Abstract
Hypernatremia is an occasionally encountered electrolyte disorder, which may lead to fatal consequences under improper management. Hypernatremia is a disorder of the homeostatic status regarding body water and sodium contents. This imbalance is the basis for the diagnostic approach to hypernatremia. We summarize the eight diagnostic steps of the traditional approach and introduce new biomarkers: exclude pseudohypernatremia, confirm glucose-corrected sodium concentrations, determine the extracellular volume status, measure urine sodium levels, measure urine volume and osmolality, check ongoing urinary electrolyte free water clearance, determine arginine vasopressin/copeptin levels, and assess other electrolyte disorders. Moreover, we suggest six steps to manage hypernatremia by replacing water deficits, ongoing water losses, and insensible water losses: identify underlying causes, distinguish between acute and chronic hypernatremia, determine the amount and rate of water administration, select the type of replacement solution, adjust the treatment schedule, and consider additional therapy for diabetes insipidus. Physicians may apply some of these steps to all patients with hypernatremia, and can also adapt the regimens for specific causes or situations.
Keywords: Correction, Evaluation, Hypernatremia, Sodium, Treatment
INTRODUCTION
Hypernatremia is defined as serum sodium (sNa) concentration exceeding 145 mmol/L and reflects serum hyperosmolality, which is an occasionally encountered electrolyte disorder in hospitalized patients, especially in elderly and critically ill patients [1–3]. The prevalence of hypernatremia varies widely depending on various clinical settings [4,5]. The prevalence among hospitalized patients has been reported to be between 0.5% and 5.0% [2,6–8]; in the emergency department, hypernatremia is rare with a prevalence of 0.2% to 1.0%, this prevalence is about 10 times higher at 2% to 6% in critically ill patients, whereas it is about 10% in intensive care units [4,9–11].
In patients with acute hypernatremia, the morbidity and mortality rates are exceedingly high [10,12]. Several studies have shown that the mortality of hypernatremia varies from 10% to 75% [1,10,12,13]. In studies with adult patient populations, sNa concentrations above 160 mmol/L (severe hypernatremia) are associated with a 75% mortality rate [3,12,14]. Neonatal hypernatremia is a potentially lethal condition, and children with acute hypernatremia have a 10% to 70% mortality rate [13,15–17]. Similar to hyponatremia [18–22], hypernatremia can be life-threatening [1], so it needs to be diagnosed and managed promptly based on practice guidelines.
PATHOPHYSIOLOGY OF HYPERNATREMIA
The human body maintains a normal osmolality between 280 and 295 mOsm/kg by water homeostasis, which is mediated by arginine vasopressin (AVP) secretion, thirst-induced water ingestion, and the renal water transport in response to AVP [1,2,23–25]. Abnormalities in water homeostasis (defects in one or more of these physiological mechanisms) are manifested as disorders in the sNa concentration—hypernatremia or hyponatremia [1,2,13,24–27]. An impairment in the urine-diluting capacity or excess water intake leads to hyponatremia [25]. By contrast, hypernatremia is caused by a defect in the ability to concentrate urine or insufficient water intake [25]. Hypernatremia usually results from a deficit of water in relation to the body’s sodium content, which can result from a net water loss or hypertonic sodium gain [12,25,27]. Because plasma sodium is a solute unable to permeate cell membranes, it contributes to tonicity and induces the movement of water across cell membranes [1,12,28]. Therefore, hypernatremia induces hypertonicity and always causes, at least, transient cellular dehydration [1,12,28]. Sustained hypertonicity caused by chronic hypernatremia promotes the accumulation of organic osmolytes (e.g., glutamate, taurine, and myo-inositol) and these adaptive changes thereby pull water into the cells and restore the cell volume [12,13,28,29]. Therefore, chronic hypernatremia is much less likely to provoke neurologic symptoms [12,28]. However, adaptive changes to chronic hypernatremia induce delayed clearance of osmolytes from the cell compared to rapid loss of potassium and sodium during cerebral swelling [12,28]. Thus, rehydration to rapidly correct chronic hypernatremia induces cerebral edema, seizures, and coma [1,12,28].
CLINICAL MANIFESTATIONS
Signs and symptoms of hypernatremia are predominantly related to disturbances of the central nervous system due to brain cell shrinkage and are prominent when the increase in sNa concentration is large or occurs rapidly [13,28]. Manifestations of hypernatremia vary from thirst, weakness, neuromuscular excitability, hyperreflexia, and lethargy to confusion, seizure, or coma (Table 1) [2,3,13,18,30]. Acute hypernatremia (within 48 hours) causes abrupt brain cell shrinkage that can result in vascular rupture, cerebral bleeding, subarachnoid hemorrhage, or even death; these vascular complications are mostly encountered in pediatric and neonatal patients [1,12,13]. The loss of consciousness level is associated with the severity of hypernatremia [3]. Patients with chronic hypernatremia (more than 48 hours or unknown time of initiation) are less likely to develop severe neurologic symptoms due to adaptive responses generating osmolytes [12,29]. However, adaptive changes to chronic hypernatremia may lead to the development of cerebral edema and seizures during overly rapid rehydration (overcorrection of plasma Na+ by ≥ 12 mmol/L or ≥ 0.5 mmol/L/ hr), especially in infants [28,31–33]. In critically ill adults, however, recent evidence does not indicate that rapid correction of hypernatremia is associated with increased mortality, seizure, or cerebral edema; therefore, if overcorrection of hypernatremia occurs, hypernatremia does not need to be reinduced [31,34]. By contrast, several adult studies have demonstrated that higher mortality results from excessively slow correction rates [11,35,36].
Table 1.
Signs and symptoms of hypernatremia
| Characteristics of hypernatremia | Symptoms related to the characteristics of hypernatremia |
|---|---|
| Cognitive dysfunction and symptoms associated with neuronal cell shrinkage | Lethargy |
| Obtundation (depression on sensorium) | |
| Confusion | |
| Abnormal speech | |
| Irritability | |
| Seizures (unusual in adults) | |
| Nystagmus | |
| Myoclonic jerks | |
| Muscle spasticity (unusual in adults) | |
| Focal neurologic deficits | |
| Nausea or vomiting | |
|
| |
| Dehydration or clinical signs of volume depletion | Orthostatic blood pressure changes |
| Tachycardia | |
| Oliguria | |
| Dry oral mucosa | |
| Abnormal skin turgor | |
| Dry axillae | |
| Intense thirst | |
|
| |
| Other clinical findings | Weight loss |
| Generalized weakness | |
| Fever | |
| Labored respiration | |
CLASSIFICATION AND ETIOLOGY OF HYPERNATREMIA
Hypernatremia is not a disease but rather a pathophysiologic process indicating disturbed water balance [20,37]. Therefore, hypernatremia should be further classified to provide evaluation and treatment directions (Table 2) [3,38]. Hypernatremia is divided into acute (within 48 hours) and chronic (more than 48 hours or unknown time of initiation) hypernatremia [13,24,39].
Table 2.
Classifications of hypernatremia
Because sustained hypernatremia can occur only when thirst perception or water access is impaired, the groups at highest risk are patients with altered mental status, intubated patients, infants, and elderly persons [12,15,27]. In geriatric and intensive care patients, hypernatremia is associated with (1) the inability to maintain an adequate volume balance (e.g., in physically or mentally impaired patients); (2) the need for parenteral nutrition (e.g., patients in intensive care units or nursing homes); and (3) an impaired thirst perception, which may be caused by the age-dependent degeneration of osmoreceptors in the brain stem [1,12,40].
Hypernatremia is generally caused by combined water and electrolyte deficit, with losses of free water in excess of Na+ [12,25,27]. This imbalance can result from (1) net water loss, which can either be pure water (absence of a sodium deficit) or hypotonic fluid (presence of a sodium deficit) loss, or (2) gain of hypertonic sodium [4]. Most cases of hypernatremia are caused by net water loss, which can result from renal and non-renal routes (insensible or gastrointestinal water loss) [20]. Common causes of renal water loss include osmotic diuresis secondary to hyperglycemia, excessive urea, postobstructive diuresis, or mannitol administration, all of which share an increase in urinary solute excretion and urine osmolality (Uosm) [1,26,41–43]. Hypernatremia due to water diuresis develops in central or nephrogenic diabetes insipidus [12]. Less frequently, hypertonic sodium gain usually results from clinical interventions or accidental sodium loading. The etiology of hypernatremia is summarized in Table 3 and Fig. 1 [12].
Table 3.
Cause of hypernatremia
| 1. Net water loss |
|
|
| 1) Euvolemia (pure water loss = water deficit) |
| ↓↓total body water, total body Na+ unchanged |
|
|
| Extrarenal losses |
|
|
| Respiratory (tachycardia) |
|
|
| Dermal (sweating, fever) |
|
|
| Renal losses |
|
|
| Central diabetes insipidus (neurogenic) |
|
|
| Post-traumatic |
|
|
| Caused by tumors, cysts, histiocytosis, tuberculosis, sarcoidosis |
|
|
| Idiopathic |
|
|
| Caused by aneurysms, meningitis, encephalitis, Guillain-Barré syndrome |
|
|
| Caused by ethanol ingestion (transient) |
|
|
| Nephrogenic diabetes insipidus |
|
|
| Congenital |
|
|
| Acquired |
|
|
| Caused by renal disease (e.g., medullary cystic disease) |
|
|
| Caused by hypercalcemia or hypokalemia |
|
|
| Caused by drugs (lithium, demeclocycline, foscarnet, methoxyflurane, amphotericin B, vasopressin V2 receptor antagonists) |
|
|
| Other |
|
|
| Inability to gain access to fluids |
|
|
| Hypodipsia or adipsia |
|
|
| Reset osmostat (essential hypernatremia) |
|
|
| 2) Hypovolemia (hypotonic fluid loss = combined water and sodium deficit) |
| ↓↓total body water, ↓total body Na+ |
|
|
| Extrarenal losses |
|
|
| Gastrointestinal losses |
|
|
| Vomiting |
|
|
| Nasogastric drainage |
|
|
| Enterocutaneous fistula |
|
|
| Diarrhea |
|
|
| Use of osmotic cathartic agents (e.g., lactulose) |
|
|
| Dermal (cutaneous causes) |
|
|
| Burns |
|
|
| Excessive sweating |
|
|
| Renal losses |
|
|
| Osmotic diuresis (mannitol, glucose, urea) |
|
|
| Loop diuretics |
|
|
| Postobstructive diuresis |
|
|
| Polyuric phase of acute tubular necrosis |
|
|
| Intrinsic renal disease |
|
|
| Others |
|
|
| 2. Hypertonic sodium gain (hypervolemia) |
| variable total body water (↓/unchanged/↑), ↑↑total body Na+ |
|
|
| Hypertonic saline (e.g, 3% normal saline) or NaHCO3 administration |
|
|
| Infants or comatose patients receiving hypertonic feeding |
|
|
| Ingestion of sodium chloride |
|
|
| Ingestion of seawater |
|
|
| Sodium chloride-rich emetics |
|
|
| Hypertonic saline enemas |
|
|
| Intrauterine injection of hypertonic saline |
|
|
| Hypertonic sodium chloride infusion |
|
|
| Hypertonic dialysis |
|
|
| Primary hyperaldosteronism |
|
|
| Cushing’s syndrome |
|
|
| Mineralocorticoid excess |
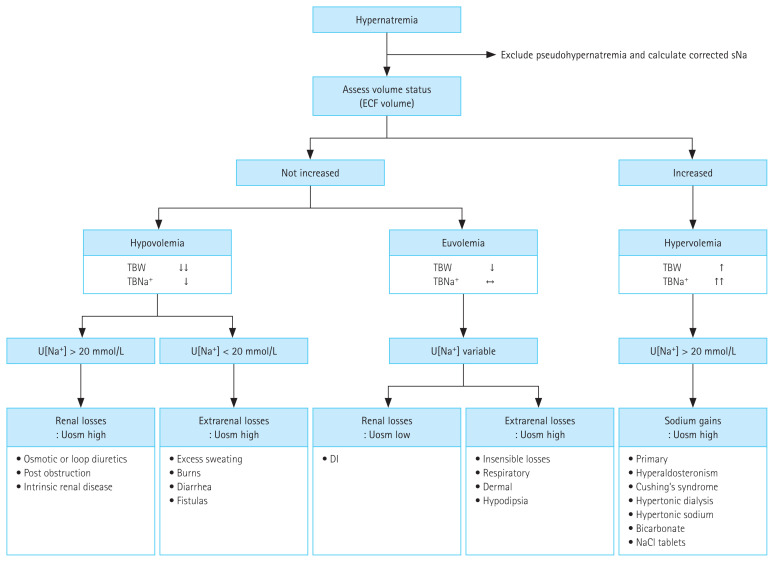
Figure 1.
Diagnostic approach to hypernatremia. sNa, serum sodium; ECF, extracellular fluid; TBW, total body water; TBNa+, total body sodium; U[Na+], urine sodium concentration; Uosm, urine osmolality; DI, diabetes insipidus; NaCl, sodium chloride.
DIAGNOSTIC APPROACH TO HYPERNATREMIA
Comprehensive consideration of clinical history, physical examination, and laboratory findings helps to determine the cause of hypernatremia (Fig. 1) [13,44]. The history should focus on predisposing factors including impaired mental states, physical handicaps, and postoperative state; the presence or absence of thirst; diuresis/oliguria; and non-renal sources of water loss, such as diarrhea, fever, and infection [13,39]. The physical examination should include a detailed neurologic evaluation and an assessment of the extracellular fluid volume; reduced jugular venous pressure and orthostatic hypotension are symptoms of hypovolemia, which indicates a considerable water deficit or an electrolyte and water deficiency [2,13]. Accurate documentation of daily fluid input and urine output is also critical for diagnosing and managing hypernatremia (Table 1) [37]. Patients with hypernatremia can be categorized into one of the following three groups depending on their volume status [2].
Hypovolemic hypernatremia (combined water and sodium deficit)
Hypovolemic hypernatremia is a condition when a patient loses both water and sodium; however, the water loss is comparatively more substantial [13]. Signs and symptoms of hypovolemia, such as low blood pressure, tachycardia, dry mucous membranes, abnormal skin turgor, orthostatic hypotension, weight loss, prerenal acute renal failure, metabolic alkalosis, hemoconcentration resulting in elevated hematocrit or serum protein, or decreased jugular venous pressure (< 5 cmH2O), are present [2,13].
Euvolemic hypernatremia (water deficit)
Patients with euvolemic hypernatremia may undergo renal or non-renal water loss without any sodium loss [2,13]. Neither hypo- nor hypervolemia is apparent in the examination [13].
Hypervolemic hypernatremia (hypertonic sodium gain)
Patients with hypervolemic hypernatremia show signs of volume overload such as peripheral or pulmonary edema [2]. Some of these patients have comorbidities such as liver dysfunction, renal dysfunction, or hypoalbuminemia that are likely to contribute to salt retention [13,45].
EIGHT DIAGNOSTIC STEPS OF HYPERNATREMIA
Hypernatremia can be divided into three different subgroups based on the extracellular fluid volume [2]. Using a urinary marker (Uosm, urine electrolyte, or urine electrolyte-free water clearance [EFWC]), the physician can determine the underlying cause of hypernatremia and adjust the treatment accordingly [2,46–48].
1. First, exclude pseudohypernatremia
Pseudohypernatremia is defined as spuriously increased plasma sodium (> 145 mmol/L) due to decreased plasma protein or lipid concentration [49,50]. There are two methods of measuring sodium concentration with an ion-selective electrode (ISE) using either an undiluted sample (direct ISE) or a diluted sample (indirect ISE) [2,51]. Because spurious sodium concentrations occur mainly in indirect ISE analyses, direct ISE has been proposed as the preferred method in clinical settings characterized by abnormal protein or lipid concentration, particularly in critically ill patients [50,51]. In patients with suspected pseudohypernatremia, measuring serum osmolality with an osmometer or sodium concentrations with a direct potentiometer can reflect the true sNa levels [2,50].
2. Second, in patients with hyperglycemia, sNa concentrations should be corrected for glucose based on Eqs. (1) and (2) [6,51,52].
3. Third, determine whether the extracellular volume is hypovolemic, euvolemic, or hypervolemic using the history and physical examinations, as described above
4. Fourth, measure urine sodium
Patients with volume depletion exhibit decreased sodium excretion in the urine (< 20 mmol/L) [2,55]. Although hypovolemia may be present, a concentrating defect and an elevated urine sodium concentration (> 20 mmol/L) might be observed in case of osmotic diuresis, the use of diuretics, postobstructive nephropathy, or the recovery phase from acute tubular necrosis [27,41,56].
5. Fifth, measure urine volume (UV) and Uosm
A Uosm < 300 mOsm/kg and polyuria (> 3 L/day or > 40 mL/kg/day) suggest the presence of diabetes insipidus [2,56–58]. Administration of exogenous AVP (typically its pharmacological analog desmopressin acetate [DDAVP]) enables the distinction between central and nephrogenic diabetes insipidus, which is associated with at least a 50% increase in Uosm along with a significant decrease in UV in central diabetes insipidus, whereas no change is seen in nephrogenic diabetes insipidus [9,28,59]. If Uosm is between 300 and 800 mOsm/kg, this may reflect partial diabetes insipidus (central or nephrogenic), central diabetes insipidus with volume depletion, or a process of osmotic diuresis [13,60]. The measurement of total solute excretion is helpful in this situation [2,13].
Eq. (3)
Excessive excretion of sodium chloride, mannitol, glucose, or urea, with a daily solute excretion of > 750–1,000 mOsm/ day (> 15 mOsm/kg body water/day), might result in an osmotic diuresis [13,61]. A Uosm > 800 mOsm/kg indicates either primary hypodipsia, excessive non-renal water loss, or saline overload [1,2,13]. In this case, the non-renal source (gastrointestinal tract, respiratory tract, or skin) of water loss may be the primary reason for the development of hypernatremia [13]. The appropriate response to hypernatremia and hyperosmolality is an increase in circulating AVP and the excretion of low volumes (< 500 mL/day) of maximally concentrated urine (Uosm > 800 mOsm/kg) [62].
6. Sixth, in patients with hypernatremia due to renal water loss, check ongoing urinary EFWC calculated based on Eq. (4).
-
Eq. (4) (UNa, urine sodium; UK, urine potassium)
EFWC can differentiate between renal or extrarenal water losses [2,63,64]. Therefore, a substantially increased positive value indicates increased renal water losses due to osmotic diuresis, furosemide administration, renal failure, or diabetes insipidus, whereas a value near zero or even (rarely) negative suggests gastrointestinal or insensible water losses [42,63,64].
7. Seventh, check AVP or copeptin levels in patients with hypotonic polyuria
A water deprivation test along with the evaluation of basal and stimulated levels of AVP or copeptin, a peptide co-secreted with AVP in the setting of hypertonicity, are required for adequate differentiation between polyuria-polydipsia syndrome, which includes primary polydipsia, as well as central and nephrogenic diabetes insipidus [24,65,66]. By definition, patients with baseline hypernatremia are hypertonic, with an adequate stimulus for AVP secretion by the posterior pituitary [20,59,67]. Basal AVP or copeptin concentrations in the serum are elevated in hypernatremic patients with nephrogenic diabetes insipidus [27,66,68–70]. Their low Uosm will also fail to respond to DDAVP, increasing by < 50% or < 150 mOsm/kg from baseline; patients with central diabetes insipidus will respond to DDAVP stimulation with a reduction in AVP or copeptin levels [68–70]. The level of circulating basal and stimulated AVP or copeptin will help distinguish the underlying etiology (central and nephrogenic diabetes insipidus, primary polydipsia) [27,62,68–70]. Patients may have a partial response to DDAVP, with a > 50% rise in Uosm that nevertheless fails to achieve 800 mOsm/ kg [60,65]. AVP is not routinely measured in clinical practice due to preanalytic instability [71–73]. Copeptin has become a surrogate marker for AVP concentration, has advantages over AVP regarding stability, and can be measured with commercially available assays with high-standard technical performance [62,68–70].
8. Eighth, check concomitant electrolyte disorders (serum potassium and calcium)
Hypokalemia (serum potassium level < 3.0 mmol/L) [74] or hypercalcemia (serum calcium concentration > 11 mg/dL or 2.75 mmol/L) [75] may induce an impairment of kidney concentrating ability via decreased collecting tubule responsiveness to vasopressin, resulting in polyuria, nephrogenic diabetes insipidus, and development of hypernatremia [9,27,61].
TREATMENT OF HYPERNATREMIA
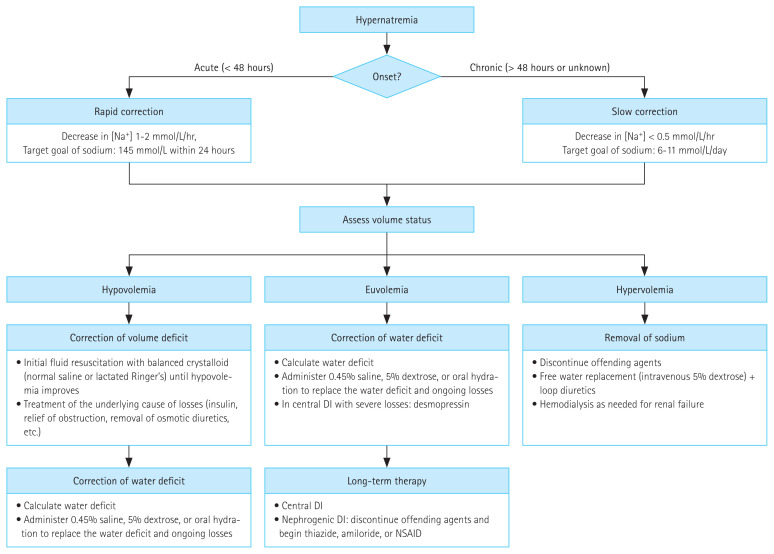
Management of hypernatremia requires two approaches: (1) identifying and resolving the underlying cause and (2) correcting the established hypertonicity (hyperosmolarity) considering the severity of neurologic symptoms, onset time (acute vs. chronic), and volume status [1,13]. A stepwise approach is summarized in the following points (Fig. 2).
Figure 2.
Treatment of hypernatremia. DI, diabetes insipidus; NSAID, nonsteroidal anti-inflammatory drug.
1. First, identify the underlying cause and initiate treatment accordingly
Identifying the underlying cause of hypernatremia and initiating treatment is important to prevent further water loss or hypertonic sodium gain [13,76]. Management of the predisposing factors may include stopping offending medication (lactulose, diuretics, or drugs associated with nephrogenic diabetes insipidus) and gastrointestinal fluid losses (vomiting or diarrhea); controlling fever, hyperglycemia, and glycosuria; relieving urinary obstruction; treating hypercalcemia and hypokalemia; and withdrawing hypertonic tube feeds [1,13,27,77].
2. Second, assess the severity of symptoms and decide whether the hypernatremia is acute or chronic
The rate of sNa lowering should be estimated based mainly on the severity of neurologic symptoms and the duration of hypernatremia [1,2,12,27]. In acute symptomatic hypernatremia (within 48 hours) due to sodium loading, a more aggressive rapid correction of plasma sodium (falling by 1–2 mmol/L/hr for the first 6–8 hours, restoring a sNa concentration of 145 mmol/L within 24 hours) improves the prognosis without increasing the risk of cerebral edema [1,9,13,28]. However, patients with hypernatremia of longer (> 48 hours) or unknown duration should be corrected at a rate of < 0.5 mmol/hr (12 mmol/L/day) based on data in pediatric patients (particularly infants) who have no neurologic sequelae [1,9,32,33,78]. However, several studies in adults have reported that rapid correction rates (> 0.5 mmol/L/hr) are not associated with a high risk of mortality and neurologic damage [31,34], whereas higher mortality results from excessively slow correction rates (< 0.25 mmol/L/hr, 6 mmol/L/day) [11,31,35]. However, if the target rate is inadvertently exceeded, therapeutic re-raising of the sNa concentration is not recommended [36]. For this reason, an ongoing study whose results will be available within 3 years examines the effects of a sNa target correction rate of 6 to 11 mmol/L/day during the first 24 hours for the treatment of hypernatremia [31].
3. Third, determine the required amount and rate of water administration
Estimate the ‘water deficit’ using Eq. (5) or (6) or devise a fluid repletion regimen (I or II) as outlined below.
Eq. (5)
For example, a female patient who weighs 60 kg and has a sNa concentration of 166 mmol/L has a water deficit of 0.5 × 60 [(166/140) − 1] = 5.6 L. With a simplified calculation, TBW is generally estimated to be 60% of the body weight for men and 50% for women, and 5% is deducted for elderly patients [12,60]. In water-depleted hypernatremic patients, lower values are usually applied (for men and women, 50% and 40% of the lean body weight, respectively) [2]. To get the plasma sodium concentration back to 140 mmol/L, use Eq. (5) to calculate how much positive water balance is needed [2,12] and administer the estimated amount of water over 48 to 72 hours (2 to 3 days) [27].
Eq. (6)
The formula in Eq. (6) proposed by Adrogue-Madias can be used in patients with hypernatremia to estimate the effect of 1 L of any infusate on the patient’s sNa concentration [79,80]. However, Eq. (6) did not accurately predict the changes in sNa levels in a group of patients with hypernatremia, severe extracellular volume depletion, and markedly reduced renal function [2,81].
The following initial fixed-dose regimens have been suggested.
Initial fixed-dose regimen with 5% dextrose water, intravenously at a rate of 1.35 mL/kg/hr [28].
Initial fixed-dose regimen with 5% dextrose water, intravenously at a rate of 3 mL/kg/hr. The rationale is that every 1 mmol/L decrease in the sNa concentration requires 3 mL/kg of electrolyte-free water [82].
When administering the calculated amount of water based on Eq. (5) or (6) (intravenously, as dextrose in water, or orally if the patient can drink) or by initial fixed-dose regimens (I or II), additional insensible loss, ongoing renal or extrarenal losses must be considered in the calculation [12].
-
Total infused amount of water/day = calculated water deficit or simplified fluid repletion regimen + insensible
Eq. (7)
‘Ongoing losses’ are classified into renal and non-renal losses. In patients with hypernatremia due to urinary losses, the amount of ongoing water loss (urine EFWC) can be calculated using Eq. (8) [64,83,84].
Eq. (8)
However, Eq. (8) is limited and impractical to make prospective decisions because of the need to measure 24-hour UV and complicated calculations [85]. A study suggested a predictive guide of water restriction based on a simple approach of the urine/plasma electrolyte ratio derived from EFWC in patients with chronic hyponatremia [85]. An ongoing study in which the urine/plasma electrolyte ratio is applied to the treatment of hypernatremia and the results regarding the usefulness of this parameter should be available in the future [31].
In contrast to ongoing urinary losses, measuring ongoing water losses in the stool (as in patients with diarrhea) is usually impractical and not routinely performed [9]. In such patients, the simplest approach is to initiate therapy without accounting for ongoing free water losses, monitor the sNa concentration, and increase the rate of fluid administration if it is not falling at the desired rate [63,86].
Extracellular fluid volume depletion or hypokalemia are common co-morbidities in patients with hypernatremia [2,13]. Because volume depletion and hypokalemia can worsen hypernatremia, they should be included in the provided ‘maintenance fluid’ or corrected by oral ingestion [2,47,87,88]. Sodium or potassium can be added to the intravenous fluid as necessary to simultaneously correct the water and electrolyte deficits [83,89]. However, the sodium and potassium content of the replacement fluid decreases the amount of free water being supplied [87,90]. Using two intravenous solutions—one for free water and the other for sodium with or without potassium as an iso-osmotic solution—instead of combining water and sodium (with or without potassium) into one solution is an alternative [88].
4. Fourth, select the type of replacement solution
A proper intravenous solution should be selected depending on the history, blood pressure, or volume status as shown in Fig. 2 [12,88]. It may be appropriate to initially treat the patient with hypotonic fluids including 5% dextrose, 0.2% or 0.45% sodium chloride (1/4 or 1/2 normal saline) [13,91]. Notably, if a hypotonic saline solution is to be given, the amount of free water in the solution should be estimated [60]. In case of shock or hypotension, 0.9% normal saline or balanced crystalloid should be used for resuscitation regardless of the sNa level until the restoration of volume has been reached [13,25,88,92]. In patients with volume overload (hypervolemic hypernatremia), the free water deficit can be replaced with 5% dextrose in water, and diuretics should be administered to promote sodium excretion and maintain a negative fluid balance [9,89].
5. Fifth, adjust the treatment schedule
For the safety and successful management of patients with hypernatremia, the treatment must be adjusted appropriately, and repeated measurements of sNa are necessary [36,46,93].
6. Sixth, consider additional therapy for diabetes insipidus
Patients with central diabetes insipidus are treated with desmopressin, either as an intranasal spray (10–20 μg per 12–24 hours) or tablets (0.1–0.8 mg orally per 12 hours) [12,25]. Nephrogenic diabetes insipidus is treated by discontinuing precipitating medications and, on occasion, beginning diuretics (e.g., thiazides, amiloride [2.5 to 10 mg/ day]), non-steroid anti-inflammatory medication, or both [12,25,27,58].
FUTURE RESEARCH
Several traditional approaches to evaluating hypernatremia and conceptual regimens for the management of hypernatremia exist. However, the level of their evidence remains low. Additional clinical studies to find new biomarkers such as copeptin may be one of the future approaches for the diagnosis of hypernatremia [62,68–70]. In terms of management, the best sNa correction rate and optimal fluid administration regimen remain to be determined. A prospective, randomized trial on hypernatremia management launched in July 2021. Its primary purpose is to find a more straightforward regimen for correcting sNa levels. There are two treatment arms, including rapid intermittent bolus and slow continuous infusion using electrolyte-free solutions in hypernatremia treatment [31], and information on the efficacy and safety of each regimen will become available in 3 years. For electrolyte disorders, we need more and challenging clinical trials in the future.
CONCLUSIONS
Hypernatremia is attributed to a deficit of water, which can result from a net water loss or hypertonic sodium gain. The detailed evaluation of the etiologies and proper choice of type and rate of intravenous solution during the treatment of hypernatremia is crucial since under- or overcorrection of hypernatremia is associated with increased morbidity and mortality.
Footnotes
CRedit authorship contributions
Giae Yun: formal analysis, methodology, visualization, writing - original draft, writing - review & editing; Seon Ha Baek: formal analysis, methodology, project administration, writing - original draft, writing - review & editing; Sejoong Kim: conceptualization, formal analysis, funding acquisition, methodology, project administration, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
The research was supported by a grant No. 2021R1C1C 1008966 from the National Research Foundation of Korea.
References
- 1.Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 2.Liamis G, Filippatos TD, Elisaf MS. Evaluation and treatment of hypernatremia: a practical guide for physicians. Postgrad Med. 2016;128:299–306. doi: 10.1080/00325481.2016.1147322. [DOI] [PubMed] [Google Scholar]
- 3.Arambewela MH, Somasundaram NP, Garusinghe C. Extreme hypernatremia as a probable cause of fatal arrhythmia: a case report. J Med Case Rep. 2016;10:272. doi: 10.1186/s13256-016-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabassi A, Tedeschi S. Severity of community acquired hypernatremia is an independent predictor of mortality: a matter of water balance and rate of correction. Intern Emerg Med. 2017;12:909–911. doi: 10.1007/s11739-017-1693-x. [DOI] [PubMed] [Google Scholar]
- 5.Maggiore U, Picetti E, Antonucci E, et al. The relation between the incidence of hypernatremia and mortality in patients with severe traumatic brain injury. Crit Care. 2009;13:R110. doi: 10.1186/cc7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsipotis E, Price LL, Jaber BL, Madias NE. Hospital-associated hypernatremia spectrum and clinical outcomes in an unselected cohort. Am J Med. 2018;131:72–82. doi: 10.1016/j.amjmed.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Ates I, Ozkayar N, Toprak G, Yilmaz N, Dede F. Factors associated with mortality in patients presenting to the emergency department with severe hypernatremia. Intern Emerg Med. 2016;11:451–459. doi: 10.1007/s11739-015-1368-4. [DOI] [PubMed] [Google Scholar]
- 8.Kang MY. Blood electrolyte disturbances during severe hypoglycemia in Korean patients with type 2 diabetes. Korean J Intern Med. 2015;30:648–656. doi: 10.3904/kjim.2015.30.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013;28:216. doi: 10.1016/j.jcrc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50:952–957. doi: 10.1053/j.ajkd.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014;15:37. doi: 10.1186/1471-2369-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SW. Hypernatemia: successful treatment. Electrolyte Blood Press. 2006;4:66–71. doi: 10.5049/EBP.2006.4.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhsin SA, Mount DB. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metab. 2016;30:189–203. doi: 10.1016/j.beem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Lam NN, Minh NTN. Risk factors and outcome of hypernatremia amongst severe adult burn patients. Ann Burns Fire Disasters. 2018;31:271–277. [PMC free article] [PubMed] [Google Scholar]
- 15.Zieg J. Diagnosis and management of hypernatraemia in children. Acta Paediatr. 2022;111:505–510. doi: 10.1111/apa.16170. [DOI] [PubMed] [Google Scholar]
- 16.Moritz ML, Ayus JC. The changing pattern of hypernatremia in hospitalized children. Pediatrics. 1999;104(3 Pt 1):435–439. doi: 10.1542/peds.104.3.435. [DOI] [PubMed] [Google Scholar]
- 17.Mujawar NS, Jaiswal AN. Hypernatremia in the neonate: neonatal hypernatremia and hypernatremic dehydration in neonates receiving exclusive breastfeeding. Indian J Crit Care Med. 2017;21:30–33. doi: 10.4103/0972-5229.198323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch CA, Fulop T. Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev Endocr Metab Disord. 2017;18:49–66. doi: 10.1007/s11154-017-9420-5. [DOI] [PubMed] [Google Scholar]
- 19.Baek SH, Kim S. Optimal treatment with hypertonic saline in patients with symptomatic hyponatremia: a perspective from a randomized clinical trial (SALSA trial) Kidney Res Clin Pract. 2020;39:504–506. doi: 10.23876/j.krcp.20.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170:G1–G47. doi: 10.1530/EJE-13-1020. [DOI] [PubMed] [Google Scholar]
- 21.Yoo BS, Park JJ, Choi DJ, et al. Prognostic value of hyponatremia in heart failure patients: an analysis of the Clinical Characteristics and Outcomes in the Relation with Serum Sodium Level in Asian Patients Hospitalized for Heart Failure (COAST) study. Korean J Intern Med. 2015;30:460–470. doi: 10.3904/kjim.2015.30.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1–S42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Singh AK. Hypernatremia. In: Mushlin SB, Greene HL, editors. Decision Making in Medicine. 3rd ed. Philadelphia (PA): Mosby; 2010. pp. 378–379. [Google Scholar]
- 24.Qian Q. Hypernatremia. Clin J Am Soc Nephrol. 2019;14:432–434. doi: 10.2215/CJN.12141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson R, Feehally J, Floege J, Tonelli M. Comprehensive Clinical Nephrology. 6th ed. Edinburgh (UK): Elsevier; 2018. [Google Scholar]
- 26.Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010;8:72–81. doi: 10.5049/EBP.2010.8.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mount DB. Fluid and electrolyte disturbances. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 21st ed. New York (NY): McGraw-Hill Education; 2022. [Google Scholar]
- 28.Sterns RH. Disorders of plasma sodium: causes, consequences, and correction. N Engl J Med. 2015;372:55–65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 29.Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992;3:12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- 30.Arieff AI, Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976;10:104–116. doi: 10.1038/ki.1976.82. [DOI] [PubMed] [Google Scholar]
- 31.Ryu JY, Yoon S, Lee J, et al. Efficacy and safety of rapid intermittent bolus compared with slow continuous infusion in patients with severe hypernatremia (SALSA II trial): a study protocol for a randomized controlled trial. Kidney Res Clin Pract. 2022;41:508–520. doi: 10.23876/j.krcp.21.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn A, Brachet E, Blum D. Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med. 1979;5:27–31. doi: 10.1007/BF01738999. [DOI] [PubMed] [Google Scholar]
- 33.Blum D, Brasseur D, Kahn A, Brachet E. Safe oral rehydration of hypertonic dehydration. J Pediatr Gastroenterol Nutr. 1986;5:232–235. [PubMed] [Google Scholar]
- 34.Chauhan K, Pattharanitima P, Patel N, et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin J Am Soc Nephrol. 2019;14:656–663. doi: 10.2215/CJN.10640918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alshayeb HM, Showkat A, Babar F, Mangold T, Wall BM. Severe hypernatremia correction rate and mortality in hospitalized patients. Am J Med Sci. 2011;341:356–360. doi: 10.1097/MAJ.0b013e31820a3a90. [DOI] [PubMed] [Google Scholar]
- 36.Sterns RH. Evidence for managing hypernatremia: is it just hyponatremia in reverse? Clin J Am Soc Nephrol. 2019;14:645–647. doi: 10.2215/CJN.02950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindner G, Schwarz C, Kneidinger N, Kramer L, Oberbauer R, Druml W. Can we really predict the change in serum sodium levels?: an analysis of currently proposed formulae in hypernatraemic patients. Nephrol Dial Transplant. 2008;23:3501–3508. doi: 10.1093/ndt/gfn476. [DOI] [PubMed] [Google Scholar]
- 38.Arzhan S, Roumelioti ME, Litvinovich I, Bologa CG, Myers OB, Unruh ML. Hypernatremia in hospitalized patients: a large population-based study. Kidney360. 2022;3:1144–1157. doi: 10.34067/KID.0000702022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Absi A, Gosmanova EO, Wall BM. A clinical approach to the treatment of chronic hypernatremia. Am J Kidney Dis. 2012;60:1032–1038. doi: 10.1053/j.ajkd.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Deubner N, Berliner D, Frey A, et al. Dysnatraemia in heart failure. Eur J Heart Fail. 2012;14:1147–1154. doi: 10.1093/eurjhf/hfs115. [DOI] [PubMed] [Google Scholar]
- 41.Popli S, Tzamaloukas AH, Ing TS. Osmotic diuresis-induced hypernatremia: better explained by solute-free water clearance or electrolyte-free water clearance? Int Urol Nephrol. 2014;46:207–210. doi: 10.1007/s11255-012-0353-3. [DOI] [PubMed] [Google Scholar]
- 42.Lindner G, Schwarz C, Funk GC. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrol Dial Transplant. 2012;27:962–967. doi: 10.1093/ndt/gfr428. [DOI] [PubMed] [Google Scholar]
- 43.Vadi S, Yim K. Hypernatremia due to urea-induced osmotic diuresis: physiology at the bedside. Indian J Crit Care Med. 2018;22:664–669. doi: 10.4103/ijccm.IJCCM_266_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Yoo KD, Baek SH, et al. Korean Society of Nephrology 2022 Recommendations on controversial issues in diagnosis and management of hyponatremia. Kidney Res Clin Pract. 2022;41:393–411. doi: 10.23876/j.krcp.33.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn T. Hypernatremia with edema. Arch Intern Med. 1999;159:93–98. doi: 10.1001/archinte.159.1.93. [DOI] [PubMed] [Google Scholar]
- 46.Harring TR, Deal NS, Kuo DC. Disorders of sodium and water balance. Emerg Med Clin North Am. 2014;32:379–401. doi: 10.1016/j.emc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Liamis G, Tsimihodimos V, Doumas M, Spyrou A, Bairaktari E, Elisaf M. Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinic. Nephrol Dial Transplant. 2008;23:136–143. doi: 10.1093/ndt/gfm376. [DOI] [PubMed] [Google Scholar]
- 48.Shah MK, Workeneh B, Taffet GE. Hypernatremia in the geriatric population. Clin Interv Aging. 2014;9:1987–1992. doi: 10.2147/CIA.S65214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimeski G, Morgan TJ, Presneill JJ, Venkatesh B. Disagreement between ion selective electrode direct and indirect sodium measurements: estimation of the problem in a tertiary referral hospital. J Crit Care. 2012;27:326. doi: 10.1016/j.jcrc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Filippatos TD, Liamis G, Christopoulou F, Elisaf MS. Ten common pitfalls in the evaluation of patients with hyponatremia. Eur J Intern Med. 2016;29:22–25. doi: 10.1016/j.ejim.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38:50–57. doi: 10.1159/000351804. [DOI] [PubMed] [Google Scholar]
- 52.Baek SH, Kim S, Na KY, Kim S, Chin HJ. Predialysis hyponatremia and mortality in elderly patients beginning to undergo hemodialysis. Korean J Intern Med. 2018;33:970–979. doi: 10.3904/kjim.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106:399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 54.Katz MA. Hyperglycemia-induced hyponatremia: calculation of expected serum sodium depression. N Engl J Med. 1973;289:843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 55.Bhave G, Neilson EG. Volume depletion versus dehydration: how understanding the difference can guide therapy. Am J Kidney Dis. 2011;58:302–309. doi: 10.1053/j.ajkd.2011.02.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mount DB. Azotemia and urinary abnormalities. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 21st ed. New York (NY): McGraw-Hill Education; 2022. [Google Scholar]
- 57.Robertson GL. Diabetes insipidus: differential diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2016;30:205–218. doi: 10.1016/j.beem.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Robertson GL, Bichet DG. Disorders of the neurohypophysis. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 21st ed. New York (NY): McGraw-Hill Education; 2022. [Google Scholar]
- 59.Saifan C, Nasr R, Mehta S, et al. Diabetes insipidus: a challenging diagnosis with new drug therapies. ISRN Nephrol. 2013;2013:797620. doi: 10.5402/2013/797620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priya G, Kalra S, Dasgupta A, Grewal E. Diabetes insipidus: a pragmatic approach to management. Cureus. 2021;13:e12498. doi: 10.7759/cureus.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose BD, Post T, Post TW. Clinical Physiology of Acid-Base and Electrolyte Disorders. New York (NY): McGraw-Hill; 2001. [Google Scholar]
- 62.Fenske W, Refardt J, Chifu I, et al. A copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med. 2018;379:428–439. doi: 10.1056/NEJMoa1803760. [DOI] [PubMed] [Google Scholar]
- 63.Lindner G, Schwarz C. Electrolyte-free water clearance versus modified electrolyte-free water clearance: do the results justify the effort? Nephron Physiol. 2012;120:p1–p5. doi: 10.1159/000336550. [DOI] [PubMed] [Google Scholar]
- 64.Bodonyi-Kovacs G, Lecker SH. Electrolyte-free water clearance: a key to the diagnosis of hypernatremia in resolving acute renal failure. Clin Exp Nephrol. 2008;12:74–78. doi: 10.1007/s10157-007-0021-6. [DOI] [PubMed] [Google Scholar]
- 65.Timper K, Fenske W, Kuhn F, et al. Diagnostic accuracy of copeptin in the differential diagnosis of the polyuria-polydipsia syndrome: a prospective multicenter study. J Clin Endocrinol Metab. 2015;100:2268–2274. doi: 10.1210/jc.2014-4507. [DOI] [PubMed] [Google Scholar]
- 66.Refardt J, Winzeler B, Christ-Crain M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin Endocrinol (Oxf) 2019;91:22–32. doi: 10.1111/cen.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Go S, Kim S, Son HE, et al. Association between copeptin levels and treatment responses to hypertonic saline infusion in patients with symptomatic hyponatremia: a prospective cohort study. Kidney Res Clin Pract. 2021;40:371–382. doi: 10.23876/j.krcp.20.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winzeler B, Cesana-Nigro N, Refardt J, et al. Arginine-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: a prospective diagnostic study. Lancet. 2019;394:587–595. doi: 10.1016/S0140-6736(19)31255-3. [DOI] [PubMed] [Google Scholar]
- 69.Christ-Crain M. Diabetes insipidus: new concepts for diagnosis. Neuroendocrinology. 2020;110:859–867. doi: 10.1159/000505548. [DOI] [PubMed] [Google Scholar]
- 70.Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12:168–176. doi: 10.1038/nrendo.2015.224. [DOI] [PubMed] [Google Scholar]
- 71.Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24:549–572. [PubMed] [Google Scholar]
- 72.Kluge M, Riedl S, Erhart-Hofmann B, Hartmann J, Waldhauser F. Improved extraction procedure and RIA for determination of arginine8-vasopressin in plasma: role of premeasurement sample treatment and reference values in children. Clin Chem. 1999;45:98–103. [PubMed] [Google Scholar]
- 73.Milles JJ, Spruce B, Baylis PH. A comparison of diagnostic methods to differentiate diabetes insipidus from primary polyuria: a review of 21 patients. Acta Endocrinol (Copenh) 1983;104:410–416. doi: 10.1530/acta.0.1040410. [DOI] [PubMed] [Google Scholar]
- 74.Yi JH, Han SW, Kim WY, Kim J, Park MH. Effects of aristolochic acid I and/or hypokalemia on tubular damage in C57BL/6 rat with aristolochic acid nephropathy. Korean J Intern Med. 2018;33:763–773. doi: 10.3904/kjim.2016.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liamis G, Kalaitzidis R, Elisaf M. Hypernatremia during correction of hypercalcemia. Nephron. 2000;86:358. doi: 10.1159/000045799. [DOI] [PubMed] [Google Scholar]
- 76.Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91:299–307. [PubMed] [Google Scholar]
- 77.Leem AY, Kim HS, Yoo BW, et al. Ifosfamide-induced Fanconi syndrome with diabetes insipidus. Korean J Intern Med. 2014;29:246–249. doi: 10.3904/kjim.2014.29.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agrawal V, Agarwal M, Joshi SR, Ghosh AK. Hyponatremia and hypernatremia: disorders of water balance. J Assoc Physicians India. 2008;56:956–964. [PubMed] [Google Scholar]
- 79.Chen S, Shieh M, Chiaramonte R, Shey J. Improving on the Adrogue-Madias formula. Kidney360. 2020;2:365–370. doi: 10.34067/KID.0005882020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berl T. The Adrogue-Madias formula revisited. Clin J Am Soc Nephrol. 2007;2:1098–1099. doi: 10.2215/CJN.03300807. [DOI] [PubMed] [Google Scholar]
- 81.Liamis G, Kalogirou M, Saugos V, Elisaf M. Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant. 2006;21:1564–1569. doi: 10.1093/ndt/gfk090. [DOI] [PubMed] [Google Scholar]
- 82.Sterns RH, Silver SM. Salt and water: read the package insert. QJM. 2003;96:549–552. doi: 10.1093/qjmed/hcg102. [DOI] [PubMed] [Google Scholar]
- 83.Shafiee MA, Bohn D, Hoorn EJ, Halperin ML. How to select optimal maintenance intravenous fluid therapy. QJM. 2003;96:601–610. doi: 10.1093/qjmed/hcg101. [DOI] [PubMed] [Google Scholar]
- 84.Rose BD. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986;81:1033–1040. doi: 10.1016/0002-9343(86)90401-8. [DOI] [PubMed] [Google Scholar]
- 85.Furst H, Hallows KR, Post J, et al. The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci. 2000;319:240–244. doi: 10.1097/00000441-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Sam R, Feizi I. Understanding hypernatremia. Am J Nephrol. 2012;36:97–104. doi: 10.1159/000339625. [DOI] [PubMed] [Google Scholar]
- 87.Frame AA, Wainford RD. Renal sodium handling and sodium sensitivity. Kidney Res Clin Pract. 2017;36:117–131. doi: 10.23876/j.krcp.2017.36.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoorn EJ. Intravenous fluids: balancing solutions. J Nephrol. 2017;30:485–492. doi: 10.1007/s40620-016-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen MK, Kurtz I. Correction of hypervolaemic hypernatraemia by inducing negative Na+ and K+ balance in excess of negative water balance: a new quantitative approach. Nephrol Dial Transplant. 2008;23:2223–2227. doi: 10.1093/ndt/gfm932. [DOI] [PubMed] [Google Scholar]
- 90.Shah SR, Bhave G. Using electrolyte free water balance to rationalize and treat dysnatremias. Front Med (Lausanne) 2018;5:103. doi: 10.3389/fmed.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adrogue HJ, Madias NE. Aiding fluid prescription for the dysnatremias. Intensive Care Med. 1997;23:309–316. doi: 10.1007/s001340050333. [DOI] [PubMed] [Google Scholar]
- 92.Van De Louw A, Shaffer C, Schaefer E. Early intensive care unit-acquired hypernatremia in severe sepsis patients receiving 0.9% saline fluid resuscitation. Acta Anaesthesiol Scand. 2014;58:1007–1014. doi: 10.1111/aas.12368. [DOI] [PubMed] [Google Scholar]
- 93.Hoorn EJ, Betjes MG, Weigel J, Zietse R. Hypernatraemia in critically ill patients: too little water and too much salt. Nephrol Dial Transplant. 2008;23:1562–1568. doi: 10.1093/ndt/gfm831. [DOI] [PubMed] [Google Scholar]