interferon-γ production and cell proliferation as shown in interleukin-1β-converting enzyme (caspase-1)-deficient mice. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 14.Fidel P L, Jr, Sobel J D. The role of cell-mediated immunity in candidiasis. Trends Microbiol. 1994;16:202–206. doi: 10.1016/0966-842x(94)90112-i. [DOI] [PubMed] [Google Scholar]
- 15.Furlan R, Martino G, Galbiati F, Poliani P L, Smiroldo S, Bergami A, Desina G, Comi G, Flavell R, Su M S, Adorini L. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- 16.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino T, Wiltrout R H, Young H A. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–5077. [PubMed] [Google Scholar]
- 19.Kapur V, Majesky M W, Li L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by introducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 21.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 22.Kremer L, Dupré L, Wolowczuk I, Locht C. In vivo immunomodulation following intradermal injection with DNA encoding IL-18. J Immunol. 1999;163:3226–3231. [PubMed] [Google Scholar]
- 23.Kuida K, Lippke J A, Harding M W, Livingston D J, Su M S, Flavell R A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F Y, Wong W, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:410–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 25.Mencacci A, Cenci E, Bistoni F, Bacci A, Del Sero G, Montagnoli C, d'Ostiani C F, Romani L. Specific and non-specific immunity to Candida albicans: a lesson from genetically modified animals. Res Immunol. 1998;149:352–361. doi: 10.1016/s0923-2494(98)80759-1. [DOI] [PubMed] [Google Scholar]
- 26.Mencacci A, Cenci E, Del Sero G, d'Ostiani C F, Montagnoli C, Bacci A, Bistoni F, Quesniaux V F J, Ryffel B, Romani L. Defective costimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-α double-deficient mice infected with Candida albicans. Int Immunol. 1998;10:37–48. doi: 10.1093/intimm/10.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Mencacci A, Cenci E, Del Sero G, d'Ostiani C F, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- 28.Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Romani L, Puccetti P, Bistoni F. Rationale for cytokine and anti-cytokine therapy of Candida albicans infection. J Mycol Med. 1995;5:25–30. [Google Scholar]
- 29.Monteleone G, Trapasso F, Parello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 30.Odds F C. Candida and candidosis. 2nd ed. London, United Kingdom: Baillière-Tindall; 1988. [Google Scholar]
- 31.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 32.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 33.Oshikawa K, Shi F, Rakhmilevich A L, Sondel P M, Mahvi D M, Yang N. Synergistic inhibition of tumor growth in a murine mammary adenocarcinoma model by combinational gene therapy using IL-12, pro-IL-18 and IL-1β converting enzyme cDNA. Proc Natl Acad Sci USA. 1999;96:13351–13356. doi: 10.1073/pnas.96.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pecyk R A, Fraser-Smith E B, Matthews T R. Efficacy of interleukin-1β against systemic Candida albicans infections in normal and immunosuppressed mice. Infect Immun. 1989;57:3257–3258. doi: 10.1128/iai.57.10.3257-3258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puccetti P, Romani L, Bistoni F. A Th1-Th2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 1995;3:237–240. doi: 10.1016/s0966-842x(00)88931-3. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi M H, Zhang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto M, Kawakami K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–649. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 38.Romani L. The T cell response to fungi. Curr Opin Immunol. 1997;9:484–490. doi: 10.1016/s0952-7915(97)80099-4. [DOI] [PubMed] [Google Scholar]
- 39.Romani L. Immunity to Candida albicans: Th1, Th2 and beyond. Curr Opin Microbiol. 1999;2:363–367. doi: 10.1016/S1369-5274(99)80064-2. [DOI] [PubMed] [Google Scholar]
- 40.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 41.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 42.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 43.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S F, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;152:5167–5175. [PubMed] [Google Scholar]
- 45.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Wolf S, Puccetti P, Bistoni F. Interleukin-12 but not interferon-γ production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur J Immunol. 1994;24:909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- 46.Romani L, Puccetti P, Bistoni F. Biological role of Th cell subsets in candidiasis. Chem Immunol. 1996;63:115–137. [PubMed] [Google Scholar]
- 47.Rothe H, Jenkins N, Copeland N, Kolb H. Active stage of autoimmune diabetes is associated with the expression of a novel cytokine, IGIF, which is located near Idd2. J Clin Investig. 1997;99:469–474. doi: 10.1172/JCI119181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaccapelo R, Romani L, Tonnetti L, Cenci E, Mencacci A, Tognellini R, Reed S G, Puccetti P, Bistoni F. TGF-β is important in determining the in vivo susceptibility or resistance in mice infected with Candida albicans. J Immunol. 1995;155:1349–1360. [PubMed] [Google Scholar]
- 49.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18 deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 50.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, Elliston K O, Ayala J M, Casano F J, Chin J, Ding G J F, et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 51.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B: functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 52.Wei X, Leung B P, Niedbala W, Piedrafita D, Feng G, Sweet M, Dobbie L, Smith A J H, Liew F Y. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–2828. [PubMed] [Google Scholar]
- 53.Wewers M D, Dare H A, Winnard A V, Parker J M, Miller D K. IL-1β-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1β release limitation is ICE independent. J Immunol. 1997;159:5964–5972. [PubMed] [Google Scholar]
- 54.Xu D, Chan W L, Leung B P, Hunter D, Schulz K, Carter R W, McInnes I B, Robinson J H, Liew F Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 56.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]