Significance
In contrast to animals and plants, the temperature and thermoregulation of fungi are relatively unknown. Our data suggest that not only mushrooms, but yeast and mold communities can maintain colder temperatures than their surroundings. Like mushrooms, unicellular fungal colonies achieve hypothermia via evaporating cooling, suggesting that this cooling process is an evolutionary ancient mechanism of thermoregulation. Similar to plants, evaporative cooling by fungi could potentially be harnessed for passive indoor air conditioning. By extending hypothermia to microscopic fungi, our data suggest that fungal hypothermia is a general characteristic of the fungal kingdom. Since fungi play essential functions in supporting terrestrial life, these results are relevant for predicting the effects of global warming on fungal biodiversity and ecology.
Keywords: fungal thermoregulation, fungal hypothermia, mushroom, fungal evapotranspiration, infrared thermography
Abstract
Fungi play essential roles in global health, ecology, and economy, but their thermal biology is relatively unexplored. Mushrooms, the fruiting body of mycelium, were previously noticed to be colder than surrounding air through evaporative cooling. Here, we confirm those observations using infrared thermography and report that this hypothermic state is also observed in mold and yeast colonies. The relatively colder temperature of yeasts and molds is also mediated via evaporative cooling and associated with the accumulation of condensed water droplets on plate lids above colonies. The colonies appear coldest at their center and the surrounding agar appears warmest near the colony edges. The analysis of cultivated Pleurotus ostreatus mushrooms revealed that the hypothermic feature of mushrooms can be observed throughout the whole fruiting process and at the level of mycelium. The mushroom’s hymenium was coldest, and different areas of the mushroom appear to dissipate heat differently. We also constructed a mushroom-based air-cooling prototype system capable of passively reducing the temperature of a semiclosed compartment by approximately 10 °C in 25 min. These findings suggest that the fungal kingdom is characteristically cold. Since fungi make up approximately 2% of Earth’s biomass, their evapotranspiration may contribute to cooler temperatures in local environments.
Fungi play a central role in balancing Earth’s ecology by breaking down decaying biological matter and providing nutrients for new growth. The fungal kingdom includes macroscopic species, such as the mushroom-producing mycorrhizal fungi as well as, microscopic unicellular molds and yeasts. Fungal organisms can be a source of food, medicines, and various biomaterials (1), but some can also be pathogenic to animal and plant flora, causing severe public health and agricultural problems (2). Given the ecological and economical importance of fungi, it is crucial to understand their temperature and thermoregulation, particularly in the face of global warming.
Temperature controls the growth, reproduction, and ecological distribution of all life forms. An organism’s temperature depends on the balance between the production, gain, and dissipation of thermal energy, as influenced by its total environment, including physical–chemical, biotic–abiotic, and micro–macrodimensions (3). In theory, if the organism gains more thermal energy than it dissipates, it becomes warmer. Conversely, if more thermal energy is lost, the organism may reach colder temperatures than its surroundings. When the organism and the environment have the same temperature, there is no heat flow, and the organism is in thermal equilibrium. Living organisms are defined as dissipative systems that exist far from thermodynamic equilibrium (4), which means that in some dimensions, they may be warmer or colder than their surroundings, but not at the same temperature.
Organisms can be classified based on their primary source of heat (endotherm/ectotherm) and their capacity to maintain their body temperatures relative to their environment (homeotherm/poikilotherm) (5). Endotherms rely on internal metabolism as their primary source of body heat. These “warm-blooded” organisms, such as birds and mammals, can maintain relatively constant internal temperatures ranging from 36 to 40 °C, regardless of fluctuations in outside temperature. The ability to maintain stable body temperatures regardless of the primary source of heat is known as homeothermy. Most life forms, however, are ectothermic (alias “cold blooded”) as their primary source of body heat is the outside environment. Ectotherms tend to be poikilotherms since their internal temperatures fluctuate with changes in external temperatures. Both endotherms and ectotherms use sweat to evaporate water at their surfaces and give off heat via evaporative cooling.
The evaporation of water is an endothermic process that consumes thermal energy to break hydrogen bonds when water goes from liquid to gas (6). Stomata, microscopic pores in some plant leaves, regulate the water transpiration process by opening and closing in response to stimuli. Depending on the thermal environmental conditions, leaves may be warmer than air temperatures (7) or can dissipate heat via evapotranspiration, becoming colder than surrounding air (8–10). Consequently, plants cool themselves and their surrounding environment by evaporating water and by collectively contributing to cloud formation. Evaporative cooling in animals occurs via cellular structures such as sweat glands that regulate the water evapotranspiration process.
The thermal biology of fungal, protist, archaeal, and bacterial communities is largely unknown and unexplored. Due to their small sizes (or high surface area-to-volume ratio), microorganisms are assumed to be ectothermic (11) with insufficient thermal mass to maintain a temperature difference from their immediate environment. However, microorganisms often exist in communities, and the identification of heat-producing bacterial colonies (12) suggests that a microbial community can produce enough internal thermal energy and maintain warmer temperatures than the surroundings. Furthermore, the report that mitochondria have temperatures close to 50 °C (13) raises the possibility of heat engines at the nanoscale, although achieving such high temperatures in organelles presents several physical conundrums(14).
Previous studies and observations have noted that mushrooms grown in the laboratory are colder than their surroundings (15–17). The biological relevance of the relatively cold temperature of mushrooms was associated with spore dispersal; a temperature difference that favors the condensation of water involved in spore ejection. Mushroom temperatures were first measured by inserting thermocouple detectors into mushroom caps, and demonstrating that their relative cold temperatures were mediated by evaporative cooling (16). Additional quantitative data on mushroom evapotranspiration were provided in subsequent studies (15, 17). Mahajan et al. quantified the transpiration rate of A. bisporous whole mushrooms and developed a mathematical model to link mushroom water loss with ambient temperature and relative humidity (RH) (15). Subsequently, Dressaire et al. quantified the rate of water loss from mushroom pilei, which can be higher than plants and enough to cool the surrounding air by several degrees Celsius (17). A most recent observation using video thermography noted the relatively cold appearance of Amanita muscaria wild mushrooms in their natural habitat (18), although no temperature values were reported.
Is the cold temperature observed in mushrooms a general characteristic of fungi? In this study, we applied infrared and thermocouple thermometry to measure and document the temperature of wild mushrooms in their natural habitat, as well as laboratory-grown colonies of microscopic fungal species. We show that yeast and mold colonies are also colder than their surroundings and use the process of evapotranspiration to give off heat. Relative coldness appears to be a general characteristic observed across the fungal kingdom.
Results
Fungi Maintain Colder Temperatures than Their Surroundings.
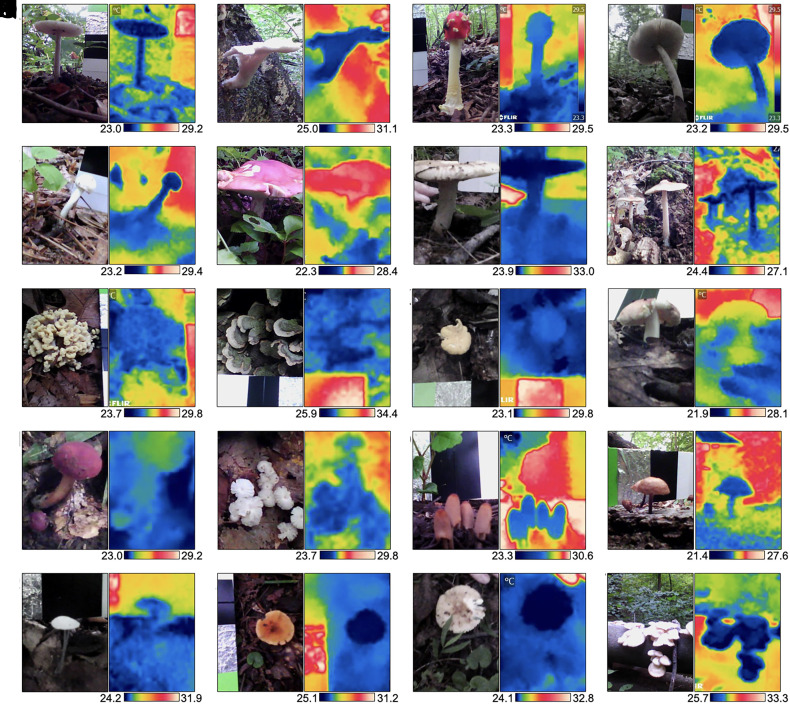
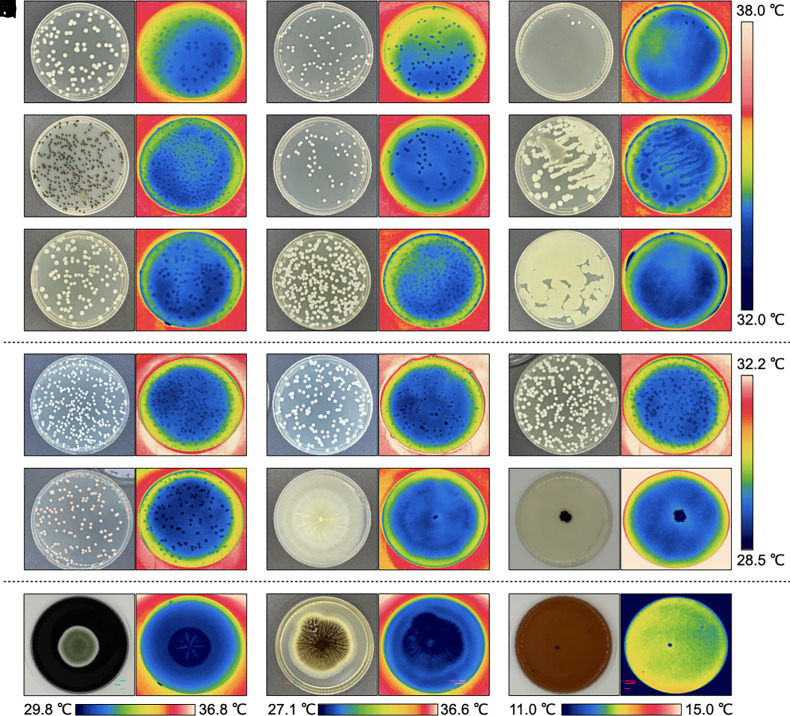
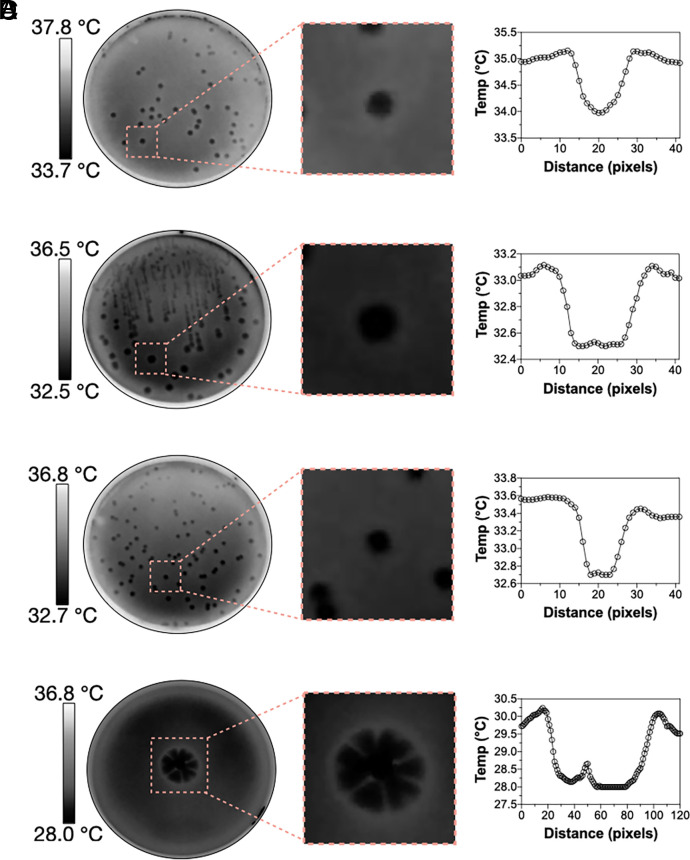
First, we investigated whether the relatively colder temperatures of cultivated mushrooms (15–17) were also observed in wild specimens in their natural habitats and while attached to their natural substrates. Thermal imaging of 20 different wild mushroom species in outdoor environments revealed colder surface apparent temperatures than surrounding air. Depending on the specie, mushrooms were 1.4 to 5.9 °C colder than the surrounding air (Fig. 1 and SI Appendix, Table S1). On average, mushrooms in the wild were 2.9 ± 1.4 °C colder than the surrounding air. Next, we looked at the surface temperature of yeast, yeast-like, and mold colonies grown on agar plates (Fig. 2). We measured the colony surface temperatures of Cryptococcus neoformans strains H99, B3501, and the cap59 acapsular mutant, Exophiala dermatitidis, Candida albicans, Candida tropicalis, Candida glabrata, Candida auris, Candida krusei, Candida haemulonii, Candida duobushaemulonii, Saccharomyces cerevisiae, Rhodotorula mucilaginosa, Gliocephalotrichum simplex, Cladosporium sphaerospermum, Penicillium spp., Aspergillus niger, and Cryomyces antarcticus. The colonies of all specimens appeared colder than the surrounding agar (Fig. 2). Depending on the specie, colony temperatures range from 0.3 to 1.9 °C colder than surrounding agar (SI Appendix, Table S2). Colonies of A. niger appeared the coldest, followed by the psychrophilic fungus C. antarcticus, R. mucilaginosa, G. simplex, Penicillium spp., and the cap59 acapsular mutant of C. neoformans. Direct-colony–contact thermometry using thermocouple detectors placed within the cap59 C. neoformans colonies confirmed the relatively cold temperatures obtained using thermography (SI Appendix, Fig. S1). Colonies of C. antarcticus and Penicillium spp. remained colder than the surrounding agar even after incubating at 4 °C, <10% RH (SI Appendix, Fig. S2). However, the temperature difference between colonies and surrounding agar was lower at 4 °C. For example, colonies of C. antarcticus were approximately 1.1 °C colder than surrounding agar when incubated at 15 °C but only 0.4 °C colder when incubated at 4 °C. We also noted that colonies of Penicillium spp. grown for 5 and 10 d became colder with increasing size, 0.8 ± 0.1 °C and 0.6 ± 0.1 °C colder than the surrounding agar, respectively. Detailed image analysis of some infrared thermographs revealed that colonies are coldest at their center and that the temperature of the agar immediately surrounding the colony was warmer than the colony or distant agar (Fig. 3). Overall, the temperatures of all fungal specimens, including those grown in the laboratory or found in their natural environment, correlated linearly with their surrounding temperatures at a slope of 1 (SI Appendix, Fig. S3).
Fig. 1.
Wild mushrooms are colder than the surrounding air. Visible images and infrared thermographs of 20 different wild mushrooms in their natural habitat while attached to their natural substrate. (A) Amanita spp.; (B) Pleurotus ostreatus; (C) Amanita muscaria; (D) Amanita brunnescens; (E) Russula spp.; (F) Boletus separans; (G) Russula spp.; (H) Amanita spp.; (I) Thelephora spp.; (J) Cerrena unicolor; (K) Cantharellus spp.; (l) Russula spp.; (M) Hortiboletus spp; (N) Marasmius capillaris; (O) Coprinellus micaceus; (P) Lactifluus spp.; (Q–S) unidentified; and (T) Pleurotus ostreatus. Temperature scale bars at the bottom of each thermograph depict °C. The average temperatures of specimens and surroundings are listed in SI Appendix, Table S1.
Fig. 2.
Yeast and mold colonies are colder than surrounding agar. Visible images and infrared thermograph examples of fungal colonies from (A) C. neoformans H99; (B) C. neoformans cap59 acapsular mutant; (C) C. neoformans B3501; (D) E. dermatitidis; (E) C. albicans; (F) C. tropicalis; (G) C. glabrata; (H) C. auris; (I) C. krusei (J) Candida haemulonii, (K) Candida duobushaemulonii, (L) Saccharomyces cerevisiae, (M) Rhodotorula mucilaginosa, (N) Gliocephalotrichum simplex, (O) Cladosporium sphaerospermum, (P) Penicillium spp., (Q) Aspergillus niger, and (R) Cryomyces antarcticus colonies. Temperature scale bars correspond to group panels A to I, J to O, and individual panels, P to R. The average temperatures of specimens and surroundings are listed in SI Appendix, Table S2.
Fig. 3.
The thermal landscape of (A) C. albicans, (B) C. neoformans H99, (C) C. neoformans cap59, and (D) Penicillium spp. colony. Close-up of a single yeast colony thermograph shows that the coldest temperature of a colony appears at its center and the warmest temperature of the surrounding agar appears near the colony edge.
Change in Mushroom Temperature during Fruiting, Heating, and Cooling.
The mushroom Pleurotus ostreatus, grown in the laboratory at 25 °C, maintained colder temperatures throughout the whole fruiting process, especially as it increased in size (SI Appendix, Fig. S4A). Even after detachment, the mushrooms flush remained relatively cold, although it was still several degrees warmer than it was when attached to the substrate (SI Appendix, Fig. S4B). The hymenium or area underneath the pileus appeared colder than the frontal side of P. ostreatus pilei. Notably, the fruiting site of mycelium also remained relatively cold after mushroom detachment, approximately 2.5 °C cooler than the rest. The relatively cold temperature of the detached P. ostreatus mushroom was maintained during heating, increasing from approximately 19 °C to 27 °C following 137 min of incubation at 37 °C and <10% RH (SI Appendix, Fig S5A). After heating, the mushroom flush was incubated at 4 °C and <10% RH, and its temperature dropped from 24 °C to 18 °C after 104 min (SI Appendix, Fig. S5B). Thermal images of the mushroom flush during heating and cooling incubations showed that different areas of the mushroom dissipate heat differently, and during cooling, the mushroom exhibited more irregular thermal gradients when compared to the heating incubation. The change in average mushroom temperature as a function of time followed an exponential curve during heating (SI Appendix, Fig. S5C) but a linear curve during cooling (SI Appendix, Fig. S5D).
Fungal Hypothermia Is Mediated via Evapotranspiration.
Evaporative cooling was confirmed in light and dark substrate-detached A. bisporus mushroom pilei by manipulating their water content, ambient temperature, and RH. Dehydrated mushrooms were unable to maintain relatively colder temperatures, irrespective of ambient temperature (SI Appendix, Fig. S6 A–C and Table S3). Both light and dark mushroom pilei exhibited similar temperature changes. The percent mass loss of light and dark A. bisporus mushroom pilei following dehydration was 93.6 ± 0.4% (m/m) (SI Appendix, Table S4), demonstrating their high-water content. Dehydration of seven additional wild unidentified mushroom specimens also shows high water content ranging from 57 to 92% by mass (SI Appendix, Table S4). Mushrooms warmed slower and reached lower absolute temperatures under dry conditions as compared to a humid environment (SI Appendix, Fig. S6 D and E). Together these results confirmed that the mushroom’s relative coldness was mediated by evaporative cooling.
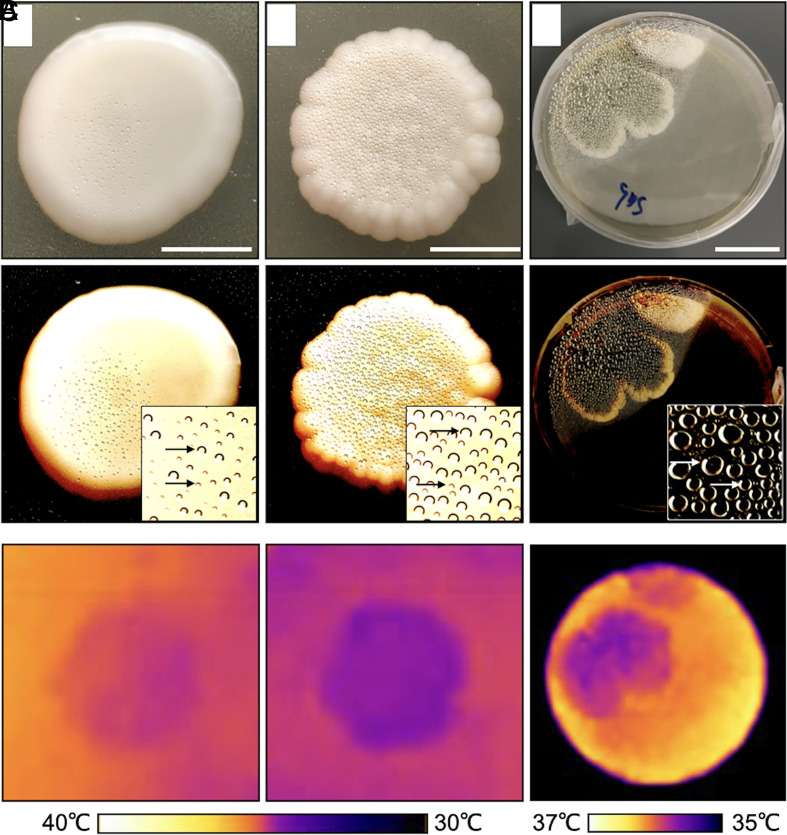
Evaporative cooling in C. neoformans and Penicillium spp. was evident from the condensation of water droplets on the lids above colonies when grown upright on agar plates (Fig. 4). Areas of the agar plate where specimens were not growing show lower levels of condensation. The acapsular mutant of C. neoformans showed more and larger water droplets than the encapsulated wildtype strain and was also ~1 °C colder (Fig. 4B). The encapsulated C. neoformans colonies are ~90% (m/m) water, while the acapsular mutant is ~82% (m/m) (SI Appendix, Table S4). Colonies of Penicillium spp. showed more water condensation compared to C. neoformans and at least ~10 times higher than the surrounding 1.5% agar medium (SI Appendix, Table S5).
Fig. 4.
Evaporative cooling in yeast and mold colonies. The evidence for evaporative cooling is observed from the condensed water droplets at the lid of the petri dish on top of the colonies. Visible (Top and Middle) and thermal images (Bottom) of (A) wildtype H99 Cryptococcus neoformans; (Scale bar, 1 cm.); (B) cap59 acapsular mutant of C. neoformans colonies; (Scale bar, 1 cm.), and (C) normal Penicillium spp.; (Scale bar 3 cm.) Visible images (Middle row) were altered to increase contrast and help visualize water droplets (arrows).
A Mushroom-Based Air-Cooling Device.
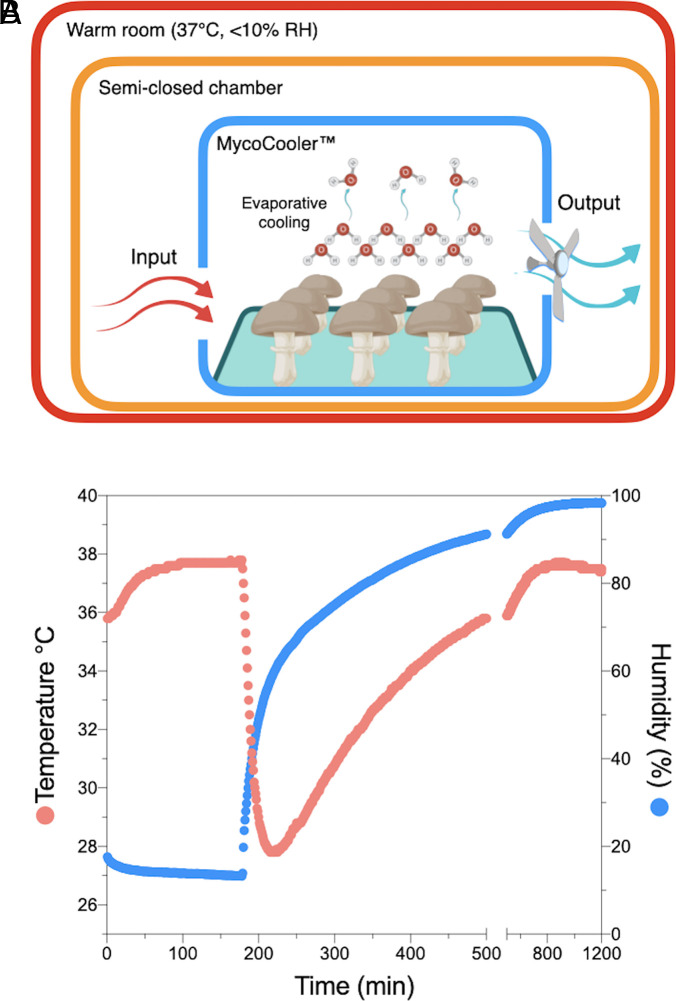
We constructed a mushroom-based air-cooling device, MycoCooler™, based on previous observations that mushrooms can cool the surrounding air via evaporative cooling (17) (Fig. 5A). The device was made from a Styrofoam box with a 1-cm–diameter inlet aperture and a 2-cm–diameter outlet aperture (SI Appendix, Fig. S7). An exhaust fan was attached outside the outlet aperture to drive airflow in and out of the box. The MycoCooler™ was loaded with ~420 g of substrate-detached A. bisporus mushrooms, closed, and placed inside a larger Styrofoam box previously equilibrated inside a warm room (37.8 °C, <10% RH). The temperature inside the closed Styrofoam box decreased from 37.8 °C to 27.8 °C, 40 min after the addition of mushrooms, cooling at approximately 10 °C, at ~0.4 °C per min (Fig. 5B). In parallel, the RH increased proportionally by ~45% at 1.3 % per min. The box interior reached the coldest temperature at ~60% RH, at which point it started to warm up again to initial temperature values as the humidity continued to increase towards saturation (Fig. 5B). As a comparison, a water-soaked sponged of equivalent mass was able to cool the air by 7.7 °C, at ~0.32 °C per min. From this data, we estimated that 420 g of A. bisporus mushroom pilei have an air-cooling capacity of approximately 20 W or 68 BTH/h. To compare the cooling capacity of mushrooms relative to water alone, we placed 19 g of water in plastic petri dishes with increasing diameter, hence surface area. As expected, the cooling capacity of water was proportional to its surface area (SI Appendix, Fig. S8). We then calculated the cooling rate of a single A. bisporus mushroom of equivalent mass, which yield 0.082 ± 0.01 °C/min (n = 3), a value 8% higher than what was obtained with water at 6,361 mm2 but lower than water at 17,671 mm2. Additional cooling rate triplicate measurements using a petri dish exposing 6,361 mm2 surface area showed that a single A. bisporus mushroom was 12.5% better at cooling air than an equivalent mass of liquid water, 20% ethanol, phosphate saline buffer, and Coca-Cola, all previously incubated for 16 h at 4 °C.
Fig. 5.
Proof-of-concept of a mushroom-based air conditioning system constructed from Styrofoam boxes and A. bisporus mushrooms. (A) Model diagram model of MycoCooler™ air conditioning system inside a semiclosed chamber. Warm air enters an insulated chamber containing mushrooms. As the warm air flows inside the chamber, mushroom-mediated evaporative cooling will cool the air. An exhaust fan will push the cooled air through a HEPA filter to limit spore dispersal and enhance air circulation. The fan can be powered via a photovoltaic cell making this system free of carbon emissions. See prototype images in SI Appendix, Fig S6. (B) Air temperature and RH as a function of time inside a Styrofoam box as the semiclosed system. The black arrow points to the time when commercially available and detached mushrooms were added inside MycoCooler™; once the temperature inside the Styrofoam box semiclosed system reached a steady state.
Discussion
This study confirms that mushrooms are hypothermic and extends that observation to molds and yeasts. Lower fungal temperatures were achieved via evapotranspiration, and the finding of hypothermia among macroscopic and microscopic fungi suggests that relative coldness is a general property of the fungal kingdom. Mushroom hypothermia occurs throughout the fruiting process, and fruiting areas of mycelium also become colder than nonfruiting areas. We also demonstrate that the process of evapotranspiration in yeast and mold biofilms can be measured from the accumulation of water droplets on agar plates. Finally, we provide a proof-of-principle demonstration for a mushroom-based air-conditioning device capable of passively cooling and humidifying the air of a closed environment. The data presented here reveal the cold nature of fungal biology and suggest evaporative cooling as a microbiological mechanism of thermoregulation.
The observations that fungal temperatures correlated with ambient temperature suggest that fungi exhibit poikilothermic properties. The extent to which fungal temperatures vary with their environmental niche likely involves multiple factors that require further study. The temperature of wild mushrooms, as well as yeast and mold colonies, relative to their surroundings varies with the genus, suggesting that there may be species-specific differences in their capacities to dissipate heat. The ability to dissipate heat could be related to a variety of factors such as differences in water content, thermal properties (i.e., heat capacity, thermal conductivity), size, morphologies, color, habitat, lifestyle, metabolic states, and/or phylogenetic relations. These and other factors are important to consider as we learn more about the thermal biology of fungi and how it relates to other kingdoms.
Fungal Hypothermia at Different Stages of the Fruiting Process.
The relative coldness of P. ostreatus becomes apparent from the appearance of the first mushroom pinheads. The observation that the mushroom flush and Penicillium spp. colonies became colder with increasing size, suggests that fungal coldness is related to fungal thermal mass and/or to an unknown age-related structural or physiological process favoring heat loss. The observation that the mushroom is coldest when still attached to the mycelium is consistent with prior observations (16, 17) and indicates that heat loss is highest when connected to its substrate via the mycelial network, which permits the flow of, and access to, water. This increase in temperature after detachment is also observed in leaves (19).
The observation that the fruiting site of mycelium remained cold after mushroom detachment suggests that fungal hypothermia is also observed at the mycelium level. The cooling of mycelium could be related to a remnant of mushroom-mediated evapotranspiration, or it could be independently regulated by the mycelium itself around the fruiting area. It is possible that fruiting areas of mycelium could become colder than nonfruiting areas by increasing evapotranspiration rates, even before the appearance of the first fruiting bodies. Further studies are needed to evaluate evapotranspiration during these early stages of the fruiting process and the potential role of mycelium-mediated evapotranspiration in reproductive success and evolution.
Regional Differences in Mushroom Heat Dissipation.
The thermal profiles of P. ostreatus mushrooms during heating and cooling suggest that heat exchange can vary in discrete areas of the mushroom flush, indicating that these dissipate heat differently. These regional temperature differences could be from fungal anatomical areas having different thermal properties, water content, water channels, or hyphal structural organizations, which could affect evapotranspiration rates. The change in P. ostreatus mushroom average temperature as a function of time during heating, followed by cooling incubations, resembles the phenomena of thermal hysteresis, a process where previous heat dissipation events influence subsequent temperature changes. The relatively cold temperatures of hymenium could be explained by the relatively high surface area exposed by lamellae or gills (20). The high surface structure and organization of hymenium lamellae are known to enhance airflow (17, 21) hence, enhance heat exchange.
Biological Implications of Fungal Hypothermia.
Having a relatively cold spore-bearing surface is considered to be important for spore detachment and release (16, 17). This is because spore discharge is known to be triggered by the mass and momentum transfer of microscopic drops (aka Buller's drop) that condense from moist air on the spore surface (22, 23). In addition to spore discharge, colder temperatures could also be relevant to fungal sporogenesis. There are multiple examples in nature where sporogenesis is associated with relatively cold temperatures. In mammals, the production of spermatozoa occurs at temperatures colder than the abdominal viscera (24). In fungi, the frequency of S. cerevisiae sporulation and tetrad formation is higher at 22 °C than at 30 °C (25). The relation between relatively cold temperatures and sporogenesis suggests that fungal coldness influences reproductive success by enhancing both the discharge and the production of spores. It also implies that evaporative cooling in fungi contributes to the thermodynamics regulating spore production and DNA duplication. The relatively cold temperature of mushrooms may also be attractive to insects, which also contributes to spore dispersal. Considering that thermoregulation is a general defense strategy to tolerate infectious diseases (26), the cold temperatures of fungi relative to their environments should be considered as a variable in understanding infections by bacteria or mycoviruses. The ability to dissipate heat via evapotranspiration may also be important for fungal temperature priming and memory (27). In this case, evapotranspiration may provide a window of opportunity for the fungus to adapt to different levels of temperature stress, where a priming temperature stress renders the fungus better prepared for more severe temperature stresses. The ability of fungal organisms to tolerate higher temperatures represents a serious public health concern considering that new pathogenic fungal species could emerge as a result of climate change (28).
Fungal Evapotranspiration.
Our indoor measurements of lightly and darkly pigmented A. bisporus detached mushroom pilei confirmed that evaporative cooling accounted for their relatively cold temperatures. Both light and dark pilei exhibited similar temperature changes, suggesting that pigmentation may not influence heat dissipation under such conditions or that the effect is too close to our limits of thermal detection. The high water content of mushrooms is consistent with previous reports (29), and the high water content of yeasts also implies that fungal thermal properties must be largely determined by water. The evapotranspiration capacity of mushrooms was reported to be higher than in some plants (15, 16). In plants, evapotranspiration occurs mainly in the leaves, and the rate of evapotranspiration is regulated at the level of stomata. An analogous cellular structure dedicated to regulating evapotranspiration in mushrooms has not been identified. It would be interesting to compare the temperature and evapotranspiration capacity of mushrooms with other plants and fruits that are also highly hydrated (e.g., aloe vera, ~98% water). Nevertheless, we are struck by the coldness of mushrooms and surmise that there must be aspects of their structure and/or composition that accounts for their relatively high capacity of evapotranspiration. Evapotranspiration in fungi provides a passive physiological mechanism for dissipating heat, and although it appears to be dominant, it may not be the only biological mechanism contributing to fungal coldness.
We propose that water condensation above fungal colonies and biofilms could be measured as a parameter to evaluate evapotranspiration, water flow, or metabolism. An index of evapotranspiration derived from water condensation may be used to screen for genetic determinants of thermoregulation in yeast. The agar surrounding fungal colonies was consistently warmer than either the colonies or distant agar. Although we did not investigate the phenomenon, we hypothesize that this temperature difference could reflect an exothermic process from the extracellular digestion of agar nutrients mediated by secreted fungal enzymes. Our observations with normal encapsulated and the nonencapsulated (cap59) mutant of C. neoformans suggest that a gene deletion can result in notable differences in both evapotranspiration and temperature. Our data show that the acapsular mutant of C. neoformans contained approximately 10% less water mass but exhibited colder temperatures and more evapotranspiration relative to the normal encapsulated strain. The water difference between the encapsulated and acapsular mutant biofilms could also be explained by the presence of the C. neoformans polysaccharide capsule, which is mostly water (30). Hydrated extracellular polysaccharides are thought to function in preventing desiccation in environmental microorganisms by retaining water (31). Cryptococcal polysaccharide is a highly hydrated structure that avidly retains and incorporates water molecules into its structure (30, 32). Such water retention may be limiting the rate of evapotranspiration in C. neoformans.
Mushroom-Based Air Cooling and Conditioning.
Our data shows that mushrooms can be used for air cooling, consistent with previous reports (17). The relatively high transpiration rate of mushrooms could be exploited to develop a natural and passive air-conditioning system. We constructed a prototype for such a mushroom-inspired cooling instrument in the form of The MycoCoolerTM. This simple prototype produced a cooling capacity of 1,002.3 BTU/h. For reference, a 100 to 150 ft2 room often requires a cooling unit with a capacity of 5,000 BTUs/h (33). Even though these kinds of traditional window air conditioners present at least five times more cooling capacity, these units also weigh >20 times more. This suggests that a mushroom-based air-conditioning device could provide superior cooling capacity per unit weight, although other features like performance, volume, or aroma may also be potential tradeoffs when comparing it to traditional air conditioning units. Mushroom-based air cooling depends on RH, and for detached A. bisporus mushroom pilei, cooling is compromised at an RH close to 60%. Better results could be achieved using mushroom species with higher transpiration rates, still attached to their mycelium, and on a design that controls the accumulation of air moisture. Mushrooms can be used not only to cool the surrounding air but also to humidify and even purify with low electrical energy consumption and/or CO2 emissions. These findings suggest the possibility of using massive myco-cultures to reduce the temperatures of locales and help mitigate local global warming trends.
Fungal Hypothermia in Psychrophiles.
While this study does not determine an optimal organismal temperature for the entire fungal kingdom, we do note that fungal thermoregulation may vary depending on the environmental temperature in which a fungus grows. The fungal kingdom is also home to several extremophilic species, including thermophiles that can survive at extremely warm temperatures (50 to 60 °C) (34) and psychrophiles that are adapted to survive at freezing temperatures (35). Psychrophilic fungi have adapted to extremely cold environments by producing cryoprotectants such as glycerol or mannitol (35), enzymes that are optimally active at low temperatures (36), and antifreeze proteins (AFP) that prevent the formation of ice crystals (37). For psychrophiles, it may not be favorable to become colder than their surroundings given that their surroundings are already cold. However, our observations that C. antarcticus colonies are colder than their surroundings when incubated at 15 or 4 °C suggests that fungal-mediated evaporative cooling is still possible at lower temperatures as long as there is sufficient hydration for evaporative cooling and a permissive ambient humidity. Examining the thermal properties of extremophilic fungi in future studies will help qualify the observation that fungi are generally colder than their surrounding environments.
Implications of Fungal Coldness in Climate Change.
The ability of certain fungi to trap carbon in soil has already pointed to their potential role in mitigating climate change (38). The identification of fungi as potential heat sinks raise additional support for their role in global and local temperature regulation. Within the realm of ecology, fungal-mediated cooling raises several questions about their effects on a microclimate, as a symbiont, and in an ecosystem. One question that could confirm this study’s findings on a larger scale would be whether ecosystems with higher fungal biomass exhibit lower ambient temperatures, as seen in areas with large amounts of plant life or water bodies, for example.
In conclusion, this study reveals the cold nature of fungi and evaporative cooling as a fundamental mechanism for thermoregulation for this kingdom. The relative cold temperature of fungi (or fungal hypothermia) implies that their heat loss is much greater than the production of heat via metabolism. Their relative cold temperatures also imply that the flow of surrounding thermal energy will move toward the fungus, turning it into some sort of heat sink. The high-water content and evapotranspiration rate of fungi implies that their molecular composition and structure enable the efficient transfer of thermal energy and water. Infrared imaging provides a powerful tool to study the thermal biology of mushrooms, mycelium, molds, and yeasts at the community level. A yeast model system to study thermal biology could allow the screening of genetic and epigenetic mechanisms regulating thermodynamics and thermal fitness. Understanding how fungal organisms dissipate heat is relevant to a world of applications, from novel biotechnologies to medical innovations, and sustainable energy.
Materials and Methods
Fungal Specimens.
All wild mushroom specimens were found in Lake Roland Park in the state of Maryland during the evenings of July 5, 6, and 9 of 2019. The identification of specimens was made based on a visual inspection and photograph analysis via crowdsourcing the Internet. Pleurotus ostreatus was purchased from The Mushroomworks (Baltimore, MD) as an already-inoculated substrate contained in a 6-pound clear filter patch bag. Fruiting was triggered by making a single 4-inch side cut on the bag and letting it standstill at 24 °C for 7 d. Mushroom flush was detached from mycelium on day 4 after it started fruiting. Light and dark Agaricus bisporus were purchased from New Moon Mushrooms (Mother Earth, LLC., Landenberg) and L. Pizzini & Son, Inc. (Landenberg), respectively.
C. neoformans strain H99 (ATCC 208821), C. neoformans B3501 (ATCC 34873), C. neoformans acapsular cap59 mutant, E. dermatitidis, C. glabrata, C. krusei, C. tropicalis, C. albicans, and C. auris were grown on Sabouraud Dextrose agar 5 d at 37 °C. C. haemulonii (CDC AR Bank# 0393), C. duobushaemulonii (CDC AR Bank# 0394), S. cerevisiae (CDC AR Bank# 0399) C. sphaerospermum (ATCC 11289), R. mucilaginosa, Penicillium spp., Gliocephalotrichum simplex (ATTC 078-1910), Aspergillus niger (ATCC 4029) and another replica of C. neoformans B3501 (ATCC 34873) were grown on Sabouraud Dextrose agar for 5 to 10 d at 30 °C. Cryomyces antarcticus (ATCC MYA-4880) was grown in Malt Dextrose agar for 20 d at 15 °C. In some cases, Penicillium spp. was grown on Sabouraud Dextrose agar supplemented with activated charcoal (0.4% m/v) to improve imaging contrast. C. glabrata, C. krusei, and C. tropicalis were kindly provided by the Mycology Laboratory, Division of Medical Microbiology at JHU School of Medicine. C. albicans, C. auris, and E. dermatitidis were kindly provided by the Noshanchuk laboratory at Albert Einstein College of Medicine. R. mucilaginosa and Penicillium were isolated from contaminated YPD agar plates in our laboratory.
Thermography.
Wild mushroom temperatures were measured in their natural habitats while still attached to their natural substrates using a FLIR C2 IR camera (FLIR Systems, Wilsonville, OR). The camera specifications are 80 × 60-pixel thermal resolution; 7.5 to 14 μm microbolometer detector; accuracy of ±2 °C or 2% of the reading; thermal sensitivity: <0.10 °C; adjusted emissivity to 0.96. The ambient temperature was derived from a card-containing black vinyl electrical tape with an emissivity of 0.96 and aluminum foil (emissivity of 0.03) (SI Appendix, Fig S9A). The black tape and aluminum foil were included in the picture as a reference for ambient and reflective temperature readings, respectively. The efficacy of the black vinyl tape in reproducing ambient temperatures was tested via thermography following ~20 min of incubation inside three temperature-controlled rooms, set to approximately 5, 25, and 38 °C. The temperature readings obtained from the black tape using the thermal camera and a mercury thermometer match clearly (SI Appendix, Fig S9B); demonstrating that black tape radiative temperature can be used as a reliable indicator of the ambient temperature.
The thermography of P. ostreatus and A. bisporus mushrooms was done with the FLIR C2 similarly as described previously (39). Thermal images of specimens were taken inside a white Styrofoam box (30 × 27 × 30 mm, and 3.5 mm wall thickness) to prevent heat loss and radiation noise from surroundings. Following the incubation period, the yeast/mold-containing plates were immediately transferred inside a Styrofoam box. Next, the box was closed with a lid having a hole fitted to the IR camera. The camera detector was set at a 25 cm distance from the specimen. The temperature of the substrate-detached P. ostreatus mushroom flush was monitored during heating and cooling by placing the mushroom inside a warm room (37 °C, <10% RH) or cold room (4 °C, ~30% RH) for 137 and 104 min, respectively. Infrared thermography of mushroom flush was taken inside the Styrofoam box at different time intervals.
The thermography of yeast and mold colonies was done using a FLIR E96 equipped with a 12-μm microbolometer detector resulting in 640 × 480-pixel thermal resolution, an accuracy of ± 2 °C or 2% of the reading, and a thermal sensitivity of <40 mK at 30 °C. All thermographs were performed using an emissivity of 0.96. For the yeasts and molds grown at 30 °C or 37 °C, the thermographs were taken inside a Styrofoam box in the warm room set at 37 °C, <10% RH. The thermography of C. antarcticus was performed inside the Styrofoam box in the cold room set at 4 °C, < 10% RH. The transport of plates between rooms was inside an thermally insulating Styrofoam box and done quickly (within minutes) to prevent heat loss/gain before thermal imaging. To evaluate the colony temperature of C. antarcticus and Penicillium spp. under 4 °C, the plates were incubated in a cold room for 5 h before thermal imaging. All apparent temperatures of fungal specimens were obtained from thermographs using the FLIR Tool analysis software Version 5.13.17214.2001. An example of single yeast colony temperature measurements is shown in SI Appendix, Fig. S10. The temperature scales in Fig. 3 were produced by correlating the temperature values with the mean gray value of grayscale thermographs using the plot profile function in the ImageJ software.
Water Condensation of Fungal Colonies.
C. neoformans yeast biofilms were prepared by spotting 25 µL of a liquid 2-d-old preculture on Sabouroaud agar medium. The liquid pre-cultures were inoculated from frozen stock and grown for 2 d at 30 °C (shaking at 180 rpm). Penicillium spp. biofilm was naturally formed by inoculating on a Sabouroaud agar plate. Yeast and mold-inoculated plates were grown upright at 24 °C for 1 to 2 wk or until water condensation on the lids became visible. The amount of condensed water at the lid above a mold’s biofilm or plain agar was collected using a Steriflip® filter vacuum unit (Millipore Sigma) connected to two 50-mL conical tubes, one at each end. Suction was achieved by connecting a small tubing across the filter into one of the conical tubes. A pipet tip connected at the end of the tubing facilitated the aspiration of condensed water droplets on the lid and its collection into one of the 50-mL tubes for weighting. The water mass was normalized by the condensation area on the lid, which was estimated from digital images using the ImageJ software.
Fungal Water Mass Percentage.
Encapsulated and acapsular Cryptococcus colonies were grown in agar plates, collected using a cell scrapper, and transfer into pre-weighted microcentrifuge tubes. Mushrooms specimens were placed inside pre-weighted 50 mL conical tubes. Tubes containing fungal specimens were connected to a freeze-drying system (Labcono, Kansas City, MO) for 5 d. The percent of water mass was calculated by measuring the mass difference of fungal content before and after dehydration.
Thermocouple Thermometry of Mushroom Pilei and Yeast Colonies.
To monitor the temperature of A. bisporus mushrooms as a function of time, mushroom pilei of equal masses, kept at 24 °C, were placed on glass trays inside ziplock clear bags (one mushroom per bag). One bag contained 40 g of desiccating-anhydrous indicating Drierite (W.A. Hammond Drierite Company, LTD), and a second contained 40 g of distilled water. Thermocouple detectors (K-type) were submerged inside each mushroom cap (centered from the top). Bags were then closed and placed inside a warm room (37 °C, <10 % RH), and temperature readings were recorded every second using the Amprobe TMD-56 thermometer (0.05% accuracy) connected to a computer. Each mushroom sample was measured individually inside the warm room. The temperatures of C. neoformans cap59 colonies and surrounding agar were also measured inside the warm room using K-type thermocouple recordings every second. Before each agar and colony measurement, the thermocouple was allowed to thermally equilibrate by submersion inside a small container with sand that was previously equilibrated in the warm room.
A Mushroom-Based Cooling Device.
The prototype device was made using a Styrofoam box with dimensions 20 × 21 × 21 cm or a total volume of 8,820 cm3. An inlet aperture of 1 cm diameter and an outlet aperture of 2 cm diameter at opposite ends of the box allowed airflow inside and outside the box containing mushrooms (SI Appendix, Fig. S7). An exhaust fan (Noctua NF-P12) was glued outside the box on top of the outlet aperture to facilitate the circulation of air (air flow rate of approximately in and out of the MycoCooler™. Approximately, 420 g of fresh and substrate-detached A. bisporus mushrooms were placed inside the MycoCooler™ box, which was then closed, and placed inside a larger Styrofoam box with dimensions (30.48 × 30.48 × 30.48 cm or a total volume of 28.32 L (28,316.85 cm3). This larger box was maintained inside a warm room (37 °C, <10 % RH) throughout the experiment. The MycoCooler™ containing mushrooms was enclosed inside the larger box once the temperature and humidity values reached a steady state. The temperature and RH inside the larger Styrofoam box (SI Appendix, Fig. S7) were recorded every minute using an Elitech GSP-6 data logger has a temperature accuracy of ± 0.5 °C (−20 ~ 40 °C) and humidity range of 10 ~ 90% and an accuracy of ±3% RH (25 °C, 20% ~ 90% RH). In some experiments, a distilled water-soaked sea wool sponge (6 to 7”) weighing 420 g was placed inside the MycoCooler™to measure cooling capacity/rates. In other experiments, plastic dishes with varying surface areas (962, 2,375, 6,361, and 17,671 mm2) containing 19 g of distilled water were placed inside the MycoCooler™ to measure cooling capacity/rates. When comparing the cooling rates of an equivalent mass (9 g) of single A. bisporus mushrooms, water, ethanol 20%, phosphate saline buffer, and Coca-Cola; all substances were pre-incubated at 4 °C for 16 h prior to the experiment.
Quantification and Statistical Analysis.
Details for each statistical analysis, precision measures, and the exact value of n (and what n represents; sample size, and the number of replicates) for all shown data can be found in the figure legends. We used an alpha level of 0.05 for all statistical tests.
To calculate the mushroom’s cooling capacity, we used the MycoCoolerTM temperature change data to estimate the cooling capacity of 420 g of A. biosporus mushroom pilei. Cooling capacity was calculated using the energy equation for heat transfer Q = mCpΔT, where m is the mass flow rate of the air in kg/s, Cp is the specific heat capacity of air in kJ/kg*K, and ΔT is the temperature difference in Kelvin. The mass flow rate was obtained by multiplying the density of air at 37 °C and 1 atm (1.138 kg/m3) by the fan flow rate taken from equipment specifications (0.0256 m3/s). This results in a mass flow rate of 0.0292 kg/s, that if multiplied by the heat capacity nominal value of air at 37 °C (1.006 kJ/kg*K) and the absolute air temperature difference of the enclosed system before and 45 min after the addition of mushrooms (27.8 °C + 273.15 K) − (37.8 °C + 273.15 K) = 10.0 K. This yields a heat transfer or cooling capacity of ~293.8 W or 1,002.3 British Thermal Units per hour (BTU/h). The cooling capacity divided by the mass of the mushrooms 0.42 kg yields 699.4 W/kg or 1,082.5 BTU/h/lb. When taking the mass of the MycoCoolerTM and mushrooms (0.71 kg) into consideration, the values become 412.2 W/kg or 638.0 BTU/h/lb. In contrast, a brief survey of popular 5000 BTU air conditioners determined that these units weigh about 18 kg, yielding a BTU/h/lb of 125. Although the MycoCoolerTM produces an impressive cooling capacity per unit weight (>20 times more), the relative volume may be a potential tradeoff in comparing it to traditional air conditioning units.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
The research was supported by the NIH (R01 AI052733). A.C. is supported in part by R01 AI152078, R01 HL059842, AI171093, and AI052733. Z.R. was supported by NSURP NSF 2149582. We thank Teporah Bilezikian for helping with fieldwork and the identification of wild mushroom species. We also thank members of The Baltimore Fungal Group and The Casadevall Laboratory, particularly Dr. Samuel dos Santos, Dr. Daniel Smith, Dr. Zach Stolp, and Dr. Livia Liporagi-Lopez for their valuable input.
Author contributions
R.J.B.C. and A.C. designed research; R.J.B.C. and E.R.M. performed research; R.J.B.C., E.R.M., and Z.R. analyzed data; A.C. laboratory principal investigator; and R.J.B.C., A.C., and E.R.M. wrote the paper.
Competing interests
R.J.B.C. and A.C. are cofounders of MelaTech, LLC, a biotech company dedicated to the manufacturing of fungal melanin and R&D of melanin-based biotechnologies. Johns Hopkins has applied for a patent on the Mycooler that is described in the paper.
Footnotes
Reviewers: J.W.B., Rutgers The State University of New Jersey; and M.S.L., NIH.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Meyer V., et al. , Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 7, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M. C., et al. , Threats posed by the fungal kingdom to humans, wildlife, and agriculture. MBio 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gates D. M., Biophysical Ecology (Springer, New York, New York, NY, 1980). [Google Scholar]
- 4.Lineweaver C. H., Egan C. A., Life, gravity and the second law of thermodynamics. Phys. Life Rev. 5, 225–242 (2008). [Google Scholar]
- 5.Clarke A., Principles of Thermal Ecology: Temperature, Energy, and Life (Oxford University Press, 2017). [Google Scholar]
- 6.Wang K., Dickinson R. E., A review of global terrestrial evapotranspiration: Observation, modeling, climatology, and climatic variability. Rev. Geophys. 50, 1–54 (2012). [Google Scholar]
- 7.Helliker B. R., Richter S. L., Subtropical to boreal convergence of tree-leaf temperatures. Nature 454, 511–514 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Jackson R. D., Advances in Irrigation (Elsevier, 1982), pp 43–85. [Google Scholar]
- 9.Doughty C. E., Goulden M. L., Seasonal patterns of tropical forest leaf area index and CO2 exchange. J. Geophys. Res. 113, 1–12 (2008). [Google Scholar]
- 10.Still C., et al. , Thermal imaging in plant and ecosystem ecology: Applications and challenges. Ecosphere 10, 1–16 (2019). [Google Scholar]
- 11.Dusenbery D. B., Living At Micro Scale: The Unexpected Physics Of Being Small (Harvard University Press, 2011). [Google Scholar]
- 12.Tabata K., et al. , Measurement of soil bacterial colony temperatures and isolation of a high heat-producing bacterium. BMC Microbiol. 13, 56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrétien D., et al. , Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol 16, e2003992 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macherel D., Haraux F., Guillou H., Bourgeois O., The conundrum of hot mitochondria. Biochim. Biophys. Acta Bioenerg 1862, 148348 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Mahajan P. V., Oliveira F. A. R., Macedo I., Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J. Food Eng. 84, 281–288 (2008). [Google Scholar]
- 16.Husher J., et al. , Evaporative cooling of mushrooms. Mycologia 91, 351–352 (1999). [Google Scholar]
- 17.Dressaire E., Yamada L., Song B., Rope M., Mushrooms use convectively created airflows to disperse their spores. Proc. Natl. Acad. Sci. U.S.A. 113, 2833–2838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul Stamets, Thermal Amanita muscaria (2016). http://paulstamets.com/videos/thermal-amanita-muscaria. Accessed 2 January 2020.
- 19.Utsumi Y., et al. , Acetic acid treatment enhances drought avoidance in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 10, 521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer M. W. F., Money N. P., Why mushrooms form gills: Efficiency of the lamellate morphology. Fungal. Biol. 114, 57–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deering R., Dong F., Rambo D., Money N. P., Airflow patterns around mushrooms and their relationship to spore dispersal. Mycologia 93, 732–736 (2001). [Google Scholar]
- 22.Turner J. C. R., Webster J., Mass and momentum transfer on the small scale: How do mushrooms shed their spores? Chem. Eng. Sci. 46, 1145–1149 (1991). [Google Scholar]
- 23.Webster J., Davey R. A., Ingold C. T., Origin of the liquid in Buller’s drop. Trans. Brit. Mycol. Soc. 83, 524–527 (1984). [Google Scholar]
- 24.Glover T. D., Young D. H., Temperature and the production of spermatozoa. Fertil. Steril. 14, 441–450 (1963). [DOI] [PubMed] [Google Scholar]
- 25.Codón A. C., Gasent-Ramírez J. M., Benítez T., Factors which affect the frequency of sporulation and tetrad formation in Saccharomyces cerevisiae baker’s yeasts. Appl. Environ. Microbiol. 61, 630–638 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieber A. M. P., Ayres J. S., Thermoregulation as a disease tolerance defense strategy. Pathog. Dis. 74, 1–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade-Linares D. R., Veresoglou S. D., Rillig M. C., Temperature priming and memory in soil filamentous fungi. Fungal. Ecol. 21, 10–15 (2016). [Google Scholar]
- 28.Casadevall A., Kontoyiannis D. P., Robert V., On the emergence of Candida auris: Climate change, azoles, swamps, and birds. MBio 10, e01397–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz J. A., et al. , Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 126, 610–616 (2011). [Google Scholar]
- 30.Maxson M. E., Cook E., Casadevall A., Zaragoza O., The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal. Genet. Biol. 44, 180–186 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Ophir T., Gutnick D. L., A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl. Environ. Microbiol. 60, 740–745 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wear M. P., et al. , Lyophilization induces physicochemical alterations in cryptococcal exopolysaccharide. Carbohydr. Polym. 291, 119547 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EnergyStar.gov Room Air Conditioner, Buying Guidance. https://www.energystar.gov/products/heating_cooling/air_conditioning_room. Accessed 31 August 2020.
- 34.Maheshwari R., Bharadwaj G., Bhat M. K., Thermophilic fungi: Their physiology and enzymes. Microbiol. Mol. Biol. Rev. 64, 461–488 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan N., Rafiq M., Hayat M., Shah A. A., Hasan F., Psychrophilic and psychrotrophic fungi: A comprehensive review. Rev. Environ. Sci. Biotechnol. 15, 147–172 (2016). [Google Scholar]
- 36.Duarte A. W. F., et al. , Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit. Rev. Biotechnol. 38, 600–619 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Pathania S., Solanki P., Putatunda C., Bhatia R. K., Walia A., Survival Strategies in Cold-adapted Microorganisms, Goel R., Soni R., Suyal D. C., khan M., Eds. (Springer Singapore, Singapore, 2022), pp. 87–111. [Google Scholar]
- 38.Averill C., Turner B. L., Finzi A. C., Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Cordero R. J. B., et al. , Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr. Biol. 28, 2657–2664.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.