Abstract
Microvascular alterations were first described in critically ill patients about 20 years ago. These alterations are characterized by a decrease in vascular density and presence of non-perfused capillaries close to well-perfused vessels. In addition, heterogeneity in microvascular perfusion is a key finding in sepsis. In this narrative review, we report our actual understanding of microvascular alterations, their role in the development of organ dysfunction, and the implications for outcome. Herein, we discuss the state of the potential therapeutic interventions and the potential impact of novel therapies. We also discuss how recent technologic development may affect the evaluation of microvascular perfusion.
Keywords: Tissue perfusion, Sepsis, Tissue oxygenation, Fluids, Vasoactive agents, COVID-19
Introduction
Septic shock is characterized by profound hemodynamic alterations that jeopardize tissue perfusion. These hemodynamic alterations include a decrease in vasomotor tone resulting in arterial and venous dilation, central hypovolemia resulting from losses (external losses as well as internal losses with plasma leakage in capillaries with increased permeability), and myocardial depression.[1] Most resuscitation strategies focus on attempts to fix these alterations by administration of fluids and vasopressor and inotropic agents. However, even when these systemic alterations seem to be controlled and values of blood pressure and cardiac output are within target, profound alterations in tissue perfusion may persist. Persistent alterations in tissue perfusion, detected by various clinical signs[2], [3], [4], [5] or biological variables such as veno-arterial differences in partial pressure of carbon dioxide (PCO2)[6] or lactate,[7], [8] have been associated with increased mortality.
Microcirculatory alterations may contribute to these alterations in tissue perfusion. The microcirculation encompasses vessels smaller than 100–150 µm and comprises resistive and distributive arterioles, capillaries, and venules. The anatomy and function differ between the different vessels, with the smallest arterioles (<20 µm) and capillaries being mostly responsible for tissue perfusion and oxygenation. In this narrative review, we discuss novelties in the assessment and management of microvascular alterations in critically ill patients, with a particular emphasis on sepsis.
Assessment of Microcirculation in Sepsis
While alterations in microvascular perfusion have long been shown in experimental models of sepsis, exploration of the microcirculation in humans is more complicated. With the development of small handheld microscopes, evaluation of human microcirculation became feasible. Twenty years ago, we first demonstrated the occurrence of microvascular alterations in the sublingual area in septic patients.[9] These alterations were characterized by a decrease in total vascular density, a decrease in the proportion of perfused capillaries, and heterogeneity between areas close by a few microns. These alterations were in line with alterations that have been described in experimental conditions. Worldwide, multiple trials have confirmed these results.[10], [11] Importantly, the severity of these alterations is associated with a poor outcome.[12], [13], [14], [15], [16]. Interestingly, it was shown that the proportion and density of perfused capillaries were lower and heterogeneity of perfusion was higher in non-survivors than survivors.[14] Further, the average velocity of red blood cells in perfused vessels did not differ between survivors and non-survivors. This finding illustrates that diffusive oxygen transport predominates over convective oxygen transport at the microcirculatory level. Importantly, the evolution of microvascular alterations differed between survivors who progressively improved their microcirculation in response to the global management of sepsis, and non-survivors who failed to improve their microcirculation or even further deteriorated it.[12], [15] Furthermore, the response of the microcirculation to these interventions was associated with evolution of organ function.[10] This finding led to the hope that these alterations are not permanent and that manipulating the microcirculation can be attempted. However, these alterations were not related to the systemic hemodynamic alterations[13], [17], [18] and are induced by multiple factors, making it unlikely that classical hemodynamic interventions or a single intervention can correct microvascular alterations. The impact of therapeutic interventions is discussed below.
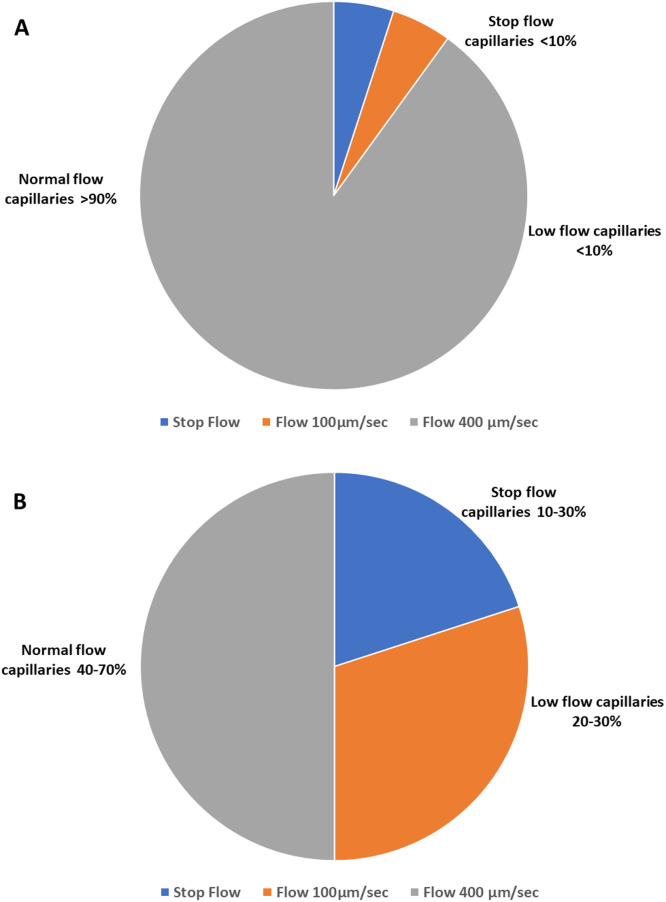
Given the enthusiasm regarding microvascular evaluation, guidelines on how to evaluate and score the microcirculation were published[19] and recently updated given the fast evolution in optics.[20] Microcirculation is usually evaluated in a semi-quantitative method, and often offline even if it can be done in a point-of-care manner.[21] More recently, software that can allow a more formal classification of vascular density and the velocity of perfused vessels has been developed.[22] By concomitantly analyzing vessel density and perfusion, Hilty et al.[23] demonstrated that it was feasible to identify different patterns that differentiate sepsis from other diseases associated with microcirculatory alterations. The authors identified that sepsis microcirculation is characterized by a significant number of capillaries perfused at low velocities (∼100 µm/s), while other capillaries have normal velocities (400–500 µm/s). Using another approach, Rovas et al.[24] further characterized the microvascular alterations in septic patients: the density of microvascular vessels was markedly decreased for vessels smaller than 8 µm, while the velocity of red blood cells in the already perfused vessels did not differ from that of the control group patients, regardless of the vessel diameter. In addition, the authors showed that red blood cell velocity in capillaries depended on the velocity in nutritive capillaries, a feature not observed in controls. This finding reflects the fact that perfused capillaries are insensitive to local variations in local oxygen demand in sepsis.
What the above studies add to previous observations of functional capillary density being decreased due to the shutting down of some capillaries, is that perfused capillaries present a huge variability in velocities, with low-flow areas in the vicinity of areas with normal velocities (Figure 1). Whether this fine-tuned characterization of the microcirculation with identification of low-flow perfusion in some capillaries offers clinical benefit over manual semi-quantitative evaluation remains to be determined.
Figure 1.
Proportion of stopped flow, low flow, and normal flow capillaries in normal situation (A) and septic shock conditions (B). The proportion of capillaries was computed from De Backer et al.[13] and Hilty et al.[23]
Another recently proposed aspect is the possibility to better evaluate the kinetics of white blood cells in the microcirculation. Intravital microscopy has long demonstrated the increase in rolling and adhesion of white blood cells to the endothelium in experimental sepsis or ischemia–reperfusion injury.[25] In humans, new advances in optics have allowed the visualization of this phenomenon.[26], [27] Patients with septic shock show greater leukocyte adhesion and rolling than healthy controls.[28] In addition, the number of adhering leukocytes is higher in non-survivors than survivors.[28] Whether leukocyte kinetics may represent a specific target for therapy remains to be determined.
Microcirculatory Alterations in other Types of Critically Ill Patients
Occurrence of microvascular alterations was also demonstrated in other conditions. In this section we discuss what is similar and what differs from the microvascular alterations in sepsis.
Assessment of the microcirculation in coronavirus disease 2019 (COVID-19) patients
COVID-19 is a viral infection with severe lung involvement in the vast majority of patients admitted to the intensive care unit (ICU). Accordingly, one may anticipate some alterations in the microcirculation in patients with severe COVID-19. Although microvascular alterations were reported in COVID-19 patients, their nature varied. Some studies showed impaired capillary perfusion,[29], [30] while others reported that the microvascular perfusion density was increased in COVID-19.[31], [32] The increase in microvascular density and perfusion observed in the latter studies resembles those observed at high altitudes.[33] The increase in vascular density was inversely proportional to the time under mechanical ventilation.[32] Hence, an integrative view of these apparently conflicting results may be that at the early stages, the microcirculation may adapt to the hypoxemia experienced by the patient, as it does at high altitudes. At later stages of COVID-19 infection, when secondary infections occur, the microvascular alterations are similar to those observed in sepsis. Importantly, microvascular reactivity has been found to be reduced in COVID-19 as in sepsis,[34] potentially as a result of endothelial dysfunction related to the increased inflammation.[29] Circulating cells may also play a role in these microvascular alterations. Leukocyte activation may be present with increased amounts of leukocytes in post-capillary venules.[35] Red blood cell deformability can also be impaired in COVID-19 patients, as in septic patients.[36] In one study, while microaggregates of white and red blood cells were noted both in recruited and de-recruited microcirculation, they were seen in higher amounts in patients with de-recruited microcirculation.[35] In a case report of a child presenting with multisystem inflammatory syndrome (MIS-C), the alterations in nailfold microvascular perfusion were very similar to those observed in sepsis.[37]
Assessment of the microcirculation in non-septic conditions
In patients with cardiogenic shock, a decrease in the proportion of perfused vessels and perfused vascular density and an increase in heterogeneity were observed.[38] Microvascular alterations were more severe in patients with poor outcomes.[38], [39], [40], [41] Differences in microvascular perfusion between survivors and non-survivors were observed not only at baseline[38], [39], [40], [41] but also at later stages when repeated measurements of the microcirculation were obtained after hemodynamic stabilization.[39] In perioperative patients, the occurrence of microvascular alterations (characterized by decreased perfused vascular density) was associated with the development of perioperative complications.[42] Anesthesia contributes to some part of these alterations; however, these effects are transient, and the impact of the superimposed surgical procedure is greater.[43] Although mortality is minimal in this type of procedure, the severity and duration of microvascular alterations are associated with hyperlactatemia and organ dysfunction.[43], [44], [45], [46] Among patients with trauma in whom bleeding was controlled and resuscitation was considered adequate based on systemic hemodynamics, more severe microvascular alterations are observed in those developing organ dysfunction.[47] In eclampsia, microcirculatory alterations can also be detected and the severity correlated with severity of disease.[48]
How can we explain the occurrence of microcirculatory alterations in these various states? Many pathways similar to those activated in sepsis can be activated in these conditions, even though the trigger is non-infectious. Among these, activation of inflammation and coagulation are the most important and these occur due to ischemia–reperfusion injury in trauma, including surgical trauma. In cardiogenic shock, activation of inflammation has been demonstrated, leading sometimes to the development of a so-called “systemic inflammatory response syndrome (SIRS)” component. Moreover, the impact of anesthetic or sedatives agents should not be neglected. In eclampsia, the common pathway is the alteration in endothelial function. Interestingly, despite the similarities observed in the type of microvascular alterations as well as in their impact on organ dysfunction, the severity of the alterations is often of lower intensity than in sepsis and septic shock.
Treating the Septic Microcirculation
Given the heterogeneous nature of microvascular alterations in sepsis, with perfused vessels in close proximity of non-perfused vessels, recruiting the microcirculation may be more beneficial than just increasing total flow to the tissue. This was well illustrated in a mathematical model that showed a higher oxygen consumption after recruiting the non-perfused vessels at baseline than a global increase in flow.[49] Nevertheless, the impact of classical resuscitation procedures should not be neglected: in sepsis, the velocity of red blood cells in capillaries is correlated with the velocity in nutritive vessels, contrary to what occurs in normal conditions.[24] Hence, restoring global perfusion may to some extend be beneficial in sepsis, even though it often fails to normalize microvascular perfusion.
Impact of fluids
In experimental models, fluids usually improve the microcirculation in septic conditions. In septic shock patients, we previously demonstrated that fluid administration improved the microcirculation.[50] Importantly, even though our results were globally significant, there was huge individual variability. While microvascular perfusion improved in most patients when fluids were administered within the first 24 h of the recognition of sepsis, fluids failed to improve the microcirculation when administered after 48 h. Another question relates to what the ideal volume of fluids would be. We do not have a large enough data set to address this question. However, in a small pilot study, the first bolus of fluid significantly improved microvascular perfusion but not the second one, even though cardiac output further increased.[51] There are several lessons from that study. First, a minimal volume of fluid is needed to improve the microcirculation, but excess volume may be useless. Some other studies have even shown that larger amount of fluids may be detrimental to the microcirculation[52] and that, at later stages when fluid accumulation had become obvious, fluid withdrawal may improve the microcirculation.[53] Second, changes in microvascular perfusion induced by fluids are independent from changes in systemic hemodynamics, a feature confirmed by other trials.[50] Interestingly, when microcirculation improved in response to fluids, this response was associated with an improvement in organ function 24 h later.[54] Given the variability in response to fluids, some authors have advised restricted fluid administration in patients with impaired microcirculation to guide fluid resuscitation based on microcirculation assessment.[54], [55]
Impact of red blood cell transfusions
While red blood cell transfusions appear to be an attractive option potentially improving diffusive capacity, results of clinical trials in sepsis have been quite variable.[56] We observed no overall beneficial effects of red blood cell transfusions, although some patients benefited from transfusions while others experienced a deterioration of their microcirculation.[57] Interestingly, baseline microcirculation evaluation helped to predict the response to transfusions, with improvement in patients with poor microcirculation at baseline and deterioration of the microcirculation in patients with close to normal microcirculation at baseline. The dependence of the effects of transfusions on the quality of microcirculation at baseline was confirmed by several trials.[58], [59], [60] While the age of transfused red blood cells does not seem to play a role, the levels of free hemoglobin in patient blood and transfused blood may play a role: microvascular perfusion increased when transfusion decreased the free hemoglobin levels in patient blood, while perfusion decreased when free hemoglobin levels increased.[59] In all trials, the microcirculatory effects of red blood cell transfusions were independent of hemoglobin level prior to transfusion.[57], [58], [59], [60]
Impact of inotropic agents
Different inotropic agents have been used in various studies to try to improve the microcirculation in sepsis. While dobutamine improved the microcirculation in experimental conditions,[61] the effects in septic patients were more variable. Low-dose dobutamine improved the microcirculation in some patients but not all,[62], [63] resulting in a decrease in lactate that was proportional to the improvement in microvascular perfusion. The microcirculatory effects of dobutamine were independent of its systemic effects.[62] Milrinone and levosimendan also showed variable results.[64]
Impact of vasopressor agents
In control conditions, vasopressors impair the microcirculation because of the combined constriction of resistive arterioles and venules, both of which decrease capillary perfusion pressure. However, vasopressors are used to restore perfusion pressure to the organs that are compromised by severe hypotension. In an experimental model of septic shock, administration of norepinephrine or vasopressin both resulted in an improvement in gut microvascular perfusion.[65] All organs may not behave similarly. In an ovine model of septic shock, correction of severe hypotension failed to improve the severely compromised cerebral microcirculation, despite slight improvements in cerebral oxygenation.[66] There are no recent data regarding the correction of severe hypotension in septic shock patients. Several trials have tested different targets of blood pressure, and although they have provided inconsistent results,[67], [68] targeting pre-disease blood pressure level is associated with a variable effect on microvascular perfusion.[69], [70]
Impact of vasodilatory agents
With the initial description of the occurrence of microvascular dysfunction in patients with sepsis, we demonstrated that topical administration of acetylcholine could completely normalize the microcirculation.[9] Shortly after, Spronk et al.[71] reported in a pilot trial that nitroglycerin improved the microcirculation. Those results were unfortunately not confirmed in a randomized trial.[17] Other vasodilatory agents have also shown promising results in experimental conditions. A randomized trial testing the impact of a prostacyclin analog is currently ongoing.[72]
Alternative therapies
Given the variability of the effects of fluids and vasoactive agents, many alternative therapies have been tested. Only a few of the most promising therapies have been discussed in this paper. Ascorbate or vitamin C has been widely studied in an experimental setting. Vitamin C improved the microcirculation in a rodent model of peritonitis even when administered 24 h after sepsis onset.[73] Interestingly, the beneficial effects of vitamin C are achieved through the action of endothelial nitric oxide synthase (NOS); the effects were absent in animals deprived of endothelial NOS, but these were still present in animals deprived of other types of NOS.[74] A pilot study reported that vitamin C administration in patients with septic shock improved the microcirculation as well as mottling scores and capillary refill time.[75] Interestingly, the beneficial effects of vitamin C were independent of plasma levels of vitamin C before infusion, which suggest that higher than normal values of vitamin C are needed to improve microvascular perfusion. In that pilot study, the sustainability and impact of repeated infusions of vitamin C were not investigated.
Another attractive pathway may be extracorporeal removal of cytokines and other substances by cartridges. In a pilot trial including nine patients with septic shock under renal replacement therapy, the addition of the CytoSorb cartridge to the system was associated with an improvement in the proportion of perfused vessels and perfused vascular density together with a decrease in certain pro-inflammatory cytokines.[76] Similar results were reported in a case report by another group.[77] These promising results require further validation in larger trials.
The Concept of Microcirculatory Reserve
As for many other aspects in the human body, the normal microvascular perfusion is not at its maximal value at rest. Using topical vasodilators, it is possible to increase the density of perfused vessels in healthy subjects with nitroglycerin but not with acetylcholine.[78] It is also possible to increase the density of perfused capillaries in healthy individuals by using enteral nitrite donors.[79] In patients with septic shock, topical administration of acetylcholine fully normalized the sublingual microcirculation.[9], [62] Similar effects of topical acetylcholine were observed in cardiogenic shock.[38] Taken together, these findings suggest that direct administration of nitric oxide (NO) recruited the microcirculation, while acetylcholine, which requires endothelial activation to release NO, is ineffective in healthy subjects but effective in disease, potentially highlighting the presence of active feedbacks preventing recruitment of the microcirculation when not required by local metabolic needs.
Is the Sublingual Microcirculation Representative of other Organs?
In experimental conditions, most organs are affected similarly with respect to both duration and severity, so that alterations in the sublingual area reflect alterations in other organs such as the gut or kidney.[80], [81] However, other studies report different degrees of alteration in the sublingual area and skin[82] or gut.[83] Considering the available evidence, one may speculate that although sepsis is associated with diffuse alterations that can be captured at the level of the sublingual area, more severe alterations may sometimes be observed in certain organs because of the specific local environment (local vasoconstriction and/or increased interstitial pressure). Given the easy access of the sublingual area and the close link with organ dysfunction and mortality, the sublingual area can be considered an excellent window to investigate the microcirculation. In patients in whom the sublingual area cannot be accessed (i.e., during non-invasive ventilation or due to lack of patients’ collaboration), the conjunctival area may be considered as a surrogate.[84]
Should We Target the Microcirculation at Bedside?
While it appears attractive to target the microcirculation at bedside given the relevance of the implication of microvascular alterations in the development of organ dysfunction, it may be premature to target the resuscitation at bedside or even in clinical trials. First, as described above, interventions that can consistently improve the microcirculation in most patients are still lacking. The effects of interventions are highly variable among individuals, even when significant results have been observed for the entire cohort. Accordingly, identifying factors associated with a positive response to the agent is crucial and not yet broadly available. Second, it is difficult to define which target value should be achieved for which variable. The question remains whether the functional vascular density or proportion of perfused vessels should be targeted, along with whether heterogeneity should be minimized. Regarding the target value, it is obvious that maximal perfusion should not be targeted, as perfusion is not at its maximal value even in healthy subjects. In that case, can the usual values observed in critically ill patients be used as target values or can some level of abnormality still be accepted? In addition, the target value may also depend on the time at which it is assessed. In the emergency department, many low acuity septic patients present microvascular alterations that rapidly resolve with basic interventions.[85] In ICU-admitted patients with more severe sepsis, the cut-off value separating survivors from non-survivors is lower just after ICU admission than 48 h later,[13] suggesting that the target value is time-dependent. Finally, and most importantly, it is challenging to know at this stage whether we should target normalization of the microcirculation or just aim for an extent of improvement and if yes, to which value. It would be beneficial to address these issues before designing and executing trials targeting microcirculation.
Conclusions
While alterations in microvascular perfusion have long been neglected owing to difficulties in bedside assessment of critically ill patients, technical developments have allowed the role of microvascular alterations in sepsis to be demonstrated. Recent developments in the analysis of images have allowed clinicians to understand that a significant number of capillaries are hypoperfused in sepsis, in addition to the already known alterations that include stop flow capillaries and heterogeneity in perfusion. Whether these new advances would better define the impact of therapeutic interventions remains to be studied further.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Managing Editor: Jingling Bao.
Footnotes
Given his role as Associate Editor, Prof. Daniel De Backer had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Prof. Olfa Hamzaoui took the responsibility for peer-review progress. Prof. Jean-Louis Teboul who is the co-editor-in-chief made the final decision.
References
- 1.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Oufella H, Bourcier S, Alves M, Galbois A, Baudel JL, Margetis D, et al. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care. 2013;3(1):31. doi: 10.1186/2110-5820-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas G, Lavillegrand JR, Joffre J, Bigé N, de-Moura EB, Baudel JL, et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. 2019;23(1):211. doi: 10.1186/s13054-019-2496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouffroy R, Saade A, Tourtier JP, Gueye P, Bloch-Laine E, Ecollan P, et al. Skin mottling score and capillary refill time to assess mortality of septic shock since pre-hospital setting. Am J Emerg Med. 2019;37(4):664–671. doi: 10.1016/j.ajem.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Lara B, Enberg L, Ortega M, Leon P, Kripper C, Aguilera P, et al. Capillary refill time during fluid resuscitation in patients with sepsis-related hyperlactatemia at the emergency department is related to mortality. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, Tafur JD, Gutiérrez A, García AF, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17(6):R294. doi: 10.1186/cc13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rimachi R, Bruzzi de Carvahlo F, Orellano-Jimenez C, Cotton F, Vincent JL, De Backer D. Lactate/pyruvate ratio as a marker of tissue hypoxia in circulatory and septic shock. Anaesth Intensive Care. 2012;40(3):427–432. doi: 10.1177/0310057X1204000307. [DOI] [PubMed] [Google Scholar]
- 8.Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the surviving sepsis campaign database. Crit Care Med. 2015;43(3):567–573. doi: 10.1097/CCM.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 10.Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34(12):2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vellinga N, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, et al. Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis. Crit Care. 2017;21(1):255. doi: 10.1186/s13054-017-1842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 13.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 14.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012;40(5):1443–1448. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 15.Massey MJ, Hou PC, Filbin M, Wang H, Ngo L, Huang DT, et al. Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care. 2018;22(1):308. doi: 10.1186/s13054-018-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanos A, Jhanji S, Vivian-Smith A, Harris T, Pearse RM. Early microvascular changes in sepsis and severe sepsis. Shock. 2010;33(4):387–391. doi: 10.1097/SHK.0b013e3181c6be04. [DOI] [PubMed] [Google Scholar]
- 17.Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38(1):93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- 18.Edul VS, Ince C, Vazquez AR, Rubatto PN, Espinoza ED, Welsh S, et al. Similar microcirculatory alterations in patients with normodynamic and hyperdynamic septic shock. Ann Am Thorac Soc. 2016;13(2):240–247. doi: 10.1513/AnnalsATS.201509-606OC. [DOI] [PubMed] [Google Scholar]
- 19.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European society of intensive care medicine. Intensive Care Med. 2018;44(3):281–299. doi: 10.1007/s00134-018-5070-7. [DOI] [PubMed] [Google Scholar]
- 21.Arnold RC, Parrillo JE, Phillip Dellinger R, Chansky ME, Shapiro NI, Lundy DJ, et al. Point-of-care assessment of microvascular blood flow in critically ill patients. Intensive Care Med. 2009;35(10):1761–1766. doi: 10.1007/s00134-009-1517-1. [DOI] [PubMed] [Google Scholar]
- 22.Hilty MP, Guerci P, Ince Y, Toraman F, Ince C. Microtools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun Biol. 2019;2:217. doi: 10.1038/s42003-019-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilty MP, Akin S, Boerma C, Donati A, Erdem Ö, Giaccaglia P, et al. Automated algorithm analysis of sublingual microcirculation in an international multicentral database identifies alterations associated with disease and mechanism of resuscitation. Crit Care Med. 2020;48(10):e864–e875. doi: 10.1097/CCM.0000000000004491. [DOI] [PubMed] [Google Scholar]
- 24.Rovas A, Sackarnd J, Rossaint J, Kampmeier S, Pavenstädt H, Vink H, et al. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: the NOSTRADAMUS study. Crit Care. 2021;25(1):112. doi: 10.1186/s13054-021-03520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann JN, Vollmar B, Inthorn D, Schildberg FW, Menger MD. A chronic model for intravital microscopic study of microcirculatory disorders and leukocyte/endothelial cell interaction during normotensive endotoxemia. Shock. 1999;12(5):355–364. doi: 10.1097/00024382-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Uz Z, van Gulik TM, Aydemirli MD, Guerci P, Ince Y, Cuppen D, et al. Identification and quantification of human microcirculatory leukocytes using handheld video microscopes at the bedside. J Appl Physiol (1985) 2018;124(6):1550–1557. doi: 10.1152/japplphysiol.00962.2017. [DOI] [PubMed] [Google Scholar]
- 27.Bauer A, Kofler S, Thiel M, Eifert S, Christ F. Monitoring of the sublingual microcirculation in cardiac surgery using orthogonal polarization spectral imaging: peliminary results. Anesthesiology. 2007;107(6):939–945. doi: 10.1097/01.anes.0000291442.69337.c9. [DOI] [PubMed] [Google Scholar]
- 28.Fabian-Jessing BK, Massey MJ, Filbin MR, Hou PC, Wang HE, Kirkegaard H, et al. In vivo quantification of rolling and adhered leukocytes in human sepsis. Crit Care. 2018;22(1):240. doi: 10.1186/s13054-018-2173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanoore Edul VS, Caminos Eguillor JF, Ferrara G, Estenssoro E, Siles DSP, Cesio CE, et al. Microcirculation alterations in severe COVID-19 pneumonia. J Crit Care. 2021;61:73–75. doi: 10.1016/j.jcrc.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grewal S, Harjo B, Aykut G, Ergin B, Nowitzky R, Ince C, et al. Case report: sublingual microcirculatory alterations in a COVID-19 patient with subcutaneous emphysema, venous thrombosis, and pneumomediastinum. Front Med (Lausanne) 2021;7 doi: 10.3389/fmed.2020.624695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Dedda U, Ascari A, Fantinato A, Fina D, Baryshnikova E, Ranucci M. Microcirculatory alterations in critically Ill patients with COVID-19-associated acute respiratory distress syndrome. J Clin Med. 2022;11(4):1032. doi: 10.3390/jcm11041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilty MP, Merz TM, Hefti U, Ince C, Maggiorini M, Pichler Hefti J. Recruitment of non-perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m. J Physiol. 2019;597(10):2623–2638. doi: 10.1113/JP277590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesquida J, Caballer A, Cortese L, Vila C, Karadeniz U, Pagliazzi M, et al. Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit Care. 2021;25(1):381. doi: 10.1186/s13054-021-03803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favaron E, Ince C, Hilty MP, Ergin B, van der Zee P, Uz Z, et al. Capillary leukocytes, microaggregates, and the response to hypoxemia in the microcirculation of coronavirus disease 2019 patients. Crit Care Med. 2021;49(4):661–670. doi: 10.1097/CCM.0000000000004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piagnerelli M, Vanderelst J, Rousseau A, Monteyne D, Perez-Morga D, Biston P, et al. Red blood cell shape and deformability in patients with COVID-19 acute respiratory distress syndrome. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.849910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamez-Rivera O, Villarreal-Treviño AV, Castañeda-Macazaga T, Britton-Robles SC, Ramos-Gómez LI, Rubio-Pérez NE. Abnormal nailfold capillaroscopy in a patient with multisystem inflammatory syndrome in children. Pediatr Infect Dis J. 2021;40(3):e113–e115. doi: 10.1097/INF.0000000000003022. [DOI] [PubMed] [Google Scholar]
- 38.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147(1):91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 39.den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, et al. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2010;31(24):3032–3039. doi: 10.1093/eurheartj/ehq324. [DOI] [PubMed] [Google Scholar]
- 40.Jung C, Ferrari M, Rödiger C, Fritzenwanger M, Goebel B, Lauten A, et al. Evaluation of the sublingual microcirculation in cardiogenic shock. Clin Hemorheol Microcirc. 2009;42(2):141–148. doi: 10.3233/CH-2009-1194. [DOI] [PubMed] [Google Scholar]
- 41.Wijntjens GW, Fengler K, Fuernau G, Jung C, den Uil C, Akin S, et al. Prognostic implications of microcirculatory perfusion versus macrocirculatory perfusion in cardiogenic shock: a CULPRIT-SHOCK substudy. Eur Heart J Acute Cardiovasc Care. 2020;9(2):108–119. doi: 10.1177/2048872619870035. [DOI] [PubMed] [Google Scholar]
- 42.Jhanji S, Lee C, Watson D, Hinds C, Pearse RM. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med. 2009;35(4):671–677. doi: 10.1007/s00134-008-1325-z. [DOI] [PubMed] [Google Scholar]
- 43.De Backer D, Dubois MJ, Schmartz D, Koch M, Ducart A, Barvais L, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg. 2009;88(5):1396–1403. doi: 10.1016/j.athoracsur.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 44.den Uil CA, Lagrand WK, Spronk PE, van Domburg RT, Hofland J, Lüthen C, et al. Impaired sublingual microvascular perfusion during surgery with cardiopulmonary bypass: a pilot study. J Thorac Cardiovasc Surg. 2008;136(1):129–134. doi: 10.1016/j.jtcvs.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 45.Greenwood JC, Jang DH, Spelde AE, Gutsche JT, Horak J, Acker MA, et al. Low microcirculatory perfused vessel density and high heterogeneity are associated with increased intensity and duration of lactic acidosis after cardiac surgery with cardiopulmonary bypass. Shock. 2021;56(2):245–254. doi: 10.1097/SHK.0000000000001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh YC, Wang MJ, Chao A, Ko WJ, Chan WS, Fan SZ, et al. Correlation between early sublingual small vessel density and late blood lactate level in critically ill surgical patients. J Surg Res. 2013;180(2):317–321. doi: 10.1016/j.jss.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42(6):1433–1441. doi: 10.1097/CCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 48.Ospina-Tascón GA, Nieto Calvache AJ, Quiñones E, Madriñan HJ, Valencia JD, Bermúdez WF, et al. Microcirculatory blood flow derangements during severe preeclampsia and HELLP syndrome. Pregnancy Hypertens. 2017;10:124–130. doi: 10.1016/j.preghy.2017.07.140. [DOI] [PubMed] [Google Scholar]
- 49.Østergaard L. Blood flow, capillary transit times, and tissue oxygenation: the centennial of capillary recruitment. J Appl Physiol (1985) 2020;129(6):1413–1421. doi: 10.1152/japplphysiol.00537.2020. [DOI] [PubMed] [Google Scholar]
- 50.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 51.Pottecher J, Deruddre S, Teboul JL, Georger JF, Laplace C, Benhamou D, et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010;36(11):1867–1874. doi: 10.1007/s00134-010-1966-6. [DOI] [PubMed] [Google Scholar]
- 52.Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol. 2013;13:17. doi: 10.1186/1471-2253-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uz Z, Ince C, Guerci P, Ince Y, P Araujo R, Ergin B, et al. Recruitment of sublingual microcirculation using handheld incident dark field imaging as a routine measurement tool during the postoperative de-escalation phase-a pilot study in post ICU cardiac surgery patients. Perioper Med (Lond) 2018;7:18. doi: 10.1186/s13741-018-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39(4):612–619. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Q, Dai C, Zhu Y, Han T, Zhou J, Zhao L, et al. The effectiveness and feasibility of fluid resuscitation directed by microcirculation monitoring in patients with septic shock: a randomized controlled trial. Ann Palliat Med. 2021;10(8):9069–9077. doi: 10.21037/apm-21-1971. [DOI] [PubMed] [Google Scholar]
- 56.Liu W, He H, Ince C, Long Y. The effect of blood transfusion on sublingual microcirculation in critically ill patients: a scoping review. Microcirculation. 2021;28(3):e12666. doi: 10.1111/micc.12666. [DOI] [PubMed] [Google Scholar]
- 57.Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35(7):1639–1644. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 58.Sadaka F, Aggu-Sher R, Krause K, O'Brien J, Armbrecht ES, Taylor RW. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1(1):46. doi: 10.1186/2110-5820-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donati A, Damiani E, Luchetti M, Domizi R, Scorcella C, Carsetti A, et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study. Crit Care. 2014;18(1):R33. doi: 10.1186/cc13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheuzger J, Zehnder A, Meier V, Yeginsoy D, Flükiger J, Siegemund M. Sublingual microcirculation does not reflect red blood cell transfusion thresholds in the intensive care unit-a prospective observational study in the intensive care unit. Crit Care. 2020;24(1):18. doi: 10.1186/s13054-020-2728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ospina-Tascón GA, García Marin AF, Echeverri GJ, Bermudez WF, Madriñán-Navia H, Valencia JD, et al. Effects of dobutamine on intestinal microvascular blood flow heterogeneity and O(2) extraction during septic shock. J Appl Physiol (1985) 2017;122(6):1406–1417. doi: 10.1152/japplphysiol.00886.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34(2):403–408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013;39(8):1435–1443. doi: 10.1007/s00134-013-2982-0. [DOI] [PubMed] [Google Scholar]
- 64.Potter EK, Hodgson L, Creagh-Brown B, Forni LG. Manipulating the microcirculation in sepsis – the impact of vasoactive medications on microcirculatory blood flow: a systematic review. Shock. 2019;52(1):5–12. doi: 10.1097/SHK.0000000000001239. [DOI] [PubMed] [Google Scholar]
- 65.Nakajima Y, Baudry N, Duranteau J, Vicaut E. Effects of vasopressin, norepinephrine, and L-arginine on intestinal microcirculation in endotoxemia. Crit Care Med. 2006;34(6):1752–1757. doi: 10.1097/01.CCM.0000218812.73741.6C. [DOI] [PubMed] [Google Scholar]
- 66.Taccone FS, Su F, He X, Peluso L, Donadello K, Scolletta S, et al. Effects of reversal of hypotension on cerebral microcirculation and metabolism in experimental sepsis. Biomedicines. 2022;10(4):923. doi: 10.3390/biomedicines10040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubin A, Pozo MO, Casabella CA, Pálizas F, Jr., Murias G, Moseinco MC, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13(3):R92. doi: 10.1186/cc7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thooft A, Favory R, Salgado DR, Taccone FS, Donadello K, De Backer D, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15(5):R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu JY, Ma SQ, Pan C, He HL, Cai SX, Hu SL, et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: a prospective open label study. Crit Care. 2015;19(1):130. doi: 10.1186/s13054-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiorese Coimbra KT, de Freitas FGR, Bafi AT, Pinheiro TT, Nunes NF, de Azevedo LCP, et al. Effect of increasing blood pressure with noradrenaline on the microcirculation of patients with septic shock and previous arterial hypertension. Crit Care Med. 2019;47(8):1033–1040. doi: 10.1097/CCM.0000000000003795. [DOI] [PubMed] [Google Scholar]
- 71.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360(9343):1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 72.Legrand M, Oufella HA, De Backer D, Duranteau J, Leone M, Levy B, et al. The I-MICRO trial, Ilomedin for treatment of septic shock with persistent microperfusion defects: a double-blind, randomized controlled trial-study protocol for a randomized controlled trial. Trials. 2020;21(1):601. doi: 10.1186/s13063-020-04549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med. 2005;33(8):1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 74.Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med. 2008;36(8):2355–2362. doi: 10.1097/CCM.0b013e31818024f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lavillegrand JR, Raia L, Urbina T, Hariri G, Gabarre P, Bonny V, et al. Vitamin C improves microvascular reactivity and peripheral tissue perfusion in septic shock patients. Crit Care. 2022;26(1):25. doi: 10.1186/s13054-022-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zuccari S, Damiani E, Domizi R, Scorcella C, D'Arezzo M, Carsetti A, et al. Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with cytosorb. Blood Purif. 2020;49(1–2):107–113. doi: 10.1159/000502540. [DOI] [PubMed] [Google Scholar]
- 77.Dilken O, Ince C, van der Hoven B, Thijsse S, Ormskerk P, de Geus H. Successful reduction of creatine kinase and myoglobin levels in severe rhabdomyolysis using extracorporeal blood purification (CytoSorb®) Blood Purif. 2020;49(6):743–747. doi: 10.1159/000505899. [DOI] [PubMed] [Google Scholar]
- 78.Hilty MP, Pichler J, Ergin B, Hefti U, Merz TM, Ince C, et al. Assessment of endothelial cell function and physiological microcirculatory reserve by video microscopy using a topical acetylcholine and nitroglycerin challenge. Intensive Care Med Exp. 2017;5(1):26. doi: 10.1186/s40635-017-0139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolb L, Orbegozo D, Creteur J, Preiser JC, Vincent JL, De Backer D. Oral nitrate increases microvascular reactivity and the number of visible perfused microvessels in healthy volunteers. J Vasc Res. 2017;54(4):209–216. doi: 10.1159/000468541. [DOI] [PubMed] [Google Scholar]
- 80.Verdant C, Bruhn A, Clausi C, Su F, Wang Z, De Backer D, et al. Sublingual microcirculation reflects intestinal mucosal microcirculation in sepsis: a quantitative assessment. Crit Care Med. 2005;33(12):A51. doi: 10.1097/00003246-200512002-00188. [DOI] [PubMed] [Google Scholar]
- 81.Lima A, van Rooij T, Ergin B, Sorelli M, Ince Y, Specht PAC, et al. Dynamic contrast-enhanced ultrasound identifies microcirculatory alterations in sepsis-induced acute kidney injury. Crit Care Med. 2018;46(8):1284–1292. doi: 10.1097/CCM.0000000000003209. [DOI] [PubMed] [Google Scholar]
- 82.Boerma EC, Kuiper MA, Kingma WP, Egbers PH, Gerritsen RT, Ince C. Disparity between skin perfusion and sublingual microcirculatory alterations in severe sepsis and septic shock: a prospective observational study. Intensive Care Med. 2008;34(7):1294–1298. doi: 10.1007/s00134-008-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boerma EC, van der Voort PH, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007;35(4):1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 84.Simkiene J, Pranskuniene Z, Vitkauskiene A, Pilvinis V, Boerma EC, Pranskunas A. Ocular microvascular changes in patients with sepsis: a prospective observational study. Ann Intensive Care. 2020;10(1):38. doi: 10.1186/s13613-020-00655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Filbin MR, Hou PC, Massey M, Barche A, Kao E, Bracey A, et al. The microcirculation is preserved in emergency department low-acuity sepsis patients without hypotension. Acad Emerg Med. 2014;21(2):154–162. doi: 10.1111/acem.12314. [DOI] [PubMed] [Google Scholar]