Abstract
Decision‐making for reimbursement and clinical guidelines (CGs) serves different purposes although the decision‐criteria and required evidence largely overlap. This study aimed to assess similarities and discrepancies between health technology assessment (HTA) reports as compared to CGs for multiple sclerosis (MS) medicines. All HTA reports and corresponding CGs for MS from the UK, France, Germany, the Netherlands, Poland, Sweden, and the European Union were assessed to identify synergies in recommendations for MS medicines (approved 1995–2020). A content analysis of HTA reports and CGs was performed to identify similarities and discrepancies in wording of treatment recommendations across documents. We assessed 132 HTA reports and 9 CGs for 16 MS treatments. Final recommendations for reimbursement and inclusion in CGs were mostly similar (90%), albeit with considerable differences in treatment lines and subindications. Since 2010, HTA reports refer to the use of CGs in 42% (55/132) and to consultations with clinicians in 43% (57/132) of cases. Six of nine CGs referred to HTA reports and two referred to HTA consultations, in one case having a formal relation to the HTA organization. CGs referenced pharmacoeconomic studies (4/9) for costs and cost‐effectiveness. To date, not all new HTA recommendations for MS treatments are included in CGs. Some synergy exists between treatment recommendations in HTA reports and CGs, although discrepancies were seen in timelines and in recommended treatment lines and subindications. More stakeholder dialogue and/or consultation of each other's publications may further improve synergy, facilitate transparency, and enhance patient access.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Little is known about synergies between health technology assessment (HTA) recommendations and clinical practice guidelines, while both HTA organizations and guideline developers often derive recommendations from similar evidence, generated from common underlying data.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study investigated the alignment of processes as well as similarities and discrepancies between treatment recommendations for multiple sclerosis in HTA reports as compared to clinical guidelines (CGs).
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
HTA organizations and clinicians developing guidelines regularly use the conclusions from each other's reports, although dialogue was found to be rare. Additionally, the timing of publication of recommendations was often misaligned, leading to contradicting recommendations. Furthermore, a wide variety in patient population definitions and treatment line recommendations was found.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The study results indicate that there is room for better alignment of HTA recommendations and CGs. Alignment of assessment timelines as well as employing systematic dialogue between HTA organizations and CG developers may facilitate more timely guideline recommendations and therewith earlier patient access. Alignment on patient definitions may provide more clarity on eligible patients for prescribing physicians as well as for HTA assessors. Alignment of underlying data sources and improving information in HTA reports to better contribute to CG development may reduce duplication of effort for both stakeholders.
INTRODUCTION
Health technology assessment (HTA) compares new health technologies to existing standard of care in order to inform market access policies and decision‐making, usually for pricing and reimbursement and in some cases for clinical practice. 1 HTA recommendations in Europe are still predominantly made on a national level. Differences in context (e.g., a country's gross domestic product), scope, and methodology in each country may cause divergence in the recommendations. 2 , 3 In the field of multiple sclerosis (MS) this divergence clearly exists. 4 , 5
Clinical treatment guidelines (CGs) are generally used as a tool to ensure guidance of evidence‐based medicine, aiming to advance quality of care and increasingly aiming to treat in a cost‐effective manner. 6 Due to a continuous increase in healthcare expenditure, researchers focused on frameworks to incorporate HTA recommendations in evidence‐based treatment practice. 7 , 8 , 9 , 10
Given the similar purpose of informing decision‐making, albeit in a different context, it is no surprise that the processes of relative effectiveness assessment (REA) in HTA and CG development contain considerable overlap. CGs, however, do not usually use the full HTA reports during development. 11 Collaboration and alignment among HTA organizations and between HTA and CG developers could prevent duplication of efforts and facilitate equality in patients' access to evidence‐based and cost‐effective treatments among European countries.
Initiatives for collaboration among HTA organizations and between HTA and CGs have emerged. The European Network for HTA (EUnetHTA) is the largest HTA collaboration in Europe, and raised the issue of translating cost‐effectiveness into CGs in 2015 as a methodological issue that requires further research. 12 GINAHTA, a collaboration between Guidelines International (G‐I‐N) and the International Network of Agencies for HTA (INAHTA), is a global initiative established in 2015, with the purpose “to explore common methods and to facilitate collaboration and sharing of products between the HTA and guideline communities”. 13
The appraisal and development of CGs were preferred services of focus for HTA organizations among decision‐makers in Spain, according to Andradas et al. in 2008. 14 Additionally, among healthcare providers there was a wide interest in assessments relevant to clinical decision‐making. The Belgian Health Care Knowledge Centre (KCE) and the UK's National Institute for Health and Care Excellence (NICE) already (co)produce CGs. However, to our knowledge there is a lack of research on the synergies between HTA reports and CGs on a broader scale. Specific knowledge on the interaction between the two decision‐makers, the alignment between assessments and processes, and the eventual recommendations is still missing.
The field of MS is of particular interest due to the many developments. Over 10 relatively expensive disease‐modifying treatments for MS have entered the market in the past decade. 15 The variety of treatment options, including different combinations and treatment sequences, with costly medication requires evidence‐based guidance for neurologists allowing them to make a personalized treatment decision, while considering the budgetary constraints. A divergence in HTA recommendations and variation in different CGs may complicate this decision. 16 For this reason, MS is one of the case studies in the European Commission‐funded (H2020) HTx project.
The HTx project is supported by the European Union (EU) lasting for 5 years from January 2019, with the aim to create a framework for the next‐generation HTA to support patient‐centred, societally oriented, real‐time decision‐making on access to and reimbursement for health technologies throughout Europe. 17 As a part of this project, this study aimed to quantify the similarities and discrepancies between reports from European HTA organizations, including their therapeutic and economic assessment, as compared to CGs for MS of the respective countries.
METHODS
This study was a document analysis for which we systematically extracted data from HTA reports and CGs. 18 The systematic data extraction focused on the references that are made to the other decision‐maker (HTA reports to CGs and CGs to HTA reports, process synergy), the final reimbursement recommendations, the recommended treatment lines and patient populations, and timelines (outcome synergies).
Country and document selection
Documents from France, Germany, the Netherlands, Poland, Sweden, and the UK were selected. These countries are larger jurisdictions, thus there is a large impact of policies, methods, and guidelines (Germany, France, Poland, UK). Additionally, these countries have pioneering HTA organizations with publicly available reports, are involved in the development of guidelines (France, Germany, the Netherlands, UK), or involved in the HTx project (the Netherlands, UK). We also aimed for a balanced geographical spread throughout the EU. Last, all included HTA organizations are directly or indirectly involved in the country's decision‐making process. All HTA reports for these treatments were included and were obtained through the websites of the respective HTA agencies and covered both initial and reassessments. All publicly available national CGs for each country were included, after searching for ‘national/country name’, ‘treatment/clinical guideline’, and multiple sclerosis' in Google, both in English as well as in the respective national language. CGs were included if they evaluated preferably all, but at least the disease‐modifying, therapies for MS and aimed to inform treatment practice. A European guideline for MS treatment and the reports for MS treatments published by EUnetHTA were additionally included as a comparison. 19 , 20 Regulatory European public assessment reports (EPARs) from the European Medicines Agency (EMA) were collected for the comparison of patient populations. Table 1 shows the selected HTA organizations and included CGs.
TABLE 1.
Overview of included countries with relevant health technology assessment organizations and clinical guidelines.
| Country | HTA organizations | Reference to website for HTA reports | Clinical guidelines | Year | Reference |
|---|---|---|---|---|---|
| France | Haute Autorité de Santé (HAS) | 21 | HAS, Actes et prestations, affection de longue durée, Sclérose en plaques | 2015 | 22 |
| Germany | Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) | 23 | DGN/KKNMS Leitlinie zur Diagnose und Therapie der MS | 2014 | 24 |
| Der Gemeinsame Bundesausschuss (G‐BA) | 25 | ||||
| The Netherlands | Dutch National Healthcare Institute (ZIN) | 26 | Nederlandse Vereniging voor Neurologie, Multipele Sclerose | 2012 | 27 |
| Addendum bij de richtlijn Multiple Sclerose | 2020 | 28 | |||
| Poland | Agency for Health Technology Assessment and Tariff System (AOTMiT) | 29 | Leczenie stwardnienia rozsianego Zalecenia Polskiego Towarzystwa Neurologicznego | 2016 | 30 |
| Sweden | Dental and Pharmaceutical Benefits Agency (TLV) | 31 | Nationella riktlinjer Socialstyrelsen, Vård vid multipel skleros och Parkinsons sjukdom | 2016 | 32 |
| Svenska MS‐sällskapet, Läkemedel | 2019–2021 | 33 | |||
| UK | National Institute for Health and Care Excellence (NICE) | 34 | National Health Service (NHS) Treatment Algorithm for Multiple Sclerosis Disease‐Modifying Therapies | 2019 | 35 |
| Association of British Neurologists: revised guidelines for prescribing disease‐modifying treatments in multiple sclerosis | 2015 | 36 | |||
| European | European Network for Health Technology Assessment (EUnetHTA) | 20 | ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis | 2018 | 19 |
Treatment selection
All pharmaceuticals in the EU with an approved indication for MS, including (active) relapsing–remitting MS (RRMS), relapsing MS, primary progressive MS (PPMS), (active) secondary progressive MS, and progressive MS, and an active market authorization in August 2020 were included. It was assumed that no HTA was performed for generics, thus they were excluded. Subsequently, all treatments that had not been assessed by any of the HTA organizations as well as withdrawn treatments were excluded.
Data extraction
A data extraction tool was developed based on two steps (Table S1). The data extraction tool is a structured Excel file (Excel for Windows, 2012; Microsoft) that helps with systematically recording the data from all the documents as well as with version control. Text in German, French, Dutch, Swedish, and Polish was translated to English before extraction. First, parameters that could show the synergies or explain potential differences were deductively included. To identify outcome‐related synergies, we included the final recommendation with corresponding arguments, treatment positioning, specified population, and additional restrictions or comments. The recommendations and corresponding arguments for the relative and cost‐effectiveness assessment were recorded separately. To identify process‐related synergies, we documented the used literature, the comparator used, references to the other stakeholder's documents, or consultation of the other stakeholder, and the time gap between the publication of the HTA reports and CGs. Second, the data extraction tool was piloted with one medicine (fingolimod) and based on this inductive approach, complemented with the reason for HTA assessment and the stakeholder initiating the assessment (the HTA organization, the pharmaceutical companies, professional organizations, etc.), the reason for guideline updates and references to pharmacoeconomic studies.
Data for France, Germany, the Netherlands, Sweden, the UK, and EU were extracted by one author (MAH). The parameters that were embedded in large texts (references to CGs, consulted physicians, the reference to HTA reports, pharmacoeconomic studies, and consulted HTA organizations) were extracted using a prespecified search strategy. A list of general search terms was developed for each language during the fingolimod pilot, consisting of the main concepts that described the extracted parameter (e.g., ‘guideline’, ‘neurologist’, ‘expert’). Two documents from each country were fully analyzed to verify the search strategy. The search terms are listed in Tables S2 and S3. A second author (RAV) duplicated the data extraction for a small selection of documents (all teriflunomide reports, n = 8 of 132), after which the results were compared, and any discrepancies were resolved through discussion. A third and fourth author checked the extracted data for completion, separately for HTA (DMD) and the CGs (BAJ). The Polish data extraction was performed separately by two native Polish authors (AZ, MZ) via a similar approach.
Data analysis
The data analysis was performed in Excel using descriptive statistics. The comparison of population definitions between decision‐makers was performed per subindication, as often used in clinical trials (i.e., clinically isolated syndrome, PPMS, RRMS, rapidly‐evolving severe (RES) MS, secondary progressive MS, and secondary progressive MS with relapses), per treatment line (first, second, third) and preferences within treatment lines, as well as per clinical or economic restrictions. 37 Qualitatively described cases were used to exemplify the descriptive statistics. A timeline of all events of market authorization, HTA assessment, and CG publication was made to visualize any time lags and the synergies in the final recommendations.
RESULTS
Included MS treatments and documents
Twenty‐one treatments for MS were identified, of which one is used off‐label (rituximab). All pharmaceuticals were approved between 1995 and 2020. 38 Five treatments were excluded due to not (yet) being assessed by any of the HTA organizations at the time of document selection (N = 4, cannabidiol, ofatumumab, ozanimod, rituximab) or due to withdrawal from the market (N = 1, daclizumab). Table 2 shows the included treatments (N = 16).
TABLE 2.
Overview of the included and excluded multiple sclerosis treatments.
| Trade name | Active substance | Authorized (EMA) | Type of treatment |
|---|---|---|---|
| Betaferon | Interferon beta‐1b | 1995 | DMT |
| Avonex | Interferon beta‐1a | 1997 | DMT |
| Rebif | Interferon beta‐1a | 1998 | DMT |
| Novantrone/Eslep | Mitoxantrone | 1998 | DMT |
| Copaxone | Glatiramer acetate | 2004 | DMT |
| Tysabri | Natalizumab | 2006 | DMT |
| Extavia | Interferon beta‐1b | 2008 | DMT |
| Gilenya | Fingolimod | 2011 | DMT |
| Sativex | Cannabidiol/delta‐9‐tetrahydrocannabinol | 2011 | Symptomatic |
| Fampyra | Fampridine | 2011 | Symptomatic |
| Lemtrada | Alemtuzumab | 2013 | DMT |
| Aubagio | Teriflunomide | 2013 | DMT |
| Tecfidera | Dimethyl fumarate | 2014 | DMT |
| Plegridy | Peginterferon beta 1‐a | 2014 | DMT |
| Mavenclad | Cladribine | 2017 | DMT |
| Ocrevus | Ocrelizumab | 2018 | DMT |
| Zinbryta | Daclizumab | 2018 | DMT |
| Mayzent | Siponimod | 2020 | DMT |
| Zeposia | Ozanimod | 2020 | DMT |
| Kesimpta | Ofatumumab | 2021? | DMT |
| Rituxan + generics | Rituximab | Not for MS | DMT |
Note: Active substances in bold type were included, the substances in light gray were excluded as they have been withdrawn from the market (daclizumab) or were not assessed by most health technology assessment organizations (all other).
Abbreviations: DMT, disease‐modifying treatment; EMA, European Medicines Agency; MS multiple sclerosis.
A total of 132 HTA reports were collected for the 16 included MS medicines. These included initial assessments (N = 70), reassessments (N = 51), and assessments of the extension of indications (N = 13) (Figure 1). The majority of these reports gave a positive recommendation, ranging from 63% to 70% for initial assessments, reassessments, and indication extensions. Most HTA reports were collected from the Haute Autorité de Santé (HAS, France, N = 46) as HAS periodically reassesses treatments. Nine guidelines published between 2014 and 2020 were identified, of which two were developed in the UK and two in Sweden.
FIGURE 1.
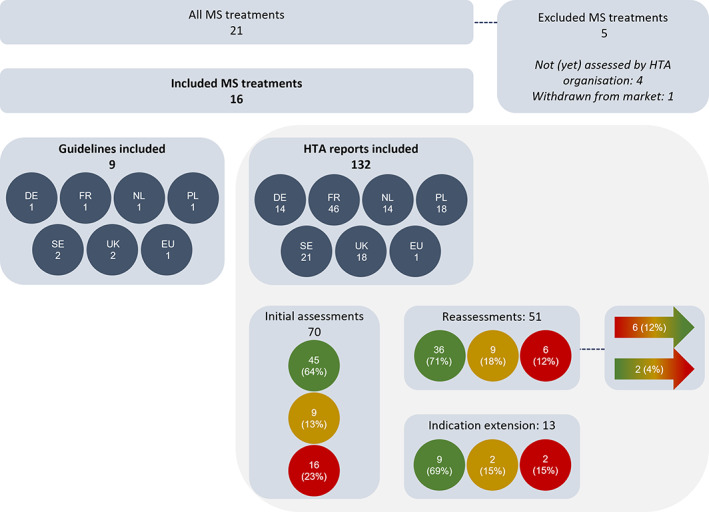
Overview of the included health technology assessment (HTA) reports and clinical guidelines. The green, yellow, and red circles represent the HTA reports with a positive, restricted, or negative recommendation, respectively. The color‐gradient arrows represent the HTA reports that turned from negative or restricted to a more positive recommendation, or from a positive or restricted to a more negative recommendation after reassessment. DE, Germany; EU, European Union; FR, France; HTA, health technology assessment; MS, multiple sclerosis; NL, the Netherlands; PL, Poland; SE, Sweden; UK, United Kingdom.
Final recommendations
A total of 82 comparisons between HTA recommendations and CGs could be made. In five cases (6%) the CG recommendations contradicted the recommendation in HTA reports. Teriflunomide (TF) and dimethyl fumarate (DMF) received a negative recommendation by the Agency for Health Technology Assessment and Tariff System (AOTMiT) for clinical and economic reasons, whereas the CG of the Polish Neurological Society did recommend its use. AOTMiT concluded that the evidence for effectiveness was available only for a smaller population than it was for comparable treatments. The economic model was considered methodological unsound (TF) and the evidence for an added clinical benefit was weak whereas costs were significantly higher than for comparators (DMF). The CG included TF and DMF as both treatments were considered safe and effective.
Similar contradictions were observed in Germany. The Deutsche Gesellschaft für Neurologie included fampridine (FP), TF, and DMF in their treatment algorithm. Arguments for inclusion of TF and DMF in the CG focused predominantly on the safety profile. All treatments were considered long‐term safe and well tolerable. In the case of FP, the CG concluded on significant effectiveness for a small subgroup of the population. Conversely, the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) found no added benefit for all three of these treatments. Data were considered insufficient for proving effectiveness on hard endpoints and did not use an appropriate comparator.
One smaller discrepancy was observed for interferons in the UK. NICE recommended three of four available interferons. Betaferon received a negative recommendation as it was valued equal to the other assessed interferon β‐1b, though at a higher price. NHS England adopted this recommendation in its treatment algorithm, whereas the Association of British Neurologists (ABN) CG recommended the use of interferon β‐1b generically, without distinguishing between brands. It is worth noting that the ABN guideline would be used not only in England, where both NHS England and NICE recommendations apply, but also to in other parts of the UK (Scotland, Wales, and Northern Ireland).
Patient populations
Many minor differences in population definitions could be noted between the EMA label, HTA reports, and CGs due to differences in wording. This included the use of a different definition of ‘treatment line’ and differences in recommendations for subindications of MS, such as clinically‐isolated syndrome (CIS) or RES MS. As shown in Figure 2, not many differences were identified between population definitions for the most prominent subindications. For the smaller subindications this was more diverse. Many of the treatments were recommended for CIS, whereas this was not explicitly included in the EMA label. For RES MS the opposite was seen. The EMA label stated use for RES whereas this was not often explicitly mentioned in CGs.
FIGURE 2.
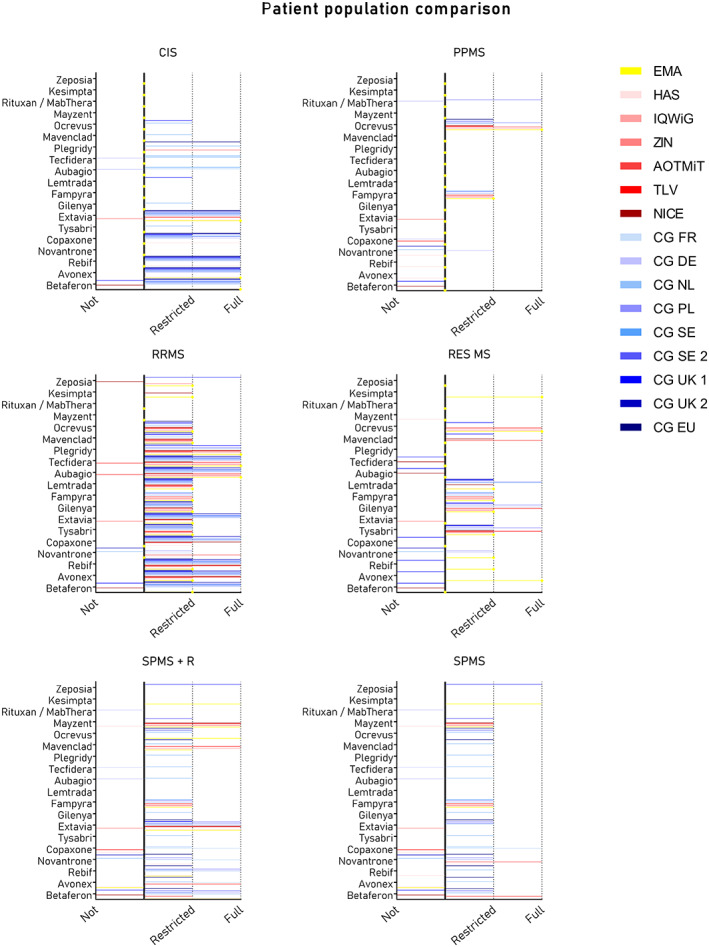
Comparison of patient population definitions. Each graph visualizes the recommendations per subindication for multiple sclerosis treatments by the European Medicines Agency (yellow), health technology assessment (HTA) organizations (red), and clinical guidelines (blue) in the six European countries. Recommendations were clustered into: Not = not recommended; Restricted = positive recommendations with restrictions; or Full = full positive recommendation. AOTMiT, Agency for Health Technology Assessment and Tariff System; CG, clinical guideline; CG DE, DGN/KKNMS Leitlinie zur Diagnose und Therapie der MS; CG EU, ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis; CG FR, HAS, Actes et prestations, affection de longue durée, Sclérose en plaques; CG NL, Nederlandse Vereniging voor Neurologie, Ziektemodulerende Behandeling van Multiple Sclerose bij volwassenen, Addendum bij de richtlijn Multiple Sclerose; CG PL, Leczenie stwardnienia rozsianego Zalecenia Polskiego Towarzystwa Neurologicznego; CG SE, Nationella riktlinjer Socialstyrelsen, Vård vid multipel skleros och Parkinsons sjukdom; CG SE 2, Svenska MS‐sällskapet, Läkemedel; CG UK 1, National Health Service Treatment Algorithm for Multiple Sclerosis Disease‐Modifying Therapies; CG UK 2, Association of British Neurologists: revised guidelines for prescribing disease‐modifying treatments in multiple sclerosis; CIS, clinically‐isolated syndrome; EMA, European Medicines Agency; HAS, Haute Autorité de Santé; IQWiG, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; NICE, National Institute for Health and Care Excellence; PPMS, primary progressive multiple sclerosis; RES MS, rapidly‐evolving severe MS; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS; SPMS‐R, SPMS with relapses; TLV, Dental and Pharmaceutical Benefits Agency; ZIN, Dutch National Healthcare Institute.
Positioning of treatments
In total, 74 comparisons of treatment position (i.e., the line of treatment) could be made. Thirty‐three (45%) compared treatment line recommendations were exactly the same in the HTA report and in the CG. In four cases (5%) the recommended treatment line started in the same line and was applied to the same subindication but was extended to further treatment lines in the CG. An example is natalizumab in the UK that was recommended by NICE as a first‐line treatment for RES MS. The National Health Service (NHS) extended this to the use of natalizumab in the second‐ and third‐line RES MS as well, after failure of previous treatments. In 30 cases (40%) the recommendations did not differ in terms of the treatment line that was recommended, though the recommended indication was extended. TF in the Netherlands was recommended by the Dutch National Health Care Institute (ZIN) as first‐line treatment for RRMS. The CG recommended this treatment for the first line as well, but extended the indication to CIS and active secondary progressive MS (SPMS). Eight cases (11%) contained one or both major differences; five recommendations (7%) differed in indication and six recommendations (8%) differed in treatment line. For example, ocrelizumab was recommended by NICE as first‐line treatment for PPMS and as second line treatment for RRMS. The NHS guidance, contrarily, included ocrelizumab as a first‐line treatment for RRMS and RES MS, but not PPMS. Additionally, fingolimod was recommended as second‐line treatment by NICE whereas the ABN CG described the use as first‐line treatment.
In Germany, all recommended treatment lines were the same across HTA reports and CGs. However, IQWIG concluded negatively in all reports, whereas the CG was positive for these same treatments in this position. This may be explained by the positive decisions from Der Gemeinsame Bundesausschuss (G‐BA), the reimbursement decision‐making body in Germany. Generally, there was no trend identified where one stakeholder was more cautious in recommending treatments in its recommendations than the other.
References in HTA reports
Of 132 HTA reports, only 55 (42%) referred to a CG (Figure 3). Half the reports that referred to a CG included multiple CGs often from different countries (N = 30, 54%). Six of 55 HTA reports (11%) mentioned CGs indirectly by referring to information that was received from the manufacturer or an external stakeholder that referred to CGs. The CGs were generally used for identification of the appropriate comparator and (diagnostic) start and stop criteria. The use of CGs in HTA seemed to have increased after 2010. The proportion of HTA assessments referring to a CG before 2010 was only 5% (N = 1/20), whereas this proportion was 48% after 2010 (N = 54) and stayed constant over time thereafter. Differences between countries were more apparent. In France (0%), Germany (29%), and Sweden (48%), HTA reports mentioned CGs less often as compared to HTA reports from the Netherlands (86%), Poland (90%), and the UK (67%).
FIGURE 3.
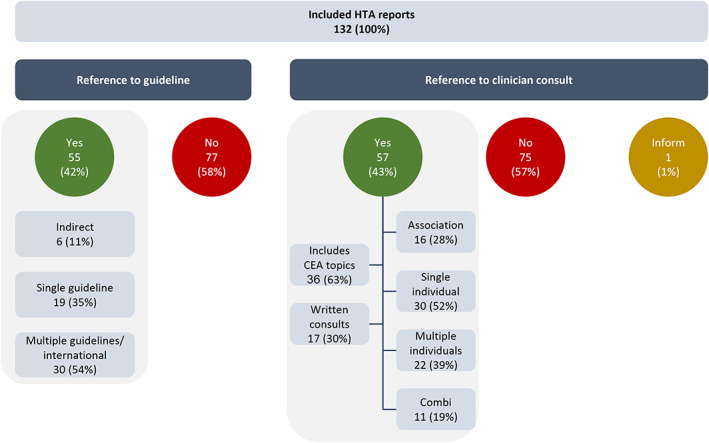
Health technology assessment reports referring to clinical guidelines and clinician consultations. CEA, cost‐effectiveness analysis; HTA, health technology assessment.
Furthermore, 43% (N = 57) HTA reports mentioned a consultation with clinicians or experts. Two‐thirds (63%, N = 36) of these consultations covered topics relevant to both the therapeutic as well as economic assessment. One‐third of the consultations (30%, N = 17) took place in a written format, namely the clinicians could provide written feedback on a draft report. HTA organizations reached out to either individuals (a single expert [52%, N = 30] or to multiple [39%, N = 22]) or clinician organizations (28%, N = 16) for consultation. Consultations were used to supplement information from CGs with practical considerations on the foreseen treatment position, specific patient populations, and (treatment, costs, prevalence of) adverse events. Similar to the trend for CG references, differences among countries were observed, although within countries the same strategy was often used for every new assessment over time.
References in clinical guidelines
Seven of nine guidelines referred to relevant HTA reports in their respective country (Figure 4). Two of these CGs also reported consulting HTA representatives, of which one was developed within the HTA organization (HAS) and the other by NHS England, which is legally obligated to fund treatments recommended by NICE. No independent guideline reported a consultation with HTA representatives during the development process. HTA reports were generally used for their final reimbursement recommendation and the specific patient population to which this recommendation applies. Four guidelines referred to pharmacoeconomic studies other than HTA reports. The pharmacoeconomic studies were used to acquire details on treatment costs and cost‐effectiveness.
FIGURE 4.
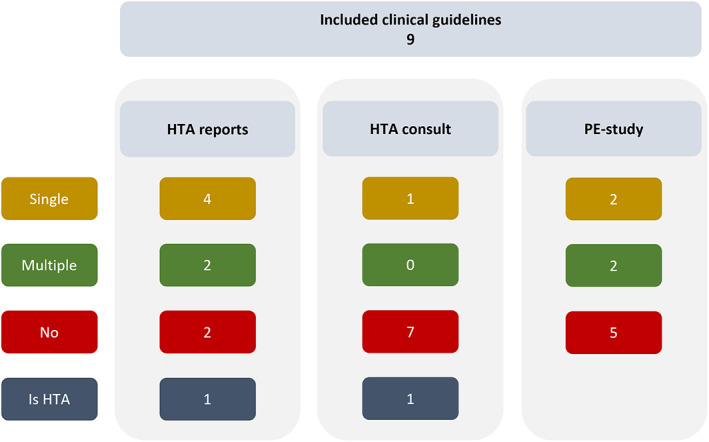
Clinical guidelines referring to health technology assessment reports, consultations and pharmacoeconomic studies. HTA, health technology assessment; PE, pharmacoeconomic.
Timing of events
Guidelines were updated periodically and included all relevant treatments at the moment of updating, whereas treatments were mostly assessed individually by HTA organizations following their marketing authorization. Consequently, there was an inevitable gap between reimbursement and uptake in CGs. In the case of MS, on average three new treatments were introduced on the market before a guideline was updated. This represents on average a lag of 2.6 years from market authorization until treatments are included in guidelines. Reassessments in HTA, similarly, did not trigger guideline updates as was exemplified by the case of alemtuzumab in France. The initial assessment of alemtuzumab by HAS resulted in a negative advice that was adopted in the HAS CG. Three positive reassessments in 2016, 2017, and 2018 had not yet been implemented in the CG at time of this study, meaning that contradicting recommendations existed. Another timing discrepancy came from the Netherlands where siponimod was positively assessed by ZIN just before publication of the CG. Siponimod was not yet included in the CG, while HTA assessment and CG development processes had at least partly been done in parallel. A similar timing issue was observed in the UK in the case of peginterferon β‐1a. The NHS guidance reported having no information on this treatment in the treatment algorithm, and listed it as “pending for NICE recommendation” until the CG would be revised. An overview events can be found in the timeline in Figure 5.
FIGURE 5.
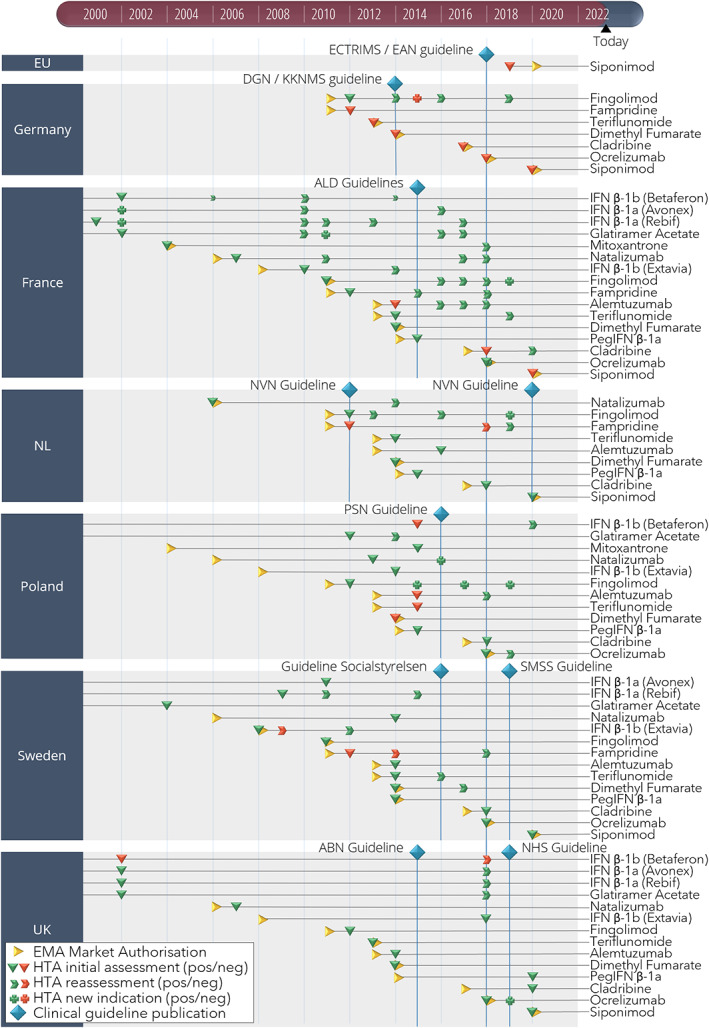
Market authorization, health technology assessment (HTA), and clinical guideline (CG) events for multiple sclerosis (MS) treatments in six countries. A timeline showing the events of market authorization, HTA assessment, and CG publication for MS treatments in six European countries between 2000 and 2021. ABN, Association of British Neurologists: revised guidelines for prescribing disease‐modifying treatments in multiple sclerosis; ALD, Haute Autorité de Santé Actes et prestations, affection de longue durée, Sclérose en plaques; DGN/KKNMS, Leitlinie zur Diagnose und Therapie der MS; ECTRIMS/EAN, guideline on the pharmacological treatment of people with multiple sclerosis; EMA, European Medicines Agency; EU, European Union; HTA, health technology assessment; NHS, National Health Service Treatment Algorithm for Multiple Sclerosis Disease‐Modifying Therapies; NL, the Netherlands; NVN, Nederlandse Vereniging voor Neurologie, Ziektemodulerende Behandeling van Multiple Sclerose bij volwassenen, Addendum bij de richtlijn Multiple Sclerose; PSN, Leczenie stwardnienia rozsianego Zalecenia Polskiego Towarzystwa Neurologicznego; Socialstyrelsen, Nationella riktlinjer Socialstyrelsen, Vård vid multipel skleros och Parkinsons sjukdom; SMSS, Svenska MS‐sällskapet, Läkemedel; UK, United Kingdom.
DISCUSSION
Our study is the first to compare recommendations in HTA reports and CGs. It shows that the final recommendations made by both decision‐makers were usually similar, and final HTA recommendations were generally described in CGs for MS. However, differences were observed in recommended treatment line and patient population definition. Additionally, there seemed to be a lack of systematic consultations between HTA organizations and CG developers, and time lags were observed between the publication of HTA reports and updates of CGs.
Relation between HTA assessments and CGs
Discrepancies in the recommended eligible patient populations may be explained by the discussion on the definition of subpopulations within MS. 39 , 40 Some argue that relapsing and progressive forms of MS are two distinct types of the diseases, as often used in trials. 39 Others argue that the manifestation of MS evolves over time rather than consisting of distinct subtypes, as age is an important predictor for the way MS is manifested and modulates the frequencies of relapse and thus phenotype presentation. 40 Similarly, different definitions of treatment lines may be used among decision‐makers and lead to differences in treatment line recommendations. Most CGs and HTA reports, however, seemed to have followed the McDonald criteria for diagnosis of MS. 41 The ABN guideline divides all treatments into moderate and high‐efficacy treatments, representing first and second line. 36 For example, fingolimod was characterized ‘moderate’ and thus first‐line treatment. NICE concluded on a second‐line treatment, as the first‐line options interferon and glatiramer acetate were considered favorable due to their safety profile. Alignment on patient definitions may provide more clarity on eligible patients for prescribing physicians when confronted with patients in a group with discrepant recommendations, as well as for HTA assessors, for example, aiming to estimate eligible patient numbers.
The low rates of consultation between HTA organizations and CG developers in the field of MS may be explained by a lack of formalized procedures. NICE seemed to be the only HTA organization that clearly describes the procedure for involvement of physician experts and guideline developers on their website. 42 The lack of description of consultation events may not necessarily mean that these do not take place. However, it may indicate that these consultations may not happen systematically, and it may be unclear for clinicians and physician expert organizations (e.g., specialist or professional organizations) how to get involved.
The delay between the publication of HTA reports and subsequent CG updates as described in our results may at least partly be explained by the financing structure of CG development. Updating guidelines may require funding that is sometimes granted only for an update relating to multiple treatments. The subsequent extensive updating process may introduce delays, despite the potential willingness among clinicians to maintain a living CG, namely dynamically updated CGs to provide real‐time information to the healthcare provider at the point of care. MAGIC (‘making GRADE the irresistible choice’) is a non‐profit foundation aiming to “increase value and reduce waste in healthcare through a digital and trustworthy evidence ecosystem”. It is one of the first examples of the trend towards living guidelines through intuitive and efficient software developed for guideline developers and users. 43 Alignment of assessment timelines as well as systematic dialogue between HTA organizations and CG developers may facilitate more timely guideline recommendations and therewith earlier patient access. However, the ever‐changing healthcare context (e.g., treatment landscape, medical need, knowledge about safety profiles, healthcare organization, economical context, etc.) requires that decisions on reimbursement or treatment positioning in guidelines evolve with the context. 44 Thus, concordance may not be achieved at any point in time.
HTA and CGs in relation to the broader patient access process
Besides HTA organizations and CG developers, regulatory bodies also make use of similar underlying data for decision‐making on market authorization. An extensive review on the synergies between regulatory authorities and HTA organizations concluded on the importance of the alignment of evidentiary requirements, for example, eligible patient populations. We believe that some of the identified facilitators for alignment in this review may be extrapolated to include CG developers, such as early stakeholder dialogue, clear post‐authorization data generation plans, and parallel review procedures. 45 These largely shared evidence requirements were also described by Woolf and colleagues in a series of article on methods to develop guidelines. 46 Existing early dialogues are usually limited to only three stakeholders: manufacturers, regulators, and HTA organizations. Extension of these dialogues into a quadripartite, giving CG developers a formal role in these dialogues, could help further alignment of the process including the step from reimbursement to update in CGs. Early dialogue could facilitate post‐authorization data generation plans for reassessments in HTA and living guidlelines. 47 Parallel reviews were suggested to increase efficiency in the process, allowing for earlier patient access. This solution could also be extended to a simultaneous review for uptake in CGs. Alternatively, HTA reports may be improved to better contribute to CG development, as was also attempted by the EMA aiming to improve the contribution of regulatory assessment reports to HTAs. Alignment of underlying data sources and improving HTA reports that better support CG developers could potentially reduce duplication of efforts, since our results showed that CGs more often refer to peer‐reviewed papers to obtain cost‐effectiveness information than to the relevant HTA reports.
Strengths and limitations of the present study
A diverse set of countries was considered in our study, including diversity in size and European region. Our results showed distinct differences among the countries’ procedures, indicating that our results are not automatically generalizable to other European countries. Available information was limited to what was actually reported and published by HTA organizations and CG developers. Practice might differ from what was reported, for example, informal moments of contact between HTA organizations and CG developers might happen without documentation. This highlights the necessity to confirm our results with data from other sources such as questionnaires or interviews.
Data were gathered from a large number of HTA reports which makes the results for the MS case strong. MS was considered an appropriate example for this analysis due to the divergence in HTA recommendations and large number of expensive disease‐modifying treatments that rapidly entered the market. 4 , 5 , 15 Results may not be transferable to other indication areas. For example, in the Netherlands, the treatment pathways for oncology products are coordinated by the BOM committee (Committee for Assessment of Oncology Products), which is closely cooperating with the Dutch National Health Care Institute (ZIN) and updates statements on new treatments regularly. 48
CONCLUSIONS
Despite the regular use of the final recommendations of CGs by HTA organizations and vice versa, substantial discrepancies were observed in population definitions and treatment lines of these recommendations and decision‐making processes for MS treatments. This example may indicate that both HTA organizations and clinicians do not structurally access each other's knowledge nor may they respond in a timely manner to each other's recommendations. We propose that more systematic dialogue between the two stakeholder groups, perhaps as part of a larger ecosystem, could facilitate increased efficiency by highlighting the needs for both parties and exchange in knowledge. Improved synergy may ultimately benefit timely patient access to the right treatments.
AUTHOR CONTRIBUTIONS
M.A.H., R.A.V., A.K.M.T., and W.G.G. designed the research. M.A.H., R.A.V., A.Z., M.Z., and D.M.D. performed the research. M.A.H., R.A.V., A.Z., M.Z., D.M.D., and B.A.J. analyzed the data. M.A.H., R.A.V., A.Z., M.Z., D.M.D., B.A.J., A.K.M.T., and W.G.G. wrote the manuscript.
FUNDING INFORMATION
This research was performed as part of the HTx project. The project received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 825162. This dissemination reflects only the authors' views and the Commission is not responsible for any use that may be made of the information it contains.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the steering committee members of the H2020 HTx project, Diana Delnoij and Ayla Lokhorst, for their careful pre‐reading and thoughtful comments.
Hogervorst MA, Vreman RA, Zawada A, et al. Synergy between health technology assessments and clinical guidelines for multiple sclerosis. Clin Transl Sci. 2023;16:835‐849. doi: 10.1111/cts.13492
REFERENCES
- 1. Kristensen FB, Nielsen CP, Panteli D. Regulating the input – health technology assessment [Internet]. Improving healthcare quality in Europe: characteristics, effectiveness and implementation of different strategies [Internet]. European Observatory on Health Systems and Policies; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549272/ [PubMed] [Google Scholar]
- 2. Vreman RA, Mantel‐Teeuwisse AK, Hövels AM, Leufkens HGM, Goettsch WG. Differences in health technology assessment recommendations among European jurisdictions: the role of practice variations. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2020;23(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 3. Vreman RA, Bouvy JC, Bloem LT, et al. Weighing of evidence by health technology assessment bodies: retrospective study of reimbursement recommendations for conditionally approved drugs. Clin Pharmacol Ther. 2019;105(3):684‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Rossum T, Quik E, Goettsch W. Divergences in reimbursement of novel multiple sclerosis treatment in Europe: a review of health technology assessment decisions on the short course, oral treatment cladribine in five European countries. Utrecht University; 2019. [Google Scholar]
- 5. Dakin HL. PND105 – multiple sclerosis: the map of patient access to disease modifying treatments in Europe. Value Health. 2018;21:S346. [Google Scholar]
- 6. Lugtenberg M, Burgers JS, Westert GP. Effects of evidence‐based clinical practice guidelines on quality of care: a systematic review. BMJ Qual Saf. 2009;18(5):385‐392. [DOI] [PubMed] [Google Scholar]
- 7. Mason J, Eccles M, Freemantle N, Drummond M. A framework for incorporating cost‐effectiveness in evidence‐based clinical practice guidelines. Health Policy. 1999;47(1):37‐52. [DOI] [PubMed] [Google Scholar]
- 8. de Folter J, Trusheim M, Jonsson P, Garner S. Decision‐components of NICE'S technology appraisals assessment framework. Int J Technol Assess Health Care. 2018;34(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 9. Chalkidou K, Lord J, Obeidat NA, et al. Piloting the development of a cost‐effective evidence‐informed clinical pathway: managing hypertension in Jordanian primary care. Int J Technol Assess Health Care. 2011;27(2):151‐158. [DOI] [PubMed] [Google Scholar]
- 10. Lord J, Willis S, Eatock J, et al. Economic modelling of diagnostic and treatment pathways in National Institute for Health and Care Excellence clinical guidelines: the modelling algorithm pathways in guidelines (MAPGuide) project. Health Technol Assess. 2013;17(58): v–vi, 1–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Velasco Garrido M, World Health Organization, European Observatory on Health Systems and Policies . Health Technology Assessment and Health Policy‐Making in Europe: Current Status, Challenges, and Potential. World Health Organization on behalf of the European Observatory on Health Systems and Policies; 2008:181 (Observatory Studies Series). [Google Scholar]
- 12. Jönsson B, Oortwijn W, Rutten A, Wailoo A. Health Technology Assessment Methodology Programme Review of External Evaluation Committee ZonMw; 2015.
- 13. Guidelines International Network (GIN) . About GINAHTA Working Group — Guidelines International Network [Internet]. https://g‐i‐n.net/working‐groups/ginahta/toolkit
- 14. Andradas E, Blasco JA, Valentín B, López‐Pedraza MJ, Gracia FJ. Defining products for a new health technology assessment agency in Madrid, Spain: a survey of decision makers. Int J Technol Assess Health Care. 2008. Jan;24(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 15. Faissner S, Gold R. Efficacy and safety of the newer multiple sclerosis drugs approved since 2010. CNS Drugs. 2018;32(3):269‐287. [DOI] [PubMed] [Google Scholar]
- 16. Marziniak M, Ghorab K, Kozubski W, et al. Variations in multiple sclerosis practice within Europe – is it time for a new treatment guideline? Mult Scler Relat Disord. 2016;8:35‐44. [DOI] [PubMed] [Google Scholar]
- 17. HTx . HTx Project|Next Generation Health Technology Assessment [Internet]. 2020. https://www.htx‐h2020.eu/
- 18. Bowen GA. Document analysis as a qualitative research method. Qual Res J. 2009;9(2):27‐40. [Google Scholar]
- 19. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96‐120. [DOI] [PubMed] [Google Scholar]
- 20. European Network for Health Technology Assessment (EUnetHTA) . EUnetHTA – European Network for Health Technology Assessment [Internet]. EUnetHTA; 2004. Available from: https://www.eunethta.eu/ [Google Scholar]
- 21. Portail HAS Professionnels [Internet] . Haute Autorité de Santé. https://www.has‐sante.fr/jcms/fc_2873790/en/professional
- 22. Haute Autorité de Santé (HAS) . Actes et prestations affection de longue durée Sclérose en plaques [Internet]. Haute Autorité de Santé; 2015. https://www.has‐sante.fr/upload/docs/application/pdf/lap_ald_25_sep_actualisation.pdf [Google Scholar]
- 23. Institute for Quality and Efficiency in Health Care|IQWiG.de [Internet]. IQWIG. https://www.iqwig.de/en/
- 24. Gold R, Hemmer B, Wiendl H. DGN/KKNMS Leitlinie zur Diagnose und Therapie der MS [Internet]. Deutsche Gesellschaft für Neurologie; 2014. Available from: https://www.kompetenznetz‐multiplesklerose.de/wp‐content/uploads/2016/02/dgn‐kknms_ms‐ll_20140813.pdf [Google Scholar]
- 25. Startseite – Gemeinsamer Bundesausschuss [Internet] . https://www.g‐ba.de/
- 26. Ministerie van Volksgezondheid W en S . Home – Zorginstituut Nederland [Internet]. Ministerie van Volksgezondheid, Welzijn en Sport; 2013. Available from: https://www.zorginstituutnederland.nl/ [Google Scholar]
- 27. Nederlandse Vereniging voor Neurologie . Multipele sclerose [Internet]. Nederlandse Vereniging voor Neurologie; 2012. https://richtlijnendatabase.nl/richtlijn/multipele_sclerose/multipele_sclerose_‐_startpagina.html [Google Scholar]
- 28. de Jong BA, van Hoeve BJA, Hoitsma E, Hoogervorst ELJ, Killestein J. Ziektemodulerende behandeling van Multiple Sclerose bij volwassenen, Addendum bij de richtlijn Multiple Sclerose 2012 [Internet]. Nederlandse Vereniging voor Neurologie; 2020. https://richtlijnendatabase.nl/richtlijn/ziektemodulerende_behandeling_van_multiple_sclerose_bij_volwassenen/radiologically_isolated_syndrome.html [Google Scholar]
- 29. AOTMiT – Agencja Oceny Technologii Medycznych i Taryfikacji [Internet]. https://www.aotm.gov.pl/
- 30. Losy J, Bartosik‐Psujek H, Członkowska A, et al. Leczenie stwardnienia rozsianego. Zalecenia Polskiego Towarzystwa Neurologicznego pol Przegląd Neurol. 2016;12(2):80‐95. [Google Scholar]
- 31. Tandvårds‐Läkemedelförmånsverket – Tandvårds‐ och läkemedelsförmånsverket TLV [Internet]. https://www.tlv.se
- 32. Socialstyrelsen . Nationella riktlinjer för vård vid multipel skleros och Parkinsons sjukdom – Stöd för styrning och ledning 2016: 116.
- 33. Läkemedel [Internet] . Svenska MS‐sällskapet. https://www.mssallskapet.se/lakemedel/
- 34. National Institute for Health and Care Excellence (NICE) [Internet] . NICE. https://www.nice.org.uk/
- 35. National Health Service (NHS) . Treatment algorithm for multiple sclerosis disease‐modifying therapies [Internet]. NHS England; 2019. https://www.england.nhs.uk/commissioning/wp‐content/uploads/sites/12/2019/03/Treatment‐Algorithm‐for‐Multiple‐Sclerosis‐Disease‐Modifying‐Therapies‐08‐03‐2019‐1.pdf [Google Scholar]
- 36. Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease‐modifying treatments in multiple sclerosis. Pract Neurol. 2015;15(4):273‐279. [DOI] [PubMed] [Google Scholar]
- 37. Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9):a028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MS Treatments . EMSP overwiew [Internet]. EMSP | European Multiple Sclerosis Platform. http://www.emsp.org/about‐ms/ms‐treatments/
- 39. Hollen CW, Paz Soldán MM, Rinker JR, Spain RI. The future of progressive multiple sclerosis therapies. Fed Pract. 2020;37(Suppl 1):S43‐S49. [PMC free article] [PubMed] [Google Scholar]
- 40. Dahlke F, Arnold DL, Aarden P, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): age is a key contributor to presentation. Mult Scler J. 2021;27:2062‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162‐173. [DOI] [PubMed] [Google Scholar]
- 42. Dillon A, Barnett D, Stevens A, Longson C, Pinwill N, Boysen M. Guide to the‐single technology appraisal process [Internet]. National Institute for Health and Care Excellence; 2009. https://www.nice.org.uk/Media/Default/About/what‐we‐do/NICE‐guidance/NICE‐technology‐appraisals/Guide‐to‐the‐single‐technology‐appraisal‐process.pdf [Google Scholar]
- 43. MAGIC – Trustworthy guidelines , evidence summaries and decision aids that we can all use and share [Internet] [cited February 12, 2021]. Available from: https://magicevidence.org/
- 44. Trowman R, Powers A, Ollendorf DA. Considering and communicating uncertainty in health technology assessment. Int J Technol Assess Health Care. 2021;37(1):e74. [DOI] [PubMed] [Google Scholar]
- 45. Ofori‐Asenso R, Hallgreen CE, De Bruin ML. Improving interactions between health technology assessment bodies and regulatory agencies: a systematic review and cross‐sectional survey on processes, progress, outcomes, and challenges. Front Med. 2020;7:582634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woolf S, Schünemann HJ, Eccles MP, Grimshaw JM, Shekelle P. Developing clinical practice guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement Sci IS. 2012;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vreman RA, HGM L, Kesselheim AS. Getting the right evidence after drug approval. Front Pharmacol. 2020;11:569535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bom C. Over de commissie BOM [Internet]. NVMO; 1999. https://www.nvmo.org/bestuur‐en‐commissies/commissie‐bom/over‐de‐commissie‐bom/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.


