Abstract
The first evidence of the interaction of Mycobacterium tuberculosis with the plasminogen system is herein reported. By FACScan analysis and affinity blotting, lysine-dependent binding of plasminogen to M. tuberculosis was demonstrated. The binding molecules were 30-, 60-, and 66-kDa proteins present in cell wall and soluble protein extracts. The activation of plasminogen, which occurred only in presence of fibrin and was not inhibited by the host serpin, α2-antiplasmin, was also demonstrated.
In humans, plasminogen is converted to plasmin by tissue type plasminogen activator and urokinase (20). Plasmin, a serine protease, plays an essential role in dissolving fibrin clots by degrading extracellular matrices, directly or indirectly, by activation of procollagenases. After the pioneer observations of Lottenberg et al. (17), the interaction of bacteria with the plasminogen system has been acknowledged as a mechanism possibly involved in bacterial pathogenesis (1, 3, 14, 18). Receptors for plasminogen have been identified on the surface of a variety of gram-positive and gram-negative bacteria, including group A streptococci (17), Staphylococcus aureus (12), Neisseria meningitidis and Neisseria gonorrhoeae (23), Haemophilus influenzae, Branhamella catarrhalis, Proteus mirabilis, Pseudomonas aeruginosa and Pseudomonas catarrhalis (22), Escherichia coli (10, 15), Salmonella enterica (10), and Borrelia burgdorferi (7).
It has been shown that plasminogen molecules captured by bacteria can be activated to plasmin by host or bacterial activators (18). Streptokinase and staphylokinase, activators produced by group A streptococci and S. aureus respectively, form 1:1 stoichiometric complexes with plasminogen; this complex, devoid of proteolytic activity, is capable of activating other plasminogen molecules to plasmin (4, 24). Surface proteases of E. coli and Yersinia pestis are further examples of plasminogen activators (14, 16). Host-derived plasmin activity, which might not be blocked by the major physiological plasmin inhibitor α2-antiplasmin (1), provides bacteria with the capacity to dissolve fibrin meshes and extracellular matrices, favoring host tissue invasion (18). In view of the above findings and knowing that Mycobacterium tuberculosis, a facultative intracellular pathogen, has the capacity to grow extracellularly, to invade host tissues, and to disseminate systematically (5), we investigated whether M. tuberculosis strain H37Rv interacts with the plasminogen system.
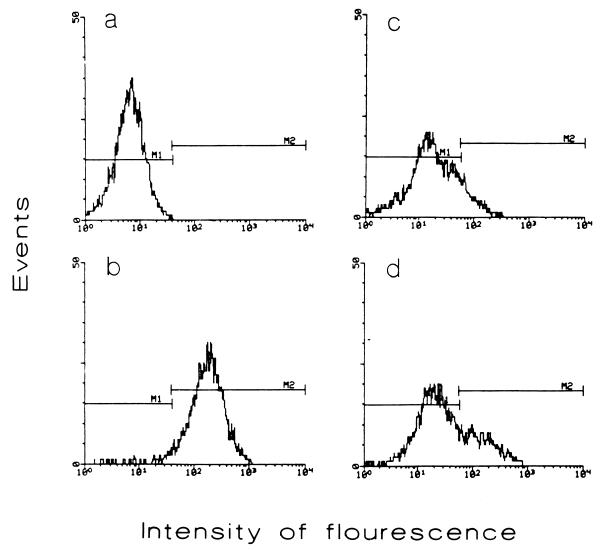
First, we investigated the presence of plasminogen receptors on the surface of whole intact bacilli by fluorescence microscopy and FACScan analysis as follows. M. tuberculosis H37Rv cells from 5-week-old cultures grown in Proskauer and Beck medium as modified by Youmans were treated with 2% sodium azide for 24 h at 37°C and harvested by centrifugation. After rinsing with phosphate-buffered saline (PBS), bacilli were passed repeatedly through a 25-gauge syringe to eliminate aggregates. Thereafter, 109 bacilli were incubated in 1 ml of PBS–3% bovine serum albumin (BSA) for 2 h at 4°C or at room temperature (22°C) with 1 or 5 μg of plasminogen from human plasma (Boehringer, Mannheim, Germany) labeled with fluorescein isothiocyanate (FITC) (Sigma Chemical Co., St. Louis, Mo.). To assess the specificity of binding and the participation of lysine residues, incubation was also done in the presence of 0.1, 1, and 2 M ɛ-aminocaproic acid (EACA; Merck, Darmstadt, Germany), a lysine analog. After extensive rinsing with PBS, bacilli were fixed in 3% cold paraformaldehyde. Fluorescence microscopy showed bacilli with a diffuse, homogeneous, fluorescent pattern (not shown). Controls which were not incubated with plasminogen were negative. The results of the FACScan (Beckton Dickinson, Palo Alto, Calif.) analysis are shown in Fig. 1. Binding of plasminogen to bacilli was better accomplished at 4°C using 5 μg of plasminogen-FITC, which resulted in the labeling of 94.7% of the bacilli. As shown for other microbes (3, 23), binding of plasminogen to M. tuberculosis receptors takes place via lysine residues. Indeed, the number of labeled bacilli was drastically reduced by EACA; the magnitude of this reduction was related to the concentration of the lysine analog in the incubation solution. The FACScan experiments were carried out several times with similar results. However, there were variations in the extent of binding and inhibition, perhaps due to different degrees of bacillus aggregation.
FIG. 1.
FACScan analysis to demonstrate plasminogen receptors on M. tuberculosis H37Rv. (a) Control bacilli incubated without plasminogen; (b) bacilli incubated at 4°C with 5 μg of plasminogen labeled with FITC; (c and d) bacilli incubated at 4°C with 5 μg of FITC-plasminogen in the presence of 2 M (c) or 1 M (d) EACA. Results of a representative experiment are presented.
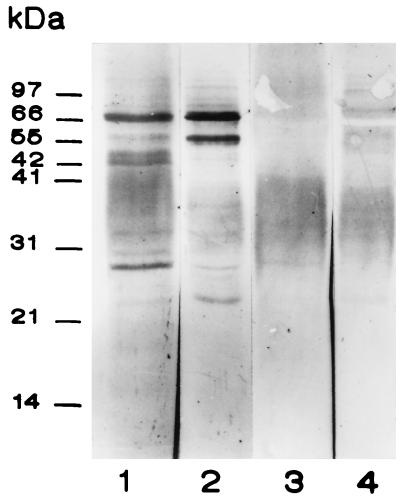
We tried next to identify M. tuberculosis plasminogen receptors by affinity blotting using soluble protein extracts (SEs) and cell wall proteins (CWPs) obtained by following the method of Hirschfield et al. (8). Bacilli were sonicated in an ice bath for 10 min and centrifuged at 27,000 × g for 20 min to obtain a supernatant representing an SE. The pellet representing the cell wall was treated with 2% sodium dodecyl sulfate for 2 h at 60°C to obtain the soluble CWPs. Ten micrograms of CWP or SE separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis under reducing conditions was transferred to Immobilon-P membranes (Millipore, Co., Bedford, Mass.) and incubated with human plasminogen (0.5 μg/ml; Boehringer) in PBS containing 0.3% Tween 20 and 1.5% BSA. After 1 h of incubation membranes were rinsed and incubated for 1 h with a rabbit anti-human plasminogen polyclonal antibody (Zymed Laboratories, Inc.) diluted 1/1,000 in PBS-Tween 20-BSA. Thereafter, the membranes were incubated 30 min with protein A labeled with peroxidase (Sigma) which was developed with 3,3-diaminobenzidine and hydrogen peroxide. This assay, repeated five times, allowed the demonstration of four plasminogen binding bands of 66, 60, 55, and 30 kDa (Fig. 2). The bands of 66 and 60 kDa were more prominent in the SE (lane 2). Control strips in which the anti-human plasminogen antibody was omitted showed no band reactivity (not shown). Incubation of membranes with plasminogen in the presence of 1 M EACA, abolished the binding of plasminogen to the mycobacterial proteins (Fig. 2, lanes 3 and 4). These results are in keeping with observations done in studies of mammalian and bacterial plasminogen receptors. It has been shown that the lysine binding site of plasminogen preferentially recognizes carboxy-terminal lysine residues; this binding can be blocked both by lysine analogues such as EACA and by treatment with carboxipeptidase B (7, 19). To date, only a few plasminogen receptor molecules of bacteria have been characterized. Examples are the glyceraldehyde-3-phosphate dehydrogenase of group A streptococci (25) and the cell surface lipoprotein of B. burgdorferi (3). Moreover, site-directed mutagenesis of the streptococus plasminogen receptor confirmed that C-terminal lysyl residues are required for binding (26). In this regard it is worth pointing out that the M. tuberculosis genome database (2) documents the existence of an α-enolase (Rv1023) and a glyceraldehyde-3-phosphate dehydrogenase (Rv2982c) with C-terminal lysyl residues.
FIG. 2.
Binding of human plasminogen to M. tuberculosis soluble CWP and SE by affinity blotting. Lanes 1 and 2, CWP and SE, respectively; lanes 3 and 4, CWP and SE incubated with plasminogen in the presence of 1 M EACA. Positions of molecular mass markers are shown on the left.
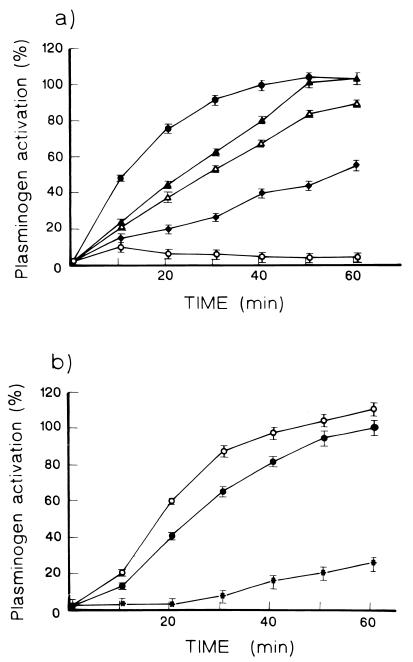
Finally, we examined the capacity of M. tuberculosis to activate plasminogen in an assay using fibrin matrices (6) and a chromogenic plasmin substrate (11). Fibrin matrix assays were done as follows. Tissue culture plates (96-well; Costar, Broadway/Cambridge, Mass.) were blocked overnight with 0.2% BSA in 50 mM sodium carbonate (pH 9.4). Wells were rinsed with PBS containing 0.05% Tween 20–0.1% BSA. Then, 100 μl of fibrinogen (1 mg/ml; Sigma) was converted to fibrin with 25 μl (3 U/ml) of human thrombin (Sigma). The fibrin matrices were dried at 37°C for 16 h. For these experiments whole sonicated suspension (WSS) and soluble proteins were used. WSS is made up of bacilli broken by sonication and the released soluble proteins. SE contained only soluble proteins released by sonication and separated by centrifugation. Five micrograms of WSS or SE, 3 μl of plasminogen (7 U/ml), and 50 μl (1.5 μM) of Chromozym (Boehringer) plasmin substrate were placed in each well. As a positive control, 5 μl of streptokinase (25 IU/ml) was placed in some wells instead of mycobacterial extracts. Wells were incubated at 37°C in a humid chamber, and absorbance was read at 405 nm every 10 min for 1 h. Results are shown in Fig. 3. Both SE and WSS could activate plasminogen only when the assay was carried out in wells covered with fibrin matrices (Fig. 3a). In wells devoid of such matrices, mycobacterial extracts were inactive. These findings suggest that plasminogen activation by M. tuberculosis requires attachment of plasminogen to a surface, in this case to the fibrin matrix. In this regard, it is worth mentioning that activation of plasminogen by tissue type plasminogen activator proceeds poorly in solution but is markedly enhanced by immobilization of plasminogen on fibrin (9, 10, 12). In this work we have not identified a plasminogen activator molecule, but we have shown that it is a heat-labile component whose activity is markedly reduced by boiling (Fig. 3a). It is also worth mentioning that recent data document the existence of proteases among M. tuberculosis molecules with the potential to behave as plasminogen activators (2, 21).
FIG. 3.
(a) Activation of plasminogen by M. tuberculosis SE (▴) and WSS (▵) in fibrin matrices and by fibrin in the absence of mycobacterial extracts (⧫). Also shown are assays with WSS carried out in wells devoid of fibrin matrices (○). Results are expressed as the percent of the maximal activation induced by streptokinase (●). (b) Assays carried out in wells covered with fibrin matrices with WSS (○), with WSS plus α2-antiplasmin (●), and with WSS after boiling (∗). Results are expressed as the percent of activation induced by WSS. Results of a representative experiment are presented. Experiments (n = 3) were carried out in triplicate, and the data are presented as means ± standard deviations (error bars).
We observed that the addition of α2-antiplasmin (3 μl; 0.5 mg/ml) did not significantly decrease activation of plasminogen by mycobacterial extracts (Fig. 3b), in keeping with studies showing that pathogen-associated plasmin activity is not blocked efficiently by host serpins (1). It has been shown that cell-bound plasmin is protected from inactivation because the plasminogen binding site is identical or in close proximity to the site targeted by α2-antiplasmin (8, 18).
Our results show that M. tuberculosis possesses plasminogen binding and activating molecules which can be solubilized by sonication and are present in the SE. It remains to be studied if binding and activation of plasminogen reside in the same molecules as those seen in group A streptococci (24). These findings could have a bearing on the pathogenesis of tuberculosis. In most individuals tuberculosis remains localized to the lungs. However, in some individuals, particularly in those with debilitating conditions or immunodeficiencies, including AIDS, the tubercle bacillus may invade blood vessels to disseminate systematically. This dissemination process could be facilitated by the interaction of M. tuberculosis with the plasminogen system herein described. Host-acquired plasmin activity might endow bacilli with the ability to break down fibrin meshes resulting from inflammatory processes and blood vessel basement membranes, thus facilitating host invasion and systemic dissemination.
Acknowledgments
This work was partially supported by Dirección General del Personal Académico grant IN-207195 from the Universidad Nacional Autónoma de México.
We thank Rafael Cervantes and Armida Baez for technical assistance. Isabel Pérez Montfort corrected of the English version of the manuscript.
REFERENCES
- 1.Boyle M D P, Lottenberg R. Plasminogen activation by invasive pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 2.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 3.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collen D, Schlott B, Engelborghs Y, Van Hoef M, Hartman H, Lijnen R, Behnke D. On the mechanism of activation of human plasminogen by recombinant staphylokinase. J Biol Chem. 1993;268:8284–8289. [PubMed] [Google Scholar]
- 5.Dannenberg A M, Rock G A W. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication. In: Bloom B R, editor. Tuberculosis, pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 459–483. [Google Scholar]
- 6.de Vries C, Veerman H, Pannekoek H. Identification of the domains of tissue-type plasminogen activator involved in the augmented binding to fibrin after limited digestion with plasmin. J Biol Chem. 1989;264:12604–12610. [PubMed] [Google Scholar]
- 7.Fuchs H R, Wallich M, Simon M, Kramer M D. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajjar K A. Cellular receptors in the regulation of plasmin generation. Thromb Haemost. 1995;74:294–301. [PubMed] [Google Scholar]
- 9.Hirschfield G R, McNeil M, Brennan P J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–1003. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kukkonen M, Saarela S, Lahteenmaki K, Hynonem U, Westerlund-Wikstrom B, Rhen M, Korhonen T. Identification of two laminin-binding fimbriae, the type 1 fimbria of Salmonella enterica serovar Typhimurium and the G fimbria of Escherichia coli, as plasminogen receptors. Infect Immun. 1998;66:4965–4970. doi: 10.1128/iai.66.10.4965-4970.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulisek E S, Holm S E, Johnston K H. A chromogenic assay for the detection of plasmin generated by plasminogen activator immobilized on nitrocellulose using a para-nitroanilide synthetic peptide substrate. Anal Biochem. 1989;15:78–84. doi: 10.1016/0003-2697(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 12.Kuusela P, Saksela O. Binding and activation of plasminogen at the surface of Staphylococcus aureus, increase in affinity after conversion to the Lys form of the ligand. Eur J Biochem. 1990;193:759–765. doi: 10.1111/j.1432-1033.1990.tb19397.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuusela P, Ullberg M, Saksela O, Kronvall G. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect Immun. 1992;60:196–201. doi: 10.1128/iai.60.1.196-201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahteenmaki K, Virkola R, Saren A, Emody L, Korhonen T. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to mammalian extracellular matrix. Infect Immun. 1998;66:5755–5762. doi: 10.1128/iai.66.12.5755-5762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahteenmaki K, Westerlund B, Kuusela P, Korhonen T. Immobilization of plasminogen on Escherichia coli flagella. FEMS Microbiol Lett. 1993;106:309–314. doi: 10.1111/j.1574-6968.1993.tb05981.x. [DOI] [PubMed] [Google Scholar]
- 16.Leytus S P, Bowles L K, Konisky J, Mangel W F. Activation of plasminogen to plasmin by a protease associated with the outer membrane of Escherichia coli. Proc Natl Acad Sci USA. 1981;78:14851–14859. doi: 10.1073/pnas.78.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lottenberg R, Broder C C, Boyle M D P. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987;55:1914–1928. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lottenberg R, Minning-Wenz D, Boyle M D. Capturing host plasmin(ogen): a common mechanism for invasive pathogens? Trends Microbiol. 1994;2:20–24. doi: 10.1016/0966-842x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 19.Miles L A, Dahlberg C M, Plescia J, Felez J, Kato K, Plow E F. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991;12:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 20.Plow E F, Herren T, Redlitz A, Miles L A, Hoover-Plot J L. The cell biology of plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 21.Skeiky Y A, Lodes M J, Guderian J A, Mohamath R, Bement T, Alderson M R, Reed S G. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun. 1999;67:3998–4007. doi: 10.1128/iai.67.8.3998-4007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullberg M, Kronvall G, Karlsson I, Wiman B. Receptors for human plasminogen on gram-negative bacteria. Infect Immun. 1990;58:21–25. doi: 10.1128/iai.58.1.21-25.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullberg M, Kuusela P, Kristiansen B E, Kronvall G. Binding of plasminogen to Neisseria meningitidis and Neisseria gonorrhoeae and formation of surface-associated plasmin. J Infect Dis. 1992;166:1329–1334. doi: 10.1093/infdis/166.6.1329. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Lottenberg R, Boyle M D. Analysis of the interaction group A streptococci with fibrinogen, streptokinase and plasminogen. Microb Pathog. 1995;18:153–156. doi: 10.1016/s0882-4010(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 25.Winram S B, Lottenberg R. The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology. 1996;142:2311–2320. doi: 10.1099/13500872-142-8-2311. [DOI] [PubMed] [Google Scholar]
- 26.Winram S B, Lottenberg R. Site-directed mutagenesis of streptococcal plasmin receptor protein (Plr) identifies the C-terminal Lys334 as essential for plasmin binding, but mutation of the plr gene does not reduce plasmin binding to group A streptococci. Microbiology. 1998;144:2025–2035. doi: 10.1099/00221287-144-8-2025. [DOI] [PubMed] [Google Scholar]