Abstract
Tendon-bone insertion injuries (TBI), such as anterior cruciate ligament (ACL) and rotator cuff injuries, are common degenerative or traumatic pathologies with a negative impact on the patient's daily life, and they cause huge economic losses every year. The healing process after an injury is complex and is dependent on the surrounding environment. Macrophages accumulate during the entire process of tendon and bone healing and their phenotypes progressively transform as they regenerate. As the “sensor and switch of the immune system”, mesenchymal stem cells (MSCs) respond to the inflammatory environment and exert immunomodulatory effects during the tendon-bone healing process. When exposed to appropriate stimuli, they can differentiate into different tissues, including chondrocytes, osteocytes, and epithelial cells, promoting reconstruction of the complex transitional structure of the enthesis. It is well known that MSCs and macrophages communicate with each other during tissue repair. In this review, we discuss the roles of macrophages and MSCs in TBI injury and healing. Reciprocal interactions between MSCs and macrophages and some biological processes utilizing their mutual relations in tendon-bone healing are also described. Additionally, we discuss the limitations in our understanding of tendon-bone healing and propose feasible ways to exploit MSC-macrophage interplay to develop an effective therapeutic strategy for TBI injuries.
The Translational potential of this article
This paper reviewed the important functions of macrophages and mesenchymal stem cells in tendon-bone healing and described the reciprocal interactions between them during the healing process. By managing macrophage phenotypes, mesenchymal stem cells and the interactions between them, some possible novel therapies for tendon-bone injury may be proposed to promote tendon-bone healing after restoration surgery.
Keywords: Tendon-bone healing, Macrophage, Mesenchymal stem cell
1. Introduction
The tendon-bone insertion (TBI, enthesis), a site where tendons or ligaments attach to bones, plays an indispensable role in movement. Its main function is to transfer complex and variable mechanical stress from the muscle to the bone or between bones [1]. As the focal aperture point of stress, the enthesis is susceptible to traumatic lesions. The incidence of enthesis injury is increasing as the interest in sporting activities grows. Anterior cruciate ligament (ACL) injuries and rotator cuff tears are the most prevalent enthesis injuries sustained in daily life and at work [2]. The incidence of full-thickness rotator cuff tears is estimated to be 20.7%, with a higher prevalence in older populations [3]. In the United States, over two million ACL injuries occur yearly, accounting for more than half of all knee injuries [4].
Surgical reconstruction has always been considered the standard therapy for enthesis injuries in the past few decades, with most patients achieving full recovery after the operation. However, undesirable outcomes such as retear, stiffness, and pain occur in some cases [5,6]. Tendon-bone healing, a complex biological process, is the most significant part of recovery from enthesis injuries. Satisfactory tendon-bone healing determines the final success of the reconstruction operation [7]. Therefore, it is important to identify promising ways to augment tendon-bone healing for successful restoration of tendon and bone functionality. Nonetheless, tendon-bone healing is a much slower process, and once formed, the enthesis's natural complex composition and structure cannot be reformed. The lack of blood vessels and bone loss at the junction site impedes the growth of the bone into the fibrovascular tissue regenerated between the tendon and bone after surgical repair. Instead, a mass of scar tissue with an inferior biomechanical structure, which is susceptible to retear, is formed due to excessive inflammation [8]. Hence, improving and augmenting tendon-bone healing remains a key challenge in clinical practice [9].
Over the last few decades, various biological strategies have emerged to facilitate and improve tendon-bone healing. These therapeutic options biologically reconstruct the complex structure and composition of the TBI using growth factors [10], stem cell therapies [2,11], platelet-rich plasma [12], and biodegradable scaffolds [13].
Mesenchymal stem cells (MSCs) have been explored for their therapeutic potential in the treatment of tendon-bone injury [2,[13], [14], [15], [16]]. MSCs are a heterogeneous group of multipotent cells that can differentiate into bone, cartilage, and fat cells [16,17]. Besides expediting angiogenesis, MSCs can regulate the inflammatory process after injury due to their immunomodulatory properties [18,19]. Furthermore, owing to their multipotent properties, MSCs have been widely applied in animal model studies to facilitate biological tendon-bone healing in vivo after reconstruction. However, the mechanisms by which MSCs accelerate tendon-bone healing are not fully understood.
MSCs are known as “sensors” and “modulators” of the immune system [20,21]. After injury, including muscle and skin injuries, MSCs gather in the injured site and interact with immune cells to initiate the repair process. As the tendon-bone healing process progresses, macrophages undergo phenotypic changes. Researchers have demonstrated that M1-type macrophages are dominant at the early stage of tendon-bone healing, whereas M2-type macrophages increasingly accumulate at the injury site over time [22]. This alteration of macrophage phenotype illustrate the fact that macrophages may play a crucial role in the healing process. In addition, it has been proven that macrophages can influence the viability and growth of MSCs [23]. The interactions between MSCs and macrophages described earlier exert a critical effect on the outcome of tendon-bone healing.
Both MSCs and macrophages are indispensable during the tendon-bone healing process. In this review, the contribution of MSCs and macrophages and their mutual interactions during tendon-bone healing after injury are discussed. A clearer understanding of the functions of these two cells and their relationship can help us develop more effective repair strategies for TBI injuries. Possible ways to utilize their reciprocal interactions will also be described.
1.1. The natural structure of enthesis
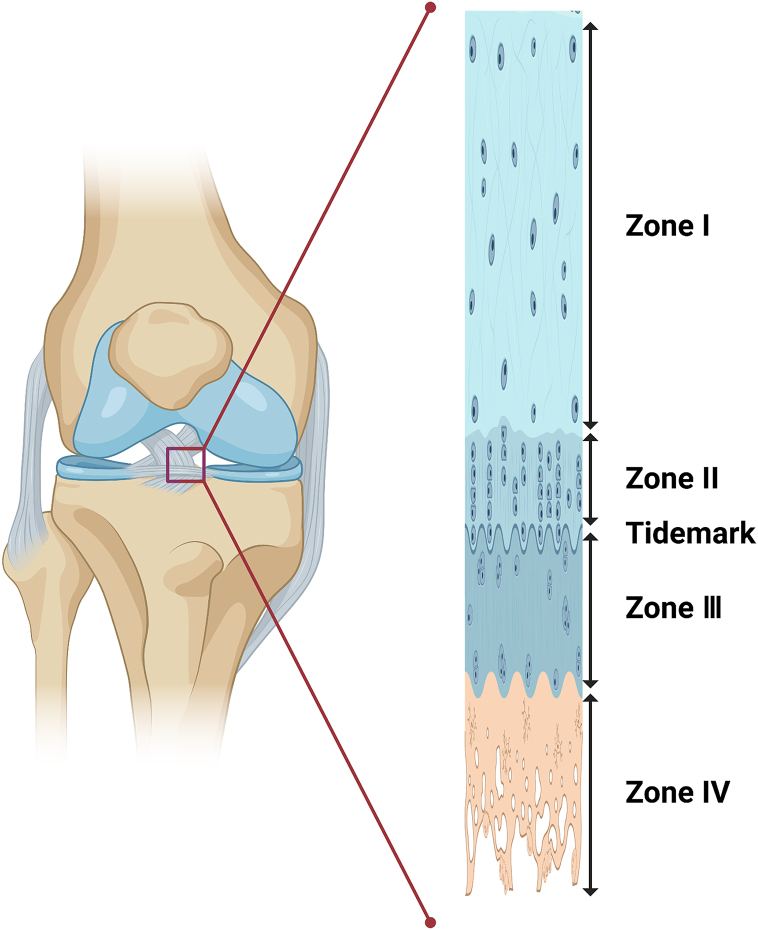
An enthesis is a unique layered transitional tissue that connects the tendon and bone, and it has two components with completely different structures, compositions, and mechanical properties. Entheses transfer the force of muscle contraction to the bone to facilitate joint movement [24] and maintain the stability of the joint [25]. As a rule, TBIs are classified into two types: direct and indirect insertions. An example of indirect insertion is the tibial insertion of the medial collateral ligament, with dense fibrous tissue connecting the ligaments to the periosteum. In comparison, direct insertions, such as the rotator cuff and anterior crucial ligament insertions, connect the fibrocartilage tissue to deeper layers of the bone [7]. The transition of direct insertion consists of four different types of tissue: tendon/ligament (I), uncalcified fibrocartilage (II), calcified fibrocartilage (III), and bone (IV) (Fig. 1) [26]. Each tendon is mainly populated by fibroblasts, with linearly arrayed type I collagen fibers as its primary component [27]. Uncalcified fibrocartilage is made up of proteoglycan aggrecan as well as types I, II, and III collagen. Fibrochondrocytes are the dominant cells in this avascular region [27,28]. Calcified fibrocartilage primarily consists of type II collagen and aggrecan as well as types I and X collagen. It is also an avascular zone that is populated by fibrochondrocytes [[27], [28], [29]]. The last zone is the bone, comprising osteocytes, osteoclasts, and osteoblasts in a matrix of mineralized type I collagen [1,28,30]. In a classical system, enthesis is divided into four dramatically different, unseparated but structurally uninterrupted structures. The transition in structure promotes a gradual change in the mechanical properties from tendon to bone, thus preventing stress concentration during mechanical conduction [31].
Fig. 1.
Transitional structure of the enthesis.
Zone I is comprised of the ligament or tendon. Zone II is composed of uncalcified fibrocartilage. Zone III contains calcified fibrocartilage. Zone IV is the bone. The tidemark is shown as the blue line between Zone II and Zone III.
1.2. Development of the tendon-bone insertion
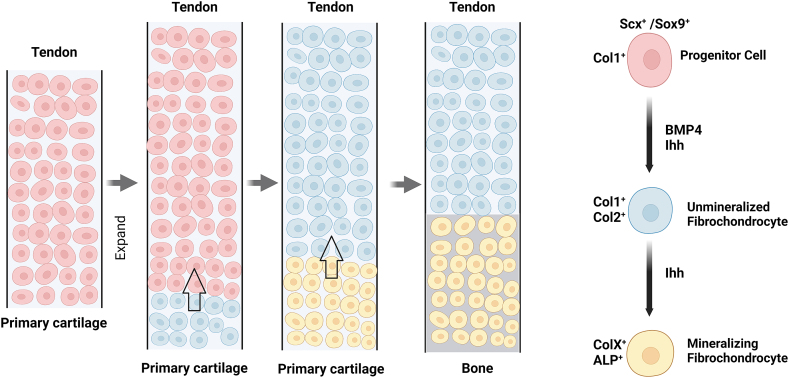
The transition structure of the TBI has important biological significance that requires a developmental process to form. With the growth and development of the fetus, the TBI arises between the bone and tendon/ligament [27]. In early entheseal development, TGF-β regulates the formation of the Scx+/Sox9+ progenitor pool that forms the bone eminence where the enthesis develops. Induced by bone morphogenic protein 4 (BMP-4), Scx+/Sox9+ cells differentiate into chondrocytes and bone eminences, forming the original connection between the tendon and the bone—the immature enthesis. With the increasing number of entheseal progenitor cells, the length of the enthesis increases, accompanied by the secretion of type I collagen. As proliferation progresses, unmineralized fibrochondrocytes are formed at the base of the developing enthesis through a mechanism regulated by BMP-4 and Indian hedgehog (Ihh). Subsequently, collagen type I and II levels start to increase. After birth, Ihh stimulates the hypertrophic differentiation of fibrochondrocytes, beginning at the bottom of the enthesis. As matrix mineralization begins, fibrochondrocytes transform into hypertrophic mineralizing fibrochondrocytes, which synthesize both type X collagen and alkaline phosphatase (ALP) (Fig. 2). [32,33] A subset of type II collagen–expressing cells undergo endochondral ossification, performing the mineralization process, which establishes a mineralized fibrocartilage region connected to the unmineralized tissue [34]. Subsequently, a mature, well-organized, transitional tissue is formed between the tendon and bone with the remodeling of the collagen fibers and minerals. After a complex developmental process, the natural structure of the enthesis is formed. An analogous process has been described for rat Achilles tendon enthesis, bovine ACL enthesis, and the deltoid-humeral tuberosity attachment [[35], [36], [37]].
Fig. 2.
Development of tendon-bone insertion.
Scx+/Sox9+ progenitor cells form an immature enthesis connecting the tendon and bone. Their continuous proliferation extends the length of the enthesis as they secrete type I collagen. Under the regulation of BMP-4 and Indian hedgehog (Ihh), the progenitor cells differentiate into unmineralized fibrochondrocytes from the bottom of the developing tendon-bone insertion and express type I and II collagen. After birth, Ihh begins to promote hypertrophic differentiation of fibrochondrocytes from the base of the enthesis. The fibrochondrocytes transform into hypertrophic mineralizing fibrochondrocytes and start to synthesize type X collagen and alkaline phosphatase (ALP) as soon as matrix mineralization begins.
1.3. Interface healing process
The unique structure of the TBI is destroyed after injury. Surgical reconstruction is recommended for the repair of TBI injuries because it is nearly impossible for the enthesis to recover by itself. Tendon-bone healing is a restorative process after injury in which inflammation plays an important role. Using the dog tendon-bone healing model, Rodeo et al. [38] demonstrated that the tendon-bone interface differentiates into a vascular, highly cellular, fibrous tissue after two weeks. Compared with the interface at two-weeks, there was an increased proportion of extracellular matrix (ECM) but decreased vascularization at four weeks. From eight to twelve weeks, the interface tissue became more mature and increasingly organized, with collagen fibers becoming aligned in the direction of the load. At twenty-six weeks, continuous collagen fibers were formed in the transitional zone between the bone and tendon. The healing process includes four phases: (1) inflammatory phase, (2) proliferative phase, (3) matrix synthesis phase, and (4) matrix remodeling phase [2,39].
After injury, hemorrhage occurs following the rupture of vessels, causing local hematoma [40]. Inflammatory cells and marrow-derived stem cells are recruited and infiltrate the tendon-bone interface, TGF-β, and PDGF. In addition, many other growth factors and cytokines are also released. Spurred by these growth factors or the hypoxic environment, the blood vessels and nerves begin to grow. After the temporary matrix is broken down by matrix metalloproteinases (MMPs) and serine proteases, a new ECM and progressive bone ingrowth are formed. TGF-β plays an important role during the matrix synthesis stage. Upon exposure to TGF-β, recruited fibroblasts initiate the synthesis of collagen I, III, and V, proteoglycans, fibronectin, and other ECM components. Finally, during the remodeling phase, the structure of the newly formed tissue is reorganized. Specifically, collagen fibers are remodeled into scar tissue by attaching to each other via molecular crosslinking [39,41]. However, excessive and continuous inflammation gives rise to superfluous scar tissue with mechanical properties inferior to those of the normal four-layered transitional structure, resulting in undesirable tendon-bone healing [8]. Therefore, maintaining the balance of the inflammatory response at the injury site and reestablishing the complex structure of the natural TBI may be a promising research direction in this area.
1.4. Key points for healing augmentation
To reestablish the natural structure of the enthesis during the interface healing process, scientists have conceived many therapeutic solutions to accelerate the healing process and validated some in practice. These ideas can be divided into four categories: anti-inflammation, osteogenesis, angiogenesis, and chondrogenesis.
2. Anti-inflammation
Inflammation is a natural response to injury and the initiation of the repair process. However, the tendon-bone healing response is usually dysregulated and chronic, which can result in pathological fibrosis and scarring [42,43]. As mentioned earlier, inflammation would produce a biomechanically weak scar if not well managed. Nonetheless, it serves a fundamental function in the tendon-bone healing process, suggesting that controlling excessive inflammation during the healing process may reduce scar formation and improve biomechanical strength during tendon-bone healing. In recent research, based on a hedging immune strategy, a microfibrous membrane (Him-MFM) carrying relevant risk receptors was fabricated that successfully mitigated inflammatory tenocyte responses, protected tenocytes in situ, and restored hierarchically arranged collagen fibers, thereby yielding a regenerative outcome rather than scarring [44]. Golman et al. demonstrated that inhibiting IKKβ can suppress undue inflammatory responses and improve tendon-bone healing after rotator cuff repair in a rat model [45]. Utilizing anti-inflammatory and pro-differentiation effects, Chen et al. developed an injectable hydrogel that enhanced tendon-bone healing [46]. The investigations mentioned above and many other in vitro or in vivo experiments have emphasized the significant role of anti-inflammation in tendon-bone healing. Therefore, anti-inflammation has been a promising research direction in tendon-bone healing and is an ideal and viable strategy for controlling inflammatory responses in the future.
3. Osteogenesis
During healing, fibrovascular interface tissue arises between the tendon and bone, and the bone gradually grows into this fibrous interface tissue. Finally, with the ingrowth of bone into the tendon, eventual reconstruction of collagen fiber continuity between the tendon and bone is achieved [47]. Several investigations have shown that the eventual result of tendon-bone healing is partly dependent on bone ingrowth, and the induction of osteogenesis is beneficial to the restoration process [[7], [8], [9],48]. Magnesium (Mg) ions can significantly facilitate the release of relevant osteoinductive cytokines. Wang et al. obtained satisfactory recovery results after wrapping the tendon graft with Mg-pretreated periosteum, and the osteointegration of the tendon graft into the bone tunnel increased, whereas peri-tunnel bone loss decreased [49]. Tie et al. affirmed that utilizing dedifferentiated bone marrow mesenchymal stem cells (BMSCs) could significantly promote bone formation at the tendon-bone junction and increase the maximal biomechanical strength [14]. The list of biological factors that induce osteogenesis in tendon-bone healing is not limited to the factors discussed here.
4. Angiogenesis
The special structure and function of the TBI makes it susceptible to damage. An adequate blood supply provides essential nutrients, minerals, and oxygen for the synthesis and mineralization of the bone matrix and maturation of the tendon matrix. However, a lack of blood supply to the TBI reduces the concentration of oxygen, growth factors, and other essential nutrients that are required for metabolism, slowing down tendon-bone healing or leading to nonunion. Therefore, there is increasing interest in promoting angiogenesis in the tendon–bone area to boost tendon–bone healing [8,15,50]. To promote restoration, Liao et al. confirmed that fabricated amorphous calcium phosphate (ACP) nanoparticles could promote angiogenesis in vitro. Furthermore, using the RCT rat model, they demonstrated that ACP nanoparticles could boost the formation of bone and blood vessels at the tendon-bone junction in vivo [8]. After the discovery of its function in facilitating angiogenesis and osteogenesis, icariin was shown to enhance tendon-bone healing by Ye et al. [51] Currently, physical treatment is widely used in medical practice to yield good curative effects. For example, low-intensity pulsed ultrasound stimulation (LIPUS) is an ideal tool for the augmentation of tendon-bone healing. One of the therapeutic mechanisms involves the promotion of angiogenesis [52]. Many animal experiments have confirmed that stimulating the formation of blood vessels is a favorable and promising idea for tendon-bone healing. However, vascularization typically decreases in the later period of the healing process [39], suggesting that angiogenesis may not always be successful and could even result in a worse outcome. Rodeo et al. [38] reported that the tendon-bone interface differentiated into vascular tissue after two weeks, but there was decreased vascularization after four weeks. Similarly, Fealy et al. [53] demonstrated that blood supply increased early after rotator cuff reconstruction, but then decreased gradually over time. In addition, they deemed that increased blood supply at the fixation point or bone groove could enhance the quality of tendon-bone healing. Consequently, inducing angiogenesis is essential for the healing process, but the timing and position are factors that should be taken into consideration.
5. Chondrogenesis
Surgical reconstruction is currently the most effective treatment for enthesis injury. During the operation, both sides of the TBI are refreshed by removing residual fibrocartilage. However, it is difficult to achieve the formation of fibrocartilage in tendon-bone healing because of its low regenerative ability [54]. Scars, instead of fibrocartilage, are produced in situ, causing a weaker junction between the tendon and the bone. It has been confirmed that the terminal biomechanical strength is increased in experimental animals treated with the appropriate methods for boosting chondrogenesis after reconstruction [[54], [55], [56], [57], [58]]. The TBI consists of four different layers: the tendon, uncalcified fibrocartilage, calcified fibrocartilage, and bone. Fibrocartilage is only a part of the complex structure, which may not be completely reestablished by expediting chondrogenesis. Moreover, the reestablishment of the intricate transitional structure of the TBI is influenced by several other factors. Therefore, promoting chondrogenesis is partly responsible for tendon-bone healing. In other words, facilitating cartilage regeneration is a promising research direction that is yet to be explored.
5.1. MSCs in tendon-bone healing
In recent years, to achieve a favorable treatment outcome, various strategies have been proposed to accelerate and promote tendon-bone healing. Among them, MSCs have attracted much attention as an ideal and superior therapeutic tool [2,11,16,59]. Friedenstein et al. firstly isolated MSCs from colonies in vitro and described them as plastic-adherent fibroblast-like cells [60]. These cells express CD105, CD73, and CD90, but not CD45, CD34, CD11b, CD19, or HLA-DR surface molecules [61,62]. Most MSCs arise from bone marrow, although other organs including the placenta, umbilical cord, amniotic fluid and membrane, skeletal muscle, adipose tissue, lung, heart, liver, and kidneys can also produce them [61,63]. MSCs have abundant sources and are multifunctional (Table 1):
Table 1.
Molecular mediators involved in MSC functions.
| MSC function | Molecular mediators | Reference |
|---|---|---|
| Multi-Lineage Differentiation Osteogenesis Chondrogenesis Adipogenesis Immune-modulation Pro-inflammation Anti-inflammation Angiogenesis |
Ascorbic acid, Dexamethasone, BMPs, WNTs, FGFs, RUNX1 Ascorbate, Insulin, Transferrin, Selenic acid, Kartogenin, TGF-β Dexamethasone, Indomethacin, Insulin, Isobutylmethylxanthine TLR ligands, TLR4 TLR ligands, TLR3 VEGF, bFGF, FGF-2, Ang-1, MCP-1, IL-6, PLGF |
[64,65,[71], [72], [73]] [56,64,74] [64,75] [21,68,76] [21,68,76] [18,19] |
BMPs, bone morphogenic proteins; FGFs, fibroblast growth factor; RUNX1, Runt-related transcription factor 1; TGF-β, transforming growth factor-β; TLR, toll-like receptor; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; FGF-2, fibroblast growth factor-2; Ang-1 angiopoietin-1; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin-6; PLGF, placental growth factor
First, MSCs can self-renew. Secondly, as multipotent cells, they can differentiate into tissue-specific cells, such as osteoblasts, chondrocytes, and adipocytes, under different physiological environments, making them ideal seed cells for regenerative medicine [64]. Utilizing a lentiviral vector with upregulated RUNX1, Kang et al. successfully fabricated RUNX1-upregulated BMSCs and injected them into the periphery of the tunnel surrounding the tendon graft after ACL reconstruction. Improved tendon-bone healing was observed, confirmed through histological analysis, biomechanical analysis, and micro-CT assessment [65]. It has been explained that Kartogenin can induce cartilage formation by promoting chondrogenesis of mesenchymal stem cells [56]. Liu et al. devised a biomimetic tendon ECM composite gradient scaffold and transplanted it into the bone tunnel to enhance tendon-bone healing. They attributed the augmentation in healing to the enhanced expression of chondrogenesis- and osteogenesis-associated genes in MSCs that were induced by the scaffold [66]. Given the great significance of osteogenesis and chondrogenesis in tendon-bone healing, MSC therapy is a feasible and potentially effective treatment; however, its differentiation mechanism needs to be elucidated to make it a viable therapeutic option in the future.
Second, the immune-modulating function of MSCs is another advantageous property, which makes them the “sensor and switch of the immune system”. [20]. Toll-like receptors (TLRs) in MSCs can recognize damage signals and activate MSCs and immune cells. Once MSCs are activated, they respond to TLR ligands and secrete anti-inflammatory factors, thereby inhibiting inflammation [67]. Furthermore, MSCs can facilitate inflammatory responses during the early stages of inflammation. Pro-inflammatory MSCs can release macrophage inflammatory protein-1 (MIP-1), C–C motif ligand 5 (CCL5), C-X-C motif ligand 9 (CXCL9), and C-X-C motif ligand 10 (CXCL10) to activate T-cells and recruit more lymphocytes [20,68]. In fact, the inflammation suppressing effect of MSCs has been applied in the augmentation of tendon-bone healing. For example, Xu et al. stated in their recent research that infrapatellar fat pad mesenchymal stromal cell-derived exosomes can exert an immunomodulatory effect on macrophages to regulate their polarization and accelerate tendon-bone healing and intra-articular graft remodeling after ACLR [69]. Based on current knowledge of tendon-bone healing, inflammatory response, which is a key process, significantly contributes to the progress. However, undue and continuous inflammation produces substantial scar tissue with poor biomechanical strength. We believe that the immune-modulating function of MSCs contributes to the augmentation of tendon-bone healing.
Lastly, several reports have claimed that MSCs contribute to angiogenesis. Many categories of cytokines and growth factors, including vascular endothelial growth factor (VEGF), bFGF, FGF-2, angiopoietin-1 (Ang-1), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and placental growth factor (PLGF), have been discovered in the secretome of MSCs. These cytokines are released by MSCs through paracrine signaling to stimulate angiogenesis [18,19]. Recent research indicated that overexpressed P311 could significantly improve their ability to promote angiogenesis by increasing VEGF production, which partly accelerates skin wound closure and improves healing quality [70]. For tendon-bone healing, the angiogenic effect of MSCs has already been exploited and utilized. Exosomes derived from BMSC have been confirmed to augment tendon-bone healing after surgical reconstruction. This augmentation is accomplished by promoting angiogenesis through the VEGF and Hippo signaling pathways [15]. In addition to their paracrine effects, MSCs have multi-differentiation potential, allowing them to differentiate into cells of the mesenchymal lineage, including osteocytes, chondrocytes, adipocytes, and hematopoiesis-supporting stromal cells. Pankajakshan et al. treated porcine MSCs with EGM-2 and 50 ng/ml VEGF, and discovered, through functional assays and mRNA and protein expression analysis of epithelial cell markers, that MSCs can differentiate into epithelial cells [62]. Promotion of angiogenesis is achieved using appropriate strategies to manage MSCs during tendon-bone healing. As blood vessels form, the blood supply increases, which means that more oxygen, growth factors, and other essential nutrients would reach the healing interface. In such advantageous conditions, the healing process of the injured enthesis would be accelerated.
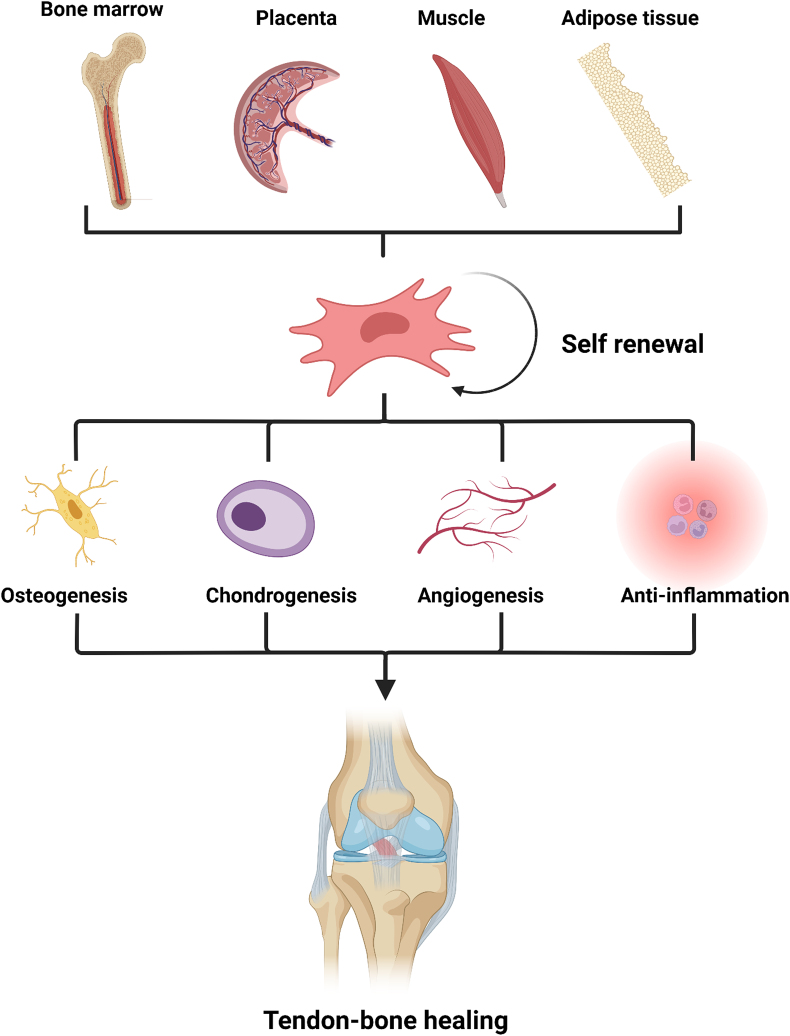
In summary, there are four noteworthy points in expediting tendon-bone healing: anti-inflammation, osteogenesis, angiogenesis, and chondrogenesis. As multi-potent seed cells, MSCs have drawn intense research interest because of their multi-lineage differentiation ability, immune-modulating function, paracrine effect, and many other advantages. Their superior multipotency offers MSCs the possibility of providing these four effects simultaneously (Fig. 3). In other words, the discovery of an ideal application method would make MSC an optimal choice for treating RCT, ACL injuries, and other TBI injuries.
Fig. 3.
Origin of MSCs and their functions in tendon-bone healing.
MSCs are mostly acquired from bone marrow and other organs like the placenta, umbilical cord, amniotic fluid and membrane, skeletal muscle, adipose tissue, lung, heart, liver, and kidneys. Because of their self-renewal ability, multi-lineage differentiation, immune-modulating function, paracrine effect, and many other advantages, MSCs can enhance anti-inflammation, osteogenesis, angiogenesis, and chondrogenesis, simultaneously benefiting tendon-bone healing.
5.2. Phenotypes of macrophages
In addition to MSCs, macrophages also play an important role in tendon-bone healing after injury. In recent years, macrophages have been increasingly characterized as multifunctional and plastic cells [77]. Macrophages can transform their phenotype according to the cellular and molecular compositional changes in their surrounding microenvironment, a phenomenon called “polarization.” [78] After transformation, macrophages with different phenotypes exhibit different expression levels of genes to secrete appropriate cytokines and express necessary receptors to adapt to the changing environment (Table 2). Most scholars agree that regarding the polarization of macrophages, two research directions should be explored: research on pro-inflammatory M1-type macrophages and anti-inflammatory M2-type macrophages [79]. Polarization occurs when macrophages are stimulated by specific signals such as microbial products and cytokines. For example, M1 macrophages are activated when exposed to activation stimuli such as LPS and IFNγ. They secrete inflammatory cytokines, including interleukin (IL) 1β, 6, and 12, tumor necrosis factor alpha (TNFα), and interferon-gamma (IFN-γ), and promote the Th1 response. The characteristics of M2-type macrophages contrast with those of the M1 type. Because M2 macrophages (M2-EXO) differ phenotypically from M1 macrophages, their activation is induced by interleukins (IL) 4, 13, 10, and 33 and macrophage colony-stimulating factor (M-CSF). The secretome of M2-EXO express anti-inflammatory molecules, such as IL4, IL10, and transforming growth factor beta (TGF-β), and growth factors that induce tissue remodeling and are responsible for their anti-inflammatory and reparative effects on the healing process after injury [79,80]. In summary, macrophages undergo phenotypic transformation through polarization. When polarized into type M1, they become pro-inflammatory and cytotoxic, whereas when polarized into type M2, they become anti-inflammatory and reparative.
Table 2.
Molecular mediators involved in macrophage polarization [42,[79], [80], [81], [82], [83], [84], [85],87,88].
| Phenotype | Inducing mediators | Released molecules | Functions |
|---|---|---|---|
| M1 | LPS, IFN-γ | IL-1β, 6, 12, TNF-α, IFN-γ | Proinflammation |
| M2 M2a M2b M2c M2d |
IL-4, IL-13 IC, IL1β, LPS IL-10, Glucocorticoids IL-6, TLR agonists |
TGF-β, IGF, Fibronectin IL-1β, IL-6, and TNF-α … IL-10, TGF-β VEGF |
Tissue repair Regulation Anti-inflammation Tumor-associated |
LPS, lipopolysaccharide; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-12 interleukin-12; IL-4, interleukin-4; IL-13, interleukin-13; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; IGF, insulin-like growth factor; IC, immune complex; IL-10, interleukin-10; TLR, toll-like receptor; VEGF, vascular endothelial growth factor
Classifying macrophages into M1 and M2 types is now a well-accepted and widely applied method for macrophage polarization typing. However, macrophages can be further subclassified into more subtypes. M2-EXO can be further divided into M2a, M2b, M2c, and M2d phenotypes based on stimulation from the surrounding environment [81,82]. M2a macrophages contribute to tissue repair and are induced by IL-4 and IL-13. They highly express CD206, decoy IL-1 receptor, and CCL17 and secrete reparative factors such as TGF-β, insulin-like growth factor (IGF), and fibronectin [42,82,83]. M2b macrophages are induced by co-exposure to immune complexes (IC) and IL1β or lipopolysaccharide and have regulatory functions in vivo. As regulatory macrophages, M2b macrophages are unique among M2-EXO because they additionally secrete proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, whereas all M2-EXO express anti-inflammatory molecules as stated above [83,84]. M2c macrophages are induced by IL-10 and glucocorticoids, and they release large amounts of IL-10 to inhibit inflammation. They can also secrete TGF-β to promote fiber proliferation [[83], [84], [85]]. Furthermore, M2c macrophages highly express Mer receptor tyrosine kinase (MerTK), which gives them the ability to phagocytose the apoptotic cells efficiently [83,86]. The M2d phenotype of macrophages, also known as tumor-associated macrophages (TAMs), has emerged as a novel M2 subset in recent years [87,88]. These cells are induced by IL-6 or TLR agonists through the adenosine receptor [81,88]. It has been proven that they contribute to angiogenesis and lymphagiogenesis in gastric cancer because of the up-regulation of VEGF and VEGF-C [89]. Subtyping macrophages helps us better understand the features and functions of macrophages. For the convenience of narration in this review, their common name—M2-EXO—is used.
5.3. Macrophages in tendon-bone healing
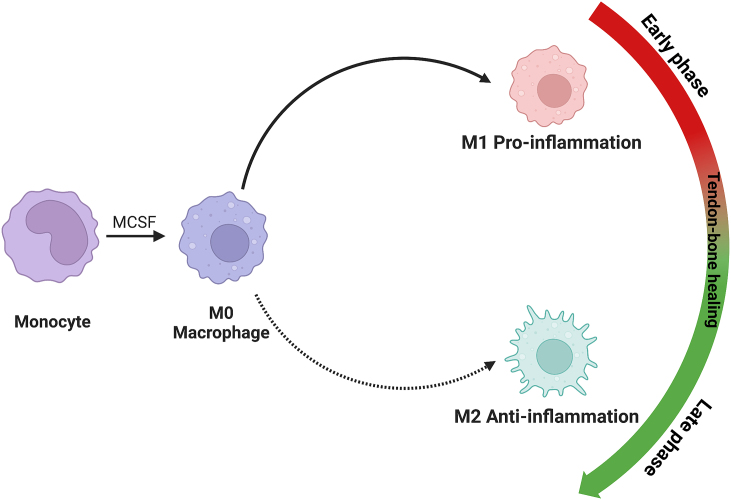
Inflammation is the primary response to tissue injury. Neutrophils are the classical inflammatory cells that first reach the damaged site, followed by monocytes, macrophages, and lymphocytes. In the earliest phase, neutrophils at the wound site secrete cytokines that simultaneously recruit other types of immune cells, such as macrophages, and promote their proliferation. Kawamura et al. discovered that M1 macrophages homed to the injured site early and remained for four weeks, whereas M2-EXO could not be detected until 11 days after surgery [22]. Subsequently, the essential function of macrophages in the tendon-bone healing process was demonstrated in a number of animal experiments [15,22,90,91]. (Fig. 4) In 2008, Hays et al. administered liposomal clodronate to Sprague–Dawley rats that had undergone ACL reconstruction with a flexor tendon autograft to decrease macrophages and TGF-β accumulation at the tendon-bone interface. They found that macrophages and TGF-β were significantly diminished at the healing site, improving the morphological and biomechanical properties at the healing tendon-bone interface [92]. More researchers have carried out experiments to uncover latent relations between tendon-bone healing and polarized macrophages. Dagher et al. showed that SD rats undergoing early immobilization after ACL reconstruction exhibited fewer M1 macrophages at the healing site but had more M2-EXO compared to the control group. They demonstrated that this kind of shift between M1 and M2-EXO contributed to the augmentation of tendon-bone healing [93]. Similarly, Gulotta et al. treated rats with TNF-α blockade after surgery to improve tendon-bone healing and found that the number of M1 macrophages decreased in the experimental group [94]. In a recent study, disulfiram (DSF) was administered to experimental animals to promote the transition of macrophages from the M1 to M2 phenotype and decrease the macrophage pro-inflammatory phenotype. The results indicated less peritendinous fibrosis and more regenerated bone and fibrocartilage at the healing sites [95]. Based on previous results, accelerating the transition of macrophages from M1 type to M2 type has clinically significant benefits for the healing process.
Fig. 4.
Phenotypes of macrophages and their temporal distribution during tendon-bone healing.
Two directions of polarization for macrophages: (1) Pro-inflammatory M1-type macrophages and (2) anti-inflammatory M2-type macrophages.
During the tendon-bone healing process, macrophages transform their phenotype into M1 in the early phase but polarize into M2 in the late phase.
Due to deficient blood supply, the necessary oxygen, growth factors, and other essential nutrients cannot be provided, thus slowing down tendon-bone healing. We believe that the induction of angiogenesis is essential for the healing process, and this view has been verified in many studies [8,15,50]. Macrophages play an important role in tendon-bone healing; in addition to clearing cell debris and activating and resolving inflammation, they also have a proangiogenic function [96,97]. Macrophages can secrete a variety of angiogenic factors including basic fibroblast growth factor (b-FGF), transforming growth factor-alpha (TGF-α), insulin-like growth factor-I (IGF-1), and human angiogenic factor (HAF) [98]. Jetten et al. demonstrated that macrophages that polarized towards an M2 phenotype have a higher angiogenic potential [99]. In a recent report, researchers isolated exosomes from M2-EXO (M2-EXO) and proved that M2-EXO could be utilized as a facilitator of angiogenesis and regeneration in vivo [100]. The proangiogenic function of macrophages, especially M2-EXO, could greatly help in promoting tendon-bone healing if used properly.
Most neutrophils undergo apoptosis and form apoptotic aggregates that contain numerous autoantigens and cytotoxic compounds. These substances induce an inflammatory response, causing excessive formation of tissue scars [101]. However, it has been reported that apoptotic cells can be endocytosed and cleared by macrophages through a process named efferocytosis. This process can terminate apoptosis and reduce the secretion of pro-inflammatory cytokines. This implies that macrophages can modulate inflammatory responses after tissue injury [102]. The role of efferocytosis in the healing process has been investigated extensively. In a recent study, SD rats that underwent ACL reconstruction were treated with milk fat globulin protein E8 (MFG-E8), a protein that can bridge macrophages and apoptotic cells during efferocytosis. MFG-E8 promoted tendon-bone healing histologically and biomechanically. This was ascribed to macrophage efferocytosis and M2 polarization [103].
5.4. Interactions between mesenchymal stem cells and macrophages
5.4.1. MSCs affect macrophages
As a sensor and switch in the immune system, MSCs play an important role in immune regulation. These cells prevent the activation and overactivation of immune and inflammatory responses [20]. Similar to the phenotypic transformation of macrophages, MSCs respond to molecules or signals in the surrounding microenvironment to acquire a polarized phenotype. Their polarization is mediated by TLRs expressed by MSCs [68]. TLRs can recognize “danger signals” released from injured cells or sites of inflammation [67]. Activation of TLRs induces the transformation of MSCs into MSC1 (pro-inflammatory phenotype) or MSC2 (anti-inflammatory phenotype) based on the type of TLRs expressed on macrophages. TLR4 recognizes pro-inflammatory signals to mediate the switch to the MSC1 phenotype. In contrast, TLR3 triggers a switch to an MSC2 phenotype in response to anti-inflammatory signals [21,68,76]. By virtue of their plasticity, MSCs communicate with the inflammatory environment to modulate immune function. Studies have shown that the macrophage phenotype is regulated by MSCs. For instance, MSCs inhibited the activation of mouse peritoneal macrophages following LPS stimulation but did not affect phagocytosis [104]. Similarly, Maggini et al. demonstrated that MSCs markedly suppressed the secretion of the pro-inflammatory cytokines TNF-α, IL-6, and IL-12p70, but increased the secretion of the anti-inflammatory cytokines IL-12p40 and IL-10 from macrophages after LPS stimulation [105]. Specifically, PGE2 released from MSCs interacts with the EP2 and EP4 receptors on macrophages, promoting the production and secretion of IL-10 and reducing inflammation. The production of PGE2 is induced by iNOS and the cyclooxygenase 2 (COX2) dependent pathway [106].
Some studies have shown that MSCs influence the polarization process of macrophages and stimulate the release of anti-inflammatory cytokines, including TGF-β, IDO, NO, TNF-inducible gene-6 (TSG-6), and prostaglandin-E2 (PGE2), which moderate macrophage metabolism. These cells also induced the transformation of macrophages into the anti-inflammatory phenotype, M2-EXO [[107], [108], [109], [110], [111], [112]]. Previously, co-culture of macrophages with pMSCs resulted in the transition of macrophages from the inflammatory M1 phenotype to the anti-inflammatory M2 phenotype [110]. Deng et al. isolated exosomes from adipose-derived MSCs (ADSCs) and used them to alleviate cardiac damage associated with myocardial infarction. The results showed that exosomes promote M2 polarization to prevent cardiac muscle injury [113]. A recent study reported that exosomes isolated from murine MSCs (βMSCs) pretreated with interleukin-1β (IL-1β) induced the M2-like polarization of macrophages [114]. This implies that MSCs can promote the polarization of macrophages to the M2 type and inhibit the M1 type. It has been proven that the shift from M1 to M2 contributes to the augmentation of tendon-bone healing [[93], [94], [95]]. Therefore, this kind of interaction between MSCs and macrophages can control dysregulated inflammatory responses, thus augmenting tendon-bone healing.
As a “sensor and switch of the immune system”, MSCs can facilitate inflammatory responses in the early stages of inflammation, and most macrophages are polarized into the M1 phenotype to induce an inflammatory response. However, if the inflammatory response is overactivated, MSCs would act as inflammation suppressors, and the induction of M2-type polarization of macrophages is one of their anti-inflammatory effects. Several studies have shown that MSCs can influence macrophage polarization to promote tendon-bone healing. For instance, BMSC-derived exosomes were isolated and injected into the tail vein after rotator cuff reconstruction in rats to promote tendon-bone healing. The results of flow cytometry and immunohistochemistry showed that the exosomes inhibited inflammation by regulating M1 macrophages, thereby accelerating the healing process [15]. In the same year, Shi et al. found that exosomes delivered using hydrogels promoted the formation of fibrocartilage by increasing M2 macrophage polarization to promote tendon-to-bone healing in an Achilles tendon reconstruction model [91]. Two years later, Xu et al. successfully extracted exosomes from infrapatellar fat pad mesenchymal stromal cells and injected them into a graft-to-bone interface. This resulted in high biomechanical strength, thin graft-to-bone healing interface, and generation of more fibrocartilage and new bone ingrowth than that in the sham and control groups. These beneficial effects were ascribed to the promotion of macrophage polarization by the exosomes [69]. These findings demonstrate a complex relationship between MSCs and macrophages and that exosomes derived from MSCs may serve as an effective tool for MSC and macrophage cross-communication during the tendon-bone healing process. However, the mechanism by which exosomes modulate macrophage polarization has not been clearly defined. This calls for further research into the pathways through which MSC-derived exosomes affect the phenotypic transformation of macrophages.
5.5. Macrophages regulate MSCs
Studies have shown that MSCs are activated by cytokines and factors secreted by immune cells under inflammatory conditions. Macrophages participate in the priming of MSCs, as an important factor involved in the inflammatory response. Moreover, TNF-α released by M1 pro-inflammatory macrophages triggers MSC activation, and this effect can be augmented by the anti-inflammatory cytokine IL-10 secreted by M2-EXO [115]. The effect of macrophages on MSCs varies with the phenotype. Macrophages of different phenotypes and their associated cytokines have been reported to affect the survival and function of hMSCs. For example, M1 macrophages and their secretome inhibit the growth and survival of MSCs. In contrast, M2-EXO and their secretome do not alter the growth and survival of MSCs, but they may promote these outcomes in some circumstances [23]. However, Vallés et al. demonstrated that M1 pro-inflammatory macrophages promoted the attachment and migration of MSCs by secreting TNF-α. Their results indicated that IL-10 released from M2 anti-inflammatory macrophages enhances the progression of MSC osteogenesis [116]. In conclusion, it seems more likely that M2-EXO enhance the growth and survival of MSCs, whereas M1 macrophages inhibit these processes.
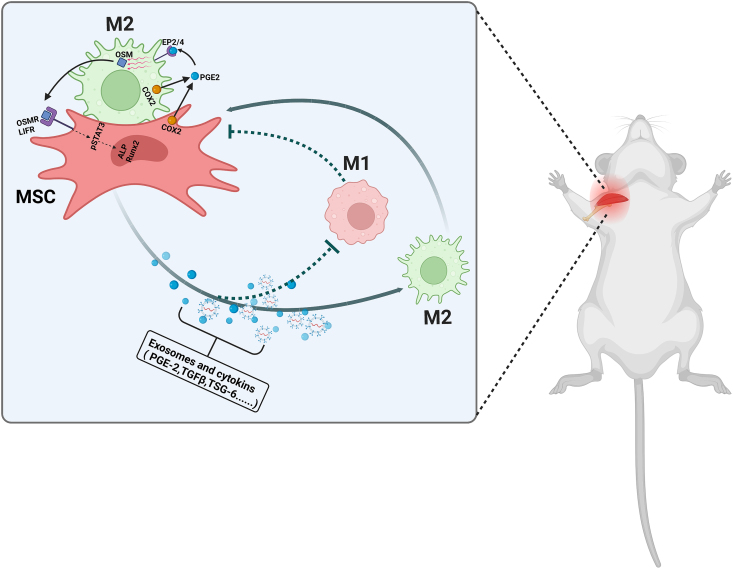
Macrophages regulate the MSC differentiation process. Cell-to-cell contact between macrophages and MSCs promotes the production of PGE2 and COX2, both of which enhance osteogenesis. PGE2 binds to its EP2/4 receptors on macrophages, triggering the production of a soluble factor, oncostatin M (OSM). This factor interacts with the OSM and LIF receptors on MSCs to enhance the expression of osteogenic genes by activating STAT3 phosphorylation [117]. (Fig. 5) Gong et al. reported that macrophage polarization can affect MSC to osteoblast differentiation. For example, co-culture of MSC with M1 macrophages decreased the levels of osteogenic markers, alkaline phosphatase (ALP), and bone mineralization, but the opposite results were obtained for M2-EXO [118]. Similarly, Zhang et al. showed that M2-EXO stimulate the proliferation and osteogenic differentiation of MSCs. They attributed this stimulatory effect to the secretion of BMP-2, OSM, and other osteogenic factors by the M2-EXO [119]. The function of extracellular vesicles secreted by macrophages in MSC osteogenic differentiation has been investigated in numerous studies [120,121]. Findings from such studies have shown that M2 anti-inflammatory macrophages can modulate BMSC chondrogenic differentiation. This effect may be attributed to the enhanced survival of BMSCs with a high chondrogenic capacity that is induced by M2-EXO in the co-culture system [122]. It has been reported that M1-phenotype macrophages inhibit MSC chondrogenesis in osteoarthritis synovium-conditioned medium [123]. However, contradictory results were reported in another study, where M1-like macrophages promoted chondrogenesis [124]. In summary, it is clear that macrophages regulate MSC differentiation, but how macrophages with different phenotypes affect MSCs remains controversial. According to most studies, more researchers hold the view that M2-EXO promote MSC differentiation while M1 macrophages do not.
Fig. 5.
Interactions between MSCs and macrophages during tendon-bone healing.
Therefore, strategies to promote the effects of macrophages on MSC differentiation may be effective in enhancing tendon-bone healing in the future. However, the recovery of the naturally complex structure of TBI is affected by many complex factors, and simply applying the effect of M2-EXO on MSC differentiation to reestablish the transitional zone is far from being advocated. Further research should be conducted to reveal the mechanisms by which macrophages modulate the MSC differentiation process.
Cytokines (PGE2, TGFβ,TSG-6) and exosomes released from MSC can promote the polarization of macrophages to the M2 type and inhibit the M1 type. In turn, M2-EXO enhance the growth and survival of MSCs whereas M1 macrophages inhibit these processes. Moreover, macrophages affect the differentiation process of MSCs. For instance, cell–cell contact results in the production of COX2 and PEG2. By acting on EP2/4 receptors, PGE2 induces the production of OSM from macrophages, which interact with OSM and LIF receptors on the MSC to activate STAT3 phosphorylation and induce the expression of osteogenic genes.
6. Conclusion and future perspectives
The healing process of TBI injuries is complicated. Numerous microenvironmental factors are involved in this process. The formation of scars, due to excessive inflammation, instead of the natural transitional structure weakens the biomechanical properties of the enthesis. Several approaches that inhibit inflammation and promote angiogenesis, osteogenesis, and chondrogenesis have been proposed to improve the outcome of tendon-bone healing. Notably, both MSCs and macrophages play an important role in the tendon-bone healing process, and several links have been reported between these two types of cells. MSCs have been widely applied in regenerative medicine because of their multidirectional differentiation potential and immunomodulatory function, especially in the tendon-bone healing field. In addition, macrophages with different phenotypes secrete diverse cytokines, molecules, and extracellular vesicles that mediate communication within the inflammatory environment, thereby influencing the healing process of tendon-bone injury. Moreover, MSCs can promote the polarization of macrophages to the M2 anti-inflammatory phenotype, thereby suppressing excessive inflammatory responses at the injury site and reducing scar formation. This theory has been applied to promote tendon-bone healing in previous studies. Additionally, M2-type macrophages can stimulate the proliferation and differentiation of MSCs. Therefore, strategies that suppress inflammation and enhance osteogenesis, chondrogenesis, and angiogenesis by utilizing the interactions between macrophages and MSCs can be effective in inducing the reestablishment of the transitional structure of the enthesis to some extent. In the future, investigation of the interactions between macrophages and MSCs to generate ideas for developing macrophage activators to promote tendon-bone healing is required.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
This work was supported by Application of Public Welfare Technology in Zhejiang Province (Grant Number: LGF21H060001), Medical Health Science and Technology Project of Zhejiang Province (Grant Number: 2021RC029) and TCM Science and Technology Project of Zhejiang Province (Grant Number: 2023ZL681). We thank Elsevier for Language Editing Services.
Contributor Information
Juntao Xu, Email: 50644554@qq.com.
Fengfeng Wu, Email: wufengfeng@zju.edu.cn.
References
- 1.Rossetti L., et al. The microstructure and micromechanics of the tendon-bone insertion. Nat Mater. 2017;16(6):664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y., et al. Stem cell therapies in tendon-bone healing. World J Stem Cell. 2021;13(7):753–775. doi: 10.4252/wjsc.v13.i7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S., Ying J.H., Xu H. Identification of diagnostic biomarkers associated with stromal and immune cell infiltration in fatty infiltration after rotator cuff tear by integrating bioinformatic analysis and machine-learning. Int J Gen Med. 2022;15:1805–1819. doi: 10.2147/IJGM.S354741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musahl V., Karlsson J. Anterior cruciate ligament tear. N Engl J Med. 2019;380(24):2341–2348. doi: 10.1056/NEJMcp1805931. [DOI] [PubMed] [Google Scholar]
- 5.Chen W., et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120714. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y., et al. Preoperative lymphocyte to monocyte ratio can Be a prognostic factor in arthroscopic repair of small to large rotator cuff tears. Am J Sports Med. 2020;48(12):3042–3050. doi: 10.1177/0363546520953427. [DOI] [PubMed] [Google Scholar]
- 7.Atesok K., et al. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am. 2014;96(6):513–521. doi: 10.2106/JBJS.M.00009. [DOI] [PubMed] [Google Scholar]
- 8.Liao H., et al. Amorphous calcium phosphate nanoparticles using adenosine triphosphate as an organic phosphorus source for promoting tendon-bone healing. J Nanobiotechnol. 2021;19(1):270. doi: 10.1186/s12951-021-01007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng C., et al. Autologous freeze-dried, platelet-rich plasma carrying icariin enhances bone-tendon healing in a rabbit model. Am J Sports Med. 2019;47(8):1964–1974. doi: 10.1177/0363546519849657. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C., et al. bFGF- and CaPP-loaded fibrin clots enhance the bioactivity of the tendon-bone interface to augment healing. Am J Sports Med. 2016;44(8):1972–1982. doi: 10.1177/0363546516637603. [DOI] [PubMed] [Google Scholar]
- 11.Hao Z.C., et al. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49(2):154–162. doi: 10.1111/cpr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao D., et al. Combination of graphene oxide and platelet-rich plasma improves tendon-bone healing in a rabbit model of supraspinatus tendon reconstruction. Regen Biomater. 2021;8(6):rbab045. doi: 10.1093/rb/rbab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q., et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Tie K., et al. Nanog/NFATc1/Osterix signaling pathway-mediated promotion of bone formation at the tendon-bone interface after ACL reconstruction with De-BMSCs transplantation. Stem Cell Res Ther. 2021;12(1):576. doi: 10.1186/s13287-021-02643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y., et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11(1):496. doi: 10.1186/s13287-020-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S., et al. Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J Orthop Translat. 2020;22:101–108. doi: 10.1016/j.jot.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 18.Bronckaers A., et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Kinnaird T., et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1) doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura S., et al. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23(6):1425–1432. doi: 10.1016/j.orthres.2005.01.014.1100230627. [DOI] [PubMed] [Google Scholar]
- 23.Freytes D.O., et al. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114(1):220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 24.Genin G.M., Thomopoulos S. The tendon-to-bone attachment: unification through disarray. Nat Mater. 2017;16(6):607–608. doi: 10.1038/nmat4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golman M., et al. Enthesis strength, toughness and stiffness: an image-based model comparing tendon insertions with varying bony attachment geometries. J R Soc Interface. 2021;18(185) doi: 10.1098/rsif.2021.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rufai A., Ralphs J.R., Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. 1995;13(4):585–593. doi: 10.1002/jor.1100130414. [DOI] [PubMed] [Google Scholar]
- 27.Lu H.H., Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apostolakos J., et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 29.Juneja S.C., Veillette C. vol. 2013. Arthritis; 2013. (Defects in tendon, ligament, and enthesis in response to genetic alterations in key proteoglycans and glycoproteins: a review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angeline M.E., Rodeo S.A. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012;31(4):645–663. doi: 10.1016/j.csm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Spalazzi J.P., et al. Elastographic imaging of strain distribution in the anterior cruciate ligament and at the ligament-bone insertions. J Orthop Res. 2006;24(10):2001–2010. doi: 10.1002/jor.20260. [DOI] [PubMed] [Google Scholar]
- 32.Blitz E., et al. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140(13):2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- 33.Jensen P.T., Lambertsen K.L., Frich L.H. Assembly, maturation, and degradation of the supraspinatus enthesis. J Shoulder Elbow Surg. 2018;27(4):739–750. doi: 10.1016/j.jse.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz A.G., et al. Mineral distributions at the developing tendon enthesis. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang I.E., et al. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24(8):1745–1755. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 36.Fujioka H., et al. Changes in the expression of type-X collagen in the fibrocartilage of rat Achilles tendon attachment during development. J Orthop Res. 1997;15(5):675–681. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- 37.Blitz E., et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17(6):861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodeo S.A., et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Lui P., et al. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5:59. doi: 10.1186/1749-799X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carofino B., Fulkerson J. Medial hamstring tendon regeneration following harvest for anterior cruciate ligament reconstruction: fact, myth, and clinical implication. Arthroscopy. 2005;21(10):1257–1265. doi: 10.1016/j.arthro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Deehan D.J., Cawston T.E. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg Br. 2005;87(7):889–895. doi: 10.1302/0301-620X.87B7.16038. [DOI] [PubMed] [Google Scholar]
- 42.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z., et al. A biomaterial-based hedging immune strategy for scarless tendon healing. Adv Mater. 2022:e2200789. doi: 10.1002/adma.202200789. [DOI] [PubMed] [Google Scholar]
- 45.Golman M., et al. Enhanced tendon-to-bone healing via IKKβ inhibition in a rat rotator cuff model. Am J Sports Med. 2021;49(3):780–789. doi: 10.1177/0363546520985203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B., et al. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg(2+)/curcumin from injectable self-healing hydrogels. Theranostics. 2021;11(12):5911–5925. doi: 10.7150/thno.56266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodeo S.A., et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89(11):2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 48.Gerber C., et al. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., et al. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120576. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y., et al. Vascular endothelial growth factor enhances tendon-bone healing by activating Yes-associated protein for angiogenesis induction and rotator cuff reconstruction in rats. J Cell Biochem. 2020;121(3):2343–2353. doi: 10.1002/jcb.29457. [DOI] [PubMed] [Google Scholar]
- 51.Ye C., et al. Icariin promotes tendon-bone healing during repair of rotator cuff tears: a biomechanical and histological study. Int J Mol Sci. 2016;17(11) doi: 10.3390/ijms17111780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai W.C., et al. Low-intensity pulsed ultrasound augments tendon, ligament, and bone-soft tissue healing in preclinical animal models: a systematic review. Arthroscopy. 2021;37(7):2318–2333. doi: 10.1016/j.arthro.2021.02.019. e3. [DOI] [PubMed] [Google Scholar]
- 53.Fealy S., et al. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34(1):120–127. doi: 10.1177/0363546505280212. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., et al. Simvastatin with PRP promotes chondrogenesis of bone marrow stem cells in vitro and wounded rat Achilles tendon-bone interface healing in vivo. Am J Sports Med. 2019;47(3):729–739. doi: 10.1177/0363546518819108. [DOI] [PubMed] [Google Scholar]
- 55.Honda H., et al. Hyaluronic acid accelerates tendon-to-bone healing after rotator cuff repair. Am J Sports Med. 2017;45(14):3322–3330. doi: 10.1177/0363546517720199. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J., et al. Fibrin glue-kartogenin complex promotes the regeneration of the tendon-bone interface in rotator cuff injury. Stem Cell Int. 2021;2021 doi: 10.1155/2021/6640424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W., et al. Type II collagen sponges facilitate tendon stem/progenitor cells to adopt more chondrogenic phenotypes and promote the regeneration of fibrocartilage-like tissues in a rabbit partial patellectomy model. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.682719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shengnan Q., et al. The role of tendon derived stem/progenitor cells and extracellular matrix components in the bone tendon junction repair. Bone. 2021;153 doi: 10.1016/j.bone.2021.116172. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y., et al. TOB1 deficiency enhances the effect of bone marrow-derived mesenchymal stem cells on tendon-bone healing in a rat rotator cuff repair model. Cell Physiol Biochem. 2016;38(1):319–329. doi: 10.1159/000438632. [DOI] [PubMed] [Google Scholar]
- 60.El Agha E., et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Friedenstein A.J., et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 62.Pankajakshan D., Kansal V., Agrawal D.K. In vitro differentiation of bone marrow derived porcine mesenchymal stem cells to endothelial cells. J Tissue Eng Regen Med. 2013;7(11):911–920. doi: 10.1002/term.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silva Meirelles L., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 64.Samsonraj R.M., et al. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang K., et al. Upregulation of Runt related transcription factor 1 (RUNX1) contributes to tendon-bone healing after anterior cruciate ligament reconstruction using bone mesenchymal stem cells. J Orthop Surg Res. 2022;17(1):266. doi: 10.1186/s13018-022-03152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H., et al. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017;56:129–140. doi: 10.1016/j.actbio.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Delarosa O., Dalemans W., Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182. doi: 10.3389/fimmu.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 69.Xu J., et al. Infrapatellar fat pad mesenchymal stromal cell-derived exosomes accelerate tendon-bone healing and intra-articular graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50(3):662–673. doi: 10.1177/03635465211072227. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z., et al. P311 facilitates the angiogenesis and wound healing function of MSCs by increasing VEGF production. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.821932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling L., et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J Biol Chem. 2010;285(34):26233–26244. doi: 10.1074/jbc.M110.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pajarinen J., et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., et al. FGFR2 accommodates osteogenic cell fate determination in human mesenchymal stem cells. Gene. 2022;818 doi: 10.1016/j.gene.2022.146199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnstone B., et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 75.Scott M.A., et al. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cell Dev. 2011;20(10):1793–1804. doi: 10.1089/scd.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waterman R.S., et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edholm E.S., Rhoo K.H., Robert J. Evolutionary aspects of macrophages polarization. Results Probl Cell Differ. 2017;62:3–22. doi: 10.1007/978-3-319-54090-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis M.J., et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio. 2013;4(3) doi: 10.1128/mBio.00264-13. e00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murray P.J. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 80.Muñoz J., et al. Macrophage polarization and osteoporosis: a review. Nutrients. 2020;12(10) doi: 10.3390/nu12102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shapouri-Moghaddam A., et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 82.Mantovani A., et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Wang L.X., et al. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106(2):345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chinetti-Gbaguidi G., Colin S., Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 85.Yang R., et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zizzo G., et al. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189(7):3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q., et al. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20(6):701–712. doi: 10.1038/cr.2010.52. [DOI] [PubMed] [Google Scholar]
- 88.Duluc D., et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110(13):4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 89.Wu H., et al. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106(4):462–468. doi: 10.1002/jso.23110. [DOI] [PubMed] [Google Scholar]
- 90.Lu J., et al. Tendon-to-Bone healing in a rat extra-articular bone tunnel model: a comparison of fresh autologous bone marrow and bone marrow-derived mesenchymal stem cells. Am J Sports Med. 2019;47(11):2729–2736. doi: 10.1177/0363546519862284. [DOI] [PubMed] [Google Scholar]
- 91.Shi Y., et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e923328. doi: 10.12659/MSM.923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hays P.L., et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 93.Dagher E., et al. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467(1):281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gulotta L.V., et al. Evaluation of tumor necrosis factor α blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy. 2011;27(10):1351–1357. doi: 10.1016/j.arthro.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Q., et al. Disulfiram suppressed peritendinous fibrosis through inhibiting macrophage accumulation and its pro-inflammatory properties in tendon bone healing. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.823933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 97.Polverini P.J., et al. Activated macrophages induce vascular proliferation. Nature. 1977;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 98.Sunderkötter C., et al. Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991;51(2):195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 99.Jetten N., et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 100.Lyu L., et al. Exosomes derived from M2 macrophages induce angiogenesis to promote wound healing. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.1008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mantovani A., et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 102.Elliott M.R., Koster K.M., Murphy P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198(4):1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geng R., et al. MFG-E8 promotes tendon-bone healing by regualting macrophage efferocytosis and M2 polarization after anterior cruciate ligament reconstruction. J Orthop Translat. 2022;34:11–21. doi: 10.1016/j.jot.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y.W., et al. Experimental study on influence of bone marrow mesenchymal stem cells on activation and function of mouse peritoneal macrophages. Zhonghua Xue Ye Xue Za Zhi. 2008;29(8):540–543. [PubMed] [Google Scholar]
- 105.Maggini J., et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5(2):e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Németh K., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho D.I., et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46(1):e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geng Y., et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5(3):80. doi: 10.1186/scrt469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou Y.Z., et al. Mesenchymal stem cell-derived conditioned medium attenuate angiotensin II-induced aortic aneurysm growth by modulating macrophage polarization. J Cell Mol Med. 2019;23(12):8233–8245. doi: 10.1111/jcmm.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abumaree M.H., et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9(5):620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 111.Hsu L.W., et al. MicroRNA-301a inhibition enhances the immunomodulatory functions of adipose-derived mesenchymal stem cells by induction of macrophage M2 polarization. Int J Immunopathol Pharmacol. 2020;34 doi: 10.1177/2058738420966092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maldonado-Lasunción I., Verhaagen J., Oudega M. Mesenchymal stem cell-macrophage choreography supporting spinal cord repair. Neurotherapeutics. 2018;15(3):578–587. doi: 10.1007/s13311-018-0629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng S., et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114 doi: 10.1016/j.biocel.2019.105564. [DOI] [PubMed] [Google Scholar]
- 114.Yao M., et al. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- 115.Kloc M., et al. Reciprocal interactions between mesenchymal stem cells and macrophages. Int J Dev Biol. 2020;64(10–11-12):465–469. doi: 10.1387/ijdb.200242jc. [DOI] [PubMed] [Google Scholar]
- 116.Vallés G., et al. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):57. doi: 10.1186/s13287-020-1578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horwood N.J. Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol. 2016;51(1):79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- 118.Gong L., et al. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46(1):65–71. [PubMed] [Google Scholar]
- 119.Zhang Y., et al. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017;369(2):273–286. doi: 10.1007/s00441-017-2598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang M., et al. Bone regeneration is mediated by macrophage extracellular vesicles. Bone. 2020;141 doi: 10.1016/j.bone.2020.115627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu A., et al. Macrophage-derived small extracellular vesicles promote biomimetic mineralized collagen-mediated endogenous bone regeneration. Int J Oral Sci. 2020;12(1):33. doi: 10.1038/s41368-020-00100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sesia S.B., et al. Anti-inflammatory/tissue repair macrophages enhance the cartilage-forming capacity of human bone marrow-derived mesenchymal stromal cells. J Cell Physiol. 2015;230(6):1258–1269. doi: 10.1002/jcp.24861. [DOI] [PubMed] [Google Scholar]
- 123.Fahy N., et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22(8):1167–1175. doi: 10.1016/j.joca.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 124.Miyamoto Y., et al. M1-like macrophage contributes to chondrogenesis in vitro. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]