Abstract
Introduction
While several European studies have reported real-world apremilast use, patient-perceived benefits, and treatment satisfaction, local reimbursement criteria for apremilast vary and data from Italy are limited.
Methods
The cross-sectional DARWIN study enrolled consecutive patients who had initiated apremilast for plaque psoriasis 6 (± 1) months prior to enrolment at a single visit across 24 Italian dermatological sites. Disease severity was assessed using body surface area (BSA) and Physician Global Assessment (PGA). Patient-reported outcomes assessed 6 (± 1) months after apremilast initiation were Dermatology Life Quality Index (DLQI), Patient Benefit Index (PBI), and 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9).
Results
Of 184 patients enrolled between July 2019 and January 2021, 180 were included in the analysis. At apremilast initiation, median (25th–75th percentile) time since psoriasis diagnosis was 8.6 (3.2–22.2) years; median BSA, 10.0% (5.0–16.0); mean (standard seviation, SD) DLQI total score, 13.5 (8.0). Over half (54.9%) of patients with available data reported psoriasis had a very or extremely large effect on their quality of life (QoL); half reported itching (50.6%) and/or special areas involvement (50.0%). Most (73.9%) had comorbidities and were biologic-naïve (81.5%). The most common reasons for initiating apremilast were lack of efficacy of previous treatment (56.7%) and contraindications to other treatments (44.4%). At 6 (± 1) months, most patients were continuing apremilast and/or reported a Global PBI score ≥ 1 (minimum clinical benefit) (86.1% and 90.0%, respectively); approximately half achieved BSA ≤ 3% and/or DLQI total score ≤ 5 (47.1% and 48.5%); 18.8% achieved PGA = 0; mean (SD) TSQM-9 global treatment satisfaction score was 59.0 (24.8). Apremilast was well tolerated; no new safety signals were identified.
Conclusions
Patients treated with apremilast for 6 months in Italian clinical practice reported improved QoL, clinically relevant improvements in symptoms, high treatment satisfaction, and high treatment persistence. Our data indicate apremilast is a valuable treatment option for moderate plaque psoriasis.
Study Registration
ClinicalTrials.gov identifier, NCT04031027.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02516-y.
Keywords: Apremilast, Dermatology Life Quality Index, Italian real-world evidence, Patient Benefit Index, Patient perspective, Patient-reported outcomes, Plaque psoriasis, Psoriasis comorbidities, Quality of life, Treatment satisfaction
Key Summary Points
| Why carry out this study? |
| While several European studies have reported real-world apremilast use, patient-perceived benefits, and treatment satisfaction, apremilast use varies by local reimbursement criteria and data from Italy are limited. |
| What was learned from this study? |
| In Italian clinical practice, apremilast is typically used in patients with moderate psoriasis, characterized by limited skin involvement and relatively short disease duration, but with a large impact on quality of life. |
| Patients treated with apremilast for 6 months reported improved quality of life, clinically relevant improvements in symptoms, and high treatment satisfaction, with high treatment persistence. |
| Our data add to the growing body of real-world evidence showing apremilast is a valuable, well-tolerated treatment option for moderate plaque psoriasis. |
Introduction
Psoriasis is a chronic inflammatory skin disease with a strong genetic predisposition and an autoimmune pathogenetic component [1, 2]. The prevalence of psoriasis in Italy is reported to range from 2% to 3%, similar to global prevalence, with plaque psoriasis the most common form [3, 4]. Psoriatic skin lesions can be severely itchy and affect visible and particularly bothersome body areas such as the face, scalp, hands and nails, and genitals. Moreover, psoriasis is associated with significant comorbidities, including arthritis, cardiovascular diseases, metabolic syndrome, and psychiatric disorders such as anxiety and depression [5, 6]. Psoriasis is known to negatively impact patients’ quality of life (QoL), which is not necessarily related to the extent of skin involvement [7, 8]. The recent UPLIFT (Understanding Psoriatic Disease Leveraging Insights for Treatment) survey reported that patients with psoriasis perceive a high disease burden, including those with limited skin involvement, persistent unmet needs, a higher severity, and a lower treatment satisfaction compared with their treating physicians [9]. Long-term treatment is required to address the skin lesions, the frequent comorbidities, and also the disease burden and impaired patient QoL [10, 11]. Current treatment recommendations are based both on physician-assessed severity measures, such as body surface area (BSA), Psoriasis Area and Severity Index (PASI), and Physician Global Assessment (PGA), and patient-reported outcomes (PROs) including QoL and treatment satisfaction indexes [12, 13].
In Europe, apremilast is approved for the treatment of moderate-to-severe chronic plaque psoriasis, and active psoriatic arthritis. Placebo-controlled randomized clinical trials demonstrated the efficacy and tolerability of apremilast in patients with moderate-to-severe plaque psoriasis previously treated with or naïve to biologics [14–16]. The retrospective, cross-sectional APPRECIATE study has reported the characteristics of patients with psoriasis treated with apremilast in routine clinical practice across nine European countries, and treatment needs and outcomes from the perspectives of both physicians and patients [17, 18]. APPRECIATE used validated disease severity scores, including PASI and BSA, and patient-reported outcomes such as the Dermatology Life Quality Index (DLQI), the 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9), and the Patient Benefit Index (PBI). The PBI assesses the patient-perceived benefits of a treatment against the patients’ individual treatment goals [19, 20]. Patients enrolled in APPRECIATE had a high baseline disease burden, and clinically relevant improvements in both disease severity and QoL 6 (± 1) months after apremilast initiation. In a subgroup analysis of APPRECIATE, the patient-perceived benefits of apremilast were higher in patients who initiated apremilast earlier versus later in the course of their disease [21]. In addition, a strong correlation was observed between the global PBI score and the TSQM-9 global and subscale scores. More recently, the APRAISAL study evaluated real-world apremilast use in a cohort of 287 biologic-naïve patients with moderate psoriasis in Greece; 68% of patients achieved a 75% reduction in their PASI score (PASI 75) and clinically relevant improvements in the severity of itching and scalp and palmoplantar involvement were observed [22]. The OTELO study assessed the real-world use and effectiveness of apremilast in patients with moderate-to-severe plaque psoriasis from the patient and physician perspectives, reporting that apremilast improved PROs, including QoL and psoriasis severity, and fulfilled patients’ expectations [23].
In Italy, apremilast is indicated for patients with moderate-to-severe chronic plaque psoriasis who fail to respond to, or are contraindicated or intolerant to other systemic therapy. Furthermore, reimbursement is restricted to patients for whom biologics are contraindicated or not tolerated. At the time our study was designed, real-world data on apremilast use for psoriasis in Italy were lacking. The DARWIN (Description of Apremilast Real-World Italian Psoriasis Network) study was designed to describe the characteristics of patients with plaque psoriasis treated with apremilast in Italian clinical practice, treatment effectiveness, and patient-perceived benefits.
Methods
Study Design and Population
The observational, retrospective, cross-sectional DARWIN study enrolled consecutive patients at a single visit between July 2019 and January 2021 across 24 Italian dermatological study sites.
Eligible patients had moderate-severe psoriasis and had initiated apremilast as part of normal clinical practice at least 6 (± 1) months prior to the enrolment visit, with hospital records available from the date of apremilast initiation. Patients were enrolled during a routine clinic visit as per ordinary clinical practice, and regardless of whether they were continuing apremilast treatment. Patients who were participating in an interventional clinical trial, who had started apremilast as part of a clinical trial, or who had received previous apremilast treatment were excluded. Patients provided written informed consent before study participation. All data were collected from medical records and PRO instruments, at the enrolment visit.
Compliance with Ethics Guidelines
This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. All participating patients received verbal and written information about the study and were given the opportunity to ask any questions to help them understand it. They provided voluntary informed consent prior to any data collection.
The DARWIN study protocol was approved by the ethics committees of all participating sites as per local requirements. Further information can be found in the Table S1 in the supplementary material. DARWIN was registered in ClinicalTrials.gov (NCT04031027).
Study Objectives and Outcome Measures
The primary objective was to describe the characteristics of patients treated with apremilast for plaque psoriasis in Italian clinical practice. Secondary objectives were to describe (i) apremilast treatment patterns in Italian clinical practice; (ii) psoriasis severity before and 6 (± 1) months after apremilast initiation; (iii) QoL before and 6 (± 1) months after apremilast initiation; (iv) apremilast tolerability; (v) patients’ satisfaction with apremilast treatment at 6 (± 1) months; (vi) patients’ perception of treatment benefits 6 (± 1) months after initiating apremilast.
Physicians assessed skin involvement and disease severity using PASI, BSA, and PGA. PASI combines the intensity of psoriatic lesions with the affected body surface area on a scale of 0–72 (larger values indicate more severe disease) and was taken retrospectively from medical records at apremilast initiation [24]. BSA measures the body surface area affected by psoriatic lesions and ranges from 0 to 100%; we assessed the percentages of patients achieving a BSA ≤ 3% or ≤ 1% at 6 (± 1) months after apremilast initiation. For PGA, several different scales are used in Italian clinical practice: a 5-point scale (0–4), a 6-point scale (0–5), and a 7-point PGA scale (0–6) [25]. All three PGA scales were permitted in our study, and the specific scale used recorded. The percentages of patients achieving a PGA = 0 (clear skin) and PGA = 1 (almost clear skin) at 6 (± 1) months from apremilast initiation were reported.
The self-administered DLQI is the most frequently used method of evaluating QoL in adult patients with different skin conditions [26]. It consists of a 10-item Likert scale assessing six areas of the patient’s life in the previous 7 days: symptoms and feelings (items 1 and 2), daily activities (items 3 and 4), leisure (items 5 and 6), work and school (item 7), personal relationships (items 8 and 9), and treatment (item 10). Each item is scored from 0 (“not at all affected”) to 3 (“very much affected”), to give a total score ranging from 0 to 30. Higher scores indicate a larger impact on the patient’s QoL.
The TSQM-9 consists of nine items across three different domains—effectiveness, convenience, and global satisfaction—each including three items and scored from 0 to 100, with higher scores indicating higher satisfaction.
The PBI assesses treatment benefits from the patients’ perspective [19, 20] and consists of two parts: the Patient Needs Questionnaire (PNQ), which assesses the relevance of 25 items to an individual patient’s treatment goals, and the Patient Benefit Questionnaire (PBQ), which measures the extent to which the treatment under analysis meets the patient’s treatment goals.
Sample Size
No formal sample size calculations were performed. According to feasibility considerations, a sample size of 375 patients was chosen.
Statistical Analysis
No formal statistical hypotheses were tested. All analyses were descriptive and excluded enrolled patients who did not meet the study inclusion criteria. Continuous variables were reported as mean (standard deviation, SD) and median (IQR, interquartile range, i.e., 25th–75th percentile); categorical variables were reported as the number and percentage in each category.
PRO measurement instruments were used in agreement with the terms and conditions of each respective license obtained prior to start the data collection. Instruments scores were calculated according to the instructions obtained from copyright holders.
Subgroups analyses were performed on the basis of (i) involvement of difficult-to-treat areas at apremilast initiation, (ii) treatment with biologic therapies before apremilast initiation, and (iii) apremilast treatment status at enrolment.
Statistical analyses were performed using SAS Enterprise Guide v. 7.1 and SAS 9.4 (SAS Institute, Cary, NC, USA). Study design, electronic case report form (eCRF) setup, site management, data management, and statistical analyses were performed by MediNeos S.U.R.L. (Modena, Italy), a company subject to the direction and coordination of IQVIA Ltd, on behalf of Amgen.
Results
Demographic and Clinical Characteristics
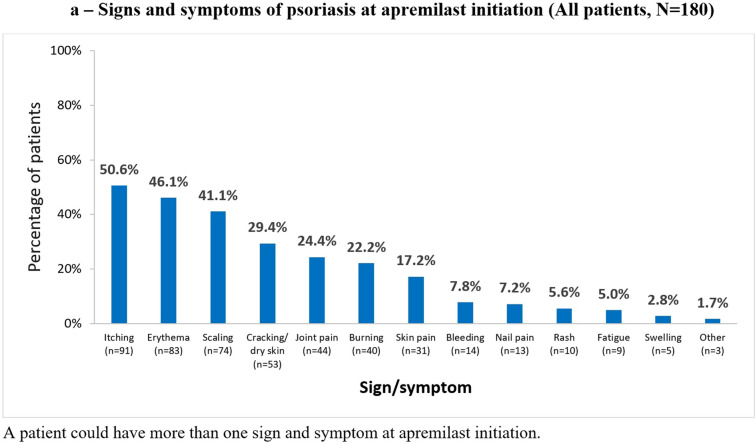
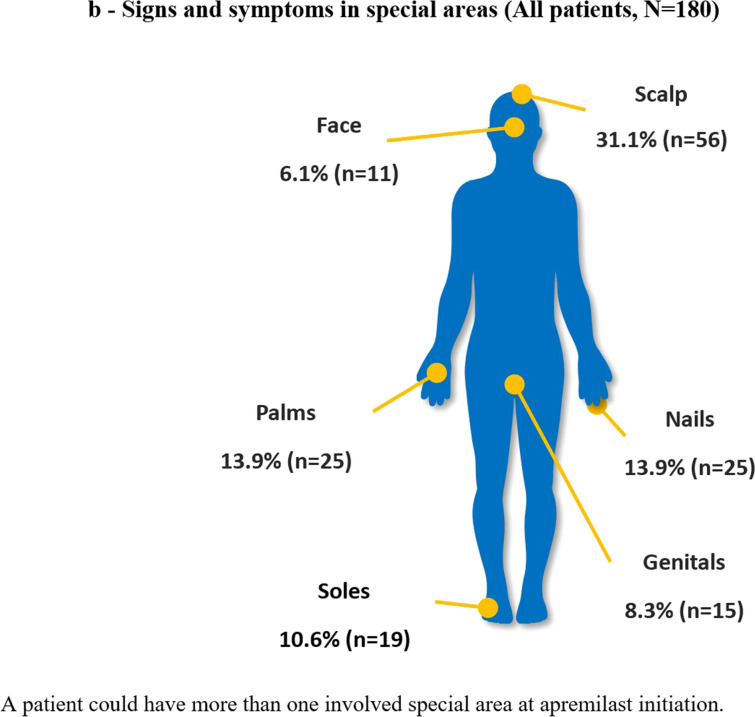
Of 184 patients enrolled, 180 (97.8%) were included in the analysis because four patients did not meet all eligibility criteria and were excluded. Table 1 and Fig. 1 summarize patient demographics and clinical characteristics at apremilast initiation. Overall, 70% (126/180) of patients had signs and symptoms of psoriasis, most commonly itching (91/180 [50.6%]) (Fig. 1a). Half (90/180 [50.0%]) had psoriasis localized in special areas, most commonly the scalp (31.1%), and approximately one-quarter (43/180 [23.9%]) had more than one special area involved (Fig. 1b). Approximately three-quarters of patients (73.9%) had comorbidities, one-third (60/180 [33.3%]) had psoriatic arthritis (Table 1).
Table 1.
Patient demographics and clinical characteristics at apremilast initiation (all patients, N = 180)
| Characteristic | Value |
|---|---|
| Gender (N = 180), n (%) | |
| Male | 103 (57.2) |
| Female | 77 (42.8) |
| Age (years) (N = 180), mean (SD) | 59.1 (12.4) |
| BMI (kg/m2) (N = 176), mean (SD) |
176 27.1 (5.1) |
| Time interval from psoriasis diagnosis to apremilast initiation (years) (N = 180), median (IQR) | 8.6 (3.2–22.2) |
| Presence of comorbidities*(N = 180), n (%) | |
| Any | 133 (73.9) |
| Hypertension | 65 (36.1) |
| Psoriatic arthritis | 60 (33.3) |
| Metabolic diseases | 59 (32.8) |
| Chronic infections | 31 (17.2) |
| Malignancies | 26 (14.4) |
N number of patients with non-missing data, n number of patients with characteristic of interest, SD standard deviation, IQR interquartile range, BMI body mass index
*Comorbidities present in ≥ 10% of patients are listed. Metabolic diseases include diabetes, hypercholesterolemia/dyslipidemia, non-alcoholic fatty liver disease (NAFLD), and obesity
Fig. 1.
Signs and symptoms of a psoriasis at apremilast initiation and b in special areas (all patients, N = 180)
Treatment Patterns
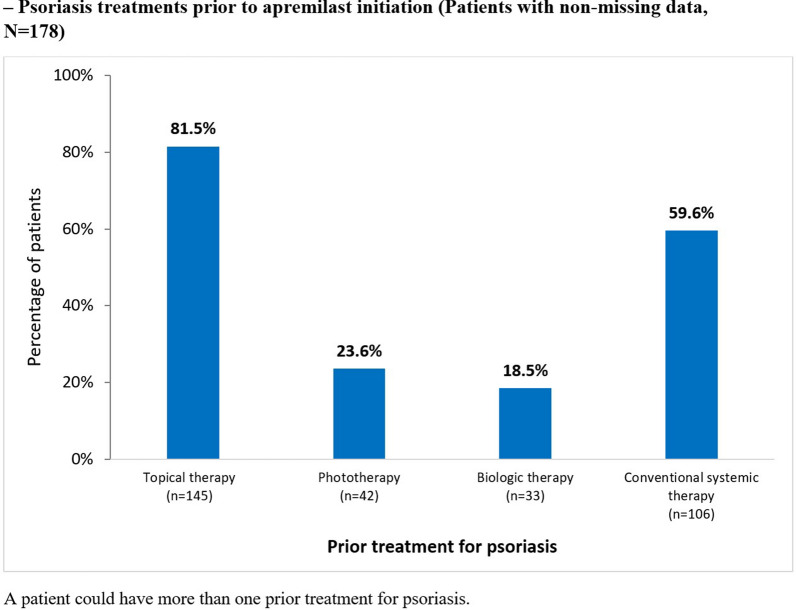
Most (169/178 [94.9%]) patients with available data had received one or more psoriasis treatments before initiating apremilast, most commonly topical therapies (145/178 [81.5%] (Fig. 2). Seventy-seven (43.3%) patients had received one biologic or conventional systemic therapy before apremilast initiation, 36 (20.2%) had received two or more. Most patients (145/178 [81.5%]) were biologic-naïve at apremilast initiation.
Fig. 2.
Psoriasis treatments prior to apremilast initiation (patients with non-missing data, N = 178)
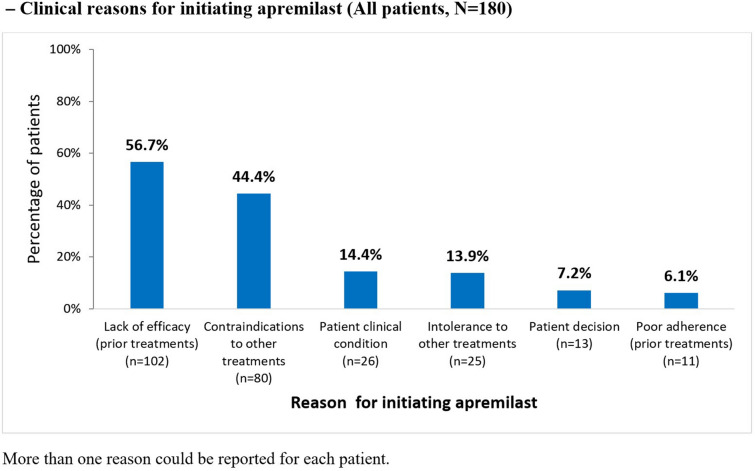
Lack of efficacy of the previous psoriasis treatment and contraindications to other treatments were the most common reasons for initiating apremilast, reported by 56.7% and 44.4% of patients, respectively (Fig. 3). Among patients with available data, 44.4% (79/178) received concomitant medications with apremilast, most commonly topical therapies (73/178 [41.0%]).
Fig. 3.
Clinical reasons for initiating apremilast (all patients, N = 180)
Most patients (155/180 [86.1%]) were continuing apremilast treatment at 6 (± 1) months; median (IQR) duration of apremilast treatment was 5.8 (5.1–6.6) months. Twenty-five (13.9%) patients had discontinued apremilast. Reasons for discontinuation were adverse events (12 [6.7%] patients), lack of efficacy (10 [5.6%]), patient’s decision (8 [4.4%]), and lack of compliance to treatment (2 [1.1%]). Most (16/25 [54.0%]) patients who had discontinued apremilast received one or more other psoriasis treatments, including conventional systemic therapy (n = 8), biological therapy (n = 7), and topical therapy (n = 2).
Clinical Outcomes
BSA and PGA are summarized in Table 2. At 6 (± 1) months, approximately one-quarter (41/157 [26.1%]) of patients with available data achieved a BSA ≤ 1% and almost half (74/157 [47.1%]) achieved a BSA ≤ 3%. Regardless of the PGA scale used, 18.8% (25/133) of patients with available data achieved a PGA score of 0 (clear skin) and 40.6% (54/133) achieved a PGA score of 1.
Table 2.
Disease severity scores at apremilast treatment initiation and 6 (± 1) months (patients with non-missing data)
| At apremilast initiation | At 6 (± 1) months | |||
|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | |
| BSA (%) | 137 | 10.0 (5.0–16.0) | 157 | 4.0 (1.0–6.0) |
| PGA (0–4 scale) | 66 | 3.0 (3.0–3.0) | 85 | 1.0 (0.0–2.0) |
| PGA (0–5 scale) | 44 | 3.0 (3.0–4.0) | 47 | 2.0 (1.0–2.0) |
| PGA (0–6 scale) | 4 | 5.0 (5.0–5.5) | 1 | 1.0 |
| PASI | 166 | 11.0 (8.0–16.0) | NA | NA |
N number of patients with non-missing data, NA not available (not collected according to the study protocol), IQR interquartile range, BSA body surface area, PASI Psoriasis Area and Severity Index, PGA Physician Global Assessment
Patient-Reported Data
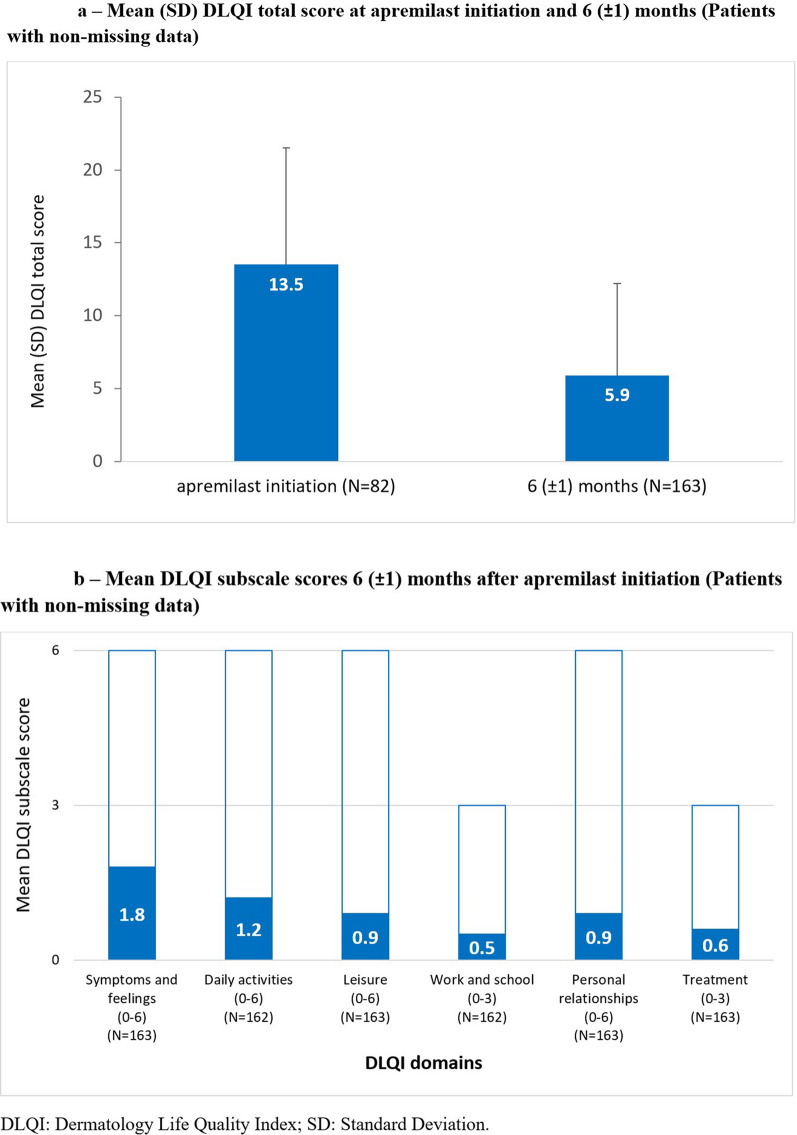
DLQI scores are summarized in Fig. 4. While fewer than half (45.6%) of all patients had DLQI scores recorded at apremilast initiation, most (90.5%) had DLQI scores recorded at 6 (± 1) months. At apremilast initiation, mean (SD) DLQI total score was 13.5 (8.0) and over half (45/82 [54.9%]) of patients with available data reported their disease had a very or extremely large impact on their QoL (total score > 10). At 6 (± 1) months, mean (SD) DLQI total score was 5.9 (6.3) and approximately half of all patients (79/163 [48.5%]) with available data reported a DLQI total score ≤ 5. DLQI subscores at 6 (± 1) months are summarized in Fig. 4b.
Fig. 4.
Mean (SD) DLQI a total score and b subscale scores 6 (± 1) months after apremilast initiation (patients with non-missing data)
Half of all patients reported itching and involvement of special areas at apremilast initiation (91/180 [50.6%] and 90/180 [50.0%], respectively), compared with 29.4% (53/180) and 24.4% (44/180) of patients at 6 (± 1) months.
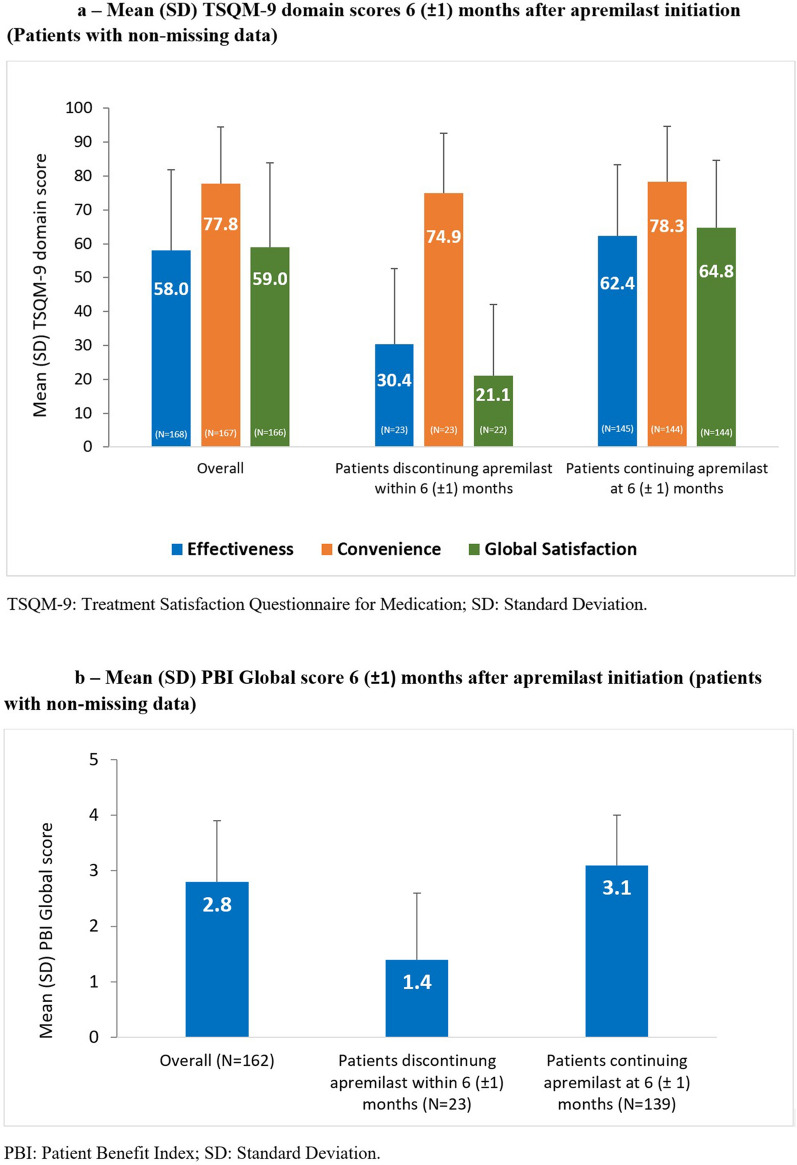
TSQM-9 scores are summarized in Fig. 5a. Most (168/180 [93.3%]) patients completed the TSQM-9 at 6 (± 1) months. Mean (SD) score was highest for the convenience domain: 77.8 (16.6; n = 167) compared with 58.0 (23.8; n = 168) and 59.0 (24.8; n = 166) for the effectiveness and global satisfaction domains, respectively. For all domains, mean (SD) scores were higher for patients continuing apremilast at 6 (± 1) months than patients who had discontinued apremilast: global satisfaction, 64.8 (19.8; n = 144) and 21.1 (20.9; n = 22), respectively; effectiveness, 62.4 (21.0; n = 145) and 30.4 (22.2; n = 23); and convenience, 78.3 (16.4; n = 144) and 74.9 (17.8; n = 23).
Fig. 5.
Mean (SD) a TSQM-9 domain scores and b PBI Global score 6 (± 1) months after apremilast initiation (patients with non-missing data)
PBI Global scores are summarized in Fig. 5b. Most (162/180 [90.0%]) patients completed the PBI at 6 (± 1) months; mean (SD) score, 2.8 (1.1). Most (146/162 [90.0%]) patients with PBI data achieved a PBI Global score ≥ 1, which corresponds to a clinically meaningful benefit. Compared with patients discontinuing apremilast, mean (SD) PBI Global score was higher in patients continuing apremilast at 6 (± 1) months: 3.1 (0.9; n = 139) versus 1.4 (1.2; n = 23).
Safety
Twenty-seven patients reported a total of 42 adverse events (AEs). No serious AEs were reported. Twenty-two patients reported 35 adverse drug reactions (ADRs), the most common being diarrhea (8/180 [4.4%]), insomnia (6/180 [3.3%]), nausea (4/180 [2.2%], anxiety (3/180 [1.7%]), and gastrointestinal symptoms (3/180 [1.7%]). Two patients reported depression; arthralgia, asthenia, confusion, dyspepsia, flushing, headache, hyporexia, panic attack, and rhinitis were reported by one patient each. One more case of headache was reported and judged by clinicians as having no suspected causal relationship with apremilast.
Discussion
The DARWIN study assessed the characteristics of patients with plaque psoriasis treated with apremilast in Italian clinical practice, treatment effectiveness, and patient-perceived benefits. Apremilast was used in psoriasis of moderate severity, with 70% of patients having active signs and symptoms of psoriasis, mainly itching, and half having skin lesions in special areas. Considering the mean age (59.1 years) and relatively short disease duration (median time from diagnosis to apremilast initiation, 8.6 years) in our patient population, the prevalence of comorbidities was higher than reported in some other real-world studies of apremilast [19, 22, 27, 28]. It is well recognized that comorbidities are more common among patients with psoriasis than in the general population [5, 6] and the established favorable safety profile of apremilast may justify its use in patients with a greater comorbidity burden in clinical practice. As expected, hypertension was the most common comorbidity in our patient population [29], followed by psoriatic arthritis and metabolic diseases, which are commonly associated with psoriasis. Almost all patients had received other psoriasis treatment before apremilast; 60% had received conventional systemic medications and less than 20% had received biologics. Apremilast persistence was high, with most patients (86.1%) continuing apremilast treatment at 6 (± 1 months) (enrolment), confirming the high persistence reported in previous real-world studies [30–33]. For example, the APRAISAL Greek real-world study reported a drug survival rate of 85% at 1 year [22] and 5-year data from a Greek tertiary care center reported a cumulative survival probability of 52.1% at 52 weeks and median time to discontinuation 58 weeks (95% CI 40.02, 75.98) [34].
In general, disease severity in DARWIN was similar to that observed in the real-world APPRECIATE [17] and the APRAISAL studies [22]. However, patients enrolled in DARWIN had shorter disease duration than those enrolled in other real-world studies, such as APPRECIATE (median 15.0 [range 0–71] years) [21], and OTELO (mean 19.7 [SD 15.6] years) [23].
The BSA improvements observed in DARWIN are consistent with ESTEEM 1 and 2 [14, 15]. Nearly half of all patients in DARWIN achieved a BSA ≤ 3%, which defines mild psoriasis [35]. As defined by a PGA of 0 or 1, approximately 60% of patients achieved clear or almost clear skin, a considerably higher proportion than reported in the ESTEEM studies [14, 15]. At 6 (± 1) months, the proportion of patients with pruritus and/or involvement of special areas had approximately halved compared with apremilast initiation. This is consistent with the APRAISAL study [21].
Despite the moderate disease observed in DARWIN, over half of patients reported a DLQI total score ≥ 10, indicative of a very or extremely strong impact. It is widely reported that patient-perceived burden can be unrelated to the severity and extent of psoriasis [7, 8, 32]. Previous real-world studies with apremilast have reported similarly high disease burden among patients with moderate skin involvement and clinically relevant improvements following apremilast treatment [17, 18, 21, 23, 36, 37]. LAPIS-PSO, a real-world study focused on QoL, reported that patients with moderate-to-severe plaque psoriasis benefited from apremilast treatment up to approximately 13 months of observation [27]. Our data from Italian clinical practice underscore the need to increase and improve the use of PROs in assessing overall psoriasis severity. The involvement of bothersome and special skin areas and high prevalence of comorbidities likely contributed to the strong impact of psoriasis on QoL reported by the patients in our study. At 6 (± 1) months after apremilast initiation, nearly half of patients had a score ≤ 5, corresponding to low or no effect on their QoL.
Overall, patient perception and satisfaction with apremilast treatment were favorable and in line with the results of the UNVEIL and APPRECIATE studies [17, 38]. As measured by the TSQM-9 at 6 (± 1) months, patients in our study reported the highest level of satisfaction for apremilast convenience, and positive scores for efficacy and satisfaction. Unsurprisingly, patients continuing apremilast at 6 (± 1) months had higher scores across all TSQM-9 domains than those who had discontinued treatment. We used the PBI to measure how well apremilast treatment met individual patients’ expectations and specific treatment goals. Ninety percent of patients achieved a PBI ≥ 1, which is considered a clinically significant benefit [20, 21], thus strengthening the data on patient-reported treatment satisfaction.
Several other authors have reported Italian real-world data from patients with psoriasis, including patients with longer disease duration than those enrolled in DARWIN and elderly patents and those with cancer [39–42]. These cohorts further support the effectiveness and tolerability of apremilast, including improvements in PASI, BSA, and DLQI, and the high prevalence of comorbidities observed in our patient population.
We observed a low rate of ADRs, which were mostly mild in nature, and no SAEs, which is consistent with the favorable safety profile previously reported for apremilast [43]. While headache is among the most commonly reported ADR for apremilast [44], only two patients in our study reported headache.
The major strength of our study is the use of real-world data, which allowed us to identify typical patient profiles and treatment patterns in Italian clinical practice, outside the strict rules of RCTs. Another strength is our study focus on PROs, such as satisfaction with and perceived benefits of apremilast, which are increasingly recognized as relevant measures of the overall effectiveness of psoriasis treatments. Our study also has some limitations. As a result of the retrospective data collection, some data were not available at apremilast initiation. In addition, the study was conducted during the SARS-CoV-2 pandemic and lockdowns and cancelled clinic visits impacted our ability to enroll patients into the study. With consideration to the ongoing pandemic, study enrolment was stopped in January 2021 before the planned sample size of 375 patients was reached. Owing to the real-world setting, we limited outcome measures to those commonly used in Italian clinical practice, such as BSA and PGA. We did not collect PASI, which is widely used in RCTs but rarely measured in Italian routine clinical practice.
Conclusions
The DARWIN study indicates that, in Italian clinical practice, apremilast is used in patients with moderate psoriasis, characterized by limited skin involvement and relatively short disease duration, but with a strong impact on QoL. Most patients continued apremilast for at least 6 months and reported improved QoL, clinically relevant improvements in disease signs and symptoms, and high treatment satisfaction. No new safety signals were observed and, collectively, our data indicate that apremilast is a valuable treatment option for moderate-to-severe psoriasis with a good balance between efficacy and patient compliance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participating patients, physicians, and study personnel. Medineos S.U.R.L., a company subject to the direction and coordination of IQVIA Ltd, acted as the contract research organization, funded by Amgen.
List of investigators and collaborators
DARWIN study group includes the Investigators involved in the study: Paolo Dapavo (Turin); Paolo Gisondi (Verona); Aurora Parodi (Genoa); Luca Bianchi (Rome); Laura Atzori (Cagliari); Gabriella Fabbrocini (Naples); Concetta Potenza (Terracina); Rocco De Pasquale (Catania); Ketty Peris (Rome); Paolo Amerio (Chieti); Rossana Tiberio (former: Novara; actual: Ravenna); Marina Venturini (Brescia); Ada Lo Schiavo (Naples); Marco Romanelli (Pisa); Antonio Richetta (Rome); Francesco Cusano (Benevento); Maria Concetta Fargnoli (L'Aquila); Annamaria Offidani (Ancona); Claudio Guarneri (Messina); Francesca Prignano (Florence); Monica Corazza (Cona); Maria Rita Bongiorno (Palermo); Claudia Giofrè (Messina); Francesco Loconsole (Bari).
MediNeos—a company subject to the direction and coordination of IQVIA Ltd—contributed to the DARWIN study conduction, scientific support, clinical operations, data management and statistical analysis (Alessandra Ori, Lucia Simoni, Christian Amici, Luca Di Palma, Daniele Andreis, Francesca Trevisan, Saide Sala, Roberto Patanè, Fabiano Mele, Andrea Pernaci, Samantha Ruberti, Mattia Trogu, Sara Pelicelli, Sara Rizzoli).
Funding
This study was sponsored by Celgene; Amgen Inc. acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019. Amgen S.r.l. a socio unico, Italy was funding the journal’s Rapid Service Fee.
Medical Writing and/or Editorial Assistance
Renata Perego, MD, provided independent medical writing assistance, funded by Amgen. Claire Desborough, Amgen UK, provided editorial assistance.
Authors’ Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Material preparation, data collection and analysis were performed by Claudia Giofrè, Gabriella Fabbrocini, Concetta Potenza, Rossana Tiberio, Luca Bianchi, Claudio Marasca, and Paolo Gisondi. The first draft of the manuscript was defined by Emiliana Benincasa, and Carmen M. A. Nuzzo. All authors were involved in reviewing the draft of the manuscript, provided critical revisions for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work.
Prior Presentations
C. Giofrè, G. Fabbrocini, E. Benincasa, A. Castiglia, D. Guerra, C. Potenza, R. Tiberio, L. Bianchi. Uso di apremilast in pazienti con un elevato livello di comorbilità: risultati ad interim dello studio DARWIN (Description of Apremilast Real World Italian psoriasis Network). Poster presented to the 58° Congresso Nazionale ADOI (Associazione dermatologi- venerologi ospedalieri ITALIANI e della sanità pubblica), Catanzaro 15–18 September 2021. C. Giofrè, G. Fabbrocini, P. Gisondi, E. Benincasa, D. Guerra, C. Potenza, R. Tiberio, L. Bianchi. Apremilastin pazienti psoriasici naïve a farmaci biologici: risultati dallo studio italiano di real world DARWIN (Description of Apremilast Real-World Italian psoriasis Network). Abstract presented to the 59° Congresso Nazionale ADOI, Riccione 26–29 October 2022; published in Dermatology Reports—BOOK OF ABSTRACTS, 2022; vol 3 n. 2.
Disclosures
Luca Bianchi served on advisory boards and received honoraria for lectures and research grants from Almirall, AbbVie, Leo Pharma, Amgen, Biogen, UCB, Eli Lilly, Janssen, Novartis, and Sanofi Genzyme. Gabriella Fabbrocini acted as speaker or consultant for AbbVie, Amgen, Eli Lilly, Janssen, Leo Pharma, Almirall, Novartis, and UCB. Paolo Gisondi served as consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo-Pharma, Eli Lilly, Novartis, Pierre Fabre, Sandoz, Sanofi, UCB. Claudia Giofrè served as consultant for Janssen, Novartis, Leo-Pharma, Amgen, AbbVie, Sanofi and Lilly. Concetta Potenza has no conflict of interest to declare. Rossana Tiberio has no conflict of interest to declare. Claudio Marasca has no conflict of interest to declare. Emiliana Benincasa and Carmen M. A. Nuzzo are Amgen Inc. employees.
Compliance with Ethics Guidelines
This study was performed in accordance with the 1964 Helsinki Declaration and its later amendments. All participating patients received verbal and written information about the study and were given the opportunity to ask any questions to help them understand it. They provided voluntary informed consent prior to any data collection. The DARWIN study protocol was approved by the ethics committees of all participating sites. A national central ethics committee or a master ethics committee were not applicable for this study, as per local requirements. DARWIN was registered in ClinicalTrials.gov (NCT04031027).
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
Footnotes
The members of the DARWIN study group are listed in Acknowledgments.
Prof. Fabbrocini prematurely passed away after the completion of this manuscript.
Contributor Information
Claudia Giofrè, Email: claudiagiofre@tiscali.it.
DARWIN study group:
Paolo Dapavo, Aurora Parodi, Laura Atzori, Rocco Pasquale, Ketty Peris, Paolo Amerio, Marina Venturini, Ada Lo Schiavo, Marco Romanelli, Antonio Richetta, Francesco Cusano, Maria Concetta Fargnoli, Annamaria Offidani, Claudio Guarneri, Francesca Prignano, Monica Corazza, Maria Rita Bongiorno, Francesco Loconsole, Alessandra Ori, Lucia Simoni, Christian Amici, Luca Di Palma, Daniele Andreis, Francesca Trevisan, Saide Sala, Roberto Patanè, Fabiano Mele, Andrea Pernaci, Samantha Ruberti, Mattia Trogu, Sara Pelicelli, and Sara Rizzoli
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 2.Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93–122. doi: 10.1146/annurev.genom.6.080604.162324. [DOI] [PubMed] [Google Scholar]
- 3.Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- 4.Prignano F, Rogai V, Cavallucci E, et al. Epidemiology of psoriasis and psoriatic arthritis in Italy—a systematic review. Curr Rheumatol Rep. 2018;20(7):43. doi: 10.1007/s11926-018-0753-1. [DOI] [PubMed] [Google Scholar]
- 5.Kimball AB, Gladman D, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58:1031–1042. doi: 10.1016/j.jaad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78:323–332. doi: 10.1016/j.jaad.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS ONE. 2012;7:e52935. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt J, Ford DE. Understanding the relationship between objective disease severity, psoriatic symptoms, illness-related stress, health-related quality of life and depressive symptoms in patients with psoriasis—a structural equations modeling approach. General Hosp Psychiatry. 2007;29(2):134–140. doi: 10.1016/j.genhosppsych.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey. Dermatol Ther (Heidelb) 2022;12(1):61–78. doi: 10.1007/s13555-021-00635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Am Acad Dermatol. 2014;70(5):871–881. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Iskandar IYK. Disease burden and quality of life in patients with moderate-to-severe psoriasis stratified according to previous systemic treatment exposure. J Eur Acad Dermatol Venereol. 2020;34:2454–2455. doi: 10.1111/jdv.16971. [DOI] [PubMed] [Google Scholar]
- 12.Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi: 10.1016/j.jaad.2020.07.087. [DOI] [PubMed] [Google Scholar]
- 13.Secrest AM, Chren MM. Incorporating patient-reported outcomes as a vital sign for dermatologic clinical care and clinical investigations. J Invest Dermatol. 2022 doi: 10.1016/j.jid.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]) J Am Acad Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 16.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31:507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2021;35(1):123–134. doi: 10.1111/jdv.16431. [DOI] [PubMed] [Google Scholar]
- 18.Cetkovská P, Dediol I, Šola M, et al. Apremilast use in severe psoriasis: real-world data from Central and Eastern Europe. Adv Ther. 2023;2:1–16. doi: 10.1007/s12325-023-02468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustin M, Radtke MA, Zschocke I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301(8):561–571. doi: 10.1007/s00403-009-0928-8. [DOI] [PubMed] [Google Scholar]
- 20.Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304(6):433–441. doi: 10.1007/s00403-012-1256-y. [DOI] [PubMed] [Google Scholar]
- 21.Klein TM, Blome C, Kleyn CE, et al. Real-world experience of patient-relevant benefits and treatment satisfaction with apremilast in patients with psoriasis: an analysis of the APPRECIATE study. Dermatol Ther (Heidelb) 2022;12(1):81–95. doi: 10.1007/s13555-021-00628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannides D, Antonakopoulos N, Chasapi V, et al. A real-world, non-interventional, prospective study of the effectiveness and safety of apremilast in bio-naïve adults with moderate plaque psoriasis treated in the routine care in Greece—the 'APRAISAL' study. J Eur Acad Dermatol Venereol. 2022;36(11):2055–2063. doi: 10.1111/jdv.18166. [DOI] [PubMed] [Google Scholar]
- 23.Ghislain PD, Lambert J, Hoai XL, et al. Real-life effectiveness of apremilast for the treatment of psoriasis in Belgium: results from the observational OTELO study. Adv Ther. 2022;39(2):1068–1080. doi: 10.1007/s12325-021-01981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 25.Duffin K. Outcome measures in psoriasis and atopic eczema. In: Yamauchi PS, editor. Biologic and systemic agents in dermatology. Cham: Springer; 2018. p. 10–12.
- 26.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 27.Reich K, Korge B, Magnolo N, et al. Quality-of-life outcomes, effectiveness and tolerability of apremilast in patients with plaque psoriasis and routine German dermatology care: results from LAPIS-PSO. Dermatol Ther (Heidelb) 2022;12(1):203–221. doi: 10.1007/s13555-021-00658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herranz P, Trasobares L, Mateu A, et al. Characterization and outcomes in patients treated with apremilast in routine clinical practice in Spain: results from the APPRECIATE study. Actas Dermosifiliogr (Engl Ed) 2021;S0001–7310(21):00202–207. doi: 10.1016/j.ad.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayba JN, Gooderham MJ. Real-world experience with apremilast in treating psoriasis. J Cutan Med Surg. 2017;21:145–151. doi: 10.1177/1203475416676030. [DOI] [PubMed] [Google Scholar]
- 31.Vujic I, Herman R, Sanlorenzo M, et al. Apremilast in psoriasis—a prospective real-world study. J Eur Acad Dermatology Venereol. 2018;32:254–259. doi: 10.1111/jdv.14598. [DOI] [PubMed] [Google Scholar]
- 32.Papadavid E, Rompoti N, Theodoropoulos K, et al. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatology Venereol. 2018;32:1173–1179. doi: 10.1111/jdv.14832. [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto M, Komine M, Kamiya K, et al. Drug survival of apremilast in a real-world setting. J Dermatol. 2019;46:615–617. doi: 10.1111/1346-8138.14943. [DOI] [PubMed] [Google Scholar]
- 34.Sotiriou E, Tsentemeidou A, Sideris N, et al. Apremilast survival and reasons for discontinuation in psoriasis: five-year experience from a Greek Tertiary Care Centre. Dermatol Pract Concept. 2022;12(2):e2022076. doi: 10.5826/dpc.1202a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122. doi: 10.1016/j.jaad.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17:221–228. [PubMed] [Google Scholar]
- 37.Papadavid E, Rompoti N, Theodoropoulos K, et al. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;23:1173–1179. doi: 10.1111/jdv.14832. [DOI] [PubMed] [Google Scholar]
- 38.Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16(8):801–808. [PubMed] [Google Scholar]
- 39.Megna M, Fabbrocini G, Camela E, Cinelli E. Apremilast efficacy and safety in elderly psoriasis patients over a 48-week period. J Eur Acad Dermatol Venereol. 2020;34(11):e705–e707. doi: 10.1111/jdv.16443. [DOI] [PubMed] [Google Scholar]
- 40.Bernardini N, Skroza N, Marchesiello A, et al. Psoriatic patients with a history of cancer: a real-life experience with apremilast treatment for 104 weeks. Dermatol Ther. 2022;35(10):e15306. doi: 10.1111/dth.15306. [DOI] [PubMed] [Google Scholar]
- 41.Radi G, Campanati A, Diotallevi F, et al. Long-term efficacy and safety of apremilast in the treatment of plaques psoriasis: a real-world, single-center experience. Dermatol Ther. 2021;34(6):e15179. doi: 10.1111/dth.15179. [DOI] [PubMed] [Google Scholar]
- 42.Filippi F, Patrizi A, Iezzi L, et al. Use of Apremilast® in the psoriasis treatment: a real-life multicenter Italian experience. Ital J Dermatol Venereol. 2022;157(4):313–317. doi: 10.23736/S2784-8671.21.07125-5. [DOI] [PubMed] [Google Scholar]
- 43.Balak DMW, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther (Heidelb) 2020;10(4):589–613. doi: 10.1007/s13555-020-00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Medicines Agency. Otezla, INN-apremilast. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf. Accessed 27 Feb 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.