Abstract
Smoking drug interaction studies represent a common approach for the clinical investigation of CYP1A2 induction. Despite this important role, they remain an “orphan topic” in the existing regulatory framework of drug interaction studies, and important methodological aspects remain unaddressed. The University of Washington Drug Interaction Database (DIDB) was used to systematically review the published literature on dedicated smoking pharmacokinetic interaction studies in healthy subjects (1990 to 2021, inclusive). Various methodological aspects of identified studies were reviewed. A total of 51 studies met all inclusion criteria and were included in the analysis. Our review revealed that methods applied in smoking interaction studies are heterogeneous and often fall short of established methodological standards of other interaction trials. Methodological deficiencies included incomplete description of study populations, poor definition and lack of objective confirmation of smoker and nonsmoker characteristics, under‐representation of female subjects, small sample sizes, frequent lack of statistical sample size planning, frequent lack of use of existing markers of nicotine exposure and CYP1A2 activity measurements, and frequent lack of control of extrinsic CYP1A2 inducing or inhibiting factors. The frequent quality issues in the assessment and reporting of smoking interaction trials identified in this review call for a concerted effort in this area, if the results of these studies are meant to be followed by actionable decisions on dose optimization, when needed, for the effects of smoking on CYP1A2 victim drugs in smokers.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Because potent P450 (CYP) 1A2 inducers are not available, smoking interaction studies have been the standard approach for investigating CYP1A2 induction liability for decades, with findings representing essential information to manage smoking‐related interactions.
WHAT QUESTION DID THIS STUDY ADDRESS?
This systematic review represents a critical appraisal of a variety of key methodological aspects reported in published smoking interaction studies with investigational or approved medicinal products that have been conducted over the last 3 decades in healthy subjects.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our review revealed a considerable methodological heterogeneity across published smoking interaction studies, which often fell short of current study design standards for drug–drug or food‐drug interaction studies. Overall, the proper implementation and standardization of important methodological aspects was found to be poor.
HOW MIGHT THIS CHANGE THE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The frequent quality issues in the assessment and reporting of smoking interaction studies call for concerted quality efforts in this area, if the results of these studies are meant to be followed by actionable decisions on dose optimization for the effects of smoking on CYP1A2 victim drugs.
INTRODUCTION
Smoking remains to represent a major public health burden. Globally, in 2019, 1.14 billion (95% uncertainty interval 1.13–1.16) individuals were current smokers. 1 The prevalence of cigarette smoking and background medical conditions together put smokers at increased risk of reduced efficacy or increased adverse effects of medicines they use. The altered pharmacology of medicines among smokers can frequently be ascribed to altered pharmacokinetics (PK) by smoking, with the latter primarily contributed by polycyclic aromatic hydrocarbons (PAHs) contained in tobacco smoke. PAHs act as potent inducers of the hepatic cytochrome P‐450 (CYP) isoenzymes 1A1, 1A2, and possibly 2E1. 2 In addition, some uridine 5′‐diphosphate (UDP)‐glucuronosyltransferases (UGT enzymes; e.g., UGT1A6, UGT1A9, and possibly some UGT 2B family members), have been shown to be inducible by PAHs. 3 , 4 , 5 , 6 , 7 , 8
As cigarette smoking interaction studies represent an essential source of smoking interaction information, such as dosing recommendations and warnings in drug labels, 9 the overall quality in study design, subject selection, study performance, data analysis, and reporting is of utmost importance to ensure that accurate, reliable and robust information on the effects (or lack of effects) of smoking are generated by smoking interaction studies, to provide informative and targeted treatment recommendations for smoking patients and patients undergoing smoking cessation.
Despite all the above, smoking studies remain an “orphan topic” in the existing regulatory framework of drug interaction studies. The US Food and Drug Administration (FDA) guidance documents only briefly refer to this topic, starting with the FDA draft guidance documents on drug interaction studies, published in 2006 and 2012. 10 , 11 However, in these documents, it was just recommended that “for a drug that is a substrate of CYP1A2, the evaluation of the effect of induction of CYP1A2 can be carried out by comparative pharmacokinetic (PK) studies in smokers vs. nonsmokers”. Current European 12 and Japanese 13 drug interaction guidelines do not refer at all to the clinical examination of smoking interactions in clinical drug development. This means that until recently, there was not any regulatory guidance available on how to design, conduct, analyze, and report such studies. Whereas the most recent and final FDA guidance on clinical drug interaction studies issued in 2020 14 now provides a few recommendations on methodological aspects that need to be considered for such studies (i.e., definition of nonsmokers, number of cigarettes smoked per day, quantification of plasma nicotine levels in both smokers and nonsmokers, and evaluation of the effects of different levels of smoking), these suggestions are far from sufficiently detailed and comprehensive, thereby leaving important methodological aspects and points to consider for such studies essentially unaddressed.
PK and pharmacodynamic interactions of smoking with medicinal products have been studied over decades, and a number of literature reviews have been published. 2 , 15 , 16 However, none of these reports put any focus on evaluating methodological aspects of such studies, leaving a surprising void in the literature in this respect.
The ultimate aim of any clinical studies investigating smoking effect is to provide a rationale for changing the dosage regimen or leaving it unaltered depending on the status of smoking. This paper provides a systematic review and analysis of a variety of methodological aspects reported in published smoking interaction studies with investigational or approved medicinal products that have been conducted over the last 3 decades in healthy subjects. The aim is to illustrate the adequacy (inadequacy) and consistency (inconsistency) of methodological practices in such studies for the intended objective of giving guidance on drug use in the absence of detailed regulatory guidance and commonly accepted scientific drug development standards.
METHODS
Search strategy
The University of Washington Drug Interaction Database (DIDB; https://druginteractionsolutions.org) was used as the primary source for a systematic review of published literature on dedicated smoking interaction studies in healthy subjects. The DIDB contains qualitative and quantitative human in vitro and clinical (in vivo) drug interaction information related to various extrinsic and intrinsic factors. These include interacting co‐medications, excipients, food products, herbals, tobacco, organ impairment, and genetics that can affect drug exposure in humans.
Healthy subject studies were chosen for the purpose of our review, because it is (i) the only reasonably homogeneous population when interaction studies with different drugs for different indications are reviewed, and (ii) strict science‐based methodological requirements (e.g., matching of populations, restrictions of concomitant medications, or experimental examinations not offering any health benefits to study participants) can be more straightforwardly implemented in healthy subject populations as compared to patient populations.
The time period covered by the search was January 1, 1990, to December 31, 2021. Key search criteria included study type (prospective clinical studies); study population (healthy subjects); perpetrator (cigarette smoking); drug interaction type (PK); full PK evaluation (at least plasma concentrations area under the curve [AUC] and/or clearance available); language (English).
The dataset of pertinent studies identified in DIDB was in a second step checked for completeness by a corresponding systematic review of published smoking interaction studies by applying a Medline In‐Process (PubMed) search with identical search criteria. The dates of the DIDB and PubMed searches were March 11, 2022, and March, 16, 2022, respectively.
Analysis of study methodologies
The original publications of the final dataset were subjected to a meticulous review of the following methodological study aspects:
Study design and objectives: criteria examined included key study design aspects, such as victim drugs evaluated, general study designs used, matching of subjects in parallel group studies (e.g., for age, sex, and body weight), single‐dose versus repeat‐dose of victim drugs; reported sample size considerations, and power calculations. It was further examined whether the smoking interaction was a primary or secondary study objective (e.g., piggybacked at one dose level in a dose‐escalation study).
Study populations: criteria examined included the overall population demographics in groups of smokers and nonsmokers (age, sex, body weight, body mass index [BMI], and ethnicity), smoker characteristics (e.g., smoking history, light vs. moderate vs. heavy smokers), definition and selection of nonsmokers, sample sizes reported for each study group by sex and smoker status, use of CYP1A2 phenotyping/activity assessments (e.g., caffeine clearance), and use of CYP1A2 genotyping.
Study methods: standardization and monitoring of perpetrator tobacco products (i.e., cigarette brands) and their daily quantity used by smoking study participants, measures in nonsmoker populations to safeguard against secondhand smoke exposure, standardization of smoking during study conduct, use of objective measures of smoke exposure in smokers and nonsmokers (e.g., cotinine, nicotine, or carboxy hemoglobin levels in serum/blood or urine), and allowed and disallowed concomitant medications.
Statistical evaluation
No formal statistical analysis was applied to the data. Descriptive statistics of numerical data (e.g., numbers of male and female smokers and nonsmokers enrolled into the dataset of studies) are presented as arithmetic means and standard deviations (SDs) as well as medians and ranges. Categorical data (e.g., BMI reported vs. not reported) are presented by numbers and percentage of the studies of the total dataset.
RESULTS
Search output and final data set
The search output and the selection of the final dataset is displayed in Figure 1. Out of the 105 publications identified with cigarette smoking evaluated as a perpetrator in healthy subjects with a change in victim AUC and/or clearance available (describing 163 individual drug interaction studies), a total of 57 publications met all selection criteria (1990–2021 publication date, prospective studies, healthy subjects, and full PK evaluation; see Methods). Original articles of these studies were retrieved and reviewed for relevance. During the review, seven publications were excluded which did not examine smoking interaction studies with medicinal products, or examined the effect of passive smoking only, or were redundant, or otherwise were not full original publications. After removing these seven studies, a total of 50 publications were included in the final dataset for analysis. As one of these publications reported two distinct smoking interaction studies with two different victim drugs, 17 eventually a total of 51 studies were included in our review.
FIGURE 1.
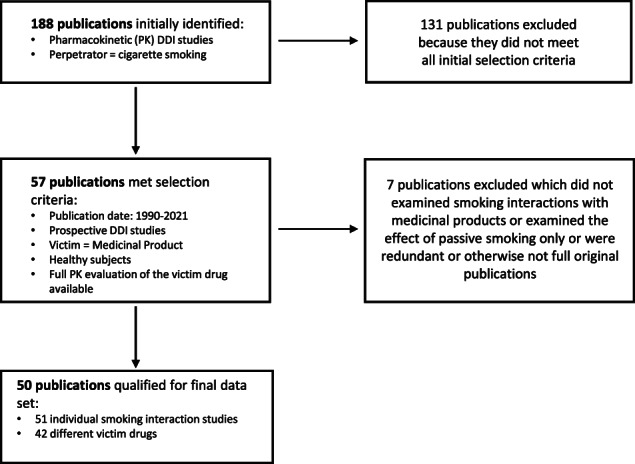
Workflow for the conducted literature searches of the University of Washington Drug Interaction Database. DDI, drug‐drug interaction.
Victim drugs studied
Forty‐two (42) victim drugs were investigated in single smoking interaction studies. The effects of smoking on two victim drugs in one study were reported in two publications. 18 , 19 Seven victim drugs were examined in more than just one study: fluvoxamine (3); inhaled insulin (2); bupropion (2); tizanidine (2); chlorzoxazone (2); antipyrine (2); and riociguat (2).
In all but four studies, the victim drugs were administered in the form of various oral dosages, whereas two victim drugs were administered by oral inhalation (inhaled insulin; 2 studies, and inhaled FK706, a neutrophil elastase inhibitor, 1 study), and one by means of a transdermal delivery system (nicotine patch).
Mechanistic rationale
A total of 18 unique victim drugs (24 studies total) are known to be at least partially metabolized by CYP1A2 and/or CYP1A1 or CYP2E1, enzymes inducible by smoking.
One further study examining codeine as the victim drug might also have had a mechanistic rationale for metabolism, because codeine is predominantly metabolized via UGTs (UGT2B4 and UGT2B7) that can be inducible by tobacco smoking. Three studies using administration of test drugs by oral inhalation, in turn, rather aimed to examine the effects of smoking on the rate and extent of pulmonary absorption of those drugs. For the remaining 23 studies (45%), a clear mechanistic rationale could not be identified. It is assumed that most of these studies were probably conducted to confirm the absence of PK interactions with smoking.
Study objectives
Not all publications clearly described and distinguished between primary and secondary study objectives. In many studies, smoking interaction objectives were combined with various other objectives, such as the effects of gender and oral contraceptives on the PK of victim drugs, or as a sub‐objective within bioequivalence objectives. Smoker and nonsmoker populations were also sometimes compared at selected dose levels in first‐in‐human dose‐escalation studies. Based on our review, we considered that the smoking interaction issue represented in 40 studies was more or less the primary or co‐primary objective, whereas in the remaining 11 studies it was considered a secondary objective.
Study designs
The most frequently applied study design of smoking interaction studies was an open‐label, parallel group (i.e., smokers vs. nonsmokers) design (46 studies, 90%), and five studies used sequential designs. The majority of the studies (i.e., 43 of the studies using parallel design) had victim drugs administered as single doses. In nine studies, repeated administrations of substrate drugs were used. Some studies followed more complex study designs (i.e., with combined parallel‐group and intra‐subject crossover elements; e.g., when smoker and nonsmoker study populations were enrolled in bioequivalence studies).
Matching of study populations in parallel‐group studies represents an important design feature, because it is believed to improve the overall comparability of study groups. However, in 38 studies (75%) no subject matching attempts for the enrolled parallel groups were reported. In six studies, smoker and nonsmoker populations were matched at least for age and body weight (BW) or BMI (12%), whereas five studies were balanced for sample size and sex, but not matched for age and BW/BMI.
Statistical sample size planning/power calculations
For the majority of studies (40 studies, 78%), no formal statistical sample size planning and power calculations were provided to capture smoking‐related differences in primary victim drugs' PK parameters (e.g., AUC and/or maximum plasma concentration [C max] values). Most of the remaining 11 studies provided formal sample size estimates aiming for a statistical power ranging between 80% and 99%, to detect 15% to 50% AUC or C max changes or a 50% change in clearance. In just four studies, the expected PK variabilities of the victim drugs were reported (e.g., as coefficient of variation for AUC or C max values, respectively).
Study populations and subjects’ demographics
As per our search criteria, the study populations consisted of healthy male and female subjects. In 50 studies (98%), healthy adult subjects were enrolled, whereas one study was conducted in healthy adolescents 13–18 years old. 20
Age
Age was, in principle, reported in all studies, but incompletely reported in two studies, in which age was not reported for the groups of smokers. In 30 studies (59%), age was separately reported for nonsmoker and smoker groups, whereas it was only given for the entire study populations in the remaining studies (41%). In 14 of 21 studies in which both male and female study participants were enrolled, age was only given for the smoker and nonsmoker groups, but not separately for male and female smokers and nonsmokers. This level of detail was only reported in six out of 17 studies (35.3%) enrolling smoking and nonsmoking study participants of both sexes.
Body weight
The BW of the study populations enrolled was not reported in 11 studies (22%) and only reported for the entire study population, but not by subject group, in 10 studies (20%). Hence, in 21 studies (42%), the information on BW was insufficient to allow the reader to make any group comparisons. BW was separately reported for each subject group enrolled to the studies (which means 4 groups in studies with male and female smokers and nonsmokers, and 2 groups in studies which either enrolled only male or female smokers and nonsmokers) in a total of 17 studies (33%). In the remaining 12 studies (24%), BW was grouped either for smokers and nonsmokers or for male and female study participants, despite the fact that four population groups (i.e., male and female smokers and nonsmokers) were enrolled into these studies. Thus, such condensed presentation of BW does not allow for a distinct group comparison of all study groups enrolled.
Body mass index
The BMI is a measurement of weight in relation to height and allows categorization of adult subjects into underweight, normal weight, and overweight or obese individuals. Besides age and body weight, the BMI is a key demographic characteristic for the proper description of study populations and is also reported as a covariate for CYP1A2 activity. 21 In the dataset of 51 studies, however, the BMI was not reported in 34 studies (67%). In five studies (9.8%), the BMI was reported just for the entire study population, which did not allow an assessment on whether the BMI of smoker and nonsmoker groups, as well as male and female study participants, were comparable. A similar issue applies to three studies (5.9%) with male and female participants, in which the BMI was just reported for smoker and nonsmoker groups, but not separately for male and female smokers and nonsmokers. In 10 studies (19.6%), BMI was separately reported for smokers and nonsmokers, and in six studies only (12%), the BMI was reported separately for each study group enrolled. In three studies (6%) an alternative metric of the nutritional status of study participants was provided instead of BMI (i.e., allowed some percent deviation from ideal body weight). In two of these studies, this information was also only provided for the entire study population, not separately for each study group.
Sex
Sex has been considered as a covariate of CYP1A2 activity, 21 , 22 , 23 although respective reports are not entirely consistent. Overall, male subjects were remarkably over‐represented. A total of 26 studies (51%) exclusively enrolled male subjects, whereas only two studies (4%) exclusively enrolled female subjects. Accordingly, 22 studies (43%) enrolled both male and female subjects. In terms of the number of study participants, 1044 and 375 male and female subjects, respectively, were enrolled in 50 studies, which translates to ~2.8‐fold over‐representation of male subjects in published smoking interaction studies. One study did not report the number of male and female subjects enrolled, 24 and two studies did not report the sex distribution of the smoker populations. 25 , 26 When studies with both male and female study participants are considered, the number of male and female subjects ranged from six to 76 (male) and two to 38 (female) individuals enrolled per study. The mean (±SD) number of male and female participants per study was calculated as 22.2 ± 14.8 and 16.3 ± 13.6 individuals, respectively. The corresponding median values were 18 and 13 male and female individuals per study, respectively.
When the enrollment of smokers is considered, then the disparity between male and female participants becomes larger. A total of 45 studies enrolled male smokers, whereas only 21 studies have female smokers enrolled. Overall, 531 and 167 male and female smokers, respectively, were enrolled (i.e., about 3.2‐fold more male smokers than female smokers). The mean (±SD) number of male and female smokers per study was calculated as 11.8 ± 10.1 and 8.0 ± 5.7 individuals, respectively. The corresponding median values and ranges were 10 (2–64) and six (4–25) male and female smokers per study, respectively.
Corresponding outcomes for nonsmokers were as follows. A total of 44 studies enrolled male nonsmokers, whereas only 21 studies enrolled female nonsmokers. As was the case with the smoker populations, the same three studies also did not report the sex distribution of the nonsmoker populations. 24 , 25 , 26 Overall, 480 and 210 male and female nonsmokers, respectively, were enrolled (i.e., about 2.3‐fold more male nonsmokers than female nonsmokers). The mean (±SD) number of male and female nonsmokers per study was calculated as 10.9 ± 9.0 and 10.0 ± 8.7 male and female individuals, respectively. The corresponding median values and ranges were nine (2–48) and seven (3–38) for male and female nonsmokers per study, respectively.
Ethnicity
Ethnicity is an important covariate of CYP1A2 activity. 27 However, in 37 of 51 smoking interaction studies (73%), information on the ethnicity of study participants was not provided. In four of these studies, presumably Japanese subjects were enrolled, because these studies were conducted by Japanese study groups in Japanese study centers. Those studies providing information on the ethnicity of study participants enrolled predominantly White subjects (5); Asian (Korean and Thai) (2); Chinese (2); Japanese (1); and individuals with various ethnic backgrounds (White, Black, and Hispanic) (3). In the vast majority of studies, the ethnic background was just reported for the entire study population, not separately per study group. In three studies only, the ethnicity was separately reported for smoker and nonsmoker populations. In one of these studies, there was an apparent mismatch between White and Black participants in the smoker and nonsmoker groups reported. 28
Smoker characteristics
As we specifically focused on cigarette smoking in our search, studies using other tobacco products (e.g., cigars, pipe, e‐vaporized products, etc.) are not considered in this review.
Smoker populations were typically characterized by self‐reported tobacco product consumption and smoking history. The studies comprised a wide range of smoking requirements per protocol, ranging from two cigarettes per week to greater than or equal to 30 cigarettes per day (Table 1). Regarding the key inclusion criterion “cigarette consumption per day,” there were not less than 15 different quantitative protocol defined inclusion criteria. Above that, there were 10 different one‐sided inclusion criteria (e.g., <20 cigarettes per day) applied in 31 studies (61%), resulting in broad ranges and thus, poor definition and ambiguity in terms of allowed cigarette consumption of the smoker populations enrolled in most of the studies. In another six studies (11.8%), no protocol requirements in terms of daily cigarette consumption were reported at all. This implies that in more than 70% of studies, there were no sufficiently specific and detailed protocol requirements defined to allow readers' judgment on commonly accepted smoker categorization (i.e., light, moderate, or heavy smokers). When the 15 different quantitative protocol inclusion criteria are grouped (to the extent possible) into these three categories, it is estimated that in five studies (9.8%) predominantly light smokers (i.e., ≤10 cigarettes per day), in 29 studies (56.9%) predominantly moderate smokers (i.e., >10 to <20 cigarettes per day), and in three studies (5.9%) heavy smokers (i.e., ≥20 cigarettes per day) were enrolled. In the remaining 14 studies (27.5%), the smoking requirements of study protocols were either not reported (6 studies, see above) or too broadly defined to allow an assignment of the respective populations to any smoker category (8 studies; 15.7%). In none of the studies, was it aimed by study design to enroll two distinctly different smoker categories (e.g., light and heavy smokers) to allow for assessment of smoke exposure‐related dose‐effects, although it is well established that these do exist. 21 , 23 , 29
TABLE 1.
Smoker characteristics
| Inclusion criteria for smokers | Number of studies |
|---|---|
| Heavy smokers not further specified | 1 |
| Current smoker not further specified | 1 |
| ≥30 cig/days | 1 |
| ≥20 cig/day | 8 |
| 10–20/25 cig/day | 10 |
| ≥15 cig/day | 4 |
| ≥12 cig/day | 2 |
| ≥10 cig/day | 12 |
| ≥5 but ≤10 cig/day | 1 |
| ≥7 cig/day | 1 |
| ≥5 cig/day | 1 |
| ≤20 cig/day | 1 |
| ≤10 cig/day | 1 |
| 2 cig/week | 1 |
| Not reported | 4 |
Note: Smoker populations comprised a wide range of smoking requirements per protocol, and actual smoking; shown are details of smoking‐related inclusion criteria along with the number of studies that applied these criteria.
In 14 publications (27.5%) only, the actual mean cigarette consumption of the smoker populations at screening was reported. Means were reported in five studies, means ± SD in nine studies, and ranges in eight studies. The mean cigarette consumption across these studies ranged from 13.4 to 28 cigarettes per day, the closest and the widest ranges were reported as 12 to 20 and four to 50 cigarettes per day, respectively, for the smoker populations in these two studies. 17 , 30
It is interesting to note that most investigators/sponsors relied on self‐reported smoking habits as the only acceptance criterion for enrollment of smokers, because, in only 10 studies (19.6%), tobacco smoke exposure was objectively confirmed by either urine (5 studies) or plasma cotinine (3 studies) measurements or both (2 studies), with cutoff values either not reported (7 studies) or greater than 500 ng/ml for the urine cotinine levels (2 studies) and 200 ng/ml for plasma cotinine levels (1 study). In two studies, 18 , 30 in addition to cotinine, carboxy hemoglobin was also quantified, and in one of these studies 18 plasma and urine nicotine concentrations were also measured. In all but one study, the identity (i.e., trade name and manufacturer) of the cotinine test products used was not reported.
In 26 studies (51%), no information on the smoking history of the smoker populations was provided, whereas in 25 studies (49%) at least some information on the smoking history is reported, albeit it was not always clear whether the authors referred to study protocol requirements (i.e., inclusion criteria) or the actual, self‐reported smoking history of the study participants.
Nonsmoker characteristics
Most investigators/sponsors entirely relied on a self‐reported nonsmoker history as the only acceptance criterion for enrollment of nonsmokers. This was the case in 41 of 51 studies (80.4%). In nine studies only (17.6%), either plasma or urine cotinine levels (or both), or serum and urine nicotine or carboxy hemoglobin concentrations were determined to objectively confirm the nonsmoker status of study participants (see above).
In 30 of 51 studies (58.2%), no nonsmoker definitions were provided at all, whereas in 21 studies (41.2%) various nonsmoker criteria in terms of nonsmoker history (e.g., former smokers or never‐smokers) and minimum time periods of smoking abstinence prior to study participation were reported. The per protocol required nonsmoking periods prior to study participation ranged within 30 days as the shortest reported nonsmoking period up to never‐smokers (3 studies). Four studies applied pre‐study nonsmoking periods ranging between 1 and 10 years.
Interestingly, little attention was paid to possible secondhand smoke exposure of nonsmokers, as evidenced by the fact that only two studies referred to the avoidance of “heavy passive smoking” in the preceding 2 months of the study 31 or to “subjects regularly exposed to passive smoking.” 32 Only one study referred to the separation of nonsmokers and smokers during study participation at the investigational site (“nonsmokers were not in the presence of smokers while on site”). 28
Information on the protection of the nonsmoker populations against environmental cigarette smoke exposure was virtually not collected and hence not reported. In none of the publications was information provided whether investigators examined nonsmokers about their living environments (e.g., smokers living in the household) or attendance in social activities typically associated with secondhand smoke exposure (e.g., joining parties or visiting Shisha lounges). In two studies only, the absence of significant secondhand smoke exposure was actually objectively confirmed during the course of the study by plasma/urine cotinine testing prior to treatment. In the remaining eight studies with objectively confirmed absence of environmental smoke exposure, plasma/urine cotinine tests were just conducted at screening visits and not during the study.
Identity of perpetrator tobacco products
A particular challenge of smoking interaction studies—as opposed to classical drug–drug interaction (DDI) or food‐drug interaction studies—is the variability in the composition and contents of the CYP1A2 inducing agents in the perpetrator products, as tobacco is a natural product derived from various tobacco plant species/cultures, factors which do not allow the specification of a well‐defined dose of the perpetrating components. It is therefore surprising that in the vast majority of smoking interaction studies not even the identity of the perpetrator products used was disclosed, but just stated that smoker populations smoked cigarettes. In 47 of 51 studies (92%), no detailed information is provided on the identity of cigarette products used before and throughout the study. In four studies (7.8%), the cigarette qualities used were at least to some extent specified (e.g., “cigarette brands containing blond tobacco”, 18 or “Cigarettes were of the domestic Chilu and Jiabin brands, containing intermediate‐tar, mixed‐type black tobacco,” 31 or the number of male and female smokers using “light brands” was reported 33 ). Only in one study, the specific cigarette brand used (“Camel”) was standardized and reported. 34
Information on smoking requirements or restrictions during study conduct
In 35 of 51 studies (69%), no information on any standardization of smoking requirements or restrictions during study conduct is reported. In 12 studies (24%), adequate and specific information on smoking restrictions or requirements during study conduct is provided, whereas, in three studies, just unspecific information is given (e.g., “smoking allowed during study”). In just five studies (10%), information on some kinds of surveillance/monitoring of smoking during the study is reported, whereas, in the remaining 46 studies (90%), no such information is provided.
Concomitant medications
Concomitant medications need to be avoided to the extent possible in interaction studies to avoid possible confounding effects on target metabolic enzymes or transporters. In healthy subject studies, any concomitant medications, including over‐the‐counter products, herbal products, vitamins, and supplements are usually prohibited for a certain period (e.g., 2–4 weeks) before enrollment and throughout the study.
However, this strict approach was followed only in 23 studies (45.1%), and only for 12 studies (23.5%) it was explicitly reported that concomitant drugs were also prohibited for a certain period (1 to 2 weeks) before subject enrollment into the studies. Oral contraceptives in female subjects were prohibited in five studies, but allowed to be taken in another five studies. The latter is noteworthy as the components of oral contraceptives are known to inhibit CYP1A2, 35 thereby potentially confounding the smoke‐induced CYP1A2 induction.
Prohibited medications during study conduct were not specified in nine studies (17.6%), and in four studies (7.8%), only few selected medications were prohibited (e.g., CYP2E1 inhibitors). Only for three studies, it was explicitly reported that enzyme inducers and inhibitors were generally prohibited. Astonishingly, in none of the studies, CYP1A2 inhibitors were explicitly prohibited.
No information at all on concomitant medications was provided in 13 publications (25.5%).
Dietary and other lifestyle restrictions
Certain dietary restrictions are key study design elements in smoking interaction studies because cruciferous vegetables (e.g., broccoli and cauliflower), caffeine, and charcoal grilled meat have been shown to induce CYP1A2 activity, 23 , 27 , 36 whereas apiaceous vegetables (e.g., carrots and celery) and grapefruit juice are reported to inhibit CYP1A2 activity. 27 , 37
Therefore, it was surprising to find in our dataset of smoking interaction studies, that, for 26 studies (51%), no dietary restrictions were reported at all. In 20 (39.2%) and 19 (37.3%) studies, caffeine‐ and methyl xanthine‐containing foods and beverages were disallowed, respectively, and, in 19 studies (37.3%), alcoholic beverages were prohibited. In five studies (9.8%) and in one study (2%), grapefruit juice and citrus fruit juice products were prohibited, respectively. Of note, in just one study (2%), cruciferous vegetables and foods prepared on charcoal were prohibited, 38 and in another study, “foods that interfere with CYP P450 enzymes” were prohibited. 19 In 20 studies (39.2%), the dietary restrictions were implemented for a certain time period before study start, ranging between 2 days and 1 week.
Apart from certain meals and beverages, heavy exercise has been reported to induce CYP1A2 activity as well. 39 , 40 However, for none of the studies, it was reported that unaccustomed strenuous physical exercise was prohibited for study participants throughout the study.
CYP1A2 activity assessment/phenotyping
From the overall dataset reviewed, a total of 22 studies used victim drugs known to be at least partially metabolized by CYP1A2 (see above). For the interpretation of the PK outcomes of these studies, a CYP1A2 activity assessment in smoker and nonsmoker populations would have been prudent. However, this was actually done in just five of these 22 studies (22.7%). In all studies using CYP1A2 phenotyping, caffeine was used as the probe drug, and, in five of six studies, caffeine clearance was applied as CYP1A2 activity metric. One study applied the use of ratios of various urinary caffeine metabolites. In 17 of 22 studies (77.3%) examining CYP1A2 substrate drugs, no CYP1A2 activity assessment was done.
CYP1A2 genotyping
None of the reviewed 22 smoking interaction studies performed with CYP1A2 victim drugs used any genotyping of study participants for genetic polymorphisms of CYP1A2.
DISCUSSION
For decades, smoking interaction studies have represented the standard approach for the clinical investigation of CYP1A1, CYP1A2, and CYP2E1 induction.
This is because there are hardly any useful and sufficiently potent inducers of CYP1A enzymes available among marketed medical products. Although the proton pump inhibitor omeprazole has been described to induce CYP1A2 in a dose‐dependent fashion, it's in vivo induction potential at a therapeutic dose of 40 mg/day only becomes apparent in poor metabolizers of CYP2C19, which represents the major metabolic pathway omeprazole. 41 Altered activity of CYP2C19 results in higher systemic omeprazole plasma concentrations in poor CYP2C19 metabolizers, which are mandatory to achieve a notable CYP1A2 induction. This exposure relationship has been later demonstrated by treatment of extensive metabolizers of CYP2C19 with a three‐fold therapeutic dose of omeprazole (120 mg/day over 7 days), which increased the caffeine clearance, as a measure of CYP1A2 induction, by about 32%. 42 However, by definition, this represents just a modest induction of CYP1A2, fairly below induction levels which are seen with various lifestyle or environmental inducers of CYP1A2, such as cigarette smoke, charcoal grilled meat, extensive caffeine consumption, 23 or heavy exercise. 39 , 40
Therefore, smoking interaction studies remained the preferred approach for in vivo studies aiming to examine CYP1A2 induction, although they are inherently associated with a number of challenges, the most important of which will be discussed in the following section. Detailed recommendations when performing a smoking interaction study are provided in Table 2.
TABLE 2.
Points to consider in design, conduct, and reporting of smoking interaction studies
| Topic/area | Proposed approach | Comments |
|---|---|---|
| Victim drugs to be examined |
Drugs undergoing CYP1A1‐, CYP1A2‐, and CYP2E1‐metabolism. Also, drugs undergoing extensive glucuronidation by UGT1A6, UGT1A9, and possibly some UGT2B family members. |
Note that even when the fraction metabolized of victim drugs suggests that these enzymes might be minor pathways, they can become major pathways under the condition of induction. |
| Study objectives |
Aim for a dedicated smoking interaction study. Consider the value of modeling and simulations if applicable. |
Avoid implementation of smoking interaction objectives as secondary objective in trials with other primary objectives. |
| Study design | Open‐label, parallel‐group design, with single‐dose administration of the victim drug. | Repeated‐dose administration may be needed for victim drugs with complex PK (in case of auto‐inhibition or auto‐induction, for example). |
| Study populations | Healthy adult subjects (except when not possible, for example for some oncology drugs), well‐characterized, male and female smoker and nonsmoker populations. | Proposed characterization of study populations detailed below; aim for a balanced representation of female subjects; aim for matching of subjects at least for age and sex. |
| Sample size | Apply a statistical (power) calculation for the estimated sample size based on the PK variability of the victim drug. | In case that a solid power calculation is not possible, enroll at least 12 subjects in each study group. |
| Subjects’ demographics | Document and report age, sex, body weight, BMI, and ethnicity of study subjects for each study population enrolled. | Note that sex, BMI, and ethnicity are covariates of CYP1A2 activity. |
| Smoker characteristics |
Document and report smoking history, self‐reported current cigarette consumption per day, and the cigarette brand used. Properly define inclusion criteria for daily cigarette consumption in a manner that allows assignment of each smoker in a well‐defined category (i.e., light, moderate, and heavy smoker), when different smoker categories are planned to be enrolled. The dose of the perpetrator should aim at reaching maximum induction to allow the evaluation of the worst‐case scenario. This implies that a group of heavy smokers (smoking ≥20 cigarettes) should always be enrolled. Confirm smoker status by objective measurements (e.g., plasma cotinine concentrations). |
Aim to standardize the tobacco products used by all smoking participants in the study. Note that the dose–response curve of CYP1A2 induction by smoking is steepest in the range of light smoking (i.e., significantly increases from smoking 1 to 5 cigarettes per day to smoking 5 to 10 cigarettes per day). Monitor, document, and report daily smoking requirements throughout the study. |
| Nonsmoker characteristics |
Obtain the self‐reported nonsmoker history. Inquire subjects for sources of second‐hand smoke exposure (e.g., smokers living in the household, etc.). Define minimum time‐period of nonsmoker status before study start. Confirm nonsmoker status by objective measurement (e.g., by quantification of plasma cotinine concentrations). |
Currently proposed cutoff to distinguish between smokers and nonsmokers is proposed with 3 ng/ml (Benowitz et al., 2009). 43 Take care of spatial separation of smokers from nonsmokers at all study days. |
| Identity of perpetrator tobacco products | Define, document, and report the perpetrator tobacco products that are to be used throughout the study, and aim for standardization across subjects to the extent possible. |
It is acknowledged that it might be a challenge to convince study participants to change their cigarette brands for the study period, but one should aim for this. It might have ethical implications when subjects are asked to switch from light cigarettes to brands containing more nicotine. This needs to be considered. |
| Information on smoking requirements or restrictions during study conduct | Provide adequate and specific information on smoking restrictions or requirements during study conduct. | Close monitoring of adherence with the protocol‐specified restrictions need to be conducted and documented. |
| Concomitant medications |
Apply strict requirements regarding prohibited concomitant medications, including OTC products, herbals, vitamins, and other supplements. Pay particular attention on the prohibition of CYP1A2‐ or multiple CYP‐enzyme inhibitors and inducers. |
There are only few CYP1A2 inducers known among marketed medicinal products (e.g., omeprazole). Oral contraceptives, fluvoxamine, ciprofloxacin, enoxacin, sertraline, methoxsalen, mexiletine, and vemurafenib are known CYP1A2 inhibitors of varying potencies. A complete list of marketed CYP1A2 inhibitors should be reviewed and addressed in the exclusion criteria of the study protocol. |
| Dietary and other lifestyle restrictions |
Cruciferous vegetables (e.g., broccoli and cauliflower), caffeine and charcoal grilled meat/foods have been shown to induce CYP1A2 activity, whereas apiaceous vegetables (e.g., carrots and celery) and grapefruit juice are reported to inhibit CYP1A2 activity. Foods and beverages containing these aliments need to be prohibited. Alcohol consumption should also be prohibited a certain time period before and throughout the study. Unaccustomed strenuous physical exercise has been shown to induce CYP1A2 activity and thus, should also be prohibited throughout the study. |
|
| CYP1A2 activity assessment/phenotyping | For the interpretation of the PK outcomes of CYP1A2 victim drugs, a CYP1A2 activity assessment by a validated phenotyping method in smoker and nonsmoker populations is recommended. | Caffeine is the most often used CYP1A2 phenotyping probe because of the predominant role of CYP1A2 in its overall metabolism, and the well‐established safety and tolerability. The assessment of the oral caffeine clearance after administration of a single 200 mg caffeine tablet is considered the current gold standard. |
| Pharmacogenetics of study populations | Consider to genotype study participants for polymorphisms known to alter activity and/or inducibility of CYP1A2. |
As this is a still evolving area, apply current knowledge based on literature search. Altered inducibility of CYP1A2 may be also due to genetic variations in the regulation of the CYP1A2 gene (e.g., AhR or by nongenetic factors modulating the intracellular inducer concentrations (e.g., cellular efflux by P‐gp etc.). |
Abbreviations: AhR, aryl hydrocarbon receptor; BMI, body mass index; OTC, over‐the‐counter; PK, pharmacokinetic.
First, smoking interaction studies, in contrast to all kinds of other interaction studies, cannot be conducted as intra‐subject crossover studies, but need to be designed as parallel‐group studies, with implications for increased total variability (i.e., intra‐ and intersubject variability) and the requirement of respective sample size considerations. The FDA guidance on bioavailability and bioequivalence 44 states that in cases when a parallel design is necessary, consideration should be given to total variability, including intersubject variability instead of just intrasubject variability. The European Guideline on bioequivalence states that the number of subjects to be included in a study should be based on an appropriate sample size calculation, but should not be less than 12 subjects. 45 Although these recommendations primarily refer to bioequivalence studies, they are essentially relevant and applicable for interaction studies aiming to have some regulatory relevance (e.g., for product labeling purposes). In view of this, it was surprising to find that for the vast majority of smoking interaction studies (78%), no formal statistical sample size calculations were done, and mean sample sizes of smoker and nonsmoker groups enrolled were low.
It goes without saying that studies lacking proper sample size calculations and actually enrolling too small sample sizes are unlikely to provide robust and generalizable information to provide sufficiently reliable dose recommendations for drug labels.
A recent comprehensive review of smoking‐related information in US drug labels indicated that quantitative effects of smoking on drug PK are specified for 18 products. 16 Dosage modifications in smokers are provided for just four drugs, namely erlotinib hydrochloride, riociguat, theophylline, and inhaled insulin. For six drugs, including cimetidine and olanzapine, dosage modifications in smokers are only recommended for multifactorial settings, which additionally may affect safety or efficacy. For seven drugs, dosage modifications in smokers were considered not required in the drug label, although the PK parameters of four of them are affected by smoking. 16 In particular for the latter examples, it cannot be entirely excluded that the absence of dose modifications, despite observed PK alterations, may be due to lack of statistical power or assay sensitivity because of inadequate sample size and/or poor control of confounding factors. Further, these data indicate that, overall, only few smoking interaction studies actually contributed to prescriber information in US product labels.
A major second challenge, as opposed to classical DDI or food‐drug interaction studies, is the variability in the composition and contents of the inducing agents in tobacco smoke (tobacco smoke contains about 500 volatile compounds and more than 3500 different particulate components). 2 This does not allow the specification of a well‐defined dose of the perpetrating PAH components in tobacco smoke. This difficulty in defining a precise dose of the perpetrator product is further aggravated by the fact that the only commonly applied exposure estimate of smoked “cigarettes per day” is an inherently imprecise indicator of actual tobacco smoke exposure, because of the variability in how smokers smoke their cigarettes (i.e., how extensively they inhale the cigarette smoke into their lungs). It is known that there is considerable individual variability in smoke inhalation, even by people smoking the same brand of cigarettes. 43
Despite the challenge of precise metrics of cigarette smoke exposure and its variability, a review of smoking interaction studies in various smoker populations indicated a quantifiable dose‐related increase in CYP1A2 activity, as measured by caffeine clearance, when the number of cigarettes smoked per day is considered. 29 As compared to nonsmokers, smokers of daily one to five cigarettes, six to 10 cigarettes, 11–20 cigarettes, and more than 20 cigarettes per day showed a monotonic dose‐related increase in caffeine clearance by 1.22‐, 1.47‐, 1.66‐, and 1.72‐fold, respectively. 29
These data should be carefully considered in future study designs, when planning the enrollment of different smoker categories. Of note, more than 70% of studies in our dataset insufficiently detailed protocol requirements to allow inferences on commonly accepted smoker categories studied (i.e., light, moderate, or heavy smokers), indicating that, overall, the smoker populations were ill‐defined in most of the studies. Broad inclusion criteria regarding daily cigarette consumption represent an important study design shortcoming, increasing the overall variability, which should be avoided. Precise definitions of cigarette consumption per day would also be an important prerequisite to meet the current recommendation in the final FDA guidance on clinical drug interaction studies, 14 and to enroll different smoker categories. Thereby important dose–response data could be captured in different groups of smokers based on which inducing effects could be modeled for smoker categories that were not studied.
Another important aspect is the lack of standardization and reporting of the identity of perpetrator tobacco products used in smoking interaction studies. Surprisingly, in 92% of studies, no detailed information was provided on the identity of cigarette products used before and throughout the study, and in one study only, it was reported that participants used a standardized cigarette brand. 34 Although it is acknowledged that it might be a challenge to convince study participants to change their cigarette brands for study participation, efforts should be made to achieve at least some standardization (e.g., with regard to the nicotine content of allowed cigarette brands) in order to be able to quantify cigarette consumption of participants by monitoring of serum cotinine concentrations. One regular cigarette contains about 1.0–1.5 mg nicotine, and thus smoking of 16 cigarettes is expected to result in about 30 ng/ml plasma cotinine concentrations. 43 Although this is a rough estimate, because the conversion of nicotine to cotinine is subject to some interindividual variability, such checks should be made to estimate the validity of the self‐reported smoking behavior of study participants.
It needs to be considered that it might have ethical implications when subjects are asked to switch for study participation from light cigarettes to regular brands containing more nicotine.
Careful definition of nonsmoker populations is another important, but widely neglected, study design issue. Most studies (80.4%) entirely relied on a self‐reported nonsmoker history as the only acceptance criterion for enrollment of nonsmokers. In just nine studies (17.6%), either serum or urine cotinine levels (or both), or carboxy hemoglobin concentrations were determined to objectively confirm the nonsmoker status of study participants. Interestingly, little attention was paid to the evaluation of possible secondhand smoke exposure of nonsmokers, and information on the protection of the nonsmoker populations against environmental cigarette smoke exposure was virtually not collected and hence not reported in most studies.
Not validating the actual nonsmoker status of study participants by objective measurements is considered an important study design deficiency, as healthy individuals aiming to volunteer for study participation may not always report truthfully about their smoking behavior when applying for study participation in nonsmoker cohorts. It is expected that the validity of self‐reporting in this particular setting may be lower than that observed in community‐based epidemiological studies. We observed during recruitment for a recent first‐in‐human study in Germany (Eudra CT Number 2018‐000324‐34) aiming to enroll exclusively nonsmokers, that about 4% of subjects applying for study participation showed positive urine cotinine test results exceeding a relatively high cutoff value of 100 ng/ml, and thus had to be excluded from study participation (personal information R.H.). It is acknowledged that this number may not always be attributable to false self‐reporting of being a nonsmoker, but may also include inadvertent secondhand smoke exposure in a young healthy study population visiting parties, clubs, etc., as part of their typical lifestyle. Therefore, ultimately the actual source of secondhand exposure in nonsmoker populations does not matter, because any exposure is expected to attenuate/obscure the group contrasts between smoker and nonsmoker populations, which may confound quantitative study outcomes regarding the actual CYP1A2 induction by smoking. Therefore, objective assessments of the nonsmoker status of study participants should be used at screening and regular intervals throughout each smoking interaction study. Subjects should also be advised how to avoid secondhand smoke exposure and about “locations of risk” that should be avoided.
For smoke‐exposure monitoring, various markers have been used, including nicotine, carboxy‐hemoglobin, or cotinine concentrations, in various biological compartments. The nicotine metabolite cotinine is the most widely used and has excellent specificity for both active use of tobacco and for secondhand smoke exposure, except in individuals using nicotine‐containing medications. 43 There is a high correlation among cotinine concentrations measured in plasma, saliva, and urine, and measurements in any one of these fluids can be used as a marker of nicotine intake. 43 Because of the long half‐life of cotinine of about 16 h, it has been used as a preferred biomarker for monitoring of tobacco smoke exposure over the past 3–4 days. 43 Benowitz reported that current optimal plasma cotinine cutoff point to distinguish smokers from nonsmokers in the general US population is 3 ng/ml, and noted that this cutoff point is much lower than that established 20 years ago, reflecting less secondhand smoke exposure due to clean air policies and more light or occasional smoking. 43
On the other hand, because of nicotine's short half‐life (2 h) the quantification of plasma nicotine is not recommended for general use. It is unclear based on which evidence or considerations the quantification of plasma nicotine levels in both smokers and nonsmokers is recommended in the final drug interaction FDA guidance, 14 rather than cotinine monitoring.
In our dataset of smoking interaction studies, concomitant medications were often not as strictly controlled as is usually standard in other healthy volunteer phase I studies. Only for three studies, it was explicitly reported that enzyme inducers and inhibitors were generally prohibited, and in one study explicitly, inhibitors of CYP2E1 were prohibited. Surprisingly, no study explicitly prohibited inhibitors of CYP1A2 as concomitant medications. Similarly, some studies authorized the use of oral contraceptives despite their known inhibitory effect on CYP1A2. 35 This resulted in enrollment of mixed populations of female subjects with and without oral contraceptive use, which is expected to increase the intersubject variability and to confound the effects of smoking on CYP1A2 induction.
It is well‐established that certain components in foods and beverages may have potential to alter the activity of CYP1A2. Dietary restrictions are therefore key study design elements in DDI and smoking interaction studies examining CYP1A2 victim drugs, meaning that foods and beverages known to either induce or inhibit CYP1A2 activity (for details, see Results: Dietary and other Lifestyle Restrictions) should be prohibited for at least 1–2 weeks before enrollment and throughout the study. Therefore, it was surprising to find in our dataset of smoking interaction studies, that for every second study (51%) no dietary restrictions were reported at all.
Apart from certain meals and beverages, heavy exercise has been reported to induce CYP1A2 activity. 39 , 40 However, for none of the studies, it was reported that unaccustomed strenuous physical exercise was prohibited for study participants throughout the study, although this is a common standard requirement for inclusion of healthy subjects into phase I studies. Compliance with this requirement can easily be monitored by regular quantification of serum creatine kinase, a muscular enzyme.
For direct in vivo phenotyping of CYP1A2 activity, a single dose of a probe drug which is predominantly metabolized by CYP1A2 needs to be administered. Proposed probe drugs include caffeine, theophylline, and melatonin. 23 Caffeine is most often used for CYP1A2 phenotyping because of the predominant role of CYP1A2 in its overall metabolism, and its well‐established safety and tolerability. Although various urinary, plasma, saliva, and breath‐based CYP1A2 caffeine metrics have been applied, the assessment of the oral caffeine clearance is considered the current gold standard. For the interpretation of the PK outcomes of studies examining CYP1A2 victim drugs, a CYP1A2 activity assessment in smoker and nonsmoker populations appears to be strongly advisable, because this also represents an internal validation of the study in terms of an assay sensitivity assessment, by providing proof that the smoker populations actually do have higher CYP1A2 activities as compared to the nonsmoker populations. However, CYP1A2 phenotyping was actually done in about 23% of studies examining CYP1A2 victim drugs, whereas in about 77% of studies, no CYP1A2 activity assessments were done.
In vivo, CYP1A2 activity exhibits a significant degree of inter‐individual variation (e.g., 14‐fold in European populations). 27 This is to a major extent due to environmental factors (such as induction by smoking), and to a minor extent, due to the existence of polymorphisms in the CYP1A2 gene. 27 A number of studies have assessed the association between genetic polymorphisms and CYP1A2 activity. However, there are still controversies as to the functional importance of CYP1A2 polymorphisms on the metabolism of CYP1A2 substrates. A recent systematic review and meta‐analysis assessing the effects of genetic polymorphisms on CYP1A2 activity, as measured by caffeine metabolism in 3570 individual subjects showed that higher enzyme activity was observed among those who were homozygous or heterozygous for the −163C>A polymorphism (rs762551), when compared to wild‐type individuals. 46 For other known CYP1A2 polymorphisms, altered caffeine metabolic ratios were not seen. These results indicate the functional importance of the −163C>A polymorphism on CYP1A2 activity.
However, regarding inducibility of CYP1A2, the matters are more complex. The origins of individual variability in CYP1A1/2 inducibility have been comprehensively reviewed earlier, 47 and key points are briefly summarized in the following. Induction of CYP1A enzymes requires intracellular access of inducing xenobiotics to the smooth endoplasmic reticulum were P4501A enzymes are expressed, and subsequent binding to the aryl hydrocarbon receptor (AhR). Thus, individual variability in intracellular concentration of inducing agents (e.g., by the action of efflux transporters, such as P‐glycoprotein and others), and the rate of intracellular metabolism by P450 and other drug‐metabolizing enzymes are known to contribute to interindividual intracellular variation of inducing agents. Further, genetic polymorphisms of the AhR have been suggested to contribute to the variable inducibility of CYP1A enzymes. It has been shown that expression of AhR in humans exhibits individual variation, and that variable expression of AhR in human populations can influence the inducibility of CYP1A genes. 47 Analyses of AhR functions in human placenta samples revealed more than 20‐fold differences in AhR affinity for ligand binding between the “high” and the “low” CYP1A1 inducibility phenotypes. This suggests that genetic factors of the nuclear AhR may determine the activity and/or the inducibility of CYP1A2 in humans. 48
Taken together, the genetics of the interindividual variability of CYP1A inducibility is complex and not just confined to genetic variations in the CYP1A gene, but is rather expected to be based on a number of upstream sources as detailed above.
For smoking interaction studies, the variable inducibility represents a challenge, as can be seen by those publications reporting individual caffeine clearance data in smokers and nonsmokers. 18 , 28 , 31 , 49 From these data, it becomes apparent that caffeine clearance only exceeds the range that is observed in the respective nonsmoker populations in a variable number of individuals of the smoker population. In the studies reported by Dong and Li, this was the case in ~50% of the smoking subjects. In the study of Benowitz, this was the case just in two of 12 smokers. This means that only about half (or less) of smokers can be considered carriers of a distinctly inducible CYP1A2 phenotype, whereas the others do not show a meaningful CYP1A2 induction due to smoking. We feel that little attention has been paid yet to these outcomes from historical smoking interaction studies and would like to suggest that this observation deserves further attention in the planning of upcoming smoking interaction studies, in particular at the level of sample size considerations. However, due to the genetic complexity of CYP1A inducibility, we do not think that this issue could be adequately addressed just by CYP1A genotyping in standard smoking interaction studies, but deserves further basic research.
Last, our review has ascertained a common drawback of many smoking interaction studies consisting of the fact that the demographic characteristics of the study populations, including, age, sex, body weight, BMI, and ethnicity were not reported in sufficient detail and often not separately for each of the study populations enrolled, although some of these (e.g., sex and ethnicity) are covariates of CYP1A2 activity.
Taken together, all these challenging methodological factors of smoking interaction studies, as discussed above, were unfortunately too often not carefully considered in the published studies over the last 3 decades, and the methodological across‐study heterogeneity is striking. The results of this review call for an improved meticulous planning and conduct of smoking interaction studies (Table 2), including enrollment of well selected and characterized study populations of smokers and nonsmokers; proper statistical sample size planning; best possible standardization of the perpetrator product(s) used; implementation of objective measures of nicotine exposure in smoker and nonsmoker populations; strict prohibition of CYP1A2 inducing/inhibiting diets, concomitant medications, and other lifestyle factors (e.g., heavy exercise); and implementation of CYP1A2 phenotyping (e.g., caffeine clearance). For selected studies, but not as a default methodology, CYP1A2 genotyping of study participants may be considered.
We feel that for a contemporary achievement of a more homogeneous method quality in smoking interaction studies the development of (a) dedicated regulatory guideline(s) would be highly desirable and beneficial, to ultimately improve smoke‐related product labeling.
There are several limitations of our analysis that need to be considered. First, we only considered studies conducted in healthy subjects, which represented only a portion of the published studies. Thus, studies with oncologic or long‐lasting immune suppressant drugs, and other studies conducted in patient populations are not represented in our review. It cannot be excluded that evidence from patient studies might have complemented the information from healthy subject studies for the purpose of product labeling. Second, in our method review, we followed a strict and standardized approach by the general assumption that measures that have not been reported in the reviewed publications were actually not done, although it is acknowledged that publications may not include all methodological details and exclusion criteria of study protocols, such as concomitant medications and dietary restrictions. We are aware that this strict approach might have the potential to bias the overall methodological quality impression of the studies. However, there was no alternative to the chosen approach because most study protocols are not available in the public domain.
CONCLUSIONS AND FUTURE DIRECTIONS
Taken together, the present review reveals a considerable methodological heterogeneity across published smoking interaction studies, which often fell short of current study design standards for DDI or food‐drug interaction studies. Overall, the proper implementation and standardization of important methodological aspects was found to be poor.
These observed methodological deficiencies may be at least in part attributable to a lack of regulatory guidance over the past 3 decades for this type of interaction studies.
Two of the most striking conceptional weaknesses of the reported smoking interaction studies – as compared to food‐interaction studies – are (a) the common lack of attempts to capture maximum effects of smoking‐related CYP1A induction (i.e., to examine a “worst case” scenario), which requires investigation of a well‐defined group of heavy smokers; and (b) the missing enrollment of at least two well‐defined smoker populations (e.g., light and heavy smokers), which would allow to examine dose‐dependencies and may form the basis for modeling and simulation of effects in populations not studied.
The management of enzyme‐based drug interactions in drug development and regulation has transitioned in the last decade from reliance of findings from clinical interaction studies to a model‐informed paradigm. Quantitative translational models such as physiologically‐based PK (PBPK) models are routinely used to prospectively predict drug interaction liabilities and could become a useful tool in the evaluation of smoking DDIs. The apparent dependence of CYP1A induction on the dose of cigarette smoking (number of daily cigarettes) 29 makes it possible to verify a PBPK model for the purpose of predicting the effect of smoking on the PK of a CYP1A substrate. Such predictions can inform the conduct of a critical smoking interaction study. Results of such study can be used to verify the PBPK model and enable confident prediction of untested scenarios.
Another approach could be perhaps removing the focus of smoking studies from specific drugs, and establishing quantitative relationships between various levels of smoking (or being exposed to environmental smoking) via probes for target enzymes and transporters. This may offer a better opportunity to estimate the impact of smoking on various drugs when the pathways for the clearance and the fractions metabolized are known. An example of this approach has already been tried by one group, 29 but was not yet replicated.
Based on our review we anticipate that improved labeling of the outcomes of smoking interaction studies may be particularly challenging for sensitive CYP1A victim drugs, because the inducibility of CYP1A shows a large interindividual variability, and appears to be unrelated to any known CYP1A2 genotype. Whereas one CYP1A2 genotype was recently identified to be associated with an increase in CYP1A2 activity, 46 it appears that rather genetic variations of the nuclear AhR may determine the inducibility of CYP1A in humans. 48
The large interindividual variability in CYP1A inducibility with “high” and “low” CYP1A inducible phenotypes implies that dose‐adjustment recommendations for smokers based on group‐mean outcomes of smoking interaction studies may not be ideal for all smoking individuals, and that individual phenotyping of CYP1A2 activity in smokers may be recommended in the future for individualized dose recommendations in smokers at least for CYP1A2 substrate drugs with a narrow therapeutic index. Considering these gene‐smoking interactions of CYP1A2 are important, as individuals with low‐inducible genotypes will have hardly any increased level of CYP1A2 activity as a result of smoking. The impact of accounting for such interaction terms of covariates has been confirmed recently. 50
In conclusion, the systematic review of the most recent smoking interaction studies in healthy subjects provides valuable insights on how regulatory smoking DDI evaluations can be improved to inform adequate dosing recommendations in smokers.
AUTHOR CONTRIBUTIONS
R.H., I.R.‐M., A.R.‐H., and P.Z. wrote the manuscript. R.H. and I.R.‐M. designed the research. R.H. and I.R.‐M. performed the research. R.H. and I.R.‐M. analyzed the data.
FUNDING INFORMATION
No funding was received for this work.
CONFLICTS OF INTEREST
The authors declared no competing interests for this work.
Hermann R, Rostami‐Hodjegan A, Zhao P, Ragueneau‐Majlessi I. Seeing what is behind the smokescreen: A systematic review of methodological aspects of smoking interaction studies over the last three decades and implications for future clinical trials. Clin Transl Sci. 2023;16:742‐758. doi: 10.1111/cts.13494
REFERENCES
- 1. GBD 2019 Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the global burden of disease study 2019. Lancet. 2021;397:2337‐2360. doi: 10.1016/S0140-6736(21)01169-7 Erratum in: Lancet. 2021; 397: 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425‐438. doi: 10.2165/00003088-199936060-00004 [DOI] [PubMed] [Google Scholar]
- 3. Grech‐Bélanger O, Gilbert M, Turgeon J, PP LB. Effect of cigarette smoking on mexiletine kinetics. Clin Pharmacol Ther. 1985;37:638‐643. doi: 10.1038/clpt.1985.103 [DOI] [PubMed] [Google Scholar]
- 4. Bock KW, Schrenk D, Forster A, et al. The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP‐glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics. 1994;4:209‐218. doi: 10.1097/00008571-199408000-00005 [DOI] [PubMed] [Google Scholar]
- 5. Walle T, Walle UK, Cowart TD, Conradi EC, Gaffney TE. Selective induction of propranolol metabolism by smoking: additional effects on renal clearance of metabolites. J Pharmacol Exp Ther. 1987;241:928‐933. [PubMed] [Google Scholar]
- 6. Hanioka N, Hayashi K, Shimizudani T, et al. Stereoselective glucuronidation of propranolol in human and cynomolgus monkey liver microsomes: role of human hepatic UDP‐glucuronosyltransferase isoforms, UGT1A9, UGT2B4 and UGT2B7. Pharmacology. 2008;82:293‐303. doi: 10.1159/000165100 [DOI] [PubMed] [Google Scholar]
- 7. Ochs HR, Greenblatt DJ, Otten H. Disposition of oxazepam in relation to age, sex, and cigarette smoking. Klin Wochenschr. 1981;59:899‐903. doi: 10.1007/BF01721923 [DOI] [PubMed] [Google Scholar]
- 8. He X, Hesse LM, Hazarika S, et al. Evidence for oxazepam as an in vivo probe of UGT2B15: oxazepam clearance is reduced by UGT2B15 D85Y polymorphism but unaffected by UGT2B17 deletion. Br J Clin Pharmacol. 2009;68:721‐730. doi: 10.1111/j.1365-2125.2009.03519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration; 2016. Content current as of: 05/07/2020. Accessed October 29, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/clinical‐pharmacology‐labeling‐human‐prescription‐drug‐and‐biological‐products‐content‐and‐format.
- 10. US Food and Drug Administration . Guidance for industry: drug interaction studies—study design, data analysis, and implications for dosing and labeling. Draft Guidance 09/12/2006. Accessed October 29, 2022. https://www.federalregister.gov/documents/2006/09/12/E6‐15058/draft‐guidance‐for‐industry‐on‐drug‐interaction‐studies‐study‐design‐data‐analysis‐and‐implications.
- 11. US Food and Drug Administration . Guidance for industry: drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations. Draft Guidance 21/02/2012. Accessed Oct 29, 2022. https://www.federalregister.gov/documents/2012/02/21/2012‐3958/draft‐guidance‐for‐industry‐on‐drug‐interaction‐studies‐study‐design‐data‐analysis‐implications‐for.
- 12. European Medicines Agency . Guideline on the Investigation of Drug Interactions. 21 June 2012; CPMP/EWP/560/95/Rev. 1 Corr. 2**; Committee for Human Medicinal Products (CHMP). Accessed Oct 29, 2022. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐investigation‐drug‐interactions‐revision‐1_en.pdf.
- 13. Pharmaceuticals and Medical Devices Agency (PMDA) . 2019 PMDA Guideline (English Translation). Guideline on drug interaction for drug development and appropriate provision of information. Accessed October 29, 2022. https://www.xenotech.com/access‐adme‐research‐resources/resources/2019‐japanese‐pmda‐guideline‐english‐translation/.
- 14. US Food and Drug Administration . Guidance for industry: Clinical Drug Interaction Studies – Cytochrome P450 Enzyme‐ and Transporter‐Mediated Drug Interactions Guidance for Industry 05/07/2020. Accessed October 29, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/clinical‐drug‐interaction‐studies‐cytochrome‐p450‐enzyme‐and‐transporter‐mediated‐drug‐interactions.
- 15. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1917‐1921. doi: 10.2146/ajhp060414 [DOI] [PubMed] [Google Scholar]
- 16. Li H, Shi Q. Drugs and diseases interacting with cigarette smoking in US prescription drug labelling. Clin Pharmacokinet. 2015;54:493‐501. doi: 10.1007/s40262-015-0246-6 [DOI] [PubMed] [Google Scholar]
- 17. Scavone JM, Greenblatt DJ, LeDuc BW, Blyden GT, Luna BG, Harmatz JS. Differential effect of cigarette smoking on antipyrine oxidation versus acetaminophen conjugation. Pharmacology. 1990;40:77‐84. doi: 10.1159/000138644 [DOI] [PubMed] [Google Scholar]
- 18. Benowitz NL, Peng M, Jacob P 3rd. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharmacol Ther. 2003;74:468‐474. doi: 10.1016/j.clpt.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 19. Mourad SS, El‐Kimary EI, Barary MA, Hamdy DA. Pharmacokinetic interaction between linagliptin and tadalafil in healthy Egyptian males using a novel LC‐MS method. Bioanalysis. 2019;11:1321‐1336. doi: 10.4155/bio-2018-0097 [DOI] [PubMed] [Google Scholar]
- 20. Stewart JJ, Berkel HJ, Parish RC, et al. Single‐dose pharmacokinetics of bupropion in adolescents: effects of smoking status and gender. J Clin Pharmacol. 2001;41:770‐778. doi: 10.1177/00912700122010564 [DOI] [PubMed] [Google Scholar]
- 21. Tantcheva‐Poór I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P‐450 CYP1A2 activity in 863 healthy Caucasians using a saliva‐based caffeine test. Pharmacogenetics. 1999;9:131‐144. Erratum in: Pharmacogenetics. 1999; 9:781. [PubMed] [Google Scholar]
- 22. Ou‐Yang DS, Huang SL, Wang W, et al. Phenotypic polymorphism and gender‐related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000;49:145‐151. doi: 10.1046/j.1365-2125.2000.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125‐134. doi: 10.1111/j.1742-7843.2005.pto_973160.x [DOI] [PubMed] [Google Scholar]
- 24. Ogilvie BW, Torres R, Dressman MA, Kramer WG, Baroldi P. Clinical assessment of drug‐drug interactions of tasimelteon, a novel dual melatonin receptor agonist. J Clin Pharmacol. 2015;55:1004‐1011. doi: 10.1002/jcph.507 [DOI] [PubMed] [Google Scholar]
- 25. Fukasawa T, Yasui‐Furukori N, Aoshima T, Suzuki A, Tateishi T, Otani K. Single oral dose pharmacokinetics of quazepam is influenced by CYP2C19 activity. Ther Drug Monit. 2004;26:529‐533. doi: 10.1097/00007691-200410000-00011 [DOI] [PubMed] [Google Scholar]
- 26. Yun HY, Seo JW, Choi JE, Baek IH, Kang W, Kwon KI. Effects of smoking on the pharmacokinetics and pharmacodynamics of a nicotine patch. Biopharm Drug Dispos. 2008;29:521‐528. doi: 10.1002/bdd.637 [DOI] [PubMed] [Google Scholar]
- 27. Perera V, Gross AS, McLachlan AJ. Influence of environmental and genetic factors on CYP1A2 activity in individuals of south Asian and European ancestry. Clin Pharmacol Ther. 2012;92:511‐519. doi: 10.1038/clpt.2012.139 [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Liu L, Wang X, et al. In vivo assessment of the effect of CYP1A2 inhibition and induction on Pomalidomide pharmacokinetics in healthy subjects. J Clin Pharmacol. 2018;58:1295‐1304. doi: 10.1002/jcph.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plowchalk DR, Rowland YK. Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Clin Pharmacol. 2012;68:951‐960. doi: 10.1007/s00228-011-1189-y [DOI] [PubMed] [Google Scholar]
- 30. Becker RH, Sha S, Frick AD, Fountaine RJ. The effect of smoking cessation and subsequent resumption on absorption of inhaled insulin. Diabetes Care. 2006;29:277‐282. doi: 10.2337/diacare.29.02.06.dc05-1913 [DOI] [PubMed] [Google Scholar]
- 31. Dong SX, Ping ZZ, Xiao WZ, et al. Effect of active and passive cigarette smoking on CYP1A2‐mediated phenacetin disposition in Chinese subjects. Ther Drug Monit. 1998;20:371‐375. doi: 10.1097/00007691-199808000-00002 [DOI] [PubMed] [Google Scholar]
- 32. Murdoch RD, Zussman B, Schofield JP, Webber DM. Lack of pharmacokinetic interactions between cilomilast and theophylline or smoking in healthy volunteers. J Clin Pharmacol. 2004;44:1046‐1053. doi: 10.1177/0091270004266488 [DOI] [PubMed] [Google Scholar]
- 33. Jennings TS, Nafziger AN, Davidson L, Bertino JS Jr. Gender differences in hepatic induction and inhibition of theophylline pharmacokinetics and metabolism. J Lab Clin Med. 1993;122:208‐216. [PubMed] [Google Scholar]
- 34. Baak LC, Ganesh S, Jansen JB, Lamers CB. Does smoking influence the pharmacokinetics and pharmacodynamics of the H2‐receptor antagonist famotidine? Br J Clin Pharmacol. 1992;33:193‐196. doi: 10.1111/j.1365-2125.1992.tb04025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Granfors MT, Backman JT, Laitila J, Neuvonen PJ. Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther. 2005;78:400‐411. doi: 10.1016/j.clpt.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 36. Peterson S, Schwarz Y, Li SS, et al. CYP1A2, GSTM1, and GSTT1 polymorphisms and diet effects on CYP1A2 activity in a crossover feeding trial. Cancer Epidemiol Biomark Prev. 2009;18:3118‐3125. doi: 10.1158/1055-9965.EPI-09-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993;35:431‐436. doi: 10.1111/j.1365-2125.1993.tb04162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Labbé L, Robitaille NM, Lefez C, et al. Effects of ciprofloxacin on the stereoselective disposition of mexiletine in man. Ther Drug Monit. 2004;26:492‐498. doi: 10.1097/00007691-200410000-00006 [DOI] [PubMed] [Google Scholar]
- 39. Vistisen K, Loft S, Poulsen HE. Cytochrome P450 IA2 activity in man measured by caffeine metabolism: effect of smoking, broccoli and exercise. Adv Exp Med Biol. 1991;283:407‐411. doi: 10.1007/978-1-4684-5877-0_55 [DOI] [PubMed] [Google Scholar]
- 40. Vistisen K, Poulsen HE, Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992;13:1561‐1568. doi: 10.1093/carcin/13.9.1561 [DOI] [PubMed] [Google Scholar]
- 41. Rost KL, Brösicke H, Brockmöller J, Scheffler M, Helge H, Roots I. Increase of cytochrome P450IA2 activity by omeprazole: evidence by the 13C‐[N‐3‐methyl]‐caffeine breath test in poor and extensive metabolizers of S‐mephenytoin. Clin Pharmacol Ther. 1992;52:170‐180. doi: 10.1038/clpt.1992.126 [DOI] [PubMed] [Google Scholar]
- 42. Rost KL, Brösicke H, Heinemeyer G, Roots I. Specific and dose‐dependent enzyme induction by omeprazole in human beings. Hepatology. 1994;20:1204‐1212. [PubMed] [Google Scholar]
- 43. Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29‐60. doi: 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration . Guidance for industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs – General Considerations; Draft Guidance. Content current as of March 2014. Accessed October 31, 2022. https://www.fda.gov/files/drugs/published/Bioavailability‐and‐Bioequivalence‐Studies‐Submitted‐in‐NDAs‐or‐INDs‐%E2%80%94‐General‐Considerations.pdf.
- 45. European Medicines Agency . Guideline on the Investigation of Bioequivalence; 2010. CPMP/EWP/QWP/1401/98 Rev. 1/Corr **; Committee for Human Medicinal Products (CHMP). Accessed Oct 31, 2022. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐investigation‐bioequivalence‐rev1_en.pdf.
- 46. Koonrungsesomboon N, Khatsri R, Wongchompoo P, Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: a systematic review and meta‐analysis. Pharmacogenomics J. 2018;18:760‐768. doi: 10.1038/s41397-017-0011-3 [DOI] [PubMed] [Google Scholar]
- 47. Ma Q, Lu AY. Origins of individual variability in P4501A induction. Chem Res Toxicol. 2003;16:249‐260. doi: 10.1021/tx0200919 [DOI] [PubMed] [Google Scholar]
- 48. Okey AB, Manchester DK, Feeley MM, Grant DL, Gilman A. Ah receptor characterization in human placenta samples. Organohalogen Compd. 1995;25:177‐184. [Google Scholar]
- 49. Backman JT, Schröder MT, Neuvonen PJ. Effects of gender and moderate smoking on the pharmacokinetics and effects of the CYP1A2 substrate tizanidine. Eur J Clin Pharmacol. 2008;64:17‐24. doi: 10.1007/s00228-007-0389-y [DOI] [PubMed] [Google Scholar]
- 50. Mostafa S, Polasek TM, Bousman C, et al. Delineating gene‐environment effects using virtual twins of patients treated with clozapine. CPT Pharmacometrics Syst Pharmacol 2022;12:168‐179. doi: 10.1002/psp4.12886 [DOI] [PMC free article] [PubMed] [Google Scholar]


